Abstract
Objective
To investigate whether bright light treatment can reduce body mass in overweight subjects irrespective of their seasonal (= light) dependence.
Methods
A crossover, placebo-controlled, randomized clinical trial was performed between November and April in Novosibirsk, Russia (55° N). The trial comprised a 3-week in-home session of morning bright light treatment using a device of light-emitting diodes and a 3-week placebo session by means of a deactivated ion generator, separated by an off-protocol period of at least 23 days. The number of placebo and light sessions was matched with respect to season. Data were obtained from 34 overweight women, aged 20–54 years, 10 were seasonal-dependent according to the Seasonal Pattern Assessment Questionnaire. Weekly measures included body weight, percentage body fat by bioimpedancemetry, and subjective scores (appetite, mood, energy levels).
Results
Motivation and expectation towards weight loss were similar for the two intervention sessions. With light, compared to the placebo session, weight did not reduce significantly, but percentage fat, fat mass, and appetite were significantly lower (average fat reduction 0.35 kg). The latter two results remained significant after excluding seasonal-dependent subjects from the analysis. Irrespective of the type of intervention, seasonal-dependent subjects had greater weight and fat mass changes during treatment (decline p ℋ 0.036) or between sessions (regain p ℋ 0.003). Photoperiod (p = 0.0041), air temperature to a lesser extent (p = 0.012), but not sunshine (p = 0.29) was associated with the weight change (greater weight reduction if the second session was in spring).
Conclusion
Morning bright light treatment reduces body fat and appetite in overweight women and may be included in weight control programs.
Key Words: Overweight, Bright light treatment, Body fat, Appetite, Seasonality
Introduction
Artificial bright light, acting via the eyes, is the treatment of choice for seasonal affective disorder [1,2,3] which presents in 2% of population at temperate and high latitudes and is characterized by depressed mood, fatigue, hypersomnia, and increased appetite/weight during fall/winter [4,5,6]. Irrespective of seasonality, each of these cohort of symptoms is separately facilitated by light therapy: mood and energy levels increase in non-seasonal depressed [2,7,8] and healthy [9,10,11] people, sleep is improved in circadian rhythm sleep disorders [12,13], and weight is reduced in obesity. However, data for the latter is scarce.
In an early controlled study performed in Norway, an 8-week hypocaloric diet combined with a 30-min walk (thrice a week), and a 30-min exposure to 10,000 lux light (daily) for the first 2 weeks reduced body mass in overweight persons by an average of 8.6 kg while in a parallel group, receiving the same treatment combination but only 500 lux light, this led to a significantly lower reduction of 2.9 kg (Thom E, 2002; abstract unpublished). There was no indication as to what extent the test volunteers were seasonally dependent (= light sensitive).
In a Canadian study published in 2007 [14], overweight/obese non-seasonal subjects were assigned to 6 weeks of moderate exercise with or without daily bright light treatment (14 vs. 11 subjects). Weight was not reduced significantly, whereas percentage body fat was.
In our previous study performed between December 2006 and April 2007 in Novosibirsk, Russia (55° N), 41 non-seasonal obese volunteers (all but 1, female) received a 3-week session of bright white light and a 3-week placebo session by means of a deactivated ion generator combined with mild hypocaloric diet. Compared to placebo, weight and appetite levels did not reduce significantly [15].
Here we report results from a subsequent crossover study of overweight subjects who used light intervention alone. The placebo intervention was a deactivated ionizer reliably used in previous light therapy trials [16,17]. In addition to weight, fat mass was measured. The subjects’ seasonality was estimated a posteriori. The aim was to investigate whether light treatment may indeed reduce body mass.
Participants and Methods
Participants
The study was performed in the winter half of the year – between January and April 2009 and between November 2009 and April 2010. The study was approved by the Ethics Committee of the Institute of Internal Medicine, Siberian Branch of the Russian Academy of Medical Sciences and registered in an international register (http://prsinfo.clinicaltrials.gov/, NCT00406770). Test participants were recruited via a patient database of the institute, advertisements, or by ‘word of mouth’ and gave written informed consent.
Inclusion criteria were as follows: women aged 20–54 years; BMI 25–30 kg/m2; a wish to decrease body weight; good general health as by entry questionnaire and study physician's enquiries; on a stable dose of medication(s), if suffering chronic disease; normal sleep regimen between 22:00–01:00 and 06:00–09:00; ability to visit investigator at the appointed dates. Exclusion criteria were as follows: regular use of substances or means for losing body weight during the 3 months prior to the study; transmeridian travel or acute illness − 2 months prior to the study; use of light therapy in the past.
No formal sample size calculation was done. Based on the previous studies performed (see ‘Introduction’), and the crossover design, we targeted to have 40 participants complete the study.
