Abstract
Chronic neuro-musculoskeletal pain is an important complication of open-heart surgery (OHS). To better understand the development and natural course of neuro-musculoskeletal pain in the immediate post-OHS period, this prospective longitudinal study assessed the prevalence and degree of pain and shoulder disability, and areas of pain pre- and post-OHS. Usual medical, nursing, and physiotherapy care was provided including early extubation, education, walking, sitting out of bed, and upper, lower limb, and trunk exercises from day 1 post-operation. Of 114 elective patients who provided consent, 98 subjects were surveyed preoperatively, and at week 6 and week 12 post-OHS. Open and closed questions encompassed numerical rating of pain scales for various body areas summed as a total pain score (TPS), the shoulder disability score (SDS), exercise compliance, and sternal clicking. Usual care comprised mobility exercises, walking program, and cardiac rehabilitation referral. Survey return rates were 100%, 88%, and 82%, respectively. Of the 76 (78%) subjects with complete data sets, 68% subjects reported a history of previous neuro-musculoskeletal injuries/conditions preoperatively while prevalence for neuro-musculoskeletal pain was 64%, 88%, and 67% and 38%, 63%, and 42% for shoulder disability, at the three assessments. In all, 11% subjects reported sternal clicking at week 6 and 7% at week 12. Pain commonly occurred in the lower back and neck preoperatively, and in front of the chest, neck, rib cage, upper back, and left shoulder at week 6. Rib cage pain alone remained significantly greater than preoperative levels by week 12 post-OHS. Preoperative SDS was positively correlated with post-OHS length of stay; women had higher SDSs than men at week 6 and week 12 and week 12 SDS was negatively correlated with height. Surgical risk score was negatively correlated with change in SDS and TPS from pre-operation to week 12. In conclusion, neuro-musculoskeletal pain and shoulder disability were common preoperatively and while prevalence increased at week 6 post-OHS, overall preoperative levels were restored by week 12.
Keywords: cardiac surgery, shoulder disability, area of pain, sternal clicking, exercise compliance, physiotherapy
Video abstract
Introduction
Neuro-musculoskeletal pain is reported in 12%–64% of patients following open-heart surgery (OHS) with chronic or persistent pain found in 11%–55% of patients.1–9
Possible causes of pain post-sternotomy include sternal retraction, first rib fracture, brachial plexus injury, cannulation of the jugular vein, patient positioning during surgery, and ischemic changes post-internal mammary artery graft (IMAG).1,10,11
While prevalence of post-OHS pain and disability has been examined in several single point-in time studies, only one retrospective and two prospective, longitudinal studies have examined preoperative levels of pain finding a 33%–35% prevalence of preexisting pain or musculoskeletal conditions including conditions such as osteoarthritis, osteoporosis, cervical spine pain, neurological conditions, and “problems” in the back, shoulder girdle, and general joints.1,2,8,9,11–13
Post-OHS pain has been described along the sternum, in the trunk, head, neck, and upper limbs and persistent pain (at 24–36 months post-OHS) in the sternum, other chest areas, arm and shoulder, and in the leg.1,2,5,10
Chronic pain of moderate intensity occurs in 9%–13% and of severe intensity in 1%–4% of patients post-OHS.3,5–9 Patients have described discomfort post-OHS as pain, stiffness, weakness, instability, numbness, tingling, and pins and needles while other studies report the levels of discomfort, disability, and dysfunction as minimal and not dissimilar to preoperative levels.1,2
Independent predictors of chronic pain have been identified as nonelective surgery, re-sternotomy, severe pain (numeric rating scale ≥4/10) on the third postoperative day, and female gender.14 Other studies conclude that persistent pain preoperatively and higher levels of preoperative anxiety predict the presence of persistent pain and pain intensity post-OHS.13 Furthermore, the presence and intensity of pain during week 1 post-OHS, the presence of pain at rest during week 4 post-OHS, the degree to which pain interfered with function, a lower intake of analgesics during hospitalization, the severity of comorbidities, younger age, and a lower education level were associated with persistent pain at 12 months post-OHS.7,8,13,15
Therefore, while neuro-musculoskeletal conditions are common pre-OHS and new and sometimes chronic pain and dysfunction occur post-OHS, few prospective, longitudinal studies have been undertaken and subsequently the prevalence, intensity, and areas of pain preoperatively and in the early postoperative period are not well defined.
As one step toward a systematic evaluation of (acute and chronic) pain and evidence-based treatment of neuro-musculoskeletal pain post-OHS, a longitudinal prospective study was designed to characterize preoperative neuro-musculoskeletal pain and shoulder disability and to study the early progression and resolution of this pain and disability in patients undergoing OHS.16 Our objectives were to determine the prevalence and area of neuro-musculoskeletal pain and shoulder disability preoperatively, at week 6 and week 12 post-OHS; to examine the intensity of pain in the various upper body areas at these three time points; to examine pre, peri, and postoperative factors that may be associated with the development of pain and shoulder disability.
Materials and methods
Participants
Ethical approval was obtained from The Prince Charles Hospital Research and Ethics Committee (approval number: EC 2373). Consecutive patients attending preadmission clinic 1 week prior to OHS in The Prince Charles Hospital, a major tertiary center in Brisbane, Australia, were invited to participate in the study over a 10-month period. Patients were included if they were booked for OHS procedures via sternotomy for coronary artery bypass grafts (CABGs), aortic, mitral, tricuspid, or pulmonary valve replacement/repair (VR), aortic root replacement, right ventricular outflow tract repair, transaortic myomectomy, atrial septal defect repair, and combination surgery for CABG and VR. The CABGs utilized included saphenous vein, radial artery, and IMAGs. Patients were excluded if they could not understand English, had a history of cerebral vascular accident with upper limb dysfunction, or developed complications preventing participation in usual postoperative physiotherapy.
Measures
Primary outcome measures were included in a preoperative survey that was repeated, excluding questions regarding preoperative pain and conditions, at week 6 and week 12 post-OHS.
