Abstract
Background
Previous studies have shown varying results in selected outcomes when directly comparing spinal anesthesia to general in lumbar surgery. Some studies have shown reduced surgical time, postoperative pain, time in the postanesthesia care unit (PACU), incidence of urinary retention, postoperative nausea, and more favorable cost-effectiveness with spinal anesthesia. Despite these results, the current literature has also shown contradictory results in between-group comparisons.
Materials and methods
A retrospective analysis was performed by querying the electronic medical record database for surgeries performed by a single surgeon between 2007 and 2011 using procedural codes 63030 for diskectomy and 63047 for laminectomy: 544 lumbar laminectomy and diskectomy surgeries were identified, with 183 undergoing general anesthesia and 361 undergoing spinal anesthesia (SA). Linear and multivariate regression analyses were performed to identify differences in blood loss, operative time, time from entering the operating room (OR) until incision, time from bandage placement to exiting the OR, total anesthesia time, PACU time, and total hospital stay. Secondary outcomes of interest included incidence of postoperative spinal hematoma and death, incidence of paraparesis, plegia, post-dural puncture headache, and paresthesia, among the SA patients.
Results
SA was associated with significantly lower operative time, blood loss, total anesthesia time, time from entering the OR until incision, time from bandage placement until exiting the OR, and total duration of hospital stay, but a longer stay in the PACU. The SA group experienced one spinal hematoma, which was evacuated without any long-term neurological deficits, and neither group experienced a death. The SA group had no episodes of paraparesis or plegia, post-dural puncture headaches, or episodes of persistent postoperative paresthesia or weakness.
Conclusion
SA is effective for use in patients undergoing elective lumbar laminectomy and/or diskectomy spinal surgery, and was shown to be the more expedient anesthetic choice in the perioperative setting.
Keywords: spinal anesthesia, general anesthesia, efficiency, expedient
Introduction
Lumbar spinal surgery can be successfully performed using various anesthetic techniques. The most commonly used technique is endotracheal general anesthesia (GA).1 This may be due to a variety of factors, including greater patient acceptance, its enabling of long surgeries, and capacity for secure airway establishment in the prone position.2,3 Despite this, many centers advocate the use of neuraxial techniques, such as spinal anesthesia (SA), for lumbar surgical techniques, such as diskectomy and laminectomy.4–8
SA, which is widely used in general orthopedic and vascular surgery, has several benefits noted in the literature, including rapid onset, less intraoperative blood loss, thrombotic events, pulmonary complications, and postoperative cognitive dysfunction.9–11 It also allows the patient to breathe spontaneously and reposition themselves to avoid compression injuries during the course of the procedure. SA for spine surgery can include epidural anesthesia via catheter infusion and SA via injection.12,13 Various studies comparing GA and SA for lumbar surgery have shown reduced surgical time, postoperative pain, time in the postanesthesia care unit (PACU), incidence of urinary retention, postoperative nausea, and more favorable cost-effectiveness.4,14
Despite encouraging results in favor of SA, SA does not come without risk, and there is (at least to date) no clear evidence to delineate the difference in morbidity and mortality between the two approaches.15 Besides considering specific risks of SA itself, one must consider the context in terms of the type of surgery to estimate the real risk better. A rare complication that may occur after lumbar decompression is symptomatic epidural hematoma. Although the reported incidence is only 0.1%–0.24%,9 prompt diagnosis is required, and thus arises the concern that any residual anesthetic effect from SA may obscure its signs and symptoms, resulting in delayed emergent evacuation of the hematoma and consequent permanent neurological deficits.
The current literature has also shown contradictory results in between-group comparisons in operative efficiency parameters, namely operative blood loss, operative time, total anesthesia time, time in the PACU, and total hospital stay.4 Many of these studies comprised relatively small numbers of patients in each cohort. In this study, we sought to elucidate the efficiency of SA in a larger retrospective cohort in comparison with GA. We hypothesized that SA is a more efficient anesthetic technique in terms of total operative time and total anesthesia time, with a postoperative complication profile analogous to that associated with GA.
Materials and methods
Following University of Pennsylvania Institutional Review Board approval for the study and a written informed consent waiver (due to the large number of patients and minimal risk), 544 consecutive patients of a single senior neurosurgeon who had undergone elective lumbar decompression at the University of Pennsylvania were retrospectively identified by current procedural terminology (CPT) codes for diskectomy (63030) and laminectomy (63047) between 2007 and 2011. Patients who had undergone lumbar spinal fusion surgery were not included. All data were abstracted from patient medical records. This manuscript adheres to the applicable Equator guidelines.
