Abstract
Photosystem II (PSII) oxidizes water to produce oxygen through a four-step photocatalytic cycle. Understanding PSII structure–function relations is important for the development of biomimetic photocatalytic systems. The quantum mechanics/molecular mechanics (QM/MM) analysis of substrate water binding to the oxygen-evolving complex (OEC) has suggested a rearrangement of water ligands in a carousel mechanism around a key Mn center. Here, we find that the most recently reported X-ray free-electron laser (XFEL) crystallographic data obtained for the dark-stable S1 state and the doubly flashed S3 state at 2.25 Å resolution support the carousel mechanism. The features in the XFEL data and QM/MM model-simulated difference Fourier maps suggest that water displacement may occur from the so-called “narrow” channel, resulting in binding of a new water molecule to the OEC, and thus provide new insights into the nature of rearrangements of water ligands along the catalytic cycle before O=O bond formation.
Photosystem II (PSII) is a large multisubunit membrane protein complex, responsible for direct solar water oxidation in higher plants, algae, and cyanobacteria.1−3 Water is oxidized at the oxygen-evolving complex (OEC) embedded in the D1 protein subunit, an oxomanganese cluster that operates by cycling through five redox states, the so-called “storage states” (or “S states”) of oxidizing equivalents (S0–S4). During each turn of the catalytic cycle, the OEC binds two water molecules and gets oxidized four times, generating the S4 state that catalyzes O–O bond formation for O2 evolution. While S0 is the most reduced state, S1 is the stable dark-adapted form of the OEC from which the S4 state is formed after three flashes of light, leading to O2 evolution and regeneration of the OEC in the S0 state.4,5 Structural models based on quantum mechanics/molecular mechanics (QM/MM) have been proposed for the S0–S3 states, consistent with known biochemical, spectroscopic, and crystallography data (Figure 1, Supporting Information (SI)).6−11
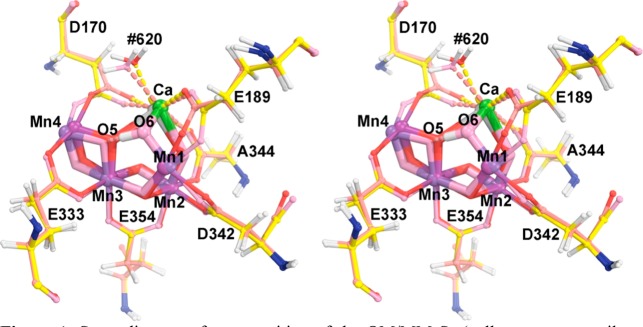
Figure 1.
Stereodiagram of superposition of the QM/MM S1 (yellow, magenta, silver, red, and blue) and S3 (salmon, green, dark magenta, pink, and blue) models, including six bidentate carboxylate ligands to highlight the moving parts to be expected in difference density features.
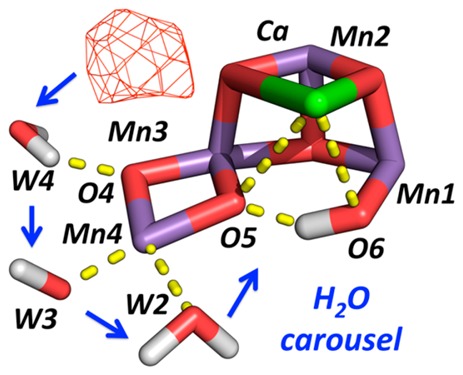
The QM/MM models suggest water binding to the cluster in the S2 → S3 transition by a carousel rearrangement of water ligands around Mn4 (Scheme 1).12 Here, we find that experimental data support such a carousel mechanism from the most recently reported X-ray free-electron laser (XFEL) crystallographic experiments,13 which are the focus of this study. As discussed previously,12 the narrow water channel has been considered by several groups as a water delivery pathway based on a variety of studies.14−20 Alternative mechanisms,21,22 including the pivot mechanism,23 have also been proposed and claimed to be consistent with the XFEL data for the S3 state.13 Reference (21) disfavored the carousel mechanism on the basis of high-energy barriers for TS6 and TS7, although the pathway of the carousel corresponds to their TS4, which has a very low barrier. At the same time, we question whether the S3 XFEL data have actually resolved the ambiguity of the water oxidation mechanism.
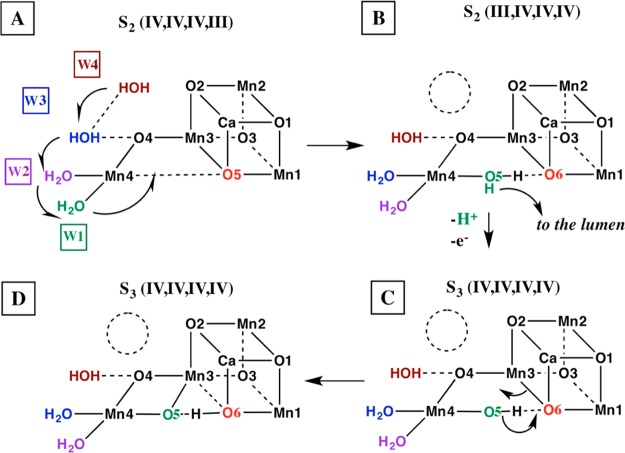
Scheme 1. Carousel Mechanism for Supply of One of the Two Water Substrates to the OEC.
Adapted from ref (12). (A) is the starting S2 state, (D) is the final S3 state, and (B) and (C) are proposed intermediate steps for the S2 to S3 state transition. W1, W2, and W3 correspond to W2, W1, and Wx in ref (12), respectively, while O5 has been relabeled as O6 following ref (13), after W1 moves into the O5 position to become O5.
