Abstract
Objective
The food contaminants bisphenol A (BPA), diethylhexylphthalate (DEHP), and tributyltin (TBT) are potent endocrine-disrupting compounds (EDC) known to interfere with adipogenesis. EDC usually act in mixtures and not as single compounds. The aim of this study was to investigate the effects of a simultaneous exposure of BPA, DEHP, and TBT on mesenchymal stem cell differentiation into adipocytes.
Methods
Multipotent murine mesenchymal stem cells (C3H10T1/2) were exposed to EDC mixtures in high concentrations, i.e. MIX-high (10 µmol/l BPA, 100 µmol/l DEHP, 100 nmol/l TBT), and in environmentally relevant concentrations, i.e. MIX-low (10 nmol/l BPA, 100 nmol/l DEHP, 1 nmol/l TBT). The exposure was performed either for the entire culture time (0-12 days) or at distinct stages of adipogenic differentiation. At day 12 of cell culture, the amount of adipocytes, triglyceride content (TG), and adipogenic marker gene expression were analyzed.
Results
MIX-high increased the development of adipocytes and the expression of adipogenic marker genes independently of the exposure window. The total TG amount was not increased. The low-concentrated EDC mixture had no obvious impact on adipogenesis.
Conclusion
In EDC mixtures, the adipogenic effect of TBT and DEHP predominates single effects of BPA. Mixture effects of EDC are not deducible from single compound experiments.
Key Words: Endocrine-disrupting compounds, EDC, Peroxisome proliferator-activated receptor γ, PPARγ, Adipogenesis, Mesenchymal stem cells, MSC
Introduction
The escalating global epidemic of overweight and obesity belongs to the most serious health challenges worldwide [1]. Its prevalence has nearly doubled within the last 30 years [2], and especially children show rising obesity rates [3]. Globally, 43 million preschool children (under 5 years of age) were overweight or obese in 2010 [4], marking an increase of 60% since 1990. It is likely that changes in fetal and early postnatal developmental conditions promote adipogenesis and obesity. Compared with diet and lifestyle, environmental factors, including exposure to xenobiotic compounds, are often neglected as potential reasons for obesity.
The increased incidence of obesity correlates with substantial changes in the chemical environment over the past 40 years [5]. The production of synthetic compounds continuously increased from 0.5 million tons per year in 1950 to 300 million tons per year today [6]. Synthetic polymers contain chemical additives like plasticizers, stabilizers, and softeners, which can easily leach from the polymer matrix. These additives are ingested by humans in daily life [7,8], and some of them show hormonal activity and may interfere with the endocrine system. Endocrine-disrupting compounds (EDC) like bisphenol A (BPA), diethylhexylphthalate (DEHP), and tributyltin (TBT) are ubiquitously detectable in human tissues and samples [9,10,11]. They affect hormonal pathways, which is a specifically critical hazard during development [12,13,14].
There are various studies which have shown that BPA, DEHP, and TBT affect the development of obesity in vivo and the differentiation of adipocytes in vitro [15,16,17,18,19]. The exposure of these EDC by maternal nutrition during gestation and nursing may program the development of obesity in utero and in early postnatal life [16,17,19]. BPA crosses the human placenta [20] and enriches as its active deconjugated form in the fetus. The capacity to metabolize BPA is reduced in the fetal liver [21]. Also, human DEHP exposure starts in utero [22]. Neonatal children in intensive care units are especially exposed to DEHP, as medical devices contain high concentrations of phthalates [23]. A specific treat of EDC are transgenerational effects due to interferences with epigenetic mechanisms [16,24,25].
In a previous study [15], we demonstrated the effects of BPA, DEHP, and TBT on mesenchymal stem cell (MSC) differentiation into adipocytes by exposing MSC to single compounds. The effects of the single compounds depended on the exposure window. Various ontogenetic stages of adipogenesis were differently sensitive to hormonal regulation and disruption. Adipogenesis can be either reduced (in the case of BPA) or enforced (DEHP, TBT) by EDC [15]. As exposure in daily life is not restricted to individual compounds, EDC are present in the environment as complex mixtures (‘real-world exposure’). The effects of individual EDC in mixtures can be additive, synergistic, or antagonistic [26].
So far, more than 95% of all toxicological research has been devoted to individual chemicals [27]. However, comparatively little is known about complex mixtures. Whereas combined effects of EDC belonging to the same category (e.g. estrogenic, antiandrogenic, or thyroid-disrupting agents) can be predicted by using dose addition, this is not true for EDC, which act by different mechanisms [27].