The survey consisted of three sections including a series of numerical rating scales identifying the area and degree of pain, the shoulder disability score (SDS), and a series of open and closed questions.17–19 Subjects were asked to list previous injuries/conditions in the upper limbs and trunk and regular pain/anti-inflammatory medication used, their regular walking distance (up to 100 m, 100–500 m, 500 m to 1 km, >1 km), walking frequency each week (daily, 4–5 times, 3 times, <3 times), need for walking assistance or walking aid (yes/no), and type of walking aid if used (free text). At week 6, subjects were additionally asked the weekly frequency (daily, few times, once, once per fortnight, never) and the duration (up to 1, 2, 3, 4, 5, or 6 weeks) during which they continued the prescribed home exercise program. Week 6 and week 12 surveys also asked subjects if they experienced any “clicking” in the rib cage or chest.
Subjects scored current pain levels (in the neck, left shoulder, right shoulder, upper back, front of chest, rib cage, lower back, left elbow/hand, and right elbow/hand) on a 0–10 numerical rating scale, where 0 = no pain and 10 = maximal pain, and identified if this pain experienced was related to their heart surgery (yes or no). Scores of 0–3 were categorized as minimal pain, 4–6 moderate pain, and 7–10 severe pain.20,21 Scores for each area were added together for each patient to provide a total pain score (TPS) (on a scale of 0–90).
The SDS required subjects to score their difficulty in performing eight regular daily activities involving different shoulder actions on a 0–10 scale (where 0 = no difficulty, and 10 = so difficult that they required help). The maximum SDS score possible at any assessment point was 80 points, with a high score indicating greater disability. To measure prevalence, the presence of shoulder disability was noted if a subject provided a score >0 on one or more of the SDS items. Box S1 lists the questions in the SDS.
The physiotherapy surgical risk score was a locally developed tool to identify higher physiotherapy postoperative treatment requirements (maximum of 10), scoring 1 (present) or 0 (not present) for each of the following factors: age >70 years; current smoker or ceased smoking <2 months ago; >35 smoking pack year history; productive cough; diagnosis of chronic obstructive pulmonary disease or on pulmonary medication; forced expiratory volume in 1 second ≤80% predicted; body mass index (BMI) >27; diabetes; unable to walk 60 m on flat ground; unable to climb a flight of stairs.22,23
Procedures
Preoperatively, following written informed consent, a physiotherapy clinical assessment was undertaken including review of the surgical risk score. Furthermore, subjects were guided on the use of the numerical rating scale for pain and the SDS and completed the preoperative survey. Patient education also included expectations for and benefits of exercises/regular walking; and incentive spirometry, breathing exercises, supported huff/cough, and the practice of shoulder flexion and abduction with the elbow extended; neck flexion, extension, rotation and unilateral flexion; with the hand on the shoulder scapular retraction and protraction, circumduction, and trunk rotation and unilateral flexion.
Following hospital admission, subjects underwent usual medical and nursing care pre- and post-OHS including planned extubation from 6 hours following their return from the theater. Postoperative physiotherapy included, but was not limited to, the exercises taught preoperatively, regular walking progression, and sitting out of bed from day 1 post-operation (post-op). Five exercise repetitions were practiced on day 1, increasing to 10 repetitions over the hospital stay. Patients were instructed to perform the exercises sitting in a chair or on the edge of the bed upon waking and 3–4 times each day. Daily supervision continued until the physiotherapist was confident the patient could safely and independently perform the tasks. Incentive spirometry using Triflo™ (TriFlo II Incentive Deep Breathing Exerciser; Hudson RCI, Teleflex Medical, Inc., Morrisville, NC, USA) was encouraged with 600 mL/s flow held for 3–5 seconds repeated 10 times each hour for 10 hours of the day.24,25 Home exercises prescribed by the physiotherapist included the aforementioned exercises, and daily walking increasing to 2 km day−1 by the end of week 4 at home. Subjects were advised to continue the exercises for 6 weeks postoperatively, or until normal movement and sensation was restored and to walk for life. While routinely offered outpatient cardiac rehabilitation, unpublished local data suggest that our patients on average commence cardiac rehabilitation 17 weeks post-cardiac event. Furthermore, as this tertiary center services metropolitan and a large rural and regional area, patients referred for surgery may not be able to access cardiac rehabilitation once discharged home.
The second and third authors, Rhonda L Lamb and Tonya D Gould, extracted data from medical records, including patient age, sex, surgery type and details, complications, and length of stay and forwarded the postal surveys at week 6 and week 12 post-OHS with return self-addressed envelopes to encourage survey retrieval and followed up by telephone if the surveys were not returned or required clarification.
Statistical analyses
Statistical analysis was conducted using Minitab version 16 (Minitab® Statistical Software version 16, Minitab Inc., State College, PA, USA), (MINITAB® and all other trademarks and logos for the Company’s products and services are the exclusive property of Minitab Inc. All other marks referenced remain the property of their respective owners. See minitab.com for more information.), SigmaPlot version 11 (SigmaPlot & SigmaStat version 11, Systat Software, Inc., Chicago, IL, USA), and Microsoft Excel 16 (Microsoft Excel 16, Microsoft Corporation, Redmond, WA, USA). The prevalence of neuro-muscular pain and sternal clicking, the pain score for each body region, the number and percentage of patients using pain medication, and the total SDS at each time point were calculated. The numerical rating scale pain scores and SDSs obtained were strongly skewed, due to many zero scores indicating no pain or disability. Therefore, while the graphs and tables report mean scores for more accurate depiction of the actual scores that occurred, statistical analysis was based on the changes in median rather than mean scores and all p-values are derived from nonparametric tests of the medians and are not adjusted for multiple comparisons. The sign test and Mann–Whitney U-test were used for pairwise comparisons (eg, pain scores between two time points for the same region) and comparisons between groups (eg, across surgical groups) were performed with Kruskal–Wallis one way analysis of variance on ranks. Tests of association between categorical variables were performed using Pearson’s chi-squared test, supplemented where required by Fisher’s exact method, while associations between numerical variables were measured using Pearson’s correlation coefficient, r.
Comparison of subjects completing all stages of the study (completers) and those who did not (non-completers) indicated no difference between groups (Table 1). Hence, case deletion method was considered a suitable method to address the missing data due to loss to follow-up.
Table 1.
