Abstract
Osteoarthritis (OA) is the most prevalent arthritis worldwide and is characterized by chronic pain and impaired physical function. We hypothesized that heightened pain in hand OA could be reduced with duloxetine or pregabalin. In this prospective, randomized clinical study, we recruited 65 participants, aged 40–75 years, with a Numerical Rating Scale (NRS) for pain of at least 5. Participants were randomized to one of the following three groups: duloxetine, pregabalin, and placebo. The primary endpoint was the NRS pain score, and the secondary endpoints included the Australian and Canadian Hand Osteoarthritis Index (AUSCAN) pain, stiffness, and function scores and quantitative sensory testing by pain pressure algometry. After 13 weeks, compared to placebo, ANOVA found significant differences between the three groups (P=0.0078). In the intention-to-treat analysis, the pregabalin group showed improvement for NRS pain (P=0.023), AUSCAN pain (P=0.008), and AUSCAN function (P=0.009), but no difference between duloxetine and placebo (P>0.05) was observed. In the per protocol analysis, NRS pain was reduced for pregabalin (P<0.0001) and duloxetine (P=0.029) compared to placebo. We conclude that centrally acting analgesics improve pain outcomes in people with hand arthritis, offering new treatment paradigms for OA pain.
Keywords: pain, hand osteoarthritis, sensitization, duloxetine, pregabalin
Introduction
Osteoarthritis (OA) is the most common form of arthritis worldwide, with hand pain and reduced function causing significant problems for people with hand OA.1 Pain is a major symptom for people with OA, with 16.7% of US adults aged 45 years and older reporting pain as a predominant problem.2 Pain and reduced function due to OA place a huge burden on patients and health care services.2,3 Although several pharmacological agents are available for OA pain management, including acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs), and opioids, a large proportion of patients continue to suffer from chronic pain despite using the agents described.4,5 Recent trials have raised questions about current treatments, suggesting that acetaminophen has poor efficacy in controlling OA pain.6,7 Pain management in OA is a huge problem, and novel approaches are urgently needed.
Pain is often characterized as having features of inflammatory nociceptive pain and neuropathic components.8,9 OA is recognized to have features of inflammatory pain and also pain sensitization. Features of pain sensitization can be evaluated using quantitative sensory testing (QST)10–13 and brain neuroimaging.14–16 Large studies have shown pain sensitization using QST in knee OA.10–13 Brain neuroimaging studies in chronic OA have also demonstrated increased central pain processing in the cingulate cortex, insula, and thalamus compared with normal controls.14,15 Many clinical trials testing new agents for OA have focused on large joint hip and knee OA, but relatively few trials have been conducted in hand OA. The ideal analgesic drug(s) in OA would achieve sustained pain relief in a dose-dependent manner with few side effects. Centrally acting analgesic drugs such as pregabalin and duloxetine could fulfill these criteria but have not been investigated in hand OA. Duloxetine is a serotonin and noradrenaline reuptake inhibitor (SNRI) that has shown efficacy for improving pain in knee OA.17 However, no previous studies have evaluated duloxetine in hand OA. Gabapentinoids are three-substituted derivatives of the neurotransmitter gamma-aminobutyric acid that blocks voltage-dependent calcium channels, used to treat epilepsy and neuropathic pain. Interest has grown in gabapentinoids for arthritis, since gabapentin inhibits pain sensitization.18,19 Arthritic pain can be improved by NSAIDs and pregabalin in OA.20,21 Ohtori et al20 found that pregabalin combined with meloxicam was more effective for knee OA pain compared to either drug alone and Arendt-Nielsen et al21 showed that pain sensitization is improved by NSAIDs in knee OA.
We hypothesized that centrally acting analgesics may alleviate arthritic pain. We conducted a proof-of-concept, randomized, placebo-controlled study comparing duloxetine and pregabalin to placebo for hand OA pain. We used validated primary endpoints for pain, with secondary endpoints for pain sensitization using QST, depression, and anxiety scores. Our report is the first proof-of-concept clinical trial comparing the effect of centrally acting analgesics duloxetine and pregabalin head-to-head vs placebo in hand OA pain with mechanistic secondary endpoints for pain threshold testing.
Methods
Study design and participants
All methods were carried out in accordance with relevant guidelines and regulations. All trial protocols were approved by the sponsors, St George’s University of London and the Medicines and Healthcare Products Regulatory Agency, UK. Ethical approval was provided by the London-Surrey Borders Ethics Committee, approval number 12/LO/0047. Written informed consent was obtained from all subjects. The clinical trial registration number is NCT02612233. Participants were eligible if they were aged 40–75 years and had hand OA diagnosed by American College of Rheumatology (ACR) criteria22,23 confirmed by a rheumatologist and experiencing pain of at least ≥5 on a Numerical Rating Scale (NRS) of 0–10. The trial protocol was followed as published, according to CONSORT guidelines and inclusion–exclusion criteria. Twenty age-matched subjects without hand OA were enrolled as controls for comparisons for pain testing and brain MRI. The brain MRI data from this study will be reported in a separate publication.