Protocol and Design
This was crossover, placebo-controlled, randomized clinical trial comprising a 3-week session of light intervention (from a light device), a 3-week session of placebo (using a deactivated ion generator) and an off-protocol period for at least 1 month in between. The devices were allocated to participants randomly from a batch of 4. Physicians knew that ion generators were deactivated, but were not aware of which device had been allocated to the participant. Participants were asked to not give any information to the supervising physician on the type of the device they used. Two physicians (SVM and EAP) recruited and supervised the test subjects independently.
Though the off-protocol duration was set to 1 month, the date to enter the second session was adjusted so that the day of menstrual cycle should be the same as at the first session ± 4 days (if present and regular). If the subject would not be able to complete the study during the current winter-spring, the second session was performed in the following winter. During each 3-week study session, subjects visited the supervising physician four times. Seasonal dependence of subjects was determined at the final visit in order to prevent possible bias by physicians towards light intervention outcomes, since seasonality with winter worsening of symptoms predicts good responsiveness to light therapy.
Intervention
Intervention devices during each 3-week session were used at home every day between 06:00 and 09:00. The subject was allowed to move away from the device briefly but the total duration of the morning exposure was required to be 45 min.
The light device was the portable panel Lumie SAD Light (size 18 × 11 × 3.5 cm, weight 0.3 kg; Lumie, Cambridge, UK). The light-emitting diodes (LEDs) of the SAD Light produced white light with enhanced blue wavelengths (peak at 461 nm) with an intensity of 1,300 lux at a distance of 41 cm. The blue light is important to exert biological effects [18]. It was not necessary to look at the screen all the time, just to allow light to freely enter both eyes.
A deactivated negative ion generator was used as placebo (model DP-240, size 11 × 11 × 18 cm, weight 0.25 kg; Dezac Group, Cheltenham, UK). After plugging in the generator, the LED on the top indicated that it was switched on, but in fact, the generator had been internally deactivated by an engineer and did not produce ions. The subjects were not aware of this and could not distinguish it, as the oxygen species produced as a result of moderately intense ionization are imperceptible to humans [17]. It was not necessary to look at the ionizer as ions enter the body with inhaled air [19], and not via the eyes.
Variables Analyzed
Body weight was documented during the subjects’ visit to the Institute. They visited between 10:00 and 12:00 following morning fast. Weight was measured by an electronic balance to the nearest 0.1 kg in indoor clothing. Percentage body fat was determined by hand-to-hand bioelectrical impedancemetry using the Omron BF 302 set (Matsusaka Co. Ltd, Matsusaka City, Japan). The method has good reproducibility [20] when hydration status is controlled (the percentage fat value declines with fluid loss [21]). Non-fat mass was estimated by subtracting the amount of body fat from total body weight.
Motivation for weight loss and expectation towards the intervention were self-assessed in the diary prior to each 3-week intervention session using a study-developed numerical scale (motivation: high = 3, moderate = 2, low = 1; expectation: no change (or increase in weight) = 1, somewhat decrease = 2, definite decrease = 3). Retrospective judgment was also rated after completion of the study session. Appetite, mood and energy levels for the past 3–4 days were each scored on an 11-point visual analogue scale once a week.
Seasonality was estimated using the Seasonal Pattern Assessment Questionnaire (SPAQ) [22] truncated to three core questions: i) months of feeling better/worse, ii) extension of the change (score 0–4) in sleep duration, social activity, mood, body weight, appetite, and energy, and iii) to what degree these changes cause a problem (from 0 = no problem to 5 = disabling). Subjects whose overall extension score was 8–9 (and problem = 1 or more) or ≥ 10 (and problem ≥ 0) were considered to be seasonal [5], and, together with the reported worsening in fall-winter, indirectly indicated subjective light dependence. Additional questions documented the amount of seasonal change in sleep duration (hours) and body weight (kg).
Data on air temperature and cloud cover were derived from local meteorological records (http://meteo.infospace.ru, Ogurtsovo station). Daytime (09:00, 12:00, 15:00, and 18:00) values of cloud cover (score ranged from 0 to 10) and air temperature were averaged to be used in the analysis. As data on daily hours of sunshine (i.e., under clear sky) were not available for these study years, the product of clear sky ((10 – cloud cover score) / 10) and day length was used instead as it highly correlated with the hours of sunshine (r > 0.95 for, e.g., November 2006 to April 2007).
Adverse events were recorded by the test subjects in the diary and by the study physician during the final visit. In the diary, the subjects also indicated time of going to bed and getting up, onset time and duration of the morning treatment, distance from the head (eyes) to the intervention device, and menstrual cycle onset dates (if applicable).
Statistical Methods
The week-to-week changes in body mass values as well as the differences between light and placebo sessions were generally normally distributed (p > 0.05, Kolmogorov-Smirnov test) and, therefore, were analyzed using analysis of variances for repeated measures (rANOVA) and Student's t-test. In rANOVA, the yielded Huynh-Feldt's corrected probability (p) was considered for the significance. The data on subjective scores were analyzed using nonparametric statistics – Friedman test and paired sign test – which are insensitive to the normality of distribution. StatView 5.0.1 and SuperANOVA 1.11 software were used. Standard deviations of the means (± SD) are reported in the text and tables while standard errors of the means are given in the figures.