A number of X-ray crystallography models of PSII have been reported in recent years, including PSII models with the OEC in the S1 state based on conventional synchrotron data.24,25 However, data collection from conventional synchrotron sources has been shown to induce radiation damage of the OEC and formation of noncatalytically relevant reduced states.24,26,28 X-ray radiation is thought to reduce the OEC and induce oxygen additions to protein side chains through mechanisms of hydroxyl free radicals.28−31 Thus, significant efforts are currently focused on XFEL crystallography.13,32,33
High-resolution XFEL diffraction data have been collected using continuous translation of unexposed parts of large single crystals of PSII,32 in an effort to achieve “diffraction-before-destruction”. However, several technical aspects remain challenging.35,36 The most effective approach for suppressing radiation damage has been the “one-shot-per-crystal” method, as in recent XFEL studies of the S1 state corresponding to the model reported for 5WS5 (of PDB accession number)13 and earlier studies of the S1 and S3 states.37−41 In one XFEL study,37 an insufficient degree of isomorphism between the S1 and S3 states required computational realignment of the resulting electron density maps before difference electron density maps could be calculated and studied.9 In other XFEL studies,38−41 computational errors introduced by data reduction based on the software cctbx.xfel were so large that structural changes due to S3 state formation were buried below the noise level.42 Nonetheless, structural information associated with the S1 to S2 transition from noisy XFEL data could still be revealed for rearrangements that involved displacement of an Mn center, found to be consistent with the simulated Fourier difference maps predicted by QM/MM models.8,38
The recent XFEL study by Shen and co-workers reported high-quality data.13 The data sets exhibit the highest possible isomorphism between the S1 and S3 states, with the overall amplitude and intensity isomorphous difference reported of only 6.8 and 5.6% at 2.35 Å resolution, respectively (see below for further discussion), ideally suitable for the observed isomorphous difference Fourier studies, that is, for calculation of the very sensitive Fobs(5WS6/S3) – Fobs(5WS5/S1) map or the Fobs – Fobs map (Figure 2A,B and SI).43−45
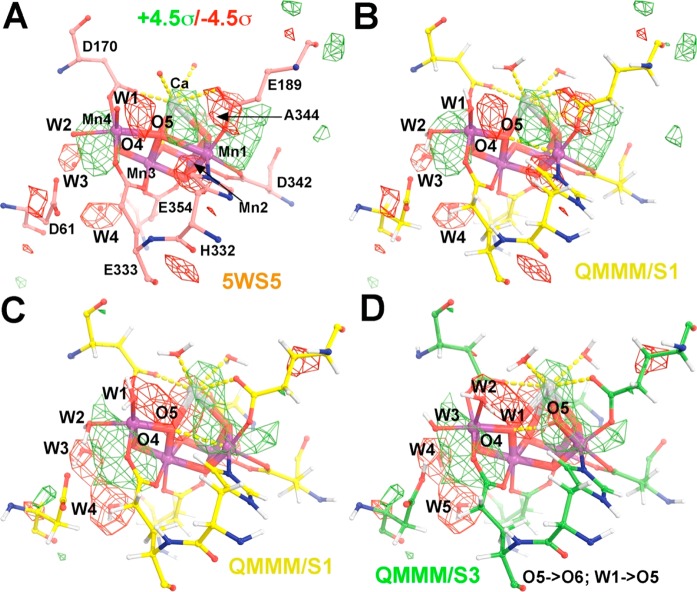
Figure 2.
Observed difference Fourier maps between the Fobs(5WS6/S3) – Fobs(5WS5/S1) XFEL data sets superimposed on the experimental S1 model (A) and on the theoretical QM/MM S1 model (B). (C,D) QM/MM-simulated difference Fourier maps between the Fsimulated(S3) – Fobs(5WS5/S1) pair, contoured at +4.5σ (green) and −4.5σ (red), superimposed onto the 5WS5 model (A, salmon), QM/MM S1 model (B,C, yellow), and QM/MM S3 model (D, green). When compared to the S1 state, the S3 model involves the following displacements: O5 → O6, W1 → O5, W2 → W1, W3 → W2, and W4 → W3. See Figures S5–S7 for additional stereodiagram views with numbering according to the PDB file reported for 5WS5.
In particular, the dark-adapted S1 structure13 (PDB access code 5WS5) is found to be most consistent with EXAFS data (SI, Figure S1) and the previously proposed EXAFS-based46 S1 QM/MM model.47 In contrast, the S3 structure (PDB access code 5WS6) is not fully consistent with S3 EXAFS data9,48 (SI, Figures S1–S3), likely due to the unavoidable mixture of S states present in the XFEL microcrystals, with only a small fraction in the S3 state. In fact, the apparently short O5–O6 distance suggested for the S3 state can be accounted for in terms of partial occupation of O5 and O6 as determined by the composition of the mixture of S1 and S3 states. Here, we provide a structural interpretation of the reported difference Fourier features13 based on QM/MM models.6−9
The OEC has four Mn ions and a Ca forming the oxomanganese cluster Mn4CaO5. The QM/MM S3 model has an additional core O atom, arising from water binding during the S2 to S3 transition.9 On the basis of data from the ammonia-bound S2 QM/MM model,12 formation of the S3 state was proposed to occur through a carousel rearrangement (Scheme 1) involving water molecules W1, W2, and W3,10 which correspond to water molecules #578, #523, and #527 in monomer A, respectively13 (the original numbering reported in 5WS5 is provided in the SI, with uppercase one-letter labeling of amino acid residues for monomer A and with lowercase for monomer B). As shown in Scheme 1, oxidation of the OEC triggers binding of a water molecule (W3) to Mn4 from the so-called “narrow” channel (W3 is #527 in monomer A or Wx in our original nomenclature;10 see the SI). W3 binding displaces W2 into the W1 position, and W1 is displaced into the O5 position. The O5 ligand is displaced toward a new position, becoming O6, using the nomenclature from Shen and co-workers.13 The role of the narrow channel as a water delivery channel during the S2–S3 transition has also been supported by Capone et al.16 and by Retegan et al.23 The rearrangement associated with the carousel mechanism slightly displaces Ca toward O6 while Mn4 is slightly displaced away from Mn1, making room for the new O5 ligand. During the rearrangement, four out of six bidentate carboxylate ligands remain largely stationary (Figures 2 and S4–S7). However, E189 bound to Ca and Mn1 and D170 bound to Ca and Mn4 undergo torsion angle displacements to accommodate the new water molecule as O5 (Figures 2 and S4).
Figure 2C,D shows that the simulated S3-minus-S1 electron density difference based on QM/MM models7,9 exhibits a positive feature (in green), extending from Ca to the new position of O5 as a ligand of Mn1 (O6 according to numbering by Shen), and a small displacement of Ca toward that new ligand. In addition, there are positive and negative features (in green and red, respectively) flanking the Mn4 center. No significant density difference features are observed at the W1 and W2 positions because there is no net change of electronic density produced by water ligand exchange. Furthermore, there is no significant negative peak behind Ca because there is concerted movement of a water molecule filling the depleted density upon Ca displacement.