Patient characteristics
Demographic data known to influence perioperative morbidity were collected. These included age, sex, body-mass index, hypertension, diabetes mellitus, urinary tract dysfunction, American Society of Anesthesiologists physical classification-system score, and previous lumbar surgery. The type of surgery (diskectomy or laminectomy) and number of levels operated on were recorded. Perioperative and physiological data were collected including heart rate and mean arterial pressure (MAP) preoperatively, intraoperatively, and in the PACU postoperatively. Maximum and minimum intraoperative systolic and diastolic pressures were recorded, as well as first and last PACU visual analogue scale pain rating.
Efficiency outcomes
The primary efficiency outcome of interest in this study was mean operative time (from incision to dressing). Secondary efficiency variables included operative blood loss, total anesthesia time (time in the operating room [OR] until transfer to PACU), length of stay in the PACU, length of overall hospital stay, mean time from patient entering the OR until incision, and mean time from bandage placement until exiting the OR.
Postoperative complication outcomes
The variables recorded to report postoperative complications included incidence of postoperative spinal hematoma and death. Incidence of post-dural puncture headache, persistent paresthesia, and paraparesis or plegia were recorded and analyzed for the SA group. Conversion from SA to GA and SA reinjections during surgery were also recorded for the SA group.
Anesthetic procedure
Patients underwent either GA or SA. Patients undergoing GA were typically given one or a combination of the following: propofol, nitrous oxide, desflurane, halothane, isoflurane, and sevoflurane. Once the patients’ tracheas had been intubated, they were placed in the prone position on a standard operating frame. When the GA course was complete, the anesthetic agents were discontinued and 100% O2 administered. Patients were then extubated when appropriate, followed by transport to the PACU. Patients were then monitored on a one-to-one basis by the PACU nursing staff until they were deemed awake, alert, responsive, and stable before transfer to the floor. Intravenous analgesia was administered to the patients during their PACU stay.
Patients receiving SA were first given a 300–500 mL infusion of lactated Ringer’s solution 10–15 minutes before institution of the spinal anesthetic. Upon arrival at the OR, these patients were placed in a seated position on a gurney. After local infiltration of 2–3 mL of 2% lidocaine, SA was achieved via lumbar puncture, using a needle size of 25 G most commonly. After spinal fluid had been observed, either bupivacaine or tetracaine was injected into the intrathecal space, sometimes in combination with epinephrine and/or fentanyl. Patients receiving bupivacaine were given a 15 mg dose of a 0.75% bupivacaine in 8.25% dextrose solution. Those patients receiving tetracaine were given a 0.5% concentration with 5% dextrose and were given a 14–16mg dose. Epinephrine (0.2 mg) was often incorporated to prevent systemic absorption and extend the duration of action. In six cases, 25 µg of fentanyl was given in combination with bupivacaine, in order to improve the antinociceptive effect of the spinal anesthetic. Once the anesthetic agent had been given, the patient was rolled into a supine position and adequate anesthesia verified on the lower back and extremities. The patient was then turned into the prone position on the operating table. Oxygen was given via nasal cannula. Light sedation was achieved with propofol infusion. At completion of the procedure, propofol was discontinued and the patient transferred to the gurney and transported to the PACU for recovery. The patients remained in the PACU until they regained adequate motor function of their lower extremities, at which time hemodynamic stability was confirmed, followed by transfer to the general neurosurgical ward.
Statistical methods
Comparisons among patient characteristics and unadjusted outcomes for the two patient groups were performed with two independent sample t-tests for continuous variables and Fisher’s exact test for categorical variables. Linear regression was used to describe the effect of anesthetic methods on outcomes.
Multivariate regression models were constructed to adjust for possible confounding preoperative and intraoperative variables. Respective simple linear regression analyses (ie, each model with a single covariate) were performed first, and variables were considered for inclusion in multivariate regression analysis if the simple linear P-value was ≤0.05. All significance tests were two-sided. Variables that were nonsignificant in the multivariate model were then removed using a backward-elimination methodology until the final model was achieved, with all variables maintaining a P-value of ≤0.05. Anesthetic technique was left in the multivariate models, regardless of its P-value, given it was the primary covariate of interest. Data were collected and analyzed by independent observers (JP, MA, and GK) in collaboration with a biostatistician (MK). Stata 13.1 software (StataCorp, College Station, TX, USA) and SAS version 9.4 (SAS Institute, Cary, NC, USA) were used for all analyses.