Results
Baseline Data
In total, 42 women entered and 39 completed the study. Drop-outs were due to non-compliance to the morning treatment duration. There seems to be an uneven distribution of placebo versus light sessions across the seasons. Among sessions in winter (last started on February 18), placebo sessions prevailed (29 vs. 24), whereas among sessions in spring (first started on March 5), light sessions prevailed (15 vs. 10). As we found a significant effect of season on the body weight dynamics (greater decrease in spring vs. winter; p = 0.0032 by rANOVA with introduced independent factor ‘season’), 5 of those 15 subjects who had light sessions in spring had to be excluded from the analysis. As also expectation towards light versus placebo intervention tended to be higher (p = 0.070, paired sign test), we excluded further 4 women with higher light versus placebo expectation ratings from those 15 subjects. Following this exclusion, expectation ratings were no longer different between the two sessions. The 5th woman to be excluded was chosen from a group of 4 with higher light versus placebo retrospective judgment ratings; she also reported an adverse event (perceiving the light too glaring).
Characteristics of the analyzed group are presented in tables 1 and 2. Only one woman had a BMI slightly below 25 kg/m2. Motivation for weight loss did not differ between placebo and light sessions. Expectation ratings also did not differ; this is not surprising as bright light therapy still is not widely known in the local population. Moreover, some of the test subjects reported to have found information in the internet on efficacy of air-negative ionization towards weight control. At post-study estimation, expectations shifted to worse ratings (p ℋ 0.0001) but this was similar between the light and placebo sessions (p = 0.27, paired sign test). Sleep onset and sleep offset times did not differ between the light and placebo sessions.
Table 1.
Characteristics of the group analyzed
| Study months | November to April |
| N (all – women) | 34 |
| SPAQ score | 6(0–18) |
| Seasonal dependence, n | 10 (worsening in winter) |
| Age, years | 37.4 ± 9.5 (20–54) |
| Body mass, kg | 78.2 ± 9.4 (59.4–100.7) |
| BMI, kg/m2 | 28.1 ± 1.7 (24.4–30.1) |
| Menstrual cycle present, n | 29 |
| Days between sessions, median (range) | 41 (23–326) |
| Number of subjects with the 2nd session started in spring | 20 (l0 with placebo, 10 with light) |
SPAQ = Seasonal pattern assessment questionnaire.
Table 2.
Baseline data differences
| Placebo | Light | |
|---|---|---|
| Motivation to weight loss, n | ||
| No change | 0 | 0 |
| Somewhat | 22 | 24 |
| Definite | 12 | 10 |
| Expectation towards weight loss, n | ||
| No change | 0 | 0 |
| Somewhat | 27 | 25 |
| Definite | 7 | 9 |
| Retrospective expectation judgment, n | ||
| No change | 16 | 9 |
| Somewhat | 18 | 25 |
| Definite | 0 | 0 |
| Time of sleep onset | 23:13 ± 38 min | 23:12 ± 36 min |
| Time of sleep offset | 7:10 ± 33 min | 7:07 ± 29 min |
| Photoperiod, h | 9.9 ± 2.2 | 9.8 ± 2.3 |
| Air temperature, ° C | −16.9 ± 12.4 | −12.6 ± 12.5 |
| Clear sky (%) × photoperiod per day | 6.4 ± 2.0 | 5.9 ± 1.9 |
| Adverse events, n | no | 2× headache |
| 2× eyestrain, glare | ||
| 2× shorter menstrual cycle |
Figures indicate mean ± SD (and range), median (and range), or number n. No significant differences between variables at placebo and light sessions were observed.