The features revealed by the simulated electron density differences of QM/MM models are consistent with features in the observed isomorphous difference Fourier maps of XFEL data for Fobs(5WS6/S3) and Fobs(5WS5/S1), originally reported by Shen and colleagues and faithfully reproduced here (Figures 2A,B, S5, and S6).13 Analogous to the QM/MM models, the XFEL difference Fourier map shows a small negative peak on W3 (#527) and a large negative peak next to W4 (#630).13 These features suggest that W4 moves into the W3 position when W3 becomes a ligand of Mn4. However, other water molecules in the narrow channel do not refill the W4 position immediately. Remarkably, these features are observed for both monomers A and B.13
By using the same method, we have assessed the correctness of the QM/MM models as just described; we have also assessed whether the atomistic models of the S1 and S3 states proposed by Shen and co-workers were consistent with the outstanding features in the observed difference Fourier map that Shen and colleagues obtained and that we faithfully produced here, that is, whether their Fcalc(5WS6/S3) – Fcalc(5WS5/S1) difference Fourier maps have reproduced the Fobs(5WS6/S3) – Fobs(5WS5/S1) maps. The observed features in the S3-minus-S1 maps as discussed above are very robust because they are contributed by the observed amplitude differences from all of the reflections so that they are not much dependent on data of the selected resolution range used nor on any given set of model-calculated phases. That is, the observed difference Fourier features remain largely the same whether the model phases are from either Fcalc(5WS6/S3) or Fcalc(5WS5/S1). This is the reason why the observed difference Fourier maps are a very sensitive method to reveal subtle structural changes and have been extensively used by the crystallographic community for many decades.43−45 However, we failed to obtain any robust features in the calculated difference Fourier maps that would be consistent with the observed features. Figure 3A shows that the calculated difference Fourier map using Fcalc(5WS5/S1) model phases, which represents one of many calculated difference Fourier maps that we have carefully examined, do not reproduce the observed map, suggesting that at least one of the two atomistic models (i.e., the S3 model) does not correspond to the observed data. We have also examined the calculated difference Fourier maps using Fcalc(5WS6/S3) model phases with a different resolution range of data, for example, by excluding some very low resolution data. An exclusion of very low resolution data is relevant here because different model refinement programs may have slightly different bulk solvent correction algorithms that can result in different calculated structure factors at very low resolution, given the fact that the calculated structure factors were not deposited in the PDB and had to be regenerated here (see the Computational Methods section).
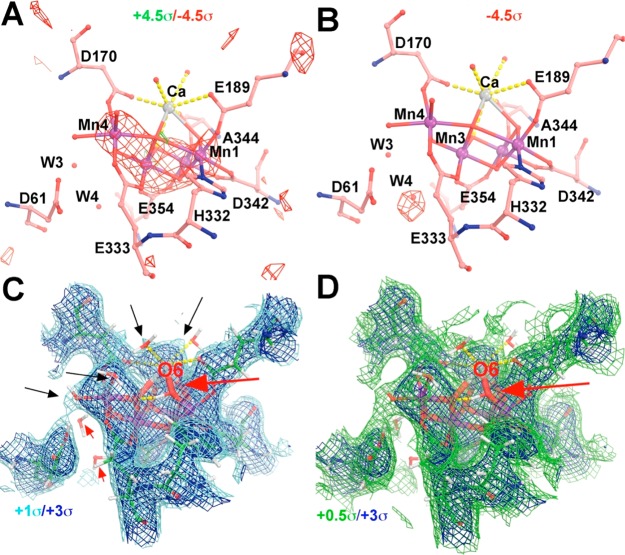
Figure 3.
Assessment of the 5WS6 S3 model using difference Fourier methods: (A) Fcalc(5WS6/S3) – Fcalc(5WS5/S1) or (B) Fcalc(5WS6/S3) – Fobs(5WS5/S1) maps contoured at +4.5σ (green) and −4.5σ (red) and σA-weighted 2Fobs – Fcalc map contoured at +1.0σ (cyan)/+3.0σ (blue) (C) and at +0.5σ (green)/+3.0σ (blue) (D). At the +1.0σ level (C), water ligands to the Ca and Mn centers (marked by black arrows) begin to emerge, but not O6 (red arrow) or W3 and W4 (small red arrows). There is no electron density visible for O6 at any contour level.
Because the 5WS5/S1 model is consistent with the S1 QM/MM model,7,13 it is likely that the quality of the XFEL-derived 5WS6/S3 model is questionable. This can be confirmed by using the difference Fourier map Fcalc(S3) – Fobs(S1) between the calculated structure factors (Fcalc) of the 5WS6/S3 model and the observed 5WS5/S1 (Fobs) data (Figure 3B). The Fcalc(S3) – Fobs(S1) difference shown in Figure 3B shows a single negative peak at the W4 position, consistent with displacement of that water molecule during the S1/S3 transition.13 However, none of the other observed features shown in Figure 2 are accounted for in Figure 3. Reciprocally, the hybrid Fobs(5WS6/S3) – Fcalc(5WS5/S1) map was also calculated, and it is found that the features in this map largely reproduced the features in the observed difference Fourier maps (data not shown). This is another way to validate that the S1 atomic model obtained by Shen and colleagues is indeed of reasonably good quality.
