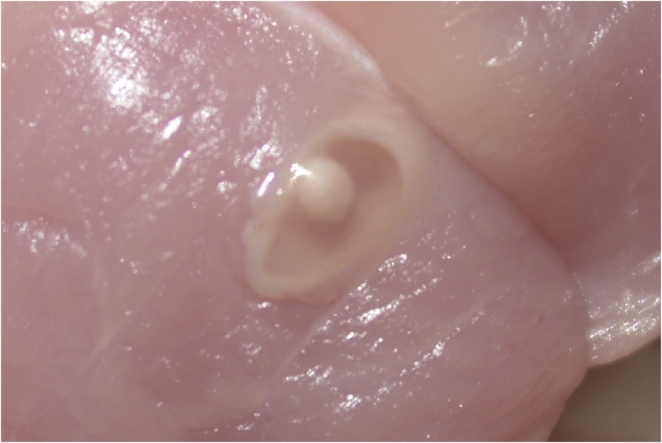
Graphical abstract
Keywords: Taenia solium, Cysticercosis, Pig, Nepal, Banke, Necropsy
Highlights
-
•
The prevalence of porcine cysticercosis was determined in pigs from the Banke District of Nepal.
-
•
Slaughter-age animals were selected at random and underwent detailed necropsy examination.
-
•
Thirty two of 110 animals were found to harbour T. solium cysticerci.
-
•
This is one of the highest levels of porcine cysticercosis described for any region in the world.
-
•
Measures are warranted to control this zoonotic disease in Nepal.
Abstract
Neurocysticercosis is a major cause of epilepsy in countries where Taenia solium is endemic and the parasite is a major cause of food-borne disease globally. Pigs are the natural intermediate host involved in transmission of the parasite. T. solium is known to be endemic in Nepal, however there is limited reliable data about the prevalence of the disease in Nepal. The aim of this study was to determine accurately the prevalence of porcine cysticercosis in slaughter age pigs in an area of Nepal where pigs are known to be free-roaming. Pigs were obtained from the Udaypur Village Development Committee (VDC) and Hirminiya & Betahani VDC of the Banke district in Nepal. One hundred and ten animals of slaughter age (approximately 8–16 months old) were purchased, slaughtered and the heart, liver, brain and half the body skeletal musculature were sliced using hand knives and the number and viability of T. solium cysts determined. Thirty two of the 110 animals were found to harbour T. solium cysticerci (29%), of which 30 (27%) were found to have viable cysticerci (93% of the infected animals). This is one of the highest prevalences of porcine cysticercosis that has been reported to date from the results of necropsy on randomly selected animals. This study highlights a high rate of transmission of T. solium in the Banke District of Nepal. It encourages further investigation of human and porcine cysticercosis in Nepal, as well as implementation of efforts to reduce transmission of the parasite and the associated human disease.
1. Introduction
Taenia solium is a zoonotic cestode parasite which causes cysticercosis in humans. Cysticercosis is a major cause of epilepsy in many developing countries; Ndimubanzi et al. (2010) identified 29% of seizure cases to be associated with neurocysticercosis in T. solium endemic regions. The disease is formally recognised by the WHO as a Neglected Tropical Disease (WHO, 2010), ranked as the most important food-borne parasitic infection from a global perspective by the Food and Agriculture Organization of the United Nations (Robertson et al., 2013) and accounts for the largest proportion of the global burden of disease caused by foodborne parasites (Havelaar et al., 2015).
T. solium is transmitted in a life cycle between humans, which act as the obligate definitive host, and pigs which act as the intermediate host. Full transmission of the life cycle of T. solium occurs in poor countries where pigs roam free and where meat hygiene and cultural practices favour the ingestion of poorly cooked pig meat harbouring the larval cysts.
Human neurocysticercosis is a frequently diagnosed condition in Nepal (Devleesschauwer et al., 2012, Joshi et al., 2007, Joshi et al., 2004, Joshi et al., 2001), where it had been estimated to be responsible for the highest burden of disease caused by a parasitic zoonosis (Devleesschauwer et al., 2014). Porcine cysticercosis is known to occur in Nepal (Joshi et al., 2007, Joshi et al., 2001) however relatively little information is available about the prevalence of T. solium infection in pigs. Devleesschauwer et al. (2013b) undertook a serological survey of porcine cysticercosis in pigs slaughtered in the Kathmandu valley, finding an infection rate of up to 28%. Maharjan and Gaihre (2010) performed serology on samples from pigs in the Syangja District of the Western Development Region in Nepal and found 23.5% to be positive. The antigen ELISA and EITB serological methods that were used in these studies are now understood to have a higher level of false positive reactions in pigs from T. solium endemic areas (Devleesschauwer et al., 2013a, Gavidia et al., 2013, Jayashi et al., 2014, Lightowlers et al., 2016) than had previously been understood, hence the reliability of these data is unclear. The only reliable and specific method currently available for determining the prevalence of porcine cysticercosis is a detailed post mortem carcase dissection that detects cysticerci in the tissues (Lightowlers et al., 2016).
