Abstract
Initial randomized trials of cholesteryl ester transfer protein (CETP) inhibitors were terminated early owing to adverse effects or futility. The REVEAL trial now shows the benefit of CETP inhibition in coronary heart disease. Despite raising HDL-cholesterol levels, the cardiovascular effect of CETP inhibitors is probably due to lowering of non-HDL-cholesterol levels.
For decades, the scientific community has been perplexed about the incongruent relationship between blood cholesterol concentrations and risk of coronary heart disease (CHD). This conflict stems from observational evidence indicating that both plasma LDL-cholesterol (LDL-C) levels and plasma HDL-cholesterol (HDL-C) levels are strongly associated with the risk of CHD. Whereas the path to show the causal role of LDL-C in CHD has been smooth, with orthogonally targeted pharmaceutical agents (statins, ezetimibe, and PCSK9 inhibitors) providing consistent evidence in randomized clinical trials (RCTs), the path has been more tortuous for drugs targeting HDL-C. Questions on the statistical robustness of the association between HDL-C and risk of CHD were raised 25 years ago1. Accordingly, investigators have sought to clarify the role of HDL-C in cardiovascular diseases with the use of genetic studies, the most recent example being the findings reported by Ference et al. in 2017, and interventional clinical trials, with the latest findings coming from the REVEAL trial2.
A causal role for LDL-C in CHD is well established, but the role of HDL-C remains much less clear. The most notable Mendelian randomization (MR) study of HDL-C by Voight and colleagues in 2012 did not provide evidence of causation in CHD. However, from a modern perspective, the approach by Voight et al. could be considered limited, as summarized in3. Nevertheless, subsequent studies using more-contemporary MR approaches (which take into account genetic pleiotropy) and larger sets of single-nucleotide polymorphisms (SNPs) identified in HDL-C genome-wide association studies (GWAS) have also shown a neutral association between plasma HDL-C levels and risk of CHD4. These findings led to the prevailing interpretation that plasma HDL-C levels are unlikely to have an important role in the aetiology of CHD.
Genetic studies of a biomarker (such as HDL-C levels) are distinct from those of a drug target, because drug targets tend not to show specificity for the exposure of interest. Cholesteryl ester transfer protein (CETP) facilitates transport of cholesterol from HDL particles to particles containing apolipoprotein B (APOB), such as very low-density lipoproteins, in exchange for triglycerides. Therefore, one way of elevating HDL-C levels is through therapeutic inhibition of CETP. Indeed, potent CETP inhibitors lead to an elevation of plasma HDL-C levels and a reduction in Friedewald-measured LDL-C levels. Early genetic studies provided weak evidence showing that CETP genetic variants were linked to the risk of CHD; however, subsequent, large-scale evidence provides robust associations, including the identification of a CETP variant associated with CHD (P = 9.8 × 10−9) in a hypothesis-free GWAS published in 2017.5 Furthermore, in a factorial MR study published in 2017, Ference et al.6 show that use of plasma LDL-C levels as a marker of drug efficacy might lead to an exaggerated estimation of the clinical benefit of CETP inhibition when combined with statin treatment, as opposed to use of plasma APOB levels, similar to findings from REVEAL2.
The first phase III RCT of a CETP inhibitor (ILLUMINATE7) showed that torcetrapib raised plasma HDL-C levels by 72% and lowered plasma LDL-C levels by 25% compared with placebo in patients at high risk of cardiovascular disease (n = 15,067). However, the trial was terminated early owing to a 25% higher risk of major vascular events in the torcetrapib group, linked to elevated systolic blood pressure (SBP)7. Of note, an association between CETP inhibition and high SBP was identified for all CETP inhibitors tested in phase III RCTs. In the dal-OUTCOMES trial8, which included 15,871 patients with a recent acute coronary syndrome, the CETP inhibitor dalcetrapib increased HDL-C levels by 31–40% from baseline, but had a minimal effect on LDL-C levels. This trial was terminated early owing to futility, with a hazard ratio (HR) for the primary end point of major vascular events of 1.04 (95% CI 0.93–1.16). In the subsequent ACCELERATE trial9, evacetrapib, an efficacious CETP inhibitor, increased HDL-C levels by 132% and lowered LDL-C levels by 37% compared with placebo in patients with established vascular disease (n = 12,092), but ACCELERATE was terminated after a median of 26 months of treatment owing to futility, with a HR for the primary end point of major vascular events of 1.01 (95% CI 0.91–1.11). In 2017, and as a surprise to the cardiovascular community, the REVEAL trial2 demonstrated a beneficial effect of anacetrapib, another potent CETP inhibitor. Compared with placebo, anacetrapib treatment led to a 104% increase in plasma HDL-C levels and a 17% or 41% reduction in LDL-C levels (measured by β-quantification or direct method, respectively), and after a median of 4.1 years of treatment, yielded a HR of 0.91 (95% CI 0.85–0.97) for major coronary events in patients with prior vascular disease (n = 30,449).
