Abstract
Objective
Both a 1- to 4-week continuous or intermittent stay and moderate exercise in hypoxia versus normoxia can lead to weight loss. We examined the reproducibility and durability of added hypoxic exposure in a feasible health program of several months.
Methods
32 obese persons, randomly assigned to either a hypoxia (age 50.3 ± 10.3 years, BMI 37.9 ± 8.1 kg/m²) or a normoxia (age 52.4 ± 7.9 years, BMI 36.3 ± 4.0 kg/m²) group, completed 52 exercise sessions within 8 months. Participants exercised for 90 min (65-70% HRpeak) either at a simulated altitude of 3,500 m or in normoxia, and rested for further 90 min at 4,500 m or normoxia. Before, after 5 weeks, after 3 months, and after the intervention, body composition and exercise capacity were determined. Risk markers (e.g., blood pressure, cholesterol) were measured before, after 3 months, and after the intervention period.
Results
Body weight, BMI, waist and hip circumference, Ppeak and BPsys improved over time (p < 0.05) but without group difference. Fat mass reductions correlated with HDL changes (r = −0.427; p < 0.05) in the entire group.
Conclusion
Long-term, moderate intensity exercise and rest in hypoxia does not lead to higher reductions in body weight than normoxia alone. Therefore, for weight loss and metabolic markers hypoxic exposure does not add effects at least when stimuli (i.e., hypoxia dose, exercise intensity/duration) are unaltered throughout the intervention.
Key Words: Hypoxia, Weight loss, Physical exercise, Obese, Overweight
Introduction
Overweight and obese individuals have an increased risk of cardiovascular disease, diabetes, and other major diseases [1,2,3,4,5]. Weight management is an important measure in public health because even small reductions in body weight (i.e., 5-7%) have been demonstrated to significantly decrease obesity-related comorbidities [5,6]. Successful weight and body fat reduction requires sustained lifestyle modifications including restricted caloric intake and physical activity [3,5,7]. When considering the latter, the health benefits of increasing physical activity are well established [8,9,10,11]. However, in order to promote weight loss large exercise volumes, ranging from 150 to 400 min/week with energy equivalents of 1,200 to >2,000 kcal/week, are necessary [3,5].
In an attempt to further increase weight loss, some studies [12,13,14,15] combined normobaric or hypobaric hypoxia, which appear to increase metabolic rate and leptin levels [15], with a regimen of intermittent exercise for 60-90 min three times a week [12,13,14] or a continuous stay for 1 week [15]. Results of these studies revealed that after a 3- to 4-week exercise period performed at the same relative intensity [12,13,14] or one continuous week at rest [15], body fat content decreased more under hypoxia conditions when compared to normoxia conditions [13,14]. Furthermore, also metabolic risk markers (e.g., triglycerides, HOMA index, and fasting insulin) improved to a greater extent under hypoxia [13]. However, whether or not the durability of these health benefits persist beyond a few weeks is not known. In order to be feasible (feasibility defined as compatible with the work and family life schedule and financing by public health care), a weight and fat loss program with moderate exercise in hypoxia should not only be more effective when compared to exercise in normoxia, but also should persist in the long term [16].
The aim of the present study was i) to investigate if hypoxia exposure combined with moderate intensity exercise over a 8-month period in obese people (BMI > 30 kg/m²) is more effective in reducing body weight and body fat when compared to exercising and resting in normoxia and ii) to investigate the long-term effects of such a feasible hypoxia health prevention program on metabolic risk markers.
We hypothesized that in the long term, hypoxia exposure combined with moderate intensity exercise is more effective in reducing body weight and body fat mass as well as metabolic risk than resting and exercising in normoxia.
Participants and Methods
Participants
Participants were recruited through a call in different local media and by means of notices at the two rehabilitation centers where the study was performed (Bad Aibling and Herxheim, Germany). Inclusion criteria for the study were BMI >30 kg/m² and the capability to complete moderate intensity exercise (e.g., adequate exercise capacity, absence of orthopedic problems). Exclusion criteria were: New York Heart Association (NYHA) class > 3, myocardial infarction or stroke within 6 months before the start of the study, instable angina pectoris, malignant hypertension, chronic kidney disease, pregnancy, and an oxygen saturation < 70% during 90 min at an simulated altitude of 4,500 m (pre-tested in a normobaric hypoxic chamber).