Subject and surgical variables
| CABG only | CABG + VR | VR only | Other OHS | p-value between OHS | Completers | Non-completers | p-value completer/non-completer | |
|---|---|---|---|---|---|---|---|---|
| Number of subjects (%) | 44 (58) | 9 (12) | 18 (24) | 5 (6) | N/A | 76 | 13 | N/A |
| Men (%) | 32 (73) | 7 (98) | 7 (39) | 4 (80) |

|
50 (66) | 13 (59) |
|
| Women (%) | 12 (27) | 2 (2) | 11 (61) | 1 (20) | 26 (34) | 9 (41) | ||
| Completers (%) | 44 (58) | 9 (12) | 18 (24) | 5 (6) |

|
76 (100) | NA | N/A |
| Non-completers (%) | 8 (36) | 3 (14) | 9 (41) | 2 (9) | N/A | 22 (100) | N/A | |
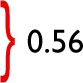
| Diabetic (%) | 9 (20) | 0 (0) | 1 (6) | 0 (0) | 0.17 | 10 (13) | 3 (14) | 0.95 |
| Using pre-op pain medication (%) | 8 (18) | 1 (11) | 1 (5) | 2 (40) | 0.27 | 12 (16) | 4 (18) | 0.79 |
| With pre-op shoulder disability (%) | 19 (43) | 3 (33) | 7 (39) | 0 (0) | 0.30 | 29 (38) | 12 (55) | 0.17 |
| With pre-op pain (%) | 26 (59) | 6 (66) | 13 (72) | 4 (80) | 0.67 | 49 (65) | 11 (50) | 0.22 |
| With previous injury/condition (%)a | 30 (68) | 6 (66) | 11 (61) | 4 (80) | 0.87 | 51 (67) | 15 (68) | 0.92 |
| Walking ≥1 km (pre-op) (%) | 17 (39) | 4 (44) | 7 (39) | 5 (100) | 0.07 | 33 (43) | 9 (41) | 0.83 |
| Walking ≥1 km (week 6) (%) | 30 (68) | 5 (56) | 10 (56) | 3 (60) | 0.76 | 48 (63) | N/A | N/A |
| Walking ≥1 km (week 12) (%) | 29 (66) | 4 (44) | 10 (56) | 4 (80) | 0.48 | 47 (62) | N/A | N/A |
| Walking 4+ times/week (week 6) (%) | 30 (68) | 5 (55) | 9 (50) | 3 (60) | 0.58 | 47 (62) | N/A | N/A |
| Walking 4+ times/week (week 12) (%) | 30 (68) | 3 (33) | 8 (44) | 3 (60) | 0.14 | 44 (58) | N/A | N/A |
| Using a walking aid (pre-op) (%)b | 2 (5) | 0 (0) | 2 (11) | 0 (0) | 0.56 | 4 (5) | 3 (14) | 0.18 |
| Using a walking aid (week 6) (%)b | 3 (7) | 0 (0) | 2 (11) | 0 (0) | 0.66 | 5 (7) | N/A | N/A |
| Using a walking aid (week 12) (%)b | 2 (5) | 0 (0) | 1 (6) | 0 (0) | 0.86 | 3 (4) | N/A | N/A |
| Trunk/limb exercises daily (%)c | 31 (71) | 4 (44) | 15 (83) | 2 (40) |
 d d
|
52 (68) | N/A | N/A |
| Trunk/limb exercises 1–4/week (%)c | 12 (27) | 5 (56) | 3 (17) | 2 (40) | 22 (29) | N/A | N/A | |
| Trunk/limb exercises once/week (%)c | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | N/A | N/A | |
| With post-op complications (%) | 24 (55) | 3 (33) | 10 (56) | 4 (80) | 0.40 | 41 (53) | 10 (46) | 0.48 |
| With post-op LOS >7 days (%) | 13 (30) | 5 (56) | 9 (50) | 2 (40) | 0.31 | 29 (38) | 9 (18)e | 0.81 |
| Sternal clicking (week 6) (%) | 3 (7) | 0 (0) | 5 (28) | 0 (0) | 0.05 | 8 (11) | N/A | N/A |
| Sternal clicking (week 12) (%) | 1 (2) | 0 (0) | 4 (22) | 0 (0) | 0.02 | 5 (7) | N/A | N/A |
| Mean age, years (SD) | 65.9 (8.7) | 69.6 (6.5) | 61.4 (13.2) | 50.4 (16.2) | 0.04f | 64.2 (11) | 59.1 (16.4) | 0.18 |
| Mean height, meters (SD) | 1.7 (0.1) | 1.7 (0.1) | 1.7 (0.1) | 1.8 (0.1) | 0.10 | 1.7 (0.1) | 1.7 (0.11) | 0.48 |
| Mean BMI, kg/m2 (SD) | 28.3 (3.8) | 26.3 (3.9) | 26.7 (6.4) | 24.5 (5.3) | 0.12 | 27.4 (4.7) | 28.7 (5.7) | 0.34 |
| Mean pre-op surgical risk score (SD) | 2 (1.4) | 2.4 (1.2) | 1.9 (1.3) | 1.8 (1.5) | 0.72 | 2 (1.3) | 2.3 (1) | 0.22 |
| Mean weeks of limb/trunk exercise (SD) | 3.5 (2) | 3.1 (1.6) | 4.2 (1.6) | 2.8 (1.3) | 0.33 | 3.6 (1.8) | N/A | N/A |
| Mean LOS, days (SD) | 8.8 (4.7) | 8.4 (1.7) | 10.4 (4.4) | 8.4 (1.1) | 0.06 | 9.12 (4.2) | 9.18 (4.8) | 0.96 |
| Mean bypass time, minutes (SD) | 74.2 (20.5) | 110 (19.9) | 103.8 (40.9) | 101.4 (44.8) | <0.001 | 87 (31.6) | 108.1 (78) | 0.23 |
| Mean X-clamp time, minutes (SD) | 52 (15.4) | 85.1 (17.7) | 61.2 (38) | 80 (39.6) | <0.001 | 60 (26.7) | 60.1 (34.8) | 0.99 |
| Mean SDS (pre-op)(SD) | 6.2 (14.7) | 2.4 (5.6) | 3.5 (6.2) | 0 (0) | 0.32 | 4.7 (11.8) | 3.1 (5.1) | 0.37 |
| Mean SDS (week 12) (SD) | 2.6 (5.5) | 1.0 (1.5) | 2.4 (3.5) | 0.8 (1.3) | 0.76 | 2.3 (4.6) | N/A | N/A |
| Mean TPS (pre-op) (SD) | 5.9 (10.2) | 4.9 (5.6) | 4.8 (7.7) | 5.6 (6.4) | 0.86 | 5.5 (8.9) | 4.7 (6.8) | 0.67 |
| Mean TPS (week 6) (SD) | 8.7 (9.8) | 8.8 (7.1) | 12.2 (14.3) | 5.4 (5.1) | 0.85 | 9.3 (10.6) | N/A | N/A |
| Mean TPS (week 12) (SD) | 4.8 (6.3) | 5.9 (8.0) | 6.5 (9.7) | 4.6 (8.1) | 0.84 | 5.3 (7.4) | N/A | N/A |
Notes: Other OHS included ascending aortic hemiarch replacement, atrial septal defect closure, redo pulmonary valve with right ventricular outflow tract repair, transaortic myomectomy. All tests between surgical groups were performed with Kruskal–Wallis test for median values. For comparisons for the completers versus non-completers, chi-squared test was used for categorical data and Student’s t-test (two-tailed for non-related groups) for numerical data.
