Abstract
Plant-parasitic nematodes are important agricultural pests and often cause serious crop losses. Novel, environmental friendly nematicides are urgently needed because of the harmful effects of some existing nematicides on human health. 5-Aminolevulinic acid (ALA) was reported as a potential biodegradable herbicide, insecticide, or plant-growth promoting agent. Lack of information on ALA against plant-parasitic nematodes prompted this investigation to determine the effects of ALA on Meloidogyne incognita, Heterodera glycines, Pratylenchus coffeae, and Bursaphelenchus xylophilus. A series of in vitro assays and one greenhouse trial were conducted to examine the nematicidal effects of ALA. The results demonstrated that ALA exhibited a strong effect of suppression against the four nematodes tested. ALA also inhibited hatching of M. incognita and H. glycines. Results from the greenhouse experiment indicated that treatment of soil with 6.0 mM ALA significantly reduced the root-gall index (RGI) and egg mass number per root system compared with the uninoculated control (P ≤ 0.05). The metabolism assays indicated that ALA treatment significantly altered the nematode metabolism including the total protein production, malondialdehyde (MDA) content, and oxidase activities. This study suggested that ALA is a promising nematicide against plant-parasitic nematodes.
Keywords: Bursaphelenchus, Heterodera, Meloidogyne, nematicide, oxidase activity, Patylenchus
Plant-parasitic nematodes are important pests for many agricultural crops, especially for the crops grown in the tropical and subtropical regions. The estimated annual crop losses caused by plant-parasitic nematodes were estimated at about US$100 billion worldwide (Abawi and Widmer, 2000; Oka et al., 2000; Chitwood, 2003). The current control strategy for plant-parasitic nematodes depends largely on applications of nematicides. However, because of environmental concerns many effective nematicides have been restricted from the market in recent years (Schneider et al., 2003).
The identification of novel and environmental friendly nematicides is of great interest to plant pathologists and the agricultural industry. For example, many current studies are now focused on the characterization of naturally occurring compounds found in bacteria that possessed nematicidal properties. A strain (PSB-01) of photosynthetic bacteria, Rhodoblastus acidophilus, was recently determined to be suppressive to root-lesion nematode (P. coffeae) on ramie (Boehmeria nivea [L.] Gaud.). A gene encoding the 5-aminolevlinate synthase was cloned from this bacterial strain (PSB-01) (Zhang et al., 2007). Preliminary study suggested that application of 3.0 mM of ALA significantly inhibited hatching of M. incognita and H. glycines, and significantly altered the expression levels of superoxide-dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and MDA in second stage juveniles (J2s) of M. incognita (Cheng et al., 2014).
ALA is an essential precursor produced by R. acidophilus during biosynthesis of porphyrins such as chlorophyll and heme (Sasaki et al., 2002). It was reported that ALA could be used as a potential biodegradable herbicide, insecticide or plant-growth promoting agent (Sasikala et al., 1994). Rebeiz et al. (1984, 1988) indicated that ALA could be used as a biodegradable herbicide, and exogenous ALA was toxic to cabbage looper (Trichoplusia ni Hubner). Amor et al. (2000) demonstrated that ALA had a strong insecticidal activity through both laboratory and field studies. Researchers reported that ALA could be used to kill locust (Oxya chinensis), an important pest for rice (Oryza sativa), maize (Zea mays), sorghum (Sorghum bicolor), and many other food crops in China (Li et al., 2005; Yin et al., 2008). Yin et al. (2008) also observed that application of ALA to O. chinensis resulted in significant changes in acetylcholinesterase (AChE), glutathione S-transferase, and GPx expression levels. The objective of this research was to examine the effects of ALA on four economically important plant-parasitic nematodes in China, namely M. incognita, H. glycines, P. coffeae, and B. xylophilus. In addition, the effects of ALA on hatching from either free eggs or egg masses/cysts, J2 development, and morphological changes on M. incognita and H. glycines were also investigated.