Outcomes and Estimation
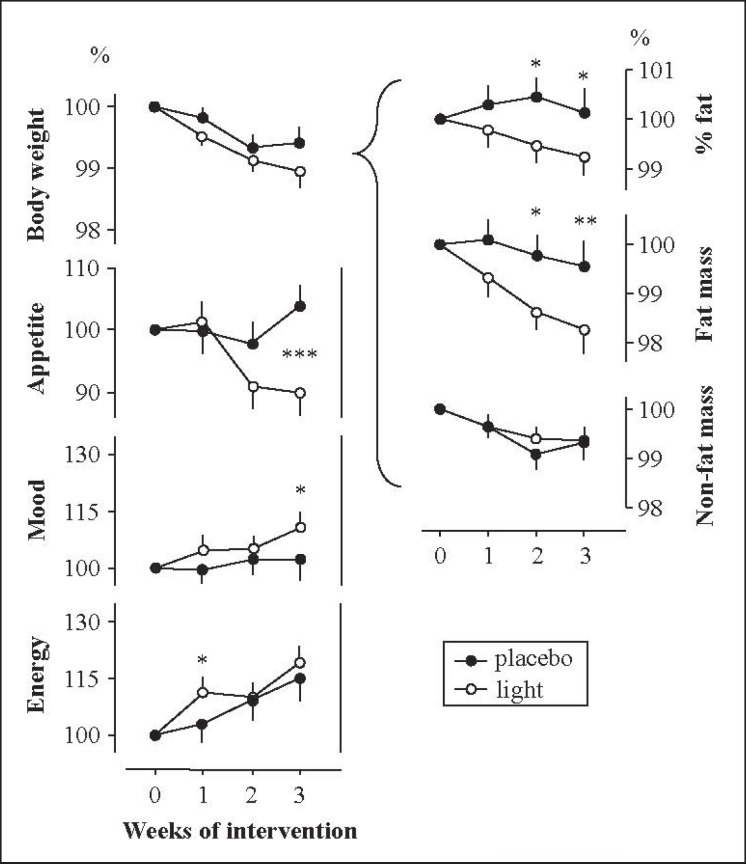
Over the 3 weeks of light session, weight (p ℋ 0.0001 by rANOVA), fat mass (p = 0.0009 by rANOVA), and appetite (p = 0.0004 by Friedman test) decreased and energy levels (p ℋ 0.0001 by Friedman test) increased. Percentage body fat scarcely reduced (p = 0.13, one-way rANOVA) and mood levels scarcely augmented (p = 0.057, Friedman test). Following the 3 weeks of placebo session, only weight and energy levels changed; the changes were in the same direction as during the light session, but with lower probability levels (p = 0.0049 and p = 0.020, respectively); the other main outcome measures did not change (p > 0.48). The overall week 3 to week 0 changes are presented in column ‘∆’ of table 3.
Table 3.
Change in body mass and subjective ratings after placebo and light sessions
| Initial value (week 0) | Δ (week 3 to week 0 difference) | ΔΔ (light to placebo difference) | ||||
|---|---|---|---|---|---|---|
| placebo | light | placebo | light | ΔΔ | 95% CI | |
| Weight, kg | 78.6 ± 9.6 | 78.3 ± 9.6 | −0.46 ± 1.19* | −0.79 ± 1.17*** | −0.33 ± 1.26 | −0.77 to 0.11 |
| % body fat | 34.7 ± 2.7 | 34.7 ± 2.7 | 0.06 ± 0.92 | −0.26 ± 0.78 | −0.31 ± 0.84* | −0.61 to −0.2 |
| Fat mass, kg | 27.4 ± 4.7 | 27.3 ± 4.7 | −0.10 ± 0.88 | −0.45 ± 0.84** | −0.35 ± 0.67** | −0.58 to −0.12 |
| Non-fat mass, kg | 51.2 ± 5.6 | 50.9 ± 5.4 | −0.36 ± 1.11 | −0.34 ± 0.89* | 0.02 ± 1.17 | −0.39 to 0.43 |
| Appetite, score | 5.5 (5; 7) | 6.0 (4.9; 7) | 0 (−1s+); 1.1) | −0.5 (–2; 0.1)** | −1.0 (−2.2; 0.2)*** | |
| Mood, score | 5.0 (4; 7) | 6.0 (5; 7) | 0 (−3; 2.1) | 1.0 (−2; 2.1)* | 1.0 (−2; 3)* | |
| Energy, score | 5.0 (3; 8) | 5.0 (4.9; 6.1) | 0.5 (−1; 2)* | 1.0 (−0.1; 2)*** | 0 (−2; 2.1) |
Figures indicate mean ± SD for body mass and median and percentiles (10%; 90%) for subjective ratings.
p <0.05
p <0.01
p <0.001, by one-sample Student t-test (for body mass) or sign test (for subjective scores).
When comparing the changes following light versus placebo session, several differences emerged (fig. 1, column ‘∆∆’ in table 3). Whereas weight loss was not greater during light sessions (p = 0.11, effect size d = 0.40), the fat mass reduced pronouncedly, resulting in a significantly lower percentage body fat after the 2nd week of light treatment already. Appetite markedly diminished to the end of light versus placebo sessions, mood somewhat improved. Energy levels were not significantly higher at the end of light versus placebo session. The differences in fat mass and appetite levels remained significant after excluding subjects with seasonal dependency from the study group (p = 0.023 and p = 0.0023, respectively; n = 24).
Fig. 1.
Dynamics of body mass and subjective scores following light and placebo interventions in 34 women wishing to lose excess weight. The week 0 value is assigned to 100%. Difference between corresponding values at light and placebo sessions: ★p ℋ 0.05, ★★p ℋ 0.01, ★★★p ℋ 0.001, by either paired Student t-test (for body mass) or paired sign test (for subjective scores), based on absolute values (see column ‘∆∆’ in table 3).
Ancillary Analyses
The following variables were investigated with respect to possible confounding effects on the above results: i) sequence of the trial sessions (light or placebo first); ii) subjects’ seasonality score, problem or category (‘seasonals’ or ‘non-seasonals’), winter-summer weight change (kg, %, or score); iii) difference between the two sessions in average day length, clear sky, and air temperature; iv) difference between the two sessions in the time of morning treatment; and v) age of the subjects. These variables, one by one, were accounted for in the two-way rANOVA.