Preoperative injury or conditions identified varied between surgical groups including: 1) CABG only: ankle and wrist, arthritis; arthritis in lower back; arthritis in spine; arthritis/muscle spasm in spine; arthritis; bilateral frozen shoulder, carpel tunnel (right); compressed spine; cut tendon hand (right); whiplash; degeneration of low back; dislocated shoulder as child; degeneration in the neck and back; disc problem; fall – fractured vertebrae; fell and hurt back; fell down a flight of stairs; fell over and damaged shoulder ligaments; football injury; forearm cut with chainsaw (left); fractured collar bone, fractured elbow; fractured metacarpal joint; fractured ribs; fractured wrist; graft surgery to left arm; hit and run – hurt back and right hand; lumbar disc problem; osteoarthritis of shoulder; osteoarthritis, pulled muscle lower back; rotator cuff shoulder (right), sciatica; scoliosis; Sherman’s disease in thoracic area; shoulder injury from fall; shoulder surgery (right); stiff neck; strained muscle; thumb and skull fracture; torn muscles; twisted back 30 years ago; whiplash. 2) CABG and valve only: finger arthritis; fractured wrist playing tennis; motor vehicle accident fractured neck/ribs + worn out disc; stiff neck, tendinitis elbow, strained back. 3) Valve only: arthritis; busted knuckle; cut tendon hand (left); cracked ribs; fell to the back/tennis elbow; long term aches and pains; neck injured in motor vehicle accident, tendon injury in shoulder; osteoarthritis back, had fallen over onto shoulder (right); osteoporosis; shoulder pain playing tennis (right), had fallen over hand and hurt shoulder (right); rotator cuff injury from fall; tennis elbow, pinched sciatic nerve. 4) Other OHS: neck injured in motor vehicle accident and tendon injury in shoulder; previous cardiac surgery; tight gluteal muscles; work injury to low back, shoulder pain (right) and upper back pain caused by heart surgery.
Walking aids included seat walkers, single sticks ×1–2; wheeled walker.
Trunk/limb exercise frequency was reported at week 6.
Missing data for one subject in the other OHS group.
Missing data for two subjects.
Pairwise comparisons of age for the surgical groups (Dunn’s method) was not significant (>0.05). Parentheses indicate that the p-value noted relates to the two lines (men and women), the two lines (completers and non-completers) and the three lines (trunk/limb exercsies - daily, 1–4 times/wk andonce/week).
Abbreviations: CABG, coronary artery bypass graft; LOS, length of stay; N/A, not applicable or not available; OHS, open-heart surgery; post-op, post-operation; pre-op, pre-operation; SDS, shoulder disability score; TPS, total pain score; VR, valve replacement/repair.
This study was observational in design, primarily examining the prevalence of pain and disability longitudinally in a single cohort, aiming to identify the most affected body areas, and to examine possible contributing factors and associations. Post hoc power and sample size calculations based upon proportions of post-OHS musculoskeletal sequelae reported in previous studies (ie, 30%–70%) indicated our study was powered to 0.88 (alpha, 0.05).1,2,14,26–28
Results
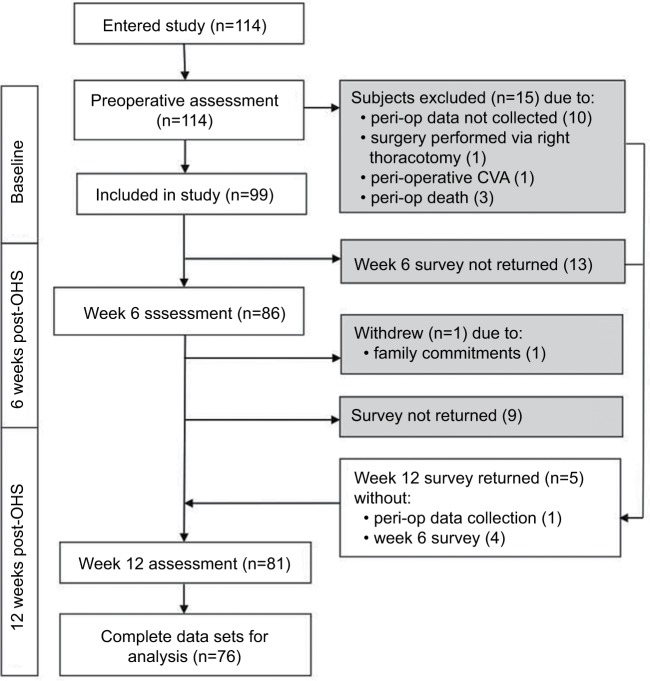
All 114 subjects consenting to participate in the study completed the initial preoperative survey. Figure 1 shows the flow of subjects through the study including exclusions and withdrawals. In all, 99 subjects were eligible for inclusion, with 1 subject formally withdrawing after the week 6 assessment. Of the remaining 98 subjects, 88% returned the week 6 survey, and 82% subjects returned the week 12 survey. Complete data sets over all times were obtained for 78% subjects (76 of 98).
Figure 1.
Patient flow through study.
Abbreviations: OHS, open-heart surgery; CVA, cerebrovascular accident; peri-op, peri-operation.
Table 1 describes the subject and surgical details including compliance with the prescribed exercise program and any complications that occurred.