The Banke district is one of 75 districts in Nepal, located in the mid-western part of the country bordering Indian Uttar Pradesh. It is a largely rural district with 85% of its approximately 0.5 million population living in rural villages (Ministry of Agriculture and Development, Nepal, 2016). Pigs are maintained under traditional husbandry conditions by the Khatik communities, where the animals are allowed to roam freely during the daylight hours and containment in a conventional enclosure at night time. Toilets are generally not present in the communities or, where they are present, they are often not used. Pigs may be kept in stys constructed using mud and bricks, the roof usually being straw. There is limited ventilation in these stys, making conditions inside damp and humid. The animals are often left to scavenge through the country side and the suburbs, but they may also be fed. The primary sources of water are boreholes and sanitation is neglected. Farmers do not use any anthelmintic for their animals. There is a paucity of awareness regarding taeniasis/cysticercosis among these communities. Commonly, the pigs are slaughtered on-farm where they were reared and the pork, without any inspection, enters into the human food chain, through local markets. Animals are also sold to slaughterhouses in regional centres where meat inspection may identify and condemn carcases heavily infected with T. solium.
In order to determine accurately the prevalence of porcine cysticercosis in a region of Nepal where T. solium transmission was thought likely to occur, a sample of pigs from the Banke district was investigated by detailed necropsy examination of selected organs and half the carcase musculature. The investigation was undertaken as part of a base-line study examining the effectiveness of a vaccination and chemotherapy intervention for porcine cysticercosis.
2. Materials and methods
2.1. Study design
A baseline survey was conducted in 184 pig rearing households in Udaypur Village Development Committee (VDC) and Hirminiya & Betahani VDC of the Banke district in Nepal (81°37′E–81°42′E, 27°90′N–28°20′N). These regions were selected because they were known to contain Dalit communities having many free-roaming pigs. The GPS coordinates of households were recorded together with household particulars, and a questionnaire completed concerning use of latrines or toilets, pig management and care, and knowledge and awareness of taeniosis and neurocysticercosis in humans and of cysticercosis in pigs. A randomized list of 110 household was obtained and one slaughter weight pig from each household was purchased in order to undertake post mortem examination. Typically, the households had only a single slaughter-weight animal that was available for purchase. The animals selected for post mortem may have been confined for part of the day, or of the year, but were not housed or confined for their entire life span. These included 55 animals from Udaipur, 31 animals from Hirmaniya and 24 animals from Betahani. The majority of the pigs were indigenous breeds. The age of the animals was recorded based on advice from the animal owners. The animal weight was estimated by experienced staff.
2.2. Post mortem procedures
The animals were transported to a licensed commercial abattoir in Nepalgunj Municipality, Banke where they were euthanized by slaughter house staff according to normal commercial practices. The viscera were removed and the heart, liver, both kidneys and the full diaphragm retained in numbered containers. The carcase was divided cranio-caudally. The organs and the right hand half of the carcase, including the complete head, were refrigerated overnight at 4 °C, after which the carcase was skinned. The head was removed and the tongue, masticatory muscles (both right and left sides) and brain removed and retained. The muscles from the right hand side of the carcase were dissected from the bones, keeping separate the muscles of the forelimb.
2.3. Examination for Taenia solium cysts
Except in cases of very heavy infection, all the retained organs and muscles were sliced by hand at intervals of approximately 3 mm and examined meticulously for the presence of T. solium cysticerci or other lesions. Cysticerci were recorded as viable where they were translucent vesicles filled with transparent fluid and having a visible white scolex. Non-viable lesions were recorded separately in cases where vesicles were non-translucent, containing a dense white or yellowish fluid and having no scolex and in cases of fibrosed or calcified lesions. In cases where it was clear that a carcase contained thousands of cysts, all of the heart, liver, kidneys, diaphragm, tongue, masticatory muscles, forelimb and brain were sliced and counted as above. The remaining carcase musculature was weighed and representative samples from different muscle sites were selected representing approximately 1 kg. This sample was weighed accurately and then sliced and counted as above.