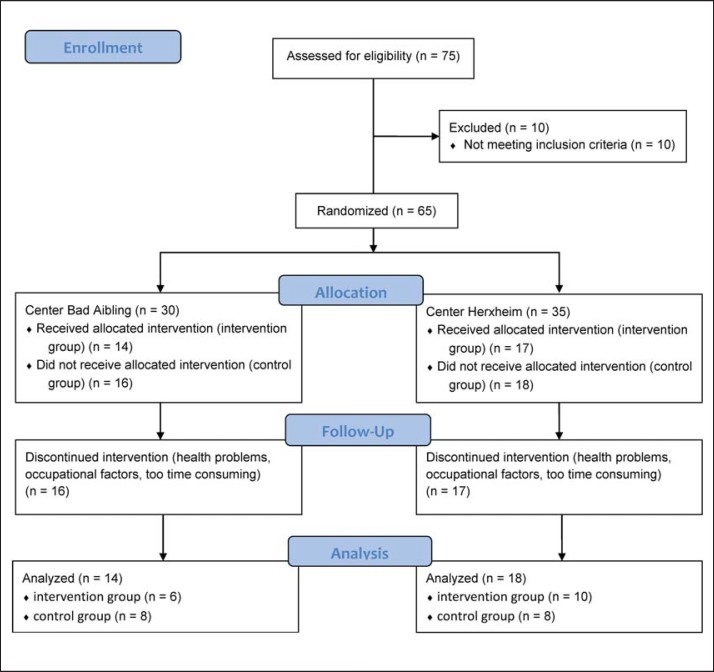
30 persons (21 females and 9 males) in Bad Aibling and 35 persons (19 females and 16 males) in Herxheim followed the call and were eligible for participation according to the inclusion and exclusion criteria. All participants were informed about the study goals and procedures and provided written informed consent for participation before any measurement was performed. Participants then were randomly assigned to a control (CG, n = 34) or an intervention group (IG, n = 31). Due to health problems (not related to the study protocol), occupational factors, or the individual perception that the intervention program is getting too time-consuming, 33 participants (18 females and 15 males) dropped out during the intervention period (fig. 1). Baseline characteristics of the participants finishing the study (22 females and 10 males) are shown in table 1 divided by group allocation (IG: 12 females and 4 males; CG: 10 females and 6 males). Despite the dropouts, group differences at the beginning of the study were not present, except for peak heart rate (HRpeak; 154 ± 16 and 141 ± 20 beats/min for IG and KG, respectively; p < 0.05). The study was carried out in conformity with the ethical standards laid down in the declaration of Helsinki, and the study protocol was approved by the ethical committees of the province of Salzburg or University of Salzburg. The study is registered as clinical trial at the WHO and the German Clinical Trial Register under the number DRKS00005479.
Fig. 1.
Flow chart of the participants throughout the study.
Table 1.
Changes of the physical fitness and body composition parameters over the 8-month intervention period a
| IG |
CG |
all |
ANOVA, p value |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | pre | 5 weeks | 3 months | post | n | pre | 5 weeks | 3 months | post | pre | 5 weeks | 3 months | post | main effect: time | main effect: sex | interaction: time × group | |
| Body weight, kg | 16 | 105.5 ± 20.0 | 103.7 ± 20.3* | 101.7 ± 20.6* t–5 | 102.2 ± 20.8* | 16 | 103.2 ± 15.1 | 102.4 ± 14.6 | 100.4 ± 13.8 # t-pre | 100.3 ± 14.2 t–5 | 104.3 ± 17.5 | 103.0 ± 17.5* | 101.1 ± 17.3* # | 101.3 ± 17.5* # | 0.005 | 0.990 | 0.376 |
|
| |||||||||||||||||
| BMI, kg/m2 | 16 | 37.9 ± 8.1 | 37.2 ± 8.1* | 36.5 ± 8.0* | 36.6 ± 7.9* | 16 | 36.3 ± 4.2 | 36.0 ± 4.4 | 35.4 ± 4.2 | 35.4 ± 4.6 | 37.1 ± 6.4 | 36.6 ± 6.5* | 35.9 ± 6.3* # | 36.0 ± 6.4* # | 0.018 | 0.977 | 0.438 |
|
| |||||||||||||||||
| Fat mass, % | 16 | 44.7 ± 8.9 | 45.3 ± 9.0 | 44.5 ± 8.9 | 44.1 ± 8.5 # | 16 | 42.4 ± 7.9 | 42.0 ± 8.9 | 41.6 ± 9.3 | 41.5 ± 8.5 | 43.6 ± 8.4 | 43.6 ± 9.0 | 43.0 ± 9.1 | 42.8 ± 8.5 | 0.439 | 0.049 | 0.626 |
|
| |||||||||||||||||
