Abstract
Background
The psychological health in obese women during pregnancy has been poorly studied.
Objective
To compare levels of anxiety and depressed mood during pregnancy in obese versus normal-weight women.
Methods
63 obese pregnant women and 156 normal-weight controls were included prospectively before 15 weeks of gestation. Levels of state and trait anxiety and depressed mood were measured during the first, second and third trimester of pregnancy. A linear mixed-effect model with repeated measures was used to evaluate group differences.
Results
The levels of state anxiety significantly increased from trimester 1 to trimester 3 in obese pregnant women (beta = 3.70; p = 0.007), while this parameter remained constant throughout pregnancy in normal-weight women. Levels of trait anxiety and depressed mood significantly decreased from trimester 1 to trimester 2 in controls, but not in obese pregnant women. Variables such as maternal education, ethnicity, marital state, psychological history and miscarriages, parity and smoking behaviour had significant effects on anxiety and/or depressed moods during pregnancy. Obese pregnant women show higher levels of anxiety and depressive symptomatology compared to normal-weight pregnant women.
Conclusion
Interventional programmes aiming at preventing the deleterious influence of maternal obesity on perinatal outcomes should include a psycho-educational program specifically tailored to this high-risk group.
Key Words: Obesity, Pregnancy, Body mass index, Anxiety, Depressed mood, Psychological aspects, Obesity management
Introduction
Obesity as a public health problem has increased in many developed countries, rising to worrying percentages. This trend is also visible in the group of women of reproductive age, including pregnant women [1,2,3]. Depending on the cohort studied and the cut-offs used for BMI, obesity in pregnancy varies between 8 and 30% [4,5,6]. Several publications report increased rates of maternal and neonatal complications in obese pregnant women, both in the short [4,5] and the long term [7].
Recent studies also show a link between antenatal maternal stress and cognitive, emotional, behavioural and neuromotor development problems in children [8,9,10]. In a recent Danish cohort study, a positive association was observed between psychosocial factors, such as feelings of depression and levels of anxiety during pregnancy, and post-partum weight retention at 6 and 18 months [11], indicating that pregnant women burdened by psychological stressors are particularly vulnerable to excessive weight retention related to childbearing.
Although several studies have looked at maternal anxiety and depression during pregnancy [12,13,14,15,16], little research has focused on these emotions in obese pregnant women. It is generally well known that obese women are more likely to suffer from higher levels of anxiety and depression compared to normal-weight women [17,18,19]. The aim of our study was to compare the levels and evolution of anxiety and depressed moods during pregnancy between obese women (study group) and normal-weight women (controls), as a first step towards the development of a global lifestyle-coaching health programme for obese pregnant women.
Material and Methods
Study Design and Participants
This study evaluates levels of anxiety and depressed mood in obese and normal-weight pregnant woman, whilst taking into account socio-demographic and medical pregnancy variables. Women were included prospectively during routine antenatal visits in three regional hospitals in Belgium (East Limburg Hospital Genk, Jessa Hospital Hasselt, Sint-Franciscus Hospital Heusden). All women received the normal standard antenatal care, and none of those hospitals offered specific antenatal care programmes for obese pregnant women at the time of the study. Obesity was defined as BMI ≥ 29 kg/m², and normal weight as BMI 19,5-26 kg/m², according to the criteria of the Institute of Medicine (IOM) [3]. During the recruitment period, IOM changed their cut-offs for maternal obesity from 29 to 30 kg/m². In order to remain consistent with the inclusion criteria and with the already collected data, we decided not to change the cut-off level in the current study. Obese and normal-weight women were recruited for participation in the study between 7 and 14 weeks of pregnancy. Women who had pre-existing type 1 diabetes, a multiple pregnancy, a primary need for nutritional advice or insufficient knowledge of the Dutch language were excluded. Socio-demographic and psychological data were obtained at the time of study entry, before 15 weeks of pregnancy (base line measurement, trimester 1). Psychological evaluation was furthermore performed between 18 and 22 weeks (trimester 2) and between 30 and 34 weeks of pregnancy (trimester 3) in both groups. None of the study women were on antidepressants. Non-responders were contacted by telephone and, if necessary, questionnaires were re-sent by post within the pre-determined timeframe. The Medical Ethics Committees of the three hospitals approved the study design, and all women provided informed consent.
Measurements
Data on maternal age, education, ethnicity, marital status, profession, employment, history of miscarriages, parity, smoking and alcohol consumption, spontaneous or assisted conception and psychological history were collected in both study groups at the time of study entry. In order to determine the participants’ psychological history, there were 3 ‘yes-or-no’ questions referring to events in the past: i) subjective feelings of depression, ii) feelings of anxiety and iii) stressful life events. The variable ‘psychological history’ was a combined variable and considered present if at least one of the questions above had been answered with ‘yes’. Present feelings of depression at the moment of inclusion in the study (yes/no) were also evaluated in both groups. Information on the pregnancy outcome, gestational diabetes mellitus (GDM), pregnancy-induced hypertension, gestational age at delivery, onset of labour, method of delivery and birth weight were obtained from the individual medical records at the delivery. The GDM was diagnosed when two or more abnormal plasma glucose values were detected (fasting > 90 mg/dl (5 mmol/l), 1 h >165 mg/dl (9.2 mmol/l), 2 h >145 mg/dl (8 mmol/l) and 3 h > 125 mg/dl (6.9 mmol/l)) [20]. The diagnosis of pregnancy-induced hypertension was defined according to the criteria of the International Society for the Study of Hypertension in Pregnancy as a blood pressure reading ≥ 140/90 mm Hg, measured at 2 occasions at least 6 h apart, after 20 weeks of pregnancy in an otherwise normotensive woman. Pre-eclampsia was defined as the presence of pregnancy induced or chronic hypertension in combination with significant proteinuria (≥300 mg / 24 h) [21].