Inclusion and exclusion criteria
Inclusion criteria were as follows: participants fulfilling the ACR criteria for the diagnosis of hand osteoarthritis, male or female, right or left handed, aged 40–75 years, and on usual care for hand OA including acetaminophen and/or NSAIDs. Exclusion criteria were another rheumatological diagnosis, eg, rheumatoid arthritis, current or planned pregnancy, contraindications to duloxetine or pregabalin such as concomitant use of monoamine oxidase inhibitors, selective serotonin reuptake inhibitors, antidepressants, oral contraceptives, St. John’s wort, history of depression, concomitant use of opioids including tramadol and pethidine, use of benzodiazepines, recent surgery, ie, <6 weeks prior to participation in the study, recent insertion of surgical implants, ie, <6 weeks before participation prior to entry, previous use of duloxetine and/or pregabalin, uncontrolled depression, estimated glomerular filtration rate <60 mL/min, hepatic impairment defined as ALT >2.5× upper limit of normal within 6 weeks of last clinical assessment, ischemic heart disease, diabetes mellitus, and regular use of alcohol or alcohol abuse (maximum limits are 28 units/week for men and 21 units/week for women, lactose intolerance). The estimated glomerular filtration rate was checked by screening blood tests, and any participants who were outside the stated range were not enrolled. Uncontrolled hypertension was checked by blood pressure in primary care, and any participants with a blood pressure >140/90 were excluded. Baseline laboratory tests of renal function and hepatic function were performed at baseline to screen for any impairment, and participants with levels outside the normal range were excluded. We checked information from all participants about a new diagnosis of diabetes, and any new cases were excluded. For the Hospital Anxiety and Depression Scale (HADS) scoring, a score of ≥12 for anxiety and/or depression was considered as too high for enrollment to the study and participants with a score of >12 were excluded.
Randomization and masking
Study drugs were supplied by Sharp Clinical Services (formerly Bilcare GCS, Powys, UK), which overencapsulated pregabalin 150 mg tablets or duloxetine 30 mg tablets and produced visually identical placebo capsules. A mid-ranging dose was selected for each of the trial medications. The random allocation sequence, with a block size of nine, was generated by the manufacturer and implemented through sequentially numbered containers. Neither participants nor investigators were aware of treatment assignment until after completion of the trial, which was performed after the last patient and last visit were conducted at the end of the trial. Emergency code breaks were administered independently by the staff from the St George’s University Hospitals NHS Foundation Trust’s Clinical Trials Pharmacy.
Clinical outcome measures
The primary endpoints were the NRS and the Australian and Canadian Hand Osteoarthritis Index (AUSCAN) rating scale 3.1 for pain,24,25 which are validated outcome measures for pain. Both NRS and AUSCAN pain endpoints are well-recognized primary endpoints in hand OA clinical trials and have been recommended in international guidelines.25,28
Prespecified secondary endpoints included the AUSCAN stiffness and function scales and HADS26 at baseline and after 12 weeks treatment. All endpoints were specified prospectively.
Pain algometry
Pain pressure thresholds (PPTs) were used to obtain objective measures of peripheral pain sensitization as we described previously.27 Briefly, a calibrated digital hand held algometer (FDX 100; Wagner Instruments, Greenwich, CT, USA) was used for all measurements. A standard operating procedure was used, which consisted of testing pain thresholds in all participants in both hands with n=30 regions for each participant, 780 regions in total. Regions tested included dorsal aspects of all distal interphalangeal, proximal interphalangeal and metacarpophalangeal joints of each digit and thumb and the dorsum of each wrist. The 1 cm2 flat rubber algometer probe was held perpendicular to the dorsal aspect of the skin, and force was applied to provide a constant increase in pressure at a rate of 1 N/cm2/s. Therefore, the algometer scores are stated as Newton per centimeter squared in all reported results. The individual was asked to say “stop” when the sensation of pressure became the first sensation of pain. The algometer was applied to each joint being examined three times in succession with an interval between applications. After all three readings were taken, the average from the last two readings was calculated as the PPT. The intervals between each algometer measurement were long enough to prohibit temporal summation.
Statistical analysis
Our sample size was based on IMMPACT guidelines25 and OARSI recommendations for RCTs in hand OA28 using NRS pain for the sample size calculation. For the NRS pain outcome, we aimed to detect a mean difference of 2.0 (SD 1.9) points between baseline and treatment after 12 weeks. With 16 participants in each group, 80% power with a 0.05 significance level (two sided) is achieved. Recruitment required up to 22 participants per treatment group, allowing a dropout rate of 25%, giving a total intervention study number of 65 participants to achieve desired statistical power.
Planned analyses included initial comparison to detect any significant differences between baseline and 13-week timepoints using primary endpoint NRS and AUSCAN pain difference between all three groups by ANOVA, with a multiple comparisons test, alpha =0.05. Following ANOVA, pairwise comparisons were performed for placebo vs pregabalin and placebo vs duloxetine. The intent-to-treat analysis was performed using the last observation at week 13 and carried forward for all participants. We present the NRS pain and AUSCAN pain, stiffness, and function outcomes as mean and confidence interval for all analyses. These are presented after checking the distribution of the data, which followed a normal distribution and were not skewed for the parameters measured. We also show the per protocol analysis for all completers.
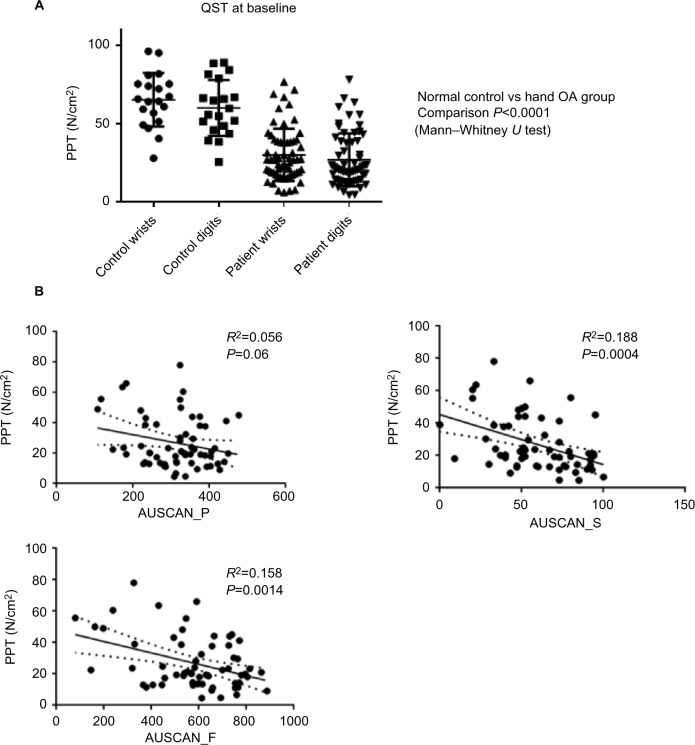
For the comparison of pain pressure algometry (PPT) in non-OA vs OA participants, Mann–Whitney U was used (Figure 1A). For correlation analyses between AUSCAN scores and PPT, an R2 correlation and P-value were calculated using GraphPad Prism (Figure 1B). In the bivariate comparisons of clinical outcome measures and PPT, SPSS was used to calculate an R2 correlation and P-value (Table 1).