We further note that the σA-weighted 2Fobs – Fcalc map based on the 5WS6/S3 model does not show any electron density attributable to O6 at any contour level (Figure 3C,D). For example, several water ligands to Ca and some Mn centers are clearly visible in this map at the 1.0σ contour level, whereas O6 is not visible at 0.5σ (Figure 3C,D) or even at 0.01σ (data not shown). This observation suggests that the fraction of PSII cores converted to the S3 state and recorded in the XFEL data is very low. Moreover, it is plausible that some of the observed density at the O5 position (Figure 2) in the Fobs(5WS6/S3) – Fobs(5WS5/S1) map may not come from the S3 state but rather from other lower states. We thus conclude that the S3 model might not actually have O5 and O6 at 1.5 Å from each other.13 In any case, there is no conclusive evidence for an O–O bond formed in the S3 state. The reason why one can see small subtle structural changes in the Fobs(S3) – Fobs(S1) difference Fourier map but not in the Fobs(S3) – Fcalc(S3) difference Fourier maps [or in the 2Fobs(S3) – Fcalc(S3) maps] is that the observed amplitude difference between the two states is only 6.8%, whereas the unbiased amplitude difference between the calculated and observed amplitudes within the S3 state (i.e., free R factor value) is 17.6% (see below for further discussion).13
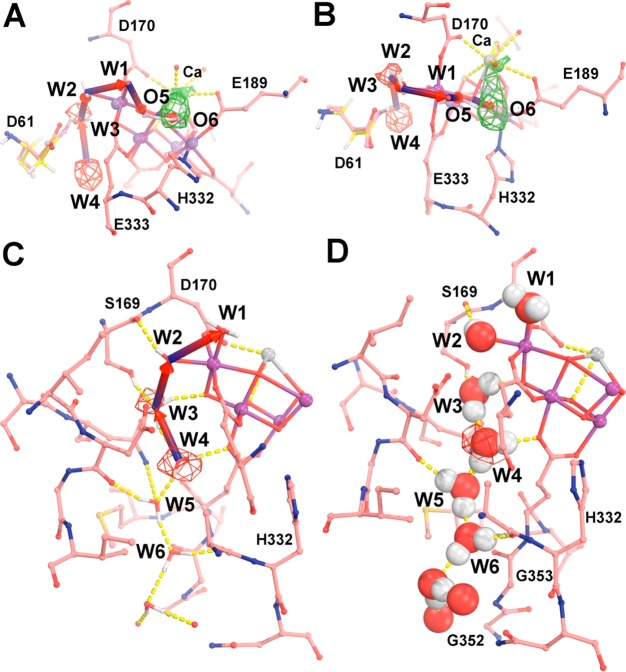
W4 likely moves to the W3 site due to the immediate proximity when W3 takes the position of W2 (Figure 4A,B). As shown by the S3 QM/MM model, the relative occupancy of each site is mostly determined by hydrogen-bonding interactions (Figures 4 and S8). W3 makes hydrogen bonds to Oγ of S169 and O4, while W2 makes bonds with D61 (Figure 4C,D). W4 makes a hydrogen bond with W5 and E354. All of these interactions are consistent with a preference of W4 to occupy the W3 site in the S3 state. W5 might not immediately move into the vacant position of W4 because W4 has three hydrogen bonds, including the carbonyl O of D61, the side chain of N87, and a water molecule (Figure S8). Thus, we conjecture that an additional conformational change is necessary for W5 to take the position of W4. The energetics of potential movement of water molecules along this channel has been discussed,19,20 although it has also been disfavored due to the limited water mobility.49
Figure 4.
Single-file narrow water channel. (A,B) Two approximately orthogonal views of the 5WS5/S1 model (salmon, red, and blue) superimposed onto the edited Fobs(5WS6/S3) – Fobs(5WS5/S1) map contoured at +4.5σ (green) and −4.5σ (red) as well as with our QM/MM S3 model (yellow, red, and blue), which contains the new O6 ligand. (C,D) Two representations of hydrogen-bonding interactions of water molecules in the single-file water channel. See Figure S8 for additional stereodiagram views.
The density feature associated with W3, next to O4, disappears during the S1 to S3 transition (Figure 2A,B). Therefore, it seems unlikely that another water molecule would move next to O4 and become a substrate. Given the geometry constraints at that site, even molecular oxygen might not fit at the O4 position. Moreover, formation of an O–O bond at this position would be thermodynamically demanding because it requires breaking two coordination interactions of the μ-O4 bridge with rather unfavorable structural changes. Thus, we disfavor O4 as the site for O–O bond formation as suggested as a secondary possibility of the two possible models put forward by Shen and co-workers (two dashed circles in their Figure 4).13 Instead, we favor the second water molecule to be located at the front end of the carousel cascade near the O5 and O6 positions, which corresponds to the first choice of Shen and colleagues but in different details,13 interacting with Ca as suggested by oxygen isotope exchange measurements.50
In the S0, S1, and S2 states, one water molecule is rapidly exchangeable, exhibiting the fastest rates within detectable experimental range, while another one is rather slow.50 The fast water molecule is likely to be very weakly associated with the OEC, such as W1 on Mn4, while the slow one might be part of the OEC, tentatively assigned as the O5 species. In the S0 state, O5 is a hydroxo, which should have a relatively higher exchangeable rate than those in the S1 and S2 states where O5 is an oxo species. However, considering that EPR signals show the presence of two S2 state structures consistent with different O5 positions (i.e., as a ligand of either Mn1 or Mn4), it is reasonable to expect a higher exchangeable rate for O5 in S2 when compared to the S1 state.50 In the S3 state, W1 moves to the O5 position, consistent with the fast exchangeable water becoming very slow. At the same time, the original O5 species becomes O6, expected to have a slightly increased exchangeable rate.50 These findings are particularly relevant to analysis of the reaction mechanism as directly compared to experimental data.
A lesson that we want to emphasize from this study is that, whereas technologies in X-ray crystallography have indeed been advanced in the last few decades, the crystallographic foundation on how to assess whether the measured data contain useful structural information (i.e., above the noise level) remains unchanged, and the isomorphous difference Fourier method remains as a sensitive method to reveal subtle structural changes recorded in the measured differences in the diffraction data.43−45 By all means, Shen and colleagues have done a superb job at data processing13 and have provided high-quality XFEL data sets for PSII intermediates with cumulative Pearson split correlation coefficients (CC1/2) of 99.4–99.7% and split intensity R factors of 5.4–6.2% for each data set that is comparable to the quality of conventional synchrotron data.36,51,52 This permits Shen and colleagues to obtain useful structural information on the S1 to S3 state transition, which is reflected in the observed intensity difference of only 5.6% between the two data sets. Although the expected structural transition signals are indeed very weak, they are consistently present in each of 35 393 reflections at 2.35 Å resolution, making them collectively powerful and useful. For comparison, in another XFEL study on ammonia binding to PSII, the authors reported cumulative CC1/2 values of 53.2% for the S1 data set at 3.0 Å resolution and 54.2% for the two-flash NH3 bound state at 2.8 Å resolution (the authors did not report intensity split R factors).41 It is clear that these authors would have to put extra effort into their data processing procedures to improve the internal consistency indexes within each data set before they could conclude with certainty whether the diffraction data recorded any reliable structural information on ammonia binding to the OEC.
Chen and colleagues have done a superb job at XFEL data processing;13 nevertheless, we do not find complete support of their interpretation, which was likely biased toward the “200 + 1 atom” open S3 model generated by Li and Siegbahn (Figure S9A),53 with a hallmark intermediate O5–H–O6 configuration where O6 is a newly added water substrate (+1). In their energy-minimized model, Li and Siegbahn relaxed all of the truncated protein side chains (and therefore all water molecules nearby) that freely reposition. The relaxation resulted in displacement of the H332 side chain by about 2.30 Å, relative to the experimental coordinates of the 5WS5 mode from our S3 model, as well as a large displacement of the Cl– ion by 0.73 Å (Figure S9) and water molecules. Unfortunately, these displacements are not consistent with the experimental features observed in the difference Fourier maps obtained by Chen and colleagues.13 While large movements of protein side chains during the S1 to S3 state transition have been suggested based on low-resolution XFEL data,37 that interpretation did not take into account possible effects of Fourier series termination.9 Furthermore, large displacements are not consistent with the much higher resolution, better-quality, new XFEL data obtained by Shen and co-workers.13 Moreover, even though the Li and Siegbahn model may have accounted for the selected Mn–Mn distances derived from the EXAFS data, their model has not been shown to reproduce the EXAFS spectra just like many other alternative models (Figures S1–S3).12 Therefore, our model and the carousel mechanism seem to remain most consistent with XFEL data and the EXAFS spectra, as compared to other suggested models.
