Abstract
Bone is one of the major organs of the human body; it supports and protects other organs, produces blood cells, stores minerals, and regulates hormones. Therefore, disorders in bone can cause serious morbidity, complications, or mortality of patients. However, despite the significant occurrence of bone diseases, such as osteoarthritis (OA), osteoporosis (OP), non-union bone defects, bone cancer, and myeloma-related bone disease, their effective treatments remain a challenge. In this review, we highlight recent progress in the development of nanotechnology-based drug delivery for bone treatment, based on its improved delivery efficiency and safety. We summarize the most commonly used nanomaterials for bone drug delivery. We then discuss the targeting strategies of these nanomaterials to the diseased sites of bone tissue. We also highlight nanotechnology-based drug delivery to bone cells and subcellular organelles. We envision that nanotechnology-based drug delivery will serve as a powerful tool for developing treatments for currently incurable bone diseases.
Introduction
Advances in the field of nanotechnology have revolutionized traditional therapies by improving the effectiveness of drug delivery strategies [1]. Given that human tissues comprise hierarchical structures at the nanoscale, nanomaterials (ranging from 1 to 100 nm in size) have the advantage of size similarity to interact with, and modulate biological components [2]. For example, nanomaterial-based drug delivery systems have improved targeting because of their small size, which allows them to traverse biological barriers, such as the small intestine, nasal and oral mucosa, and skin, for more efficient delivery [3]. As such, nanomaterials can sequentially target from tissues to cells to organelles to deliver drugs [4]. In addition, nanotechnology-based drug delivery systems can be functionalized to sense, diagnose, image, and deliver therapeutics by conjugating moieties, such as peptides, aptamers, and small molecules [5].
These nanotechnology-based drug delivery strategies can be applied for treating bone diseases that are difficult to treat with conventional clinical therapies. These diseases encompass several skeletal-related disorders, such as OA, OP, nonunion defects, bone cancer (metastatic and primary bone cancer), and myeloma-related bone disease [6–12]. In the USA, approximately 52.5 million adults have clinical OA, which has become the leading bone disease causing disabilities [6,7]. OP is another concern, because approximately 10.2 million adults aged 50 years and older had OP in 2014, and 43.4 million adults who have low bone mass are under increased risk of developing OP [8]. Bone defects and non-union bone defects remain a challenge, because up to 100000 fractures end with non-union in the USA [9]. Primary bone cancer is the bone disease with the highest mortality; according to the report of cancer statistics in 2015, approximately 1490 deaths occurred in the 2970 cases of newly diagnosed bone and joint cancer [10]. In addition, according to data provided by the American Cancer Society, approximately 11170 men and 9010 women are diagnosed with multiple myeloma every year, and associated myeloma-related bone disease makes treatment more complex because of the accompanying dysfunction of bone formation and resorption [11,12].
Several limitations of current treatments for bone diseases include: wide excisions of tissue [13] (in cases using amputation surgery), low targeting efficiency and adverse effects on other organs and tissues (in cases using chemotherapy and radiation therapy) [14], short plasma half-life and poor physicochemical stability in biodistribution (in cases using biomacromolecular drugs), and insufficient bone graft sources and risk of infection and host immune responses (in cases using bone grafts) [15].
To overcome the limitations of previous approaches for treating orthopedic diseases, targeted delivery using nanomaterials is a potential solution to increase therapeutic efficiency and reduce adverse effects. Generally, the advantages of applying nanomaterials include: (i) enlarged drug-loading capacity with large surface area:volume ratios [16,17]; (ii) increased drug solubility in conjugation with delivery vehicles [18,19]; (iii) promoted drug stability by providing a protective shield to increase drug retention time [20]; (iv) targeted delivery and on-demand release after fabrication with stimuli-responsive functional groups and reduced systemic adverse effects on other tissues or organs [21–23]; and (v) enhanced transport ability across cell membranes, enabling intracellular drug delivery or delivery to specific organelles [24,25].
Nanomaterials also have unique advantages in treating bone diseases. Given that bone tissue comprises inorganic minerals and organic matrices that are assembled at the nanoscale, nanoparticles can assimilate into the bone microenvironment to approach and cure diseased bone [26,27]. In addition, the nanodrug delivery vehicles, such as calcium phosphate (CaP) nanoparticles (CPNs), gold nanoparticles (GNPs), and nanodiamonds (NDs), can stimulate new bone growth by stimulating mineralization or promoting bone cell activity [28,29]. Furthermore, the intracellular targeting drug delivery based on nanotechnology can enhance the treatment efficiency of bone diseases via the precise delivery of drugs to subcellular regions. As a result, intracellular targeted delivery shows great potential to solve multidrug resistance, a longstanding challenge for cancer chemotherapy [30,31]. Taken together, functionalized nanomaterials can specifically accumulate in diseased bone and target cells and further translocate to target organelles, delivering therapeutic agents to the pathological site with high efficacy and specificity.
In this review, we discuss the recent progress in the development of nanodelivery systems for treating bone diseases. First, we review the classification and applications of using different types of nanomaterial-based drug delivery system to heal damaged/diseased bone. Then we discuss nanotechnology-based drug delivery strategies that can benefit the local drug accumulation and retention in the bone tissue. We go on to emphasize nanotechnology-based intercellular targeting and delivery strategies that depend on the functionalities of organelles. Lastly, to improve clinical translation in this field, we discuss the need for an improved understanding of the safety and toxicity of nanomaterials.
Types of nanomaterials
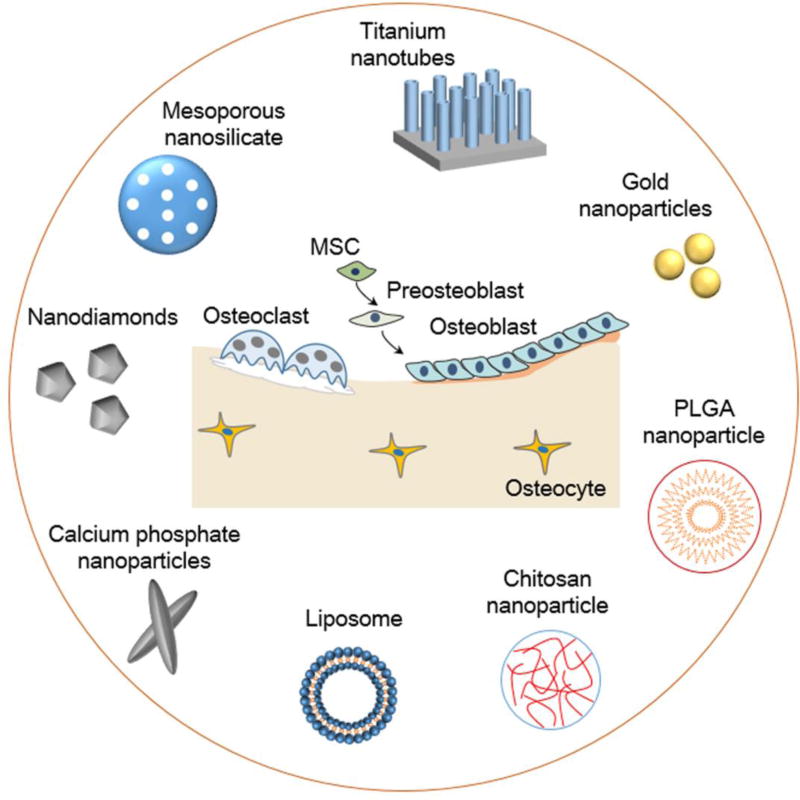
Nanomaterials have unique physical and chemical properties for drug delivery applications, including: high surface:volume ratio for efficient drug loading, ease of functionalization with biological targeting moieties, and small size to overcome tissue barriers for more effective targeting [32]. In recent years, the development of inorganic and organic nanomaterials has led to novel drug delivery strategies for treating bone diseases (Figure 1).
Figure 1.
Examples of nanomaterials for bone drug delivery. The inorganic nanomaterials include titanium nanotubes, gold nanoparticles, calcium phosphate nanoparticles, and mesoporous silica nanoparticles. The organic nanomaterials include chitosan nanoparticles, poly(L-lactide-co-glycolide) (PLGA) nanoparticles, and liposomes. These nanomaterials can selectively target bone tissues and cells to deliver drugs. Abbreviation: MSC, mesenchymal stem cell.
Inorganic nanomaterials
Given that bone largely comprises inorganic materials, such as hydroxyapatite [HA: Ca10(PO4)6(OH)2] and whitlockite [Ca18Mg2(HPO4)2(PO4)12] [33], inorganic nanomaterials have gained attention for bone drug delivery for their structural and mechanical similarity to the target tissue [32]. Inorganic nanomaterials, such as mesoporous silica nanoparticles (MSNs), titanium nanotubes (TNTs), GNPs, NDs, and CPNs, have unique physicochemical properties for novel applications in drug delivery.
Mesoporous silica nanoparticles
MSNs have advantageous properties for drug delivery applications, especially given their unique mesoporous structure (up to 10–20 nm pore size [34]), allowing for a high surface area for efficient drug loading [35]. In particular, MSNs are a promising therapeutic for the treatment of OP because they can act as both a drug carrier [36] and a bone bioactive agent [37]. The treatment of this disease can be divided into two approaches: accelerating bone formation by upregulating osteoblast (OB) activity, and reducing bone resorption by downregulating osteoclast (OC) activity [38]. MSNs can be incorporated with calcium ions to form mesoporous bioactive glass nanospheres (MBGs), using well-established sol-gel and supramolecular surfactant chemistry techniques [37]. For example, Fan et al. used an alkali morphological catalyst in a multi-step sol-gel synthesis method to obtain bioactive glass nanoparticles of uniform shape and size of <60 nm in diameter [39]. These MBGs can promote bone formation, because the release of calcium ions has been shown to support OB proliferation and differentiation [37]. In addition, MSNs can reduce OC activity by delivering silencing RNA (siRNA) to downregulate genes related to osteoclastogenesis [40]. For instance, the work of Kim et al. highlights the use of MBGs to deliver siRNA to downregulate the expression of the OC inducer receptor activator of nuclear factor kappa B (RANK) [40]. The in vitro release profile of MBGs demonstrated that the nanoparticles could release siRNA linearly for a period of over 3 days. In addition, RAW 264.7 cells treated with siRNA-MBGs showed significantly improved siRNA uptake efficiency (>72%) compared with free siRNA (<1%), which showed almost no fluorescent signal of intracellular localization. Furthermore, genes involved in osteoclastogenesis [cfos, TRAP, NFATc1, and cathepsin-K (CTSK)] were significantly downregulated, demonstrating MBGs as a tool to decrease excessive OC-mediated bone loss for future therapeutics [40].
Titanium nanotubes
Titanium metals are commonly used for the fabrication of bone implants in the form of screws, plates, and pins, based on their mechanical strength, high biocompatibility, and resistance to corrosion [41]. The integration of the bone implant with the surrounding tissue is crucial for tissue repair, because a lack of osseointegration can lead to bone infection requiring resurgery or amputation. Using nanofabrication techniques, titanium bone implants can be modified with hierarchical structures to achieve nanoscale surface roughness to increase the interaction with the surface adherent cells and, thus, to promote osseointegration [42]. This nanoscale architecture can further improve bone tissue formation by promoting cell adhesion, proliferation, cell spreading, and OB differentiation [43]. To enhance the surface roughness of titanium implants, the surface of these metals can be modified to produce TNTs, using a simple and scalable anode oxidation treatment method [44]. Owing to their high surface area and drug-loading capacity, TNTs have also gained attention for drug delivery applications [45].