Figure 1.
Pain sensitization characteristics of study population.
Notes: (A) Data from the DUPRO clinical trial demonstrating reduced pain thresholds globally in the wrist and finger joints in hand OA participants compared to normal age- and sex-matched controls. (B) Graphs demonstrating correlation for PPT in Newton per centimeter squared at baseline with clinical measures for AUSCAN_P, AUSCAN_S, and AUSCAN_F in all groups.
Abbreviations: AUSCAN_P, Australian and Canadian Hand Osteoarthritis Index pain; AUSCAN_S, Australian and Canadian Hand Osteoarthritis Index stiffness; AUSCAN_F, Australian and Canadian Hand Osteoarthritis Index function; OA, osteoarthritis; PPT, pain pressure threshold; QST, quantitative sensory testing.
Table 1.
Bivariate correlation analysis of clinical scores in study
| AUSCAN_P | AUSCAN_S | AUSCAN_F | QST | HADS_A | HADS_D | |
|---|---|---|---|---|---|---|
| NRS | 0.606 | 0.268 | 0.471 | −0.167 | −0.010 | 0.158 |
| 0.000 | 0.034 | 0.000 | 0.203 | 0.938 | 0.216 | |
| AUSCAN_P | 0.251 | 0.716 | −0.234 | 0.171 | 0.328 | |
| 0.047 | 0.000 | 0.072 | 0.179 | 0.009 | ||
| AUSCAN_S | 0.331 | −0.429 | 0.230 | 0.213 | ||
| 0.008 | 0.001 | 0.070 | 0.093 | |||
| AUSCAN_F | −0.409 | 0.132 | 0.294 | |||
| 0.001 | 0.304 | 0.019 | ||||
| QST | −0.285 | −0.187 | ||||
| 0.027 | 0.152 | |||||
| HADS_A | 0.692 | |||||
| 0.000 |
Abbreviations: AUSCAN_P, Australian and Canadian Hand Osteoarthritis Index pain; AUSCAN_S, Australian and Canadian Hand Osteoarthritis Index stiffness; AUSCAN_F, Australian and Canadian Hand Osteoarthritis Index function; HADS, Hospital Anxiety and Depression Scale; NRS, Numerical Rating Scale; QST, quantitative sensory testing.
We used IBM SPSS Statistics 21.0 for all analyses. Graphs were plotted using SPSS or GraphPad Prism Version 7.
Results
Characteristics of patient population
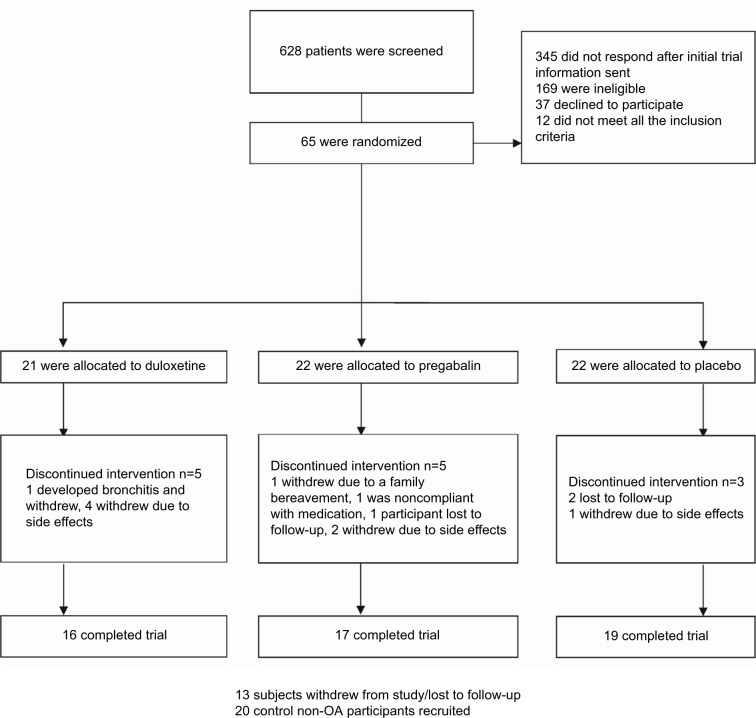
Between April 2013 and April 2016, we recruited 65 participants (Figure 2). A total of 21 participants were randomized to duloxetine, a further 22 participants were randomized to pregabalin, and 22 participants were randomized to placebo, respectively. There were 20 age-matched healthy volunteer participants enrolled for the comparison of pain scores using algometry and brain MRI (MRI data from this study will be reported separately). All 65 participants who were randomized to treatment were included in the intention-to-treat (ITT) analysis. A total of 52 participants completed the trial procedures after 13 weeks and were included in the per protocol analyses (Figure 3). Baseline characteristics show that the three treatment groups were well-matched for demographic data (Table 2). The mean disease duration was 3.5 years (SD 4.2), which was measured from the time that the participant was first told that they had a diagnosis of hand OA. For prior analgesic use, there was slightly less acetaminophen use at baseline before enrollment in the duloxetine group than in the pregabalin and placebo groups, but for other NSAIDs and opiates, analgesic use was similar in all three groups.
Figure 2.