Levels of anxiety were measured using a Dutch standardized version of the State-Trait Anxiety Inventory (STAI) [22,23]. The STAI comprises two self-report scales for measuring two distinct anxiety aspects: state anxiety and trait anxiety. Both scales contain 20 statements asking the pregnant women to describe how they feel. The state anxiety scale includes statements about the intensity of feelings at a particular moment in time, whereas the trait anxiety scale includes statements about the frequency of general feelings. Items are rated on a Likert scale, ranging from a score of 1 to 4. For state anxiety, 1’ means ‘not at all’, 2 means ‘somewhat’, 3 means ‘moderately so’ and 4 means ‘very much so’. Similarly, for trait anxiety 1 means ‘almost never’, 2 ‘sometimes’ 3 ‘often’ and 4 ‘almost always’. A composite score is generated for each subscale after a reversal of the negative items, ranging for each scale from a minimum of 20 to a maximum of 80. High scores mean more state or trait anxiety. Although a cut-off point for high anxiety has not been properly defined, most studies consider a score above 40 as highly anxious [24,25]. The STAI is a reliable and valid self-report measure that can be used for pregnant women [24]. The Cronbach's alpha analyses for these measurements for state and trait anxiety were high, at 0.94 and 0.93 respectively. To calculate the Cronbach's alpha, one measurement for each score was taken at random for all pregnant women to ascertain independent measurements.
Feelings of depression, i.e. depressed moods, during pregnancy were assessed using the 10-item Edinburgh Postnatal Depression Scale (EPDS). This scale was originally designed as a screening instrument for postnatal depression but was also validated later for usage during pregnancy [26,27,28] and named Edinburgh Depression Scale (EDS). Items are rated on a 4 point Likert scale (0-3) and address the intensity of depressive symptoms in the previous seven days. A cut-off of 13 normally discriminates between minor and major depression [29]. Recently, a cut-off score of 11 in the first trimester of pregnancy and 10 in the second and third trimester was recommended to predict a major depression, although the obtained positive predictive values ranged up to 51%, 39% and 29% for the first, second and third trimester respectively [30]. Cronbach's alpha for EDS was 0.83, reflecting a high internal consistency [31]. In this study, we only used cut-offs for descriptive statistics. For the multivariate analysis, we used the continuous measure.
Statistical Analysis
Linear mixed-effect models with repeated measures were used to analyse group differences for levels of anxiety and depressed mood, checking for the effects of those co-variates that correlated significantly with state or trait anxiety, or depressed mood. The co-variates considered were: maternal age and education, marital state, employment, profession, ethnicity, parity, history of miscarriages, gravidity, cigarette smoking and alcohol consumption, spontaneous or assisted conception, psychological history and diabetes and hypertensive disorders in the current pregnancy. Three separate models were constructed for each of the following three dependent variables: state anxiety, trait anxiety and depressed mood. For each of these dependent variables, two steps were included in the final model. Initially, the impact of a co-variate on the dependent variable was measured by a small multi-variate variable model, including only three variables: presence or absence of obesity, trimester of pregnancy and the co-variate under consideration. If a co-variate was significant in the small three-variate model, this variable was then included in the multi-variate model to identify the variables that remained significant. We assumed that if respondents left the study before giving birth, they were ‘missing at random’ (i.e. respondents who stop co-operating are comparable with respondents who continue co-operating) and therefore incomplete cases were also retained in the analysis. All analyses were performed using the statistical software SAS Enterprise Guide 4.2, (SAS Institute Inc., Cary, NC, USA).
Results
Of the 206 eligible normal-weight women, 29 decided not to participate. Three were excluded because of language problems, one because of termination of pregnancy (hypoplastic left heart) and one suffered an intra-uterine death. Six women had a miscarriage, and 4 were lost to follow-up. Of the remaining 162 women, 6 were excluded because their BMI was <19,5 kg/m². This left 156 normal-weight women in the study and analysis. Eleven of the 74 eligible obese women chose not to participate, leaving 63 obese pregnant women in the analysis.
Descriptive Analysis
Obese pregnant women were significantly less educated, had higher parity, more medically assisted conceptions and more history of psychological stress compared to normal-weight women (table 1). Significant differences in perinatal outcomes were found between obese and normal-weight women for pregnancy-induced hypertension, GDM and method of delivery (table 2). In the total sample, state and trait anxiety scores ranged from 20 to 69, with a mean of 36 and 35.3 for state and trait anxiety respectively. These scores covered the range of scores observed in a non-clinical female Dutch community sample. The mean level for normal-weight and obese women in our sample equalled decile 5 in a female community sample [23]. A high state anxiety (i.e. score ≥ 40) was reported in 44% and a high trait anxiety in 40% of pregnant women in at least one trimester of pregnancy. In the total cohort of pregnant women, the EDS scores ranged from 0 to 21 and the mean level was 6.3. When a cut-off point of 13 [29] was used for all trimesters, 15% of the total cohort were at risk of major depression in at least one trimester of pregnancy, compared to 29% if a cut-off point of 11 was used for the first trimester and 10 [30] for the second and third trimesters.
Table 1.