Results
This retrospective review comprised 544 patients in the study sample. GA was used in 183 patients and SA in 361 patients. One patient received a reinjection of local anesthetic during the procedure. Clinical characteristics of the study population stratified by anesthesia type are summarized in Table 1. The proportion of female patients, prevalence of urinary dysfunction, and previous lumbar surgery were similar between the two groups. Perioperative and physiological characteristics stratified by anesthetic technique are summarized in Table 2. The GA and SA groups had approximately equal preoperative MAP, intraoperative minimum diastolic pressure, number of vasopressors used, and incidence of nausea and/or vomiting. The GA group had slightly higher but clinically insignificant preoperative heart rate than the SA group (74.9 vs 73.5, respectively; P=0.23), intraoperative MAP (80.6 vs 79.7, respectively; P=0.09), and intraoperative maximum systolic (145.7 vs 141.0, respectively; P=0.008) and diastolic (79.9 vs 76.3, respectively; P<0.001) blood pressures. The first PACU pain ratings (2.5 vs 0.7, respectively; P<0.001) and last PACU pain ratings (3.2 vs 2.6, respectively; P=0.015) were significantly higher in the GA group. Urinary retention was significantly higher in the GA group as well (51.9% vs 11.9%, respectively; P<0.001). The SA group experienced one postoperative spinal hematoma, arising after discharge from the PACU. This patient complained of persistent and worsening leg pain 24 hours postsurgery. Magnetic resonance imaging was used to confirm soft-tissue edema. This patient was taken back to surgery for an epidural hematoma evacuation. It was not associated with long-term neurological deficits.
Table 1.
Baseline patient characteristics
| Variable | Anesthetic technique
|
||
|---|---|---|---|
| Spinal (n=361) |
General (n=183) |
P-value | |
| Mean age (SD) | 56.0 (16.1) | 60.5 (14.3) | <0.002b |
| Female, n (%) | 170 (47.1) | 89 (48.6) | 0.79a |
| Mean BMI (SD) | 28.3 (5.2) | 31.5 (7.0) | <0.001c |
| Hypertension, n (%) | 132 (36.6) | 94 (51.4) | 0.001a |
| Diabetes, n (%) | 45 (12.5) | 45 (24.6) | 0.001a |
| Previous lumbar surgery, n (%) | 61 (16.9) | 56 (30.6) | <0.001a |
| Type of surgery | |||
| Diskectomy, n (%) | 203 (56.2) | 74 (40.4) | <0.001a |
| Laminectomy, n (%) | 181 (50.1) | 127 (69.4) | <0.001a |
| History of urinary dysfunction, n (%) | 17 (4.7) | 12 (6.6) | 0.42a |
| Mean levels operated (SD) | 1.7 (1.3) | 2.3 (1.4) | <0.001b |
| ASA score, n (%) | <0.001a | ||
| 1 | 38 (11.0) | 9 (5.1) | |
| 2 | 232 (66.9) | 84 (47.2) | |
| 3+ | 77 (22.2) [14 missing] |
85 (47.8) [5 missing] |
|
Notes:
Association between the two groups using Fisher’s exact test;
mean differences, pooled method (assuming equal variance across the groups);
mean differences, Satterthwaite method (assuming unequal variance across the groups).
Abbreviations: BMI, body-mass index; ASA, American Society of Anesthesiologists.
Table 2.
Perioperative and physiologic characteristics
| Variable | Anesthetic technique
|
||
|---|---|---|---|
| Spinal (n=361) |
General (n=183) |
P-value | |
| Preoperative MAP (SD) | 93.2 (13.0) [1 missing] |
92.8 (11.8) | 0.74b |
| Preoperative HR (SD) | 73.5 (13.0) [1 missing] |
74.9 (12.4) | 0.23b |
| Mean intraoperative MAP (SD) | 79.7 (6.5) [1 missing] |
80.6 (5.1) | 0.09c |
| Intraoperative HR (SD) | 76.6 (12.5) | 79.0 (12.7) | 0.034b |
| Intraoperative maximum Ps (SD) | 141.0 (19.3) [1 missing] |
145.7 (18.9) | 0.008b |
| Intraoperative maximum Pd (SD) | 76.3 (10.4) [1 missing] |
79.9 (9.6) | <0.001c |
| Intraoperative minimum Ps (SD) | 101.8 (11.1) [1 missing] |
99.8 (9.4) | 0.028c |
| Intraoperative minimum Pd (SD) | 49.4 (6.9) [1 missing] |
49.1 (6.1) | 0.56c |
| First PACU pain rating (SD) | 0.7 (2.1) | 2.5 (3.6) | <0.001c |
| Last PACU pain rating (SD) | 2.6 (2.5) | 3.2 (2.8) | 0.015c |
| Postoperative mean MAP (SD) | 88.9 (13.2) [3 missing] |
85.6 (12.8) | 0.005b |
| Postoperative mean HR (SD) | 77.8 (13.6) [3 missing] |
82.2 (14.5) | 0.001b |
| Nausea/vomiting, n (%) | 45 (12.5) | 18 (9.8) | 0.40a |
| Urinary retention, n (%) | 43 (11.9) | 95 (51.9) | <0.001a |
| Vasopressor used, n (%) | 90 (24.9) | 30 (16.4) | 0.028a |
| Postoperative hematoma, n (%) | 1 (0.3) | 0.0 | 1.00a |
Notes:
Association between the two groups using Fisher’s exact test;
mean differences, pooled method (assuming equal variance across the groups);
mean differences, Satterthwaite method (assuming unequal variance across the groups).