Here we show that the mixture of BPA, DEHP, and TBT affects adipogenesis at the stage of mesenchymal cell fate commitment of embryonic stem cells (C3H10T1/2). The impact on adipogenesis was dose-dependent and not predictable from the effects of each individual compound.
Material and Methods
Cell Lines
Prior to current exposure studies, we have tested that the cells express the peroxisome proliferator-activated receptors (PPAR) α and γ as well as the estrogen receptors ERα, ERβ, and G protein-coupled estrogen receptor 1 (data not shown).
Cell Culture and Reagents
The murine MSC line C3H10T1/2, purchased from ATCC-LGC (Wesel, Germany), was cultured and differentiated as described before [15]. All cell culture reagents were purchased from Invitrogen (Darmstadt, Germany) or Millipore (Schwalbach/Taunus, Germany). BPA, DEHP, and TBT were obtained from Sigma-Aldrich (Taufkirchen, Germany) and diluted in DMSO for exposure during in vitro culture. All studies were performed as n = 3 independent replicates. The concentrations of each EDC used in this study were not cytotoxic and did not affect cellular proliferation [15].
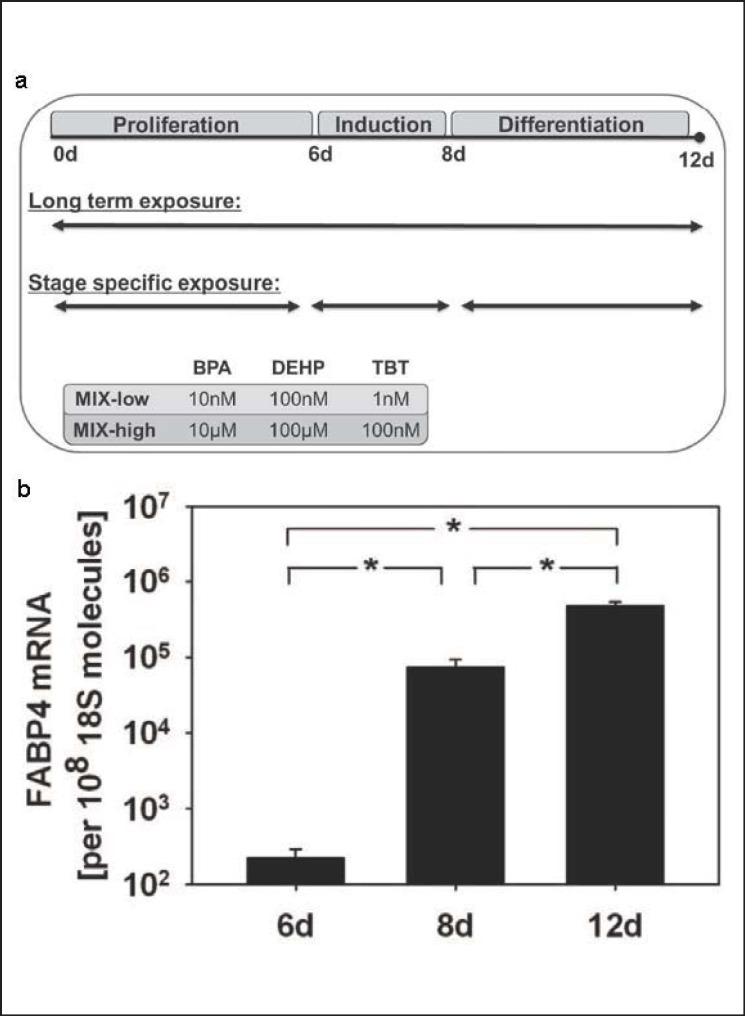
Two different experimental approaches were chosen (fig. 1a): i) long-time exposure of MSC during the entire differentiation protocol and ii) stage-specific exposures during proliferation (0-6 days), induction (6-8 days), or differentiation (8-12 days). Cells were exposed to a combination of the EDC in a low environmentally relevant concentration range (MIX-low; 10 nmol/l BPA, 100 nmol/l DEHP, 1 nmol/l TBT), a high LOAEL (lowest observed adverse effect level)-orientated range (MIX-high; 10 µmol/l BPA, 100 µmol/l DEHP, 100 nmol/l TBT), or 0.05% DMSO (solvent control) [15]. The medium was replaced every 2 days, and cells were washed with PBS.
