Highlights
-
•
For the women the older age increased the risk of developing diabetic foot.
-
•
The claw toes and calluses increased the risk of developing diabetic foot in woman.
-
•
For the men, the use of insulin increased the risk of developing diabetic foot.
-
•
The presence of sensory comorbidities and ulcers also increased the risk for men.
Keywords: Elderly, Diabetes mellitus, Gender, Risk factors, Diabetic foot
Abstract
Aims
This trial aims to identify differences between genders in relation to factors associated with the risk of diabetic foot in elderly persons.
Methods
We evaluated 187 older adults diagnosed with diabetes type 2. The variables investigated were sociodemographic data, clinical history of diabetes mellitus and complaints about the feet. The plantar sensitivity was evaluated on both feet, with the use of Semmes–Weinstein monofilaments. For data analysis we used chi-square test and binary logistic regression (p < 0.05; 95% CI).
Results
We included 174 elderly people who had no history of stroke and peripheral vascular disease. Most (58.6%) were female and among them the risk factors for diabetic foot were older age (p < 0.021; OR 6.0), presence of calluses (p < 0.046; OR 2.83) and claw toes (p < 0.041; OR 3.18). And among men, the risk factors for diabetic foot were insulin use (p < 0.008; OR 5.22), presence of sensory comorbidities (p < 0.007; OR 5.0), ulcers (p < 0.001), numbness (p < 0.002; OR 6.6) and stiffness in the feet (p < 0.009; OR 5.44).
Conclusion
The factors associated with the development of diabetic foot were presented differently in women and men, so a targeted and more specific preventive approach is required.
Introduction
The prevalence of diabetes mellitus (DM) has increased worldwide [1], reaching 13.8% in Spain [2] and 8% in Costa Rica [3]. In England, for example, more than 10% of individuals over 75 years old have DM [4]. In Brazil, about 20% of people over 65 years old are already diabetic [5]. And the National Health Survey data of 2013 show a prevalence of diabetes of 19.9% for people aged from 65 to 74 years old [6]. DM type 2 (DM2) is the most common, especially among elderly persons [7] and its development may be associated with increased population life expectancy, sedentary lifestyle and increased obesity [2].
A telephone survey held with 318 people with DM in southern Brazil pointed out that the age group, regardless of other factors, shows association with the presence of general complications, especially with retinopathy and nephropathy [8]. The DM2 complications can be micro and/or macro vascular and are resulting from risk factors such as age, time of diagnosis of disease, hyperglycemia, sedentary behavior, obesity and lack care with feet [9], as well as hereditary factors [10].
The lack of control of these factors can trigger numerous complications, including diabetic foot, a condition that meets a set of clinical and physical symptoms at the end of the lower limbs, more precisely in the foot and ankle [11]. Initially, there is an accumulation of fat in the vessels, thus reducing the blood flow [12], then a decrease in peripheral sensitivity, rapidly evolving to less vascularized areas and higher pressure, susceptible to ulcers and risk of amputations [13].
Data for people enrolled in Medicare in 2007, a health insurance system run by the United States, showed that 24.4% of total expenditure on health in the country were spent in the care of individuals with DM2 who had diabetic foot [14]. Thus, the prevention of complications in the feet should be emphasized, through the control of blood glucose and cardiovascular risk factors [15], nutritional counseling and food control, healthy lifestyle habits [16], [17] associated with more specific guidelines, such as the use of proper footwear and correct handling of the feet and nails [12].
Other demographic factors, such as sex, make the difference in the prevalence of DM [6], but can also influence the behavior of the individual in relation to foot care. Women and men with DM may differ in the way they face the disease and the way they adhere to the care necessary to keep the disease under control. Men, for example, care less for their feet [18], [19], [20], resulting in a higher proportion of amputations among them [3]. On the other hand, women have higher difficulty in maintaining glycemic and lipid control due to the difficulty of change in lifestyle, especially the adoption of an eating plan and regular physical activity [9], [21].
Given these factors, the present trial aims to answer the following question: Are there sociodemographic and clinical characteristics related to feet, which are associated with risk of developing diabetic foot differently in women and men with DM2?
