Abstract
Background
Severe postprandial hypoglycemia after bariatric surgery is a rare but invalidating complication. Our aim was to describe the different tests performed for its diagnosis and their outcomes as well as the response to the prescribed pharmacological and surgical treatments.
Methods
Multicenter, retrospective systematic review of cases with recurrent severe postprandial hypoglycemia.
Results
Over 11 years of follow-up, 22 patients were identified. The test most used to provoke hypoglycemia was the oral glucose load test followed by the mixed meal test which was the least standardized test. With pharmacological treatment, 3 patients were symptom-free (with octreotide) and in 12 patients hypoglycemic episodes were attenuated. Seven patients had persistent hypoglycemic episodes and underwent surgery. Partial pancreatectomy was performed in 3 patients who had positive selective arterial calcium stimulation, and nesidioblastosis was confirmed in 2 patients. Reconversion to normal anatomy was performed in 3 patients, and 1 patient underwent a resection of the ‘candy cane’ roux limb, with resolution of hypoglycemia in all cases.
Conclusions
There is high heterogeneity in the evaluation and treatment options for postoperative hypoglycemia. In patients that do not respond to pharmacological treatment, reconstruction of gastrojejunal continuity may be the safest and most successful procedure.
Key Words: Postprandial hypoglycemia, Bariatric surgery, Nesiodioblastosis, Neuroglycopenic hypoglycemia, Revisional surgery
Introduction
Postprandial hyperinsulinemic hypoglycemia after bariatric surgery has been described as a new entity which is characterized by severe neuroglycopenic symptoms such as coma and seizures that can be disabling for the patient [1]. It is difficult to know the exact incidence as most studies describe patients with hypoglycemic events not depending on their severity and without a clear differentiation from the early and late dumping syndrome. However, according to the Swedish Nationwide cohort study, 0.2% of patients undergoing Roux-en-Y gastric bypass (RYGB) suffer from this complication [2].
Postprandial hyperinsulinemic hypoglycemia was initially attributed to hyperplasia of pancreatic islets consistent with nesiodioblastosis [3,4]. Exaggerated GLP-1 response to meals after gastric bypass was proposed as the key factor promoting β-cell proliferation [1]. However, other authors observed an enlarged nuclear diameter of the β cell suggesting increased secretory activity [5]. Therefore, a functional rather than a structural pancreatic alteration has been hypothesized. However, the exact etiology has still to be elucidated [6,7,8,9]. The published data regarding this entity include small series of patients, and not all of them were affected by severe hypoglycemia [10]. Moreover, among authors there is high heterogeneity in the definition of severe hypoglycemia, the diagnostic approach, and its treatment because to date there is no established consensus. In this sense, we collected data from patients with recurrent severe hypoglycemic episodes after bariatric surgery, events which brought them to the emergency room, not responding either to diet or adequately to α-glucosidase inhibitors. Our aim was to describe the different biochemical tests performed for the diagnosis of this entity and their outcomes, as well as the response to the prescribed pharmacological and surgical treatments.
Material and Methods
This is a multicenter, retrospective a systematic review of cases with severe postprandial hypoglycemia after bariatric surgery. Subjects were selected from registered cohorts from Spanish referral hospitals followed by the Obesity Group of the Spanish Society of Endocrinology and Nutrition (GOSEEN) [11,12].
Detailed clinical and biochemical data were retrospectively collected by reviewing medical charts of all patients who had previously undergone bariatric surgery (RYGB and malabsorptive procedures) during the period from January 2002 to December 2013 in the 12 tertiary reference public hospitals in Spain.
A severe episode of hypoglycemia was defined as a hypoglycemic event (venous glucose < 50 mg/dl) [13] requiring attendance by the emergency services or hospitalization that occurred after bariatric surgery and was accompanied by neuroglycopenic symptoms in the absence of a pharmacological treatment which might explain their appearance. Patients were only included if they had undergone recurrent episodes of severe hypoglycemia that had not responded to diet modifications and showed an incomplete response to α-glucosidase inhibitors. Precocious and late dumping syndrome was clinically discarded since they usually respond to low-carbohydrate diet and frequent small split meals.
Data collected in the questionnaire included demographic and anthropometric characteristics of patients and a description of the hypoglycemic events. The laboratory tests, the radiological studies, and the performance of an arterial calcium stimulation test were registered. Medical treatments and pancreatic or reconstructive surgery used to manage the hypoglycemic events and their outcomes were recorded.
Meal test : In all centers, the patients arrived at the laboratory in the morning after an overnight fast. An antecubital venous cannula was inserted, and blood was extracted. Baseline glucose, insulin and C-peptide values were obtained in the hospital laboratory and at times 0, 30, 60, 90, 120, 150, 180 and 240 min after the liquid meal ingestion. In some centers, sample collection was finished after 120 min and in others after 180 min. Different products were used as a liquid test meal containing approximately 16% protein, 30% lipids and 54% carbohydrates with 250 kcal.