2.4. Estimation of the burden of Taenia solium in each carcase
For carcases where all the musculature from the right half of the carcase was sliced, the numbers of cysticerci in the whole carcase were estimated by doubling the number recorded in the carcase half that was sliced, and adding the number recorded for the full diaphragm, tongue, masticatory muscles, heart, liver, kidneys and brain. For carcases having very heavy levels of infection, the total carcase burden was estimated by adding the numbers for the diaphragm, tongue, masticatory muscles, heart, liver, kidneys, and brain plus twice the number found in the foreleg, plus the numbers found in the 1 kg sample multiplied by the total weight of carcase musculature that was sampled.
2.5. Definition of a case of confirmed porcine cysticercosis
An animal was determined to be a confirmed case of porcine cysticercosis if one or more viable cysticerci were found in the muscle and or the brain, or if more than one non-viable lesion was detected in the muscles and/or brain.
3. Results
3.1. Survey
The survey of 110 households found that they held a total of 578 pigs. The majority of households (80.9%) did not have access to latrine and 95% of the households confirmed that their pigs had access to human faeces. Most of the pigs (92.7%) were of indigenous breeds and 7.3% were crossbreed. Most pigs were free ranging and 89% penned the animals at night, 2.7% allowed their animals to be permanently free ranging, 2.7% tethered the pigs and 5.5% of the animals were reared intensively. The major reason for rearing pigs was for sale to the local market (85.5%) with 13.6% being for both home consumption and sale. An awareness regarding tapeworm infection was found in 34.5% of the households and 16.4% recognised a relationship between eating raw/undercook pork and being infected with tapeworm. Almost all households (91.8%) affirmed that they had found cysts in pig meat during meat preparation. Most households (88.2%) were aware of epilepsy, headache and skin nodules and 16% were able to relate tapeworm infection with symptoms such as epilepsy, headache and skin nodules.
3.2. Post mortem examination
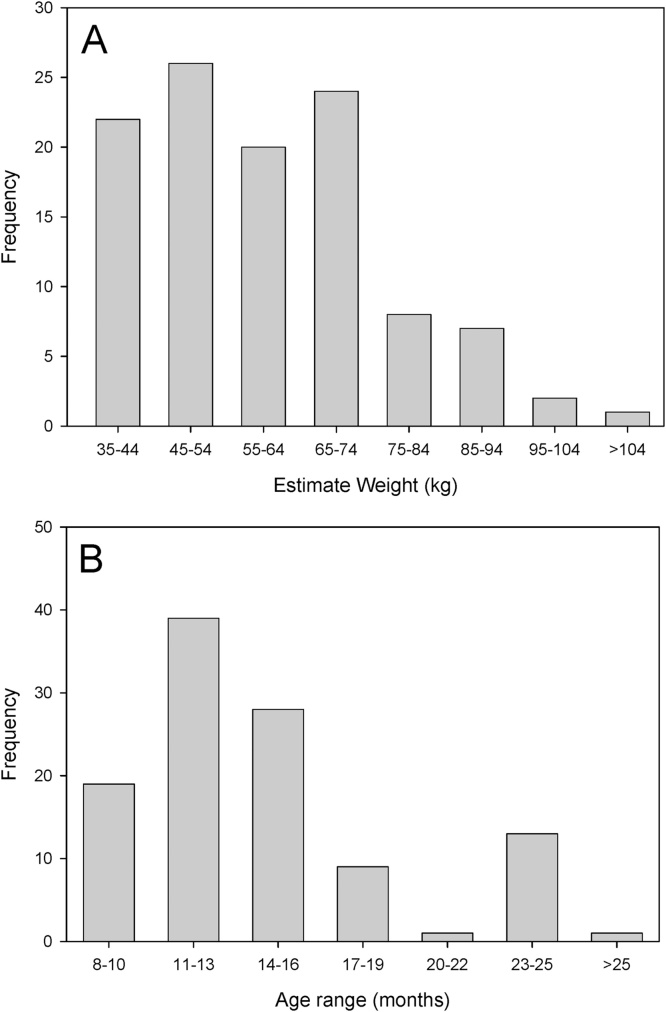
Animals selected for slaughter ranged between 35 and 107 kg in weight and 8–25 months of age (Fig. 1A and B). Necropsy findings are detailed in Table 1, Table 2. Thirty two of the 110 animals that were examined were found to harbour T. solium cysticerci (29%), of which 30 (27%) were found to have viable cysticerci (93% of the infected animals). Four animals were found to have lesions but were not recorded as being confirmed cases of T. solium infection; three were recorded to have a single non-viable lesion in the muscle tissue and one was found to have two viable lesions in the liver only.