Socio-demographic baseline characteristics in normal-weight (n =156) and obese (n =63) pregnant women
| Normal-weight women BMI 19.5–26 kg/m2 | Obese women BMI ≥ 29 kg/m2 | p value a | |
|---|---|---|---|
| Continuous variables, mean, (SD) | |||
| Prepregnancy BMI, kg/m2 | 22.0 (1.4) | 34.4 (4.1) | <0.0001 |
| Age, years | 28.7 (3.8) | 28.7 (4.2) | 0.98 |
| Mean parity | 0.6 (0.8) | 0.8 (1.0) | 0.02 |
|
| |||
| Categorical variables, n (%) b | |||
| Miscarriage | 25 (16.0) | 14 (22.2) | 0.28 |
| Multigravidae c | 75 (48.1) | 42 (66.7) | 0.01 |
| Ethnicity | 0.50 | ||
| Belgian/Dutch | 129 (82.7) | 49 (77.8) | |
| Italian | 8 (5.1) | 3 (4.8) | |
| Moroccan/Turkish | 12 (7.7) | 9 (14.3) | |
| Other | 7 (4.4) | 2 (3.2) | |
| Higher education | 0.0008 | ||
| Master degree | 25 (16.0) | 2 (3.2) | |
| Bachelor degree | 72 (46.2) | 21 (33.3) | |
| None | 59 (37.8) | 40 (63.5) | |
| Employment at inclusion | 0.15 | ||
| Full-time | 68 (43.9) | 24 (38.7) | |
| Part-time | 31 (20.0) | 20 (32.3) | |
| Unemployed | 56 (36.1) | 18 (29.0) | |
| Profession | 0.19 | ||
| Self-employment | 12 (7.7) | 5 (7.9) | |
| Employee | 107 (68.6) | 35 (55.6) | |
| Worker | 20 (12.8) | 15 (23.8) | |
| No outdoor profession | 17 (10.9) | 8 (12.7) | |
| Marital state e | 0.70 | ||
| Married/cohabiting | 151 (96.8) | 60 (95.2) | |
| Single | 5 (3.2) | 3 (4.8) | |
| Method of conception e | 0.05 | ||
| Medical assistance | 8 (5.1) | 9 (14.3) | |
| Alcohol and smoking consumption | |||
| Alcohol | 14 (9.0) | 7 (11.3) | 0.61 |
| Smoking | 19 (12.2) | 10 (15.9) | 0.47 |
| Psychological history | |||
| Depression | 15 (9.7) | 9 (14.5) | 0.30 |
| Anxiety | 17 (11.0) | 8 (12.9) | 0.67 |
| Stressful family events | 32 (20.6) | 21 (33.9) | 0.04 |
| Psychological history, combined d | 40 (25.8) | 30 (48.4) | 0.001 |
| Depression at inclusion e | 11 (7.0) | 2 (3.3) | 0.36 |
p values were calculated by chi-square for categorical variables and by ANOVA for continuous variables (BMI, age and parity).
All percentages are column percentages.
Multigravidity = being once or more pregnant, independent of pregnancy outcome (miscarriage and/or delivery) – combination of miscarriage and parity.
Psychological history, combined is a combined variable and considered present if at least one of the 3 questions regarding depression, anxiety or stressful family events in history had been answered with ‘YES’.
Fisher's exact test was used for analyzing categorical variables if small expected counts per cel.
Table 2.
Pregnancy and obstetrical outcomes a
| Normal-weight women BMI 19.5–26 kg/m2 (n = 156) |
Obese women BMI ≥ 29 kg/m2 (n = 63) |
p value b | |
|---|---|---|---|
| Categorical variables, n (%) c | |||
| PET | 3 (1.9) | 4 (6.3) | 0.20 |
| PIH | 4 (2.6) | 6 (9.5) | 0.04 |
| GDM | 3 (1.9) | 7 (11.1) | 0.007 |
| Induction of labour | 24 (15.6) | 15 (24.2) | 0.14 |
| Method of delivery | |||
| Vaginal delivery | 122 (87.1) | 37 (66.1) | 0.0007 |
| Caesarean delivery | 18 (12.9) | 19 (34) | |
|
| |||
| Continuous variables, mean (SD) | |||
| Gestational age at delivery, weeks | 39.3 (1.6) | 39.5 (1.8) | 0.49 |
| Birth weight, kg | 3.378 (523) | 3.504 (583) | 0.12 |
PET= Pre-eclamptic toxicosis; PIH= pregnancy-induced hypertension; GDM = gestational diabetes mellitus.
Missing data: PET/PIH normal weight n = 2; GDM normal weight n = 1; Induction of labour normal weight n = 2, obese women n = 1.; Method of delivery/pregnancy duration/birth weight normal weight n = 2.
p values were calculated by chi-square (Fisher exact test if small expected counts per cell, for PET, PIH, GDM) for categorical variables and by ANOVA for continuous variables (birth weight and duration of pregnancy).
All percentages are column percentages.
Anxiety during Pregnancy in Obese and Normal-Weight Women
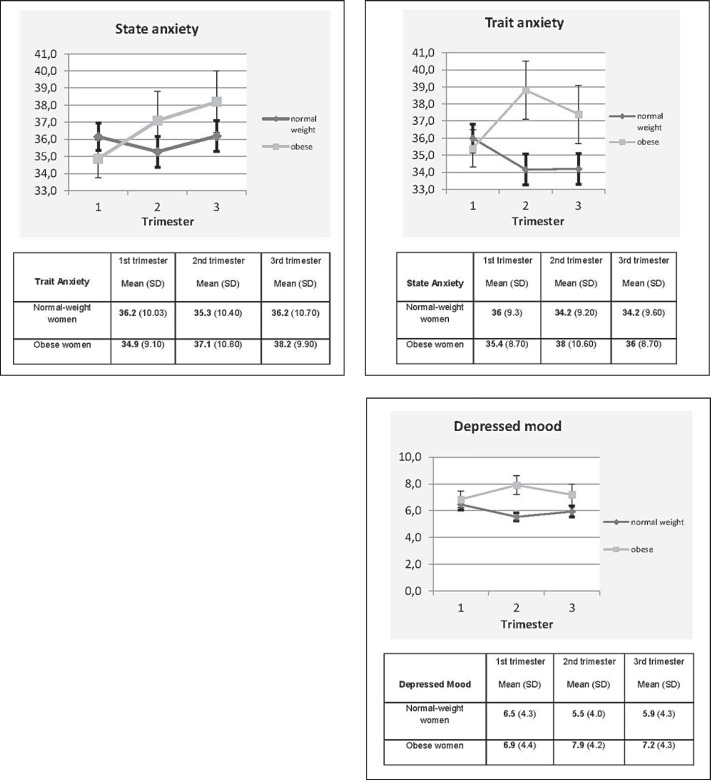
In figure 1, interaction effects during pregnancy between obese and normal-weight pregnant women, based on a model with repeated measures using only group and trimester as outcome predictors, are visualized. At baseline (trimester 1), obese pregnant women showed significant lower state and trait anxiety (β estimate = −3.89; p = 0.005 and β estimate = −2.75; p = 0.03 respectively) than normal-weight pregnant women (tables 3 and 4). For state anxiety, a significant increase from trimester 1 to trimester 2 (β estimate = 2.39; p = 0.04) and from trimester 1 to trimester 3 (β estimate = 3.70; p = 0.007) is observed in obese pregnant women. By contrast, the results for normal-weight women revealed no significant differences in state anxiety throughout pregnancy (fig. 1, table 3).