Abbreviations: Ps, systolic pressure; Pd, diastolic pressure; PACU, postanesthesia care unit; MAP, mean arterial pressure; HR, heart rate.
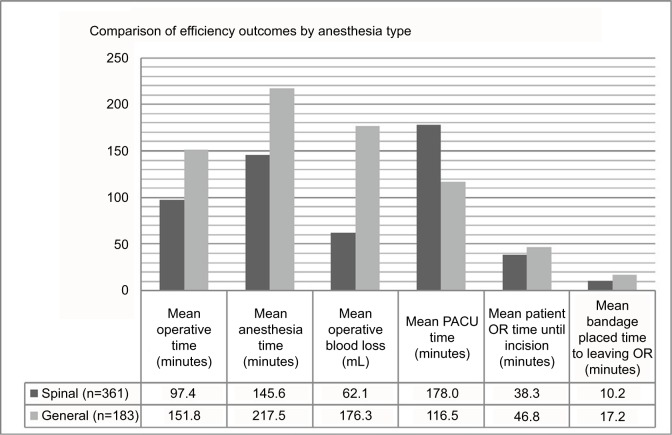
Efficiency outcomes between anesthesia groups are summarized in Figure 1. Simple linear regression analysis and multivariate adjustment of SA and association with efficiency outcomes are summarized in Table 3. Operative time was shorter for patients receiving SA than the GA group (97.4 vs 151.8 minutes, respectively; P<0.001). A final multivariate model that adjusted for potential explanatory variables of anesthesia type, body-mass index, history of spine surgery, number of levels operated on, and urinary retention showed the greatest moderating effects on the association of anesthesia type with outcome. After full adjustment, the association of SA with lower operative time remained statistically significant.
Figure 1.
Efficiency outcomes by anesthesia type.
Notes: Direct comparison of mean operative time, anesthesia time, operative blood loss, PACU time, time from patient entering the OR until incision, and the time from bandage placement until the patient leaves the OR for patients who underwent lumbar laminectomy and diskectomy spinal surgery with spinal or general anesthesia. All P-values <0.001.
Abbreviations: PACU, postanesthesia care unit; OR, operating room.
Table 3.
Simple linear regression analysis and multivariate adjustment of association of spinal anesthesia and efficiency outcomes
| Variable | β | SE | 95% CI
|
P-value | |
|---|---|---|---|---|---|
| Low | High | ||||
| Operative time | |||||
| Simple [7 missing] | −54.4 | 4.3 | −62.9 | −45.9 | <0.001 |
| Multivariate (final model) [7 missing] | −31.0 | 4.4 | −39.7 | −22.2 | <0.001 |
| Anesthesia time | |||||
| Simple [11 missing] | −71.9 | 4.1 | −81.8 | −62.0 | <0.001 |
| Multivariate (final model) [30 missing] | −48.3 | 5.3 | −58.7 | −37.9 | <0.001 |
| Estimated blood loss | |||||
| Simple [4 missing] | −114.3 | 13.9 | −141.6 | −86.9 | <0.001 |
| Multivariate (final model) [6 missing] | −80.2 | 15.1 | −109.8 | −68.6 | <0.001 |
| PACU time | |||||
| Simple [11 missing] | 61.6 | 6.7 | 48.3 | 74.8 | <0.001 |
| Multivariate (final model) [11 missing] | 55.4 | 6.7 | 42.4 | 50.5 | <0.001 |
| Duration of hospital stay | |||||
| Simple | −1.6 | 0.2 | −1.9 | −1.3 | <0.001 |
| Multivariate (final model) [2 missing] | −1.2 | 0.2 | −1.5 | −0.9 | <0.001 |
| OR time until incision | |||||
| Simple [6 missing] | −8.5 | 1.3 | −10.9 | −6.0 | <0.001 |
| Multivariate (final model) [7 missing] | −6.1 | 1.4 | −8.8 | −3.3 | <0.001 |
| Bandage placed, time to leaving OR | |||||
| Simple [12 missing] | −7.0 | 0.8 | −8.6 | −5.4 | <0.001 |
| Multivariate (final model) [13 missing] | −6.9 | 0.8 | −8.4 | −5.3 | <0.001 |
Abbreviations: PACU, postanesthesia care unit; OR, operating room.