Fig. 1.
Characteristics of pigs necropsied for Taenia solium infection. Weight (A) and age (B) distributions of pigs from the Banke Region, Nepal, which underwent detailed necropsy examination for Taenia solium infection.
Table 1.
Numbers of Taenia solium cysticerci in individual, infected pigs among 110 animals subjected to post mortem examination and slicing of selected carcase sites, including all the carcase skeletal musculature from the right hand side of the carcase. Animals were 8–25 (mean 14.5) months of age from the Nepaljung region of Nepal. Cysts were detected by slicing predilection sites and half the remaining body skeletal musculature. Numbers of viable cysts are shown for various carcase locations.
| Animal ID | Age (months) | Estimated total cysts (full carcass V+ NV) | Viable cysts (% all sites) | Cysts detected at necropsy (specified sites) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tongue | Masseter | Diaphragm | Heart | Liver | Brain | Right forelimb | Remaining half carcass | ||||
| U-20 | 10 | 3245 | 90.0 | 33 | 212 | 17 | 4 | 1 | 2 | 518 | 970 |
| U-24 | 18 | 1318 | 99.5 | 0 | 30 | 27 | 0 | 0 | 17 | 253 | 369 |
| U-13 | 12 | 1235 | 100.0 | 7 | 85 | 5 | 1 | 0 | 41 | 171 | 377 |
| U-71 | 18 | 737 | 100.0 | 11 | 76 | 2 | 1 | 1 | 0 | 97 | 226 |
| B-77 | 13 | 647 | 10.7 | 0 | 18 | 0 | 0 | 2 | 5 | 157 | 154 |
| B-80 | 16 | 500 | 96.6 | 2 | 13 | 6 | 0 | 1 | 0 | 136 | 103 |
| U-9 | 15 | 430 | 100.0 | 2 | 6 | 0 | 0 | 0 | 0 | 94 | 117 |
| H-72 | 15 | 49 | 100.0 | 2 | 6 | 0 | 0 | 0 | 1 | 9 | 11 |
| U-14 | 25 | 45 | 0.0 | 1 | 0 | 0 | 0 | 28 | 0 | 4 | 4 |
| H-39 | 12 | 31 | 100.0 | 0 | 0 | 1 | 0 | 0 | 0 | 8 | 7 |
| B-89 | 12 | 21 | 23.8 | 0 | 0 | 0 | 0 | 1 | 0 | 4 | 6 |
| H-110 | 12 | 17 | 76.5 | 1 | 3 | 0 | 0 | 0 | 1 | 4 | 2 |
| H-57 | 8 | 16 | 100.0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 4 |
| U-8 | 15 | 13 | 100.0 | 1 | 0 | 0 | 0 | 0 | 0 | 3 | 3 |
| U-11 | 12 | 12 | 100.0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 5 |
| U-65 | 20 | 11 | 90.9 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 5 |
| H-37 | 11 | 11 | 100.0 | 0 | 0 | 1 | 0 | 0 | 0 | 4 | 1 |
| B-86 | 12 | 9 | 0.0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 2 |
| U-12 | 15 | 6 | 100.0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 |
| U-15 | 8 | 4 | 100.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| U-67 | 24 | 4 | 100.0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| B-87 | 12 | 4 | 100.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| U-70 | 18 | 3 | 100.0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 |
| U-99 | 15 | 3 | 100.0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| B-78 | 24 | 3 | 33.3 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| U-34 | 12 | 2 | 100.0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| U-66 | 24 | 2 | 100.0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 |
| U-4 | 12 | 2 | 100.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| U-30 | 11 | 2 | 100.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| U-64 | 15 | 2 | 100.0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| H-49 | 10 | 2 | 100.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| B-85 | 12 | 2 | 100.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Animal ID: first letter corresponds to source (Udaypur, Hirminiya or Betahani); V: viable; NV: non-viable.
Table 2.
Intensity of T. solium infection in the muscle tissues of individual pigs from the Banke Region, Nepal.
| Intensity of infection (number of cysticerci in muscle tissues) | Number of animals | Proportion (%) |
|---|---|---|
| 1–10 | 15 | 49 |
| 11–50 | 9 | 29 |
| 51–200 | 0 | 0 |
| 201–500 | 2 | 6 |
| 501–2000 | 4 | 13 |
| 2001–5000 | 1 | 3 |
| >5000 | 0 | 0 |
The prevalences of infection in the three VDC were 35% in Udaipur (19/55 animals), 19% in Hirmaniya (6/31 animals) and 29% in Betahani (7/24 animals). The differences between the VDC were not statistically significantly different (Fisher’s Exact test p > 0.05).