Oral glucose challenge testing was performed in some centers with 75 and in others with 100 g of glucose administered orally to evoke postprandial hypoglycemia. After an overnight fast, blood was extracted for hospital laboratory determination of glucose, insulin, C-peptide baseline values and at times 0, 30, 60, 90, 120, 150, 180 and 240 min. In some centers, sample collection was finished after 120 min and in others after 180 min.
This study was approved by the Ethic Committees of each hospital and is in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All patients signed a written informed consent which specified that data collected from their medical charts could be potentially used in an anonymous way for investigation and publication.
Statistical Analysis
Data were expressed as mean ± SD for parametric variables and as frequencies for categorical variables. For categorical variables differences between groups were assessed with the chi-square test for independent samples. Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS/Windows version 18; SPSS Inc., Chicago, IL, USA). Statistical significance was set at p < 0.05.
Results
Clinical Characteristics
The total number of cases of hypoglycemia after bariatric surgery reported amounted to 22, with a total of 4,645 bariatric surgery interventions (RYGB and malabsorptive procedures) performed in the retrospective period analyzed (January 2002 to December 2013). Therefore, the estimated prevalence found in our series was 0.47%. However, this is a conservative estimate as it cannot be discarded that cases from our cohort had been attended in other hospitals.
21 women and 1 man, aged 51 ± 10 years, were included. The clinical characteristics of patients are given in table 1. 19 patients (86.3%) had undergone a standard RYGB and 3 a malabsorptive procedure. Elapsed time since bariatric surgery was 31.8 ± 26 months (range 7-120 months). At the time of hypoglycemic episodes, 77% of patients had regained weight (10.7 ± 16% from their minimal weight after surgery). The hypoglycemic events were mainly postprandial, but 27% of patients presented with mixed episodes pre- and postprandially. Data relevant to the episodes of hypoglycemia are shown in table 2.
Table 1.
Clinical characteristics of patients and laboratory values during episodes of postprandial hypoglycemia
| N | |
|---|---|
| Sex (M/F) | 1/21 |
|
| |
| Age, years | 51 ±10 |
|
| |
| Type of surgery, n (%) | |
| Roux-en-Y gastric bypass | 19 (86.3%) |
| Biliopancreatic diversion | 2 (9.2 %) |
| Duodenal switch | 1 (4.5%) |
|
| |
| Time after surgery, months | 31.8 ± 26 |
|
| |
| Frequency of hypoglycemias, n (%) | |
| Daily episodes | 13 (59.0%) |
| 2–3 episodes/week | 2 (9.2%) |
| Weekly episodes | 7 (31.8%) |
|
| |
| Serum glucose during episode of hypoglycemia, mg/dl | 39.2 ± 9.3 |
|
| |
| Serum insulin during episode of hypoglycemia (normal range 2.3 – 16.7 μUI/ml), μUI/ml | 15.9 ± 13.7 |
|
| |
| Effects of hypoglycemia | |
| Falls and contusions | 54.7% |
| Coma | 13.6% |
| Continuous invalidating faints | 22.7% |
| Seizures | 9.0% |
Table 2.
Clinical characteristics of the episodes of spontaneous postprandial hypoglycemia
| N (%) | |
|---|---|
| Type of hypoglycemias | |
| Postprandial | 16 (72.7%) |
| Mixed | 6 (27.3%) |
|
| |
| Frequency of hypoglycemias | |
| Daily episodes | 13 (59.0%) |
| 2 – 3 episodes/week | 2 (9.2%) |
| Weekly episodes | 7 (31.8%) |
|
| |
| Acute treatment of the episode | |
| Simple carbohydrates (oral) | 7 (31.8%) |
| Glucose (iv) | 4 (18.2%) |
| Glucagon (subcut) | 1 (4.5%) |
| Simple carbohydrates +glucose (iv) | 4 (18.2%) |
| Simple carbohydrates + glucagon (subcut) | 2 (9.1%) |
| All previous treatments on different episodes | 2 (9.1%) |
|
| |
| Effects of hypoglycemia | |
| Falls and contusions | 54.7% |
| Coma | 13.6% |
| Continuous invalidating faints | 22.7% |
| Seizures | 9.0% |
Regarding the characteristics of patients, none suffered from hypoglycemic episodes before surgery, and type 2 diabetes was present in 9% of them remitting after the procedure, without requiring any hypoglycemic drugs.