Method
Trial participants
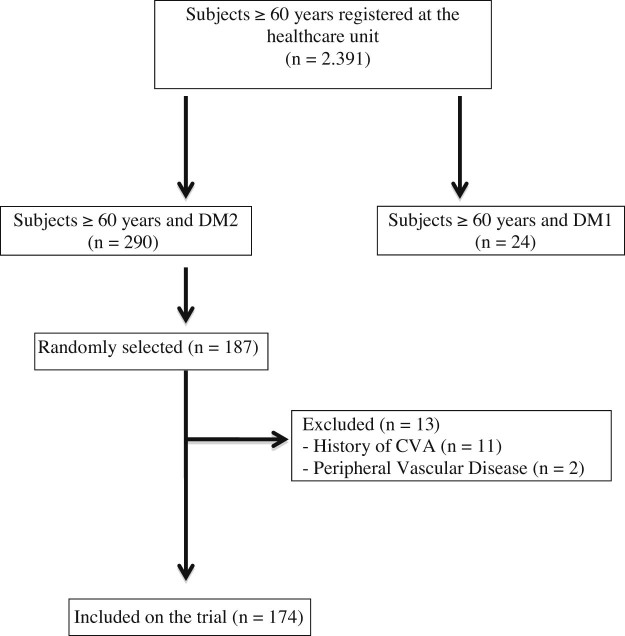
A total of 187 subjects with DM2 ≥ 60 years old were evaluated in a healthcare facility in the city of Maringá, Paraná, Brazil. Elderly people with cerebrovascular accident (CVA) history and peripheral vascular disease (PVD) were excluded, totaling 174 elderly persons for the trial (Fig. 1).
Figure 1.
Sample flowchart.
Reviews and interviews were scheduled by phone and individually performed at the facility, during 20–30 minutes. In the interview, data were collected regarding the clinical characteristics of DM2, disease history and lifestyle habits. Clinical evaluation of the feet and plantar sensitivity were conducted, aiming to trace the risk of diabetic foot development. The development of the trial met national and international standards of ethics in research involving human subjects and was approved by the Standing Committee on Ethics and Human Research of State University Maringá (No. 353 067).
Socio-demographic and clinical evaluation of DM2
Data were collected regarding age, marital status, education, number of residents in the household, individual income and health insurance. We also investigated the time of diagnosis of disease, presence of DM2 in the family (father, mother and siblings), insulin use, lifestyle habits (smoking, alcohol consumption and physical activity), and comorbidities.
We asked about the frequent use of cigarette as well as alcoholic drink. The physical activity was assessed using the International Physical Activity Questionnaire (IPAQ), which classifies the elderly person in levels of activity according to the energy expenditure during a typical week and the work-related activities, transportation, housekeeping, leisure and time sitting. When the elderly person spends more than 150 minutes in such activities, he is classified as active; when he spends less than 150 minutes is classified as insufficiently active, and when he does not perform any physical activity for at least 10 continuous minutes during the week, he is considered sedentary [22]. And the comorbidities were grouped into: Cardiovascular (heart attack history, hypertension and arrhythmia); Osteomioarticular (osteoarthritis, osteoarthritis, low back pain, osteoporosis) and Sensory (visual, auditory and vestibular impairment).
The nutritional status was determined based on the body mass index (BMI = weight/height2), which was measured in anthropometric mechanical scale and classified the elderly person as normal (BMI = 18.5 to 24.9 kg/m2), overweight (BMI = 25.0 to 29.9 kg/m2) and obese (BMI ≥ 30.0 kg/m2) [23].
Evaluation of the feet
In the evaluation of the feet, it was verified the presence or absence of corns, keratosis, cracks, mycosis, claw toes, hollow foot, flat foot and ulcers. We searched for the presence of clinical complaints from subjective data such as pain, numbness, burning, tingling in the feet, stiffness in the foot and ankle, lack of balance and lameness.
The risk of diabetic foot development was determined by plantar sensitivity, which was measured by Semmes–Weinstein monofilaments that, when applied to the foot surface, exert a force of 0.2; 2.0; 4.0; 10 and 300 g [24]. They were evaluated six plantar regions of the foot: 1st, 3rd and 5th toe, medial and lateral tarsal regions and a central point on the calcaneus [25]. The evaluation was initiated by less dense monofilament and, facing the absence of response, continued with the next and so on, until the individual accused some sensitivity [26]. It was considered loss of plantar sensitivity when the individual did not show sensitivity to the 10 g filament in one of the points assessed on any toes [27].
Defining the degree of risk for the development of diabetic foot by six-point scale: 0 – intact and preserved sensitivity, 1 – loss of plantar protective sensation, 2 – loss of plantar protective sensation and deformity/increase of plantar pressure, 3 – loss of protective sensation and plantar prior ulcer history, 4 – loss of protective sensation and history of prior ulcer with deformity or increase of plantar pressure and 5 – presence of neuropathic fracture [28].
For analysis purposes, the risk for developing diabetic foot was considered when we identified plantar sensitivity impairment (rating 1 to 5).
Statistical analysis
Sample calculation
To calculate the sample size, a prevalence of peripheral neuropathy of 50% was considered [29], an error of 5% and 1.0 of effect of design for an infinite sample, resulting in a minimum sample size of 151 elderly patients with DM2.