| Muscle mass, % | 16 | 24.4 ± 3.7 | 24.2 ± 3.9 | 24.5 ± 4.0 | 24.8 ± 3.8 | 16 | 25.7 ± 3.5 | 26.0 ± 4.1 | 26.1 ± 4.3 | 26.1 ± 3.9 | 25.1 ± 3.6 | 25.1 ± 4.1 | 25.3 ± 4.2 | 25.4 ± 3.8 | 0.522 | 0.060 | 0.582 |
|
| |||||||||||||||||
| Waist circumference, cm | 16 | 113.0 ± 12.6 | 112.3 ± 14.5 | 109.1 ± 14.2* | 16 | 112.7 ± 11.4 | 108.6 ± 11.2* | 108.6 ± 10.4* | 112.8 ± 11.8 | 110.5 ± 12.9* | 108.8 ± 12.2* | 0.001 | 0.884 | 0.230 | |||
|
| |||||||||||||||||
| Hip circumference, cm | 16 | 123.7 ± 14.0 | 121.5 ± 16.0 | 121.4 ± 15.8 | 16 | 120.1 ± 9.5 | 117.8 ± 10.2* | 117.0 ± 10.6* | 121.9 ± 11.9 | 119.6 ± 13.4* | 119.2 ± 13.4* | 0.016 | 0.380 | 0.343 | |||
|
| |||||||||||||||||
| WHR | 16 | 0.92 ± 0.08 | 0.93 ± 0.07 | 0.90 ± 0.08 | 16 | 0.94 ± 0.11 | 0.93 ± 0.11 | 0.93 ± 0.10 | 0.93 ± 0.09 | 0.93 ± 0.09 | 0.92 ± 0.09 | 0.116 | 0.372 | 0.167 | |||
|
| |||||||||||||||||
| Ppeak, W | 16 | 186.8 ± 52.8 | 193.3 ± 47.1 | 200.1 ± 50.0 | 14 | 168.9 ± 39.3 | 184.9 ± 41.8 | 187.6 ± 48.9* | 178.5 ± 47.1 | 189.4 ± 44.2 | 194.2 ± 49.0* | 0.005 | 0.279 | 0.542 | |||
|
| |||||||||||||||||
| Ppeak, W/kg | 16 | 1.79 ± 0.46 | 1.96 ± 0.54 | 2.01 ± 0.54 | 14 | 1.66 ± 0.43 | 1.87 ± 0.50 | 1.91 ± 0.55* | 1.73 ± 0.44 | 1.92 ± 0.51 | 1.96 ± 0.54* | 0.002 | 0.372 | 0.833 | |||
|
| |||||||||||||||||
| VO2peak, l/min | 16 | 2.0 ± 0.7 | 2.1 ± 0.7 | 2.0 ± 0.7 | 14 | 2.0 ± 0.6 | 1.8 ± 0.5 | 1.9 ± 0.6 | 2.0 ± 0.7 | 2.0 ± 0.6 | 2.0 ± 0.7 | 0.858 | 0.880 | 0.050 | |||
|
| |||||||||||||||||
| VO2peak, ml/min/kg | 16 | 19.3 ± 6.2 | 20.9 ± 6.2 | 19.8 ± 6.8 | 14 | 19.1 ± 5.8 | 18.5 ± 5.3 | 19.7 ± 6.3 | 19.2 ± 5.9 | 19.8 ± 5.8 | 19.8 ± 6.5 | 0.592 | 0.785 | 0.027 | |||
|
| |||||||||||||||||
| HRpeak, b/min | 16 | 154 ± 16 | 161 ± 14 | 161 ± 16 | 15 | 142 ± 19 | 145 ± 17 | 145 ± 21 | 149 ± 18 | 153 ± 17 | 153 ± 20 | 0.163 | 0.485 | 0.849 | |||
IG = Intervention group; CG = control group; Ppeak = peak power output; VO2peak = peak oxygen uptake; HRpeak = peak heart rate.
Except for HRpeak, no group differences at the beginning of the study were detected.
Indicates differences with respect to pre (post hoc t-test with Bonferroni correction),
Indicates differences with respect to 5 weeks (post hoc t-test with Bonferroni correction).
t-Pre: Different by trend with respect to pre (post hoc t-test with Bonferroni correction).
Different by trend with respect to 5 weeks (post hoc t-test with Bonferroni correction).
Procedures
The study was designed as a randomized, placebo-controlled single blinded intervention study and was performed simultaneously in two different centers. One center was located in Bad Aibling, Germany, (at approximately 490 m above sea level) and one center in Herxheim, Germany, (at approximately 130 m above sea level). Both centers were equipped with the same devices and had access to accredited testing laboratories.
