Abstract
Finasteride is used to treat male pattern baldness and benign prostatic hyperplasia. This study investigated the toxicity of finasteride and recovery by DA-9401 using Sprague Dawley (SD) rats. Forty adult male SD rats were assigned to four groups: control (CTR), finasteride 1 mg/kg/day (F), finasteride 1 mg/kg + DA-9401 100 mg/kg/day (F + DA 100) and finasteride 1 mg/kg + DA-9401 200 mg/kg/day (F + DA 200). Treatments were by oral delivery once daily for 90 consecutive days. The gross anatomical parameters assessed included: genital organ weight; vas deferens sperm count and sperm motility; testosterone, dihydrotestosterone (DHT) and malondialdehyde levels; and histological and terminal deoxynucleotidyl transferase enzyme mediated dUTP nick-end labeling (TUNEL) staining of testis for spermatogenic cell density, Johnsen’s score and apoptosis. Testicular tissue was also used for evaluating endoplasmic reticulum (ER) stress and apoptotic proteins. Epididymis weight, seminal vesicle weight, prostate weight, penile weight and vas deferens sperm motility showed significant differences between the F group and the CTR, F + DA 100 and F + DA 200 groups. There was no significant change in the testosterone level. DHT level decreased significantly in the F group compared with the CTR group. Testis tissue revealed significant changes in spermatogenic cell density, Johnsen’s score and apoptotic index. Western blot showed significant changes in the ER stress and apoptotic markers. Finasteride resulted in reduced fertility and increased ER stress and apoptotic markers, which were recovered by administration of DA-9401 in the SD rats.
Keywords: finasteride, DA-9401, infertility, sperm, dihydrotestosterone, endoplasmic reticulum stress, apoptosis
Introduction
Finasteride and dutasteride are 5α-reductase inhibitors that are commonly used to treat male pattern baldness and benign prostatic hyperplasia (BPH).1 Androgens, especially testosterone, increase libido, but the drug interferes with the action of androgens and so has been implicated in infertility.2 Recently, Ribeiro et al reported that adult rat males treated with finasteride during perinatal life exhibited increased rate of preimplantation loss.3 The subfertility seems to be due to a defect in sperm morphology.3 Finasteride may also alter sperm motility and function of sex glands, including changes in fructose, vitamins and enzymes content. 5α-reductase inhibition might have caused an increased sensitivity of Leydig cell to luteinizing harmone (LH) in adulthood. Therefore, finasteride could interfere with spermatogenesis by affecting hypothalamic–pituitary–testicular axis.
A review of 73 papers on medical therapies for BPH focused on the effects of different pharmacological agents on sexual function.4 A number of isolated case reports have also been published on the effect of low-dose finasteride on DNA changes in sperm and on sperm motility and sperm counts.5 Another small study reported on three cases of young men who had used finasteride for 5 years and who were investigated for male infertility.6 Semen quality was investigated by light microscopy to evaluate sperm concentration and motility, sperm morphology by transmission electron microscopy (TEM), presence of Y microdeletions by PCR and meiotic segregation by fluorescence in situ hybridization (FISH). TEM analysis revealed altered sperm morphology consistent with necrosis, and FISH data revealed elevated diploidy and sex chromosome disomy frequencies.6
Accumulation of free radicals coupled with an increase in oxidative stress has been implicated in the pathogenesis of several disease states. Sperm damage occurs when oxidative stress overcomes natural antioxidant defenses.7 Oxidative stress is a crucial regulator of endoplasmic reticulum (ER) function and activation of the unfolded protein response (UPR) in disease conditions, as ER stress and increased oxidative stress production occur concurrently.8 Reactive oxygen species (ROS)-linked ER stress has been associated with phosphorylated inositol requiring kinase 1 (p-IRE1) and phosphorylated c-Jun-N-terminal kinase (p-JNK) signaling transduction.9,10
Antioxidants are widely available and inexpensive when compared to other fertility treatments and are used by many men to improve their fertility. Oral antioxidant supplements may improve sperm quality by reducing oxidative stress.11 DA-9401 is a novel compound that is a mixture of extracts of three medicinal plants: Morinda officinalis, Allium cepa and Cuscuta chinensis. DA-9401 acts as an antioxidant and is under development for the treatment of male subfertility. However, no data are available on the effects of DA-9401 on ROS-based finasteride-related infertility. In this study, we evaluated the pathophysiology, efficacy and safety of DA-9401 in a Sprague Dawley (SD) rat model of finasteride-induced infertility.
Materials and methods
Animals
All animal procedures were conducted in accordance with the Principles of Laboratory Animal Care of Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC) of Chonbuk National University Hospital (approval no: CUH-IACUC-151201-34). Institutional Animal Care and Use Committees (IACUC) of Chonbuk National University Hospital was accredited by AAALAC on October 28, 2016.
Sixty sexually mature male SD rats (weight, 300–320 g; age, 10–12 weeks) were fed standard rat chow and had free access to water. They were maintained in an animal facility under constant environmental conditions (room temperature, 20°C±2°C; relative humidity, 50%±10%; and a 12-hour light-dark cycle).
Experimental protocol
The rats were randomly divided into four groups with 10 rats in each group. The control group (CTR) was not treated. A second group received finasteride (Sigma-Aldrich Co., St Louis, MO, USA) 1 mg/kg/day (F). A third group received finasteride 1 mg/kg + DA-9401 100 mg/kg/day (F + DA 100). A fourth group received finasteride 1 mg/kg + DA-9401 200 mg/kg/day (F + DA 200). Finasteride and both doses of DA-9401 were mixed in normal saline. Treatments were given orally each day using a Zonde needle (JD-S-124; Jeungdo, Seoul, Korea) for 90 days. After 90 days, rats were anesthetized using a mixture of ketamine (100 mg/mL) and 2% xylazine (20 mg/mL). Testis, epididymis, seminal vesicle and prostate were immediately removed and placed in liquid nitrogen for further analysis.