Data analysis
Descriptive data were used, represented by absolute and relative frequencies (average and standard deviation), besides applying Pearson's chi-square test to verify associations between covariates (sociodemographic, clinical and lifestyle characteristics of the feet and clinical complaints) and the dependent variable (risk ratings for diabetic foot), according to each gender, but the adjustment variables were not assessed. As association measure, we adopted the odds ratio (OR), and to estimate it, and its 95% confidence interval, we used the simple logistic regression function. In the analysis of contingency tables, when the expected values were less than 5, we used Fisher's exact test to estimate the p-value, but without presenting the OR and the confidence interval. All analyses were conducted using IBM SPSS Statistics 2.0 and p < 0.05 was considered for significance level.
Results
From the 174 elderly persons included in the trial, more than a half (58.6%) was female with an average age of 69.6 (±7.16) years old. The average age for men was 70.4 (±7.26) years old. In relation to sociodemographic factors, we found that women aged 80 years old and over had six times higher chance for developing diabetic foot, than those aged between 60 and 69 years old (OR: 6.0 and p < 0.021) (Table 1).
Table 1.
Association of sociodemographic factors with the risk of diabetic foot in women and men with DM2
| Sociodemographic variables | Diabetic foot risk | |||||
|---|---|---|---|---|---|---|
| Women (n = 102) | Men (n = 72) | |||||
| N (%) | P | OR (95% CI) | N (%) | P | OR (95% CI) | |
| Age (years) | ||||||
| 60–69 | 6(10.0) | – | 1 | 8(20.5) | – | 1 |
| 70–79 | 8(25.0) | 0.064 | 3.0(0.93to9.59) | 4(19.0) | 0.892 | 0.91(0.24to3.47) |
| ≥80 | 4(40.0) | 0.021* | 6.0(1.31to27.44) | 4(33.3) | 0.365 | 1.93(0.46to8.09) |
| Marital status | ||||||
| With spouse | 6(11.5) | 0.105 | 0.41(014to1.20) | 13(21) | 0.527 | 0.62(0.14to2.73) |
| Without spouse | 12(24.0) | 3(30) | ||||
| Education | ||||||
| Illiterate | 5(29.4) | 0.071** | – | 0(0) | 0.007** | – |
| IES | 13(21.0) | 8(16.7) | ||||
| CES | 0(0) | 6(66.7) | ||||
| HS/HE | 0(0) | 2(16.7) | ||||
| No. residents per domicile | ||||||
| Alone | 4(19.0) | – | 1 | 1(20.0) | – | 1 |
| With one(1) | 1(2.9) | 0.077 | 0.13(0.01to1.24) | 8(25.8) | 0.782 | 1.39(0.13to14.36) |
| With two or more | 13(27.7) | 0.451 | 1.62(0.46to5.74) | 7(19.4) | 0.977 | 0.96(0.09to10.04) |
| Income(MW) | ||||||
| 0–3 | 16(20.0) | 0.248 | 0.4(0.08to1.89) | 13(29.5) | 0.072 | 0.28(0.07to1.11) |
| ≥4 | 2(9.1) | 3(10.7) | ||||
| Health Insurance | ||||||
| Yes | 12(18.5) | 0.775 | 1.17(0.39to3.42) | 11(25.0) | 0.479 | 1.53(0.47to5.0) |
| No | 6(16.2) | 5(17.9) | ||||
IES, incomplete elementary school; CES, complete elementary school; HS/HE, high school/higher education; MW, minimum wages.
1 variable reference.
P value in Fisher's exact
Fisher's exact.
Among the clinical characteristics and lifestyle, women who reported family history of DM2 (OR: 0.22 p < 0.009) and women physically active (OR: 0.09 p < 0.002) had these conditions as a protective factor for the risk of developing diabetic foot. Among men, the use of insulin (OR: 5.22 p < 0.008) and the presence of sensory changes (OR: 5.0 p < 0.007) were associated with the risk for diabetic foot development, with equal or five times higher chances compared to those who did not use insulin and who had no sensory changes (Table 2).
Table 2.