Computational Methods
Crystallographic analysis was carried out using the program CCP4 suite and displayed using the graphics Coot.54,55 When the OEC and its protein ligands from the QM/MM model were reinserted into an experimental atomic model, the uniform atomic B factor was kept the same for the mean B factor for the replaced part of the model. The QM level of our original S3 QM/MM model did not include W3 and W4. Therefore, reoptimization of the S3 QM/MM with W4 displaced to the W3 position led to a model that is even in better agreement with the published EXAFS data for the S3 state (Figures S2 and S3). The calculated structure factors were obtained from atomic models using Refmac5 by setting the zero cycle rigid-body refinement option.56 The correctness of the structure factor calculation has been verified by visual inspection of both 2Fobs – Fcalc and Fobs – Fcalc maps and by comparison with the reported statistical values such as amplitude differences.
For comparisons with experiments, we note that the features of the difference Fourier maps do not provide how much S3 was formed by the two flashes. Difference features of the same kind would be observed regardless of whether the transition is 100% complete or, for example, only 20% because the unchanged portion between the two structures cancels out in both cases. The only difference between 100 or 20% completion would be that the peak heights in the latter would be reduced 5-fold relative to the former. In the PDB 5WS6/S3 atomic coordinate file, Shen and colleagues modeled two alternative conformations of 20:80% for a mixed CaMn4O5 cluster in each monomer.13 Yet, the occupancy of O6 was modeled as 40%, which did not correspond to either state of the cluster. The basis of this discrepancy was not discussed in their publication and remains unknown. If O6 is part of part of a minor species, it has an extra O6 relative to the species by 20%. If O6 is part of the major species (which the coordinates imply), 40% of the species lacks O6, which leads to another question: can the S3 state exist without O6 binding? Another aspect that should be noted is that XFEL crystallography is a :diffraction-while-destruction” technique.36 Therefore, the atomic scattering factors do not necessarily correspond to those of conventional synchrotron radiation. Nevertheless, the isomorphous Fobs – Fobs difference Fourier maps should partially cancel some of the systematic errors due to a change in atomic scattering factors.
Acknowledgments
The authors acknowledge support by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, Division of Chemical Sciences, Geosciences, and Biosciences, Photosynthetic Systems. Experimental work was funded by Grant DE-FG02-05ER15646 (G.W.B.), computational work was funded by Grant DESC0001423 (V.S.B), and crystallographic study was supported by National Institutes of Health Grant P01 GM022778 (J.W).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsenergylett.7b00750.
Description of QM/MM models, description of EXAFS analysis, electron density map analysis, additional references, and updated S3 state coordinates (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- McEvoy J. P.; Brudvig G. W. Water-splitting chemistry of photosystem II. Chem. Rev. 2006, 106 (11), 4455–4483. 10.1021/cr0204294. [DOI] [PubMed] [Google Scholar]
- Cox N.; Pantazis D. A.; Neese F.; Lubitz W. Biological Water Oxidation. Acc. Chem. Res. 2013, 46 (7), 1588–1596. 10.1021/ar3003249. [DOI] [PubMed] [Google Scholar]
- Shen J. R. The Structure of Photosystem II and the Mechanism of Water Oxidation in Photosynthesis. Annu. Rev. Plant Biol. 2015, 66 (66), 23–48. 10.1146/annurev-arplant-050312-120129. [DOI] [PubMed] [Google Scholar]
- Joliot P.; Kok B., Oxygen evolution in photosynthesis. In Bioenergetics of Photosynthesis; Govindjee, Ed. Academic Press: New York, 1975; pp 387–412. [Google Scholar]
- Kok B.; Forbush B.; Mcgloin M. Cooperation of Charges in Photosynthetic O2 Evolution-I. A Linear Four Step Mechanism. Photochem. Photobiol. 1970, 11 (6), 457–475. 10.1111/j.1751-1097.1970.tb06017.x. [DOI] [PubMed] [Google Scholar]
- Pal R.; Negre C. F. A.; Vogt L.; Pokhrel R.; Ertem M. Z.; Brudvig G. W.; Batista V. S. S-0-State Model of the Oxygen-Evolving Complex of Photosystem II. Biochemistry 2013, 52 (44), 7703–7706. 10.1021/bi401214v. [DOI] [PubMed] [Google Scholar]
- Luber S.; Rivalta I.; Umena Y.; Kawakami K.; Shen J. R.; Kamiya N.; Brudvig G. W.; Batista V. S. S-1-State Model of the O-2-Evolving Complex of Photosystem II. Biochemistry 2011, 50 (29), 6308–6311. 10.1021/bi200681q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askerka M.; Wang J.; Brudvig G. W.; Batista V. S. Structural Changes in the Oxygen-Evolving Complex of Photosystem II Induced by the S1 to S2 Transition: A Combined XRD and QM/MM Study. Biochemistry 2014, 53 (44), 6860–6862. 10.1021/bi5011915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askerka M.; Wang J.; Vinyard D. J.; Brudvig G. W.; Batista V. S. S3 State of the O2-Evolving Complex of Photosystem II: Insights from QM/MM, EXAFS, and Femtosecond X-ray Diffraction. Biochemistry 2016, 55 (7), 981–4. 10.1021/acs.biochem.6b00041. [DOI] [PubMed] [Google Scholar]