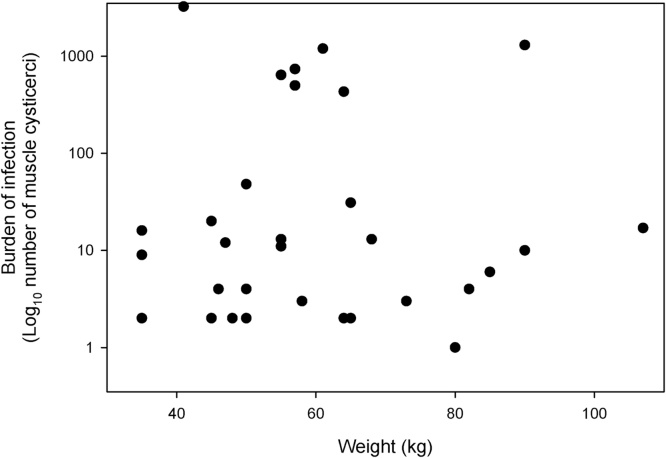
There was no significant relationship between the burden of T. solium cysticerci in the muscle tissues and the weight of each animal (r = −0.07558, p = 0.68; Pearson correlation coefficient; Fig. 2) or the age of each animal (r = −0.11637, p = 0.53; Pearson correlation coefficient)
Fig. 2.
Pig age vs burden of Taenia solium infection relationship. Relationship between the burden of T. solium infection in the muscle tissues of individual pigs from the Banke Region, Nepal and the estimated body weight of the animals.
4. Discussion
The Banke Region in Nepal was found to have one of the highest prevalences of T. solium infection in pigs of any region of the world where prevalence has been determined by detailed necropsy. More than a quarter of the slaughter age pigs (27%) which were necropsied were found to harbour viable T. solium cysts in their muscles. The great majority of the animals were reared for sale to the regional centre, Nepalgunj, a city of approximately 130,000 inhabitants. These animals present a serious risk for transmission of T. solium to the local communities and clearly reflect a significant level of taeniasis, and very likely neurocysticercosis, in the villages from which the animals were derived.
The survey conducted among the households from which pigs were purchased for necropsy identified many risk factors for transmission of T. solium, including a lack of availability or use of latrines, free-ranging pigs and little knowledge about the relationship between personal hygiene, T. solium infections in pigs or the symptoms of neurocysticercosis in humans. The Khatik community are predominantly farmers who rely heavily on keeping pigs as a source of food and income. The majority of these communities do not use latrines. Other communities similar to the Khatik, are present throughout the Southern parts of Nepal and the bordering area of Bihar,Uttar Pradesh, India. Traditionally, they keep indigenous breed of pig and feed mostly through scavenging. Pigs are slaughtered and consumed locally as well as sold to enter the formal meat market. Dom, Dushadh, and Mushahar are communities known to practice similar pig rearing practices like the Khatik and are found from east to west southern part of Nepal as well as in Uttar Pradesh and Bihar.
In determining the prevalence of porcine cysticercosis in this study, the assessment was made on the basis of detailed post mortem examination. Five similar assessments have been published of the prevalence of porcine cysticercosis in randomly selected, naturally exposed pigs. The highest prevalence of porcine cysticercosis was recorded in Zambia where 31 of 61 (47.7%) pigs were found to have cysticercosis (Dorny et al., 2004). In Mexico 19 of 120 animals (15.8%) were identified with T. solium cysticerci (Huerta et al., 2001), while in Cameroon 20 of 102 animals were found to be infected (19.6%) (Assana et al., 2010) and in Peru 18 of 107 pigs (16.8%) (Jayashi et al., 2012) and 18 of 326 pigs (5.5%) were found infected (Garcia et al., 2016). These data, which ware based on detailed necropsy examinations, provide reliable values on the prevalence of porcine cysticercosis. Recent evidence has cast doubt on the reliability of reports describing a high prevalence of porcine cysticercosis where the reports had been based on use of serological methods (Lightowlers et al., 2016). For example, in an extensive study conducted in northern Peru, 55.2% of 326 pigs were found to be positive by serology whereas only 5.5% were confirmed as being infected when the entire carcase musculature, liver and brain were sliced to reveal cysticerci (Garcia et al., 2016). Other studies have found a high rate of positive reactions in serological tests for porcine cysticercosis with sera from uninfected pigs (Devleesschauwer et al., 2013a, Gavidia et al., 2013, Jayashi et al., 2014).