Dynamic Tests
Only 3 (13.6%) of patients had plasma insulin concentrations determined during a spontaneous episode of hypoglycemia; in the remaining subjects, insulin and glucose levels were analyzed along with venous glucose sampling during the provocative test. The laboratory findings confirmed hyperinsulinemia or an inappropriate normal insulinemia in all patients and a concomitant venous glucose level of less than 50 mg/dl (mean 39.1 ± 9.3 mg/dl).
The test most used to provoke a hypoglycemic episode was the oral glucose load, which was performed in 16 (72%) patients. In 11 (50%) patients, a mixed meal test was done as an exclusive test (n = 3) and in addition to an oral glucose load (n = 8). During the oral glucose load, 81% of patients experienced a hypoglycemic event. In 2 patients, this test was not assessable because of vomiting. During the mixed meal test, 54% of patients had a hypoglycemic event. Comparing the 8 patients evaluated both with an oral glucose load and with a liquid mixed meal test, a greater proportion showed a positive result (presence of hypoglycemia) after the oral glucose load test (66% vs. 33%, p = 0,049). The mixed meal was the best tolerated test, although the least standardized. Different times of sample collection and total duration (ranging from 120 to 240 min) were reported. Also, different liquid meals were used containing 33-50 g of carbohydrates (mean 39.5 ± 5.3 g). A 72-hour fast was performed in 10 (45%) patients, mainly in patients with mixed pre- and postprandial episodes, and in 70% of them hypoglycemia was observed, but with low plasma insulin concentrations.
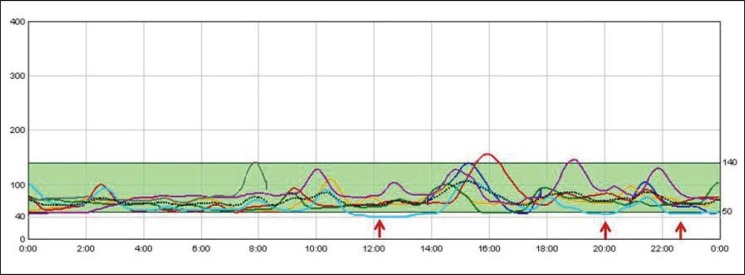
An exploratory glucose sensor was placed in only 3 patients, and hypoglycemic events were detected in all of them during a 5-day period. The continuous glucose monitoring of one of these patients (patient number 4) with persistent episodes of hypoglycemia can be seen in figure 1.
Fig. 1.
Continuous glucose monitoring pre-pharmacological treatment. Each line represents an individual day. Y-axes represent glucose (mg/dl), X-axes represent hour of the day. Glucose values below 50 mg/dl are indicated in red and they represent 8% of all glycemic values.
Therapy Strategies: Pharmacological Treatment
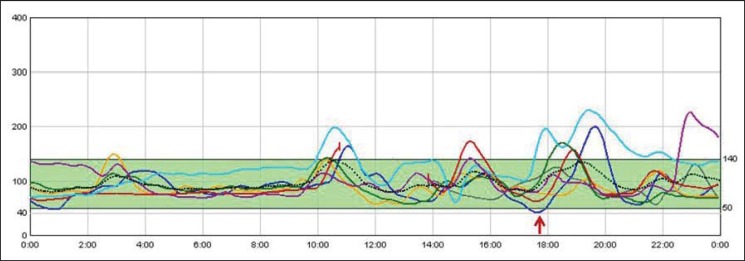
The first step in the pharmacological treatment was the use of α-glucosidase inhibitors, only 4 patients (18%) showed a partial response (table 3). A reduction of 50% in the number of hypoglycemic events and in their severity was considered a partial response. The second step was the use of a calcium channel blocker in 10 (45.4%) patients (nifedipine and verapamil at doses of 20 mg and 80 mg, respectively), and of diazoxide (mean dose 168.7 ± 94 mg/day) in 6 (27.2%). The results obtained with both previous treatments were similar, achieving an initial reduction of symptoms in 50% of patients. Octreotide was used as a second pharmacological treatment step after α-glucosidase inhibitors in 2 patients and as a third step in 11 patients (8 without response and 3 with partial response after calcium channel blockers / diazoxide). Among patients receiving octreotide, 5 (38.4%) showed a partial response and in 3 (23%) the hypoglycemic episodes resolved. Figure 2 shows the continuous glucose monitoring of patient number 4 under octreotide treatment. In the follow-up, 2 patients with partial response under calcium channel blockers and diazoxide experienced a worsening of their symptoms. Therefore, with pharmacological treatment, 3 patients were symptom-free (with octreotide) and 12 experienced an attenuation of their hypoglycemic episodes (with α-glucosidase inhibitors n = 4, with calcium channel blockers n = 3, with octreotide n = 5). Seven patients with persistent severe hypoglycemic episodes underwent further studies.
Table 3.