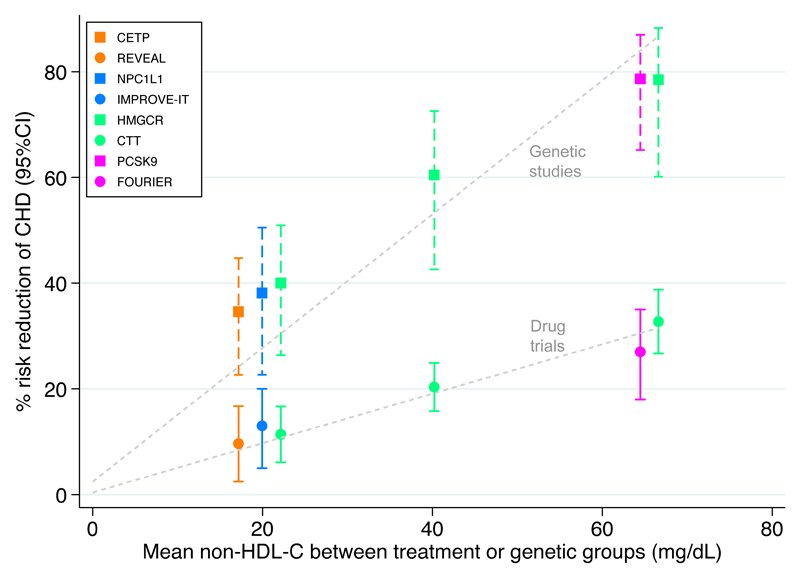
How do we explain the seemingly conflicting findings from the multiple trials of CETP inhibitors and the genetic studies of HDL-C and CETP? First, the REVEAL2 findings do not change the notion that circulating HDL-C levels are unlikely to have an important role in the aetiology of CHD: CETP inhibitors that did not have a large effect on the levels of atherogenic lipoproteins, as measured by LDL-C or APOB, showed no association with CHD8. Second, the magnitude of effect on non-HDL-C levels and the corresponding risk of CHD reported in REVEAL are entirely consistent with those reported in trials of statins, ezetimibe, and PCSK9 inhibitors (Figure 1); the genetic associations that correspond to these drug targets scaled to the same difference in non-HDL-C line up on a steeper slope, a result that is expected given that the effect of atherogenic lipoproteins on the risk of cardiovascular disease accumulates over a lifetime. Third, the neutral finding in ACCELERATE9 with evacetrapib — a drug that had a similar lipid profile to anacetrapib in REVEAL2, including strong non-HDL-C lowering — is likely to arise from the premature termination of the trial: in REVEAL, the HR for major coronary events at 2 years of follow-up was 0.96 (95% CI 0.84–1.10)2, which overlaps with the estimation for major vascular events in ACCELERATE9. Furthermore, in REVEAL, the HR for major coronary events after 4.1 years was stronger than for major vascular events (HR 0.93, 95% CI 0.88–0.99), indicating that the composite primary outcome in ACCELERATE might have included end points that attenuated the association.
Figure 1. Non-HDL-cholesterol levels and risk of coronary heart disease in drug trials and genetic studies.