Medication intervention, ingredients and HPLC analysis of DA-9401
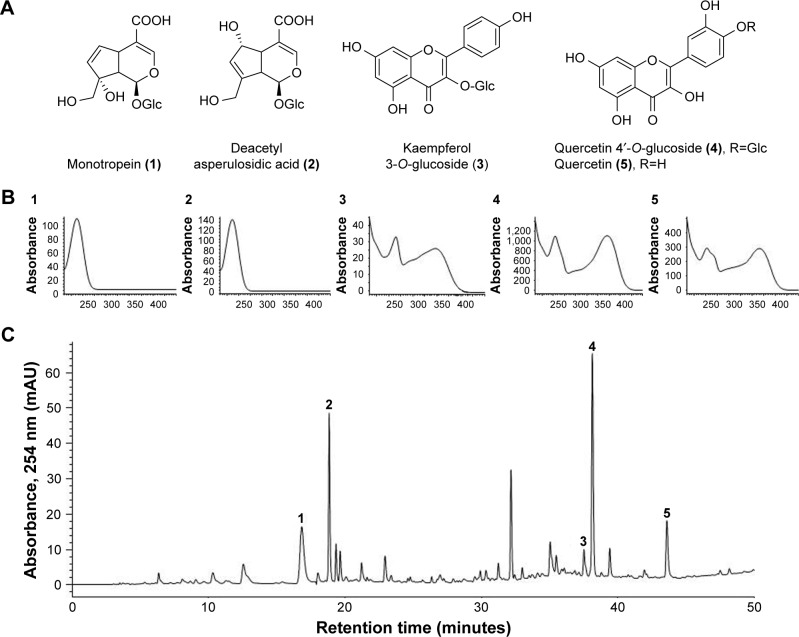
DA-9401 is a medication to treat infertility and is being developed by the Dong-A Pharmaceutical Company (Kyoungi, South Korea) to treat infertility. It is a mixture of natural extracts of three medicinal herbs (roots of Morinda officinalis, outer scales of Allium cepa and seeds of Cuscuta chinensis). M. officinalis root (Rubiaceae) is used to treat rheumatoid arthritis and impotence in traditional Oriental medicine. Monotropein (1) and deacetyl asperulosidic acid (2) are the major iridoid compounds in M. officinalis. Monotropein has anti-nociceptive and anti-inflammatory activities.12,13 Kaempferol 3-O-glucoside (3) is a major flavonoid found in the seeds of C. chinensis, which is an important Oriental traditional medicine widely used to improve sexual function and prevent and treat cardiovascular diseases.14,15 The outer scales of onion (A. cepa) are enriched in quercetin (5) and quercetin 4′-O-glucoside (4), which are effective antioxidants. Other reported pharmacological activities include liver protection, immune enhancement, anti-infection, anti-stress and anticancer effects.16
The herbs were ground and extracted with ethanol under reflux for 3 hours at 70°C–80°C and the procedure was repeated for three times. The combined filtrate was concentrated in a rotary evaporator, freeze-dried and stored at −20°C until required. The quality of DA-9401 was determined using high-performance liquid chromatography (HPLC). DA-9401 (20 mg) was dissolved in 30 mL of methanol and filtered through a 0.45 μM membrane filter. Ten microliters of the filtrate was injected into the HPLC system for analysis. The peaks in the DA-9401 HPLC profile were identified by comparison with the retention times and ultraviolet (UV) spectra of standard compounds. The HPLC profile of DA-9401 and its identified compounds are shown in Figure 1. Quality control of DA-9401 as a mixture of herbal extracts requires marker compounds. Peak identification was performed by comparison of HPLC retention times and UV spectra of purified standard compounds. Monotropein (1) and deacetyl asperulosidic acid (2) in M. officinalis, kaempferol 3-O-glucoside (3) in the seeds of C. chinensis, quercetin 4′-O-glucoside (4) and quercetin (5) in A. cepa were purified by repeated chromatography and preparative HPLC. Their chemical structures were elucidated by nuclear magnetic resonance and comparison with data reported previously.12,17
Figure 1.
HPLC chromatogram of DA-9401 and UV spectra of major marker components of the herbal ingredients.
Notes: (A) Chemical structures, (B) UV spectra of marker compounds (1–5) and (C) HPLC chromatogram of DA-9401. Monotropein (1) and deacetyl asperulosidic acid (2) in Morinda officinalis, kaempferol 3-O-glucoside (3) in the seeds of Cuscuta chinensis, quercetin 4′-O-glucoside (4) and quercetin (5) in Allium cepa. Each peak of M in the HPLC chromatogram was identified by comparison with the retention times and UV spectra of standard compounds.
Abbreviations: HPLC, high-performance liquid chromatography; UV, ultraviolet.
Sperm motility and sperm counts in the vas deferens
The vas deferens were removed and placed in separate 1.5 mL microcentrifuge tubes, minced and suspended in normal saline at 37°C for 5 minutes. Sperm motility was evaluated by observing a sperm suspension within 3–5 minutes after it was placed on the sperm counting chamber (Makler/Sperm Meter; SEFI-Medical Instruments, Haifa, Israel). This method mitigates errors that result from the tendency of spermatozoa to migrate from the periphery. The number of motile spermatozoa within the 10 central squares of the grid was counted using an Axio Imager 2 light microscope (Carl Zeiss Meditec AG, Jena, Germany), and mean sperm counts were recorded. The percentage of motile spermatozoa was determined as the (mean number of motile spermatozoa/total number of spermatozoa) × 100%.