Response to pharmacological treatment
| Medication | Dose | Number of patients | No response | Partial response | Complete response |
|---|---|---|---|---|---|
| Alpha glucosidase inhibitors | 50 mg / 8 h orally | 22 | 18 (82%) | 4 (18%) | 0 |
|
| |||||
| Nifedipine plus verapamil | 20 mg/day 80 mg/day orally | 10 | 5 (50%) | 5 (50%) | 0 |
|
| |||||
| Diazoxide | 168.7 ± 94 mg/day orally | 6 | 3 (50%) | 3 (50%) | 0 |
|
| |||||
| Octreotide | 50/100 μg / 12 h subcutaneously | 13* | 5 (38,4%) | 5 (38,4%) | 3 (23%) |
N= 2 patients after alpha glucosidase inhibitors + N = 11 after calcium channel blocker / diazoxide.
Fig. 2.
Continuous glucose monitoring under octreotide treatment. Each line represents an individual day. Y-axes represent glucose (mg/dl), X-axes represent hour of the day. Glucose values below 50 mg/dl are indicated in red. Only one value of glucose below 50 mg/dl was detected. On the contrary, due to side effects of the medication, hyperglycemia (glucose > 150 mg/dl) was detected on a few ocasions.
Radiological Studies
Abdominal CT and ecoendoscopy were performed in the former 7 patients, and octreotide scintigraphy was performed in 3 patients, without pathological findings. Selective arterial calcium stimulation was undertaken, and it was positive in 3 patients.
Surgical Treatment
Partial pancreatectomy was performed in 3 patients with positive selective arterial calcium stimulation. In 2 of them, neosidioblastosis was confirmed. After the intervention they were symptom-free, 1 or 2 years after surgery. In 1 patient, no histologically abnormal findings were observed, and hypoglycemic episodes persisted, requiring a reconversion of duodenal switch to original anatomy. However, 2 years later hypoglycemic episodes recurred and were controlled with α-glucosidase inhibitors during the next 3 years. After this period of time, type 2 diabetes mellitus was diagnosed and required treatment with insulin and metformin.
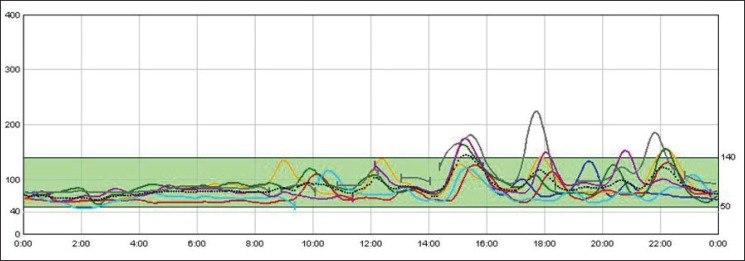
Redo surgery (revisional or conversional surgery) was chosen as a first surgical therapeutical option in 4 patients with previous RYGB. One of them underwent a ‘kissing operation’ with performance of an anastomosis between the alimentary limb and the antral remnant. However, 2 years later, because of recurrence, the alimentary limb was removed and the gastric pouch was anastomosed to the gastric remnant to restore the gastroduodenal tract continuity. The patient persisted with hypoglycemic episodes that could be controlled with α-glucosidase inhibitors. Two other patients underwent a complete reversal of RYGB to normal anatomy. The procedure consisted in dismantling the previous gastrojejunostomy and jejunojejunostomy, re-anastomosing the gastric pouch to the gastric remnant and the proximal alimentary limb end to the distal biliary limb end. The 2 patients have been without evidence of hypoglycemia for 1 year or for 3 months after the intervention. The last patient with RYGB (patient number 4) showed recurrence of hypoglycemic episodes after an initial resolution with octreotide. She underwent a gastric pouch restriction, reducing the anastomosis diameter and a resection of the nonfunctional Roux limb proximal to the jejunostomy in order to avoid the ‘candy cane’ Roux syndrome associated with hypoglycemia. The patient is symptom-free 1 year after the procedure (fig. 3).
Fig. 3.
Continuous glucose monitoring after revisional surgery. Each line represents an individual day. Y-axes represent glucose (mg/dl), X-axes represent hour of the day. Six months after the resection of the ‘candy cane’ roux limb no hypoglycemia was observed.
Discussion
Our series of 22 well-characterized patients with recurrent severe episodes of postprandial hypoglycemia, not responding to diet, is the largest published to date. In spite of the limitations, the estimated prevalence of 0.47% is a little higher than previously reported [2,14] although the fact that we are referral hospitals could explain this difference. Nevertheless, our findings conformed the infrequence of this entity.