Association of clinical characteristics and lifestyle with the risk of diabetic foot in women and men with DM2
| Clinical variables | Diabetic foot risk | |||||
|---|---|---|---|---|---|---|
| Women (n = 102) | Men (n = 72) | |||||
| N (%) | P | OR (95% CI) | N (%) | P | OR (95% CI) | |
| Diagnostic time | ||||||
| 0–4 | 3(12.0) | – | 1 | 3(20.0) | – | 1 |
| 5–9 | 4(17.4) | 0.599 | 1.54(0.30to7,78) | 3(15.0) | 0.699 | 0,70(0.12to4.11) |
| ≥10 | 11(20.4) | 0.370 | 1.87(0.47to7.42) | 10(27.0) | 0.597 | 1.48(0.34to6.37) |
| Family history | ||||||
| Yes | 5(8.6) | 0.009* | 0.22(0.07to0.69) | 5(14.7) | 0.153 | 0.42(0.13to1.37) |
| No | 13(29.5) | 11(28.9) | ||||
| Use of insulin | ||||||
| Yes | 3(12.0) | 0.399 | 0.56(0.15to2.13) | 8(47.1) | 0.008* | 5.22(1.55to17.54) |
| No | 15(19.5) | 8(14.5) | ||||
| Comorbidities | ||||||
| Yes | 15(15.6) | 0.051 | 0.18(0.03to1.00) | 13(20.0) | 0.183 | 0.33(0.06to1.67) |
| No | 3(50.0) | 3(42.9) | ||||
| Cardiovascular | ||||||
| Yes | 15(17.0) | 0.690 | 0.75(0.18to3.03) | 11(18.6) | 0.129 | 0.36(0.10to1.34) |
| No | 3(21.4) | 5(38.5) | ||||
| Osteomioarticular | ||||||
| Yes | 9(14.5) | 0.305 | 0.58(0.21to1.63) | 4(16.0) | 0.358 | 0.55(0.15to1.94) |
| No | 9(22.5) | 12(25.5) | ||||
| Sensory | ||||||
| Yes | 4(17.4) | 0.977 | 0.97(0.28to3.32) | 10(41.7) | 0.007* | 5.0(1.53to16.25) |
| No | 14(17.7) | 6(12.5) | ||||
| BMI | ||||||
| Normal | 6(22.2) | – | 1 | 5(18.5) | – | 1 |
| Overweight | 6(15.4) | 0.481 | 0.63(0.18to2.23) | 7(75.0) | 0.562 | 1.46(0.40to5.35) |
| Obesity | 6(16.7) | 0.579 | 0.70(0.19to2.47) | 4(23.5) | 0.689 | 1.35(0.30to5.96) |
| IPAQ | ||||||
| Sedentary | 8(42.1) | – | 1 | 2(20.0) | – | 1 |
| Insufficient active | 7(20.0) | 0.089 | 0.34(0.10to1.17) | 7(25.9) | 0.710 | 1.4(0.23to8.24) |
| Active | 3(6.3) | 0.002* | 0.09(0.02to0.40) | 7(20.0) | 1.0 | 1.0(0.17to5.79) |
| Smoker | ||||||
| Yes | 1(16.7) | 0.948 | 0.93(0.10to8.47) | 4(22.2) | 1.0** | – |
| No | 17(17.7) | 12(22.2) | ||||
| Alcoholic | ||||||
| Yes | 0(0) | – | – | 3(21.4) | 0.937 | 0.94(0.23to3.89) |
| No | 18(17.6) | 13(22.4) | ||||
BMI, body mass index; IPAQ, International Physical Activity Questionnaire.
1 variable reference.
P value in Fisher's exact
Fisher's exact.
Regarding the characteristics of the feet, the presence of callus (OR 2.83 p < 0.046) and claw toes (OR 3.18 p < 0.041) increased risk of diabetic foot in women. In men, the presence of ulcers was significantly associated with the risk of developing diabetic foot (Table 3).
Table 3.
Association of the characteristics of the feet with the risk of diabetic foot in women and men with DM2
| Variables related to the feet | Diabetic foot risk | |||||
|---|---|---|---|---|---|---|
| Women (n = 102) | Men (n = 72) | |||||
| N (%) | P | OR (95% CI) | N (%) | P | OR (95% CI) | |
| Calluses | ||||||
| Yes | 11(26.8) | 0.046* | 2.83(0.99to8.06) | 10(28.6) | 0.212 | 2.0(0.66to6.46) |
| No | 7(11.5) | 6(16.2) | ||||
| Keratosis | ||||||
| Yes | 11(17.5) | 0.950 | 0.96(0.34to2.75) | 12(24.0) | 0.586 | 1.42(0.40to5.02) |
| No | 7(17.9) | 4(18.2) | ||||
| Crack | ||||||
| Yes | 12(23.1) | 0.148 | 2.20(0.75to6.41) | 11(26.8) | 0.284 | 1.90(0.58to6.20) |
| No | 6(12.0) | 5(16.1) | ||||
| Mycoses | ||||||
| Yes | 12(21.4) | 0.273 | 1.81(0.62to5.29) | 11(22.0) | 0.945 | 0.96(0.29to3.18) |
| No | 6(13.0) | 5(22.7) | ||||
| Claw toes | ||||||
| Yes | 7(33.3) | 0.041* | 3.18(1.05to9.63) | 4(26.7) | 0.643 | 1.36(0.36to5.05) |
| No | 11(13.6) | 12(21.1) | ||||
| Pes cavus | ||||||
| Yes | 1(7.1) | 0.290 | 0.32(0.04to2.62) | 4(30.8) | 0.417 | 1.74(0.45to6.63) |
| No | 17(19.3) | 12(20.3) | ||||
| Flat foot | ||||||
| Yes | 4(25.0) | 0.405 | 1.71(0.48to6.09) | 0(0) | 0.215** | – |
| No | 14(16.3) | 16(23.9) | ||||
| Ulcer | ||||||
| Yes | 1(50.0) | 0.271 | 4.88(0.29to81.95) | 3(100.0) | 0.001*,** | – |
| No | 17(17.0) | 13(18.8) | ||||
P value in Fisher's exact
Fisher's exact.