- Askerka M.; Brudvig G. W.; Batista V. S. The O2-Evolving Complex of Photosystem II: Recent Insights from Quantum Mechanics/Molecular Mechanics (QM/MM), Extended X-ray Absorption Fine Structure (EXAFS), and Femtosecond X-ray Crystallography Data. Acc. Chem. Res. 2017, 50 (1), 41–48. 10.1021/acs.accounts.6b00405. [DOI] [PubMed] [Google Scholar]
- Shoji M.; Isobe H.; Nakajima T.; Shigeta Y.; Suga M.; Akita F.; Shen J. R.; Yamaguchi K. Large-scale QM/MM calculations of the CaMn4O5 cluster in the S3 state of the oxygen evolving complex of photosystem II. Comparison between water-inserted and no water-inserted structures. Faraday Discuss. 2017, 198, 83–106. 10.1039/C6FD00230G. [DOI] [PubMed] [Google Scholar]
- Askerka M.; Vinyard D. J.; Brudvig G. W.; Batista V. S. NH3 Binding to the S2 State of the O2-Evolving Complex of Photosystem II: Analogue to H2O Binding during the S2 --> S3 Transition. Biochemistry 2015, 54 (38), 5783–6. 10.1021/acs.biochem.5b00974. [DOI] [PubMed] [Google Scholar]
- Suga M.; Akita F.; Sugahara M.; Kubo M.; Nakajima Y.; Nakane T.; Yamashita K.; Umena Y.; Nakabayashi M.; Yamane T.; Nakano T.; Suzuki M.; Masuda T.; Inoue S.; Kimura T.; Nomura T.; Yonekura S.; Yu L. J.; Sakamoto T.; Motomura T.; Chen J. H.; Kato Y.; Noguchi T.; Tono K.; Joti Y.; Kameshima T.; Hatsui T.; Nango E.; Tanaka R.; Naitow H.; Matsuura Y.; Yamashita A.; Yamamoto M.; Nureki O.; Yabashi M.; Ishikawa T.; Iwata S.; Shen J. R. Light-induced structural changes and the site of O=O bond formation in PSII caught by XFEL. Nature 2017, 543 (7643), 131–135. 10.1038/nature21400. [DOI] [PubMed] [Google Scholar]
- Cox N.; Messinger J. Reflections on substrate water and dioxygen formation. Biochim. Biophys. Acta, Bioenerg. 2013, 1827 (8–9), 1020–30. 10.1016/j.bbabio.2013.01.013. [DOI] [PubMed] [Google Scholar]
- Retegan M.; Pantazis D. A. Interaction of methanol with the oxygen-evolving complex: atomistic models, channel identification, species dependence, and mechanistic implications. Chem. Sci. 2016, 7 (10), 6463–6476. 10.1039/C6SC02340A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capone M.; Narzi D.; Bovi D.; Guidoni L. Mechanism of Water Delivery to the Active Site of Photosystem II along the S(2) to S(3) Transition. J. Phys. Chem. Lett. 2016, 7 (3), 592–6. 10.1021/acs.jpclett.5b02851. [DOI] [PubMed] [Google Scholar]
- Perez Navarro M.; Ames W. M.; Nilsson H.; Lohmiller T.; Pantazis D. A.; Rapatskiy L.; Nowaczyk M. M.; Neese F.; Boussac A.; Messinger J.; Lubitz W.; Cox N. Ammonia binding to the oxygen-evolving complex of photosystem II identifies the solvent-exchangeable oxygen bridge (mu-oxo) of the manganese tetramer. Proc. Natl. Acad. Sci. U. S. A. 2013, 110 (39), 15561–6. 10.1073/pnas.1304334110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmiller T.; Krewald V.; Navarro M. P.; Retegan M.; Rapatskiy L.; Nowaczyk M. M.; Boussac A.; Neese F.; Lubitz W.; Pantazis D. A.; Cox N. Structure, ligands and substrate coordination of the oxygen-evolving complex of photosystem II in the S2 state: a combined EPR and DFT study. Phys. Chem. Chem. Phys. 2014, 16 (24), 11877–92. 10.1039/c3cp55017f. [DOI] [PubMed] [Google Scholar]
- Vassiliev S.; Zaraiskaya T.; Bruce D. Molecular dynamics simulations reveal highly permeable oxygen exit channels shared with water uptake channels in photosystem II. Biochim. Biophys. Acta, Bioenerg. 2013, 1827 (10), 1148–55. 10.1016/j.bbabio.2013.06.008. [DOI] [PubMed] [Google Scholar]
- Vassiliev S.; Zaraiskaya T.; Bruce D. Exploring the energetics of water permeation in photosystem II by multiple steered molecular dynamics simulations. Biochim. Biophys. Acta, Bioenerg. 2012, 1817 (9), 1671–8. 10.1016/j.bbabio.2012.05.016. [DOI] [PubMed] [Google Scholar]
- Guo Y.; He L. L.; Zhao D. X.; Gong L. D.; Liu C.; Yang Z. Z. How does ammonia bind to the oxygen-evolving complex in the S2 state of photosynthetic water oxidation? Theoretical support and implications for the W1 substitution mechanism. Phys. Chem. Chem. Phys. 2016, 18 (46), 31551–31565. 10.1039/C6CP05725J. [DOI] [PubMed] [Google Scholar]
- Guo Y.; Li H.; He L. L.; Zhao D. X.; Gong L. D.; Yang Z. Z. The open-cubane oxo-oxyl coupling mechanism dominates photosynthetic oxygen evolution: a comprehensive DFT investigation on O-O bond formation in the S4 state. Phys. Chem. Chem. Phys. 2017, 19 (21), 13909–13923. 10.1039/C7CP01617D. [DOI] [PubMed] [Google Scholar]
- Retegan M.; Krewald V.; Mamedov F.; Neese F.; Lubitz W.; Cox N.; Pantazis D. A. A five-coordinate Mn (iv) intermediate in biological water oxidation: spectroscopic signature and a pivot mechanism for water binding. Chem. Sci. 2016, 7, 72–84. 10.1039/C5SC03124A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umena Y.; Kawakami K.; Shen J.-R.; Kamiya N. Crystal structure of oxygen-evolving Photosystem II at a resolution of 1.9 angstrom. Nature 2011, 473 (7345), 55–60. 10.1038/nature09913. [DOI] [PubMed] [Google Scholar]
- Tanaka A.; Fukushima Y.; Kamiya N. Two Different Structures of the Oxygen-Evolving Complex in the Same Polypeptide Frameworks of Photosystem II. J. Am. Chem. Soc. 2017, 139 (5), 1718–1721. 10.1021/jacs.6b09666. [DOI] [PubMed] [Google Scholar]
- Vogt L.; Ertem M. Z.; Pal R.; Brudvig G. W.; Batista V. S. Computational insights on crystal structures of the oxygen-evolving complex of photosystem II with either Ca(2+) or Ca(2+) substituted by Sr(2+). Biochemistry 2015, 54 (3), 820–5. 10.1021/bi5011706. [DOI] [PubMed] [Google Scholar]