Amongst the pigs that were examined from the Banke region, four animals in which lesions were identified were not included among those that were considered to have T. solium infection. These included three animals in which a single non-viable lesion was identified in the tissues examined. The study did not include histological or molecular analyses that may have been able to confirm the nature of these lesions. Little attention has been paid to the nature of rare fibrotic or calcified lesions in the muscle tissues of pigs, however in cattle, muscle lesions with causes other than cestode cysticerci have been described (Bockeler and Friedland, 1990, Gracey and Collins, 1992, Ogunremi et al., 2004, van der Logt and Gottstein, 2000). A similar situation is likely to be the case in pigs and, for this reason, animals detected with only a single non-viable lesion in the muscle tissues were not classified as having been infected with T. solium. Two other animals were found with only non-viable lesions, one with 9 lesions and one with 17 lesions. Given the number of lesions and the low likelihood that these could have any other cause than T. solium, these animals were included among those considered to have been infected with T. solium. One animal was recorded as having two viable cysticerci in the liver only. T. hydatigena, T. saginata and T. asiatica and T. solium are all known to have the capacity to encyst in the liver of pigs (discussed in Lightowlers et al., 2016). Testing to confirm the identity of liver lesions was not undertaken in this study, hence cysticerci in the liver were not recorded as being T. solium and the one individual animal having only cysticerci in the liver was not recorded as having T. solium infection.
The prevalence of porcine cysticercosis in the animals that were investigated in this study is likely to have been underestimated because not all the carcase skeletal musculature was sliced, due to limitations in resources that were available to undertake the post mortem studies. Vargas Mendez et al. (1986) found no difference in the distribution of T. solium cysts between the left and right sides of a pig carcase, hence some of the pigs from the Banke region that were found to have no cysts may have had light infections which, by probability, were present only in the carcase musculature which was not examined.
The prevalence of porcine cysticercosis described here in pigs from the Banke District of Nepal indicates that there is a high rate of T. solium transmission in the region and, very likely, a high rate of human cysticercosis. Further studies are warranted to determine the burden of human neurocysticercosis in the region as well as implementation of control measures to reduce transmission of the parasite (Lightowlers, 2013, Lightowlers and Donadeu, 2017).
Acknowledgements
The authors acknowledge the logistic supports made available by the Heifer Nepal team and administrative support from District Livestock Service Office (DLSO), Nepalgunj, Banke. Valuable contributions were made by the following government veterinary officers: Dr Bed Bahadur KC Senior Veterinary Officer, District Livestock Service Office, Banke, Nepal, Dr Krishna Raj Pandey Senior Veterinary Officer, Regional Veterinary Laboratory, Surkhet, Nepal, Dr Sankar Pandey Senior Veterinary Officer, Animal Quarantine Office, Nepalgunj, Nepal and Dr Dinesh Kumar Yadav Animal Scientist, National Agriculture Research Council, Khajura, Nepal. Participants in the necropsy team included Rakesh Chand, Niraj Dhakal, Manish Gautum, Bedika Ghising, Jitendra Lama, Sikesh Manandhar, Sanjay Poudel, Saroj Shrestha, Saurav Shrestha and Kaberi Sing. The project was funded in part by the Bill & Melinda Gates Foundation and the UK Government. The findings and conclusions contained within are those of the authors and do not necessarily reflect positions or policies of the Bill & Melinda Gates Foundation nor the UK Government. Funding to MWL is acknowledged from Australian National Health and Medical Research Council grant GTN1105448. The funding bodies played no role in design of the project, collection, analysis or interpretation of data, in writing the report or in the decision to submit the article for publication.