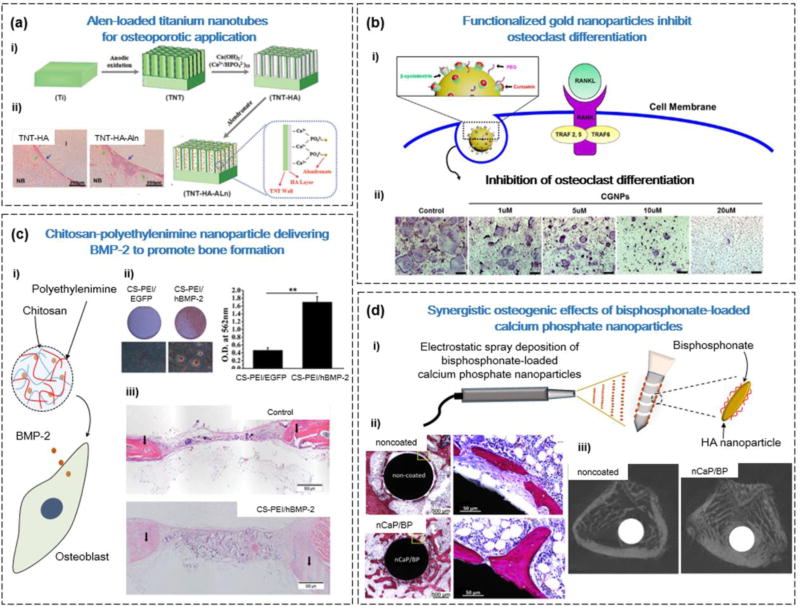
Previous studies have demonstrated that the antiresorptive drug alendronate (Alen) can promote new bone formation by stimulating OB proliferation and/or differentiation, and inhibiting OC function [46]. However, there is a need to develop novel materials for the controlled release of this drug because of toxicity issues at high doses [46]. Shen et al. sought to use TNTs loaded with Alen to improve osseointegration and bone formation for diseases such as OP (Figure 2a) [47]. The surface of TNTs was coated with nano-HA (nHA) to facilitate cell attachment and improve drug-loading efficiency through the formation of covalent bonds between Ca2+ ions of HA and PO34− groups in Alen. The in vitro results demonstrated that the Alen-loaded TNT-nHA substrate could sustain the release of Alen for over 500 h, which in turn promoted OB proliferation and/or differentiation and downregulated OC differentiation. To confirm these effects in vivo, TNT-nHA-Alen implants were implanted in an OP rabbit model. After 3 months, the bone volume and total trabecular thickness were significantly improved in TNTs functionalized with nHA and Alen compared with control (TNTs only) [47].
Figure 2.
Types of nanomaterial for applications in bone drug delivery. (a) Alendronate (Alen)-loaded titanium nanotubes (TNTs) were able to promote bone formation in a rabbit osteoporosis model. (i) Fabrication of hydroxyapatite (HA)-coated TNTs for local delivery of Alen. (ii) Hemotoxylin and eosin (H&E) images of TNT-HA and TNT-HA-Alen, showing more newly formed bone in the TNT-HA-Alen group (blue arrows). (b) Delivery of the drug curcumin using βCD-functionalized gold nanoparticles (GNPs) to inhibit osteoclast differentiation. (i) Schematic of the GNPs with βCD-curcumin inclusion complexes for intracellular delivery of the drug. (ii) Dose-dependent reduction of TRAP expression with administration of GNPs functionalized with βCD-curcumin. (c) Delivery of the gene encoding bone morphogenetic protein 2 (BMP2) using chitosan (CS)-polyethylenimine (PEI) nanoparticles to promote bone formation. (i) CS-PEI nanoparticles can efficiently transfect osteoblasts with the BMP2 gene. (ii) CS-PEI/BMP-2 promotes mineralization, determined by Alizarin red staining 21 days after transfection. Mineralization was quantified by adding cetylpyridinium chloride. (iii) Implantation of the CS-PEI/BMP-2 collagen scaffolds promoted new bone formation, determined by H&E staining. (d) The coating of bone implants with calcium phosphate (CaP) nanoparticles (CPNs) and bisphosphonates (BPs) can improve their integration with bone tissue. (i) Using the electrospray deposition technique, the surface of bone implants can be functionalized with CPNs and BP. (ii) Histological sections of bone tissue demonstrated that CPN and/or BP functionalized implants form direct contact with bone tissue. Scale bars = 500 Mm (left) and 50 Mm (right). (iii) Micro-CT images of bone tissue after 4 weeks with noncoated and coated bone implants demonstrated improved bone volume percentage. Reproduced, with permission, from [47] (a), [50] (b), [67] (c), and (d) [110].
Gold nanoparticles
GNPs show potential for bone-targeted drug delivery because of their ease of functionalization with drugs, targeting moieties, and polymers on gold-thiol bonds [48], as well as their intrinsic ability to upregulate OB activity [49] and downregulate OC differentiation [50,51]. In particular, GNPs can downregulate OC formation by inhibiting the function of the osteoclastogenesis promoter, RANK ligand (RANKL), and by reducing reactive oxygen species (ROS) levels [52]. In addition, GNPs can apply mechanical stress on the membranes of mesenchymal stem cells (MSCs) to activate the mechanosensitive p38 mitogen-activated protein kinase (MAPK) pathway to induce osteogenic differentiation [49]. Therefore, in cases where OC activity and bone resorption are pathologically increased, such as in OP, GNPs can be used to promote homeostasis in bone remodeling.
In addition to the osteogenic capabilities of GNPs, they can also be functionalized with drugs or other biological molecules for drug delivery applications [48]. For instance, the drug curcumin (CUR) has a range of pharmacological effects, including the suppression of osteoclastogenesis by inhibiting RANKL-induced nuclear factor (NF)-κB [53]. However, CUR has poor stability in vivo and can readily degrade at physiological pH [54]. To improve CUR delivery, Heo et al. developed GNPs functionalized with β-cyclodextrin (βCD), which can stabilize CUR through the formation of βCD-CUR inclusion complexes (Figure 2b) [50]. The in vitro results demonstrated that the GNP-βCD-CUR could inhibit the OC differentiation of bone marrow-derived macrophages (BMM). Furthermore, in an ovariectomy-induced (OVX-induced) OP mouse, the application of GNP-βCD-CUR prevented bone loss and increased bone dimensions in trabecular bone, because of the improved intracellular delivery of GNP-loaded CUR [50].
To improve tissue-specific delivery and reduce toxicity issues, GNPs can also be functionalized with biological targeting moieties. Lee et al. sought to functionalize GNPs with Alen to target drugs specifically to bone tissue [51]. The ex vivo results demonstrated the specific binding of GNPs linked to Alen (GNP-Alen), because a large percentage of the GNPs immobilized to the tibia of mice compared with GNPs alone. They also demonstrated that GNP-Alen had a greater inhibitory effect on OC-related genes (TRAP, OSCAR, cFos, and NFATc1) compared with GNPs or Alen alone [51].
Nanodiamonds
NDs are octahedral, nanoscale carbon allotropes that have gained attention for applications in regenerative medicine because of properties such as: high surface area, ease of surface functionalization, and excellent biocompatibility [55]. The surface of NDs can be modified with various functional groups (e.g., carboxyl groups) without compromising the ND core, allowing for the linkage of proteins and polymers [55]. Similar to GNPs, NDs have been suggested to have a positive role in OB proliferation and differentiation [56]. To leverage these results, Ryu et al. designed ND conjugated with Alen to deliver the drug to enhance bone cell activity and tissue repair [57]. To determine the applicability of NDs for bone drug delivery, they conducted affinity tests and determined that Alen-ND could bind favorably to the bone mineral HA, through interaction with HA and Alen. The in vitro results also demonstrated that Alen-NDs could preferentially bind to MC3T3-E1 murine OBs compared with HepG2 hepatocytes or NIH3T3 fibroblasts, and also enhanced the alkaline phosphatase (ALP) expression of the OBs. Also, when Alen-NDs were injected intravenously into the rat tail vein, Alen-NDs deposited in bone tissue with high targeting efficiency. The authors hypothesized that the accumulation of NDs in bone to increase mechanical strength, and the delivery of Alen, could promote synergistic bone formation for future treatments of OP [57].
Calcium phosphate nanoparticles
CPNs have gained special interest for bone-related applications because of their superior biocompatibility, biodegradability, and similarity in structure to the inorganic composition of bone minerals [58]. CPNs have been widely used for applications in gene delivery, because they can stabilize DNA or RNA through electrostatic interactions between the positive calcium ions and negatively charged nucleotides of these molecules [59]. This stabilizing structure can prevent the degradation of nucleotides by plasma nucleases, and promotes the internalization of the former into cells [58]. However, a major disadvantage of CPNs is that their spontaneous aggregation can result in reduced transfection efficiency for gene therapy applications [60].
Using an aqueous, low-temperature, rapid precipitation technique, Curtin et al. formulated homogenous (<200 nm), nonagglomerating nHA to bind and carry the gene encoding bone morphogenetic protein 2 (BMP2) for stem-cell mediated bone formation [61,62]. Compared with Lipofectamine 2000, a polymer-based transfection method, nHA was less cytotoxic. In addition, nHA showed superior transfection efficiency compared with commercial CaP transfection kits. In 2D culture, the transfection of nHA-BMP-2 in rabbit MSCs (rMSCs) resulted in a significant increase in calcium deposition compared with controls (nHA or cells alone). As an application of these nHA particles, the authors developed a novel local gene delivery platform by incorporating nHA-BMP2 into a porous collagen scaffold to act as a ‘gene-activated matrix’. The results demonstrated an improved osteogenic capability of this scaffold, as indicated by increased osteocalcin expression and calcium deposition [61].
Organic nanomaterials
Polymeric nanoparticles are a widely used organic nanomaterial for drug delivery applications, particularly because of their tunable biodegradability and release kinetics [32]. Examples of organic nanomaterials for bone drug delivery include chitosan [CS: β-(1–4)-2-amino-2-deoxy-D-glucose] and poly(L-lactide-co-glycolide) (PLGA) nanoparticles. Lipid nanoparticles (LNPs) represent another class of biodegradable, biocompatible, and functional nanomaterials comprising solid and liquid-state lipids for use in bone drug-delivery applications [63].
Chitosan nanoparticles
CS is a naturally occurring polysaccharide, derived from the deacetylated form of chitin [64]. CS-based nanoparticles are effective gene carriers, because the positively charged amine backbone of CS can permit strong electrostatic interactions with negatively charged nucleotides to protect it from nuclease degradation [65]. However, because of the low cell internalization efficiency of CS nanoparticles, they must be modified before use as an effective gene therapy vector [66]. To accomplish this, Zhao et al. (Figure 2c) combined CS nanoparticles with polyethylenimine (PEI) to improve the delivery of the human BMP2 gene (hBMP2) [67]. PEI is an efficient gene carrier because it can escape lysosomal degradation by hyperosmotic rupturing through the proton sponge effect [68], but is disadvantaged because of its cytotoxicity [67]. To overcome this limitation of PEI, Zhao et al. determined that an ideal ratio of 20:1 of CS to PEI retained the low cytotoxicity of CS, as well as the benefits of increased transfection efficiency of PEI. To determine its physiological effects, the authors transfected MC3T3-E1 murine OBs with CS-PEI/hBMP-2, and demonstrated that markers of osteogenesis (ALP, Sp7, and Col1) were upregulated at various time points. In addition, an in vivo bone defect model in rats demonstrated that CS-PEI/hBMP-2 could promote greater bone formation 12 weeks post implantation, compared with the nontreatment control [67].
In addition, CS can be modified with sulfate groups to produce a polysaccharide similar in structure to heparin, which can bind favorably to the basic amino acid stretches of BMP-2. This interaction can enhance the bioactivity of BMP-2 for bone repair, by improving its encapsulation and sustained release [69,70]. For instance, Cao et al. devised a photocrosslinked hydrogel incorporated with 2-N,6-O-sulfated CS nanoparticles for the controlled delivery of rhBMP-2 [69]. The aim of this system was to sustain the release of BMP-2 over a long period of time, because ineffective release can cause chronic inflammation and heterotopic ossification. By combining BMP-2/CS and photocrosslinked hydrogel, the delivery of BMP-2 could be sustained for over 42 days in vivo, and new bone formation significantly increased as early as 2 weeks [69].
Poly(L-lactide-co-glycolide) nanoparticles
PLGA nanoparticles are widely used for drug delivery applications because of their ease of functionalization with targeting ligands [71], tunable drug release kinetics [72], and low toxicity [73]. PLGA nanoparticles can also be used for the delivery of bone-related hormones and proteins, such as parathyroid hormone (PTH) [74] and BMP-2 [75], by providing protection against enzymatic degradation in vivo and improving diffusion across biological carriers [76]. PLGA nanoparticles can also reduce the systemic adverse effects associated with the administration of antiresorptive drugs, such as Alen, through controlled delivery of small doses at the site of administration [77]. The rate of drug release from PLGA nanoparticles can also be regulated by controlling the co-polymer ratio between lactide and glycolide, and the molecular weight of the polymer [72].