Materials and Methods
Culture of plant-parasitic nematode inoculum:
M. incognita was isolated from roots of tomato plants grown in the Hunan Province, China, and maintained on roots of tomato grown in sterile soil in a greenhouse at 25 ± 2°C, 12 hr light of day light (Cheng et al., 2010). H. glycines was obtained from Deliang Peng (Institute of Plant Protection, Chinese Academy of Agricultural Sciences, Beijing, China) and maintained in sterile soil on soybean (Glycine max) in a greenhouse. The roots of tomato or soybean infected with M. incognita and H. glycines, respectively, were removed from the pots and washed several times in water. Egg masses of M. incognita or brown cysts of H. glycines were hand-picked from the infected roots and sterilized in a 0.5% NaOCl solution for 3 min. After three washes in sterile water, the egg masses and brown cysts were placed separately on 200-mesh sieves submerged in water and incubated for 3 d inside a 25 ± 2°C incubator to collect J2s daily and stored at 13°C. J2s younger than 3 d after hatch were used for an in vitro assay experiment. In another in vitro assay experiment, eggs of M. incognita and H. glycines were extracted from egg masses or brown cysts using the method published by Hussey and Barker (1973). The total number of J2s or free eggs in a 100-μl suspension was determined by counting each sample four times under an inverted microscope. P. coffeae was isolated from ramie and cultured on carrot (Daucus carota linn. var. sativa Hoffm) callus cultures inside the laboratory as described by Moody et al. (1973). B. xylophilus was provided by Xinyue Cheng from Beijing Normal University, Beijing, China and cultured on Botrytis cinerea mats grown on 1% potato-dextrose agar plates at 25°C (Cheng et al., 2008). P. coffeae and B. xylophilus were harvested separately using the Baermann funnel technique (Mamiya, 1975). Juveniles and adults of P. coffeae and B. xylophilus were mixed and used for the in vitro assay experiment.
Toxicity of ALA on vermiform stages:
ALA obtained from Sigma (Sigma-Aldrich, St. Louis, MO) was dissolved in sterile water to prepare six concentrations of ALA at 1.5, 3.0, 6.0, 12.0, 24.0, and 48 mM. The motility assay was then carried out in wells of 24-well plates by mixing 1 ml ALA solution at a specific concentration with 1 ml nematode suspension containing about 100 nematodes in a single well (Thus, the concentration of ALA tested were 0.75, 1.5, 3.0, 6.0, 12.0, and 24.0 mM). The plates were incubated under 25 ± 2°C and a relative humidity of 60% (Li et al., 2009). Nematode suspensions mixed with sterile water were used as the controls. All treatments were replicated four times, and the experiment was performed three times. The number of active and dead nematode was counted at 48 hr post ALA treatment under an inverted optical microscope. Nematodes were considered dead if they did not move on probing with a pin. Mortality rate was calibrated as (X − Y)/(100 − Y) × 100, where X and Y indicated the mortality rates for the ALA-treated samples and water-treated controls, respectively.
Effect of ALA on hatching of free egg:
Suspensions of M. incognita or H. glycines free eggs were chosen for this study. One milliliter of 3.0, 6.0, 12.0, or 24.0 mM ALA solution was mixed with an equal volume of free egg suspension in wells of a 24-well plate. The plates were incubated inside an incubator at 25 ± 2°C and 60% RH. The number of freshly hatched J2s was recorded for each treatment at 0, 3, 6, 9, and 12 d after incubation. The percentage of egg hatch was calculated as (J2Dx/eggs at D0) × 100, where D0 was the number of eggs at day zero, and J2Dx was the number of freshly hatched J2s at day x. The inhibition rate of hatch was calculated based on ([the percentage of the hatched J2s for the control − the percentage of the hatched J2s for a particular ALA treatment]/the percentage of the hatched J2s for the control) × 100. Four biological replicates were used for each treatment in each experiment, and the experiment was repeated three times.
Effect of ALA on hatching of egg masses or brown cysts:
Ten mature egg masses of M. incognita were handpicked from pure culture of M. incognita on tomato roots and placed in a well of 24-well plate containing 2 ml of water (control) or 1.5, 3.0, 6.0, or 12.0 mM ALA solution. In a separate assay for the cyst of H. glycines, 10 brown cysts were handpicked from the pure culture of H. glycines on soybean roots and incubated in the same solutions as described for the M. incognita egg masses assay. After 5 d of incubation, the ALA solutions were removed, and the egg masses or brown cysts were washed in sterile water and then allowed to develop in deionized water. The cumulative J2 hatch for each treatment was recorded over 25 d, and hatch inhibition rate was calculated as ([the number of hatched J2s from the control − the number of hatched J2s from a particular treatment]/the number of J2s in the control) × 100. The experiment was repeated three times, and each treatment had four replications.