The total sperm count was calculated using two or three drops of each specimen to increase the reliability of the count. The number of sperm heads in 10 squares was counted. The recorded sperm count represented the concentration of spermatozoa as millions of spermatozoa per mL, and the mean value was reported. Spermatozoa were counted using the 20X magnification objective of the light microscope.
Testosterone and DHT
Blood was collected from the vena cava. For testosterone estimation, 10 μL of heparin was added to 1 mL of blood and centrifuged at 3,500 rpm for 10 minutes. Plasma was transferred to a 5 mL tube and sealed with paraffin film. Samples were sent to the hospital laboratory for testosterone assay. DHT was analyzed using a DHT ELISA kit (Cat # 11-DHTHU-E01; BioCat, Salem, NH, USA).
Malondialdehyde
Malondialdehyde (MDA) is a marker for oxidative stress. Homogenized testis tissue was used for MDA analysis based on its reaction with thiobarbituric acid to form a pink complex with absorption maximum at 535 nm.
Histology
For histological estimations, small pieces of testis were fixed in formalin and stained with hematoxylin and eosin. Sections were examined by light microscopy for spermatogenic cell density measurements. Spermatogenic cell density was determined by measuring the thickness of the germinal cell layer and the diameter of the seminiferous tubules. Seminiferous tubules were graded by Johnsen’s scoring. In this system of classification, seminiferous tubules are assessed according to the presence of spermatogenic cells and each is given a score from 1–10. Complete spermatogenesis with many spermatozoa present is given a score of 10.
Apoptotic index
The DeadEnd™ Colorimetric TUNEL System (G7130 and G7360; Promega Corporation, Fitchburg, WI, USA) was used to quantify apoptosis in the testis tissue according to the manufacturer’s instructions. Dark brown cells were considered as apoptotic seminiferous tubules. For image analysis, five randomly selected fields per testis were examined to evaluate seminiferous epithelial quality; these fields were photographed at 200× magnification. The apoptotic index (AI) was determined as 100× number of terminal deoxynucleotidyl transferase enzyme mediated dUTP nick-end labeling (TUNEL)-positive cell nuclei/total number of cell nuclei.
Western blot
Glucose-regulated protein-78 (GRP-78), phosphorylated inositol-requiring transmembrane kinase/endoribonuclease 1 (p-IRE1), p-JNK, procaspase-3 and cleaved caspase-3 measurements in testis tissue were conducted with tissue that had been washed with cold phosphate-buffered saline (PBS). Lysis buffer with protease inhibitor was added to tissue, a cordless motor pellet pestle was used to pestle and the mixture was centrifuged at 12,000 rpm for 30 minutes at 4°C. The samples were used for 10%, 12% and 15% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis, and the resolved proteins were transferred to polyvinylidene fluoride membranes using a Trans-blot® SD semi-dry electrophoretic transfer cell (Bio-Rad Laboratories Inc., Hercules, CA, USA). After transfer, each membrane was blocked with 10% bovine serum albumin for 1 hour and incubated with phosphorylated antibody to p-IRE1 (ab-48187; Abcam, Cambridge, UK) or p-JNK (SC-6254; Santa Cruz Biotechnology Inc., Dallas, TX, USA), or blocked with 10% nonfat milk before exposure to antibodies to nonphosphorylated GRP-78 (SC-376768; Santa Cruz Biotechnology Inc.), procaspase-3 and cleaved caspase-3 (CST-9662S and CST-9664S, respectively; Cell Signaling Technology, Danvers, MA, USA). Each membrane was washed with Tris-buffered saline Tween (TBST) three times prior to the addition of 1:5,000 dilution of secondary antibody for 1 hour. Each membrane was washed three times with TBST and processed using enhanced chemiluminescence substrate.
Statistical analysis
All the data are expressed as mean ± standard error. The statistical analysis was carried out using SigmaPlot 12.0 (Systat Software, San Jose, CA, USA) with P<0.05 being considered statistically significant.
Results
Body and organ weights
Body weight, testis weight and epididymis weight showed no significant changes in the F group, but body and epididymis weights were significantly lowered in the F + DA 100 group compared to the CTR and F groups. Seminal, prostate and penile weights were lowered in F, F + DA 100, and F + DA 200 groups compared to the CTR group, and were reduced more in the F + DA 100 and 200 groups compared to the F group (Table 1).
Table 1.
Effect of finasteride and DA-9401 on body, testicular, epididymis, seminal vesicle, prostate and penile weights
| Variables | CTR | F | F + DA 100 | F + DA 200 |
|---|---|---|---|---|
| Body wt at sacrifice | 510.10±20.23 | 480.30±11.54 | 430±14.11*,# | 450.3±13.4 |
| Testis wt | 2.21±0.05 | 2.24±0.06 | 2.21±0.05 | 2.18±0.04 |
| Epididymis wt | 0.75±0.03 | 0.70±0.01 | 0.66±0.01*,# | 0.68±0.02 |
| Seminal vesicle wt | 2.23±0.08 | 1.61±0.06** | 1.01±0.06**,## | 1.05±0.06**,## |
| Prostate wt | 1.48±0.10 | 0.86±0.05** | 0.66±0.04**,# | 0.62±0.05**,# |
| Penile wt | 0.43±0.02 | 0.35±0.02* | 0.34±0.01* | 0.33±0.02*,# |
Notes: Data are presented in mean ± SE;
P<0.05 versus CTR group,
P<0.001 versus CTR group,
P<0.05 versus F group,
P<0.001 versus F group.