Previous reports described smaller cohorts of up to 9 cases, and they included patients suffering from late dumping [15,16]. Our study only included patients with severe symptomatic hyperinsulinemic hypoglycemia with consistent biochemical and clinical data. Hyperinsulinemic severe hypoglycemia should be differentiated from early and late dumping syndrome that respond well to dietary manipulation and appear early in the postoperative period. More severe hypoglycemia, associated with neuroglycopenia, typically occurs 1-3 years after gastric bypass [3]. Indeed, in our patients hypoglycemic symptoms presented 32 months after surgery.
A greater prevalence of postoperative dumping syndrome among type 2 diabetic patients has been described in one previous paper [17]. However, this finding has not been confirmed in our series in which only 9% of patients had a prior history of type 2 diabetes.
In our study, different methods of diagnosing hyperinsulinemic hypoglycemic events were used. The easiest test is to determine venous glucose and insulin concentrations during a spontaneous episode of low plasma glucose. However, patients are usually attended at home or at the hospital emergency room, and insulin concentrations during the hypoglycemic episode cannot be easily determined. The tests used to induce hypoglycemia show low specificity, and, taking all the data together, it is not possible to recommend only one of them. The oral glucose tolerance test (OGTT) has historically been used for reactive hypoglycemia; however, it may not be well tolerated after gastric bypass and has been found to be positive (episode of hypoglycemia) in 10% of normal people [18] and 33% of patients with gastric bypass without hypoglycemia [19,20]. The liquid meal test seems to be a more physiological stimulus with lower glucose load, but it can be positive in 30% of asymptomatic patients undergoing gastric bypass [21]. In our study, a greater proportion of patients experienced a hypoglycemic event during the OGTT than during the mixed meal. However, with the former test not all patients received 50 g of carbohydrates which could be the major physiological stimulus.
A glucose sensor can register low glucose episodes in free-living conditions. A recent study has described better detection rates of hypoglycemia with continuous glucose monitoring compared to mixed meal test [21]. However, its use is limited because it is not available to all centers, and the results obtained have to be supplemented by laboratory parameters [22].
The etiology of this entity is still unclear. Some authors have attributed it to the presence of nesidioblastosis but others have alternatively supported increased activity of β cells, probably induced by GLP-1 [1,23]. However, the use of GLP-1 receptor antagonist, has not shown a different effect on insulin secretion in patients with and without recurrent hypoglycemia [24]. The case of a patient has been published in whom hypoglycemia was completely ameliorated by feeding via gastrostomy tube through the bypassed gut [25]. This suggested that in some cases there is a functional pancreatic alteration that may be resolved when food does not rapidly bypass the duodenum and arrives to the ileum. In this sense, this entity has only been described in gastric bypass and malabsorptive surgeries that exclude the proximal gut.
There is no ‘gold-standard’ medical treatment for severe hyperinsulinemic hypoglycemia [26,27,28,29]. The pharmacological treatment is directed to reduce carbohydrate absorption, reduce insulin secretion, and slow gastric emptying. When the symptoms are refractory to diet the first pharmacological treatment used in our series is α-glucosidase inhibitors that are able to improve but not resolve symptoms in 18% of cases. In our experience, the second step is a calcium channel blocker because of its low cost and easy administration. One of the main mechanisms of action of calcium antagonists is a direct inhibitory effect on the pancreatic β-cell glucose-induced insulin release [30]. Some clinicians prefer a β-cell inhibitor diazoxide [10,31,32,33] even though in our country it can only be prescribed in the hospital pharmacy as a foreign drug, with similar results compared to calcium antagonists in our experience (partial effect in 50% of patients and ineffective in the rest). The third step and increasingly being used as a second step is the secretory inhibitor octreotide. In our series, with this treatment 23% of patients had complete resolution of the hypoglycemic episodes. Therefore, octreotide could be the treatment of choice when diet, α-glucosidase and calcium channel blockers fail to control hypoglycemic episodes. Although it is expensive, needs subcutaneous administration and can only be prescribed in our country as compassionate use for the treatment of hyperinsulinemic hypoglycemia, different reports have shown how it specifically inhibits GLP-1 secretion [34].
Surgical therapy has to be considered when pharmacological treatment is unsuccessful. In our experience, reconversion to normal anatomy in one step is a safe process and the most successful technique. However, our follow-up is relatively short, and other authors have recently described persistence of hypoglycemia after reconversion [35]. Other surgical approaches have been used, such as restoring gastric restriction with an adjustable gastric band [36] or providing nutrition through a gastrostomy tube [25]. However, to date there is insufficient experience to recommend any of the previous approaches.
Partial or subtotal pancreatic resection is reserved when a gradient is found in venous sampling. In our series, 3 patients underwent pancreatic resection, and in 2 nesidioblastosis was confirmed with resolution of symptoms [37]. In another patient the histology showed no abnormalities and required restoring of gastrointestinal continuity with a final development of type 2 diabetes a few years later. Partial pancreatectomy carries morbimortality [38] and in our opinion should be reserved for cases with high suspicion of nesidioblastosis with a detailed radiologic study and a positive venous sampling. For the remaining cases, reconversion to original anatomy might be a safer procedure.