References
- Assana E., Kyngdon C.T., Gauci C.G., Geerts S., Dorny P., De Deken R., Anderson G.A., Zoli A.P., Lightowlers M.W. Elimination of Taenia solium transmission to pigs in a field trial of the TSOL18 vaccine in Cameroon. Int. J. Parasitol. 2010;40:515–519. doi: 10.1016/j.ijpara.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockeler W., Friedland T. Is bovine cysticercosis due only to infection with Taenia saginata? [Ist eine Rinderzystizerkose allein auf Taenia saginata-infektionen zuruckzufuhren?] Mitteilungen der Osterreichischen Gesellschaft fur Tropenmedizin und Parasitologie. 1990;12:81–85. [Google Scholar]
- Devleesschauwer B., Aryal A., Joshi D.D., Rijal S., Sherchand J.B., Praet N., Speybroeck N., Duchateau L., Vercruysse J., Dorny P. Epidemiology of Taenia solium in Nepal: is it influenced by the social characteristics of the population and the presence of Taenia asiatica? Trop. Med. Int. Health. 2012;17:1019–1022. doi: 10.1111/j.1365-3156.2012.03017.x. [DOI] [PubMed] [Google Scholar]
- Devleesschauwer B., Aryal A., Tharmalingam J., Joshi D.D., Rijal S., Speybroeck N., Gabriel S., Victor B., Dorny P. Complexities in using sentinel pigs to study Taenia solium transmission dynamics under field conditions. Vet. Parasitol. 2013;193:172–178. doi: 10.1016/j.vetpar.2012.12.010. [DOI] [PubMed] [Google Scholar]
- Devleesschauwer B., Pruvot M., Joshi D.D., De Craeye S., Jennes M., Ale A., Welinski A., Lama S., Aryal A., Victor B., Duchateau L., Speybroeck N., Vercruysse J., Dorny P. Seroprevalence of zoonotic parasites in pigs slaughtered in the Kathmandu Valley of Nepal. Vector Borne Zoonotic Dis. 2013;13:872–876. doi: 10.1089/vbz.2013.1313. [DOI] [PubMed] [Google Scholar]
- Devleesschauwer B., Ale A., Torgerson P., Praet N., Maertens de Noordhout C., Pandey B.D., Pun S.B., Lake R., Vercruysse J., Joshi D.D., Havelaar A.H., Duchateau L., Dorny P., Speybroeck N. The burden of parasitic zoonoses in Nepal: a systematic review. PLoS Negl. Trop. Dis. 2014;8:e2634. doi: 10.1371/journal.pntd.0002634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorny P., Phiri I.K., Vercruysse J., Gabriel S., Willingham A.L., III, Brandt J., Victor B., Speybroeck N., Berkvens D. A Bayesian approach for estimating values for prevalence and diagnostic test characteristics of porcine cysticercosis. Int. J. Parasitol. 2004;34:569–576. doi: 10.1016/j.ijpara.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Garcia H.H., Gonzalez A.E., Tsang V.C., O’Neal S.E., Llanos-Zavalaga F., Gonzalvez G., Romero J., Rodriguez S., Moyano L.M., Ayvar V., Diaz A., Hightower A., Craig P.S., Lightowlers M.W., Gauci C.G., Leontsini E., Gilman R.H., Cysticercosis Working Group in P Elimination of Taenia solium transmission in Northern Peru. N. Engl. J. Med. 2016;374:2335–2344. doi: 10.1056/NEJMoa1515520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavidia C.M., Verastegui M.R., Garcia H.H., Lopez-Urbina T., Tsang V.C., Pan W., Gilman R.H., Gonzalez A.E., Cysticercosis Working Group in Peru Relationship between serum antibodies and Taenia solium larvae burden in pigs raised in field conditions. PLOS Negl. Trop. Dis. 2013;7:e2192. doi: 10.1371/journal.pntd.0002192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracey J.F., Collins D.F. Ballière Tindall; London, UK: 1992. Meat Hygiene. [Google Scholar]
- Havelaar A.H., Kirk M.D., Torgerson P.R., Gibb H.J., Hald T., Lake R.J., Praet N., Bellinger D.C., de Silva N.R., Gargouri N., Speybroeck N., Cawthorne A., Mathers C., Stein C., Angulo F.J., Devleesschauwer B., World Health Organization Foodborne Disease Burden Epidemiology Reference Group World Health Organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med. 2015;12:e1001923. doi: 10.1371/journal.pmed.1001923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta M., de Aluja A.S., Fragoso G., Toledo A., Villalobos N., Hernandez M., Gevorkian G., Acero G., Diaz A., Alvarez I., Avila R., Beltran C., Garcia G., Martinez J.J., Larralde C., Sciutto E. Synthetic peptide vaccine against Taenia solium pig cysticercosis: successful vaccination in a controlled field trial in rural Mexico. Vaccine. 2001;20:262–266. doi: 10.1016/s0264-410x(01)00249-3. [DOI] [PubMed] [Google Scholar]