Effect of ALA against infection of M. incognita on greenhouse tomato:
Seeds of tomato cv. L3708 were germinated in sterilized sand:soil mix (1:2). The seedlings were transplanted individually into pots containing 2.5 kg mixed soil per pot. When the seedlings reached 3- to 4-leaf stage, 100 ml of 1.5, 3.0, or 6.0 mM ALA solution was drenched into each pot. Pots supplied with 100 ml of 1:1000 diluted 1.8% avermectin emulsifiable concentrate (Syngenta Crop Protection, Inc., China) solution or water were used as the positive and negative controls, respectively. Two days after the application of ALA, avermectin, or water, 500 freshly hatched M. incognita J2s were introduced into the soil near the root per pot. Experiment was arranged in a randomized complete block design, with three replicated blocks of 10 tomato pots per block. The pots were maintained inside a greenhouse set at 25 ± 2°C. Two month later, roots of each assayed plant were harvested. The number of galls per root was counted using a handheld lens, and six strains of each treatment were selected randomly for counting the number of egg masses per root under a microscope after 15 to 20 min staining in a phloxine-B solution (0.15 g/liter tap water) followed by a quick rinse in water. The RGI was recorded in a scale of 0 to 4 as described by Barker (1978). The experiment was performed twice.
Microscopic examination:
Approximately 100 J2s of M. incognita were immersed in 3.0 mM ALA solution in a 15-mm diameter glass dish or in sterile water as a control. The J2s were then maintained at 25 ± 2°C. Each treatment had three biological replicates, and the experiment was performed twice. Morphology and behavior of M. incognita J2s were examined under a Zeiss Observer A1 inverted microscope (Carl Zeiss AG, Oberkochen, Germany) at 2-hr intervals for 2 d.
Transmission electron microscopy (TEM):
Nematode samples for TEM were prepared based on the method described by Baldwin and Hirschmann (1975) with slight modification. Approximately 1,000 M. incognita J2s were placed in a 2-ml centrifuge tube containing 1 ml of 3.0 mM ALA solution or sterile water (control), and the tubes were kept inside an incubator (25 ± 2°C) for 48 hr. After 10 min centrifugation at 3,000 g, the pelleted M. incognita J2s were collected followed by three rinses in sterile water. The J2s were picked up with needles under an inverted microscope and placed in 200 μl 2.5% glutaraldehyde solution overnight at 4°C. After 10 min centrifugation at 3,000 g, the fixative solution was removed, and the J2 samples were rinsed three times in a phosphate-buffered saline (PBS) buffer of pH 7.0. The rinsed J2s were further fixed in a 1% buffered osmium tetroxide solution for 2 hr. After three rinses in PBS and dehydration in a serial diluted ethanol solutions, the J2s were embedded in Araldite (Sigma-Aldrich). Ultrathin sections were cut, mounted on EM grids and examined under an electron microscope. The morphological changes of 10 J2s from each treatment were examined, and the experiment was performed twice.
Sample preparation for metabolism assays:
M. incognita J2s were obtained as described previously. Approximately 6 × 106 M. incognita J2s were pelleted by 10 min centrifugation at 3,000 g, weighed, and used for each treatment. The resulting J2s were immersed in 3.0 mM ALA solution or in sterile water (control) followed by 24 hr incubation at 25 ± 2°C. The J2s were pelletized again for 10 min at 3,000 g, rinsed three times in sterile water, and resuspended in 500 μl PBS containing 0.3% Triton-100, pH 7.8. The J2 samples were stored at −80°C till use.
Effect of ALA on AChE activity:
J2s obtained previously were frozen and thawed several times, homogenized with a glass rod, and centrifuged for 15 min at 5,000 g and 4°C. Supernatant of each sample was used for the AChE activity and protein content assays. Activity of AChE was determined using a published method (Ellman et al., 1961). After the reaction was terminated by addition of 50 μl 2.5 mM TCA, the absorbance at 412 nm was measured using a Shimadzu UV-1601 spectrophotometer (Shanghai, China). Each treatment had three biological replicates, and the experiment was performed twice.