The staff in both centers was experienced in exercise training and was retrained together in guiding training in hypoxia and sham hypoxia as well as performing anthropometric measurements (weight, fat mass etc.). Blood samples were taken by one experienced physician and assistant in both centers. The study protocol consisted of a baseline examination, an 8-month intervention, whereby the IG exercised and rested in hypoxia and the CG in normoxia, with an intermediate and a final examination of all subjects. Blinding of the subjects was done by covering any display in the hypoxic rooms and running the ventilation system at the same power with closed windows in both study groups. The success of blinding was assessed by interview of subjects during intervention.
During the study period, participants were advised to use this training regimen and not to change their usual physical activity and nutritional habits. Before the start of the study, after 5 weeks, after 3 months, and at the end of the intervention anthropometry and body composition were assessed. Laboratory assessment and performance testing were performed at the start, after 3 months, and at the end of the intervention. Anthropometric data comprised weight and height measures to the nearest 0.1 kg and 0.5 cm, respectively, and hip and waist circumference measures to the nearest 0.5 cm. Body composition was determined by bioelectrical impedance analysis (OMRON BF511 T Monitor, Healthcare Co., Kyoto, Japan) and included estimation of total body fat and muscle mass. For percent body fat estimation the standard error of the estimate (SEE) of the device is 3.5% (instruction manual, OMRON BF511, technical data). Measurements were completed according to the manufacturer's guidelines.
Laboratory measurements from venous blood samples were performed in accredited laboratories and included the determination of glucose concentration (G), glycated hemoglobin (HbA1c), high-density lipoprotein (HDL), total cholesterol (CHOL) and triglycerides (TG). Resting ECG records and blood pressure measurement were taken before participants did an incremental exercise test to exhaustion on a cycle ergometer (Ergo-Fit Cycle 3000 MED, Ergo Fit, Pirmasens, Germany). The exercise test consisted of cycling for 2 min at 5 W; then work rate was increased by 5 W every 12 s. During the test, heart rate was measured and gas exchange variables, including ventilation (VE), oxygen uptake (VO2) and carbon dioxide production (VCO2) were recorded using an ergospirometric device (MetaVital, Cortex Biophysik Gmbh, Leipzig, Germany).
Intervention
During the 8-month study period, 52 intervention sessions had to be completed. Participants exercised twice a week for 90 min and rested for additional 90 min in normobaric hypoxic chambers. The IG exercised at a FiO2 of 14.0 ± 0.2%, corresponding to approximately 3,500 m and rested at a FiO2 of 12.2 ± 0.3%, equivalent to approximately 4,500 m. The CG followed the same protocol, in the same chambers but under normoxia conditions. Both groups exercised at a moderate intensity of 65-70% of the maximal heart rate, approximately corresponding to the intensity that elicits maximal fat oxidation rates in the untrained (i.e., 47-52% of VO2max) [17]. Participants could exercise either on a cycle ergometer, a treadmill, or a cross trainer. When treadmill or cross trainer was used, the target heart rate was increased by 10 beats/min [18]. After 3 months, target heart rate was adapted according to the outcome of an additional incremental exercise test.
Statistical Analysis
A power analysis based on the results of our preliminary 3- week investigation [12] revealed that a minimum of 16 persons in each group would have enough statistical power to show significance in differences of body weight and laboratory parameters between hypoxia and sham hypoxia.
Unpaired t-tests were used to investigate baseline differences between groups. To check if the double center approach might have had any influence on the main outcome parameter (body weight), we performed an analysis of variance (ANOVA) with repeated measurement design with center as between-subject and measurement time points as within-subject factor. To investigate the influence of hypoxia and to check for a possible gender effect, ANOVA was also done for repeated measurements with 2 between-subjects factors (hypoxia vs. normoxia, men vs. women) and one within-subject factor (measurement time before, in between, and after the exercise period). When significant time effects were found post hoc t-tests with Bonferroni correction were applied to locate the differences. Pearson's correlation analyses were applied to investigate relationships between physical fitness parameter and body composition changes and changes of the metabolic risk markers. Changes were calculated as post-values minus pre-values. Results are presented as means ± SD. Significance was set at p ≤ 0.05.
Results
No center difference for the main outcome parameter weight was found via ANOVA. Blinding was successful as more than 60% of subjects guessed their group incorrectly. There were no serious adverse health effects recorded in both groups during the study period.
Participants exercised at a mean intensity corresponding to 68.3 ± 6.3% of the HRpeak. Changes for parameters of the physical fitness level and body composition over the 8-month intervention period are shown in table 1. Body weight, BMI, waist and hip circumference, and peak power output (Ppeak) improved over time (p < 0.05), with no differences between groups and no gender effect.