These nanoparticles can also be combined with biodegradable scaffolds to deliver therapeutics at the site of injection for an extended period of time. For example, Posadowska et al. combined PLGA nanoparticles encapsulating Alen within a gellan gum (GG) hydrogel [78]. They demonstrated that the GG-PLGA-Alen hydrogel was injectable and capable of restoring mechanical properties after extrusion. This GG-PLGA-Alen hydrogel also promoted drug release over a period of 25 days. In addition, GG-PLGA-Alen could reduce OC formation by inhibiting the ability of RAW264.7 to fuse and flatten, demonstrating that it could act as a nanocarrier for Alen [78].
Lipid nanoparticles
LNPs are rigid, uniform nanocarriers comprising solid and liquid state lipids, which can be formed into a core-shell or homogenous particle structure. LNPs represent a diverse class of lipid-based nanocarriers, including: solid lipid nanoparticle (SLN) carriers, nanostructured lipid carriers (NLCs), lipid drug conjugate (LDC) carriers, and lipid nanocapsule carriers (LNCs) [63]. SLN carriers are produced using hot pressure homogenization methods and are the most commonly used formulation of LNPs [79]. NLCs contain both liquid and solid phase lipids, which can promote increased payload storage [63,80]. LDC carriers can also promote increased drug storage through the covalent linkage of the drug to the lipid backbone [81]. Finally, LNCs have a core-shell structure, with a semiliquid core and solid external lipid layer [82]. Owing to their superior biocompatibility, kinetic and physical stability, LNPs have been widely applied as nanocarriers for drug delivery [63].
LNPs are an effective tool for the delivery of nucleic acids for in vivo gene therapy because of their small size (1– 100 nm), which allows them to cross bone sinusoids and to deliver a large quantity of nucleotides into the cell through endocytosis [83,84]. For example, Basha et al. investigated LNPs for the delivery of siRNA to downregulate the expression of sclerostin (SOST) [85]. The protein SOST is secreted by osteocytes and inhibits OB differentiation by inhibiting the function of the BMP-Wnt pathway. Previous studies have shown that administration of antibodies against SOST increased bone mineral density and bone formation [85]. To validate the physiological effects of the LNP-siRNA system, these authors administered these nanoparticles to primary mouse embryonic fibroblasts, and determined that SOST was downregulated, and the expression of ALP increased over 1600-fold. The knockdown efficiency of the LNPs was confirmed in vivo, because a systemic injection in mice reduced SOST expression [85].
Strategies for delivery
Nanomaterials show promise for bone tissue repair and regeneration, because they are able to efficiently load drugs and target the diseased site. The methods of drug delivery to bone tissue include: oral delivery, implant-based delivery, injectable delivery, and transdermal delivery (Figure 3).
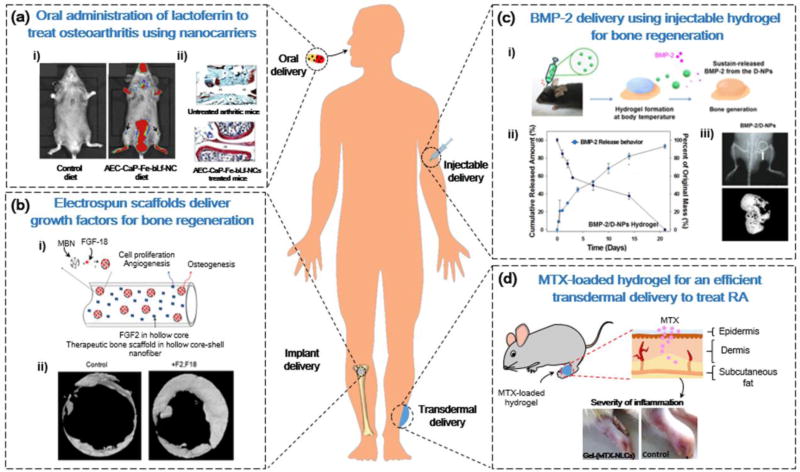
Figure 3.
Nanotechnology-based administration strategies for bone drug delivery. (a) Chitosan (CS) nanocarriers for the oral delivery of iron-saturated bovine lactoferrin for osteoarthritis treatment. (i) Fluorescence intensity of a Cy5.5-labeled nanocarrier comprising an alginate-enclosed CS-calcium phosphate (CaP) nanocarrier, encapsulated with Fe-bLf (AEC-CaP-Fe-bLf-NC) demonstrated localization in joint cartilage (arrows). (ii) Histological analysis of joints illustrated that AEC-CaP-Fe-bLf-NC improved cartilage regeneration and had antiarthritic effects. (b) Electrospun nanofibrous scaffolds for the dual delivery of growth factors to promote bone regeneration. (i) Sequential release of FGF-2 from the core-shell structure of nanofibrous scaffold, followed by release of FGF-18 from mesoporous silica nanoparticles. (ii) Micro-CT analysis of improved bone formation after dual delivery of FGF-2 and FGF-18 from a nanofibrous scaffold. (c) Injectable delivery of bone morphogenetic protein 2 (BMP-2) using thermosensitive hydrogels. (i) Thermosensitive poly (phosphazene) hydrogels promoted BMP-2 release from dual-interacting polymeric nanoparticles (D-NPs) at physiological temperatures. (ii) Cumulative release of BMP-2 from D-NPs in vitro. (iii) X-ray of the site of injection of hydrogels, and micro-CT analysis of new bone formation. (d) Transdermal delivery of methotrexate (MTX)-loaded lipid nanocarriers (LNCs) for rheumatoid arthritis treatment. Local application of gel containing MTX-loaded LNCs on rat paws reduced the severity of inflammation. Reproduced, with permission, from [92] (a), [104] (b), [113] (c), and [115] (d).
Oral delivery
Oral delivery is a convenient and noninvasive route for the administration of drugs, but issues such as enzymatic degradation in the gastrointestinal (GI) tract, and limited permeation across the mucosal layer need to be solved to realize the full benefits of this approach [86]. Nanoparticles have been explored as a potential solution because of their ability to cross the intestinal epithelium, either passively or using GI-specific ligands, such as lectin and M cell targeted antibodies [86].
Celecoxib is an orally administered, anti-inflammatory drug, used to treat OA and rheumatoid arthritis (RA) [87]. However, high doses of this drug are required to circumvent its low solubility and variable absorption rate, which can lead to toxicity issues [88]. To solve this issue, Bachar et al. devised a celecoxib-loaded β-casein nano-sized micelle to reduce the toxicity of this drug and to improve its dispersibility [87]. By loading celecoxib in β-casein micelles, the authors improved its solubility and stability, and demonstrated a loading efficiency of over 25% by weight [87]. Given that β-casein is defined as generally recognized as safe (GRAS), it is applicable for oral delivery in future clinical applications to treat bone diseases. β-casein can also naturally degrade in the stomach or through interaction with the GI tract [87].
Another challenge of oral administration is the poor bioavailability of peptide and protein drugs caused by reduced stability, poor uptake across epithelial cells of the GI tract, and limited lipid solubility [86]. To solve these issues, SLNs were introduced as a drug carrier system for oral delivery because of their stabilizing effect on peptides [89]. For instance, salmon calcitonin (sCT), a peptide-based drug, is used for the treatment of postmenopausal OP and Paget’s disease. However, the nasal administration of sCT can cause irritation, and the alternative use of oral bisphosphonates can cause adverse intestinal effects [90]. Chen et al. sought to develop an oral delivery system by loading SLNs with sCT using a micelle-double emulsion technique using the lipids stearic acid (SA), tripalmitin (TP), or a combination of both [91]. They observed in vivo that the SLN-sCT prepared with SA and TP had increased bioavailability when administered to the duodenum of mice, demonstrating its applicability for oral delivery to the GI tract [91].
In another approach, Samarasinghe et al. developed a nanocarrier to deliver iron-saturated bovine-lactoferrin (Fe-bLf) in an oral route using natural biomaterials, such as alginate, CS, and CaP (Figure 3a) [92]. The protein Fe-bLf has previously demonstrated anti-inflammatory and immunomodulatory properties by inhibiting the activity of natural killer cells, increasing antioxidant levels, and replenishing lymphocyte levels [93]. Therefore, Fe-bLf could reduce the progression of OA by modulating the inflammatory response. By combining Fe-bLf with an alginate-CS-CaP nanocarrier (Fe-bLf-Alg-CS-CaP-NC), the authors controlled the release of Fe-bLf for a period of 24 h [92]. The application of Fe-bLf-Alg-CS-CaP-NC to chondrocytes pretreated with interleukin (IL)-1β (to induce apoptosis and mitochondrial stress) reduced hypoxia-inducible factor (HIF-2α) levels by 20%, demonstrating its immunomodulatory effects. In addition, the disease-modifying effects were demonstrated in vivo in mice, because oral supplements of Fe-bLf-Alg-CS-CaP-NC downregulated the expression of the proinflammatory cytokines IL-6, IL-1β, and tumor necrosis factor (TNF)-α by 4.3-fold, 8.3-fold, and 33.3-fold, respectively [92]. These results demonstrate that the oral delivery of therapeutic peptides using biocompatible NC is a favorable approach for the treatment of OA.
Implant-based delivery
Although bone tissue is a dynamic structure that has the ability to heal after fractures, larger defects resulting from critical injuries, disease, or malformations often require bone grafts [94]. The current gold standard for bone grafts is an autologous graft from the iliac crest, but disadvantages of this approach include donor site morbidity and limited tissue availability [94]. These limitations have prompted the development of biodegradable and bioactive 3D nanostructured scaffolds that mimic bone and, thus, can induce the recruitment and migration of host cells for tissue repair [32]. In addition, these scaffolds can serve as a drug delivery vehicle for controlled drug release to guide cellular processes [32]. However, because of their large volume, they must be implanted to the target site in an invasive manner [32]. Several materials have been developed for the fabrication of these scaffolds, including: peptide amphiphiles, electrospun nanofibres, and CaP nanomaterials.
Peptide amphiphiles (PAs) are short peptide sequences attached to a hydrophobic alkyl tail that can self-assemble into nanofibres in solution. This process is driven by hydrophobic interactions of the alkyl trail and the hydrogen bonding of the peptides [95]. PAs can be formulated with epitopes for the binding of proteins [96] or drugs [97] on the peptide sequence. This can allow for the presentation of a high density of ligands for cell receptor binding, as well as for controlling the release of covalently bound drugs [97–100].
PAs can promote the controlled delivery of growth factors from synthetic grafts by providing a platform for ligand-specific binding [101]. To improve the bone-forming abilities of collagen sponges, Lee et al. devised a heparin-binding PA that can bind to the heparin-sulfate domains of BMP-2 [101]. Collagen sponges cannot specifically bind to BMP-2 or BMP-7; thus, their use as a delivery agent is limited because of issues of rapid burst release, potentially causing soft-tissue hematomas [102]. The authors demonstrated that the PA nanofibres could reduce the proportion of BMP-2 released over 24 h (22.7%) compared with gels without heparin sulfate functionalization (45.4%). They also applied this nanofiber gel to collagen sponges in vivo, and determined that the volume of new bone formation in a rat defect gap increased significantly compared with collagen sponges and BMP-2 alone [101].
The electrospinning of polymers to produce nanofibres is another technique for developing scaffolds for drug delivery applications. The process of electrospinning involves applying an electrical solution to draw a polymer solution from a nozzle onto a collector plate [32]. This solution can then be used to fabricate a variety of structures, with properties such as high surface area, high porosity, and structural similarity to extracellular matrix (ECM) [103]. For instance, Kang et al. fabricated polycaprolactone (PCL) scaffolds with a core-shell morphology to sequentially deliver fibroblast growth factor-2 (FGF-2) and fibroblast growth factor-18 (FGF-18) for bone repair (Figure 3b) [104]. The core-shell morphology of these scaffolds produces a hollow shell with high surface area for the efficient loading of drugs. In the scaffold, the authors added mesoporous bioactive nanoparticles (MBNs) loaded with FGF-18, as well as free FGF-2 in the core shell. The FGF-2 was released initially from the core shell to stimulate proliferation of cells for repair, followed by FGF-18 from the MBNs, which promoted osteogenesis through the upregulation of BMP-2 expression. After implanting this scaffold in rat calvarium defects, the authors determined that both bone volume and bone surface density increased compared with controls [104].
Implants have been successfully applied for promoting the rehabilitation of bone tissue, notably in cases such as hip replacements [105]. However, this method is limited for patients with diseases such as OP, which limits the osseointegration and biological fixation of implants [106]. To promote these factors, implants can be coated with materials such as CaP, which can promote a healing response [107] and the colonization of MSCs [108]. In particular, the electrospray deposition technique can be used to coat CPNs on implants, which can then be combined with drugs, peptides, or growth factors [109]. For instance, Alghamdi et al. fabricated titanium implants sprayed with CPNs and Alen to promote implant integration and fixation. At 4 weeks post implantation in an OP rat model, the combination of CPNs and Alen improved bone volume formation and bone-implant contact, compared with CPNs or Alen alone (Figure 2d) [110].