The weight dynamics were influenced by the sequence of the sessions (p = 0.0069), a difference in day length (p = 0.0041) and a difference in air temperature (p = 0.012). All three factors interrelated: the 2nd-order session was generally carried out under longer days (except for two subjects), and the difference in air temperature was also well correlated with the difference in day length (r = 0.67, p ℋ 0.0001). Photoperiod was the strongest and, of course, primary of these three variables in the effect. Figure 2 illustrates the effect of photoperiod on weight change during the light versus placebo sessions. A similar, but less consistent dependence was found for non-fat body mass. Amount of sunshine (as approximated by clear sky) appeared to be not a confounding factor (p = 0.29). The difference in clear sky did not correlate with the difference in day length (r = 0.18): it happened that January and February were very sunny compared to the months before or after.
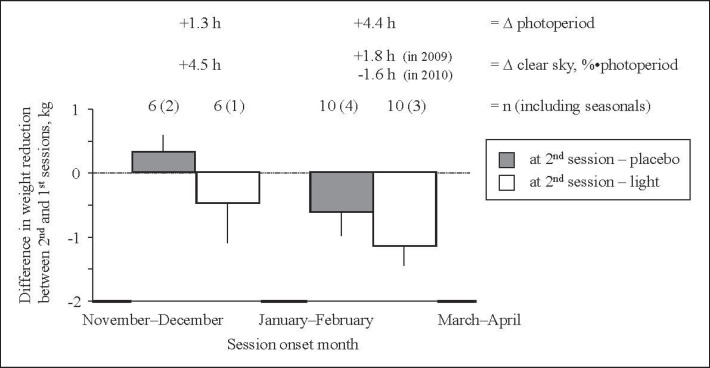
Fig. 2.
Difference in weight reduction between the 2nd and the 1st trial sessions depending on the time of year (downward bars indicate that the 2nd session was more efficacious than the 1st one). The difference was greater when the 2nd session occurred in spring than in winter (first two vs. last two bars combined, effect of season). However, the light session was consistently more efficacious towards weight loss than placebo session (open vs. dark bars combined, effect of intervention). Two subjects who performed the study over two different winters, were not included in the diagram; both were non-seasonals. The effect of seasons is obviously attributable to a change (∆) in photoperiod, not ambient light (as January and February happened to be very sunny during both years).
Fat body mass, percentage body fat (and subjective ratings) were not affected by any of the tested variables (nonsignificant influence on the intervention-by-weeks interaction in rANOVA).
Irrespective of the type of intervention, ‘seasonals’ (n = 10), compared to ‘non-seasonals’ (n = 24), lost more weight and fat mass (group Ч time interaction, p ℋ 0.036). Additionally, seasonals regained weight and fat mass more easily between the sessions (p ℋ 0.003 compared to non-seasonals), even to a greater level than pre-study. The greatest rebound was observed in three seasonals who started the 2nd session after New Year vacations. The mild weight rebound was characteristic for the non-seasonal group also (p = 0.032), but mainly due to non-fat mass (p = 0.052), but not fat mass (p = 0.53).
The changes from week 0 to week 3 in weight, percentage fat, appetite, and mood were poorly intercorrelated (r ℋ 0.26). The strongest correlation for the percentage fat (and fat mass) change was with appetite change: r = 0.25, p = 0.08 (Spearman test, n = 68).
Adverse events were not observed in the placebo intervention. During the light intervention, 4 of the 34 subjects reported headache or visual discomfort (table 2). Two of the additional 5 subjects excluded from the main analysis also reported glare from the SAD Light device, a relatively common complaint with a small lighting unit [23]. Nevertheless, all these immediate side effects were mild and usually transient, and did not require discontinuation. Two women spontaneously reported a 2–3 days shorter menstrual cycle following the light treatment – a phenomenon that has been previously noted [24].
Discussion
Our placebo-controlled crossover study showed that morning bright light reduces body mass, specifically fat mass, in women who wished to lose excess weight. The effect, relative to placebo, is mild − 0.35 kg of fat reduction in 3 weeks.
Weight mass did not change significantly, whereas percentage fat and fat mass did. Such discrepancies have also been demonstrated previously [25,26] and suggests some kind of redistribution of the tissues affected; for instance, fat-free (lean, muscle) mass may, oppositely, somewhat increase due to physical exercise [27,28]. However, non-fat body mass in our study did not increase, it was reduced similarly following light and placebo session, and was not linked to greater physical activity in women during the light session, but the relationship between the changes in fat and non-fat masses appeared to be slightly inversed, explaining on a statistical level why the body weight, unlike fat mass, did not reduce significantly.