The present study has several limitations, the most important being that it is retrospective. However, a systemized revision of cases was performed, and only patients with clinical severe recurrent hypoglycemia with confirmed biochemical parameters were included. Different diagnostic tests and medical treatments were used as there are no established guidelines regarding the management of this complication, highlighting the need for their elaboration.
Conclusions
Postoperative hypoglycemia is an uncommon, yet troublesome, side effect after RYGB. There is high heterogeneity in the initial evaluation and treatment options, and most authors follow their own experience as there is no established consensus. Approximately 30% of these episodes will not respond to pharmacological treatment involving α-glucosidase, calcium channel antagonists, diazoxide, and octreotide. In these cases radiological studies and a calcium stimulation test should be performed. Reconstruction of gastrojejunal continuity might be the safest and most successful option. Partial or subtotal pancreatic resection has high morbimortality, can lead to the onset of diabetes and should be applied only when a clear gradient is found in venous sampling.
Disclosure Statement
The authors declare that they have no conflict of interest.
Acknowledgements
We would like to thank The Group of Obesity of the Spanish Society of Endocrinology and Nutrition (GOSEEN) for participating and providing data for this study. This work was supported by ‘Ajuts per a projectes de recerca clínica de l'Hospital Universitari de Bellvitge (2011-PR143/11)’. The authors thank Dr. Jonathan Rogerson for his valuable help with the English version of the manuscript.
References
- 1.Goldfine AB, Mun EC, Devine E, Bernier R, Baz-Hecht M, Jones DB, Schneider BE, Holst JJ, Patti ME. Patients with neuroglycopenia after gastric bypass surgery have exaggerated incretin and insulin secretory responses to mixed meal. J Clin Endocrinol Metab. 2007;92:4678–4685. doi: 10.1210/jc.2007-0918. [DOI] [PubMed] [Google Scholar]
- 2.Marsk R, Jonas E, Rasmussen F, Näslund E. Nationwide cohort study of post-gastric bypass hypoglycaemia including 5040 patients undergoing surgery for obesity in 1986-2006 in Sweeden. Diabetologia. 2010;53:2307–2311. doi: 10.1007/s00125-010-1798-5. [DOI] [PubMed] [Google Scholar]
- 3.Service GJ, Thompson GB, Service FJ, Andrews JC, Collazo-Clavell ML, Lloyd RV. Hyperinsulinemic hypoglycaemia with nesiodioblastosis after gastric-bypass surgery. N Engl J Med. 2005;353:249–254. doi: 10.1056/NEJMoa043690. [DOI] [PubMed] [Google Scholar]
- 4.Patti ME, McMahon G, Mun EC, Bitton A, Holst JJ, Goldsmith J, Hanto DW, Callery M, Arky R, Nose V, Bonner-Weir S, Goldfine AB. Severe hypoglycaemia post-gastric bypass requiring partial pancreatectomy: evidence for inappropriate insulin secretion and pancreatic islet hyperplasia. Diabetologia. 2005;48:2236–2240. doi: 10.1007/s00125-005-1933-x. [DOI] [PubMed] [Google Scholar]
- 5.Meier J, Butler A, Galasso R, Butler P. Hyperinsulinemic hypoglycaemia after gastric bypass surgery is not accompanied by islet hyperplasia or increased β-cell turn-over. Diabetes Care. 2006;29:1554–1559. doi: 10.2337/dc06-0392. [DOI] [PubMed] [Google Scholar]
- 6.Cui Y, Elahi D, Andersen D. Advances in the etiology and management of hyperinsulinemic hypoglycaemia after Roux-en-Y gastric bypass. J Gastrointest Surg. 2011;15:1879–1888. doi: 10.1007/s11605-011-1585-8. [DOI] [PubMed] [Google Scholar]
- 7.Patti ME, Goldfine AB. Hypoglycaemia after gastric bypass: the dark side of GLP-1. Gastroenterology. 2014;146:605–608. doi: 10.1053/j.gastro.2014.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vidal J, Nicolau J, Romero F, Casamitjana R, Momblan D, Conget I, Morínigo R, Lacy AM. Long-term effects of Roux-en-Y gastric bypass surgery on plasma glucagon-like peptide 1 and islet function in morbidly obese subjects. J Clin Endocrinol Metab. 2009;94:884–891. doi: 10.1210/jc.2008-1620. [DOI] [PubMed] [Google Scholar]