Abbreviations: wt, weight in grams; CTR, control; F, finasteride 1 mg/kg (po); F + DA 100, finasteride 1 mg/kg + DA-9401 100 mg/kg (po); F + DA 200, finasteride 1 mg/kg + DA-9401 200 mg/kg (po); po, per oral; SE, standard error.
Sperm count, motility and percentage of pregnant females
Vas deferens sperm count showed no changes among the groups. Vas deferens sperm motility showed significant increase in the F + DA 100 group than in the F group. The percentage of pregnant female rats were also low in the F group compared to the CTR group and slightly recovered in the F + DA 100 and F + DA 200 groups (Table 2). Female rats were only used to find the number of pregnancies.
Table 2.
Effect of finasteride and DA-9401 on vas deferens, sperm count, sperm motility and percent of pregnancy
| Variables | CTR | F | F + DA 100 | F + DA 200 |
|---|---|---|---|---|
| Vas deferens sperm count (106) | 24.85±2.69 | 18.85±1.76 | 24.60±2.45 | 22.65±2.35 |
| Vas deferens sperm motility (%) | 47.44±4.34 | 44.10±3.89 | 58.50±4.14* | 45.79±3.52 |
| Pregnant females (%) | 100 | 70 | 90 | 80 |
Note: Data are presented in mean ± SE;
P<0.05 versus CTR group.
Abbreviations: CTR, control; F, finasteride 1 mg/kg (po); F + DA 100, finasteride 1 mg/kg + DA-9401 100 mg/kg (po); F + DA 200, finasteride 1 mg/kg + DA-9401 200 mg/kg (po); po, per oral; SE, standard error.
Testosterone and DHT
The testosterone level was increased in all finasteride groups compared to the CTR group but not significantly. DHT was significantly decreased in the F, F + DA 100 and F + DA 200 groups compared to the CTR group (Table 3).
Table 3.
Effect of finasteride and DA-9401 on testosterone and dihydrotestosterone
| Variables | CTR | F | F + DA 100 | F + DA 200 |
|---|---|---|---|---|
| Testosterone (ng/mL) | 2.76±0.33 | 3.86±0.54 | 5.57±1.42 | 3.27±0.09 |
| Dihydrotestosterone (pg/mL) | 2,058.02±246.80 | 563.77±209.30* | 744.63±240.90* | 1,032.008±194.04* |
Notes: Data are presented in mean ± SE.
P<0.05 versus CTR group.
Abbreviations: CTR, control; F, finasteride 1 mg/kg (po); F + DA 100, finasteride 1 mg/kg + DA-9401 100 mg/kg (po); F + DA 200, finasteride 1 mg/kg + DA-9401 200 mg/kg (po); po, per oral; SE, standard error.
MDA
The tissue MDA level was decreased in the F + DA 100 and F + DA 200 groups compared to the CTR group but was not significant. MDA was increased in the F group but the change was not significant (Table 4).
Table 4.
Effect of finasteride and DA-9401 on testis tissue malondialdehyde
| CTR | F | F + DA 100 | F + DA 200 | |
|---|---|---|---|---|
| MDA (nM/mg) | 5.14±0.54 | 6.16±0.62 | 4.36±1.03 | 4.72±0.46 |
Note: Data are presented in mean ± SE.
Abbreviations: CTR, control; F, finasteride 1 mg/kg (po); F + DA 100, finasteride 1 mg/kg + DA-9401 100 mg/kg (po); F + DA 200, finasteride 1 mg/kg + DA-9401 200 mg/kg (po); po, per oral; SE, standard error; MDA, malondialdehyde.
Testis histology
The testicular tissue showed a proper arrangement of germinal cells, Sertoli cells and Leydig cells in the CTR group, with no histopathologic lesions. These appeared improper in the F group, but improved with administration of DA-9401 (Figure 2A). Spermatogenic cell density and Johnson’s score were significantly lower in the F group than in the CTR group, which was improved in DA-9401 treated F + DA 100 and F + DA 200 groups compared to the F group (Table 5, Figure 2A).
Figure 2.
Light micrographs of hematoxylin and eosin (H&E) staining.
Notes: (A) The testis, spermatogenic cell density and Johnsen’s scores of the seminiferous tubules. (B) Apoptotic index of testis seminiferous tubules. DA-9401 attenuates finasteride in testis. Data are presented in mean ± SE.
Abbreviations: CTR, control; F, finasteride 1 mg/kg (po); F + DA 100, finasteride 1 mg/kg + DA-9401 100 mg/kg (po); F + DA 200, finasteride 1 mg/kg + DA-9401 200 mg/kg (po); po, per oral; SE, standard error.
Table 5.
Effect of finasteride and DA-9401 on spermatogenic cell density, Johnsen’s score and apoptosis index of seminiferous tubules
| Variables | CTR | F | F + DA 100 | F + DA 200 |
|---|---|---|---|---|
| Spermatogenic cell density | 0.45±0.02 | 0.17±0.02* | 0.39±0.04# | 0.42±0.05# |
| Johnsen’s score | 8.33±0.33 | 5.67±0.33* | 6.67±0.33# | 7.33±0.33# |
| Apoptosis index (%) | 9.67±0.33 | 67.70±1.45* | 32.33±1.45# | 17.70±1.45# |
Notes: Data are presented in mean ± SE.
P<0.05 versus CTR group,
P<0.05 versus F group.