Fig. 1.
a Schematic time schedule for adipogenic differentiation and EDC exposure of C3H10T1/2-MSC. Cells were cultured for 6 days until reaching 2 days post-confluence (proliferation). Adipogenic differentiation was induced by insulin 10 (µg/ml), dexamethasone (1 µmol/l), and IBMX (500 µmol/l) for 48 h (induction) and cells were terminally differentiated for 4 days including supplementation of insulin for 48 h (differentiation). Cells were exposed during the whole adipogenic differentiation period (long-term exposure) or stage-specifically during proliferation, induction, or differentiation to MIX-low (10 nmol/l BPA, 100 nmol/l DEHP, and 1 nmol/l TBT), MIX-high (10 µmol./l BPA, 100 µmol/l DEHP, and 100 nmol/l TBT), or 0.05% DMSO (vehicle control). b Verification of FABP4 as adipogenic marker gene. FABP4 mRNA amounts were quantified at day 6, day 8, and day 12 and presented as absolute transcript numbers related to the housekeeping gene 18S. All values are given as mean ± SEM; n = 3 replicates; *p < 0.05.
Assessment of Adipocyte Differentiation
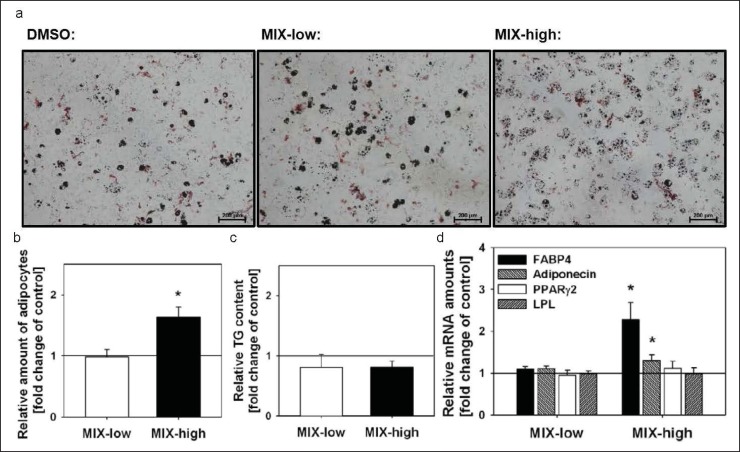
Lipid droplets were visualized after oil-red-o staining at day 12 as described by Wdziekonski et al. [28] (fig. 2a). For quantitative determination of the amount of adipocytes, flow cytometric analysis was used. Differentiated C3H10T1/2 were separated by using a solution of 0.25% (m/v) trypsin and 0.53 mmol/l EDTA and stained with Nile Red. The percentage of adipocytes was assessed using FACSCalibur (BD Biosciences, Heidelberg, Germany) as described before [29].
Fig. 2.
MIX-high exposure during the entire differentiation protocol increased the development of adipocytes but not the TG content. a Adipocytes were visualized at day 12 by oil-red-o staining, scale bar = 200 µm and b quantified by flow cytometry. c Total TG content. d MIX-high increased the mRNA amounts of FABP4 and adiponectin, but not PPARγ2 and LPL. No influence was observed in MIX-low treated cells. Data are presented as relative to the corresponding vehicle control, which is set 1 and indicated by a straight line. All values are given as mean ± SEM; n = 3 replicates; *p < 0.05.
Determination of Triglyceride Amount
2 × 105 cells/ml PBS were counted (Neubauer counting chamber) and sonicated. Lipids were extracted with chloroform and dissolved in Triton X-100. The triglyceride (TG) amounts were determined using Triglyceride FS (DiaSys, Holzheim, Germany).
Real-Time Polymerase Chain Reaction
Cells were homogenized using Precellys 24 tissue homogenizer (Bertin Technologies, Montigny le Bretonneux, France). Total RNA extraction and cDNA synthesis were performed as described before [30]. Quantitative real-time polymerase chain reaction (RT PCR) (StepOnePlus; Applied Biosystems, Darmstadt, Germany) was performed with primer sets for 18S, PPARγ2, adiponectin, fatty acid-binding protein 4 (FABP4), and lipoprotein lipase (LPL). The levels of mRNA encoding the indicated genes were normalized with ribosomal 18S rRNA and are presented as relative fold change to control. To assess the FABP4 mRNA expression pattern, the mRNA was quantified absolutely by use of standard curves of cDNA plasmid clones. A calibration curve of specific DNA probes, generated from cDNA plasmid clones, was used as an external standard in each run.