CONSORT flow diagram for the DUloxetine or PRegabalin for Osteoarthritis pain (DUPRO) clinical trial.
Figure 3.
Study flow diagram and outcome measures.
Abbreviations: AUSCAN, Australian and Canadian Hand Osteoarthritis Index; HADS, Hospital Anxiety and Depression Scale; NRS, Numerical Rating Scale; OA, osteoarthritis.
Table 2.
Baseline characteristics of study patients (ITT analysis)
| Characteristics | Pregabalin (n=22) | Duloxetine (n=21) | Placebo (n=22) |
|---|---|---|---|
| Age (years), mean (SD) | 64.0 (5.0) | 62.3 (7.3) | 62.4 (8.7) |
| Women | 19 (86.4) | 14(66.7) | 19 (86.4) |
| White | 20 (90.9) | 20 (95.2) | 18 (81.8) |
| Black | 2 (9.1) | 1 (4.8) | 1 (4.6) |
| Asian | 3 (13.6) | ||
| Body mass index, mean (SD) | 27.1 (6.3) | 28.4 (5.9) | 27.0 (4.3) |
| NRS, mean (SD) | 6.1 (1.2) | 6.4 (1.5) | 6.4 (1.4) |
| AUSCAN pain score, mean (SD) | 317.0 (81.4) | 296.0 (105.2) | 320.3 (66.2) |
| HADS, mean (SD) | 11.6 (7.4) | 10.3 (6.1) | 12.2 (6.2) |
| Most common analgesics before inclusion | |||
| Acetaminophen | 15 | 8 | 15 |
| Other NSAID oral/topical | 7 | 5 | 5 |
| Codeine-based analgesic | 3 | 4 | 6 |
Note: Values are numbers (percentages) unless stated otherwise.
Abbreviations: AUSCAN, Australian and Canadian Hand Osteoarthritis Index; HADS, Hospital Anxiety and Depression Scale; ITT, intention-to-treat; NRS, Numerical Rating Scale; NSAID, nonsteroidal anti-inflammatory drug.
Patient-reported outcomes
ITT analysis
Participants in all three groups receiving duloxetine, pregabalin, or placebo reported improvement in pain at the end of the trial. Comparison of the three groups by ANOVA showed a significant difference at the end of treatment for NRS pain (P=0.035) and AUSCAN pain (P=0.0078) at the end of the trial. Following the primary analysis, pairwise comparisons were performed.
Pregabalin
Comparison of pregabalin vs placebo showed a significant improvement in the pregabalin group for primary outcomes of NRS pain (P=0.023), AUSCAN pain (P=0.008), and AUSCAN function (P=0.009) but not AUSCAN stiffness (P=0.22) scores (Table 3 and Figure 4).
Table 3.
Primary and secondary outcomes in ITT population
| Outcome at 13 weeks | Pregabalin (N=22) | Duloxetine (N=21) | Placebo (N=22) |
|---|---|---|---|
| NRS | |||
| Baseline (95% CI) | 6.1 (5.6 to 6.7) | 6.4 (5.7 to 7.1) | 6.4 (5.7 to 6.9) |
| 13 weeks (95% CI) | 3.4 (2.4 to 4.4) | 4.3 (2.6 to 5.9) | 5.4 (4.1 to 6.8) |
| Mean difference (95% CI) | −2.7 (−3.5 to −1.9) | −2.3 (−3.8 to −0.9) | −0.9 (−0.2 to 0.2) |
| P-value | 0.023* | 0.19 | |
| AUSCAN pain score | |||
| Baseline (95% CI) | 317.0 (280.8 to 353.1) | 296.0 (248.2 to 343.9) | 320.3 (290.9 to 349.6) |
| 13 weeks (95% CI) | 176.5 (123.9 to 229.1) | 248.1 (162.3 to 333.9) | 273.5 (218.0 to 329.0) |
| Mean difference (95% CI) | −132.1 (−181.1 to −82.9) | −35.8 (−119.7 to 48.2) | −46.61 (−93.9 to 0.75) |
| P-value | 0.008* | 0.59 | |
| AUSCAN stiffness | |||
| Baseline (95% CI) | 60.18 (51.7 to 68.7) | 60.95 (46.98 to 74.9) | 55.5 (45.2 to 65.8) |
| 13 weeks (95% CI) | 36.5 (23.0 to 49.9) | 48.25 (29.87 to 66.6) | 50.0 (36.0 to 64.0) |
| Mean difference (95% CI) | −18.7 (−33.1 to −4.3) | −13.5 (−26.5 to −0.6) | −5.67 (−16.8 to 5.5) |
| P-value | 0.22 | 0.96 | |
| AUSCAN function | |||
| Baseline (95% CI) | 576.2 (499.1 to 653.4) | 577.2 (478.0 to 676.4) | 582.3 (509.1 to 655.5) |
| 13 weeks (95% CI) | 362.2 (281.7 to 442.7) | 496.4 (342.4 to 650.5) | 508.7 (379.5 to 637.9) |
| Mean difference (95% CI) | −246.4 (−341.7 to −151.0) | −101.8 (−248.4 to −44.7) | −67.3 (−156.4 to −21.8) |
| P-value | 0.009* | >0.05 | |
| Consumption of rescue medication (total number of days) | 9 | 5 | 56 |
| HADS | |||
| Anxiety | |||
| Baseline (95% CI) | 6.5 (4.6 to 8.4) | 5.9 (4.3 to 7.6) | 7.2 (5.4 to 9.0) |
| 13 weeks (95% CI) | 5.2 (2.9 to 7.5) | 4.3 (2.2 to 6.3) | 8.2 (6.4 to 9.9) |
| Mean difference (95% CI) | −0.82 (−2.1 to 0.5) | −1.3 (−3.1 to 0.5) | 0.5 (−0.4 to 1.4) |
| P-value | 0.15 | 0.07 | |
| Depression | |||
| Baseline (95% CI) | 5.1 (3.6 to 6.7) | 4.4 (2.9 to 5.8) | 4.9 (3.6 to 6.2) |
| 13 weeks (95% CI) | 4.1 (2.6 to 5.6) | 3.8 (1.9 to 5.7) | 5.1 (3.9 to 6.3) |
| Mean difference (95% CI) | −1.1 (−2.1 to −0.02) | −0.3 (−1.9 to 1.2) | 0.05 (−1.3 to 1.4) |
| P-value | 0.66 | 0.99 |
Notes:
Indicates significant at <0.05
Abbreviations: AUSCAN, Australian and Canadian Hand Osteoarthritis Index; HADS, Hospital Anxiety and Depression Scale; ITT, intention-to-treat; NRS, Numerical Rating Scale.