Abbreviations: CTR, control; F, finasteride 1 mg/kg (po); F + DA 100, finasteride 1 mg/kg + DA-9401 100 mg/kg (po); F + DA 200, finasteride 1 mg/kg + DA-9401 200 mg/kg (po); po, per oral; SE, standard error.
Assessment of apoptosis in the testis
Apoptotic cells were identified by the TUNEL assay. The AI was significantly increased in the F group compared to the CTR group, and was significantly improved in DA-9401 treated F + DA 100 and F + DA 200 groups compared to the F group (Table 5, Figure 2B).
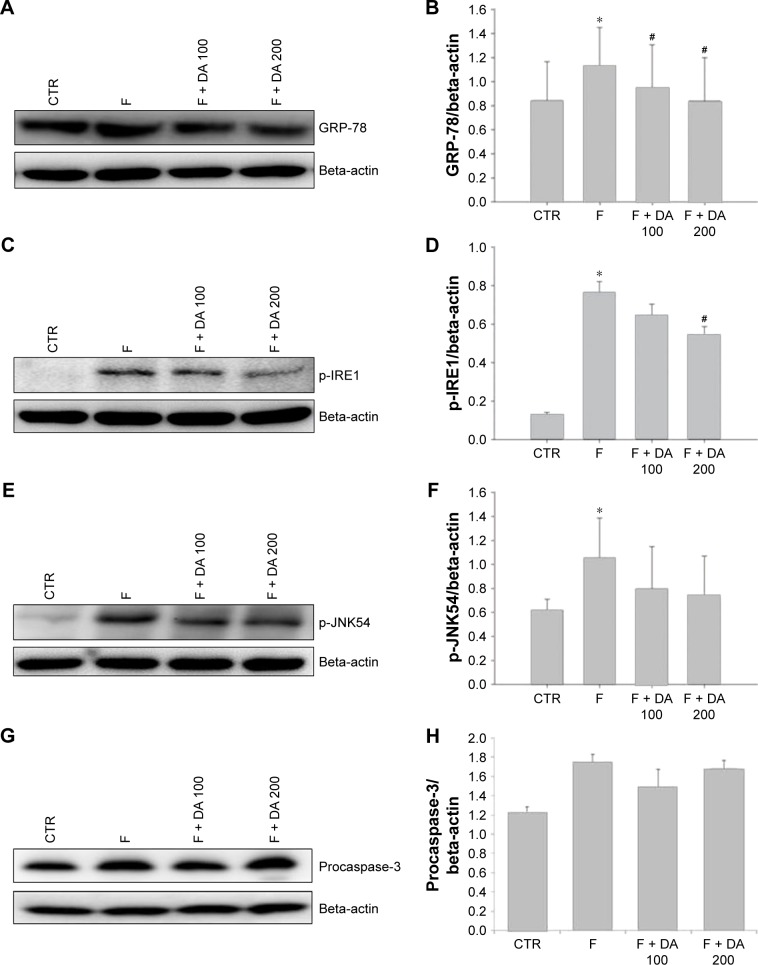
ER stress and apoptosis markers
The levels of the main proteins related to ER stress were affected by finasteride. We evaluated the levels of ER stress-related molecules, including GRP-78, p-IRE1 and p-JNK. Apoptotic proteins procaspase-3 and cleaved caspase-3 were also checked. The GRP-78 level was significantly higher in the F group compared to the CTR group. The level was lowered in the F + DA 100 and F + DA 200 groups compared to the F group (Figure 3A and B). The p-IRE1 level was increased significantly in the F group compared with the CTR group. It was significantly lowered in the F + DA 200 group compared to the F group (Figure 3C and D). The p-JNK54 level was significantly increased in the F group compared to the CTR group (Figure 3E and F). The level of procaspase-3 showed no difference among all the groups (Figure 3G and H). Similarly, cleaved caspase-3 was significantly increased in the F group compared to the CTR group, which was correlated with apoptosis in testicular tissue induced by finasteride, and treatment with DA 200 significantly reduced apoptosis compared to F group (Figure 3I and J).
Figure 3.
Effect of DA-9401 on the finasteride-induced endoplasmic reticulum (ER) stress response.
Notes: (A, B) GRP-78; (C, D) p-IRE1; (E, F) p-JNK54; (G, H) procaspase-3; (I, J) cleaved caspase-3. Data are presented in mean ± SE. *P<0.05 versus CTR group, #P<0.05 versus F group.
Abbreviations: GRP-78, glucose-regulated protein-78; p-IRE1, phosphorylated inositol requiring kinase 1; p-JNK54, phosphorylated c-Jun-N-terminal kinase; CTR, control; F, finasteride 1 mg/kg (po); F + DA 100, finasteride 1 mg/kg + DA-9401 100 mg/kg (po); F + DA 200, finasteride 1 mg/kg + DA-9401 200 mg/kg (po); po, per oral; SE, standard error.
Discussion
The effect of finasteride on testis remains a point of concern. The influence of finasteride on testis and spermatogenesis has varied in different studies. Testosterone, DHT, estradiol and progesterone all feedback at the hypothalamus and pituitary level to control gonadotropin secretion. Uygur et al reported that continued administration of 5 mg finasteride for 3 months resulted in a 15% increase in testosterone levels as well as a 24% decrease in follicle stimulating hormone (FSH) levels and a 16% decrease in LH levels.18 Clark et al reported that DHT levels were markedly suppressed, testosterone levels increased and LH levels mildly decreased within the normal range, with suppression of 5α-reductase with finasteride.19 The changes in the plasma levels of FSH and LH in previous studies suggest that finasteride may have a central effect on the brain, possibly at the hypothalamic level. However, in an another report, 5α-reductase inhibition in man has been shown to have little effect on serum gonadotropin levels or the LH response to GnRH.20 The interaction of 5α-reductase inhibitors with hypothalamic–pituitary–testicular axis in the human is not clearly known.