Statistical Analyses
The levels of significance between groups were calculated using the unpaired two-tailed Student's t-test after proving normal distribution by means of Shapiro-Wilk test (SigmaPlot v.11.0; Systat Software GmbH, Erkrath, Germany). Differences between groups were considered as statistically significant if p < 0.05. Data are presented as mean ± SEM (standard error of the mean); n = 3.
Results
Adipogenesis of MSC can be divided into three stages, i.e. i) discriminating undifferentiated growth, from day 0 to day 6, ii) hormonal adipogenic induction, from day 6 to day 8, and iii) final adipogenic differentiation, from day 8 to day 12 (fig. 1a). The exposure with MIX-low or MIX-high was performed either for the entire culture time from day 0 to day 12 or separately within the specified stages (fig. 1a). At day 12, the amount of adipocytes was visualized by oil-red-o staining and quantified by flow cytometry. The TG was determined and adipogenic marker gene expression was measured.
FABP4 was used and confirmed as marker for adipogenic differentiation of MSC. The mRNA amounts of FABP4 increased exponentially during the differentiation process (fig. 1b). During proliferation, when cells possess multipotent characteristics, the mRNA level was low. FABP4 mRNA transcripts increased by 1,000-fold during hormonal induction and by a further 10-fold during subsequent differentiation.
Effect of Low-Concentrated Mixture of EDC on Adipogenesis in MSC
Exposure with MIX-low during the entire differentiation protocol or during distinct exposure windows did not affect the resulting amount of adipocytes (fig. 2a, 2b, 3a), total TG accumulation (fig. 2c, 3b), and adipogenic marker gene expression (fig. 2d, 3c).
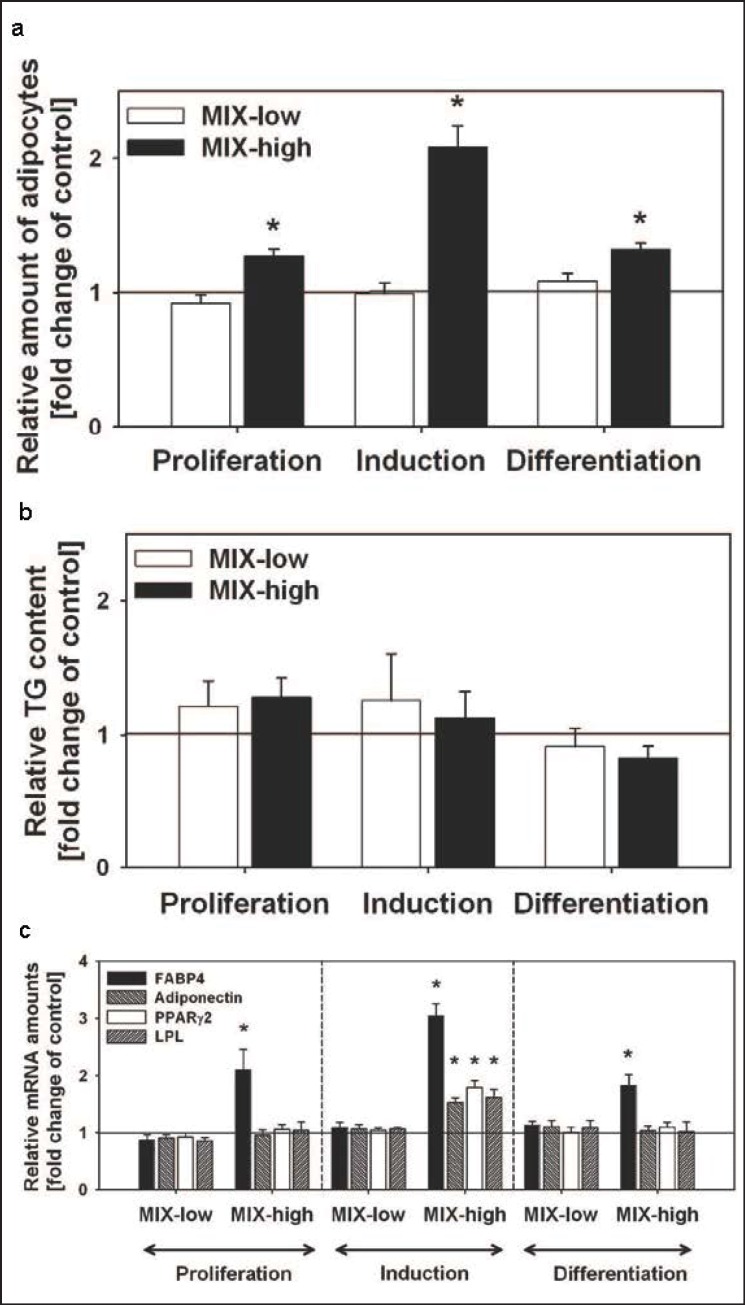
Fig. 3.