Figure 4.
(A, B) Plots for change in primary outcome measures in all treatment groups (ITT analysis).
Abbreviations: AUSCAN, Australian and Canadian Hand Osteoarthritis Index; ITT, intention-to-treat; NRS, Numerical Rating Scale.
Duloxetine
For NRS pain and AUSCAN pain, function, and stiffness outcomes in patients receiving duloxetine compared to placebo, none of these outcomes were significantly different to placebo (Table 3).
Use of rescue medication
Average use of acetaminophen as rescue medication was much lower in the pregabalin and duloxetine groups than in the placebo group (Table 3). The use of rescue medication in the placebo group was higher, amounting to 56 days.
Per protocol analysis
There was a reduction in reporting pain in all three groups at the end of the trial. A significant difference between the three groups at the end of treatment for NRS pain score (P=0.04) was found by ANOVA. Pairwise comparisons between duloxetine and placebo, pregabalin, and placebo were then performed (Table 4).
Table 4.
Summary table for per protocol analysis
| Outcome at 13 weeks (imputed data on per protocol set) | Pregabalin | Duloxetine | Placebo |
|---|---|---|---|
| NRS | |||
| Baseline (95% CI) | 6.1 (5.4 to 6.7) | 6.6 (5.7 to 7.4) | 6.3 (5.6 to 6.9) |
| 13 weeks (95% CI) | 3.4 (2.4 to 4.4) | 4.3 (2.6 to 5.9) | 5.4 (4.1 to 6.6) |
| Mean difference (95% CI) | −2.7 (−3.5 to −1.9) | −2.3 (−3.8 to −0.9) | −0.9 (−2.3 to 0.2) |
| P-value | <0.0001* | 0.029* | |
| AUSCAN pain score | |||
| Baseline (95% CI) | 308.5 (262.6 to 354.5) | 310.6 (254.3 to 367.0) | 321.1 (288.7 to 353.4) |
| 13 weeks (95% CI) | 176.5 (123.9 to 229.1) | 248.1 (162.3 to 333.9) | 273.5 (218.0 to 329.0) |
| Mean difference (95% CI) | −132.0 (−181.1 to −82.9) | −62.5 (−141.6 to 16.6) | −47.1 (−93.8 to 11.7) |
| P-value | 0.013* | 0.9 | |
| AUSCAN stiffness | |||
| Baseline (95% CI) | 59.9 (51.8 to 67.9) | 61.8 (45.8 to 77.8) | 56.1 (44.5 to 67.7) |
| 13 weeks (95% CI) | 36.5 (23.0 to 49.9) | 48.3 (29.9 to 66.6) | 50.0 (36.0 to 64.0) |
| Mean difference (95% CI) | −23.4 (−35.7 to −11.1) | −13.5 (−26.5 to −0.6) | 5.7 (−16.8 to 5.5) |
| P-value | 0.06 | 0.46 | |
| AUSCAN function | |||
| Baseline (95% CI) | 608.5 (541.3 to 675.7) | 598.3 (481.2 to 715.3) | 580.0 (494.6 to 665.4) |
| 13 weeks (95% CI) | 362.2 (281.7 to 442.7) | 496.4 (342.4 to 650.5) | 508.7 (379.5 to 637.9) |
| Mean difference (95% CI) | −246.3 (−341.7 to −151.0) | −101.9 (−248.4 to −44.8) | −69.7 (−158.3 to −18.9) |
| P-value | 0.02* | 0.93 | |
| Consumption of rescue medication (total number of days) | 9 | 5 | 56 |
| HADS | |||
| Anxiety | |||
| Baseline (95% CI) | 6.1 (3.8 to 8.3) | 5.6 (3.6 to 7.5) | 7.6 (5.8 to 9.5) |
| 13 weeks (95% CI) | 5.2 (2.9 to 7.5) | 4.3 (2.2 to 6.3) | 8.2 (6.4 to 9.9) |
| Mean difference (95% CI) | −0.82 (−2.1 to 0.5) | −1.3 (−3.1 to 0.5) | 0.5 (−0.4 to 1.4) |
| P-value | 0.52 | 0.21 | 0.71 |
| Depression | |||
| Baseline (95% CI) | 5.1 (3.3 to 6.9) | 4.1 (2.3 to 5.9) | 5.4 (4.0 to 6.7) |
| 13 weeks (95% CI) | 4.1 (2.6 to 5.6) | 3.8 (1.9 to 5.7) | 5.1 (3.9 to 6.3) |
| Mean difference (95% CI) | −1.1 (−2.1 to −0.02) | −0.3 (−1.8 to 1.2) | 0.05 (−1.3 to 1.4) |
| P-value | 0.41 | 0.54 | 0.93 |
Notes:
Indicates significant at <0.05
Abbreviations: AUSCAN, Australian and Canadian Hand Osteoarthritis Index; HADS, Hospital Anxiety and Depression Scale; NRS, Numerical Rating Scale.
Pregabalin
For NRS pain, pregabalin was more effective than placebo (P<0.0001). Similarly, compared to placebo, there was a significant improvement in the pregabalin group for AUS-CAN pain (P=0.013), AUSCAN function (P=0.02) but not AUSCAN stiffness (P=0.06).