Prevalence and area of neuro-musculoskeletal pain and shoulder disability preoperatively, at week 6 and week 12 post-OHS
Preoperatively, 52/76 (68%) subjects reported a history of previous neuro-musculoskeletal injury or conditions, including osteoarthritis (n=13), falls resulting in shoulder injury or fractures to ribs or upper extremities (n=12), low back pain (n=11), other muscle tears/strains/orthopedic conditions (n=9), neck pain (n=5), osteoporosis (n=1), and cardiac surgery (n=1).
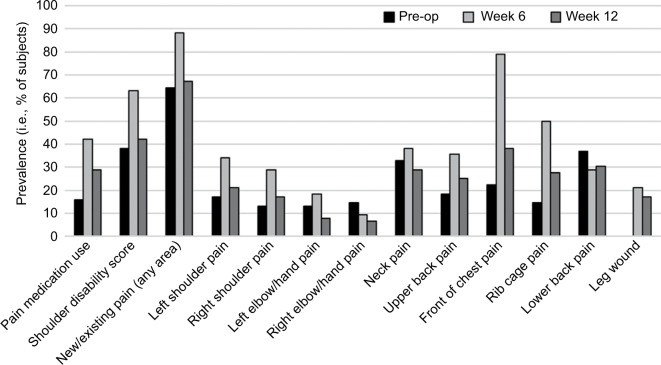
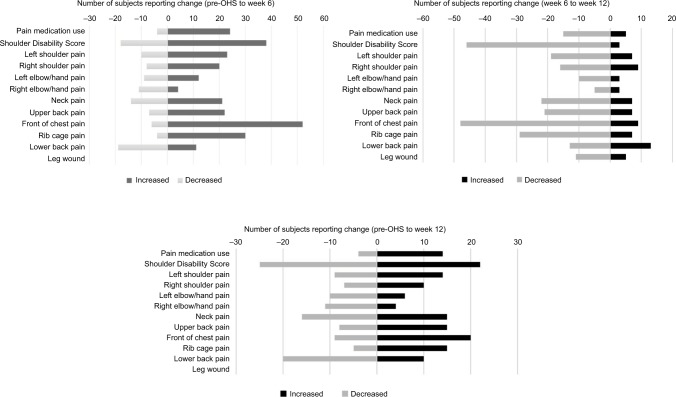
Although 68% subjects reported prior neuro-musculoskeletal injuries or conditions, the preoperative prevalence of neuro-musculoskeletal pain, that is, the percentage of subjects reporting current pain, was only 64%. The prevalence of pain (including new and old pain) increased to 88% in week 6 and reduced to 67% by week 12 (Figure 2), whereas new pain was reported in 67% (51/76) of subjects at week 6 postoperatively. A similar prevalence pattern was noted for pain in all the various body areas across assessment points except for the right elbow/hand and low back area (Figure 2). It was noted that 18% (14/76) of subjects demonstrated an increase or new pain between week 6 and week 12, while the majority, 71% (54/76), of subjects reported a decrease in pain (Figure 3).
Figure 2.
Prevalence of pain, shoulder disability, and pain medication use.
Abbreviation: pre-op, pre-operation.
Figure 3.
Subjects reporting increase or decrease in pain medication use, shoulder disability score, and pain in body areas.
Note: The figure showing different scales applied to each forest plot.
Abbreviation: OHS, open-heart surgery.
The prevalence of shoulder disability in the cohort was 38% preoperatively, 63% at week 6, and 42% at week 12.
Intensity of pain and degree of shoulder disability preoperatively, at week 6, and week 12 post-OHS
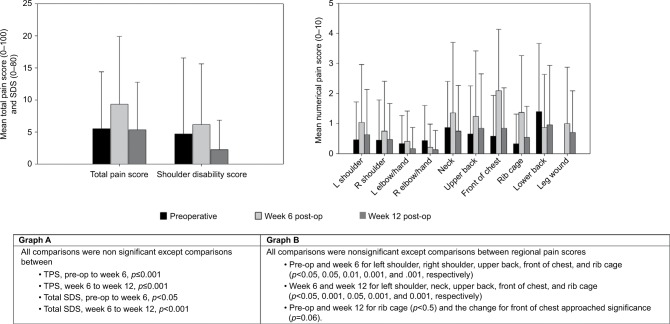
Figure 4A shows the changes in the overall intensity of pain, as represented by the TPS, and the degree of shoulder disability (SDS) at the preoperative, week 6, and week 12 post-OHS assessment points while Figure 4B shows the intensity of pain in the various upper body areas over these assessment points. TPS and SDS increased from the preoperative assessment to week 6 for the entire cohort and there was a subsequent decrease at week 12, resulting in no significant difference between week 12 and preoperative levels for TPS and SDS (Figure 4A). The only body region to demonstrate significantly higher levels of pain at week 12 than preoperatively was the rib cage (p=0.04) (Figure 4B).
Figure 4.
Pain and SDSs pre- and post-OHS.
Notes: (A) TPS and total SDS; (B) mean numerical pain scores by body region.
Abbreviations: OHS, open-heart surgery; SDS, shoulder disability score; R, right; L, left; TPS, total pain score; pre-op, pre-operation; post-op, post-operation.
While overall, pain was well controlled and minimal in intensity, the prevalence of regular pain medication use increased from 16% preoperatively to 42% at week 6 (p=0.0003) reducing by week 12 to 29% (p=0.01, week 12 vs week 6) and nonsignificantly different to preoperative levels (p=0.052) (Figure 2).
Pre, peri, postoperative factors associated with neuro-musculoskeletal pain and shoulder disability
To assess potential differences between the surgical procedures, subjects were categorized into four groupings (CABG only, CABG + VR, VR only, other OHS) and differences in patient parameters for these surgical groups are shown in Table 1. Subjects who had undergone CABG were further categorized based upon graft sites and the number of grafts performed. Other factors considered as potentially associated with pain and shoulder disability postoperatively were also examined.
Presence of preoperative pain, musculoskeletal injury, or other conditions
Previous injury or conditions was not a factor in the levels of preoperative or postoperative pain or SDSs. However, for subjects reporting preoperative pain, TPS increased for 55% (27/49) and decreased for 43% (21/49) of subjects from pre-OHS to week 6; and similarly, 55% (27/49) subjects reported a reduction and 37% (18/49) an increase in TPS from pre-OHS to week 12.
Conversely, for subjects without preoperative pain, 93% (25/27) reported an increase in TPS and 0% (0/27) a decrease at week 6; while 60% (16/27) reported an increase and 0% (0/27) a decrease in TPS from pre-OHS to week 12.