Independently of the exposure interval, MIX-high increased the development of adipocytes but not the TG content. a Adipocytes were quantified by flow cytometry. b Total TG content. c MIX-high increased the mRNA amounts of FABP4 in all investigated stages. Adiponectin, PPARγ2, and LPL mRNA amounts were only increased when cells were exposed during induction. No influence was observed in MIX-low treated cells. Data are presented as relative to the corresponding vehicle control, which is set 1 and indicated by a straight line. All values are given as mean ± SEM; n = 3 replicates; *p < 0.05.
Effect of High-Concentrated Mixture of EDC on Adipogenesis in MSC
C3H10T/2 cells were exposed during the entire differentiation protocol to MIX-high (fig. 1a). In the amounts of adipocytes, the treatment led to an increase to 163 ± 16% (fig. 2b). Accordingly, the adipogenic markers FABP4 and adiponectin were significantly increased to 227 ± 40% and 130 ± 13%, respectively (fig. 2d). There were no changes in total TG and mRNA expression of LPL and PPARγ2 (fig. 2c, 2d).
Adipogenic Effect of High-Concentrated Mixture of EDC Depending on the Exposure Interval
To discriminate EDC-susceptible differentiation stages, the adipogenic differentiation protocol was split into three exposure intervals (fig. 1a). In all separately studied windows, MIX-high led to an increase in the resulting number of adipocytes at day 12 (fig. 3a). The increased rate of adipocytes was closely correlated with the measured FABP4 mRNA amounts (fig. 3c). Exposure during proliferation enhanced the resulting amount of adipocytes to 127 ± 5% and FABP4 mRNA expression to 210 ± 35%, during hormonal induction to 208 ± 15% and 305 ± 21%, and during final differentiation to 132 ± 4% and 183 ± 18%, respectively. The adipogenic effect of MIX-high was mostly pronounced during hormonal induction, as exposure during that stage led to increased mRNA amounts for the adipogenic marker genes adiponectin (152 ± 8%), LPL (161 ± 15%), and PPARγ2 (179 ± 12%). None of the studied exposure windows affected the resulting total TG content of the cells (fig. 3b).
Discussion
C3H10T1/2 MSC have the ability to differentiate into the adipose lineage as well as into cartilage, bone, or muscle [31]. During adipogenesis, these cells pass through different ontogenetic stages (undifferentiated growth, adipogenic induction, terminal adipogenic differentiation) and are therefore a suitable model to study adipogenesis in vitro.
Recently, we have reported on the concentration-, stage-, and compound-specific effects of BPA, DEHP, and TBT as single compounds on adipogenic MSC differentiation [15]. Exposure of C3H10T1/2 during proliferation with BPA (10 µmol/l) decreased the resulting number of adipocytes, the total TG content, and the mRNA expression of adipogenic marker genes, whereas DEHP (100 µmol/l), during induction, and TBT (100 nmol/l), in all investigated stages, enhanced adipogenesis. In contrast to these LOAEL-orientated concentrations, environmentally relevant concentrations of 10 nmol/l BPA, 100 nmol/l DEHP, or 1 nmol/l TBT did not affect adipogenesis.
Taking into account that real-life EDC exposure occurs more likely as a ‘cocktail’ of chemical reagents than in the form of single compounds, the effect of a simultaneous exposure to a mixture of BPA, DEHP, and TBT on adipogenic MSC differentiation was investigated in this study. Independently of the exposure interval, the LOAEL-orientated EDC mixture, MIX-high, led to an enhanced development of adipocytes. The antiadipogenic effect of BPA during proliferation [15], when MSC commit to the adipogenic lineage, was overridden by the adipogenic action of DEHP and TBT. In contrast to MIX-high, treatment with the environmentally relevant mixture, MIX-low, did not show adipogenic or antiadipogenic effects.
FABP4, a classical adipogenic marker, whose protein expression is mostly controlled at the transcriptional level [32,33,34], correlated closely with the adipogenic differentiation process and the formation of adipocytes. Treatment of multipotent C3H10T1/2 with MIX-high during the entire differentiation protocol as well as during distinct ontogenetic stages revealed enhanced mRNA amounts of FABP4 as compared to corresponding controls. The adipogenic effect of MIX-high was mostly pronounced during hormonal induction. Treatment during this stage also led to increased mRNA amounts of the adipogenic marker genes PPARγ2, adiponectin, and LPL.