- 9.Rabiee A, Magruder JT, Salas-Carrillo R, Carlson O, Egan JM, Askin FB, Elahi D, Andersen DK. Hyperinsulinemic hypoglycemia after Roux-en-Y gastric bypass: unraveling the role of gut hormonal and pancreatic endocrine dysfunction. J SurgRes. 2011;167:199–205. doi: 10.1016/j.jss.2010.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathavan VK, Arregui M, Davis C, Singh K, Patel A, Meacham J. Management of postgastric bypass noninsulinoma pancreatogenous hypoglycaemia. Surg Endosc. 2010;24:2547–2555. doi: 10.1007/s00464-010-1001-6. [DOI] [PubMed] [Google Scholar]
- 11.Caixàs A, Lecube A, Morales MJ, Calañas A, Moreiro J, Cordido F, Díaz MJ, Masmiquel L, Moreno B, Vidal J, Goday A, Arrizabalaga JJ, García-Luna PP, Iglesias P, Burguera B, Rubio MA, Monereo S. Weight-related quality of life in Spanish obese subjects suitable for bariatric surgery is lower than in their North American counterparts: a case-control study. Obes Surg. 2013;23:509–514. doi: 10.1007/s11695-012-0791-0. [DOI] [PubMed] [Google Scholar]
- 12.González I, Rubio MA, Cordido F, Bretón I, Morales MJ, Vilarrasa N, Monereo S, Lecube A, Caixàs A, Vinagre I, Goday A, García-Luna PP. Maternal and perinatal outcomes after bariatric surgery: a Spanish Multicenter Study. Obes Surg. 2015;25:436–442. doi: 10.1007/s11695-014-1387-7. [DOI] [PubMed] [Google Scholar]
- 13.Permutt MA. Postprandial hypoglycemia. Diabetes. 1976;25:719. doi: 10.2337/diab.25.8.719. [DOI] [PubMed] [Google Scholar]
- 14.Sarwar H, Chapman WH, Pender JR, Ivanescu A, Drake AJ, Pories WJ, Dar MS. Hypoglycemia after Roux-en-Y gastric bypass: the BOLD experience. Obes Surg. 2014;24:1120–1124. doi: 10.1007/s11695-014-1260-8. [DOI] [PubMed] [Google Scholar]
- 15.Ceppa EP, Ceppa DP, Omotosho PA, Dickerson JA, 2nd, Park CW, Portenier DD. Algorithm to diagnose etiology of hypoglycaemia after Roux-en-Y gastric bypass for morbid obesity: case series and review of literature. Surg Obes Relat Dis. 2012;8:641–647. doi: 10.1016/j.soard.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Mordes JP, Alonso LC. Evaluation, medical therapy, and course of adult persistent hyperinsulinemic hypoglycemia after Roux-en-Y gastric bypass surgery: a case series. Endocr Pract. 2015;3:237–246. doi: 10.4158/EP14118.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vontobel A, Galvao M, Moretto M, Barancelli F, Eckerdt C, Cora C. Obese patients with type 2 diabetes submitted to banded gastric bypass: greater incidence of dumping syndrome. Obes Surg. 2009;19:1481–1484. doi: 10.1007/s11695-009-9943-2. [DOI] [PubMed] [Google Scholar]
- 18.Lev-Ran A, Anderson RW. The diagnosis of postprandial hypoglycaemia. Diabetes. 1981;30:996–999. doi: 10.2337/diab.30.12.996. [DOI] [PubMed] [Google Scholar]
- 19.Kim SH, Liu TC, Abbasi F, Lamendola C, Morton JM, Reaven GM, McLaughlin TL. Plasma glucose and insulin regulation is abnormal following gastric bypass surgery with or without neurogycopenia. Obes Surg. 2009;19:1550–1556. doi: 10.1007/s11695-009-9893-8. [DOI] [PubMed] [Google Scholar]
- 20.Pigeyre M, Vaurs C, Raverdy V, Hanaire H, Ritz P, Pattou F. Increased risk of OGTT-induced hypoglycemia after gastric bypass in severely obese patients with normal glucose tolerance. Surg Obes Relat Dis. 2015;11:573–577. doi: 10.1016/j.soard.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Kefurt R, Langer FB, Schindler K, Shakeri-Leidenmühler S, Ludvik B, Prager G. Hypoglycemia after Roux-En-Y gastric bypass: detection rates of continuous glucose monitoring (CGM) versus mixed meal test. Surg Obes Relat Dis. 2015;11:564–569. doi: 10.1016/j.soard.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Halperin F, Patti ME, Skow M, Bajwa M, Goldfine AG. Continuous glucose monitoring for evaluation of glycemic excursions after gastric bypass. J Obes. 2011;2011:869536. doi: 10.1155/2011/869536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patti ME, Li P, Goldfine AB. Insulin response to oral stimuli and glucose effectiveness increased in neuroglycopenia following gastric bypass. Obesity (Silver Spring) 2015;23:798–807. doi: 10.1002/oby.21043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salehi M, Prigeon RL, D'Alessio DA. Gastric bypass surgery enhances glucagon-like peptide 1-stimulated postpradial insulin secretion in humans. Diabetes. 2011;60:2308–2314. doi: 10.2337/db11-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mc Laughlin T, Peck M, Holst J, Deacon C. Reversible hyperinsulinemic hypoglycemia after gastric bypass: a consequence of altered nutrient delivery. J Clin Endocrinol Metab. 2010;95:1851–1855. doi: 10.1210/jc.2009-1628. [DOI] [PubMed] [Google Scholar]