Fig. 1.
Mean levels and error bars (SEM) of state and trait anxiety and depressed mood in obese and normal-weight pregnant women by trimester
Table 3.
Multivariate model of state anxiety in normal-weight and obese pregnant women
| B estimate | Standard error | t value | p value | |
|---|---|---|---|---|
| Intercept | 36.00 | 1.18 | 3.04 | <0.0001 |
| Trimester 1 (ref) | ||||
| Normal-weight women | 0 | |||
| Obese women | −3.89 | 1.38 | −2.82 | 0.005 |
| Trimester 2 | ||||
| Normal-weight women | −0.63 | 0.64 | −0.99 | 0.32 |
| Obese women | 2.39 | 1.17 | 2.05 | 0.04 |
| Trimester 3 | ||||
| Normal-weight women | 0.01 | 0.69 | 0.02 | 0.98 |
| Obese women | 3.70 | 1.37 | 2.70 | 0.007 |
| Education | ||||
| Until 18 years | 0 | |||
| Bachelor degree | −4.44 | 1.25 | −3.56 | 0.0004 |
| Master degree | −1.13 | 1.86 | −0.61 | 0.54 |
| Ethnicity | ||||
| Belgian/Dutch | 0 | |||
| Italian | 2.64 | 2.66 | 0.99 | 0.32 |
| Moroccan/Turkish | 4.67 | 1.97 | 2.37 | 0.02 |
| Other | 5.85 | 2.85 | 2.05 | 0.04 |
| Marital state, single | 10.11 | 2.98 | 3.40 | 0.0008 |
| History of miscarriage, yes | 3.61 | 1.46 | 2.47 | 0.01 |
| History of stressful family events, yes | 5.08 | 1.33 | 3.83 | 0.0002 |
Table 4.
Multivariate model of trait anxiety in normal-weight and obese pregnant women
| B estimate | Standard error | t value | p value | |
|---|---|---|---|---|
| Intercept | 34.82 | 1.22 | 2.85 | <0.0001 |
| Trimester 1 (ref) | ||||
| Normal-weight women | 0 | |||
| Obese women | −2.75 | 1.28 | −2.15 | 0.03 |
| Trimester 2 | ||||
| Normal-weight women | −2.20 | 0.63 | −3.48 | 0.0006 |
| Obese women | 1.74 | 1.04 | 1.68 | 0.09 |
| Trimester 3 | ||||
| Normal-weight women | −2.36 | 0.78 | −3.01 | 0.003 |
| Obese women | 0.40 | 1.35 | 0.30 | 0.77 |
| Education | ||||
| Until 18 years | 0 | |||
| Bachelor degree | −2.59 | 1.17 | −2.21 | 0.03 |
| Master degree | −2.17 | 1.71 | −1.27 | 0.20 |
| Ethnicity | ||||
| Belgian/Dutch | 0 | |||
| Italian | 2.51 | 2.42 | 1.04 | 0.30 |
| Moroccan/Turkish | 5.59 | 1.83 | 3.05 | 0.002 |
| Other | 5.25 | 2.60 | 2.02 | 0.04 |
| Multiparae, yes* trimester 1 | 0.65 | 1.14 | 0.57 | 0.57 |
| Multiparae, yes trimester 2 | 2.25 | 0.91 | 2.48 | 0.01 |
| Multiparae, yes trimester 3 | 2.41 | 1.15 | 2.10 | 0.04 |
| Marital state, single | 9.09 | 2.69 | 3.37 | 0.0008 |
| Smoking, yes | 3.08 | 1.57 | 1.96 | 0.05 |
| History of stressful family events, yes | 5.58 | 1.22 | 4.58 | <.0001 |
ref = nulliparae.
For trait anxiety, no significant difference throughout pregnancy was observed in the obese pregnant women, while normal-weight controls showed a decrease from trimester 1 to trimester 2 (β estimate = −2.20; p = 0.0006) and from trimester 1 to trimester 3 (β estimate = −2.36; p = 0.003) (table 4).
Depressed Mood during Pregnancy in Obese and Normal-Weight Women
At baseline (trimester 1), no difference in depressive symptoms was found between obese women and controls. For normal-weight women, a significant decrease was found in trimester 2 compared to trimester 1 (β estimate = −0.85; p = 0.02) while obese pregnant women reported no difference in depressive symptomatology throughout the pregnancy (data not shown).
Influences of Covariates on the Levels of Anxiety and Depressed Mood in the Three Multivariate Models
Apart from the BMI, we also found maternal education level, marital state, ethnicity and history of stressful family events to be significantly related to levels of state and trait anxiety (tables 3 and 4). A history of miscarriage also influenced state anxiety (table 3) while parity and smoking behaviour had positive effects on trait anxiety (table 4). Variables such as multigravidity (β estimate = 1.67; p = 0.0004), ethnicity (β estimate = 1.63; p = 0.04 for Moroccan/Turkish women), history of stressful family events (β estimate = 2.35; p < 0.0001) and depression (β estimate = 1.91; p = 0.01), were associated with an increased rate of depressed mood throughout the pregnancy (data not shown). GDM and pregnancy-induced hypertension were not retained in multivariate models.