Women showed no association between clinical complaints and the risk for diabetic foot, but in men, complaints about numbness (OR: 6.60 p < 0.002) and stiffness (OR: 5.44 p < 0.009) were associated with the risk for diabetic foot development, with a chance five times higher than those who did not have such complaints (Table 4).
Table 4.
Association of clinical complaints with the risk of diabetic foot in women and men with DM2
| Clinical complaints | Diabetic foot risk | |||||
|---|---|---|---|---|---|---|
| Women (n = 102) | Men (n = 72) | |||||
| N (%) | P | OR (95% CI) | N (%) | P | OR (95% CI) | |
| Pain | ||||||
| Yes | 10(18.2) | 0.878 | 1.08(0.39to3.01) | 5(25.0) | 0.725 | 1.24(0.37to4.17) |
| No | 8(17.0) | 11(21.2) | ||||
| Dormancy | ||||||
| Yes | 8(18.6) | 0.829 | 1.12(0.40to3.12) | 11(44.0) | 0.002* | 6.60(1.95to22.30) |
| No | 10(16.9) | 5(10.6) | ||||
| Burning | ||||||
| Yes | 7(17.5) | 0.975 | 0.98(0.34to2.79) | 5(25.0) | 0.725 | 1.24(0.37to4.17) |
| No | 11(17.7) | 11(21.2) | ||||
| Pricking | ||||||
| Yes | 10(21.7) | 0.329 | 1.66(0.59to4.64) | 6(31.6) | 0.258 | 1.98(0.60to6.50) |
| No | 8(14.3) | 10(18.9) | ||||
| Stiffening | ||||||
| Yes | 4(25.0) | 0.405 | 1.71(0.48to6.09) | 7(50.0) | 0.009* | 5.44(1.53to19.31) |
| No | 14(16.3) | 9(15.5) | ||||
| Lack of balance | ||||||
| Yes | 8(21.6) | 0.429 | 1.51(0.54to4.26) | 4(25.0) | 0.762 | 1.22(0.33to4.48) |
| No | 10(15.4) | 12(21.4) | ||||
| Lameness | ||||||
| Yes | 3(42.9) | 0.089 | 4.0(0.81to19.7) | 0(0) | 0.590** | – |
| No | 15(15.8) | 16(22.5) | ||||
P value in Fisher's exact
Fisher's exact.
Discussion
In this trial, factors significantly associated with the risk for diabetic foot were different in each gender. In women, older age (≥80 years), presence of calluses and claw toes were determinant in the development of diabetic foot, with a chance at least two times higher than those who did not present these factors, and, for them, the family history and physical activity level constituted protective factors. Among men, use of insulin, presence of sensory comorbidities (auditory, visual and vestibular), ulcers, numbness and stiffness in the feet were associated with a chance at least five times higher than that presented by men without the presence of these factors.
Although the average age of men was higher, women had the higher association between age and risk of developing diabetic foot (six times higher for those with 80 years or more). This follows from the fact that men have shown twice the risk for diabetic foot from the first category of age (20.5% in men aged 60–69 years old), while in women, the risk in this age group was 10%, but it increased as the age increased. Age is an important triggering factor for complications, increasing the likelihood of developing the disease [2]. In the trial conducted by Yu et al. [9], men also tended to be older.
Among women, the fact of having a family member with DM2 and practice regular physical activity was a protective factor for the risk of diabetic foot, since women typically tend to be more inactive than men [30]. However, we note that women taking part in the present trial could be in better physical/motor conditions for physical activity, whereas among men, sensory change and change in the plantar sensitivity could hinder the practice of regular physical activity.
In a trial of 207 people with type 2 diabetes and aged between 40 and 75 years old, it was observed that the average number of days of physical activity of men is significantly higher than that of women, which differs from the findings in this trial. This is probably due to the higher age, the significant presence of sensory changes and increased plantar sensitivity among men. However, the women reported performing some care, such as: examine their feet, look inside the shoes and dry the interdigital spaces, in average days higher than men [31].
The use of insulin in men increased more than five times the risk of diabetic foot. It could be due to the difficulty of adhesion to diet recommendations, glycemic control and care with the feet [9].
When care related to lifestyle, self-monitoring, pharmacological management are not adopted, the disease tends to haywire and only oral medication cannot correct insulin deficits [7]. The lack of care and seriousness with glycemic control increases the need for insulin use, which in turn can be associated with the risk of diabetic foot development. A cohort trial in Costa Rica found that menwho used insulin had 16.8 times more incidence of lower limb amputations [3].
Although the use of insulin has represented a significant factor in the risk of diabetic foot among men, it is emphasized that probably this association arises, not from an insulin-related causal effect, but rather the fact that men using insulin may have a glycemic control uncompensated for their habits and behavior, and thus, the use of insulin becomes a necessary tool for controlling the disease.