Injectable delivery
Hydrogels are soft, elastic, 3D polymer networks that have found many applications in regenerative medicine for their similarity to the ECM, such as scaffolds for cells, barriers for wound healing, and cell transplantation [111]. One of these applications includes drug delivery, because of their tunable characteristics, such as cross-linking density, degradation rate, and swelling rate, allowing for controlled drug release kinetics [111]. In particular, there has been considerable interest in developing in situ-forming hydrogels for local, injectable delivery of drugs [112]. These materials can be applied in the clinic in a minimally invasive injection method, and can take on the shape of the wound cavity to which they are applied. Therefore, injectable in situ-forming hydrogels could solve the disadvantage of implant-based delivery methods, which require invasive surgeries to be placed in the target site [112].
For instance, Seo et al. devised an in situ-forming thermosensitive poly (organophosphazene) hydrogel with dual-interacting nanoparticles (D-NPs) loaded with BMP-2 for bone delivery (Figure 3c) [113]. The D-NPs can interact with BMP-2 through two mechanisms: (i) hydrophobic interactions with the hydrophobic loops of BMP-2; and (ii) electrostatic interactions between the negatively charged ionic groups of D-NPs, and the positively charged BMP-2 protein. This strong interaction can allow for more sustainable BMP-2 release from the hydrogel. This sustainable release was demonstrated in vitro, because the BMP-2/D-NP hydrogels slowed the BMP-2 release rate compared with BMP-2 linked to nanoparticles that could not bind electrostatically. In addition, because of the thermosensitive properties of the hydrogel, the hydrogel–nanoparticle–protein composite was able to form a gel when exposed to physiological temperatures in the body. The authors demonstrated in vivo that an orthotopic injection to the calvarial bone of mice could increase bone volume and thickness [113].
Transdermal delivery
NLCs have been studied for applications in transdermal delivery because of their physical stability, ability to penetrate skin efficiently, and drug release capabilities [114]. The work of Garg et al. demonstrated the ability of NLCs to deliver methotrexate (MTX) transdermally as a treatment for RA (Figure 3d) [115]. The drug MTX can reduce proinflammatory cytokine levels that can cause joint destruction in RA, but intra-articular delivery of this drug limits its concentration at RA sites because of the rapid exit of the drug from joint cavity sites. To improve local delivery of this drug, the authors formulated a gel incorporating NLC-MTX and the chemical permeation enhancer (CE), α-terpineol [Gel-(MTX-NLC+CE)]. The application of this gel to the paws of RA mice significantly reduced inflammation, paw thickness, and levels of proinflammatory cytokines [IL-1β, IL-6, (Matrix metalloproteinase-1) MMP-1, and TNF-α] in serum and synovial fluid of RA mice [115].
Approaches for cellular delivery
For drug delivery applications to bone, nanomaterials can be targeted to specific cells (i.e., Obs or OCs) or to subcellular organelles [i.e., autophagosomes, endoplasmic reticulum (ER), or lysosomes] to enhance their therapeutic efficiency while reducing adverse effects. Therefore, the modification of nanomaterials with biological targeting ligands (e.g., peptides, aptamers, and small molecules) can improve the targeting efficacy of drugs [116].
Peptide ligands
The modification of nanomaterials with small peptides is a promising approach for cell targeting because of their high ligand avidity, easy bioconjugation, and low immunogenicity [117]. For instance, Sun et al. used the phage display technique to identify a five amino-acid long motif (SDSSD) with high binding affinity to human and mouse OBs [118]. Specifically, they used mass spectrometry and pulldown assays to determine that the SDSDD peptide could bind to periostin, an OB-specific cell surface protein. To apply this for the OB-specific delivery of drugs, the authors linked this peptide to a polyurethane (PU) nanomicelle to deliver an oligonucleotide to block the effects of miRNA-214 (anti-miRNA-214), which is known to have OB-inhibiting effects [119]. The effect of cellular internalization of the SDSDD-PU nanomicelle in OBs was confirmed by the co-localization of DMP1 (an OB marker) and fluorescently labeled FAM-siRNA. To determine the physiological effects of SDSDD-PU-anti-miRNA in vivo, they injected the nanomicelles into OVX-induced OP rats, and demonstrated that it could improve bone microarchitecture and bone mineral density [118].
Another approach for OB-specific targeting is demonstrated in the work of Zhang et al., who used (AspSerSer)6 as a targeting moiety to bind small, misaligned HA crystals on bone-forming surfaces [120]. Given that cells of the osteogenic lineage are favorably recruited to bone-forming surfaces, this approach could be used to deliver drugs specifically to these cell types [120]. In addition, the authors linked (AspSerSer)6 to DOTAP-based cationic liposomes [DOTAP-siRNA-(AspSerSer)6] to deliver a siRNA to downregulate the expression of casein kinase-2 interacting protein-1 (CKIP-1). The protein CKIP-1 has been shown to interact cooperatively with Smad ubiquitylation regulatory factor 1 (Smurf1), which is a negative regulator of osteogenesis [121]. Additionally, DOTAP-siRNA-(AspSerSer)6 nanoparticles co-localized in vivo with ALP, Runx2, and Col1A1 (markers of osteogenic cells) expression in rats, but not with OC-associated receptor (OSCAR) expression found in pre-OC and mature OC. To determine the physiological effects of this system in vivo, the authors injected DOTAP-siRNA-(AspSerSer)6 into the tail vein of OVX-induced OP rats, and found that it significantly improved bone mineral density and the 3D architecture of the proximal tibia. Furthermore, the DOTAP-siRNA nanoparticles also increased cellular internalization of the siRNA compared with siRNA alone, enhancing its physiological effects by improving knockdown efficiency of the target gene. The work of Zhang et al. offers an advantage over bone targeting using Alen, a traditional bisphosphonate that is not able to differentiate between bone-forming and resorbing surfaces [120].
In addition to OB targeting, peptide moieties can also be used to target OCs. The D-Asp8 peptide has previously been demonstrated to bind favorably to bone-resorbing surfaces, where OCs are responsible for degrading the bone matrix [122]. Liu et al. sought to use this D-Asp8 peptide linked to DOTAP-based liposomes to deliver a modulator of miR-148a (antagomiR-148a), for the treatment of OP [123]. One of the functions of miR-148a is to regulate osteoclastogenesis [124], and downregulation of this miRNA with antagomiR-148a can reduce bone resorption. The authors determined that the D-Asp8-conjugated liposomes could more effectively target bone tissues compared with liposomes without the peptide moiety. These liposomes also demonstrated cell-specific delivery of antagomiR-148a to OCs. Using the fluorescence-activated cell sorting technique, they determined that the miR-148a knockdown efficiency was significantly greater in OSCAR-positive cells (i.e., OCs) compared with OSCAR-negative cells (i.e., non-OCs). The in vivo results demonstrated that the linked liposomes with antagomiR-148a significantly attenuated the decrease of bone mineral density and bone volume in OVX-induced mice [123].
Aptamer ligands
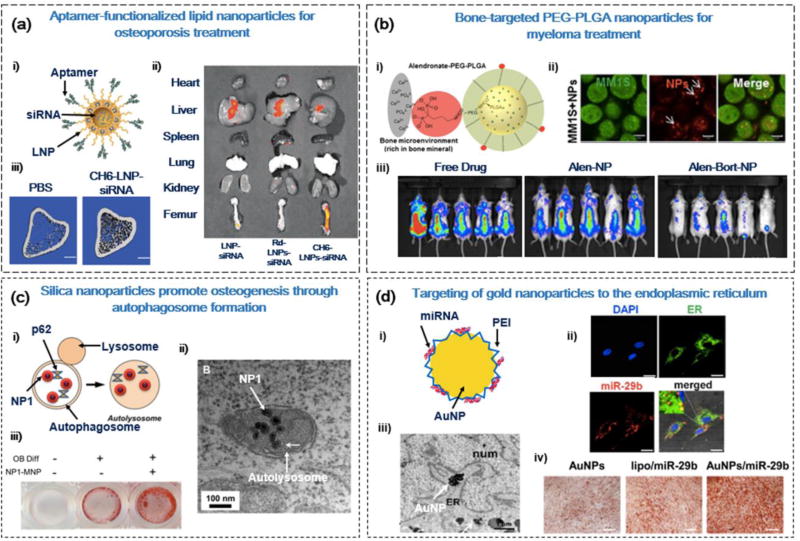
Aptamers are short, single-stranded DNA or RNA sequences that can be applied as targeting moieties because of their versatility and specificity. Their versatility allows them to bind to a variety of targets, including peptides, small molecules, or whole cells. Their specificity arises from the Systematic Evolution of Ligands by Exponential Enrichment (SELEX) method used to purify aptamers for ligand-specific binding [116]. A study by Liang et al. explored the ability of aptamers to target OBs at a cell-specific level (Figures 4a and 5) [125]. Using the SELEX method, they positively selected aptamers that could bind to OBs with high affinity. The specificity of the aptamer to OBs ensures that endothelial cells and lymphocytes residing at the bone surface are not targeted, reducing adverse effects on nonskeletal tissues. In addition, the authors conjugated the OB-specific aptamer (CH6) to LNPs for the delivery of siRNA to downregulate CKIP-1. The conjugation of the CH6 aptamer to LNPs also promoted its internalization by OBs through both clathrin-mediated endocytosis and macropinocytosis. To determine the physiological effects of the CH6-siRNA-LNP system, the authors administered the LNPs into OVX-induced OP rats. The CH6-siRNA-LNP treatment group showed significantly enhanced mineral apposition rate, bone formation rate, and OB number compared with siRNA or LNPs administered alone [125].
Figure 4.
Cellular and subcellular delivery strategies for bone disease therapy. (a) Aptamer-functionalized lipid nanoparticles (LNPs) for the anabolic treatment of osteoporosis (OP). (i) LNPs functionalized with aptamer (CH6) for osteoblast (OB)-specific delivery of casein kinase-2 interacting protein-1 (CKIP-1) small interfering (si)RNA. (ii) CH6-LNP-siRNA can preferentially accumulate in bone tissue (femur), indicated by fluorescent intensity of Cy3-labeled siRNA. (iii) The 3D microarchitecture of proximal tibia was improved 14 weeks after the addition of CH6-LNP-siRNA in OP rats. Scale bar = 1 mm. (b) Bone microenvironment-targeted delivery of bortezomib for myeloma therapy. (i) Poly(L-lactide-co-glycolide) (PLGA)-polyethylene glycol (PEG) nanoparticles functionalized with alendronate (Alen) for bone-specific targeting. (ii) Intracellular uptake of Alexa647-labeled nanoparticles in GFP+MM1S cells. Scale bar = 5 Mm (iii) Pretreatment of bortezomib-load PLGA-PEG-Alen nanoparticles reduced tumor burden in mice after 29 days. (c) Silica nanoparticles promoted osteogenesis via the stimulation of autophagy. (i) Silica nanoparticles interact with autophagy factors (p62) to stimulate autolysosome formation. (ii) TEM images of silica nanoparticles (NP1) localized in autolysosome structures. Scale bar = 1 Mm (iii) Alizarin red S staining of MC3T3-E1 OBs after 14 days of treatment with NP1. (d) Targeting of miR-29b-loaded gold nanoparticles (GNPs) to the endoplasmic reticulum (ER) for promoting osteogenic differentiation. (i) GNPs functionalized with polyethylenimine (PEI) for intracellular delivery of miR-29b. (ii) Immunofluorescence staining of nuclei (blue), ER (green), and Cy3-miR-29b (red), illustrating the localization of GNPs/Cy3-miR-29b to the ER of hMSCs. Scale bar = 25 Mm. (iii) TEM images of GNPs agglomerated in the ER of hMSCs after 14 days. Scale bar = 1 Mm. (iv) Alizarin red staining of MC3T3-E1 OBs after 21 days of treatment with GNPs/miR-29b. Reproduced, with permission, from [125] (a), [129] (b), [131] (c), and [138] (d).
Figure 5.