Discussion
In this study, we discovered that obese pregnant women demonstrate higher levels of anxiety and depressed mood in comparison with normal-weight women. A mean increase of more than 3 points on the STAI for state anxiety from trimester 1 to trimester 3 was found in obese women. Normal-weight women, on the contrary, did not show any significant difference on state anxiety levels throughout pregnancy. The significant decrease in trait anxiety, demonstrated over the course of the pregnancy in normal-weight women, was not observed in obese women. Most studies concerning anxiety and depression in pregnancy consist of a general pregnant population [16,32], and only a few [33,34,35] have studied those in the context of obesity. One of those studies [33] used a cross-sectional design to detect a significant association between an increased pre-pregnancy weight category and increasing levels of anxiety and depression. We believe that the longitudinal design used in our study is more appropriate to evaluate the psychological health of women during pregnancy. Indeed, in each trimester of pregnancy, specific events may induce feelings of anxiety [36,37]. The positive association between obesity and anxiety in general [19] can become more explicit during the course of the pregnancy. A lot of studies demonstrated higher risks for specific congenital malformations [38], hypertension and GDM [4,39] in obese pregnant women; knowledge of these risks may induce anxiety [40]. Our study, however, was not powered to detect a relationship between the presence of specific complications like GDM and hypertensive disorders in pregnancy and levels of anxiety and depressed mood. Future in-depth qualitative and quantitative research should explore the exact reasons for the increasing levels of anxiety/depressed mood in obese pregnant women.
Another longitudinal study [34] demonstrates a strong positive association between the pre-pregnancy BMI and the likelihood of a major depressive disorder during the second and third trimester of pregnancy. Our study did not identify an increase in depressed mood in obese pregnant women, but did highlight a significant decrease in the control normal-weight women between trimester 1 and 2; this was not shown in obese pregnant women, neither after controlling for pre-conceptional psychological stress factors. A study [35] investigating psychological well-being among obese pregnant women attending a weight gain restriction programme compared with a control group of obese women receiving traditional care reported the same risk of experiencing anxiety/depressive symptoms in both groups of obese pregnant women. Although the authors did not use a control group of normal-weight pregnant women themselves, they concluded that – based on various other studies measuring levels of anxiety/depressed mood in a general pregnant population – obese pregnant women did not run a higher risk for anxiety/depressive symptoms compared with a general pregnant population, which is in contrast with our own findings.
Other studies have described a decreasing or stable level of anxiety and depressive mood as the pregnancy progresses [16,41,42,43,44]. In our study, this decrease is not present in obese pregnant women and encourages us to think that they seem to be rather insensitive to the ‘physiological’ increase in psychological comfort of confidence that we find in non-obese pregnant women.
Interestingly, at the point of inclusion in the study, levels of anxiety were significantly lower in obese than in normal-weight women. No difference at baseline was observed between obese and normal-weight pregnant women with regard to depressed mood. We have no clear explanation for this, but some factors could be involved. First, obese women often have to deal with fertility problems [45], as was also the case in our cohort, and therefore have more intensive contacts with health care providers. The lower baseline levels can be explained by the final relief obese women experience by becoming pregnant. Also, the mode of administration of the psychological scales could have interfered to some extent. In normal-weight women, all questionnaires were sent by post, while in the obese pregnant women, the first measurement took place in the antenatal care unit in the presence of the research midwife. All further questionnaires were sent by post from then on. A recent study using the STAI in a pregnant population showed an impact of the location of the completion of the questionnaire (hospital vs. local community clinic) on levels of state but not trait anxiety, with higher scores obtained in high-risk surroundings like hospitals [46]. This is in contradiction with our results, where lower levels were obtained in a hospital setting, but, at the same time, these results suggest that the anxiety scale can be sensitive to a situation-specific environment.
Another strength of our current study is the fact that we also identified maternal and pregnancy-related variables which influence levels of anxiety and depressed mood. These findings are important to understand the specific psychological context of pregnancy and obesity. As reported in earlier studies [23], maternal education in this sample was associated negatively with levels of anxiety. Women dealing with stressful family events in the past as well as single mothers and mothers of non-Caucasian origin showed significantly higher levels of state and trait anxiety. Other studies [14,47,48] produced similar results. Smoking behaviour as well as a high parity were associated with higher levels of trait anxiety in our cohort. High parity was associated with a statistically significant positive influence on trait anxiety in each trimester. A prior negative perinatal experience and/or an imbalance in parenting roles may provoke anxiety in multiparous women and make obese women especially more vulnerable to the effects of perinatal stress [32].
Some limitations can be pointed out. First, we have to consider the voluntary basis for participation in this study, which can be selective in the sense that those who participated may be in fact more happy and satisfied with their pregnancy compared to those who declined to participate. Secondly, we were faced with drop-outs, which are inevitable in a longitudinal design. Linear mixed-effect models with repeated measures allow the retention of participants for as long as data are available. Hence, data of women who participated only in the first pregnancy trimester can be used to estimate parameters for the first trimester. This assumes that women who stop co-operating are comparable with the women that continue co-operating. This assumption is supported by the fact that, from all the variables for which normal-weight pregnant women with missing data were compared with normal-weight pregnant women without missing data, only one variable was significant. Normal-weight pregnant women with missing data had a higher BMI (22.9 kg/m² vs. 21.9 kg/m²; p = 0.0007) compared to women with complete data. No significant differences were observed in obese pregnant women with missing data versus those with complete data. Furthermore, obese and normal-weight pregnant women with missing data did not differ significantly from women with complete data for levels of anxiety and depressed mood, from which we can conclude that the possible attrition bias was minimal.
Our data demonstrate a different psychological state of well-being for obese women throughout pregnancy. Given the positive predictive value of antenatal anxiety and depression for postnatal depression [49] and given their impact on child development [8,50], a systematic antenatal psychological evaluation seems justified for obese women. Although validated scales with suggested cut-offs can only detect a potential risk and cannot replace a diagnostic tool (e.g., a psychiatric interview), they may indicate which pregnant women may benefit from further intensive counselling for psychosocial vulnerability [51].