The frequency of comorbidities, often concomitant – cardiovascular, musculoskeletal or sensory – was high in both genders (over 94% in women and 90% in men). Many people with DM2 have other chronic diseases that affect the management of DM2 and the results of treatment [2]. Men with sensory impairments (hearing, visual and vestibular) had a five times higher chance to develop diabetic foot. The presence of these sensory comorbidities damages the feedback necessary to maintain the balance and motor stability during the walk and the development of some activities of daily living [32].
In women, the presence of calluses and claw toes formed factors associated with increased risk of diabetic foot. A guidance regarding this risk is necessary because these deformities alter the plantar distribution, leaving the area more susceptible to ulcers [10]. Thus, the irregular monitoring of HbA1c and use of inappropriate shoes may increase the predisposition to the development of foot complications in women with DM2 [13].
The women in this trial showed no association between clinical complaints of the feet and the risk of developing diabetic foot and although the presence of pain in the feet has appeared in more than a half of them, there was no significant relation of this variable with the loss of plantar sensitivity. This can be understood by the fact that women commit more to the care with the feet [9]. On the other hand, among men, more attention should be given to those with complaints about numbness and stiffness in the feet, who should be encouraged for the effectiveness of self-care with their health, especially regarding to care with the feet. Thus, the care with feet is an effective measure that can prevent the risk of diabetic foot and lower public spending on complications from this condition because the elderly people with DM2 have a higher risk of falls and a worse quality of life index [33].
To minimize the specific risk factors identified in this trial, it is necessary to consider the existence of differences in the practice of foot care for men and women. Even though there is no consensus on the influence of gender on self-care in diabetes [34], [35], it is possible to use different approaches to guidance and evaluation of this population in order to provide an expert assistance, able to encourage self-care and thus improve the quality of life and health of the elderly ones.
In this regard, a trial conducted in southern Brazil, with 1515 people with diabetes over 40 years old, found that among men the low-frequency of drying of the interdigital spaces is significantly higher, the non-periodic evaluation of feet, the habit of staying barefoot, the poor foot hygiene and inadequate nail cutting. Among women, most frequently not only the practice of scalding feet was observed, but also the use of inappropriate footwear [36].
Thus, it is necessary to weigh the factors that act as potential barriers to self-care in DM for men and women. It must be considered that such issues involve cultural aspects, and these in turn can influence the demand for health services. Therefore, the way different groups of people behave and self-assess their own health impacts on their morbidity and mortality profile [37].
For men, despite the major change observed in recent decades, the responsibility for the financial support of the family and the image of invulnerability and strength still falls on them. These characteristics, accepted by common sense, can influence the detachment of men from activities related to health care and even slow down or even inhibit the demand for health services, which would be closer to the female universe of habits [37]. Thus, the difficulties encountered by males are linked to man's negligence with their own health culture, which can contribute to lower effectiveness of these in performing self-care activities in DM [38].
Despite some limitations, such as the fact of evaluating elderly people belonging to a single health unit and the sample size, which did not allow a logistic regression analysis of associated factors, this trials advanced as identified clinical changes of the feet and the differences between the genders regarding the risk for the development of diabetic foot among the elderly people, pointing variables associated with each gender. Therefore, it pointed out the need for specific actions in primary care, for men and women, to prevent diabetic foot, in opposition to decontextualized and general educational approaches.
Conclusion
Based on the trial findings, there is a need for greater attention to the care performed with the feet, for elderly patients with DM. Therefore, educational programs should be implemented, aimed at the empowerment of people with DM and the individualization of treatment. The assistance plan should be centered on the person and involves the development of education activities for health based on clinical aspects of diabetic foot, and also gender and health issues. Finally, further researches, especially of qualitative nature, will enable greater understanding of the possible emotional and socio-cultural factors that impact the health and self-care of elderly men and women with diabetes.
Conflict of interest
The authors declare that they have no competing interests and declare that they have no funding.
Acknowledgments
We are grateful to all the participants in the study and health professionals, who conducted the evaluations.
References
- 1.Nyanzi R., Wamala R., Atuhaire L.K. Diabetes and quality of life: a Ugandan perspective. J Diabetes Res. 2014;1:1–9. doi: 10.1155/2014/402012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alonso-Morán E., Orueta J.F., Esteban J.I.F., Axpe J.M.A., Gonzáles M.L.M., Polanco N.T. The prevalence of diabetes-related complications and multimorbidity in the population with type 2 diabetes mellitus in the Basque Country. BMC Public Health. 2014;14:1059–1066. doi: 10.1186/1471-2458-14-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lacle A., Valero-Juan L.F. Diabetes-related lower-extremity amputation incidence and risk factors: a prospective seven-year study in Costa Rica. Rev Panam Salud Publica. 2012;32:192–198. doi: 10.1590/s1020-49892012000900004. [DOI] [PubMed] [Google Scholar]
- 4.Demakakos P., Hamer M., Stamatakis E., Steptoe A. Low-intensity physical activity is associated with reduced risk of incident type 2 diabetes in older adults: evidence from the English Longitudinal Study of Ageing. Diabetologia. 2010;53:1877–1885. doi: 10.1007/s00125-010-1785-x. [DOI] [PubMed] [Google Scholar]
- 5.Portal Brasil Elderly health. Brasília (DF) 2012. http://www.brasil.gov.br/sobre/saude/saude-do-idoso/diabetes Available in: accessed 28.2.14.