However, in contrast to the individual substances [15], MIX-high did not affect TG accumulation, although the development of adipocytes was clearly enhanced. This observation was made during the entire differentiation protocol as well as during the indicated exposure windows.
It has been shown that TBT and DEHP induce the adipogenic differentiation process by activating PPARγ [12,13,15]. As master regulator, this transcription factor regulates the final differentiation of adipocytes [35]. The interaction of DEHP and TBT during the adipogenic differentiation process may therefore target the same signaling cascade (PPARγ) and act synergistically, implying that individual substances of a chemical cocktail can ‘act together’ [27].
Regarding its mode of action, BPA belongs to a different class of EDC than DEHP and TBT, which share their hormonal action in terms of PPARγ activation. BPA is an estrogenic compound whose impact on adipogenesis is controversially discussed [36]. Masuno et al. [18] have shown that BPA triggers the terminal differentiation of 3T3 preadipocytes mediated by the PI3-kinase pathway. In the same cell line, it was reported that the enhanced development of adipocytes by BPA was not accompanied by changes in the total TG content [37]. Another study observed that BPA mediated a reduction in the LPL gene of human adult stem cells, which resulted in a decreased TG accumulation during adipogenic differentiation [38]. This is in accordance with our observation in C3H10T1/2 MSC [15] and with other in vitro studies, which have shown that estrogens and phytoestrogens inhibit adipogenic differentiation of mesenchymal precursor cells [39,40]. Furthermore, estrogenic signaling in female ovariectomized mice and in 3T3 preadipocytes was shown to blunt the adipogenic effects of PPARγ activators, which resulted in a reduced TG accumulation by a decreased DNA binding of PPARγ [40]. We therefore hypothesize that the interaction with the estrogenic compound BPA suppressed the adipogenic effects of DEHP and TBT during adipogenesis induced by MIX-high. This might explain why the adipogenic effects of MIX-high were far less pronounced than those induced by the individual compounds DEHP and TBT.
Considering the results of this study and the assumed modes of action of the single compounds, we suppose that MIX-high influences adipogenic differentiation by affecting at least two mechanisms, i.e. estrogenic [36,40] and PPAR-mediated signaling [12,13,15]. However, additional studies are necessary to elucidate the underlying mechanisms responsible for the findings that DEHP and TBT predominate single effects of BPA and that BPA in turn suppressed the adipogenic effects of DEHP and TBT within the mixture MIX-high.
In conclusion, we have shown that a mixture of selected endocrine-disrupting chemicals, representing different classes of EDC and affecting different mechanisms of hormonal actions, revealed an impact on adipogenesis which was not predictable by the effects of the individual compounds itself. The increasing appearance of EDC in our environment, their interactions, and the resulting influence on early cell fate development may contribute to the worldwide rising obesity rates.
Disclosure Statement
The authors declare no conflict of interest.
Acknowledgments
This work was supported by the German United Association for Clinical Chemistry and Laboratory Medicine (DGKL), the Wilhelm Roux Program of the Martin Luther University Faculty of Medicine, Halle (Saale), Germany, and the European Community's Seventh Framework Program under Grant Agreement No. 212885 (REEF). The authors thank Sabine Schroetter, Franziska Knoefel, and Christine Froehlich for excellent technical assistance.