Finasteride showed no detectable effects on the quantitative and qualitative analysis of spermatogenesis in rats and did not affect the testicular weight in one study,21 but finasteride reduced the weight of testis, decreased DHT and inhibited spermatogenesis in rats in another study.22 Results of our study are similar to the existing findings. In this study, we observed that the levels of the main proteins related to ER stress were affected by finasteride. In addition, apoptosis of testis was induced by finasteride. In the present study, body weight and epididymis weight were reduced in the finasteride treated groups but both were nonsignificant compared with the CTR group. Also, there was no significant change in the testis weight. In contrast, seminal vesicle, prostate and penile weights were reduced significantly, similar to prior studies.22,23
Sperm count and motility were reduced but not significantly. Sperm motility in both F + DA groups was significantly increased compared to the F group. Percentage of pregnant female rats was also reduced in the F group (70%) compared to the CTR group (100%); the decrease was recovered by DA-9401 administration. Finasteride-induced infertility was reported previously,24 but no effects on mating were noted. In this study, testosterone was slightly increased in the F + DA 100 group compared to the F group, but DHT (the more biologically active androgen) was significantly reduced in the F group. In prior studies, DHT improved in the DA-9401 treated groups compared to the finasteride group.2,25
Histological findings showed that the seminiferous tubule was disrupted significantly in the F group compared to the CTR group and was recovered by DA-9401 administration. Similar findings were found in TUNEL analysis. These findings are contrary to those of Rhoden et al21 who reported no changes in seminiferous tubules. But our findings are similar to the descriptions of Wang et al25 and Cukierski et al24 who reported finasteride-induced infertility with inhibited spermatogenesis.
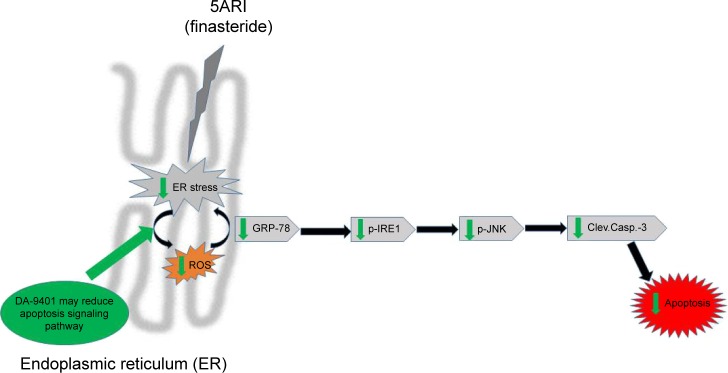
MDA was increased in the F compared to the CTR group, but the increase was not significant. MDA is a biomarker of oxidative stress.26 The present data indicate a relationship between finasteride, oxidative stress and ER stress-induced apoptosis. The relationship between oxidative stress and ER stress is bidirectional, and the accumulation of unfolded proteins in the ER lumen is sufficient to trigger the production of ROS.11,27,28 ER stress can also lead to ROS production, and this also can occur subsequent to the accumulation of unfolded proteins in the ER, among the classical ER stress signaling molecules, p-IRE1, protein kinase RNA-like endoplasmic reticulum kinase (PERK) and activating transcription factor-6 (ATF6). p-IRE1 and p-JNK have garnered a great deal of attention with regard to ROS.12,28,29 However, no data regarding the role of ER stress and UPR effectors in the effect of finasteride are available. The present study reveals an important relationship between ROS-mediated ER stress and finasteride (Figure 4). We investigated the ability of ROS to evoke ER stress in a finasteride model and found that the UPR effectors (GRP-78, p-IRE1 and p-JNK54) and apoptotic markers (procaspase-3 and cleaved caspase-3) were triggered in a ROS-dependent manner. There are three signaling pathways initiated by ER stress sensors: ATF6, PERK and IRE1.30 The IRE1 pathway is activated by prolonged ER stress. The IRE1 complex may lead to activation of the JNK pathway. ER stress-induced activation of the JNK pathway may trigger apoptosis.31 Excessive and prolonged stresses lead cells to apoptosis.32 The cleaved caspase-3 antibody is popular in apoptosis research.33 Redox imbalance decreased activation of the UPR pathway and related proteins, such as GRP-78, p-IRE1, p-JNK54, procaspase-3 and cleaved caspase-3 after DA-9401 treatment in finasteride treated rats.
Figure 4.
Correlation between finasteride-induced oxidative injury and endoplasmic reticulum (ER) stress and apoptosis.
Abbreviations: GRP-78, glucose-regulated protein-78; p-JNK, phosphorylated c-jun-N-terminal kinase; p-IRE1, phosphorylated inositol-requiring transmembrane kinase/endoribonuclease; Clev.Casp.-3, cleaved caspase-3; ROS, reactive oxygen species.
Regarding the relationship between finasteride and reproductive organs, evidences linking finasteride with apoptosis in prostate cells have been published.34 In contrast to these results, we found apoptosis markers procaspase-3 and its active form cleaved caspase-3 in the F group, showing that finasteride may cause apoptosis in testicular tissue.