Earlier studies have demonstrated that pregnant women with elevated levels of stress and anxiety consume more food, such as fats, oils, sweets and snacks, but also have a decreased intake of vitamins [52]. Subjects with such an eating behaviour are often described as ‘emo eaters’. The quality of diet in general decreases with an increasing BMI in pregnant women [53. M]ore specifically, the intake of fibreand calcium decreases, and the intake of fat increases [54]. Earlier research also showed a positive correlation between the pre-pregnancy BMI, gestational weight gain and anxiety and depressive symptoms, suggesting a correlation between (pre-)pregnancy weight status and psychosocial vulnerability [33,34]. Interventional programmes for obese pregnant women aimed at obtaining an adequate gestational weight gain between 5-9 kg [55], and optimal maternal and neonatal outcomes do not seem to be very effective [56,57,58]. One of the problems these intervention programmes face is that their aims and methods are not compatible with the specific needs and psychological characteristics of obese pregnant women. The promotion of the well-being and the psychological health for this higher-risk group is an important but often neglected aspect of educational interventions. A review [59] provides some evidence that pregnant women's health benefits from psycho-education as it reduces the women's level of stress and anxiety, although evidence is limited and should be interpreted with caution. Controlled trials targeting specific risk groups [60], i.e., obese pregnant women, should be set up in order to study effects of psycho-education on perinatal outcomes more thoroughly.
Funding
AB was supported by a PWO project from Flanders. RD is senior clinical researcher for FWO Flanders (2010-2015), and BVDB is funded by a grant of the European Science Foundation (EuroSTRESSS programme ‘Stress and Mental Health’, 2008-2011), by the Netherlands Organisation for Scientific Research (NWO, Brain and Cognition Program, 2008-2012), and by EU 7th Framework program (FP7-Health-2011; BrainAGE 2012-2017).
Contributors
AB, RD, IW and BVDB designed the study and wrote the protocol. AB took care of the literature searches, did the inclusions and managed the analysis. EN undertook the statistical analysis. AB wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Disclosure Statement
The authors declare no conflict of interest.
Acknowledgements
We thank all the pregnant women for their participation and the gynaecologists and midwives from East Limburg Hospital Genk, Jessa Hospital Hasselt and Sint Franciscus Hospital Heusden-Zolder, Belgium, for their co-operation and support. We are grateful to Marianne Mead for reading this manuscript.
References
- 1.Kerrigan AM, Kingdon C. Maternal obesity and pregnancy: a retrospective study. Midwifery. 2010;26:138–146. doi: 10.1016/j.midw.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Shaikh H, Robinson S, Teoh TG. Management of maternal obesity prior to and during pregnancy. Semin Fetal Neonatal Med. 2010;15:77–82. doi: 10.1016/j.siny.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Institute of Medicine . Weight Gain during Pregnancy: Re-Examining the Guidelines. Washington DC: National Academy Press; 2009. [Google Scholar]
- 4.Guelinckx I, Devlieger R, Beckers K, Vansant G. Maternal obesity: pregnancy complications, gestational weight gain and nutrition. Obes Rev. 2008;9:140–150. doi: 10.1111/j.1467-789X.2007.00464.x. [DOI] [PubMed] [Google Scholar]
- 5.Heslehurst N, Simpson H, Ells LJ, Rankin J, Wilkinson J, Lang R, Brown TJ, Summerbell CD. The impact of maternal BMI status on pregnancy outcomes with immediate short-term obstetric resource implications: a meta-analysis. Obes Rev. 2008;9:635–683. doi: 10.1111/j.1467-789X.2008.00511.x. [DOI] [PubMed] [Google Scholar]
- 6.Briese V, Voigt M, Wisser J, Borchardt U, Straube S. Risks of pregnancy and birth in obese primiparous women: an analysis of German perinatal statistics. Arch Gynecol Obstet. 2010;283:249–253. doi: 10.1007/s00404-009-1349-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nelson SM, Matthews P, Poston L. Maternal metabolism and obesity: modifiable determinants of pregnancy outcome. Hum Reprod Update. 2010;16:255–275. doi: 10.1093/humupd/dmp050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van den Bergh BR, Mulder EJ, Mennes M, Glover V. Antenatal maternal anxiety and stress and the neurobehavioural development of the fetus and child: links and possible mechanisms. A review. Neurosci Biobehav Rev. 2005;29:237–258. doi: 10.1016/j.neubiorev.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 9.van Batenburg-Eddes T, de Groot L, Huizink AC, Steegers EA, Hofman A, Jaddoe VW, Verhulst FC, Tiemeier H. Maternal symptoms of anxiety during pregnancy affect infant neuromotor development: the generation R study. Dev Neuropsychol. 2009;34:476–493. doi: 10.1080/87565640902964508. [DOI] [PubMed] [Google Scholar]
- 10.Gutteling BM, de Weerth C, Willemsen-Swinkels SH, Huizink AC, Mulder EJ, Visser GH, Buitelaar JK. The effects of prenatal stress on temperament and problem behavior of 27-month-old toddlers. Eur Child Adolesc Psychiatry. 2005;14:41–51. doi: 10.1007/s00787-005-0435-1. [DOI] [PubMed] [Google Scholar]
- 11.Pedersen P, Baker JL, Henriksen TB, Lissner L, Heitmann BL, Sorensen TIA, Nohr EA. Influence of psychosocial factors on postpartum weight retention. Obesity. 2011;19:639–646. doi: 10.1038/oby.2010.175. [DOI] [PubMed] [Google Scholar]
- 12.Andersson L, Sundstrom-Poromaa I, Wulff M, Astrom M, Bixo M. Depression and anxiety during pregnancy and six months postpartum: a follow-up study. Acta Obstet Gynecol Scand. 2006;85:937–944. doi: 10.1080/00016340600697652. [DOI] [PubMed] [Google Scholar]