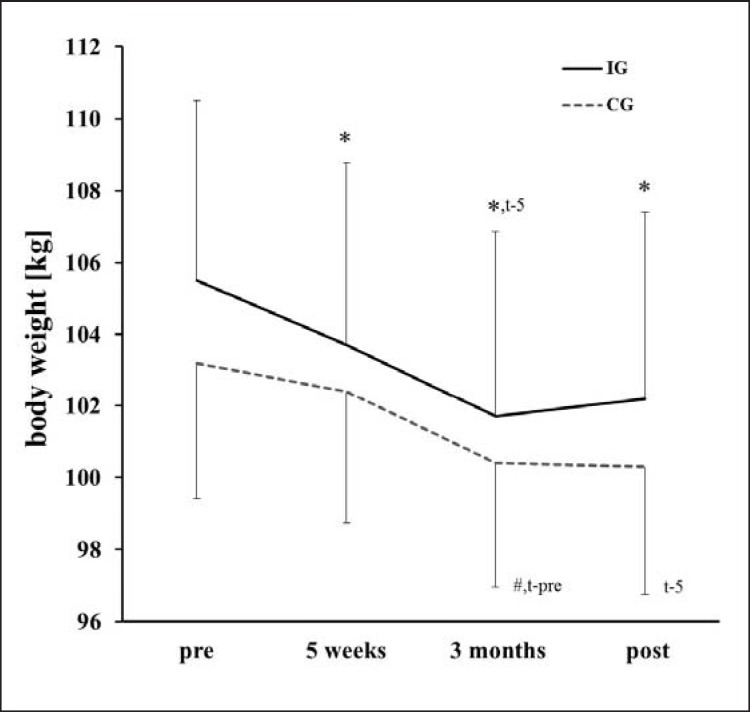
With respect to the body weight, post hoc tests showed that until month 3 values decreased and then stabilized (fig. 2). Table 2 shows changes for the cardiovascular risk factors. Systolic blood pressure improved over the course of the intervention, with no group difference and no gender effect. No correlation was found for the improvements in Ppeak and metabolic risk factors. Reductions in fat mass and increases in muscle mass correlated with changes in HDL (r = −0.427 and r = 0.421, respectively; p < 0.05) Changes in waist circumference and waist-to-hip ratio tended to correlate with changes in TG (r = 0.378 and r = 0.346, respectively; 0.05 < p < 0.1).
Fig. 2.
Weight loss over the course of the intervention for the intervention (IG) and the control group (CG). *Indicates differences with respect to pre (post hoc t-test with Bonferroni correction). #Indicates differences with respect to 5 weeks (post hoc t-test with Bonferroni correction). t-preDifferent by trend with respect to pre (post hoc t-test with Bonferroni correction). t-5Different by trend with respect to 5 weeks (post hoc t-test with Bonferroni correction).
Table 2.
Changes of the cardiovascular risk factor parameters over the 8-month intervention period a
| IG |
CG |
All |
ANOVA, p value |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | pre | 3 months | post | n | pre | 3 months | post | pre | 3 months | post | main effect: time | main effect: sex | interaction: time × group | |
| BPsys, mm Hg | 13 | 134.2 ± 9.7 | 129.6 ± 12.1 | 129.5 ± 11.5 | 14 | 125.8 ± 15.2 | 123.0 ± 21.4 | 118.6 ± 14.3 | 129.8 ± 13.4 | 126.2 ± 17.5 | 123.9 ± 14.0 t-pre | 0.033 | 0.344 | 0.577 |
|
| ||||||||||||||
| BPdia, mm Hg | 13 | 91.7 ± 11.0 | 88.7 ± 8.1 | 89.8 ± 8.2 | 14 | 86.8 ± 9.4 | 84.6 ± 12.2 | 82.4 ± 7.8 | 89.1 ± 10.3 | 86.6 ± 10.5 | 86.0 ± 8.7 | 0.061 | 0.531 | 0.489 |
|
| ||||||||||||||
| HDL, mg/dl | 12 | 49.4 ± 14.3 | 50.3 ± 18.0 | 50.4 ± 13.0 | 15 | 53.1 ± 16.0 | 52.1 ± 13.7 | 52.7 ± 13.3 | 51.5 ± 15.1 | 51.3 ± 15.4 | 51.7 ± 13.0 | 0.845 | 0.411 | 0.939 |
|
| ||||||||||||||
| Cholesterol, mg/dl | 12 | 206.9 ± 29.7 | 208.3 ± 34.0 | 213.8 ± 33.0 | 15 | 208.7 ± 37.5 | 208.1 ± 47.2 | 197.7 ± 37.7 | 207.9 ± 33.6 | 208.1 ± 41.1 | 204.8 ± 36.9 | 0.818 | 0.423 | 0.123 |
|
| ||||||||||||||
| Triglyceride, mg/dl | 12 | 210.5 ± 100.2 | 205.4 ± 115.0 | 224.3 ± 105.0 | 15 | 176.1 ± 62.0 | 176.3 ± 68.0 | 154.1 ± 51.6 | 191.4 ± 81.4 | 189.3 ± 91.1 | 185.2 ± 85.8 | 0.862 | 0.814 | 0.467 |
|
| ||||||||||||||
| HbA1c, % | 16 | 5.8 ± 0.8 | 5.5 ± 0.8 | 6.0 ± 0.6 | 15 | 5.9 ± 0.9 | 5.9 ± 1.2 | 5.8 ± 0.4 | 5.9 ± 0.8 | 5.7 ± 1.0 | 5.9 ± 0.5 | 0.053 | 0.110 | 0.117 |
|
| ||||||||||||||
| Glucose, mg/dl | 12 | 123.0 ± 41.3 | 111.1 ± 18.6 | 120.1 ± 40.4 | 15 | 117.3 ± 39.0 | 116.3 ± 40.1 | 111.7 ± 16.8 | 119.8 ± 39.4 | 114.0 ± 32.0 | 115.4 ± 29:3 | 0.853 | 0.341 | 0.411 |
IG = Intervention group; CG = Control group; BPsys = systolic blood pressure; BPdia = diastolic blood pressure; HDL = high-density lipoprotein; HbA1c = glycated hemoglobin.