Total anesthesia time (time from the patient entering the OR to the patient being transferred to the PACU) was also shorter for the SA group than the GA group (145.6 vs 217.5 minutes, respectively; P<0.001). A multivariate model that adjusted for potential explanatory variables, ie, anesthesia type, history of spine surgery, number of levels operated on, and urinary retention, showed the greatest moderating effects on the association of anesthesia type with outcome. After full adjustment, the association of SA with lower anesthesia time remained statistically significant.
Estimated blood loss was less in the SA group than the GA group (62.1 vs 176.3 mL, respectively; P<0.001). A final multivariate model that adjusted for potential explanatory variables, ie, anesthesia type, number of levels operated on, last PACU pain rating, and urinary retention, showed the greatest moderating effects on the association of anesthesia type with outcome. After full adjustment, the association of SA with lower estimated blood loss remained statistically significant.
The mean PACU length of stay was longer in the SA group than the GA group (178.0 vs 116.5 minutes, respectively; P<0.001). A multivariate model that adjusted for potential explanatory variables, ie, anesthesia type, hypertension, laminectomy, and first PACU pain rating, showed the greatest moderating effects on the association of anesthesia type with outcome. After full adjustment, the association of SA with longer PACU stay remained statistically significant.
The mean total hospital stay was shorter in the SA group than the GA group (1.5 vs 3.1 days, respectively; P<0.001). A multivariate model that adjusted for potential explanatory variables, ie, anesthesia type, age at surgery, urinary issues, number of levels operated on, last PACU pain rating, and urinary retention, showed the greatest moderating effects on the association of anesthesia type with outcome. After full adjustment, the association of SA with shorter hospital stay remained statistically significant.
The time from the patient entering the OR until incision was made was shorter in the spinal group than the general group (38.3 vs 46.8 minutes, respectively; P<0.001). A multivariate model that adjusted for potential explanatory variables, ie, anesthesia type, age at surgery, urinary issues, number of levels operated on, last PACU pain rating, and urinary retention, showed the greatest moderating effects on the association of anesthesia type with outcome. After full adjustment, the association of SA with time from entering the OR until incision remained statistically significant.
The time from the bandage being placed to the patient leaving the OR was shorter in the SA group than the GA group (10.2 vs 17.2 minutes, respectively; P<0.001). A multivariate model that adjusted for potential explanatory variables, ie, anesthesia type, age at surgery, urinary issues, number of levels operated on, last PACU pain rating, and urinary retention, showed the greatest moderating effects on the association of anesthesia type with outcome. After full adjustment, the association of SA with the time from the bandage being placed to the patient exiting the OR remained statistically significant.
Intraoperative SA characteristics of the 361 patients in the SA group are summarized in Table 4. Patients were all given one or a combination of bupivacaine, tetracaine, epinephrine, or fentanyl. A total of 337 (96.6%) patients received bupivacaine, 128 (36.7%) intrathecal epinephrine, 12 (3.4%) tetracaine, and six (1.7%) intrathecal fentanyl. A needle size of 25 G was used in 264 (91.7%) of the procedures. No patients experienced persistent paresthesia or weakness, paraparesis or plegia, or a post-dural puncture headache. An intraoperative second dose was given in one case (0.3%). There was no mortality in either group.
Table 4.
Intraoperative spinal anesthesia characteristics (spinal only)
| Variable | Spinal only (n=361) |
|---|---|
| Type of local anesthetic used (%)* | [12 missing/unknown] |
| Bupivacaine | 337 (96.6) |
| Tetracaine | 12 (3.4) |
| Epinephrine | 128 (36.7) |
| Fentanyl | 6 (1.7) |
| Needle size (%) | [73 missing/unknown] |
| 22 G | 14 (4.9) |
| 25 G | 264 (91.7) |
| Other | 10 (3.5) |
| Paresthesia (%) | 0.0 |
| Intraoperative second dose (%) | 1 (0.3) [2 missing/unknown] |
| PDPH (%) | 0.0 |
| Paraparesis or plegia (%) | 0.0 |
Note:
Including multiple-anesthetic use (sums to greater than 100%).