- 13.Faisal-Cury A, Rossi MP. Prevalence of anxiety and depression during pregnancy in a private setting sample. Arch Womens Ment Health. 2007;10:25–32. doi: 10.1007/s00737-006-0164-6. [DOI] [PubMed] [Google Scholar]
- 14.Breitkopf CR, Primeau LA, Levine RE, Olson GL, Wu ZH, Berenson AB. Anxiety symptoms during pregnancy and postpartum. J Psychosom Obstet Gynaecol. 2006;27:157–162. doi: 10.1080/01674820500523521. [DOI] [PubMed] [Google Scholar]
- 15.Austin MP, Tully L, Parker G. Examining the relationship between antenatal anxiety and postnatal depression. J Affect Disord. 2007;101:169–174. doi: 10.1016/j.jad.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 16.Heron J, O'Connor TG, Evans J, Golding J, Glover V. The course of anxiety and depression through pregnancy and the postpartum in a community sample. J Affect Disord. 2004;80:65–73. doi: 10.1016/j.jad.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Zhao G, Ford ES, Dhingra S, Li C, Strine TW, Mokdad AH. Depression and anxiety among US adults: associations with body mass index. Int J Obes (Lond) 2009;33:257–266. doi: 10.1038/ijo.2008.268. [DOI] [PubMed] [Google Scholar]
- 18.Zender R, Olshansky E. Women's mental health: depression and anxiety. Nurs Clin North Am. 2009;44:355–364. doi: 10.1016/j.cnur.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Gariepy G, Nitka D, Schmitz N. The association between obesity and anxiety disorders in the population: a systematic review and meta-analysis. Int J Obes (Lond) 2010;34:407–419. doi: 10.1038/ijo.2009.252. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman L, Nolan C, Wilson JD, Oats JJ, Simmons D. Gestational diabetes mellitus – management guidelines. The Australasian Diabetes in Pregnancy Society. Med J Aust. 1998;169:93–97. doi: 10.5694/j.1326-5377.1998.tb140192.x. [DOI] [PubMed] [Google Scholar]
- 21.Brown MA, Lindheimer MD, de Swiet M, Van AA, Moutquin JM. The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP) Hypertens Pregnancy. 2001;20:IX–XIV. doi: 10.1081/PRG-100104165. [DOI] [PubMed] [Google Scholar]
- 22.Spielberger CD, Gorsuch RL, Lushene RE. STAI Manual for the State-Trait Anxiety Inventory. Palo Alto: Consulting Psychologists Press; 1970. [Google Scholar]
- 23.van der Ploeg HM. Een Nederlandse bewerking van de Spielberger State-Trait Anxiety Inventory STAI-DY. Lisse: Swets & Zeitlinger B.V. Test Publishers; 2000. Handleiding bij de Zelf-Beoordelings Vragenlijst. [Google Scholar]
- 24.Grant KA, McMahon C, Austin MP. Maternal anxiety during the transition to parenthood: a prospective study. J Affect Disord. 2008;108:101–111. doi: 10.1016/j.jad.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Britton JR. Maternal anxiety: course and antecedents during the early postpartum period. Depress Anxiety. 2008;25:793–800. doi: 10.1002/da.20325. [DOI] [PubMed] [Google Scholar]
- 26.Rich-Edwards JW, Kleinman K, Abrams A, Harlow BL, McLaughlin TJ, Joffe H, Gillman MW. Sociodemographic predictors of antenatal and postpartum depressive symptoms among women in a medical group practice. J Epidemiol Commun Health. 2006;60:221–227. doi: 10.1136/jech.2005.039370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 28.Pop VJ, Komproe IH, van Son MJ. Characteristics of the Edinburgh Post Natal Depression Scale in The Netherlands. J Affect Disord. 1992;26:105–110. doi: 10.1016/0165-0327(92)90041-4. [DOI] [PubMed] [Google Scholar]
- 29.Murray D, Cox JL. Screening for depression during pregnancy with the Edinburgh Depression Scale (EPDS) J Reprod Infant Psychol. 1990;8:99–107. [Google Scholar]
- 30.Bergink V, Kooistra L, Lambregtse-van den Berg MP, Wijnen H, Bunevicius R, van Baar A, Pop V. Validation of the Edinburgh Depression Scale during pregnancy. J Psychosom Res. 2011;70:385–389. doi: 10.1016/j.jpsychores.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 31.Polit DE, Beck CT. Nursing Research, Generating and Assessing Evidence for Nursing Practice. ed 8. Philadelphia: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 32.Dipietro JA, Costigan KA, Sipsma HL. Continuity in self-report measures of maternal anxiety, stress, and depressive symptoms from pregnancy through two years postpartum. J Psychosom Obstet Gynaecol. 2008;29:115–124. doi: 10.1080/01674820701701546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laraia BA, Siega-Riz AM, Dole N, London E. Pregravid weight is associated with prior dietary restraint and psychosocial factors during pregnancy. Obesity (Silver Spring) 2009;17:550–558. doi: 10.1038/oby.2008.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bodnar LM, Wisner KL, Moses-Kolko E, Sit DK, Hanusa BH. Prepregnancy body mass index, gestational weight gain, and the likelihood of major depressive disorder during pregnancy. J Clin Psychiatry. 2009;70:1290–1296. doi: 10.4088/JCP.08m04651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Claesson IM, Josefsson A, Sydsjo G. Prevalence of anxiety and depressive symptoms among obese pregnant and postpartum women: an intervention study. BMC Public Health. 2010;10:766. doi: 10.1186/1471-2458-10-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huizink AC, Mulder EJ, Robles de Medina PG, Visser GH, Buitelaar JK. Is pregnancy anxiety a distinctive syndrome? Early Hum Dev. 2004;79:81–91. doi: 10.1016/j.earlhumdev.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 37.Rosenberg KB, Monk C, Glickstein JS, Levasseur SM, Simpson LL, Kleinman CS, Williams IA. Referral for fetal echocardiography is associated with increased maternal anxiety. J Psychosom Obstet Gynaecol. 2010;31:60–69. doi: 10.3109/01674821003681472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rasmussen SA, Chu SY, Kim SY, Schmid CH, Lau J. Maternal obesity and risk of neural tube defects: a metaanalysis. Am J Obstet Gynecol. 2008;198:611–619. doi: 10.1016/j.ajog.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 39.Nohr EA, Vaeth M, Baker JL, Sorensen TI, Olsen J, Rasmussen KM. Combined associations of prepregnancy body mass index and gestational weight gain with the outcome of pregnancy. Am J Clin Nutr. 2008;87:1750–1759. doi: 10.1093/ajcn/87.6.1750. [DOI] [PubMed] [Google Scholar]