In the present study, the oxidative stress levels were higher in the F group and were lowered by DA-9401. Thus, finasteride has adverse effects on the testes, which may be attenuated by DA-9401. However, there are several limitations. Although the plant combination was made based on the theory of herb–herb combination in Chinese medicine, the exact mechanism is not yet discovered and further study is needed. In addition, it is necessary to identify the active ingredient of each plant. Also, we observed functional and molecular improvement of infertility by reducing antioxidative effect; however, the precise mechanism is not yet defined to support the treatment effect. There is a need to conduct further studies for greater understanding of the interaction between 5α-reductase inhibitors and DA-9401 in hypothalamic–pituitary–testicular axis in the human.
In conclusion, we are the first to report that finasteride given for 90 days may cause infertility due to disrupted seminiferous tubules. TUNEL analysis revealed that apoptosis in testicular tissue by ROS-mediated activation of GRP-78, p-IRE1, p-JNK, procaspase-3 and cleaved caspase-3 path-ways was recovered by administration of DA-9401.
Acknowledgments
The authors thank the members of the Laboratory of Experimental Urology for helpful discussions.
Footnotes
Author contributions
KKS, YSS, BRC and JKP had full access to all of the data and are responsible for the integrity and accuracy of the data analysis. The study concept and design were developed by JKP and HKK. Acquisition of data was performed by KKS and BRC. Drafting of the manuscript was done by KKS, YSS and JKP. Critical revision of the manuscript and important intellectual content was done by KKS and JKP. Statistical analysis was conducted by KKS and KKK. All authors were involved in the critical revision of the manuscript and approved the final version.
Disclosure
This study was supported by grants from the Korean Healthcare Technology R&D Project, Ministry for Health, Welfare, & Family Affairs, Republic of Korea (HI14C0018). The Korean Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the Korean Healthcare Technology R&D Project, Ministry for Health, Welfare, & Family Affairs, Republic of Korea. All researchers received support from this grant, but there are no financial or other potential conflicts of interest to declare.
Jong Kwan Park is a consultant and speaker for Dong-A Pharmaceutical Company, Yong-in, Kyoung-gi, Republic of Korea, and has received unconditional research grants from that company. However, the company had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript. The authors report no other conflicts of interest in this work.
References
- 1.Olsen EA, Hordinsky M, Whiting D, et al. The importance of dual 5alpha-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride. J Am Acad Dermatol. 2006;55:1014–1023. doi: 10.1016/j.jaad.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 2.Mysore V. Finasteride and sexual side effects. Indian Dermatol Online J. 2012;3:62–65. doi: 10.4103/2229-5178.93496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ribeiro CM, Pereira OC. 5alpha-reductase 2 inhibition impairs brain defeminization of male rats: reproductive aspects. Pharmacol Biochem Behav. 2005;82:228–235. doi: 10.1016/j.pbb.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 4.Carbone DJ, Jr, Hodges S. Medical therapy for benign prostatic hyperplasia: sexual dysfunction and impact on quality of life. Int J Impot Res. 2003;15:299–306. doi: 10.1038/sj.ijir.3901017. [DOI] [PubMed] [Google Scholar]
- 5.Tu H, Zini A. Finasteride-induced secondary infertility associated with sperm DNA damage. Fertil Steril. 2011;95:2125.e13–e14. doi: 10.1016/j.fertnstert.2010.12.061. [DOI] [PubMed] [Google Scholar]
- 6.Collodel G, Scapigliati G, Moretti E. Spermatozoa and chronic treatment with finasteride: a TEM and FISH study. Arch Androl. 2007;53:229–233. doi: 10.1080/01485010701426471. [DOI] [PubMed] [Google Scholar]
- 7.Tremellen K. Oxidative stress and male infertility – a clinical perspective. Hum Reprod Update. 2008;14:243–258. doi: 10.1093/humupd/dmn004. [DOI] [PubMed] [Google Scholar]
- 8.Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal. 2007;9:2277–2293. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 9.Zeeshan HM, Lee GH, Kim HR, Chae HJ. Endoplasmic reticulum stress and associated ROS. Int J Mol Sci. 2016;17:327. doi: 10.3390/ijms17030327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soni KK, Kim HK, Choi BR, et al. Dose-dependent effects of cisplatin on the severity of testicular injury in Sprague Dawley rats: reactive oxygen species and endoplasmic reticulum stress. Drug Des Devel Ther. 2016;10:3959–3968. doi: 10.2147/DDDT.S120014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lombardo F, Sansone A, Romanelli F, Paoli D, Gandini L, Lenzi A. The role of antioxidant therapy in the treatment of male infertility: an overview. Asian J Androl. 2011;13:690–697. doi: 10.1038/aja.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi J, Lee KT, Choi MY, et al. Antinociceptive anti-inflammatory effect of Monotropein isolated from the root of Morinda officinalis. Biol Pharm Bull. 2005;28:1915–1918. doi: 10.1248/bpb.28.1915. [DOI] [PubMed] [Google Scholar]