Duloxetine
For the comparison between placebo and duloxetine treatment, duloxetine was more effective as measured by NRS after 13 weeks (P=0.029). For AUSCAN pain, stiffness, and function outcomes in patients receiving duloxetine, these outcomes did not reach statistical significance.
Adverse events
Side effects were recorded prospectively throughout the study (Table 5). The placebo group showed fewer adverse events with a total of 22 recorded, with no difference in adverse events between the three groups (P=0.73). The highest reporting of adverse events was observed in the pregabalin and duloxetine groups: 55 adverse events were recorded with pregabalin, the most common of which were mental disturbance, headaches, sleepiness, dizziness, and dry mouth. In the duloxetine group, a total of 57 adverse events were recorded; there were a total of four withdrawals due to drug side effects and one participant withdrew due to the development of bronchitis, as shown in the CONSORT flow diagram (Figures 3 and 5). For the pregabalin group, there were two withdrawals due to drug side effects, one withdrawal due to a family bereavement, one withdrawal due to noncompliance, and one withdrawal due to loss to follow-up.
Table 5.
Side effect profile in all three treatment groups from ITT analysis
| System | Pregabalin (N=22) | Duloxetine (N=21) | Placebo (N=22) |
|---|---|---|---|
| Cardiovascular | 3 | 2 | 1 |
| Digestive | 7 | 18 | 5 |
| ENT | 2 | ||
| Endocrine/metabolic | 1 | ||
| Genitourinary | 1 | ||
| Hematological | |||
| Mental | 9 | 9 | 9 |
| Nervous system | |||
| Dry mouth | 6 | 6 | 4 |
| Headaches | 3 | 8 | |
| Dizziness | 7 | 3 | |
| Sleepiness | 5 | 3 | |
| Loss of balance | 7 | ||
| Ophthalmological | 4 | 2 | 1 |
| Respiratory | 2 | 3 | |
| Skin | 1 | 2 | |
| Total | 55 | 57 | 22 |
Abbreviations: ENT, ear, nose and throat; ITT, intention-to-treat.
Figure 5.
CONSORT 2010 checklist of information for the DUloxetine or PRegabalin for Osteoarthritis pain (DUPRO) randomized controlled trial.
Note: *Page numbers optional depending on journal requirements. Hopewell S, Clarke M, Moher D, Wager E, Middleton P, Altman DG, et al. CONSORT for reporting randomised trials in journal and conference abstracts. Lancet. 2008;371:281–283.38
Pain sensitization by PPT and relation to clinical scores
Using PPT testing as a measure for pain sensitization, compared to non-OA controls, the hand arthritis group had globally reduced pain thresholds (P<0.0001) across all finger joints at baseline, even at the metacarpophalangeal joints and wrists where there was little evidence of radiographic OA (Figure 1A). We investigated the correlation between the various clinical scores at baseline with age as a covariate (Table 1). Measurements for the PPT modality of QST showed a significant correlation with AUSCAN stiffness (R2=0.188, P=0.0004) and function (R2=0.158, P=0.0014) for all patients at baseline (Figure 1B). There was a trend for lower PPT correlated with higher AUSCAN pain scores, although this trend did not reach statistical significance (P=0.06). NRS pain correlated strongly with AUSCAN pain and function (P<0.0001). PPT measures did not change significantly in any of the three groups after 3 months treatment. We found that all participants with hand OA had lower PPT scores at baseline compared to healthy controls at inclusion and demonstrated a reduction of NRS at follow-up. HADS anxiety and depression scores were significantly correlated after Bonferroni correction. There were weaker correlations (significant without correction) between HADS depression and AUSCAN pain (P=0.009) and between AUSCAN function and stiffness scores (P=0.008).
Discussion
Principal findings
Our clinical study provides the first evidence in chronic painful hand OA that pregabalin and duloxetine are analgesics with potential for use in OA pain, with pregabalin providing the best treatment response and sustained effects beyond the reduction in dose. Second, we observed by QST that hand arthritis subjects have pain sensitization, which may include peripheral and central mechanisms. Third, the central but distinct actions of pregabalin and duloxetine could therefore be exerting an effect on central pain sensitization, which we and others have demonstrated as a significant component of arthritic pain.14,15 Finally, we observed improvement for pregabalin in NRS for the ITT analysis, but not for duloxetine, with improvement in NRS for both active drugs only in the per protocol analysis. The results of our trial have strong clinical relevance, since many patients report lack of efficacy or side effects on NSAIDs and other patients have important safety concerns.
Study strengths and limitations
The lack of new analgesic targets for OA in this most common arthritic disease, coupled with recent data from animal models,18,19 prompted us to investigate the use of the gabapentinoid pregabalin and the SNRI duloxetine. Pregabalin is licensed for neuropathic pain29 and duloxetine for depression and diabetic neuropathic pain.30 Our proof-of-concept trial demonstrated an improvement in pain for pregabalin and also for duloxetine after 13 weeks treatment. We enrolled subjects who had an NRS pain rating of at least 5 to ensure that clinically meaningful improvements in pain could be detected. There were some differences in our ITT and per protocol analysis: in ITT, pregabalin, but not duloxetine, showed a significant improvement in pain compared to placebo; and in the per protocol analysis, both agents showed an improvement in pain. Although we observed an improvement in pain reporting for both centrally acting agents, pregabalin was more effective after 13 weeks. In our secondary endpoint analyses, we did not see any significant improvement in depression or anxiety scores in any treatment group.
Since this was a proof-of-concept analgesic endpoint study, we did not collect structural outcome data including joint damage progression changes by plain radiograph and synovitis by ultrasound, as described in other studies,31–34,37 which could be addressed in future work. We also recognize that our study has been conducted in hand arthritis pain, whereas several large previous datasets have focused on knee OA,35–37 and there may be some differences in pain characteristics including loading effects due to structural joint differences between the hand and knee.