Abbreviation: PDPH, post-dural puncture headache.
Discussion
To our knowledge, this is the largest retrospective cohort to analyze the effect of SA vs GA on operative efficiency variables. The main finding of this study of 544 consecutive cases was a reduction in operative time in patients receiving SA. Further, total anesthesia time, operative blood loss, duration of hospital stay, time of patient entering the OR until incision, and time from bandage placement until patient exit from the OR were all lower in the SA group. The SA group experienced one spinal hematoma, which was diagnosed postoperatively after PACU discharge and evacuated without the development of subsequent neurological deficits. Though these cohorts were identified retrospectively and the GA group was more complex medically and surgically, these main findings persisted after statistical adjustment. This sample reflects a typical population undergoing lumbar spinal surgery that might be seen at any large medical center.
Both GA and SA are sensible anesthetic choices for lumbar surgery. Though many studies have compared the two, there has been no clearly superior technique in terms of morbidity and mortality.12,16 Nevertheless, multiple studies have supported the findings here that there are short-term benefits of SA over GA. Meng et al performed a systematic meta-analysis of eight randomized, controlled trials of SA vs GA in lumbar spine surgery. They found those patients receiving SA had a reduction in intraoperative hypertension and tachycardia, reduced hospital length of stay, reduced PACU pain scores, and reduced nausea and vomiting.17 McLain et al reported a case-controlled study of 400 consecutive patients undergoing lumbar spine surgery in which SA was as safe and effective as GA and offered additional benefits, including less postoperative nausea, less need for analgesia, better perioperative hemodynamics, and shorter anesthesia time.22
Multiple studies have shown that heart rate and blood pressure are lower with SA group than GA.19–22 It has been surmised that the reduced operative blood loss we observed may be due to lower heart rate and MAP from sympathetic blockade.13,22 There are other studies that have not found a difference between the two methods. Sadrolsadat et al, for example, did not find a significant difference in operative time or blood loss between the two, and suggested that operative blood loss is confounded by shorter operative time.16 This is not in accordance with our study, which not only found shorter operative time and less blood loss but also multivariate regression showing that each of these parameters remained significantly lower when adjusting for the other.
In the present study, there was also shorter total anesthesia time in the SA group. Though operative time is a large component of this parameter, results remained significant when adjustment was made for operative time. This finding is consistent with previous studies,18 and may be due in part to the fact that the patient is not required to recover from a surgical plane of GA for extubation before leaving the OR, as is standard for GA.
To elucidate further on total anesthesia time, we collected two additional time points: time from when the patient enters the OR until incision is made, and time from when the surgeon places the final bandage until the patient exits the OR. Both times were significantly shorter for the SA group. Notably, this study was not done in a hospital with an anesthesiology-training program. These shorter times, in this context, contribute to why SA has shorter associated total anesthesia time than GA. These times also demonstrate less time spent in the OR, both before incision and after bandage placement, leading to quicker OR turnover rate and cost-effectiveness. It may be that our efficiency results would not be duplicated in an anesthesiology department with a teaching mission.
We found that patients in the SA group had a longer PACU stay than the GA group. This is in accordance with several studies,5,18 and is most likely due to the fact that patients are only discharged from the PACU when adequate sensorimotor function is regained. This policy may not be standard across all hospitals, which may explain why this is not a consistent finding across all studies.3,19–21,23
Taken together, less operative time and anesthesia time suggest a faster turnover rate and more efficient use of the OR. This suggests SA may be the more cost-effective method of anesthesia. Indeed, several studies have reported that SA is a more cost-effective alternative to GA, including a retrospective analysis by our group.13–15,19 We have previously found that when controlling for patient and procedure characteristics, SA use was associated with a 41.1% lower direct operating cost, 36.6% lower indirect cost, and a 39.6% lower total cost compared to GA.19 Though PACU time was higher in the SA group in the present study, overall hospital stay was significantly less. Lengthy hospital stays increase the risk of hospital-acquired infections, pressure ulcers, and other adverse events, increased hospital costs, and further prolonging hospital stay. Though our results favor SA, it should be noted that anesthetic choice is not unilateral, and should be tailored to each patient’s specific needs and concerns.