The mean (SD) TPSs over the three assessment periods were 8.5 (9.9), 11.8 (12.2), and 6.8 (8.2) for those with pain preoperatively (p=0.17 for change pre-operation (pre-op) to week 6 and p=0.19 pre-op to week 12) and 0 (0), 6.5 (6.0), and 3.8 (5.8) for those reporting no pain preoperatively (p<0.001 for both pre-op to week 6 and pre-op to week 12).
Across all subjects, Pearson’s correlation coefficient for TPS was r=0.40 preoperative to week 6; r=0.48 preoperative to week 12; and r=0.65 week 6 to week 12 (p<0.001 in all cases).
The SDS was higher for subjects with pain pre-OHS than those without pain at the preoperative assessment, with mean (SD) SDS of 6.72 (14.04) and 0.60 (1.23), respectively (p=0.017). However, there was no significant difference in SDS between these groups at week 6 or week 12.
Preoperative SDS for all subjects was positively correlated with postoperative length of stay (r=0.29, p=0.012) and with SDS at week 12 (r=0.42, p=0.0001) while SDS at week 6 was positively correlated with SDS at week 12 (r=0.66, p<0.000001).
Patient variables and surgical factors
Across the entire cohort, no significant correlations were found between age, weight, BMI, bypass time, cross-clamping time, number of grafts, or length of preoperative stay, and pain scores for body regions or for the TPS at any time point. However, in the “CABG only” group the number of bypass grafts performed was moderately correlated with total pain preoperatively (r=0.33, p=0.03) and negatively correlated with total pain change in pre-op to week 6 (r=−0.31, p=0.037).
The surgical risk score was found to correlate with preoperative TPS (r=0.23, p=0.04) and SDS (r=0.31, p=0.005) and with change in SDS preoperative to week 6 (r=−0.29, p=0.01) and in TPS and SDS preoperative to week 12 (r=−0.24, p=0.04, −0.33, and 0.003, respectively).
Correlations between SDS and age, weight, BMI, bypass time, cross-clamping time, or preoperative length of stay were not significant. However, median (25%, 75%) SDS was higher for women than men at week 6 (6.0 [2.5, 10.0] vs 1.5 [0.0, 6.0], p<0.009) and at week 12 (1.0 [0.0, 3.3] vs 0.0 [0.0, 1.0], p=0.049), and was negatively associated with height at week 12 (r=−0.24, p=0.036).
While some differences were noted in the pattern of change for TPS, pain scores in the body areas, and for SDS across the entire cohort and when analyzed by surgical groups, graft sites, and by the number of grafts overall, similar low pain scores were demonstrated in all analyses.
Sternal clicking/use of IMAG
The prevalence of sternal clicking in the entire cohort was 11% at week 6 and 7% at week 12. Within our cohort, subjects who underwent CABGs, that is, those in the “CABG only” and “CABG + VR” groups, commonly received both IMAG and saphenous vein graft (SVG)/radial artery graft (RAG) and these subjects demonstrated a significantly lower prevalence of sternal clicking at week 6 (3/53 patients, 6%) than subjects in the “VR only” and “other OHS” groups combined (5/23, 22%), p=0.04.
However, when examining the entire cohort, that is, all 76 subjects, there was no significant difference in the prevalence of clicking between IMAG recipients and non-IMAG recipients, with a prevalence of 7% (3/43) at both week 6 and week 12 in IMAG recipients and 14% (5/33) and 6% (2/33), respectively, in non-IMAG recipients (p=0.71).
TPS and SDS comparisons preoperatively, and at week 6 and week 12 post-op demonstrated no significant difference between IMAG graft recipients and non-IMAG recipients (p=0.45, 0.09, and 0.69 for TPS and p=0.76, 0.29, and 0.95 for SDS); or for IMAG graft recipients versus SVG/RAG recipients (p=0.77, 0.34, and 0.43 for TPS and p=0.73, 0.41, and 0.49 for SDS, respectively).
Discussion
To our knowledge, this is the first study to examine the prevalence of pain and shoulder disability preoperatively, and at week 6 and week 12 postoperatively to examine the resolution of neuro-musculoskeletal pain early post-OHS and make comparisons with preoperative data. Overall, pain and SDS were minimal in intensity and degree, with TPS and SDS increasing at week 6 and returning to preoperative levels by week 12, with the rib cage being the only area with a significantly greater pain score at week 12 than preoperatively. Risk factors identified for pain and shoulder disability postoperatively were shorter height, being a woman, and preoperative SDS. Preoperative SDS was also found to positively correlate with post-OHS length of stay.
While we found a higher prevalence of preexisting musculoskeletal injury or conditions than reported previously (68% vs 33%) with less osteoarthritis and osteoporosis, the prevalence of new neuro-musculoskeletal pain and disability at week 6 in our study was similar to that reported by others.1,2 However, while some studies have reported that 30% of subjects experience increased musculoskeletal pain and/or decreased function between weeks 8 and 10 post-OHS, we found TPS was only 3% and SDS 4% above the preoperative level by week 12 suggesting that in our cohort most new pain and shoulder disability post-OHS were transitory and resolved by week 12.1,2 While home exercise compliance in our study was similar to other studies, our treatment regime was of higher intensity and frequency compared to that reported in previous research.2 Further, our study’s end point was at week 12 post-OHS, whereas others had an endpoint between week 3 and week 10.1,2 These differences in assessment protocols, including questions asked, timing, and frequency of assessments, may account for the greater symptom resolution noted in our cohort (final prevalence of pain and disability 3%–4% above pre-OHS levels vs 30% in other studies).1,2
Neuro-musculoskeletal pain was most prevalent in the lower back and neck preoperatively and in front of the chest, rib cage, neck, and upper back at week 6. At week 12, while the front of the chest was the most prevalent area of pain, neck and lower back (noted to be the most prevalent areas preoperatively) and rib cage pain were the next most prevalent. Others reported that 26% of subjects had anterior chest pain 3–6 weeks post-CABG.1 In our study, front of chest pain was found in 79% of subjects at week 6, compared to 22% of subjects preoperatively. Pain levels in the current study were generally low, with the front of the chest at week 6 being the only area to have a mean pain score >1.5. By week 12, the average pain score was not above 1/10 for any region.