References
- 1.Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132:2087–2102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 2.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, Farzadfar F, Riley LM, Ezzati M. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y, Lobstein T. Worldwide trends in childhood overweight and obesity. Int J Pediatr Obes. 2006;1:11–25. doi: 10.1080/17477160600586747. [DOI] [PubMed] [Google Scholar]
- 4.de Onis M, Blossner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr. 2010;92:1257–1264. doi: 10.3945/ajcn.2010.29786. [DOI] [PubMed] [Google Scholar]
- 5.Casals-Casas C, Desvergne B. Endocrine disruptors: from endocrine to metabolic disruption. Annu Rev Physiol. 2011;73:135–162. doi: 10.1146/annurev-physiol-012110-142200. [DOI] [PubMed] [Google Scholar]
- 6.Thompson RC, Moore CJ, vom Saal FS, Swan SH. Plastics, the environment and human health: current consensus and future trends. Philos Trans R Soc Lond B Biol Sci. 2009;364:2153–2166. doi: 10.1098/rstb.2009.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson NK, Chuang JC, Morgan MK, Lordo RA, Sheldon LS. An observational study of the potential exposures of preschool children to pentachlorophenol, bisphenol-A, and nonylphenol at home and daycare. Environ Res. 2007;103:9–20. doi: 10.1016/j.envres.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Kannan K, Takahashi S, Fujiwara N, Mizukawa H, Tanabe S. Organotin compounds, including butyltins and octyltins, in house dust from Albany, New York, USA. Arch Environ Contam Toxicol. 2010;58:901–907. doi: 10.1007/s00244-010-9513-6. [DOI] [PubMed] [Google Scholar]
- 9.Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect. 2005;113:391–395. doi: 10.1289/ehp.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silva MJ, Barr DB, Reidy JA, Malek NA, Hodge CC, Caudill SP, Brock JW, Needham LL, Calafat AM. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999-2000. Environ Health Perspect. 2004;112:331–338. doi: 10.1289/ehp.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nielsen JB, Strand J. Butyltin compounds in human liver. Environ Res. 2002;88:129–133. doi: 10.1006/enrs.2001.4321. [DOI] [PubMed] [Google Scholar]
- 12.Feige JN, Gelman L, Rossi D, Zoete V, Metivier R, Tudor C, Anghel SI, Grosdidier A, Lathion C, Engelborghs Y, Michielin O, Wahli W, Desvergne B. The endocrine disruptor monoethyl-hexyl-phthalate is a selective peroxisome proliferator-activated receptor gamma modulator that promotes adipogenesis. J Biol Chem. 2007;282:19152–19166. doi: 10.1074/jbc.M702724200. [DOI] [PubMed] [Google Scholar]
- 13.Kanayama T, Kobayashi N, Mamiya S, Nakanishi T, Nishikawa J. Organotin compounds promote adipocyte differentiation as agonists of the peroxisome proliferator-activated receptor gamma/retinoid x receptor pathway. Mol Pharmacol. 2005;67:766–774. doi: 10.1124/mol.104.008409. [DOI] [PubMed] [Google Scholar]
- 14.Rubin BS, Soto AM. Bisphenol A: perinatal exposure and body weight. Mol Cell Endocrinol. 2009;304:55–62. doi: 10.1016/j.mce.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biemann R, Navarrete Santos A, Riemann D, Knelangen J, Bluher M, Koch H, Fischer B. Endocrine disrupting chemicals affect the adipogenic differentiation of mesenchymal stem cells in distinct ontogenetic windows. Biochem Biophys Res Commun. 2012;417:747–752. doi: 10.1016/j.bbrc.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 16.Chamorro-Garcia R, Sahu M, Abbey RJ, Laude J, Pham N, Blumberg B. Transgenerational inheritance of increased fat depot size, stem cell reprogramming, and hepatic steatosis elicited by prenatal obesogen tributyltin in mice. Environ Health Perspect. 2013;121:359–366. doi: 10.1289/ehp.1205701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heindel JJ, vom Saal FS. Role of nutrition and environmental endocrine disrupting chemicals during the perinatal period on the aetiology of obesity. Mol Cell Endocrinol. 2009;304:90–96. doi: 10.1016/j.mce.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 18.Masuno H, Iwanami J, Kidani T, Sakayama K, Honda K. Bisphenol A accelerates terminal differentiation of 3T3-L1 cells into adipocytes through the phosphatidylinositol 3-kinase pathway. Toxicol Sci. 2005;84:319–327. doi: 10.1093/toxsci/kfi088. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt JS, Schaedlich K, Fiandanese N, Pocar P, Fischer B. Di(2-ethylhexyl) phthalate (DEHP) impairs female fertility and promotes adipogenesis in C3H/N mice. Environ Health Perspect. 2012;120:1123–1129. doi: 10.1289/ehp.1104016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balakrishnan B, Henare K, Thorstensen EB, Ponnampalam AP, Mitchell MD. Transfer of bisphenol A across the human placenta. Am J Obstet Gynecol. 2010;202:391–397. doi: 10.1016/j.ajog.