No group differences at the beginning of the study were detected.
t-pre: Different by trend with respect to pre (post hoc t-test with Bonferroni correction.
Discussion
To the best of our knowledge, this was the first long-term (several months) and two-center randomized sham controlled study for health improvement as well as weight and fat loss in simulated hypoxia. It was also the first study where a financially responsible and acceptable time-consuming hypoxia training prevention program was performed. The main findings refute the hypothesis in that the present study found that long-term exposure and moderate intensity exercise in hypoxia does not enhance loss of body weight or improve metabolic markers more than with resting and exercising in normoxia alone. Thus the health prevention strategy against obesity using hypoxia has no advantage over regular obesity prevention and healthy lifestyle programs with moderate exercise. Independent of hypoxia, however, the level of moderate intensity exercise led to beneficial reductions in body weight, BMI, waist and hip circumference as well as improvements in Ppeak and systolic blood pressure. With respect to the body composition, the greatest effects were found within the first 3 months of the intervention, with only slight changes thereafter.
There is a limited number of studies investigating the influence of 4 weeks of hypoxia exercise on body composition and metabolic risk markers in the obese. The authors showed that fat mass and body weight was reduced to a greater extent and diastolic blood pressure reduction tended to be higher after 4 weeks of hypoxia exercise (FiO2 approximately 15%) performed at the same relative intensity when compared to normoxia conditions [12,14,19]. Furthermore, in a non-obese population Haufe et al. [13] showed better improvements in body fat content, triglycerides, HOMA index, and fasting insulin when exercising for 4 weeks under hypoxia conditions compared to normoxia. The authors of these studies speculated that altered catecholamine, thyroid hormone, and/or leptin levels might be involved in changing substrate metabolism and food intake patterns and thus could have affected body composition [12,14]. In contrast, the present investigation found no added effects of hypoxia on body composition after 5 weeks.
With respect to the long-term effects (i.e., 8 months), hypoxia exercise had no additional effect on body composition and metabolic risk factors when compared to normoxic exercise. This results are in accordance with those of Engfred et al. [20], who suggested that there could be an upper limit for the degree of endocrine adaptation to exercise and that hypoxia and normoxia training elicit comparable adaptations of the glucoregulatory hormones and of metabolites reflecting fat and carbohydrate metabolism. Interestingly after 3 months of exercise no further weight loss was found (fig. 2). It could be suggested that after 3 months the adaptations to the same stimulus might be abolished and leads to a stabilization of the effects.