Main results in context of other literature
In knee OA, Chappell et al17 showed that duloxetine was effective for pain, Ohtori et al20 found that pregabalin with meloxicam was more effective than pregabalin alone, and our data show that both agents have efficacy in chronic hand OA pain with pregabalin showing superiority over duloxetine.
Recent concepts in novel therapeutic agents for OA have included potential therapeutics for structural changes in the joint including synovitis31 and bone marrow lesions (BML).32 However, such studies have not been without difficulty since recent trials targeting the inflammatory component of OA have not shown improved outcomes33 and the use of bisphosphonates potentially for reducing BML-related pain need to define significant clinical and structural endpoints.34 It is possible that patients demonstrating a largely “inflammatory” phenotype are likely to benefit from agents such as NSAIDs, and when there are features of sensitization with ongoing pain, patients may require additional treatment such as centrally acting agents including pregabalin and duloxetine. In the clinic, patients may also require additional treatments if NSAIDs are linked to side effects, lack of efficacy, and ongoing pain.
There is recognition that pain sensitization occurs in people with OA.11–15,35–37 The main indication from our data of peripheral sensitization in hand OA is that the control subjects had significantly higher PPTs than the hand OA group. We noted that PPTs did not change significantly after treatment, suggesting that pathways which led to sensitization in hand arthritis may continue to exist in the patients even after drug treatment.
Implications for practice and future research
Pregabalin and duloxetine had efficacy in hand OA pain in our clinical study, with pregabalin showing greater effect than duloxetine for validated pain endpoints. In our study, one or more of the following analgesics had been used by more than half of the participants prior to enrollment in the study: acetaminophen, NSAID, or codeine-based analgesics. When such analgesics had not previously been effective, our trial showed that pregabalin, and to a less significant degree duloxetine, may provide a realistic alternative to pain management in OA. In future, clinical trials that examine the efficacy of centrally acting analgesics over a longer treatment period of >12 weeks in chronic arthritic pain should be conducted. Further studies measuring peripheral and central sensitization will be crucial to understand how pain, loss of function, comorbid conditions, and medication use contribute to the development of arthritic pain.
Acknowledgments
This work was supported by The Rosetrees’ Trust, grant number M11-F1, by the UK National Institute of Health (NIHR) Clinical Research Network and an NIHR Clinical Academic Fellowship to MR. The views expressed are those of the authors and not necessarily those of the NHS, NIHR, or Department of Health.
Footnotes
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Dahaghin S, Bierma-Zeinstra SMA, Reijman M, Pols HAP, Hazes JMW, Koes BW. Prevalence and determinants of one month hand pain and hand related disability in the elderly (Rotterdam study) Ann Rheum Dis. 2005;64(1):99–104. doi: 10.1136/ard.2003.017087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Felson DT, Lawrence RC, Dieppe PA, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2005;13(8):635–646. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 3.Bijlsma JW, Berenbaum F, Lafaber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;18(377):2115–2126. doi: 10.1016/S0140-6736(11)60243-2. [DOI] [PubMed] [Google Scholar]
- 4.NICE [webpage on the Internet] NICE Guidelines ‘Osteoarthritis: Care and Management’. 2014. [Accessed May 18, 2017]. Available from: https://www.nice.org.uk/guidance/cg177.
- 5.Zhang W, Doherty M, Leeb BF, et al. EULAR evidence based recommendations for the management of hand osteoarthritis: report of a Task Force of the EULAR Standing Committee for international clinical studies including therapeutics (ESCISIT) Ann Rheum Dis. 2007;66(3):377–388. doi: 10.1136/ard.2006.062091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Da Costa BR, Reichenbach S, Keller N, et al. Effectiveness of nonsteroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2016;387:2093–2105. doi: 10.1016/S0140-6736(16)30002-2. [DOI] [PubMed] [Google Scholar]
- 7.Williams CM, Maher CG, Latimer J, et al. Efficacy of paracetamol for acute low-back pain: a double-blind, randomised controlled trial. Lancet. 2014;384(9954):1586–1596. doi: 10.1016/S0140-6736(14)60805-9. [DOI] [PubMed] [Google Scholar]
- 8.Woolf CJ. Pain: Moving from symptom control toward mechanism-specific pharmacologic management. Ann Inter Med. 2004;140:441–451. doi: 10.7326/0003-4819-140-8-200404200-00010. [DOI] [PubMed] [Google Scholar]
- 9.Kidd BL, Urban LA. Mechanisms of inflammatory pain. Br J Anaesth. 2001;87:3–11. doi: 10.1093/bja/87.1.3. [DOI] [PubMed] [Google Scholar]
- 10.Suokas AK, Walsh DA, McWilliams DF, et al. Quantitative sensory testing in painful osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2012;20(10):1075–1085. doi: 10.1016/j.joca.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Arendt-Nielsen L, Nie H, Laursen MB, et al. Sensitization in patients with painful knee osteoarthritis. Pain. 2010;149(3):573–581. doi: 10.1016/j.pain.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Sofat N, Ejindu V, Kiely P. What makes OA painful? The evidence for peripheral and central pain processing. Rheumatology. 2011;50:2157–2165. doi: 10.1093/rheumatology/ker283. [DOI] [PubMed] [Google Scholar]