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 21.Nahar MS, Liao C, Kannan K, Dolinoy DC. Fetal liver bisphenol A concentrations and biotransformation gene expression reveal variable exposure and altered capacity for metabolism in humans. J Biochem Mol Toxicol. 2013;27:116–123. doi: 10.1002/jbt.21459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Latini G, De Felice C, Presta G, Del Vecchio A, Paris I, Ruggieri F, Mazzeo P. In utero exposure to di-(2-ethylhexyl)phthalate and duration of human pregnancy. Environ Health Perspect. 2003;111:1783–1785. doi: 10.1289/ehp.6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koch HM, Preuss R, Angerer J. Di(2-ethylhexyl)phthalate (DEHP): human metabolism and internal exposure – an update and latest results. Int J Androl. 2006;29:155–165. doi: 10.1111/j.1365-2605.2005.00607.x. discussion 181-185. [DOI] [PubMed] [Google Scholar]
- 24.Bernal AJ, Jirtle RL. Epigenomic disruption: the effects of early developmental exposures. Birth Defects Res A Clin Mol Teratol. 2010;88:938–944. doi: 10.1002/bdra.20685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci U S A. 2007;104:13056–13061. doi: 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rajapakse N, Silva E, Kortenkamp A. Combining xenoestrogens at levels below individual no-observed-effect concentrations dramatically enhances steroid hormone action. Environ Health Perspect. 2002;110:917–921. doi: 10.1289/ehp.02110917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kortenkamp A. Ten years of mixing cocktails: a review of combination effects of endocrine-disrupting chemicals. Environ Health Perspect. 2007;115(suppl 1):98–105. doi: 10.1289/ehp.9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wdziekonski B, Villageois P, Dani C. Development of adipocytes from differentiated ES cells. Methods Enzymol. 2003;365:268–277. doi: 10.1016/s0076-6879(03)65019-6. [DOI] [PubMed] [Google Scholar]
- 29.Schaedlich K, Knelangen JM, Navarrete Santos A, Fischer B. A simple method to sort ESC-derived adipocytes. Cytometry A. 2010;77:990–995. doi: 10.1002/cyto.a.20953. [DOI] [PubMed] [Google Scholar]
- 30.Navarrete Santos A, Tonack S, Kirstein M, Kietz S, Fischer B. Two insulin-responsive glucose transporter isoforms and the insulin receptor are developmentally expressed in rabbit preimplantation embryos. Reproduction. 2004;128:503–516. doi: 10.1530/rep.1.00203. [DOI] [PubMed] [Google Scholar]
- 31.Pinney DF, Emerson CP., Jr 10T1/2 cells: an in vitro model for molecular genetic analysis of mesodermal determination and differentiation. Environ Health Perspect. 1989;80:221–227. doi: 10.1289/ehp.8980221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glatz JF, van der Vusse GJ. Cellular fatty acid-binding proteins: their function and physiological significance. Prog Lipid Res. 1996;35:243–282. doi: 10.1016/s0163-7827(96)00006-9. [DOI] [PubMed] [Google Scholar]
- 33.Tontonoz P, Graves RA, Budavari AI, Erdjument-Bromage H, Lui M, Hu E, Tempst P, Spiegelman BM. Adipocyte-specific transcription factor ARF6 is a heterodimeric complex of two nuclear hormone receptors, PPAR gamma and RXR alpha. Nucleic Acids Res. 1994;22:5628–5634. doi: 10.1093/nar/22.25.5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang VW, Christy RJ, Cook JS, Kelly TJ, Lane MD. Mechanism of regulation of the 422(aP2) gene by cAMP during preadipocyte differentiation. Proc Natl Acad Sci U S A. 1989;86:3629–3633. doi: 10.1073/pnas.86.10.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- 36.Andersen HR, Andersson AM, Arnold SF, et al. Comparison of short-term estrogenicity tests for identification of hormone-disrupting chemicals. Environ Health Perspect. 1999;107(suppl 1):89–108. doi: 10.1289/ehp.99107s189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phrakonkham P, Viengchareun S, Belloir C, Lombes M, Artur Y, Canivenc-Lavier MC. Dietary xenoestrogens differentially impair 3T3-L1 preadipocyte differentiation and persistently affect leptin synthesis. J Steroid Biochem Mol Biol. 2008;110:95–103. doi: 10.1016/j.jsbmb.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 38.Linehan C, Gupta S, Samali A, O'Connor L. Bisphenol A-mediated suppression of LPL gene expression inhibits triglyceride accumulation during adipogenic differentiation of human adult stem cells. PLoS One. 2012;7:e36109. doi: 10.1371/journal.pone.0036109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim MH, Park JS, Seo MS, Jung JW, Lee YS, Kang KS. Genistein and daidzein repress adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells via Wnt/beta-catenin signalling or lipolysis. Cell Prolif. 2010;43:594–605. doi: 10.1111/j.1365-2184.2010.00709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jeong S, Yoon M. 17beta-estradiol inhibition of PPARgamma-induced adipogenesis and adipocyte-specific gene expression. Acta Pharmacol Sin. 2011;32:230–238. doi: 10.1038/aps.2010.198. [DOI] [PMC free article] [PubMed] [Google Scholar]