Beside the unaltered exercise stimulus, the rather moderate exercise intensity applied in the present investigation might also have influenced outcomes. As mentioned, the intervention improved body composition, Ppeak and systolic blood pressure, independently of the hypoxia stimulus, but did not enhance VO2peak. This could indicate that exercise intensity was too low to improve maximal aerobic capacity. Accordingly Powell et al. [9] stated that vigorous activity seems to induce larger improvements in physical performance and to be more cardioprotective than moderate intensity exercise. In the present study the exercise program was not designed to improve performance but to enhance fat and body weight loss [17], and as such improvements in body composition, though not to a dramatic extent, were achieved. Nonetheless, higher exercise intensity might have been necessary to achieve enhancements of maximal aerobic capacity and to reveal a cardioprotective effect. It has to be mentioned that when exercising at the same relative intensity the cardiovascular strain is the same in hypoxia or normoxia even though the mechanical strain is reduced under hypoxia [14]. Therefore, the finding of a comparable body weight loss under both conditions could be of particular importance for the obese patient who cannot attain a given work load because of orthopedic or spinal comorbidities.
Some points of the study protocol have to be critically discussed as limitations. During the 8-month intervention period, no information on food intake and physical activity patterns were collected. Even though participants were instructed not to change their usual physical activity and nutritional habits, some favorable behavioral alterations might have occurred outside the study protocol. However, as such alterations would be desirable in real life settings, their influence, which should be low, should not constrain present conclusions. The dropout rate of our study population (51.7%) is comparable to earlier long-term clinical trials of exercise [21] and behavioral life changing studies dealing mainly with nutritional intervention [22,23,24], but is somewhat lower than current studies reporting adherence rates of approximately 75% [25]. Importantly, according to the pre-study power analysis the number of finishing participants suggested statistical power was achieved. Nevertheless, the loss of participants might have led to a selection bias with those already more motivated and fit continued and those, who could have even more gained from the program because of a less physical fitness and higher body weight, dropped out. However, we do not think that this changed the outcome of the study to a critical extent. The assessment of percent body fat with a relatively large SEE of approximately 3.5% represents a further limitation of the study and could explain why percent body fat did not significantly decrease.
Conclusions
In conclusion, short- and/or long-term exercise at a moderate intensity led to reductions in body weight, BMI, and waist and hip circumferences as well as to improvements in Ppeak and systolic blood pressure. Furthermore, data from the present study indicate no added effect by hypoxia in the long term, at least when stimuli (i.e., hypoxia dose, exercise intensity, and duration) are unaltered. Nonetheless, overweight persons with orthopedic problems might profit from such training as comparable adaptations, despite lower absolute workloads and thus lower mechanic strain, can be obtained.
Disclosure Statement
The authors declared that have no conflict of interest.
References
- 1.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 2.Scaglione R, Argano C, Di Chiara T, Licata G. Obesity and cardiovascular risk: the new public health problem of worldwide proportions. Expert Rev Cardiovasc Ther. 2004;2:203–212. doi: 10.1586/14779072.2.2.203. [DOI] [PubMed] [Google Scholar]
- 3.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK, American College of Sports Medicine American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459–471. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 4.Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. doi: 10.1186/1471-2458-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laddu D, Dow C, Hingle M, Thomson C, Going S. A review of evidence-based strategies to treat obesity in adults. Nutr Clin Pract. 2011;26:512–525. doi: 10.1177/0884533611418335. [DOI] [PubMed] [Google Scholar]