Similarly, low levels of shoulder disability were recorded and total SDS at week 12 was lower than at week 6 and preoperative levels. While these changes in disability align with changes in pain and the expected healing processes post-operatively, the finding that SDS at week 12 was lower than baseline level suggests some improvement in function due to the surgical procedure or to the postoperative management such as shoulder/trunk exercises or walking. Preoperative SDS was found to be positively correlated with post-OHS length of stay suggesting that shoulder function may be an important factor to be managed proactively preoperatively and postoperatively.
Use of the IMAG has been previously associated with increased musculoskeletal complications compared to use of the SVG (78% vs 45%, respectively) and when compared to SVG/RAG or VR OHS (88% vs 76%), while others found no increase in new musculoskeletal problems or chronic pain in patients undergoing IMAG.1–3,11 In our study, pain levels and SDS were no greater for IMAG graft recipients compared to non-IMAG recipients. Indeed, pain at week 6 was not correlated with surgery type, graft site, or the number of grafts. Similarly, in contrast to others who reported 9% sternal instability in the IMAG group and 3% in the SVG group, we found similar prevalence of sternal clicking in subjects who underwent CABG “with IMAG” and “without IMAG” and a lower prevalence in subjects who underwent CABG compared to those without CABG.1 Differences in surgical technique or the higher levels of osteoporosis and osteoarthritis in the previous IMAG study group may have led to higher pain levels, delayed healing, and sternal instability in this group.1
Risk factors previously associated with post-OHS pain include cardiopulmonary bypass time >60 minutes and pain levels during the first week post-OHS; women are more at risk of pain 3 months post-OHS and may continue for 12 months.8–10,12–14 In addition, a higher BMI and preoperative angina have been identified as being risk factors for chronic post-OHS pain.6 In our cohort, while cardiopulmonary bypass time and BMI were not found to be associated with pain or SDS, women were more likely to have a higher SDS at week 6 and SDS was negatively associated with height at week 12. An additional and potentially useful tool examined was the surgical risk score which was found to correlate with preoperative TPS, preoperative SDS, and with the change in TPS and SDS over time.
Several study limitations are noted, which may have overestimated or underestimated the outcomes assessed. Complete data sets were not obtained for all subjects; however, return rates were comparable to other studies; patients attending preadmission may not represent all adult OHS patients; some surgical subgroups included small numbers; our study relied on self-report of sternal clicking and did not include a physical examination of patients reporting pain; the study did not capture the prevalence of angina preoperatively or of chronic pain of >12 weeks’ duration.2,8,13 Further, subjects may have had difficulty in differentiating between pain of cardiac (angina) and neuro-musculoskeletal origin. However, the similar pattern reported by subjects for the SDS indicates that subjects may have been successful in this task. Moreover, the fact that preoperative SDS was associated with postoperative length of stay may indicate a link to functional tasks rather than angina.
Conclusion
Neuro-musculoskeletal pain and shoulder disability were found to be common preoperatively and the prevalence increased at week 6 post-OHS. However, pain and disability scores at week 12 were similar to preoperative levels in patients undertaking usual post-OHS walking, upper limb and trunk exercise. Only rib cage pain remained above preoperative levels at week 12 post-OHS. At all assessment points, the degree of pain and shoulder disability were minimal. Preoperative SDS was positively correlated with post-OHS length of stay; women had a higher SDS than men at week 6 and week 12; and week 12 SDS was negatively correlated with height. Surgical risk score was negatively correlated with change in SDS and in TPS from pre-op to week 12. Future research could examine patients with ongoing pain at week 12 to explore the most appropriate management and prevention strategies of chronic pain and disability.
Supplementary material
Box S1. Shoulder disability scale.
How much difficulty do you have in carrying out the following activities?
Circle the number that best describes your experience, where 0 = no difficulty and 10 = so much difficulty that you require help.
Washing your hair
Washing your back
Putting on an undershirt or jumper
Putting on a shirt that buttons down the front
Putting on your pants
Placing an object on a high shelf
Carrying a heavy object of 4.5 kg
Removing something from your back pocket
Note: Data from Roach K et al.1
Reference
- 1.Roach K, Budiman-Mak E, Songsirideg N, Yongsuk L. Development of a shoulder pain and disability index. Arthritis Res. 2001;4:143–149. [PubMed] [Google Scholar]
Acknowledgments
The authors wish to thank Mr Oystein Tronstad (BPthy), Advanced Physiotherapy Clinical Lead at The Prince Charles Hospital who assisted by updating the literature review for this study. This research was supported in kind by the Physiotherapy Department of The Prince Charles Hospital, Brisbane Australia.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.El-Ansary D, Adams R, Ghandi A. Musculoskeletal and neurological complications following coronary artery bypass graft surgery: a comparison between saphenous vein and internal mammary artery grafting. Aust J Physiother. 2000;46(1):19–25. doi: 10.1016/s0004-9514(14)60310-x. [DOI] [PubMed] [Google Scholar]
- 2.Stiller K, McInnes M, Huff N, Hall B. Do exercises prevent musculoskeletal complications after cardiac surgery? Physiother Theory Pract. 1997;13:117–126. [Google Scholar]
- 3.Meyerson J, Thelin S, Gordh T, Karlsten R. The incidence of chronic post-sternotomy pain after cardiac surgery – a prospective study. Acta Anaesthesiol Scand. 2001;45(8):940–944. doi: 10.1034/j.1399-6576.2001.450804.x. [DOI] [PubMed] [Google Scholar]