These efficiency measures suggest that SA may be preferred for accepting patients and surgeons. However, before drawing any such conclusions, it is important to consider comparative postoperative complications, which we also included in our assessment. Fourteen patients were converted from SA to GA. Eleven of the 14 patients did not have adequate anesthetic effect at the desired dermatome. The three others had a failed lumbar puncture before positioning and underwent standard GA. All 14 patients were converted to GA before incision was made. The records did not specify if they were turned supine for this, but this is assumed. One patient received a second dose of intrathecal local anesthetic. The first dose given was 15 mg of bupivacaine and epinephrine. The second dose administered was 12.5 mg of bupivacaine given intrathecally by the surgeon 177 minutes after the initial dose.
The strength of the conclusions from this data set is limited by a number of factors. This investigation was a retrospective study of a single senior surgeon’s practice at a single institution. The decision to administer SA or GA is at the discretion of the surgeon, the anesthesiologist, and ultimately the patient, which introduces possible selection bias. In this respect, there is no difference from previous studies investigating differences between the two groups.6,23 There were some differences in baseline characteristics between the two groups, which we adjusted for statistically but may nonetheless be a concern. There still is a risk of bias that would be avoided with a prospective randomized study. A large, randomized prospective study should be performed in the future to limit possible biases. The information was retrieved from patient medical records, and not all data points were able to be extracted for each variable.
Conclusion
In this retrospective study of 544 patients undergoing lumbar spinal surgery, SA was associated with shorter operative time, less operative blood loss, shorter anesthesia time, shorter time from entering the OR until incision, shorter time from bandage placement until exit from the OR, and shorter duration of hospital stay than GA; however, it was associated with longer duration in the PACU. SA had a postoperative incidence profile similar to GA and SA experienced one postoperative spinal hematoma, which was evacuated in a timely manner. The SA group had zero cases of post-dural puncture headaches, episodes of persistent paresthesia or weakness, paraparesis or plegia, and mortality. SA is effective for use in patients undergoing elective lumbar laminectomy and/or diskectomy spinal surgery, and was shown to be the more expedient anesthetic choice in the perioperative setting.
Acknowledgments
The authors would like to thank Eileen Maloney-Wilensky, MSN, ACNP-BC and the Neurosurgery Clinical Research Division at the University of Pennsylvania for their support of this project. They would also like to thank Dr Frederick Simeone of the Department of Neurosurgery at Pennsylvania Hospital for providing the impetus to drive this study. This work was funded by the Department of Neurosurgery, University of Pennsylvania.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Demirel CB, Kalayci M, Ozkocak I, Altunkaya H, Ozer Y, Acikgoz B. A prospective randomized study comparing perioperative outcome variables after epidural or general anesthesia for lumbar disc surgery. J Neurosurg Anesthesiol. 2003;15:185–192. doi: 10.1097/00008506-200307000-00005. [DOI] [PubMed] [Google Scholar]
- 2.De Rojas JO, Syre P, Welch WC. Regional anesthesia versus general anesthesia for surgery on the lumbar spine: a review of the modern literature. Clin Neurol Neurosurg. 2014;119:39–43. doi: 10.1016/j.clineuro.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 3.Pflug AE, Halter JB. Effect of spinal anesthesia on adrenergic tone and the neuroendocrine responses to surgical stress in humans. Anesthesiology. 1981;55:120–126. doi: 10.1097/00000542-198108000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Chen HT, Tsai CH, Chao SC, et al. Endoscopic discectomy of L5-S1 disc herniation via an interlaminar approach: prospective controlled study under local and general anesthesia. Surgical Neurol Int. 2011;2:93. doi: 10.4103/2152-7806.82570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jellish WS, Thalji Z, Stevenson K, Shea J. A prospective randomized study comparing short- and intermediate-term perioperative outcome variables after spinal or general anesthesia for lumbar disk and laminectomy surgery. Anesth Analg. 1996;83:559–564. doi: 10.1097/00000539-199609000-00021. [DOI] [PubMed] [Google Scholar]