Comparison of the effects of drugs (circles) and corresponding genetic proxies (squares) on the risk of coronary heart disease (CHD) according to their treatment and genetic effects on non-HDL-cholesterol (HDL-C) levels. The three values from the Cholesterol Treatment Trialists’ collaboration (CTT; green circles) are derived, from left to right, from: five trials of more versus less statin; 17 trials of statin versus placebo with <50 mg/dl average difference in non-HDL-C levels; and four trials of statin versus placebo with >50 mg/dl average difference in non-HDL-C levels. These data and the data from REVEAL were obtained from2; estimates obtained using PlotDigitizer (http://plotdigitizer.sourceforge.net/). Data on genetic variants were obtained from ref. 6 and scaled to match the corresponding differences in non-HDL-C achieved from drug trials, using apolipoprotein B as a proxy for non-HDL-C. CHD end points: coronary death or myocardial infarction (MI) in REVEAL and CCT; MI in IMPROVE-IT and FOURIER; and MI, coronary revascularization, stroke, or coronary death in Ference et al.6. CETP, cholesteryl ester transfer protein; HMGCR, 3-hydroxy-3-methylglutaryl-CoA reductase; NPC1L1, Niemann–Pick C1-like protein 1.
Moving forward, crucial questions include the mechanisms underlying the increase in SBP seen with CETP inhibition (with the exception of the disproportionate effect seen with torcetrapib, the modest SBP increase seen with CETP inhibitors seems to be correlated with the degree of increase in HDL-C levels and might therefore be target-mediated); whether therapeutic CETP inhibition leads to age-related macular degeneration, as predicted by genetic studies10 (which REVEAL was underpowered to detect); whether CETP inhibitors alter the risk of diabetes mellitus (a modest beneficial effect was seen in both REVEAL and ACCELERATE); which patients might derive clinical benefit from CETP inhibitors; and the cost-efficiency of CETP inhibitor treatment. Certainly, the findings from REVEAL bring to a close the long-standing discordance between findings from MR studies (which anticipated cardiovascular benefit from therapeutic inhibition of CETP) and phase III RCTs (which, before REVEAL2, corresponding showed no such benefit). For lipidoligists, the accumulating data point towards a unifying theory of APOB driving CHD, and, for HDL, it might be back to the drawing board.
Acknowledgements
M.V.H. works in a unit that receives funds from the University of Oxford and the UK Medical Research Council. G.D.S. works in a unit that receives funds from the University of Bristol and the UK Medical Research Council (MC_UU_12013/1). The funders had no role in study design, decision to publish, or preparation of the manuscript.
Footnotes
Competing interests statement
The authors declare no competing interests.
References
- 1.Phillips AN, Davey Smith G. How independent are “independent” effects? Relative risk estimation when correlated exposures are measured imprecisely. J Clin Epidemiol. 1991;44:1223–1231. doi: 10.1016/0895-4356(91)90155-3. [DOI] [PubMed] [Google Scholar]
- 2.HPS3/TIMI55-REVEAL Collaborative Group. Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med. 2017 doi: 10.1056/NEJMoa1706444. [DOI] [PubMed] [Google Scholar]
- 3.Holmes MV, Ala-Korpela M, Davey Smith G. Mendelian randomization in cardiometabolic disease: challenges in evaluating causality. Nat Rev Cardiol. 2017;14:577–590. doi: 10.1038/nrcardio.2017.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White J, et al. Association of lipid fractions with risks for coronary artery disease and diabetes. JAMA Cardiol. 2016;1:692–699. doi: 10.1001/jamacardio.2016.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Webb TR, et al. Systematic evaluation of pleiotropy identifies 6 further loci associated with coronary artery disease. J Am Coll Cardiol. 2017;69:823–836. doi: 10.1016/j.jacc.2016.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ference BA, et al. Association of genetic variants related to CETP inhibitors and statins with lipoprotein levels and cardiovascular risk. JAMA. 2017;318:947–956. doi: 10.1001/jama.2017.11467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barter PJ, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz GG, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–2099. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 9.Lincoff AM, et al. Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N Engl J Med. 2017;376:1933–1942. doi: 10.1056/NEJMoa1609581. [DOI] [PubMed] [Google Scholar]
- 10.Burgess S, Davey Smith G. Mendelian randomization implicates high-density lipoprotein cholesterol-associated mechanisms in etiology of age-related macular degeneration. Ophthalmology. 2017;124:1165–1174. doi: 10.1016/j.ophtha.2017.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]