- 13.Neogi T, Frey-Law L, Scholz J, et al. Multicenter Osteoarthritis (MOST) Study Sensitivity and sensitisation in relation to pain severity in knee osteoarthritis: trait or state? Ann Rheum Dis. 2015;74(4):682–688. doi: 10.1136/annrheumdis-2013-204191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sofat N, Smee C, Hermansson M, et al. Functional MRI demonstrates pain perception in hand osteoarthritis has features of central pain processing. J Biomed Graph Comput. 2013;3(4):20–26. doi: 10.5430/jbgc.v3n4p20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gwilym S, Filippini N, Douaud G, Carr A, Tracey I. Thalamic atrophy associated with painful osteoarthritis of the hip is reversible after arthroplasty: a longitudinal voxel-based morphometric study. Arthritis Rheum. 2010;62(10):2930–2940. doi: 10.1002/art.27585. [DOI] [PubMed] [Google Scholar]
- 16.Abaei M, Sagar DR, Stockley EG, et al. Neural correlates of hyperalgesia in the monosodium iodoacetate model of osteoarthritis pain. Mol Pain. 2016;12:1–12. doi: 10.1177/1744806916642445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chappell AS, Ossanna MJ, Liu-Seifert H, et al. Duloxetine, a centrally acting analgesic in the treatment of patients with osteoarthritis knee pain: a 13 week, randomized, placebo-controlled trial. Pain. 2009;146:253–260. doi: 10.1016/j.pain.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 18.Park J, Yu YP, Zhou CY, et al. Central mechanisms mediating thrombospondin-4-induced pain states. J Biol Chem. 2016;291(25):13335–13348. doi: 10.1074/jbc.M116.723478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan B, Guo Y, Wu HE, et al. Thrombospondin-4 divergently regulates voltage-gated Ca2+ channel subtypes in sensory neurons after nerve injury. Pain. 2016;157(9):2068–2080. doi: 10.1097/j.pain.0000000000000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohtori S, Inoue G, Orita S, et al. Efficacy of combination of meloxicam and pregabalin for pain in knee osteoarthritis. Yonsei Med J. 2013;54(5):1253–1258. doi: 10.3349/ymj.2013.54.5.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arendt-Nielsen L, Lindhardt Egshard L, Petersen KK. Evidence for a central mode of action for COX-2 inhibitor in patients with painful knee osteoarthritis. Pain. 2016;157:1634–1644. doi: 10.1097/j.pain.0000000000000562. [DOI] [PubMed] [Google Scholar]
- 22.Altman R, Alarcon G, Appelrouth D, et al. The American College of Rheumatology Criteria for the classification and reporting of osteoarthritis of the hand. Arthritis Rheum. 1990;33(11):1601–1610. doi: 10.1002/art.1780331101. [DOI] [PubMed] [Google Scholar]
- 23.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellamy N, Hochberg M, Tubach F, et al. Development of multinational definitions of minimal clinically important improvement and patient acceptable symptomatic state in osteoarthritis. Arthritis Care Res (Hoboken) 2015;67(7):972–980. doi: 10.1002/acr.22538. [DOI] [PubMed] [Google Scholar]
- 25.Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 26.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale: an updated literature review. J Psychosom Res. 2002;52(2):69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 27.Wajed J, Hermansson M, Axford J, Sofat N. Pain threshold testing using algometers is a reliable measure of pain in hand osteoarthritis and correlates with patient-reported pain scores. Rheumatology. 2011;50:iii91. [Google Scholar]
- 28.Kloppenburg M, Maheu E, Kraus VB, et al. OARSI clinical trials recommendations for RCTs. Osteoarthritis Cartilage. 2015;23:772–786. doi: 10.1016/j.joca.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 29.Freynhagen R, Serpell M, Emir B, et al. A comprehensive drug safety evaluation of pregabalin in peripheral neuropathic pain. Pain Pract. 2015;15(1):47–57. doi: 10.1111/papr.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gierthmulen J, Baron R. Neuropathic pain. Semin Neurol. 2016;36(5):462–468. doi: 10.1055/s-0036-1584950. [DOI] [PubMed] [Google Scholar]
- 31.Kingsbury SR, Tharmanathan P, Arden NK, et al. Pain reduction with oral methotrexate in knee osteoarthritis, a pragmatic Phase III trial of treatment effectiveness (PROMOTE): study protocol for a randomized controlled trial. Trials. 2015;16:77. doi: 10.1186/s13063-015-0602-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bruyere O, Cooper C, Arden N, et al. Can we identify patients with high risk of osteoarthritis progression who will respond to treatment? A focus on epidemiology and phenotype of osteoarthritis. Drugs Aging. 2015;32:179–187. doi: 10.1007/s40266-015-0243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basoki N, Lee W, Ruijgrok L, et al. Efficacy of hydroxychloroquine in primary hand osteoarthritis: a randomized, double-blind, placebo controlled trial. Ann Rheum Dis. 2015;74:188. [Google Scholar]
- 34.Davis AJ, Smith TO, Hing CB, Sofat N. Are bisphosphonates effective in the treatment of osteoarthritis pain? A research synthesis and meta-analysis. PLoS One. 2013;8:e72714. doi: 10.1371/journal.pone.0072714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wylde V, Palmer S, Learmonth ID, Dieppe P. Test-retest reliability of quantitative sensory testing in knee osteoarthritis and healthy participants. Osteoarthritis Cartilage. 2011;19(6):655–658. doi: 10.1016/j.joca.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 36.Arendt-Nielsen L, Skou ST, Nielsen TA, Petersen KK. Altered central sensitization and pain modulation in the CNS in chronic joint pain. Curr Osteoporosis Rep. 2015;13(4):225–234. doi: 10.1007/s11914-015-0276-x. [DOI] [PubMed] [Google Scholar]
- 37.Felson DT, Niu J, Neogi T, et al. MOST Investigators Group Synovitis and the risk of knee osteoarthritis: the MOST study. Osteoarthritis Cartilage. 2016;24(3):458–464. doi: 10.1016/j.joca.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hopewell S, Clarke M, Moher D, Wager E, Middleton P, Altman DG, et al. CONSORT for reporting randomised trials in journal and conference abstracts. Lancet. 2008;371:281–283. doi: 10.1016/S0140-6736(07)61835-2. [DOI] [PubMed] [Google Scholar]