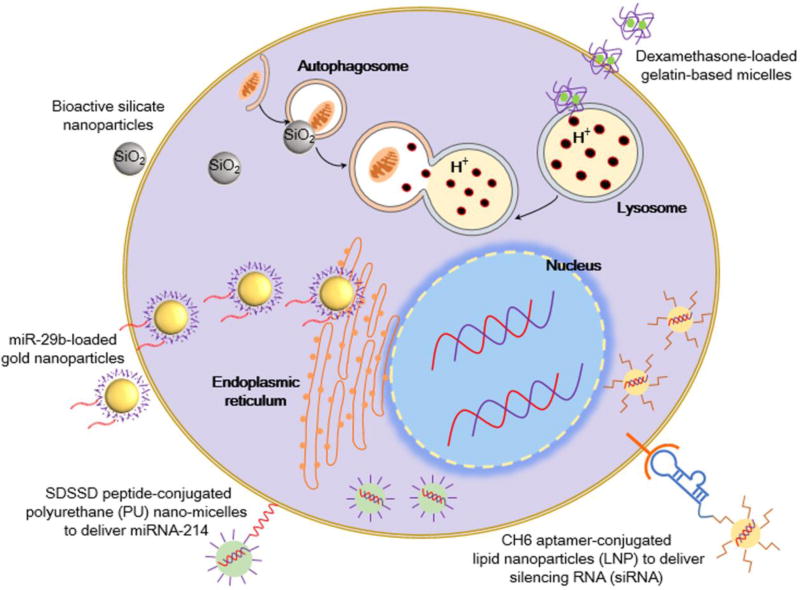
Intracellular delivery strategies for bone drug delivery. Nanoparticles can be functionalized with ligands, such as the SDSDD peptide or CH6 aptamer, to promote cell-specific delivery through ligand–receptor binding at the cell surface [118, 125]. The use of pH-sensitive nanomaterials, such as gelatin-based micelles, can promote targeted drug release after internalization in acidic lysosomes [143]. Gold nanoparticles can deliver miRNA and accumulate in the endoplasmic reticulum for enhanced osteogenesis [138]. The stimulation of autophagosome formation by bioactive silica nanoparticles can also promote osteogenesis [131].
Small molecule ligands
The class of small molecules known as bisphosphonates can be applied as bone-targeting moieties, particularly because of their ability to bind bone through the interaction of the P-C-P bonds of bisphosphonates to the calcium crystals in HA [84]. Bisphosphonates are also approved for clinical usage to treat bone disorders, such as OP, Paget’s disease [126], and metastatic bone tumors [127]. Bisphosphonates can also act as a therapeutic agent to interfere with the resorption activity of OCs by blocking the mevalonate pathway, which is essential for OC function [128]. Therefore, the efficient drug-loading capacity of nanoparticles can be combined with the targeting ability of bisphosphonates to improve the pharmacokinetics and bioavailability of bone-related drugs.
For instance, Swami et al. sought to improve the efficacy of bortezomib, an antitumor agent with toxic and neuropathic adverse effects, to treat multiple myeloma and bone metastasis (Figure 4b) [129]. They loaded the bortezomib drug into PLGA-polyethylene glycol (PEG) nanoparticles functionalized with Alen. The nanoparticles could efficiently bind to HA crystals and enter myeloma cells, as determined using flow cytometry of fluorescently labeled nanoparticles. In addition, the tumor growth slowed significantly in rats with the application of PLGA-PEG-Alen nanoparticles, which also localized more specifically to bone tissue compared with free drug or PLGA-PEG nanoparticles [129].
Subcellular targets
Nanotechnology has the potential to reduce the systemic adverse effects associated with traditional medicine by targeting drugs and therapeutics to their site of action. In addition to targeting specific organs and tissues, current research has moved towards subcellular targeting, which has potential to increase the drug therapeutic index through delivery to specific organelles. For example, the targeting of organelles, such as the autophagosome, ER, and lysosome, can improve the treatment efficacy of bone diseases (Figure 4).
Autophagosome
The autophagosome is a double-membrane vesicle compartment formed by at least 32 different autophagy-related proteins (Atg) that work together to sequester and degrade waste to reutilize molecules and energy [130,131]. During the process of autophagy, cytoplasmic contents, such as dysfunctional organelles, intracellular pathogens, or targeted proteins, are enclosed in the autophagosome, which then fuses with the acidic lysosomal compartment to break down these cellular products for recycling [132,133]. Recent studies have demonstrated that autophagy is closely linked to bone cell function in both normal physiology and disease. For instance, the autophagy factors Atg5/7/4B and LC3 regulate the generation of the OC ruffled border [134], and a knockdown of the autophagy regulatory protein FIP2000 in mice leads to OP [135].
Given that autophagy is linked to bone physiology, Ha et al. sought to formulate bioactive silica nanoparticles that can stimulate osteogenic activity in OBs through interaction with autophagy factors (Figures 4c and 5) [131]. At the size of approximately 50 nm, these bioactive silica nanoparticles can efficiently enter mesenchymal-derived cells through caveolae-dependent endocytosis. This process, mediated by caveolin proteins, occurs through the formation of caveosomes at the cell surface to internalize cargo to the smooth ER or Golgi [136]. When caveolae-dependent endocytosis was blocked by methyl-beta-cyclodextrin and nystatin inhibitors, internalization of these nanoparticles in OBs was completely prevented. In addition, transmission electron microscopy (TEM) imaging analysis clearly showed autophagosome and autolysosome localization of nanoparticles. These silica nanoparticles had strong affinity with key autophagy-related proteins: microtubule-associated protein 1 light chain 3 (LC3β-II) and signaling adaptor/scaffold protein (p62). The interaction between these nanoparticles and proteins was reduced when the early stage of autophagosome formation was blocked using a 3-methyladenin (3-MA) inhibitor. The sequestration of nanoparticles to autophagy vesicles also stimulated the formation of autophagosomes by the ERK1/2 signaling pathway. When preosteoblasts were grown in the presence of these silica nanoparticles, nanoparticles were localized in autolysosome structures and the gene expression levels of LC3bII and p62 increased. Furthermore, osteogenic differentiation was accelerated with increased ALP activity and mineralization [131].
Endoplasmic reticulum
The ER is an organelle structure involved in the synthesis, maturation, and export of proteins [137,138]. In the presence of unfolded proteins in the ER lumen, ER stress transducers clear these peptides via the unfolded protein response (UPR) pathway [139]. The protein cAMP-responsive element binding protein 3 like 1 (OASIS) was identified as one of these stress transducers, and has been closely linked to the regulation of osteogenesis. OASIS stimulates the production of Col1A1, and a knockdown of OASIS in mice leads to severe OP and reduction in OB activity [139]. Furthermore, studies have shown that nanoparticles (e.g., silver nanoparticles) can also transduce ER stress [140]. To leverage these results, Pan et al. developed miR-29b-loaded GNPs that can agglomerate and exert stress in the ER of human MSCs (hMSCs) and MC3T3-E1 OBs, as confirmed by TEM and fluorescent imaging (Figures 4d and 5) [138]. The GNPs were also functionalized with miR-29b, a miRNA that can downregulate known inhibitors of osteogenesis (e.g., HDAC4, TGF-β3, CTNNBIP1, etc.). The miR-29b-loaded GNPs showed a positive osteogenic effect on hMSCs and MC3T3-E1 cells, with improved ALP activity and calcium deposition. The authors hypothesized that miR-29b loaded GNPs could have a synergistic effect on osteogenesis by the combined effect of miR-29b and ER stress [138].
Lysosome
The lysosome has a crucial role in the cell for clearing waste, regulating plasma membrane repair, and maintaining cholesterol homeostasis. The lysosome is also the most common end point for internalized extracellular debris during endocytosis and, therefore, is a preferred intracellular target for drug delivery using nanoparticles [141,142]. Given that the lysosome has an acidic environment compared with its neutral surroundings, nanocarriers can be modified to provide the pH-sensitive release of drugs once they are internalized by cells [141]. For example, Santo et al. devised a pH-sensitive gelatin-based micelle loaded with dexamethasone, an anti-OP drug (Figure 5) [143]. When placed in a citrate buffer of pH ~4.0 in vitro, the gelatin micelles exhibited a significant increase in particle distribution index (PDI) and destabilization of the micelle structure, suggesting its use for pH-sensitive drug release. Furthermore, in vitro results demonstrated that close to 100% of the gelatin micelles were internalized in rat bone marrow MSCs (rBMSCs) and MC3T3-E1 OBs. The biological assessment of the nanomicelles in vivo showed a dose-dependent effect on new bone volume, mineralized tissue, and the production of osteoid and ECM.
In another study, Sun et al. formulated pH-sensitive MSNs loaded with doxorubicin (DOX) for the treatment of bone cancer metastasis [144]. In addition, they linked the MSNs to zoledronate (ZOL), a previously established moiety for bone metastasis targeting. The DOX-MSN-ZOL exhibited pH-sensitive drug release, because there was an improved cumulative drug release of 38% at pH 5.0, compared with 10% at pH 7.4. The authors hypothesized that this accelerated drug release was caused by the protonation of the amino group of DOX at acidic pH. Furthermore, fluorescent staining demonstrated that DOX-MSN-ZOL co-localized in lysosomes, and that this signal intensity increased with incubation time in A549 cells in vitro. As a model of cancer cell metastasis, the authors performed a wound-healing migration assay, and determined that DOX-MSN-ZOL had the greatest ability to reduce cancer cell migration [144].
Concluding remarks and outlook
Nanomaterial drug delivery systems have demonstrated their tremendous potential to treat bone diseases because of their versatility for conjugating secondary functional groups, the ability to traverse to the diseased site in bone, and tunable drug release kinetics. These promising delivery systems can be built on a spectrum of organic and inorganic materials, and fabricated with a plethora of techniques based on surface modification and bioconjugation. As a result, multiple choices of delivery vehicles and administration strategies are emerging to treat bone diseases, providing many possibilities for future personalized medication. Simultaneously, a higher therapeutic index can also be expected, because functionalized nanomaterials have the ability to target and deliver drugs precisely into subcellular regions.
Nanomaterial-based drug delivery systems are revolutionizing traditional drug delivery in orthopedic disorders both in terms of efficacy and safety based on their high targeting efficiency. Despite the impressive progress made by nanotechnology for treating bone disease, most of the outcomes reviewed here are in the early research stages. Critical challenges remain, such as a lack of understanding of nanotoxicity, insufficient drug-loading capacity, low delivery efficiency, and inflexibility of drug release kinetics, which are making nanomaterial-based drug delivery systems hard to translate into the clinic.
Understanding of nanotoxicity
The toxicity of nanomaterials remains a longstanding challenge, because nanoparticles can cause both chemical and physical damage to healthy cells. For instance, overproduction of ROS can result in vitro apoptosis by free radical formation, peroxidative product accumulation, and cell antioxidant depletion [145]. Nanomaterials can also cause cell dysfunction by physically binding to biological molecules in an uncontrolled manner, inducing cell death by random membrane insertion, and physically blocking microcirculation [145]. Therefore, a more comprehensive and systematic understanding of cellular responses when cells encounter nanoparticles is required.
Enhancing drug-loading capacity, release kinetics, and delivery efficiency
Although nanomaterials have a large surface area for high loading capacity, the weight proportion of the drug is usually <5% of the carrier [146]. In this regard, either the drug concentration is not sufficient to reach a therapeutic level, or a greater amount of carrier nanomaterials is required, which can lead to systemic toxicity. In addition, when the drug molecules are only physically absorbed or weakly bonded to the surface of the nanomaterial, rapid release of the incorporated drug can occur immediately after administration. Thus, a large fraction of loaded drugs will be dispersed and cannot efficiently reach the pharmacological target in vivo. Consequently, low therapeutic efficiency requires repeated administration and larger doses, resulting in more adverse effects. Therefore, there is an urgent need to increase the loading efficiency and promote the physiochemical stability of the drug–nanocarrier complex. Recently, a report of the efficiency of administered nanoparticles determined that only 0.7% (median) of administered nanoparticles could be found at the tumor site [147]. The authors hypothesized that most of the injected nanoparticles are trapped and eliminated in the liver, spleen, and kidneys as foreign substances. Therefore, future research should also put more effort into understanding the interactions of nanoparticles with other organs to reduce nanoparticle filtration before they arrive at bone tissue.