The reduction in fat may be explained by lower appetite (leading to less food intake): appetite lowering during the light versus placebo session was quite obvious, and the lowering tended to correlate with the change in fat. Light stimulates serotonin synthesis and turnover in the human brain [29]; by this action light has neurobiological parallels to serotoninergic drugs that attenuate appetite through central mechanisms [30]. Light in humans also activates the sympathetic nervous system as demonstrated by (a transient) increase in peroneal nerve sympathetic activity [31], heart rate [32], and norepinephrine blood release [33] or excretion [34]. This activation causes, in analogy with noradrenergic anti-obesity drugs [31], an increase in energy expenditure (in fact, light therapy increases oxygen consumption in healthy subjects [10]), although the design of our study does not permit definition of the involvement of energy expenditure to the light effects.
The chronobiological effect of light – via normalization of daily feeding behavior and biochemistry behind it [35,36] – may also be accounted for, as anomalies in circadian rhythmicity are considered to contribute to metabolic syndrome and obesity [37]. Our pre-study assumption was that light-induced elevated mood could potentiate the drive for dieting and physical activity in women who wished to lose weight. However, the change in mood was borderline, it did not correlate with the change in body mass, and physical activity did not increase at all (no evidence for increased lean mass), suggesting that the elevated mood is not causally linked to the body mass decline in the study.
The effect of light on body mass (total or fat) was observed irrespective of the woman's seasonality trait. Seasonals, though, showed greater body mass changes (loss and regain), irrespective of placebo or light intervention, in accordance with their greater natural weight variability across the seasons [4,5]. As for many non-human mammals, it is the change in photoperiod – not sunshine or air temperature – that triggers seasonally related changes [38]. This also emerged in our study: a change in day length, rather than air temperature and definitely not in sunshine, was associated with a reduction of body mass with spring coming, this as a geophysical background to the experimental manipulations.
As in our study, in a study done in Canada [14], in which light and exercise was assigned in combination to overweight/obese non-seasonal subjects, the researchers exploited a similar LED device at a similar time of the year (though not in the early morning), and found similar results, namely, a decrease by light versus non-light in percentage fat, but not in weight (only a trend) and a light-induced elevation of mood that did not correlate with the change in body fat. Advantageously, they did not inform test subjects that the study concerned weight loss. However, light intervention alone was not studied, nor were appetite levels estimated.
The strengths of our study include the crossover design that accounted for an interindividual variation in the changeability of the body mass. Limitations of our study were the use of biompedancemetry for the fat measurements since the method reflects both fat and hydration status [21], only few weeks of light treatment that does not predict any long-term effects, and a removal of several subjects from the dataset. Further limitations were a relatively low number of non-seasonal subjects in the group (n = 24), and the fact that the start of intervention sessions did not match by dates (e.g. starting sessions at 1 day before New Year and 1 day after New Year), since both seasonality trait and photoperiod were found post hoc to be confounders for the weight loss.
A reduction in fat mass and percentage body fat following light intervention, nevertheless, was independent of seasonality trait and photoperiod, allowing us to conclude that bright light was the primary cause of fat reduction. Based on our own study and that of Dunai and co-workers [14], light therapy may provide a simple adjuvant to weight control programs for overweight and obese women.
Author Contributions
KVD designed and supervised the study, analyzed the data, and wrote the manuscript. SVM and EAP recruited and supervised the test participants, prepared data files and were involved in the discussion of the study design, the interpretation of the findings, and the literature survey. All authors read and approved the final manuscript.
Disclosure Statement
The authors declare no conflict of interest.
Acknowledgements
The study was supported by Lumie (Cambridge, UK). We thank Thor Helge Bergan for providing results of the early Norway studies, Elena Danilenko for deactivating ion generators, Ekaterina Tolstykh for randomization and instructing study participants on how to use the intervention devices, Evgeniy Verevkin for processing meteorological data, Anna Wirz-Justice for English editing and comments on the manuscript, Eryl Price of Lumie for English editing of the final version of the manuscript.