- Jayashi C.M., Kyngdon C.T., Gauci C.G., Gonzalez A.E., Lightowlers M.W. Successful immunization of naturally reared pigs against porcine cysticercosis with a recombinant oncosphere antigen vaccine. Vet. Parasitol. 2012;188:261–267. doi: 10.1016/j.vetpar.2012.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayashi C.M., Gonzalez A.E., Neyra R.C., Rodriguez S., Garcia H.H., Lightowlers M.W. Validity of the enzyme-linked immunoelectrotransfer blot (EITB) for naturally acquired porcine cysticercosis. Vet. Parasitol. 2014;199:42–49. doi: 10.1016/j.vetpar.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi D.D., Poudyal P.M., Jimba M., Mishra P.N., N L.A., Maharjan M. Epidemiological status of Taenia/cysticercosis in pigs and humans in Nepal. J. Inst. Med. 2001;23:1–12. [Google Scholar]
- Joshi D.D., Maharjan M., Johansen M.V., Willingham A.L., Gaihr Y., Sharma M. Taeniasis/cysticercosis situation in Nepal. Southeast Asian J. Trop. Med. Public Health. 2004;35(Suppl. 1):252–258. [Google Scholar]
- Joshi D.D., Bista P.R., Ito A., Yamasaki H. Present situation of procine taeniasis and human cysticercosis in Nepal. Southeast Asian J. Trop. Med. Public Health. 2007;38(Suppl. 1):144–150. [Google Scholar]
- Lightowlers M.W., Donadeu M. Designing a minimal intervention strategy to control Taenia solium. Trends Parasitol. 2017;33:426–434. doi: 10.1016/j.pt.2017.01.011. (in press) [DOI] [PubMed] [Google Scholar]
- Lightowlers M.W., Garcia H.H., Gauci C.G., Donadeu M., Abela-Ridder B. Monitoring the outcomes of interventions against Taenia solium: options and suggestions. Parasite Immunol. 2016;38:158–169. doi: 10.1111/pim.12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightowlers M.W. Control of Taenia solium taeniasis/cysticercosis: past practices and new possibilities. Parasitology. 2013;140:1566–1577. doi: 10.1017/S0031182013001005. [DOI] [PubMed] [Google Scholar]
- Maharjan M., Gaihre K. Procine cysticercosis in the Magar community of Syangja District. Lifesci. Journo-Mag. 2010;2:20–26. https://www.researchgate.net/publication/215562580_Porcine_Cysticercosis_in_the_Magar_Community_of_Syangja_District, (Accessed 28 April 2017) [Google Scholar]
- Ministry of Agriculture and Development, Nepal . Agriculture Management Information System, Government of Nepal; Kathmandu: 2016. Banke District Profile. Mid-baneshwor. 24p. http://www.namis.gov.np/downloadfile/Banke_District%20Profile_1466675653.pdf, (Accessed 28 April 2017) [Google Scholar]
- Ndimubanzi P.C., Carabin H., Budke C.M., Nguyen H., Qian Y.J., Rainwater E., Dickey M., Reynolds S., Stoner J.A. A systematic review of the frequency of neurocyticercosis with a focus on people with epilepsy. PLoS Negl. Trop. Dis. 2010;4:e870. doi: 10.1371/journal.pntd.0000870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogunremi O., MacDonald G., Geerts S., Brandt J. Diagnosis of Taenia saginata cysticercosis by immunohistochemical test on formalin-fixed and paraffin-embedded bovine lesions. J. Vet. Diagn. Invest. 2004;16:438–441. doi: 10.1177/104063870401600513. [DOI] [PubMed] [Google Scholar]
- Robertson L.J., van der Giessen J.W., Batz M.B., Kojima M., Cahill S. Have foodborne parasites finally become a global concern? Trends Parasitol. 2013;29:101–103. doi: 10.1016/j.pt.2012.12.004. [DOI] [PubMed] [Google Scholar]
- van der Logt P.B., Gottstein B. Unidentified parasitic cysts in cattle. Vet. Rec. 2000;146:610–612. doi: 10.1136/vr.146.21.610. [DOI] [PubMed] [Google Scholar]
- Vargas Mendez, G.D., Saldierna, U., Navarro Fierro, R., Acevedo Hernandez, A., Flisser de, M.A., Aluja, A.S.D., 1986. Localizacion del cisticerco de la Taenia solium en diferentes regiones musculares del cerdo y su importancia para la inspeccion sanitaria (Localization of Taenia solium cysticerci in different muscular regions of swine and its significance in meat inspection). Veterinaria Mexico 17, 275–279.
- World Health Organization . In: Working to Overcome the Global Impact of Neglected Tropical Diseases. First WHO Report on Neglected Tropical Diseases. Crompton D.W.T., editor. WHO/HTM/NTD/2010.1; Geneva, Switzerland: 2010. p. 172. http://apps.who.int/iris/bitstream/10665/44440/1/9789241564090_eng.pdf, (Accessed 28 April 2017) [Google Scholar]