Achieving controllable multiphase drug release kinetics
The development of nanomaterials for sequential and temporally controlled delivery is gaining popularity, particularly because multiphase drug release kinetics can improve the therapeutic index. This approach is based on the principle that sequential release and scheduling of combinatorial drugs in situ can improve drug synergism [148]. Multiphasic drug delivery systems can respond to external cues through self-regulated stimuli for responsive drug delivery. The most common types of external stimulus that can be applied include: temperature, electricity, magnetic fields, as well as ultrasound [23,149], which can be applied to treat other disease, but will be particularly beneficial for bone treatment in terms of stimulation efficiency. This is because bone is deeply embedded into soft tissue and the hard bone minerals require a stimulus with excellent penetration ability to reach the deep diseased site. By contrast, self-regulated delivery systems are designed to automatically respond to information in dynamic biological systems [150,151], such as endogenous pH, temperature, or specific molecular presence in the local microenvironment. However, delays in sensor response should be addressed between actual diseased site and the values of information sensed in the tissues [152], especially in the delicate bone microenvironment.
Designing multifunctional nanoparticles
Multifunctional nanoparticles can combine various therapeutic agents to act together on the biological target, enhancing the therapeutic index compared with the addition of an individual drug. For instance, co-delivery of an anticancer drug and DNA intercalating agent could harness the synergistic therapeutic activity of drug and gene loads [153]. By using a single delivery vehicle, the co-delivery strategy can have advantages, such as synchronized pharmacokinetics, as well as the delivery of refined doses of drug and genes to the same cell subpopulation [153]. However, to realize the potential of co-delivery systems, several scientific challenges still need to be addressed. For example, it should be ensured that multiple therapeutic agents work cooperatively while avoiding inhibitory effects in terms of functionality. Multifunctional nanoparticles also refer to the associations of diagnostic and therapeutic agents [154], wherein additional functionalities, such as image contrast enhancement, are included in the nanoparticles. This strategy, termed ‘theragnostics’, can accomplish the precise diagnosis of disease and delivery of therapeutic agents to the disease site simultaneously. Furthermore, there are several limitations to overcome, including sophisticated fabrication steps to create additional functionality, more complicated effects and behavior in vivo to be understood, and greater regulatory hurdles [154].
Despite the many hurdles in the road to clinical translation, nanomaterial-based drug delivery systems remain a promising approach to cure bone disease based on their high targeting and delivery efficiency. The main advantage of applying nanomaterials is that they can be designed and devised with precise functional moieties to deliver to the unique bone microenvironment and subcellular compartment. We are looking forward to the continued efforts and sharing of expertise from a multidisciplinary team of clinicians and scientists to overcome the current limitations. In the coming decades, nanotechnology will remain a rapidly expanding field and more promising outputs will be translated. With continually emerging novel technologies, nanomaterial-based drug delivery systems have the potential to cure currently incurable bone diseases.
Highlights.
The most representative nanomaterials for bone drug delivery are summarized.
Nanomaterials can target bone by oral, implant, injectable, and transdermal route.
Nanodelivery systems can target bone cells and subcellular organelles.
Outlooks and hurdles for clinical translation of nanodelivery systems are discussed.
Acknowledgments
This work was supported by the National Institutes of Health (AR057837, DE021468, HL099073, AR070647), and the Presidential Early Career Award for Scientists and Engineers (PECASE).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baglioni P, et al. Nanomaterials in art conservation. Nat. Nanotechnol. 2015;10:287–290. doi: 10.1038/nnano.2015.38. [DOI] [PubMed] [Google Scholar]
- 2.Jiang W, et al. Nanoparticle-mediated cellular response is size-dependent. Nat. Nanotechnol. 2008;3:145–150. doi: 10.1038/nnano.2008.30. [DOI] [PubMed] [Google Scholar]
- 3.Hubbell JA, Chilkoti A. Nanomaterials for drug delivery. Science. 2012;337:303–305. doi: 10.1126/science.1219657. [DOI] [PubMed] [Google Scholar]
- 4.Ma X, et al. Future of nanotherapeutics: targeting the cellular sub-organelles. Biomaterials. 2016;97:10–21. doi: 10.1016/j.biomaterials.2016.04.026. [DOI] [PubMed] [Google Scholar]
- 5.Lee DE, et al. Multifunctional nanoparticles for multimodal imaging and theragnosis. Chem. Soc. Rev. 2012;41:2656–2672. doi: 10.1039/c2cs15261d. [DOI] [PubMed] [Google Scholar]
- 6.Neogi T, Zhang Y. Epidemiology of osteoarthritis. Rheum. Dis. Clin. North Am. 2013;39:1–19. doi: 10.1016/j.rdc.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbour KE, et al. Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation: United States. MMWR Morb. Mortal. Wkly Rep. 2013;62:869–873. [PMC free article] [PubMed] [Google Scholar]
- 8.Wright NC, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J. Bone Miner. Res. 2014;29:2520–2526. doi: 10.1002/jbmr.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hak DJ, et al. Delayed union and nonunions: epidemiology, clinical issues, and financial aspects. Injury. 2014;45:S3–S7. doi: 10.1016/j.injury.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Siegel RL, et al. Cancer statistics, 2015. CA Cancer J. Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 11.Jemal A, et al. Cancer statistics, 2010. CA Cancer J. Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 12.Silbermann R, Roodman GD. Myeloma bone disease: pathophysiology and management. J. Bone Oncol. 2013;2:59–69. doi: 10.1016/j.jbo.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rajani R, et al. Giant cell tumors of the foot and ankle bones: high recurrence rates after surgical treatment. J. Foot Ankle Surg. 2015;54:1141–1145. doi: 10.1053/j.jfas.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 14.Baines CR, et al. An integrative review of skin assessment tools used to evaluate skin injury related to external beam radiation therapy. J. Clin. Nurs. 2016;26:1137–1144. doi: 10.1111/jocn.13430. [DOI] [PubMed] [Google Scholar]
- 15.Figueiredo A, et al. Inflammatory reaction post implantation of bone graft materials. Exp. Pathol. Health Sci. 2012;6:15–18. [Google Scholar]
- 16.Ma P, et al. Inorganic nanocarriers for platinum drug delivery. Mater. Today. 2015;18:554–564. [Google Scholar]
- 17.Lee BK, et al. PLA micro-and nano-particles. Adv. Drug Deliv. Rev. 2016;107:176–191. doi: 10.1016/j.addr.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dai L, et al. A novel self-assembled targeted nanoparticle platform based on carboxymethylcellulose co-delivery of anticancer drugs. J. Mater. Chem. B. 2015;3:6605–6617. doi: 10.1039/c5tb00900f. [DOI] [PubMed] [Google Scholar]
- 19.Liechty WB, et al. Polymers for drug delivery systems. Annu. Rev. Chem. Biomol. Eng. 2010;1:149–173. doi: 10.1146/annurev-chembioeng-073009-100847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gan L, et al. Recent advances in topical ophthalmic drug delivery with lipid-based nanocarriers. Drug Discov. Today. 2013;18:290–297. doi: 10.1016/j.drudis.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Yang K, et al. Stimuli responsive drug delivery systems based on nano-graphene for cancer therapy. Adv. Drug Deliv. Rev. 2016;105:228–241. doi: 10.1016/j.addr.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Q, et al. Biocompatible, uniform, and redispersible mesoporous silica nanoparticles for cancer-targeted drug delivery in vivo. Adv. Funct. Mater. 2014;24:2450–2461. [Google Scholar]
- 23.Mura S, et al. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013;12:991–1003. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- 24.Narasimhan B, et al. Rational design of targeted next-generation carriers for drug and vaccine delivery. Annu. Rev. Biomed. Eng. 2016;18:25–49. doi: 10.1146/annurev-bioeng-082615-030519. [DOI] [PubMed] [Google Scholar]
- 25.Feng K, et al. Modular design of poly (norbornenes) for organelle-specific imaging in tumor cells. Biomacromolecules. 2016;17:538–545. doi: 10.1021/acs.biomac.5b01450. [DOI] [PubMed] [Google Scholar]
- 26.Rajesh R, Ravichandran YD. Development of a new carbon nanotube-alginate-hydroxyapatite tricomponent composite scaffold for application in bone tissue engineering. Int. J. Nanomedicine. 2015;10:7–15. doi: 10.2147/IJN.S79971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang L, Webster TJ. Nanotechnology controlled drug delivery for treating bone diseases. Expert Opin. Drug Deliv. 2009;6:851–864. doi: 10.1517/17425240903044935. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen TBL, et al. Nanoparticle biphasic calcium phosphate loading on gelatin-pectin scaffold for improved bone regeneration. Tissue Eng. Part A. 2015;21:1376–1387. doi: 10.1089/ten.TEA.2014.0313. [DOI] [PubMed] [Google Scholar]
- 29.Xing Z, et al. Biological effects of functionalizing copolymer scaffolds with nanodiamond particles. Tissue Eng. Part A. 2013;19:1783–1791. doi: 10.1089/ten.tea.2012.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moncharmont C, et al. Targeting a cornerstone of radiation resistance: cancer stem cell. Cancer Lett. 2012;322:139–147. doi: 10.1016/j.canlet.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 31.Chong CR, Jänne PA. The quest to overcome resistance to EGFR-targeted therapies in cancer. Nat. Med. 2013;19:1389–1400. doi: 10.1038/nm.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang L, Webster TJ. Nanotechnology controlled drug delivery for treating bone diseases. Expert Opin. Drug Deliv. 2009;6:851–864. doi: 10.1517/17425240903044935. [DOI] [PubMed] [Google Scholar]
- 33.Jang HL, et al. Revisiting whitlockite, the second most abundant biomineral in bone: nanocrystal synthesis in physiologically relevant conditions and biocompatibility evaluation. ACS Nano. 2014;8:634–641. doi: 10.1021/nn405246h. [DOI] [PubMed] [Google Scholar]
- 34.Kim MH, et al. Facile synthesis of monodispersed mesoporous silica nanoparticles with ultralarge pores and their application in gene delivery. ACS Nano. 2011;5:3568–3576. doi: 10.1021/nn103130q. [DOI] [PubMed] [Google Scholar]
- 35.Argyo C, et al. Multifunctional mesoporous silica nanoparticles as a universal platform for drug delivery. Chem. Mater. 2014;26:435–451. [Google Scholar]
- 36.El-Fiqi A, et al. Capacity of mesoporous bioactive glass nanoparticles to deliver therapeutic molecules. Nanoscale. 2012;4:7475–7488. doi: 10.1039/c2nr31775c. [DOI] [PubMed] [Google Scholar]
- 37.Wu C, Chang J. Mesoporous bioactive glasses: structure characteristics, drug/growth factor delivery and bone regeneration application. Interface Focus. 2012;2:292–306. doi: 10.1098/rsfs.2011.0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Larsson S, Fazzalari NL. Anti-osteoporosis therapy and fracture healing. Arch. Orthop. Trauma Surg. 2014;134:291–297. doi: 10.1007/s00402-012-1558-8. [DOI] [PubMed] [Google Scholar]
- 39.Fan JP, et al. In vitro response of human osteoblasts to multi-step sol-gel derived bioactive glass nanoparticles for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2014;36:206–214. doi: 10.1016/j.msec.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 40.Kim TH, et al. Inhibition of osteoclastogenesis through siRNA delivery with tunable mesoporous bioactive nanocarriers. Acta Biomater. 2016;29:352–364. doi: 10.1016/j.actbio.2015.09.035. [DOI] [PubMed] [Google Scholar]