- 6.Seagle HM, Strain GW, Makris A, Reeves RS, American Dietetic Association Position of the American Dietetic Association: weight management. J Am Diet Assoc. 2009;109:330–346. doi: 10.1016/j.jada.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 7.Casazza K, Fontaine KR, Astrup A, Birch LL, Brown AW, Bohan Brown MM, Durant N, Dutton G, Foster EM, Heymsfield SB, McIver K, Mehta T, Menachemi N, Newby PK, Pate R, Rolls BJ, Sen B, Smith DL, Jr, Thomas DM, Allison DB. Myths, presumptions, and facts about obesity. N Engl J Med. 2013;368:446–454. doi: 10.1056/NEJMsa1208051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burtscher M, Gatterer H, Kunczicky H, Brandstätter E, Ulmer H. Supervised exercise in patients with impaired fasting glucose: impact on exercise capacity. Clin J Sport Med. 2009;19:394–398. doi: 10.1097/JSM.0b013e3181b8b6dc. [DOI] [PubMed] [Google Scholar]
- 9.Powell KE, Paluch AE, Blair EN. Physical activity for health: What kind? How much? How intense? On top of what? Annu Rev Public Health. 2011;32:349–365. doi: 10.1146/annurev-publhealth-031210-101151. [DOI] [PubMed] [Google Scholar]
- 10.Blair SN, Kampert JB, Kohl HW, Barlow CE, Macera CA, Paffenbarger RS, Jr, Gibbons LW. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA. 1996;276:205–210. [PubMed] [Google Scholar]
- 11.Myers J, Kaykha A, George S, Abella J, Zaheer N, Lear S, Yamazaki T, Froelicher V. Fitness versus physical activity patterns in predicting mortality in men. Am J Med. 2004;117:912–918. doi: 10.1016/j.amjmed.2004.06.047. [DOI] [PubMed] [Google Scholar]
- 12.Netzer NC, Chytra R, Küpper T. Low intense physical exercise in normobaric hypoxia leads to more weight loss in obese people than low intense physical exercise in normobaric sham hypoxia. Sleep Breath. 2008;12:129–134. doi: 10.1007/s11325-007-0149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haufe S, Wiesner S, Engeli S, Luft FC, Jordan J. Influences of normobaric hypoxia training on metabolic risk markers in human subjects. Med Sci Sports Exerc. 2008;40:1939–1944. doi: 10.1249/MSS.0b013e31817f1988. [DOI] [PubMed] [Google Scholar]
- 14.Wiesner S, Haufe S, Engeli S, Mutschler H, Haas U, Luft FC, Jordan J. Influences of normobaric hypoxia training on physical fitness and metabolic risk markers in overweight to obese subjects. Obesity (Silver Spring) 2010;18:116–120. doi: 10.1038/oby.2009.193. [DOI] [PubMed] [Google Scholar]
- 15.Lippl FJ, Neubauer S, Schipfer S, Lichter N, Tufman A, Otto B, Fischer R. Hypobaric hypoxia causes body weight reduction in obese subjects. Obesity (Silver Spring) 2010;18:675–681. doi: 10.1038/oby.2009.509. [DOI] [PubMed] [Google Scholar]
- 16.Yank V, Stafford RS, Rosas LG, Ma J. Baseline reach and adoption characteristics in a randomized controlled trial of two weight loss interventions translated into primary care: a structured report of real-world applicability. Contemp Clin Trials. 2013;34:126–135. doi: 10.1016/j.cct.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Achten J, Jeukendrup AE. Optimizing fat oxidation through exercise and diet. Nutrition. 2004;20:716–727. doi: 10.1016/j.nut.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Millet GP, Vleck VE, Bentley DJ. Physiological differences between cycling and running: lessons from triathletes. Sports Med. 2009;39:179–206. doi: 10.2165/00007256-200939030-00002. [DOI] [PubMed] [Google Scholar]
- 19.Kong Z, Zang Y, Hu Y. Normobaric hypoxia training causes more weight loss than normoxia training after a 4-week residential camp for obese young adults. Sleep Breath. 2013;18:591–597. doi: 10.1007/s11325-013-0922-4. [DOI] [PubMed] [Google Scholar]
- 20.Engfred K, Kjaer M, Secher NH, Friedman DB, Hanel B, Nielsen OJ, Bach FW, Galbo H, Levine BD. Hypoxia and training-induced adaptation of hormonal responses to exercise in humans. Eur J Appl Physiol Occup Physiol. 1994;68:303–309. doi: 10.1007/BF00571448. [DOI] [PubMed] [Google Scholar]
- 21.Oldridge NB. Compliance and exercise in primary and secondary prevention of coronary heart disease: a review. Prev Med. 1982;11:56–70. doi: 10.1016/0091-7435(82)90005-6. [DOI] [PubMed] [Google Scholar]
- 22.Dalle Grave R, Calugi S, Molinari E, Petroni ML, Bondi M, Compare A, Marchesini G, QUOVADIS Study Group Weight loss expectations in obese patients and treatment attrition: an observational multicenter study. Obes Res. 2005;13:1961–1969. doi: 10.1038/oby.2005.241. [DOI] [PubMed] [Google Scholar]
- 23.Busetto L, Mazza M, Salvalaio S, De Stefano F, Marangon M, Calò E, Sampietro S, Enzi G. Obesity treatment in elderly outpatients: predictors of efficacy and drop-out. Eat Weight Disord. 2009;14:e56–65. doi: 10.1007/BF03327801. [DOI] [PubMed] [Google Scholar]
- 24.Colombo O, Ferretti VV, Ferraris C, Trentani C, Vinai P, Villani S, Tagliabue A. Is drop-out from obesity treatment a predictable and preventable event? Nutr J. 2014;13:13. doi: 10.1186/1475-2891-13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller FL, O'Connor DP, Herring MP, Sailors MH, Jackson AS, Dishman RK, Bray MS. Exercise dose, exercise adherence, and associated health outcomes in the TIGER study. Med Sci Sports Exerc. 2014;46:69–75. doi: 10.1249/MSS.0b013e3182a038b9. [DOI] [PMC free article] [PubMed] [Google Scholar]