- 13.Shin JS, Yun KJ, Chung KS, et al. Monotropein isolated from the roots of Morinda officinalis ameliorates proinflammatory mediators in RAW 264.7 macrophages and dextran sulfate sodium (DSS)-induced colitis via NF-kappaB inactivation. Food Chem Toxicol. 2013;53:263–721. doi: 10.1016/j.fct.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 14.Löffler C, Czygan FC, Proksch P. Phenolic constituents as taxonomic markers in the genus Cuscuta (Cuscutaceae) Biochem Syst Ecol. 1997;25:297–303. [Google Scholar]
- 15.Yang L, Chen Q, Wang F, Zhang G. Antiosteoporotic compounds from seeds of Cuscuta chinensis. J Ethnopharmacol. 2011;135:553–560. doi: 10.1016/j.jep.2011.03.056. [DOI] [PubMed] [Google Scholar]
- 16.Singh BN, Singh BR, Singh RL, et al. Polyphenolics from various extracts/fractions of red onion (Allium cepa) peel with potent antioxidant and antimutagenic activities. Food Chem Toxicol. 2009;47:1161–1167. doi: 10.1016/j.fct.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Lee HJ, Lee JY, Kim SM, et al. Inhibitory effects of dicaffeoylquinic acids from Artemisia dubia on aldo-keto reductase family 1b10. J Korean Soc Appl Biol Chem. 2010;53:826–830. [Google Scholar]
- 18.Uygur MC, Arik AI, Altuğ U, Erol D. Effects of the 5 alpha-reductase inhibitor finasteride on serum levels of gonadal, adrenal, and hypophyseal hormones and its clinical significance: a prospective clinical study. Steroids. 1998;63:208–213. doi: 10.1016/s0039-128x(98)00005-1. [DOI] [PubMed] [Google Scholar]
- 19.Clark RV, Hermann DJ, Cunningham GR, Wilson TH, Morrill BB, Hobbs S. Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5alpha-reductase inhibitor. J Clin Endocrinol Metab. 2004;89:2179–2184. doi: 10.1210/jc.2003-030330. [DOI] [PubMed] [Google Scholar]
- 20.Rittmaster RS, Lemay A, Zwicker H, et al. Effect of finasteride, a 5 alpha-reductase inhibitor, on serum gonadotropins in normal men. J Clin Endocrinol Metab. 1992;75:484–488. doi: 10.1210/jcem.75.2.1322427. [DOI] [PubMed] [Google Scholar]
- 21.Rhoden EL, Gobbi D, Menti E, Rhoden C, Teloken C. Effects of the chronic use of finasteride on testicular weight and spermatogenesis in Wistar rats. BJU Int. 2002;89:961–963. doi: 10.1046/j.1464-410x.2002.02785.x. [DOI] [PubMed] [Google Scholar]
- 22.Wang XD, Jia Y, Cui YG, et al. Effect of large-dosage of 5alpha-reductase inhibitors on the spermatogenesis of male rats. Zhonghua Nan Ke Xue. 2005;11:652–654. [PubMed] [Google Scholar]
- 23.Laroque PA, Prahalada S, Gordon LR, et al. Effects of chronic oral administration of a selective 5 alpha-reductase inhibitor, finasteride, on the dog prostate. Prostate. 1994;24:93–100. doi: 10.1002/pros.2990240207. [DOI] [PubMed] [Google Scholar]
- 24.Cukierski MA, Sina JL, Prahalada S, et al. Decreased fertility in male rats administered the 5 alpha-reductase inhibitor, finasteride, is due to deficits in copulatory plug formation. Reprod Toxicol. 1991;5:353–362. doi: 10.1016/0890-6238(91)90094-v. [DOI] [PubMed] [Google Scholar]
- 25.Wang D, Zha X, Nagase K, et al. Effects of the 5alpha-reductase inhibitor dutasteride on rat prostate alpha1A-adrenergic receptor and its mediated contractility. Urology. 2015;85:704.e9–e14. doi: 10.1016/j.urology.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen F, Mikkelsen BB, Nielsen JB, Andersen HR, Grandjean P. Plasma malondialdehyde as biomarker for oxidative stress: reference interval and effects of life-style factors. Clin Chem. 1997;43:1209–1214. [PubMed] [Google Scholar]
- 27.Kaufman RJ, Back SH, Song B, Han J, Hassler J. The unfolded protein response is required to maintain the integrity of the endoplasmic reticulum, prevent oxidative stress and preserve differentiation in beta-cells. Diabetes Obes Metab. 2010;12(Suppl 2):99–107. doi: 10.1111/j.1463-1326.2010.01281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ozgur R, Uzilday B, Sekmen AH, Turkan I. The effects of induced production of reactive oxygen species in organelles on endoplasmic reticulum stress and on the unfolded protein response in arabidopsis. Ann Bot. 2015;116:541–553. doi: 10.1093/aob/mcv072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhandary B, Marahatta A, Kim HR, Chae HJ. An involvement of oxidative stress in endoplasmic reticulum stress and its associated diseases. Int J Mol Sci. 2012;14:434–456. doi: 10.3390/ijms14010434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kadowaki H, Nishitoh H. Signaling pathways from the endoplasmic reticulum and their roles in disease. Genes (Basel) 2013;4:306–333. doi: 10.3390/genes4030306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishitoh H, Matsuzawa A, Tobiume K, et al. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 2002;16:1345–1355. doi: 10.1101/gad.992302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shiraishi H, Okamoto H, Yoshimura A, Yoshida H. ER stress-induced apoptosis and caspase-12 activation occurs downstream of mitochondrial apoptosis involving Apaf-1. J Cell Sci. 2006;119:3958–3966. doi: 10.1242/jcs.03160. [DOI] [PubMed] [Google Scholar]
- 33.Fan Y, Bergmann A. The cleaved-Caspase-3 antibody is a marker of Caspase-9-like DRONC activity in Drosophila. Cell Death Differ. 2010;17:534–539. doi: 10.1038/cdd.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsujimura A, Fukuhara S, Soda T, et al. Histologic evaluation of human benign prostatic hyperplasia treated by dutasteride: a study by xenograft model with improved severe combined immunodeficient mice. Urology. 2015;85:274.e1–e8. doi: 10.1016/j.urology.2014.09.024. [DOI] [PubMed] [Google Scholar]