- 26.Frankhouser SY, Ahmad AN, Perilli GA, Quintana BJ, Vengrove MA. Post-gastric-bypass hypoglycemia successfully treated with alpha-glucosidase inhibitor therapy. Endr Pract. 2013;19:511–514. doi: 10.4158/EP12281.RA. [DOI] [PubMed] [Google Scholar]
- 27.Moreira RO, Moreira RB, Machado NA, Gonçalves TB, Coutinho WF. Post-prandial hypoglycaemia after bariatric surgery: pharmacological treatment with verapamil and acarbose. Obes Surg. 2008;18:1618–1621. doi: 10.1007/s11695-008-9569-9. [DOI] [PubMed] [Google Scholar]
- 28.Guseva N, Phillips D, Mordes JP. Successful treatment of persisenthyperinsulinemic hypoglycaemia with nifedipine in an adult patient. Endocr Pract. 2010;16:107–111. doi: 10.4158/EP09110.CRR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Molina M, García J, Civera M, Ortega J, Martínez-Valls JF, Martínez-Hervás S, Real JT, Carmena R. Hypoglycemia after Roux-en-Y gastric bypass surgery. Endocrinol Nutr. 2011;58:197–199. doi: 10.1016/j.endonu.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 30.Sanke T, Nanjo K, Kondo M, Nishi M, Moriyama Y, Miyamura K. Effect of calcium antagonists on reactive hypoglycemia associated with hyperinsulinemia. Metabolism. 1986;35:924–927. doi: 10.1016/0026-0495(86)90055-7. [DOI] [PubMed] [Google Scholar]
- 31.Won JG, Tseng HS, Yang AH, Tang KT, Jap TS, Lee CH, Lin HD, Burcus N, Pittenger G, Vinik A. Clinical features and morphological characterization of 10 patients with noninsulinoma pancreatogenous hypoglycaemia syndrome (NIPHS) Clin Endocrinol (Oxf) 2006;65:566–578. doi: 10.1111/j.1365-2265.2006.02629.x. [DOI] [PubMed] [Google Scholar]
- 32.Spanakis E, Gragnoli C. Successful medical management of status post-Roux-en-Y-gastric-bypass hyperinsulinemic hypoglycemia. Obes Surg. 2009;19:1333–1334. doi: 10.1007/s11695-009-9888-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonzalez-Gonzalez A, Delgado M, Fraga-Fuentes MD. Use of diazoxide in management of severe postprandial hypoglycemia in patient after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2013;9:e18–19. doi: 10.1016/j.soard.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 34.Myint KS, Greenfield JR, Farooqi IS, Henning E, Holst JJ, Finer N. Prolonged successful therapy for hyperinsulinaemic hypoglycaemia after gastric bypass: the pathophysiological role of GLP-1 and its response to a somatostatin analogue. Eur J Endocrinol. 2012;166:951–955. doi: 10.1530/EJE-11-1065. [DOI] [PubMed] [Google Scholar]
- 35.Lee CJ, Brown T, Magnuson TH, Egan JM, Carlson O, Elahi D. Hormonal response to a mixed-meal challenge after reversal of gastric bypass for hypoglycemia. J Clin Endocrinol Metab. 2013;98:1208–1212. doi: 10.1210/jc.2013-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Z'graggen K, Guweidhi A, Steffen R, Potoczna N, Biral R, Walther F, Komminoth P, Horber F. Severe recurrent hypoglycemia after gastric bypass surgery. Obes Surg. 2008;18:981–988. doi: 10.1007/s11695-008-9480-4. [DOI] [PubMed] [Google Scholar]
- 37.García BF, Peromingo R, Galindo J, Arrieta F, Sánchez J, Vázquez C, Botella-Carretero JI. Case report subtotal pancreatectomy as treatment for severe hypoglycemia after gastric bypass. Endocrinol Nutr. 2014;61:391–393. doi: 10.1016/j.endonu.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 38.Patti ME, Goldfine AB. Hypoglycaemia following gastric bypass surgery-diabetes remission in the extreme? Diabetologia. 2010;53:2276–2279. doi: 10.1007/s00125-010-1884-8. [DOI] [PubMed] [Google Scholar]