- 6.Rung GW, Williams D, Gelb DE, Grubb M. Isobaric spinal anesthesia for lumbar disk surgery. Anesth Analg. 1997;84:1165–1166. doi: 10.1097/00000539-199705000-00045. [DOI] [PubMed] [Google Scholar]
- 7.Scott NB, Kehlet H. Regional anaesthesia and surgical morbidity. Br J Surg. 1988;75:299–304. [PubMed] [Google Scholar]
- 8.Tetzlaff JE, Dilger JA, Kodsy M, al-Bataineh J, Yoon HJ, Bell GR. Spinal anesthesia for elective lumbar spine surgery. J Clin Anesth. 1998;10:666–669. doi: 10.1016/s0952-8180(98)00112-3. [DOI] [PubMed] [Google Scholar]
- 9.McLain RF, Bell GR, Kalfas I, Tetzlaff JE, Yoon HJ. Complications associated with lumbar laminectomy: a comparison of spinal versus general anesthesia. Spine (Phila Pa 1976) 2004;29:2542–2547. doi: 10.1097/01.brs.0000144834.43115.38. [DOI] [PubMed] [Google Scholar]
- 10.McLain RF, Tetzlaff JE, Bell GR, Uwe-Lewandrowski K, Yoon HJ, Rana M. Microdiscectomy: spinal anesthesia offers optimal results in general patient population. J Surg Orthop Adv. 2007;16:5–11. [PubMed] [Google Scholar]
- 11.Rodgers A, Walker N, Schug S, et al. Reduction of postoperative mortality and morbidity with epidural or spinal anaesthesia: results from overview of randomised trials. BMJ. 2000;321:1493. doi: 10.1136/bmj.321.7275.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Attari MA, Mirhosseini SA, Honarmand A, Safavi MR. Spinal anesthesia versus general anesthesia for elective lumbar spine surgery: a randomized clinical trial. J Res Med Sci. 2011;16:524–529. [PMC free article] [PubMed] [Google Scholar]
- 13.Brown MJ. Anesthesia for elective spine surgery in adults. 2015. [Accessed July 26, 2017]. Available from: https://www.uptodate.com/contents/anesthesia-for-elective-spine-surgery-in-adults.
- 14.Greenbarg PE, Brown MD, Pallares VS, Tompkins JS, Mann NH. Epidural anesthesia for lumbar spine surgery. J Spinal Disord. 1988;1:139–143. [PubMed] [Google Scholar]
- 15.Kao FC, Tsai TT, Chen LH, et al. Symptomatic epidural hematoma after lumbar decompression surgery. Eur Spine J. 2015;24:348–357. doi: 10.1007/s00586-014-3297-8. [DOI] [PubMed] [Google Scholar]
- 16.Sadrolsadat SH, Mahdavi AR, Moharari RS, et al. A prospective randomized trial comparing the technique of spinal and general anesthesia for lumbar disk surgery: a study of 100 cases. Surg Neurol. 2009;71:60–65. doi: 10.1016/j.surneu.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Meng T, Zhong Z, Meng L. Impact of spinal anesthesia vs. general anesthesia on peri-operative outcome in lumbar spine surgery: a systematic review and meta-analysis of randomised, controlled trials. Anaesthesia. 2017;72:391–401. doi: 10.1111/anae.13702. [DOI] [PubMed] [Google Scholar]
- 18.Kahveci K, Doger C, Ornek D, Gokcinar D, Aydemir S, Ozay R. Perioperative outcome and cost-effectiveness of spinal versus general anesthesia for lumbar spine surgery. Neurol Neurochirurg Pol. 2014;48:167–173. doi: 10.1016/j.pjnns.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Agarwal P, Pierce J, Welch WC. Cost analysis of spinal versus general anesthesia for lumbar discectomy and laminectomy spine surgery. World Neurosurg. 2016;89:266–271. doi: 10.1016/j.wneu.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 20.Davis FM, Laurenson VG, Lewis J, Wells JE, Gillespie WJ. Metabolic response to total hip arthroplasty under hypobaric subarachnoid or general anaesthesia. Br J Anaesth. 1987;59:725–729. doi: 10.1093/bja/59.6.725. [DOI] [PubMed] [Google Scholar]
- 21.Edgcombe H, Carter K, Yarrow S. Anaesthesia in the prone position. Br J Anaesth. 2008;100:165–183. doi: 10.1093/bja/aem380. [DOI] [PubMed] [Google Scholar]
- 22.McLain RF, Bell GR, Kalfas I, Tetzlaff JE, Yoon HJ. Complications associated with lumbar laminectomy: a comparison of spinal versus general anesthesia. Spine (Phila Pa 1976) 2004;29:2542–2547. doi: 10.1097/01.brs.0000144834.43115.38. [DOI] [PubMed] [Google Scholar]
- 23.Modig J, Karlström G. Intra- and post-operative blood loss and haemodynamics in total hip replacement when performed under lumbar epidural versus general anaesthesia. Eur J Anaesthesiol. 1987;4:345–355. [PubMed] [Google Scholar]