References
- 1.Lam R, Levitt AJ. Canadian Consensus Guidelines for the Treatment of Seasonal Affective Disorder. Vancouver: Clinical and Academic Publishing; 1999. [Google Scholar]
- 2.Golden RN, Gaynes BN, Ekstrom RD, Hamer RM, Jacobsen FM, Suppes T, Wisner KL, Nemeroff CB. The efficacy of light therapy in the treatment of mood disorders: a review and meta-analysis of the evidence. Am J Psychiatry. 2005;162:656–662. doi: 10.1176/appi.ajp.162.4.656. [DOI] [PubMed] [Google Scholar]
- 3.Wirz-Justice A, Benedetti F, Terman M. Chronotherapeutics for Affective Disorders: A Clinician's Manual for Light and Wake Therapy. Basel: Karger; 2009. [DOI] [PubMed] [Google Scholar]
- 4.Rosenthal NE, Sack DA, Gillin JC, Lewy AJ, Goodwin FK, Davenport Y, Mueller PS, Newsome DA, Wehr TA. Seasonal affective disorder: a description of the syndrome and preliminary findings with light therapy. Arch Gen Psychiatry. 1984;41:72–80. doi: 10.1001/archpsyc.1984.01790120076010. [DOI] [PubMed] [Google Scholar]
- 5.Kasper S, Wehr TA, Bartko JJ, Gaist PA, Rosenthal NE. Epidemiological findings of seasonal changes in mood and behavior. A telephone survey of Montgomery County, Maryland. Arch Gen Psychiatry. 1989;46:823–833. doi: 10.1001/archpsyc.1989.01810090065010. [DOI] [PubMed] [Google Scholar]
- 6.Danilenko KV, Levitan RD. Seasonal affective disorder. Handb Clin Neurol. 2012;106:279–289. doi: 10.1016/B978-0-444-52002-9.00017-6. [DOI] [PubMed] [Google Scholar]
- 7.Wirz-Justice A, Bader A, Frisch U, Stieglitz RD, Alder J, Bitzer J, Hцsli I, Jazbec S, Benedetti F, Terman M, Wisner KL, Riecher-Rцssler A. A randomized, double-blind, placebo-controlled study of light therapy for antepartum depression. J Clin Psychiatry. 2011;72:986–993. doi: 10.4088/JCP.10m06188blu. [DOI] [PubMed] [Google Scholar]
- 8.Lieverse R, Van Someren EJ, Nielen MM, Uitdehaag BM, Smit JH, Hoogendijk WJ. Bright light treatment in elderly patients with nonseasonal major depressive disorder: a randomized placebo-controlled trial. Arch Gen Psychiatry. 2011;68:61–70. doi: 10.1001/archgenpsychiatry.2010.183. [DOI] [PubMed] [Google Scholar]
- 9.Partonen T, Lonnqvist J. Bright light improves vitality and alleviates distress in healthy people. J Affect Disord. 2000;57:55–61. doi: 10.1016/s0165-0327(99)00063-4. [DOI] [PubMed] [Google Scholar]
- 10.Pinchasov BB, Shurgaja AM, Grischin OV, Putilov AA. Mood and energy regulation in seasonal and non-seasonal depression before and after midday treatment with physical exercise or bright light. Psychiatry Res. 2000;94:29–42. doi: 10.1016/s0165-1781(00)00138-4. [DOI] [PubMed] [Google Scholar]
- 11.Putilov AA, Danilenko KV. Antidepressant effects of light therapy and ‘natural’ treatments for winter depression. Biol Rhythm Res. 2005;36:389–403. [Google Scholar]
- 12.Lack LC, Wright HR. Treating chronobiological components of chronic insomnia. Sleep Med. 2007;8:637–644. doi: 10.1016/j.sleep.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Morgenthaler TI, Lee-Chiong T, Alessi C, Friedman L, Aurora RN, Boehlecke B, Brown T, Chesson AL, Jr, Kapur V, Maganti R, Owens J, Pancer J, Swick TJ, Zak R. Practice parameters for the clinical evaluation and treatment of circadian rhythm sleep disorders. An American Academy of Sleep Medicine report. Sleep. 2007;30:1445–1459. doi: 10.1093/sleep/30.11.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunai A, Novak M, Chung SA, Kayumov L, Keszei A, Levitan R, Shapiro CM. Moderate exercise and bright light treatment in overweight and obese individuals. Obesity (Silver Spring) 2007;15:1749–1757. doi: 10.1038/oby.2007.208. [DOI] [PubMed] [Google Scholar]
- 15.Mustafina SV, Pechyonkina EA, Simonova GI, Bergan TH, Danilenko KV. Bright light therapy for weight loss. Soc Light Treatment Biol Rhythms Abstracts. 2007;19:7. [Google Scholar]
- 16.Eastman CI, Young MA, Fogg LF, Liu L, Meaden PM. Bright light treatment of winter depression: a placebo-controlled trial. Arch Gen Psychiatry. 1988;55:883–889. doi: 10.1001/archpsyc.55.10.883. [DOI] [PubMed] [Google Scholar]
- 17.Terman M, Terman JS, Ross DC. A controlled trial of timed bright light and negative air ionization for treatment of winter depression. Arch Gen Psychiatry. 1998;55:875–882. doi: 10.1001/archpsyc.55.10.875. [DOI] [PubMed] [Google Scholar]
- 18.Hatori M, Panda S. The emerging roles of melanopsin in behavioral adaptation to light. Trends Mol Med. 2010;16:435–446. doi: 10.1016/j.molmed.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwama H, Ohmizo H, Obara S. The relaxing effect of negative air ions on ambulatory surgery patients. Can J Anaesth. 2004;51:187–188. doi: 10.1007/BF03018784. [DOI] [PubMed] [Google Scholar]