- 4.Eisenberg E, Pultorak Y, Pud D, Bar-El Y. Prevalence and characteristics of post coronary artery bypass graft surgery pain (PCP) Pain. 2001;92(1–2):11–17. doi: 10.1016/s0304-3959(00)00466-8. [DOI] [PubMed] [Google Scholar]
- 5.Kalso S, Mennander T, Tasmuth T, Nilsson E. Chronic post-sternotomy pain. Acta Anaesthesiol Scand. 2001;45:935–939. doi: 10.1034/j.1399-6576.2001.450803.x. [DOI] [PubMed] [Google Scholar]
- 6.Bruce J, Drury N, Poobalan A, Jeffrey R, Smith W, Chambers W. The prevalence of chronic chest and leg pain following cardiac surgery: a historical cohort study. Pain. 2003;104:265–273. doi: 10.1016/s0304-3959(03)00017-4. [DOI] [PubMed] [Google Scholar]
- 7.Gjeilo KH, Klepstad P, Wahba A, Lydersen S, Stenseth R. Chronic pain after cardiac surgery: a prospective study. Acta Anaesthesiol Scand. 2010;54(1):70–78. doi: 10.1111/j.1399-6576.2009.02097.x. [DOI] [PubMed] [Google Scholar]
- 8.Bjornnes AK, Parry M, Lie I, et al. Pain experiences of men and women after cardiac surgery. J Clin Nurs. 2016;25(19–20):3058–3068. doi: 10.1111/jocn.13329. [DOI] [PubMed] [Google Scholar]
- 9.Marcassa C, Faggiano P, Greco C, Ambrosetti M, Temporelli PL, Italian Association of Cardiovascular Prevention, Rehabilitation A retrospective multicenter study on long-term prevalence of chronic pain after cardiac surgery. J Cardiovasc Med (Hagerstown) 2015;16(12):857. doi: 10.2459/JCM.0000000000000271. [DOI] [PubMed] [Google Scholar]
- 10.van Leersum NJ, van Leersum RL, Verwey HF, Klautz RJ. Pain symptoms accompanying chronic poststernotomy pain: a pilot study. Pain Med. 2010;11(11):1628–1634. doi: 10.1111/j.1526-4637.2010.00975.x. [DOI] [PubMed] [Google Scholar]
- 11.Kamalipour H, Vafaei A, Parviz Kazemi A, Khademi S. Comparing the prevalence of chronic pain after sternotomy in patients undergoing coronary artery bypass grafting using the internal mammary artery and other open heart surgeries. Anesth Pain Med. 2014;4(3):e17969. doi: 10.5812/aapm.17969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raksamani K, Wongkornrat W, Siriboon P, Pantisawat N. Pain management after cardiac surgery: are we underestimating post sternotomy pain? J Med Assoc Thai. 2013;96(7):824–828. [PubMed] [Google Scholar]
- 13.Choiniere M, Watt-Watson J, Victor JC, et al. Prevalence of and risk factors for persistent postoperative nonanginal pain after cardiac surgery: a 2-year prospective multicentre study. CMAJ. 2014;186(7):E213–E223. doi: 10.1503/cmaj.131012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Gulik L, Janssen LI, Ahlers SJ, et al. Risk factors for chronic thoracic pain after cardiac surgery via sternotomy. Eur J Cardiothorac Surg. 2011;40(6):1309–1313. doi: 10.1016/j.ejcts.2011.03.039. [DOI] [PubMed] [Google Scholar]
- 15.Lahtinen P. Pain after Coronary Artery Bypass Grafting Surgery: Clinical Studies of Acute and Persistent Postoperative Pain. Kuopio: Faculty of Health Sciences, University of Eastern Finland; 2012. [Google Scholar]
- 16.Cogan J. Pain management after cardiac surgery. Semin Cardiothorac Vasc Anesth. 2010;14(3):201–204. doi: 10.1177/1089253210378401. [DOI] [PubMed] [Google Scholar]
- 17.Roach K, Budiman-mak E, Songsirideg N, Yongsuk L. Development of a shoulder pain and disability index. Arthritis Res. 2001;4:143–149. [PubMed] [Google Scholar]
- 18.Abramson J, Abramson Z. Survey Methods in Community Medicine. 5th ed. London: Churchill Livingstone; 1999. [Google Scholar]
- 19.Hjermstad MJ, Fayers PM, Haugen DF, et al. Studies comparing numerical rating scales, verbal rating scales, and visual analogue scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manage. 2011;41(6):1073–1093. doi: 10.1016/j.jpainsymman.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 20.Collins S, Moore R, McQuay H. The visual analogue pain intensity scale: what is moderate pain in millimeters? Pain. 1997;72:95–97. doi: 10.1016/s0304-3959(97)00005-5. [DOI] [PubMed] [Google Scholar]
- 21.Ostelo RWJG, Deyo RA, Stratford P, et al. Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine. 2008;33(1):90–94. doi: 10.1097/BRS.0b013e31815e3a10. [DOI] [PubMed] [Google Scholar]
- 22.Whinney CM. Perioperative Evaluation. 2009. [Accessed April 29, 2017]. Available from: http://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/preventive-medicine/perioperative-evaluation/
- 23.Mohabir PK, Gurney J. Preoperative Evaluation. 2015. [Accessed April 29, 2017]. Available from: http://www.merckmanuals.com/professional/special-subjects/care-of-the-surgical-patient/preoperative-evaluation.
- 24.Bartlett RH. Postoperative pulmonary prophylaxis. Breathe deeply and read carefully. Chest. 1982;81(1):1–3. doi: 10.1378/chest.81.1.1. [DOI] [PubMed] [Google Scholar]
- 25.Craven JL, Evans GA, Davenport PJ, Williams RH. The evaluation of the incentive spirometer in the management of postoperative pulmonary complications. Br J Surg. 1974;61(10):793–797. doi: 10.1002/bjs.1800611012. [DOI] [PubMed] [Google Scholar]
- 26.Werner MU, Mjobo HN, Nielsen PR, Rudin A. Prediction of postoperative pain: a systematic review of predictive experimental pain studies. Anesthesiology. 2010;112(6):1494–1502. doi: 10.1097/ALN.0b013e3181dcd5a0. [DOI] [PubMed] [Google Scholar]
- 27.Williams JW, Jr, Holleman DR, Jr, Simel DL. Measuring shoulder function with the shoulder pain and disability index. J Rheumatol. 1995;22(4):727–732. [PubMed] [Google Scholar]
- 28.Leegaard M, Naden D, Fagermoen MS. Postoperative pain and self-management: women’s experiences after cardiac surgery. J Adv Nurs. 2008;63(5):476–485. doi: 10.1111/j.1365-2648.2008.04727.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Box S1. Shoulder disability scale.
How much difficulty do you have in carrying out the following activities?
Circle the number that best describes your experience, where 0 = no difficulty and 10 = so much difficulty that you require help.
Washing your hair
Washing your back
Putting on an undershirt or jumper
Putting on a shirt that buttons down the front
Putting on your pants
Placing an object on a high shelf
Carrying a heavy object of 4.5 kg
Removing something from your back pocket
Note: Data from Roach K et al.1