- 6.IBGE (Instituto Brasileiro de Geografia e Estatística) Pesquisa Nacional de Saúde. 2013. http://biblioteca.ibge.gov.br/visualizacao/livros/liv91110.pdf accessed 20.6.16.
- 7.Stopa S.R., César C.L.G., Segri N.J., Goldbaum M., Guimarães V.M.V., Alves M.C.G.P. Self-reported diabetes in older people: comparison of prevalences and control measures. Rev Saude Publica. 2014;48:554–562. doi: 10.1590/S0034-8910.2014048005219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santos A.L., Cecílio H.P.M., Teston E.F., Arruda G.O., Peternella F.M.N., Marcon S.S. Microvascular complications in type 2 diabetes and associated factors: a telephone survey of self-reported morbidity. Cien Saude Colet. 2015;20:761–770. doi: 10.1590/1413-81232015203.12182014. [DOI] [PubMed] [Google Scholar]
- 9.Yu M.K., Lyles C.R., Bent-Shaw L.A., Young B.A. Sex disparities in diabetes process of care measures and self-care in high-risk patients. J Diabetes Res. 2013 doi: 10.1155/2013/575814. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Formosa C., Gatt A., Chockalingam N. Diabetic foot complications in Malta: prevalence of risk factors. Foot (Edinb) 2012;22:294–297. doi: 10.1016/j.foot.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Lazo M.A., Bernabe-Ortiz A., Pinto M.E., Ticse R., Malaga G., Sacksteder K. Diabetic peripheral neuropathy in ambulatory patients with type 2 diabetes in a general hospital in a middle income country: a cross-sectional study. PLoS ONE. 2014;9(5):e95403. doi: 10.1371/journal.pone.0095403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paton J., Bruce G., Jonesa R., Stenhousea E. Effectiveness of insoles used for the prevention of ulceration in the neuropathic diabetic foot: a systematic review. J Diabetes Complications. 2011;25:52–62. doi: 10.1016/j.jdiacomp.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Kogani M., Monsournia M.A., Doosti-Irani A., Holokouie-Naiemi K. Risk factors for amputation in patients with diabetic foot ulcer in South West Iran: a matched case-control study. Epidemiol Community Health. 2015;37:1–6. doi: 10.4178/epih/e2015044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sargen M.R., Hoffstad O., Margolis D.J. Geographic variation in Medicare spending and mortality for diabetic patients with foot ulcers and amputations. J Diabetes Complications. 2013;27:128–133. doi: 10.1016/j.jdiacomp.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li R., Zhang P., Barker L.E., Chowdhury F.M., Zhang X. Cost-effectiveness of interventions to prevent and control diabetes mellitus: a systematic review. Diabetes Care. 2010;33:1872–1894. doi: 10.2337/dc10-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bantle J.P., Wylie-Rosett J., Albright A.L., Apovian C.M., Clark N.G., Franz M.J., Hoogwerf B.J. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2008;31:S61–78. doi: 10.2337/dc08-S061. [DOI] [PubMed] [Google Scholar]
- 17.Imayama I., Plotnikoff R.C., Courneya K.S., Johnson J.A. Determinants of quality of life in adults with type 1 and type 2 diabetes. Health Qual Life Outcomes. 2011;9:115–120. doi: 10.1186/1477-7525-9-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pscherer S., Dippel F.W., Lauterbach S., Kostev K. Amputation rate and risk factors in type 2 patients with diabetic foot syndrome under real-life conditions in Germany. Prim Care Diabetes. 2012;6:241–246. doi: 10.1016/j.pcd.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Monteiro-Soares M., Boyko E.J., Ribeiro J., Ribeiro I., Dinis-Ribeiro M. Predictive factors for diabetic foot ulceration: a systematic review. Diabetes Metab Res Rev. 2012;28:574–600. doi: 10.1002/dmrr.2319. [DOI] [PubMed] [Google Scholar]
- 20.Tang Z.Q., Chen H.L., Zhao F.F. Gender differences of lower extremity amputation risk in patients with diabetic foot: a meta-analysis. Int J Low Extrem Wounds. 2014;13:197–204. doi: 10.1177/1534734614545872. [DOI] [PubMed] [Google Scholar]
- 21.Schroeder E.B., Bayliss E.A., Daugherty S.L., Steiner J.F. Gender differences in cardiovascular risk factors in incident diabetes. Women's Health Issues. 2014;24:61–68. doi: 10.1016/j.whi.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsudo S., Araujo T., Matsudo V., Andrade D., Andrade E., Oliveiro L.C. International Physical Activity Questionnaire IPAC: study validity and reproducibility in Brazil. Phys Activity Health. 2001;6:5–18. [Google Scholar]