- Yano J.; Kern J.; Irrgang K. D.; Latimer M. J.; Bergmann U.; Glatzel P.; Pushkar Y.; Biesiadka J.; Loll B.; Sauer K.; Messinger J.; Zouni A.; Yachandra V. K. X-ray damage to the Mn4Ca complex in single crystals of photosystem II: a case study for metalloprotein crystallography. Proc. Natl. Acad. Sci. U. S. A. 2005, 102 (34), 12047–52. 10.1073/pnas.0505207102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. X-ray radiation-induced addition of oxygen atoms to protein residues. Protein Sci. 2016, 25 (8), 1407–19. 10.1002/pro.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meents A.; Dittrich B.; Gutmann S. A new aspect of specific radiation damage: hydrogen abstraction from organic molecules. J. Synchrotron Radiat. 2009, 16 (2), 183–90. 10.1107/S0909049509002192. [DOI] [PubMed] [Google Scholar]
- Meents A.; Gutmann S.; Wagner A.; Schulze-Briese C. Origin and temperature dependence of radiation damage in biological samples at cryogenic temperatures. Proc. Natl. Acad. Sci. U. S. A. 2010, 107 (3), 1094–9. 10.1073/pnas.0905481107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga M.; Akita F.; Hirata K.; Ueno G.; Murakami H.; Nakajima Y.; Shimizu T.; Yamashita K.; Yamamoto M.; Ago H.; Shen J. R. Native structure of photosystem II at 1.95 A resolution viewed by femtosecond X-ray pulses. Nature 2014, 517 (7532), 99–103. 10.1038/nature13991. [DOI] [PubMed] [Google Scholar]
- Young I. D.; Ibrahim M.; Chatterjee R.; Gul S.; Fuller F. D.; Koroidov S.; Brewster A. S.; Tran R.; Alonso-Mori R.; Kroll T.; Michels-Clark T.; Laksmono H.; Sierra R. G.; Stan C. A.; Hussein R.; Zhang M.; Douthit L.; Kubin M.; de Lichtenberg C.; Vo Pham L.; Nilsson H.; Cheah M. H.; Shevela D.; Saracini C.; Bean M. A.; Seuffert I.; Sokaras D.; Weng T. C.; Pastor E.; Weninger C.; Fransson T.; Lassalle L.; Brauer P.; Aller P.; Docker P. T.; Andi B.; Orville A. M.; Glownia J. M.; Nelson S.; Sikorski M.; Zhu D.; Hunter M. S.; Lane T. J.; Aquila A.; Koglin J. E.; Robinson J.; Liang M.; Boutet S.; Lyubimov A. Y.; Uervirojnangkoorn M.; Moriarty N. W.; Liebschner D.; Afonine P. V.; Waterman D. G.; Evans G.; Wernet P.; Dobbek H.; Weis W. I.; Brunger A. T.; Zwart P. H.; Adams P. D.; Zouni A.; Messinger J.; Bergmann U.; Sauter N. K.; Kern J.; Yachandra V. K.; Yano J. Structure of photosystem II and substrate binding at room temperature. Nature 2016, 540 (7633), 453–457. 10.1038/nature20161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin M.; Badawi A.; Obayya S. S. Radiation Damage in XFEL: Case study from the oxygen-evolving complex of Photosystem II. Sci. Rep. 2016, 6, 36492. 10.1038/srep36492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Destruction-and-diffraction by X-ray free-electron laser. Protein Sci. 2016, 25 (9), 1585–92. 10.1002/pro.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupitz C.; Basu S.; Grotjohann I.; Fromme R.; Zatsepin N. A.; Rendek K. N.; Hunter M. S.; Shoeman R. L.; White T. A.; Wang D.; James D.; Yang J. H.; Cobb D. E.; Reeder B.; Sierra R. G.; Liu H.; Barty A.; Aquila A. L.; Deponte D.; Kirian R. A.; Bari S.; Bergkamp J. J.; Beyerlein K. R.; Bogan M. J.; Caleman C.; Chao T. C.; Conrad C. E.; Davis K. M.; Fleckenstein H.; Galli L.; Hau-Riege S. P.; Kassemeyer S.; Laksmono H.; Liang M.; Lomb L.; Marchesini S.; Martin A. V.; Messerschmidt M.; Milathianaki D.; Nass K.; Ros A.; Roy-Chowdhury S.; Schmidt K.; Seibert M.; Steinbrener J.; Stellato F.; Yan L.; Yoon C.; Moore T. A.; Moore A. L.; Pushkar Y.; Williams G. J.; Boutet S.; Doak R. B.; Weierstall U.; Frank M.; Chapman H. N.; Spence J. C.; Fromme P. Serial time-resolved crystallography of photosystem II using a femtosecond X-ray laser. Nature 2014, 513 (7517), 261–5. 10.1038/nature13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern J.; Alonso-Mori R.; Tran R.; Hattne J.; Gildea R. J.; Echols N.; Glockner C.; Hellmich J.; Laksmono H.; Sierra R. G.; Lassalle-Kaiser B.; Koroidov S.; Lampe A.; Han G.; Gul S.; Difiore D.; Milathianaki D.; Fry A. R.; Miahnahri A.; Schafer D. W.; Messerschmidt M.; Seibert M. M.; Koglin J. E.; Sokaras D.; Weng T. C.; Sellberg J.; Latimer M. J.; Grosse-Kunstleve R. W.; Zwart P. H.; White W. E.; Glatzel P.; Adams P. D.; Bogan M. J.; Williams G. J.; Boutet S.; Messinger J.; Zouni A.; Sauter N. K.; Yachandra V. K.; Bergmann U.; Yano J. Simultaneous femtosecond X-ray spectroscopy and diffraction of photosystem II at room temperature. Science 2013, 340 (6131), 491–5. 10.1126/science.1234273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern J.; Alonso-Mori R.; Hellmich J.; Tran R.; Hattne J.; Laksmono H.; Glockner C.; Echols N.; Sierra R. G.; Sellberg J.; Lassalle-Kaiser B.; Gildea R. J.; Glatzel P.; Grosse-Kunstleve R. W.; Latimer M. J.; McQueen T. A.; DiFiore D.; Fry A. R.; Messerschmidt M.; Miahnahri A.; Schafer D. W.; Seibert M. M.; Sokaras D.; Weng T. C.; Zwart P. H.; White W. E.; Adams P. D.; Bogan M. J.; Boutet S.; Williams G. J.; Messinger J.; Sauter N. K.; Zouni A.; Bergmann U.; Yano J.; Yachandra V. K. Room temperature femtosecond X-ray diffraction of photosystem II microcrystals. Proc. Natl. Acad. Sci. U. S. A. 2012, 109 (25), 9721–6. 10.1073/pnas.1204598109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern J.; Tran R.; Alonso-Mori R.; Koroidov S.; Echols N.; Hattne J.; Ibrahim M.; Gul S.; Laksmono H.; Sierra R. G.; Gildea R. J.; Han G.; Hellmich J.; Lassalle-Kaiser B.; Chatterjee R.; Brewster A. S.; Stan C. A.; Glockner C.; Lampe A.; DiFiore D.; Milathianaki D.; Fry A. R.; Seibert M. M.; Koglin J. E.; Gallo E.; Uhlig J.; Sokaras D.; Weng T. C.; Zwart P. H.; Skinner D. E.; Bogan M. J.; Messerschmidt M.; Glatzel P.; Williams G. J.; Boutet S.; Adams P. D.; Zouni A.; Messinger J.; Sauter N. K.; Bergmann U.; Yano J.; Yachandra V. K. Taking snapshots of photosynthetic water oxidation using femtosecond X-ray diffraction and spectroscopy. Nat. Commun. 