- 40.Furber CM, McGowan L. A qualitative study of the experiences of women who are obese and pregnant in the UK. Midwifery. 2011;27:437–444. doi: 10.1016/j.midw.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 41.Teixeira C, Figueiredo B, Conde A, Pacheco A, Costa R. Anxiety and depression during pregnancy in women and men. J Affect Disord. 2009;119:142–148. doi: 10.1016/j.jad.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 42.Lee AM, Lam SK, Sze Mun Lau SM, Chong CS, Chui HW, Fong DY. Prevalence, course, and risk factors for antenatal anxiety and depression. Obstet Gynecol. 2007;110:1102–1112. doi: 10.1097/01.AOG.0000287065.59491.70. [DOI] [PubMed] [Google Scholar]
- 43.Skouteris H, Wertheim EH, Rallis S, Milgrom J, Paxton SJ. Depression and anxiety through pregnancy and the early postpartum: an examination of prospective relationships. J Affect Disord. 2009;113:303–308. doi: 10.1016/j.jad.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 44.Felice E, Saliba J, Grech V, Cox J. Prevalence rates and psychosocial characteristics associated with depression in pregnancy and postpartum in Maltese women. J Affect Disord. 2004;82:297–301. doi: 10.1016/j.jad.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 45.Brannian JD. Obesity and fertility. S D Med. 2011;64:251–254. [PubMed] [Google Scholar]
- 46.Gunning MD, Denison FC, Stockley CJ, Ho SP, Sandhu HK, Reynolds RM. Assessing maternal anxiety in pregnancy with the State-Trait Anxiety Inventory (STAI): issues of validity, location and participation. J Reprod Infant Psychol. 2010;28:266–273. [Google Scholar]
- 47.Karacam Z, Ancel G. Depression, anxiety and influencing factors in pregnancy: a study in a Turkish population. Midwifery. 2009;25:344–356. doi: 10.1016/j.midw.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 48.Edwards B, Galletly C, Semmler-Booth T, Dekker G. Antenatal psychosocial risk factors and depression among women living in socioeconomically disadvantaged suburbs in Adelaide, South Australia. Aust N Z J Psychiatry. 2008;42:45–50. doi: 10.1080/00048670701732673. [DOI] [PubMed] [Google Scholar]
- 49.Austin MP, Hadzi-Pavlovic D, Saint K, Parker G. Antenatal screening for the prediction of postnatal depression: validation of a psychosocial pregnancy risk questionnaire. Acta Psychiatr Scand. 2005;112:310–317. doi: 10.1111/j.1600-0447.2005.00594.x. [DOI] [PubMed] [Google Scholar]
- 50.Raikkonen K, Seckl JR, Pesonen AK, Simons A, Van den Bergh BR. Stress, glucocorticoids and liquorice in human pregnancy: programmers of the offspring brain. Stress. 2011;14:590–603. doi: 10.3109/10253890.2011.602147. [DOI] [PubMed] [Google Scholar]
- 51.Austin MP. Psychosocial assessment and management of depression and anxiety in pregnancy. Key aspects of antenatal care for general practice. Aust Fam Physician. 2003;32:119–126. [PubMed] [Google Scholar]
- 52.Hurley KM, Caulfield LE, Sacco LM, Costigan KA, Dipietro JA. Psychosocial influences in dietary patterns during pregnancy. J Am Diet Assoc. 2005;105:963–966. doi: 10.1016/j.jada.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 53.Rifas-Shiman SL, Rich-Edwards JW, Kleinman KP, Oken E, Gillman MW. Dietary quality during pregnancy varies by maternal characteristics in Project Viva: a US cohort. J Am Diet Assoc. 2009;109:1004–1011. doi: 10.1016/j.jada.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guelinckx I, Devlieger R, Vansant G. Pregnancies complicated by obesity: clinical approach and nutritional management. Verh K Acad Geneeskd Belg. 2010;72:253–276. [PubMed] [Google Scholar]
- 55.Rasmussen KM, Abrams B, Bodnar LM, Butte NF, Catalano PM, Maria Siega-Riz A. Recommendations for weight gain during pregnancy in the context of the obesity epidemic. Obstet Gynecol. 2010;116:1191–1195. doi: 10.1097/AOG.0b013e3181f60da7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guelinckx I, Devlieger R, Mullie P, Vansant G. Effect of lifestyle intervention on dietary habits, physical activity, and gestational weight gain in obese pregnant women: a randomized controlled trial. Am J Clin Nutr. 2010;91:373–380. doi: 10.3945/ajcn.2009.28166. [DOI] [PubMed] [Google Scholar]
- 57.Streuling I, Beyerlein A, von Kries R. Can gestational weight gain be modified by increasing physical activity and diet counseling? A meta-analysis of interventional trials. Am J Clin Nutr. 2010;92:678–687. doi: 10.3945/ajcn.2010.29363. [DOI] [PubMed] [Google Scholar]
- 58.Wolff S, Legarth J, Vangsgaard K, Toubro S, Astrup A. A randomized trial of the effects of dietary counseling on gestational weight gain and glucose metabolism in obese pregnant women. Int J Obes (Lond) 2008;32:495–501. doi: 10.1038/sj.ijo.0803710. [DOI] [PubMed] [Google Scholar]
- 59.Beddoe AE, Lee KA. Mind-body interventions during pregnancy. J Obstet Gynecol Neonatal Nurs. 2008;37:165–175. doi: 10.1111/j.1552-6909.2008.00218.x. [DOI] [PubMed] [Google Scholar]
- 60.Jaddoe VW. Antenatal education programmes: do they work? Lancet. 2009;374:863–864. doi: 10.1016/S0140-6736(09)61610-X. [DOI] [PubMed] [Google Scholar]