- 41.Tschernitschek H, et al. Nonalloyed titanium as a bioinert metal—a review. J. Prosthet. Dent. 2006;96:12. [PubMed] [Google Scholar]
- 42.Mendonca G, et al. Advancing dental implant surface technology-from micron- to nanotopography. Biomaterials. 2008;29:3822–3835. doi: 10.1016/j.biomaterials.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 43.Gittens RA, et al. The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials. 2011;32:3395–3403. doi: 10.1016/j.biomaterials.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roy P, et al. TiO2 nanotubes: synthesis and applications. Angew. Chem. Int. Ed. Engl. 2011;50:2904–2939. doi: 10.1002/anie.201001374. [DOI] [PubMed] [Google Scholar]
- 45.Losic D, et al. Titania nanotube arrays for local drug delivery: recent advances and perspectives. Expert Opin. Drug Deliv. 2015;12:103–127. doi: 10.1517/17425247.2014.945418. [DOI] [PubMed] [Google Scholar]
- 46.Komatsu K, et al. Alendronate promotes bone formation by inhibiting protein prenylation in osteoblasts in rat tooth replantation model. J. Endocrinol. 2013;219:145–158. doi: 10.1530/JOE-13-0040. [DOI] [PubMed] [Google Scholar]
- 47.Shen X, et al. Alendronate-loaded hydroxyapatite-TiO2 nanotubes for improved bone formation in osteoporotic rabbits. J. Mater. Chem. B. 2016;4:1423–1436. doi: 10.1039/c5tb01956g. [DOI] [PubMed] [Google Scholar]
- 48.Ghosh P, et al. Gold nanoparticles in delivery applications. Adv. Drug Deliv. Rev. 2008;60:1307–1315. doi: 10.1016/j.addr.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 49.Yi C, et al. Gold nanoparticles promote osteogenic differentiation of mesenchymal stem cells through p38 MAPK pathway. ACS Nano. 2010;4:6439–6448. doi: 10.1021/nn101373r. [DOI] [PubMed] [Google Scholar]
- 50.Heo DN, et al. Inhibition of osteoclast differentiation by gold nanoparticles functionalized with cyclodextrin curcumin complexes. ACS Nano. 2014;8:12049–12062. doi: 10.1021/nn504329u. [DOI] [PubMed] [Google Scholar]
- 51.Lee D, et al. Inhibition of osteoclast differentiation and bone resorption by bisphosphonate-conjugated gold nanoparticles. Sci. Rep. 2016;6:27336. doi: 10.1038/srep27336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sul OJ, et al. Gold nanoparticles inhibited the receptor activator of nuclear factor-kappab ligand (RANKL)-induced osteoclast formation by acting as an antioxidant. Biosci. Biotechnol. Biochem. 2010;74:2209–2213. doi: 10.1271/bbb.100375. [DOI] [PubMed] [Google Scholar]
- 53.Moon HJ, et al. Antioxidants, like coenzyme Q10, selenite, and curcumin, inhibited osteoclast differentiation by suppressing reactive oxygen species generation. Biochem. Biophys. Res. Commun. 2012;418:247–253. doi: 10.1016/j.bbrc.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 54.Tomren MA, et al. Studies on curcumin and curcuminoids XXXI. Symmetric and asymmetric curcuminoids: stability, activity and complexation with cyclodextrin. Int. J. Pharm. 2007;338:27–34. doi: 10.1016/j.ijpharm.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 55.Mochalin VN, et al. The properties and applications of nanodiamonds. Nat. Nanotechnol. 2011;7:11–23. doi: 10.1038/nnano.2011.209. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Q, et al. Fluorescent PLLA-nanodiamond composites for bone tissue engineering. Biomaterials. 2011;32:87–94. doi: 10.1016/j.biomaterials.2010.08.090. [DOI] [PubMed] [Google Scholar]
- 57.Ryu TK, et al. Bone-targeted delivery of nanodiamond-based drug carriers conjugated with alendronate for potential osteoporosis treatment. J. Control. Release. 2016;232:152–160. doi: 10.1016/j.jconrel.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 58.Bose S, Tarafder S. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: a review. Acta Biomater. 2012;8:1401–1421. doi: 10.1016/j.actbio.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen WY, et al. Studies of the interaction mechanism between single strand and double-strand DNA with hydroxyapatite by microcalorimetry and isotherm measurements. Colloids Surf. Physicochem. Eng. Aspects. 2007;295:274–283. [Google Scholar]
- 60.Olton D, et al. Nanostructured calcium phosphates (NanoCaPs) for non-viral gene delivery: influence of the synthesis parameters on transfection efficiency. Biomaterials. 2007;28:1267–1279. doi: 10.1016/j.biomaterials.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 61.Curtin CM, et al. Innovative collagen nano-hydroxyapatite scaffolds offer a highly efficient non-viral gene delivery platform for stem cell-mediated bone formation. Adv. Mater. 2012;24:749–754. doi: 10.1002/adma.201103828. [DOI] [PubMed] [Google Scholar]
- 62.Cunniffe GM, et al. The synthesis and characterization of nanophase hydroxyapatite using a novel dispersant-aided precipitation method. J. Biomed. Mater. Res. A. 2010;95:1142–1149. doi: 10.1002/jbm.a.32931. [DOI] [PubMed] [Google Scholar]
- 63.Battaglia L, Gallarate M. Lipid nanoparticles: state of the art, new preparation methods and challenges in drug delivery. Expert Opin. Drug Deliv. 2012;9:497–508. doi: 10.1517/17425247.2012.673278. [DOI] [PubMed] [Google Scholar]
- 64.Shukla SK, et al. Chitosan-based nanomaterials: a state-of-the-art review. Int. J. Biol. Macromol. 2013;59:46–58. doi: 10.1016/j.ijbiomac.2013.04.043. [DOI] [PubMed] [Google Scholar]
- 65.Mao S, et al. Chitosan-based formulations for delivery of DNA and siRNA. Adv. Drug Deliv. Rev. 2010;62:12–27. doi: 10.1016/j.addr.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 66.Riva R, et al. Chitosan and chitosan derivatives in drug delivery and tissue engineering. 2011;244:19–44. [Google Scholar]
- 67.Zhao L, et al. Effective delivery of bone morphogenetic protein 2 gene using chitosan-polyethylenimine nanoparticle to promote bone formation. RSC Adv. 2016;6:34081–34089. [Google Scholar]
- 68.Hu YC. Gene Therapy for Cartilage and Bone Tissue Engineering. Springer; Berlin Heidelberg: 2014. Gene therapy for bone tissue engineering; pp. 33–54. [Google Scholar]
- 69.Cao L, et al. Bone regeneration using photocrosslinked hydrogel incorporating rhBMP-2 loaded 2-N, 6-O-sulfated chitosan nanoparticles. Biomaterials. 2014;35:2730–2742. doi: 10.1016/j.biomaterials.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 70.Zhou H, et al. Enhanced bioactivity of bone morphogenetic protein-2 with low dose of 2-N, 6-O-sulfated chitosan in vitro and in vivo. Biomaterials. 2009;30:1715–1724. doi: 10.1016/j.biomaterials.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 71.Betancourt T, et al. PEGylation strategies for active targeting of PLA/PLGA nanoparticles. J. Biomed. Mater. Res. A. 2009;91:263–276. doi: 10.1002/jbm.a.32247. [DOI] [PubMed] [Google Scholar]
- 72.Vert M, et al. Biodegradation of PLA/GA polymers: increasing complexity. Biomaterials. 1994;15:1209–1213. doi: 10.1016/0142-9612(94)90271-2. [DOI] [PubMed] [Google Scholar]
- 73.Kumari A, et al. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces. 2010;75:1–18. doi: 10.1016/j.colsurfb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 74.Gentile P, et al. Influence of parathyroid hormone-loaded PLGA nanoparticles in porous scaffolds for bone regeneration. Int. J. Mol. Sci. 2015;16:20492–20510. doi: 10.3390/ijms160920492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yilgor P, et al. Sequential BMP-2/BMP-7 delivery from polyester nanocapsules. J. Biomed. Mater. Res. A. 2010;93:528–536. doi: 10.1002/jbm.a.32520. [DOI] [PubMed] [Google Scholar]
- 76.Danhier F, et al. PLGA-based nanoparticles: an overview of biomedical applications. J. Control. Release. 2012;161:505–522. doi: 10.1016/j.jconrel.2012.01.043. [DOI] [PubMed] [Google Scholar]
- 77.Wang YH, et al. PLGA-linked alendronate enhances bone repair in diaphysis defect model. J. Tissue Eng. Regen. Med. 2016 doi: 10.1002/term.2160. Published online June 3, 2016. http://onlinelibrary.wiley.com/doi/10.1002/term.2160/full. [DOI] [PubMed]
- 78.Posadowska U, et al. Injectable nanoparticle-loaded hydrogel system for local delivery of sodium alendronate. Int. J. Pharm. 2015;485:31–40. doi: 10.1016/j.ijpharm.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 79.Muller RH, et al. Solid lipid nanoparticles (SLN) for controlled drug delivery - a review of the state of the art. Eur. J. Pharm. Biopharm. 2000;50:161–177. doi: 10.1016/s0939-6411(00)00087-4. [DOI] [PubMed] [Google Scholar]
- 80.Muller RH, et al. Nanostructured lipid matrices for improved microencapsulation of drugs. Int. J. Pharm. 2002;242:121–128. doi: 10.1016/s0378-5173(02)00180-1. [DOI] [PubMed] [Google Scholar]
- 81.Olbrich C, et al. Lipid-drug-conjugate (LDC) nanoparticles as novel carrier system for the hydrophilic antitrypanosomal drug diminazenediaceturate. J. Drug Target. 2002;10:387–396. doi: 10.1080/1061186021000001832. [DOI] [PubMed] [Google Scholar]
- 82.Huynh NT, et al. Lipid nanocapsules: a new platform for nanomedicine. Int. J. Pharm. 2009;379:201–209. doi: 10.1016/j.ijpharm.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 83.del Pozo-Rodriguez A, et al. Lipid nanoparticles as vehicles for macromolecules: nucleic acids and peptides. Recent Pat. Drug Deliv. Formul. 2011;5:214–226. doi: 10.2174/187221111797200515. [DOI] [PubMed] [Google Scholar]
- 84.Dang L, et al. Targeted delivery systems for molecular therapy in skeletal disorders. Int. J. Mol. Sci. 2016;17:428. doi: 10.3390/ijms17030428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Basha G, et al. Lipid nanoparticle delivery of siRNA to osteocytes leads to effective silencing of SOST and inhibition of sclerostin in vivo. Mol. Ther. Nucleic Acids. 2016;5:e363. doi: 10.1038/mtna.2016.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mitragotri S, et al. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat. Rev. Drug Discov. 2014;13:655–672. doi: 10.1038/nrd4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bachar M, et al. Development and characterization of a novel drug nanocarrier for oral delivery, based on self-assembled beta-casein micelles. J. Control. Release. 2012;160:164–171. doi: 10.1016/j.jconrel.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 88.Kearney PM, et al. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ. 2006;332:1302–1308. doi: 10.1136/bmj.332.7553.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Olbrich C, et al. Lipase degradation of Dynasan 114 and 116 solid lipid nanoparticles (SLN)-effect of surfactants, storage time and crystallinity. Int. J. Pharm. 2002;237:119–128. doi: 10.1016/s0378-5173(02)00035-2. [DOI] [PubMed] [Google Scholar]
- 90.Cheng WP, et al. In vitro and in vivo characterisation of a novel peptide delivery system: amphiphilic polyelectrolyte -salmon calcitonin nanocomplexes. J. Control. Release. 2010;147:289–297. doi: 10.1016/j.jconrel.2010.07.128. [DOI] [PubMed] [Google Scholar]
- 91.Chen C, et al. Orally delivered salmon calcitonin-loaded solid lipid nanoparticles prepared by micelle-double emulsion method via the combined use of different solid lipids. Nanomedicine. 2013;8:1085–1100. doi: 10.2217/nnm.12.141. [DOI] [PubMed] [Google Scholar]
- 92.Samarasinghe RM, et al. The effect of oral administration of iron saturated-bovine lactoferrin encapsulated chitosan-nanocarriers on osteoarthritis. Biomaterials. 2014;35:7522–7534. doi: 10.1016/j.biomaterials.2014.04.109. [DOI] [PubMed] [Google Scholar]
- 93.Kanwar JR, et al. ‘Iron-saturated’ lactoferrin is a potent natural adjuvant for augmenting cancer chemotherapy. Immunol. Cell Biol. 2008;86:277–288. doi: 10.1038/sj.icb.7100163. [DOI] [PubMed] [Google Scholar]
- 94.Gusic N, et al. Nanobiotechnology and bone regeneration: a mini-review. Int. Orthop. 2014;38:1877–1884. doi: 10.1007/s00264-014-2412-0. [DOI] [PubMed] [Google Scholar]
- 95.Stendahl JC, et al. Intermolecular forces in the self-assembly of peptide amphiphile nanofibers. Adv. Funct. Mater. 2006;16:499–508. [Google Scholar]