2014, 5, 4371. 10.1038/ncomms5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young I. D.; Ibrahim M.; Chatterjee R.; Gul S.; Fuller F. D.; Koroidov S.; Brewster A. S.; Tran R.; Alonso-Mori R.; Kroll T.; Michels-Clark T.; Laksmono H.; Sierra R. G.; Stan C. A.; Hussein R.; Zhang M.; Douthit L.; Kubin M.; de Lichtenberg C.; Vo Pham L.; Nilsson H.; Cheah M. H.; Shevela D.; Saracini C.; Bean M. A.; Seuffert I.; Sokaras D.; Weng T. C.; Pastor E.; Weninger C.; Fransson T.; Lassalle L.; Brauer P.; Aller P.; Docker P. T.; Andi B.; Orville A. M.; Glownia J. M.; Nelson S.; Sikorski M.; Zhu D.; Hunter M. S.; Lane T. J.; Aquila A.; Koglin J. E.; Robinson J.; Liang M.; Boutet S.; Lyubimov A. Y.; Uervirojnangkoorn M.; Moriarty N. W.; Liebschner D.; Afonine P. V.; Waterman D. G.; Evans G.; Wernet P.; Dobbek H.; Weis W. I.; Brunger A. T.; Zwart P. H.; Adams P. D.; Zouni A.; Messinger J.; Bergmann U.; Sauter N. K.; Kern J.; Yachandra V. K.; Yano J. Structure of photosystem II and substrate binding at room temperature. Nature 2016, 540, 453–457. 10.1038/nature20161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.; Askerka M.; Brudvig G. W.; Batista V. S. Insights into Photosystem II from Isomorphous Difference Fourier Maps of Femtosecond X-ray Diffraction Data and Quantum Mechanics/Molecular Mechanics Structural Models. ACS Energy Lett. 2017, 2 (2), 397–407. 10.1021/acsenergylett.6b00626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rould M. A.; Carter C. W. Jr. Isomorphous difference methods. Methods Enzymol. 2003, 374, 145–63. 10.1016/S0076-6879(03)74007-5. [DOI] [PubMed] [Google Scholar]
- Kraut J. Structural Studies with X-Rays. Annu. Rev. Biochem. 1965, 34, 247–68. 10.1146/annurev.bi.34.070165.001335. [DOI] [PubMed] [Google Scholar]
- Stryer L.; Kendrew J. C.; Watson H. C. The Mode of Attachment of the Azide Ion to Sperm Whale Metmyoglobin. J. Mol. Biol. 1964, 8, 96–104. 10.1016/S0022-2836(64)80152-2. [DOI] [PubMed] [Google Scholar]
- Dau H.; Grundmeier A.; Loja P.; Haumann M. On the structure of the manganese complex of photosystem II: extended-range EXAFS data and specific atomic-resolution models for four S-states. Philos. Trans. R. Soc., B 2008, 363 (1494), 1237–1243. 10.1098/rstb.2007.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askerka M.; Vinyard D. J.; Wang J. M.; Brudvig G. W.; Batista V. S. Analysis of the Radiation-Damage-Free X-ray Structure of Photosystem II in Light of EXAFS and QM/MM Data. Biochemistry 2015, 54 (9), 1713–1716. 10.1021/acs.biochem.5b00089. [DOI] [PubMed] [Google Scholar]
- Haumann M.; Muller C.; Liebisch P.; Iuzzolino L.; Dittmer J.; Grabolle M.; Neisius T.; Meyer-Klaucke W.; Dau H. Structural and oxidation state changes of the Photosystem II manganese complex in four transitions of the water oxidation cycle (S0 -> S1, S1 -> S2, S2 -> S3, and S3,4 -> S0) characterized by X-ray absorption spectroscopy at 20 K and room temperature. Biochemistry 2005, 44 (6), 1894–1908. 10.1021/bi048697e. [DOI] [PubMed] [Google Scholar]
- Saito K.; Rutherford A. W.; Ishikita H. Energetics of proton release on the first oxidation step in the water-oxidizing enzyme. Nat. Commun. 2015, 6, 8488. 10.1038/ncomms9488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillier W.; Wydrzynski T. O-18-Water exchange in photosystem II: Substrate binding and intermediates of the water splitting cycle. Coord. Chem. Rev. 2008, 252 (3–4), 306–317. 10.1016/j.ccr.2007.09.004. [DOI] [Google Scholar]
- Wang J.; Wing R. A. Diamonds in the rough: a strong case for the inclusion of weak-intensity X-ray diffraction data. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2014, 70, 1491–7. 10.1107/S1399004714005318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karplus P. A.; Diederichs K. Assessing and maximizing data quality in macromolecular crystallography. Curr. Opin. Struct. Biol. 2015, 34, 60–8. 10.1016/j.sbi.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. C.; Siegbahn P. E. M. Alternative mechanisms for O-2 release and O-O bond formation in the oxygen evolving complex of photosystem II. Phys. Chem. Chem. Phys. 2015, 17 (18), 12168–12174. 10.1039/C5CP00138B. [DOI] [PubMed] [Google Scholar]
- Winn M. D.; Ballard C. C.; Cowtan K. D.; Dodson E. J.; Emsley P.; Evans P. R.; Keegan R. M.; Krissinel E. B.; Leslie A. G.; McCoy A.; McNicholas S. J.; Murshudov G. N.; Pannu N. S.; Potterton E. A.; Powell H. R.; Read R. J.; Vagin A.; Wilson K. S. Overview of the CCP4 suite and current developments. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2011, 67, 235–242. 10.1107/S0907444910045749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P.; Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2004, 60, 2126–2132. 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- Murshudov G. N.; Skubak P.; Lebedev A. A.; Pannu N. S.; Steiner R. A.; Nicholls R. A.; Winn M. D.; Long F.; Vagin A. A. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2011, 67, 355–367. 10.1107/S0907444911001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.