- 20.Vasudev S, Mohan A, Mohan D, Farooq S, Raj D, Mohan V. Validation of body fat measurement by skinfolds and two bioelectric impedance methods with DEXA – the Chennai Urban Rural Epidemiology Study [CURES-3] J Assoc Physicians India. 2004;52:877–881. [PubMed] [Google Scholar]
- 21.Saunders MJ, Blevins JE, Broeder CE. Effects of hydration changes on bioelectrical impedance in endurance trained individuals. Med Sci Sports Exerc. 1998;30:885–892. doi: 10.1097/00005768-199806000-00017. [DOI] [PubMed] [Google Scholar]
- 22.Rosenthal NE, Genhart MJ, Sack DA, Skwerer RG, Wehr TA. Seasonal affective disorder and its relevance for the understanding and treatment of bulimia. In: Hudson JI, Pope HG Jr, editors. The Psychology of Bulimia. Washington: American Psychiatric Press; 1987. pp. 205–228. [Google Scholar]
- 23.Terman M, Terman JS. Light therapy for seasonal and nonseasonal depression: efficacy, protocol, safety, and side effects. CNS Spectr. 2005;10:647–663. doi: 10.1017/s1092852900019611. [DOI] [PubMed] [Google Scholar]
- 24.Danilenko KV. Shortening of the menstrual cycle following light therapy in seasonal affective disorder. Psychiatry Res. 2007;153:93–95. doi: 10.1016/j.psychres.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 25.Thom E, Wadstein J, Gudmundsen O. Conjugated linoleic acid reduces body fat in healthy exercising humans. J Int Med Res. 2001;29:392–396. doi: 10.1177/147323000102900503. [DOI] [PubMed] [Google Scholar]
- 26.Avila JJ, Gutierres JA, Sheehy ME, Lofgren IE, Delmonico MJ. Effect of moderate intensity resistance training during weight loss on body composition and physical performance in overweight older adults. Eur J Appl Physiol. 2010;109:517–525. doi: 10.1007/s00421-010-1387-9. [DOI] [PubMed] [Google Scholar]
- 27.Josse AR, Atkinson SA, Tarnopolsky MA, Phillips SM. Increased consumption of dairy foods and protein during diet- and exercise-induced weight loss promotes fat mass loss and lean mass gain in overweight and obese premenopausal women. J Nutr. 2011;141:1626–1634. doi: 10.3945/jn.111.141028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weinheimer EM, Sands LP, Campbell WW. A systematic review of the separate and combined effects of energy restriction and exercise on fat-free mass in middle-aged and older adults: implications for sarcopenic obesity. Nutr Rev. 2010;68:375–388. doi: 10.1111/j.1753-4887.2010.00298.x. [DOI] [PubMed] [Google Scholar]
- 29.Lambert GW, Reid C, Kaye DM, Jennings GL, Esler MD. Effect of sunlight and season on serotonin turnover in the brain. Lancet. 2002;7:1840–1842. doi: 10.1016/s0140-6736(02)11737-5. [DOI] [PubMed] [Google Scholar]
- 30.Bello NT, Liang NC. The use of serotonergic drugs to treat obesity – is there any hope? Drug Des Devel Ther. 2011;5:95–109. doi: 10.2147/DDDT.S11859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saito Y, Shimizu T, Takahashi Y, Mishima K, Takahashi K, Ogawa Y, Kogawa S, Hishikawa Y. Effect of bright light exposure on muscle sympathetic nerve activity in human. Neurosci Lett. 1996;219:135–137. doi: 10.1016/s0304-3940(96)13171-2. [DOI] [PubMed] [Google Scholar]
- 32.Rьger M, Scheer FA. Effects of circadian disruption on the cardiometabolic system. Rev Endocr Metab Disord. 2009;10:245–260. doi: 10.1007/s11154-009-9122-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parker JS, Flory RK, Everhart DE, Denbow DM. Case report: neurochemical, physiological, and behavioral effects of bright light therapy on a cortically blind patient. Int J Neurosci. 1996;88:273–282. doi: 10.3109/00207459609000620. [DOI] [PubMed] [Google Scholar]
- 34.Stoica E, Enulescu O. Catecholamine response to light in migraine. Cephalalgia. 1988;8:31–36. doi: 10.1046/j.1468-2982.1988.0801031.x. [DOI] [PubMed] [Google Scholar]
- 35.Goel N, Stunkard AJ, Rogers NL, Van Dongen HP, Allison KC, O'Reardon JP, Ahima RS, Cummings DE, Heo M, Dinges DF. Circadian rhythm profiles in women with night eating syndrome. J Biol Rhythms. 2009;24:85–94. doi: 10.1177/0748730408328914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crispim CA, Waterhouse J, Dвmaso AR, Zimberg IZ, Padilha HG, Oyama LM, Tufik S, de Mello MT. Hormonal appetite control is altered by shift work: a preliminary study. Metabolism. 2011;60:1726–1735. doi: 10.1016/j.metabol.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 37.Garaulet M, Madrid JA. Chronobiological aspects of nutrition, metabolic syndrome and obesity. Adv Drug Deliv Rev. 2010;62:967–978. doi: 10.1016/j.addr.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 38.Young MA, Meaden PM, Fogg LF, Cherin EA, Eastman CI. Which environmental variables are related to the onset of seasonal affective disorder? J Abnorm Psychol. 1997;106:554–562. doi: 10.1037//0021-843x.106.4.554. [DOI] [PubMed] [Google Scholar]