- 23.WHO . World Health Organization.; Geneva: 1998. World Health Organization obesity. Preventing and managing the global epidemic: report of a WHO consultation. (Technical report series, 894). [PubMed] [Google Scholar]
- 24.Simmons R.W., Richardson C., Pozos R. Postural stability of diabetics patients with and without cutaneous sensory deficit in the foot. Diabetes Res Clin Pract. 1997;36:153–160. doi: 10.1016/s0168-8227(97)00044-2. [DOI] [PubMed] [Google Scholar]
- 25.Souza A., Nery C.A.S., Marciano L.H.S.C., Garbino J.A. Evaluation of peripheral neuropathy: correlation between skin sensitivity of the feet, clinical and electromyographic. Acta Fisiátrica. 2005;12:87–93. [Google Scholar]
- 26.Semmens J., Weinstein S., Ghent L., Teuber H.L. 2000. Somatosensory changes after penetrating brain wounds in man. Cambridge, MA: Harvard University Press, 1960 apud Dellon AL. Evaluation of sensibility and re-education of sensation in the hand. Baltimore: Williams & Wilkins, 99–175. [Google Scholar]
- 27.Tuttle L.J., Hastings M.K., Mueller M.J. A moderate-intensity weight-bearing exercise program for a person with type 2 diabetes and peripheral neuropathy. Phys Ther. 2013;92:133–141. doi: 10.2522/ptj.20110048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sims D.S., Cavanagh P.R., Ulbrecht J.S. Risk factors in the diabetic foot. Recognition and management. Phys Ther. 1988;68:1887–1902. doi: 10.1093/ptj/68.12.1887. [DOI] [PubMed] [Google Scholar]
- 29.Guangren L., Chenglin S., Yanjun W., Yujia L., Xiaokun G., Ying G. A clinical and neuropathological study of Chinese patients with diabetic peripheral neuropathy. PLoS ONE. 2014;9:1–5. doi: 10.1371/journal.pone.0091772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mendes T.A.B., Goldbaum M., Segri N.J., Barros M.B.A., Cesar C.L.G., Carandina L. Diabetes mellitus: factors associated with prevalence in the elderly, control measures and practices, and health services utilization in São Paulo, Brazil. Cad Saude Publica. 2011;27:1233–1243. doi: 10.1590/s0102-311x2011000600020. [DOI] [PubMed] [Google Scholar]
- 31.Daniele T.M.C., Vasconcelos J.P., Coutinho F.G. Self-care assessment of patients with type 2 diabetes in a primary care unit. Cinergis. 2014;15(3):135–139. [Google Scholar]
- 32.Lin S.I., Chen Y.R., Chou C.W. Association between sensorimotor function and forward reach in patients with diabetes. Gait Posture. 2010;32:581–585. doi: 10.1016/j.gaitpost.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Chiba Y., Kimbara Y., Kodera R., Tsuboi Y., Sato K., Tamura Y. Risk factors associated with falls in elderly patients with type 2 diabetes. J Diabetes Complications. 2015;29:898–902. doi: 10.1016/j.jdiacomp.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 34.Tol A., Shojaeezadeh D., Sharifirad G., Alhani F., Tehrani M.M. Determination of empowerment score in type 2 diabetes patients and its related factors. J Pak Med Assoc. 2012;62:16–20. [PubMed] [Google Scholar]
- 35.Clark M.L., Utz S.W. Social determinants of type 2 diabetes and health in the United States. World J Diabetes. 2014;5:296–304. doi: 10.4239/wjd.v5.i3.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rossaneis M.A., Haddad M.C.F.L., Mathias T.A.F., Marcon S.S. Differences in foot self-care and lifestyle between men and woman with diabetes mellitus. Rev Lat Am Enfermagem. 2016;24:e2761. doi: 10.1590/1518-8345.1203.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levorato C.D., Mello L.M., Silva A.S., Nunes A.A. Fatores associados a procura por serviços de saúde numa perspectiva relacional de gênero. Cien Saude Colet. 2014;19:1263–1274. doi: 10.1590/1413-81232014194.01242013. [DOI] [PubMed] [Google Scholar]
- 38.Cortez D.N. 2016. Avaliação da efetividade do programa de empoderamento para o autocuidado em diabetes mellitus na atenção primária. Tese de doutorado; Programa de pós graduaçao em enfermagem. Universidade Estadual de Minas Gerais. [Google Scholar]