- 96.Webber MJ, et al. Development of bioactive peptide amphiphiles for therapeutic cell delivery. Acta Biomater. 2010;6:3–11. doi: 10.1016/j.actbio.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Matson JB, et al. Nanostructure-templated control of drug release from peptide amphiphile nanofiber gels. Soft Matter. 2012;8:3586–3595. doi: 10.1039/C2SM07420F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Matson JB, Stupp SI. Drug release from hydrazone-containing peptide amphiphiles. Chem. Commun. 2011;47:7962–7964. doi: 10.1039/c1cc12570b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Aida T, et al. Functional supramolecular polymers. Science. 2012;335:813–817. doi: 10.1126/science.1205962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Matson JB, et al. Peptide self-assembly for crafting functional biological materials. Curr. Opin. Solid State Mater. Sci. 2011;15:225–235. doi: 10.1016/j.cossms.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee SS, et al. Bone regeneration with low dose BMP-2 amplified by biomimetic supramolecular nanofibers within collagen scaffolds. Biomaterials. 2013;34:452–459. doi: 10.1016/j.biomaterials.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gautschi OP, et al. Bone morphogenetic proteins in clinical applications. ANZ J. Surg. 2007;77:626–631. doi: 10.1111/j.1445-2197.2007.04175.x. [DOI] [PubMed] [Google Scholar]
- 103.Goldberg M, et al. Nanostructured materials for applications in drug delivery and tissue engineering. J. Biomater. Sci. Polym. Ed. 2007;18:241–268. doi: 10.1163/156856207779996931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kang MS, et al. Therapeutic-designed electrospun bone scaffolds: mesoporous bioactive nanocarriers in hollow fiber composites to sequentially deliver dual growth factors. Acta Biomater. 2015;16:103–116. doi: 10.1016/j.actbio.2014.12.028. [DOI] [PubMed] [Google Scholar]
- 105.Learmonth ID, et al. The operation of the century: total hip replacement. Lancet. 2007;370:1508–1519. doi: 10.1016/S0140-6736(07)60457-7. [DOI] [PubMed] [Google Scholar]
- 106.Marco F, et al. Peri-implant osteogenesis in health and osteoporosis. Micron. 2005;36:630–644. doi: 10.1016/j.micron.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 107.Junker R, et al. Effects of implant surface coatings and composition on bone integration: a systematic review. Clin. Oral Implants Res. 2009;20:185–206. doi: 10.1111/j.1600-0501.2009.01777.x. [DOI] [PubMed] [Google Scholar]
- 108.Anselme K. Osteoblast adhesion on biomaterials. Biomaterials. 2000;21:667–681. doi: 10.1016/s0142-9612(99)00242-2. [DOI] [PubMed] [Google Scholar]
- 109.de Jonge LT, et al. Organic-inorganic surface modifications for titanium implant surfaces. Pharm. Res. 2008;25:2357–2369. doi: 10.1007/s11095-008-9617-0. [DOI] [PubMed] [Google Scholar]
- 110.Alghamdi HS, et al. Synergistic effects of bisphosphonate and calcium phosphate nanoparticles on peri-implant bone responses in osteoporotic rats. Biomaterials. 2014;35:5482–5490. doi: 10.1016/j.biomaterials.2014.03.069. [DOI] [PubMed] [Google Scholar]
- 111.Slaughter BV, et al. Hydrogels in regenerative medicine. Adv. Mater. 2009;21:3307–3329. doi: 10.1002/adma.200802106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kretlow JD, et al. Injectable matrices and scaffolds for drug delivery in tissue engineering. Adv. Drug Deliv. Rev. 2007;59:263–273. doi: 10.1016/j.addr.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 113.Seo BB, et al. Sustained BMP-2 delivery and injectable bone regeneration using thermosensitive polymeric nanoparticle hydrogel bearing dual interactions with BMP-2. J. Control. Release. 2015;209:67–76. doi: 10.1016/j.jconrel.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 114.Andrade LM, et al. Impact of lipid dynamic behavior on physical stability, in vitro release and skin permeation of genistein-loaded lipid nanoparticles. Eur. J. Pharm. Biopharm. 2014;88:40–47. doi: 10.1016/j.ejpb.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 115.Garg NK, et al. Effective transdermal delivery of methotrexate through nanostructured lipid carriers in an experimentally induced arthritis model. Colloids Surf. B. Biointerfaces. 2016;147:17–24. doi: 10.1016/j.colsurfb.2016.07.046. [DOI] [PubMed] [Google Scholar]
- 116.Chou LY, et al. Strategies for the intracellular delivery of nanoparticles. Chem. Soc. Rev. 2011;40:233–245. doi: 10.1039/c0cs00003e. [DOI] [PubMed] [Google Scholar]
- 117.Field LD, et al. Peptides for specifically targeting nanoparticles to cellular organelles: quo vadis? Acc. Chem. Res. 2015;48:1380–1390. doi: 10.1021/ar500449v. [DOI] [PubMed] [Google Scholar]
- 118.Sun Y, et al. Osteoblast-targeting-peptide modified nanoparticle for siRNA/microRNA delivery. ACS Nano. 2016;10:5759–5768. doi: 10.1021/acsnano.5b07828. [DOI] [PubMed] [Google Scholar]
- 119.Wang X, et al. miR-214 targets ATF4 to inhibit bone formation. Nat. Med. 2013;19:93–100. doi: 10.1038/nm.3026. [DOI] [PubMed] [Google Scholar]
- 120.Zhang G, et al. A delivery system targeting bone formation surfaces to facilitate RNAi-based anabolic therapy. Nat. Med. 2012;18:307–314. doi: 10.1038/nm.2617. [DOI] [PubMed] [Google Scholar]
- 121.Lu K, et al. Targeting WW domains linker of HECT-type ubiquitin ligase Smurf1 for activation by CKIP-1. Nat. Cell Biol. 2008;10:994–1002. doi: 10.1038/ncb1760. [DOI] [PubMed] [Google Scholar]
- 122.Wang D, et al. Osteotropic peptide that differentiates functional domains of the skeleton. Bioconjug. Chem. 2007;18:1375–1378. doi: 10.1021/bc7002132. [DOI] [PubMed] [Google Scholar]
- 123.Liu J, et al. A delivery system specifically approaching bone resorption surfaces to facilitate therapeutic modulation of microRNAs in osteoclasts. Biomaterials. 2015;52:148–160. doi: 10.1016/j.biomaterials.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 124.Cheng P, et al. miR-148a regulates osteoclastogenesis by targeting V-maf musculoaponeurotic fibrosarcoma oncogene homolog B. J. Bone Miner. Res. 2013;28:1180–1190. doi: 10.1002/jbmr.1845. [DOI] [PubMed] [Google Scholar]
- 125.Liang C, et al. Aptamer-functionalized lipid nanoparticles targeting osteoblasts as a novel RNA interference-based bone anabolic strategy. Nat. Med. 2015;21:288–294. doi: 10.1038/nm.3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science. 2000;289:1508–1514. doi: 10.1126/science.289.5484.1508. [DOI] [PubMed] [Google Scholar]
- 127.Singh T, et al. The critical role of bisphosphonates to target bone cancer metastasis: an overview. J. Drug Target. 2015;23:1–15. doi: 10.3109/1061186X.2014.950668. [DOI] [PubMed] [Google Scholar]
- 128.Roelofs AJ, et al. Bisphosphonates: molecular mechanisms of action and effects on bone cells, monocytes and macrophages. Curr. Pharm. Des. 2010;16:2950–2960. doi: 10.2174/138161210793563635. [DOI] [PubMed] [Google Scholar]
- 129.Swami A, et al. Engineered nanomedicine for myeloma and bone microenvironment targeting. Proc. Natl. Acad. Sci. U. S. A. 2014;111:10287–10292. doi: 10.1073/pnas.1401337111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tanida I. Autophagosome formation and molecular mechanism of autophagy. Antioxid. Redox Signal. 2011;14:2201–2214. doi: 10.1089/ars.2010.3482. [DOI] [PubMed] [Google Scholar]
- 131.Ha SW, et al. Bioactive silica nanoparticles promote osteoblast differentiation through stimulation of autophagy and direct association with LC3 and p62. ACS Nano. 2014;8:5898–5910. doi: 10.1021/nn5009879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kim PK, et al. Ubiquitin signals autophagic degradation of cytosolic proteins and peroxisomes. Proc. Natl. Acad. Sci. U. S. A. 2008;105:20567–20574. doi: 10.1073/pnas.0810611105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Deter RL, et al. Participation of lysosomes in cellular autophagy induced in rat liver by glucagon. J. Cell Biol. 1967;35:C11–C16. doi: 10.1083/jcb.35.2.c11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.DeSelm CJ, et al. Autophagy proteins regulate the secretory component of osteoclastic bone resorption. Dev. Cell. 2011;21:966–974. doi: 10.1016/j.devcel.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liu F, et al. Suppression of autophagy by FIP200 deletion leads to osteopenia in mice through the inhibition of osteoblast terminal differentiation. J. Bone Miner. Res. 2013;28:2414–2430. doi: 10.1002/jbmr.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sakhrani NM, Padh H. Organelle targeting: third level of drug targeting. Drug Des. Devel. Ther. 2013;7:585–599. doi: 10.2147/DDDT.S45614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pan T, et al. miR-29b–loaded gold nanoparticles targeting to the endoplasmic reticulum for synergistic promotion of osteogenic differentiation. ACS Appl. Mater. Interfaces. 2016;8:19217–19227. doi: 10.1021/acsami.6b02969. [DOI] [PubMed] [Google Scholar]
- 139.Murakami T, et al. Signalling mediated by the endoplasmic reticulum stress transducer OASIS is involved in bone formation. Nat. Cell Biol. 2009;11:1205–1211. doi: 10.1038/ncb1963. [DOI] [PubMed] [Google Scholar]
- 140.Zhang R, et al. Endoplasmic reticulum stress signaling is involved in silver nanoparticles-induced apoptosis. Int. J. Biochem. Cell Biol. 2012;44:224–232. doi: 10.1016/j.biocel.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 141.Ma X, et al. Future of nanotherapeutics: targeting the cellular sub-organelles. Biomaterials. 2016;97:10–21. doi: 10.1016/j.biomaterials.2016.04.026. [DOI] [PubMed] [Google Scholar]
- 142.Appelqvist H, et al. The lysosome: from waste bag to potential therapeutic target. J. Mol. Cell. Biol. 2013;5:214–226. doi: 10.1093/jmcb/mjt022. [DOI] [PubMed] [Google Scholar]
- 143.Santo VE, et al. Cell engineering by the internalization of bioinstructive micelles for enhanced bone regeneration. Nanomedicine. 2015;10:1707–1721. doi: 10.2217/nnm.15.11. [DOI] [PubMed] [Google Scholar]
- 144.Sun W, et al. Bone-targeted mesoporous silica nanocarrier anchored by zoledronate for cancer bone metastasis. Langmuir. 2016;32:9237–9244. doi: 10.1021/acs.langmuir.6b02228. [DOI] [PubMed] [Google Scholar]
- 145.Wang Q, et al. Nanomaterials promise better bone repair. Mater. Today. 2016;19:451–463. [Google Scholar]
- 146.Couvreur P. Nanoparticles in drug delivery: past, present and future. Adv. Drug Del. Rev. 2013;65:21–23. doi: 10.1016/j.addr.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 147.Wilhelm S, et al. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016;1:16014. [Google Scholar]
- 148.Lavi O, et al. The dynamics of drug resistance: a mathematical perspective. Drug Resist. Updat. 2012;15:90–97. doi: 10.1016/j.drup.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Paris JL, et al. Polymer-grafted mesoporous silica nanoparticles as ultrasound-responsive drug carriers. ACS Nano. 2015;9:11023–11033. doi: 10.1021/acsnano.5b04378. [DOI] [PubMed] [Google Scholar]
- 150.Ge Z, Liu S. Functional block copolymer assemblies responsive to tumor and intracellular microenvironments for site-specific drug delivery and enhanced imaging performance. Chem. Soc. Rev. 2013;42:7289–7325. doi: 10.1039/c3cs60048c. [DOI] [PubMed] [Google Scholar]
- 151.Shastri A, et al. An aptamer-functionalized chemomechanically modulated biomolecule catch-and-release system. Nat. Chem. 2015;7:447–454. doi: 10.1038/nchem.2203. [DOI] [PubMed] [Google Scholar]
- 152.Siegel RA. Stimuli sensitive polymers and self regulated drug delivery systems: a very partial review. J. Control. Release. 2014;190:337–351. doi: 10.1016/j.jconrel.2014.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Teo PY, et al. Co-delivery of drugs and plasmid DNA for cancer therapy. Adv. Drug Del. Rev. 2016;98:41–63. doi: 10.1016/j.addr.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 154.Cheng Z, et al. Multifunctional nanoparticles: cost versus benefit of adding targeting and imaging capabilities. Science. 2012;338:903–910. doi: 10.1126/science.1226338. [DOI] [PMC free article] [PubMed] [Google Scholar]