Effect of ALA on oxidase activities and MDA content:
M. incognita J2s prepared for the metabolism assay were grinded in the chilled PBS buffer followed by 15 min centrifugation at 5,000 g and 4°C. Each supernatant was adjusted to 1 ml with the PBS buffer (pH 7.0) and used immediately for the SOD, CAT, and GPx activity assays, and the MDA content assay. Each treatment was repeated three times, and the experiment was performed twice. SOD activity was measured by a modified method described by Aebi (1974). Inhibition of nitro blue-tetrazolium (NBT) reduction was monitored at 560 nm. Fifty microliter supernatant from a treatment was added into a tube containing 3 ml reaction mixture (1 × PBS, pH 7.8, with 50 mM methionine, 750 μM NBT, 20 μM riboflavin, and 0.1 mM EDTA). The reaction was allowed to occur for 10 min at 25°C and under visible light. Control reaction was performed in the same manner without the supernatant. SOD absorbance of individual samples was read at 560 nm using a spectrophotometer (Mettler Toledo, Switzerland). One unit activity was defined based on the amount of supernatant needed to achieve 50% inhibition of the NBT reduction rate. The SOD activity was presented as units (U) per milligram total protein in the supernatant and was calculated using the formula:
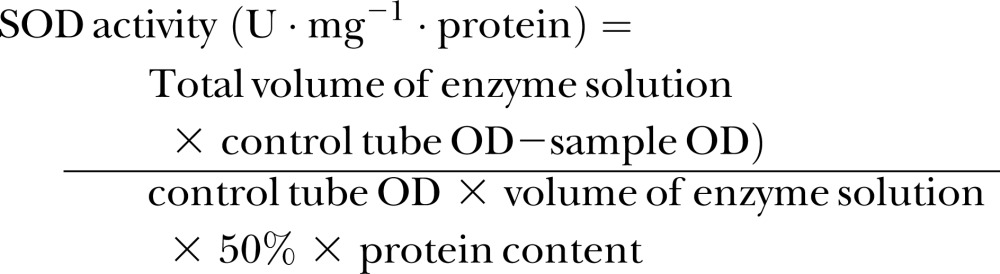 |
where OD was the optical density.
CAT activity was assayed by spectrophotometry, and the H2O2 dismutation monitored at 240 nm. A freshly made mixture containing 0.1 ml H2O2 and 0.1 ml supernatant was measured immediately. One unit of CAT activity was defined as a reduction of 0.01 OD value per minute. GPx activity was measured following the instructions (Nanjing Institute of Biological Engineering Co., Ltd., Nanjing, China). The activity was presented in units per milligram protein. One unit was defined as the amount of GPx needed to oxidase 1 μmol glutathione per minute.
MDA content was determined by the thiobarbituric acid reaction method as described by Draper and Hadley (1990). Briefly, 0.5 ml of supernatant was mixed with 1.5 ml 5% trichloroacetic acid (TCA) in a tube. After addition of 0.5 ml 0.5% thiobarbituric acid into the tube, the mixture was boiled for 30 min. The reaction was then stopped by chilling the tubes on ice followed by 10 min centrifugation at 5,000 g. Supernatant was collected from each tube, and their light absorbance at 532 and 450 nm were measured. Concentration of MDA in each sample was estimated by subtracting the base absorbance at 450 nm from the absorbance at 532 nm and calculated using the extinction coefficient of 155 mM−1 cm−1.
Effect of ALA on protein content:
Second-stage juvenile samples were ground individually in the chilled PBS buffer (pH 7.0). After 10 min centrifugation at 3,000 g, the supernatant was collected for the soluble protein content assay. For insoluble protein content, 0.5 ml deionized water was added to a pellet, and the tube was incubated at 65°C for 15 min followed by 15 min centrifugation at 5,000 g. The resulting pellet was resuspended in 0.5 ml deionized water and 1 ml 10% TCA, incubated for 10 min, and centrifuged for 10 min at 3,000 g. The supernatant was discarded and the pellet was dissolved in 1 ml of 1 M NaOH for the insoluble protein content assay. The protein content was measured by the Bradford test (Bradford, 1976). In addition, the sizes of proteins in the sample were estimated in 12.5% SDS-polyacrylamide gels through electrophoresis (Beeley et al., 1996). Each treatment was repeated three times and the experiment was performed twice.
Statistical analysis:
Results from all the experiments were analyzed using the SPSS (version 20.0; International Business Machines Corporation, Armomk, NY). All data were subjected to one-way analysis of variance. Mean separation was conducted using the Waller–Duncan k-ratio t-test (k = 100, P ≤ 0.05) wherever appropriate. The 95% fiducial limits were calculated by the probit analysis.
Results
Toxicity of ALA on vermiform stages:
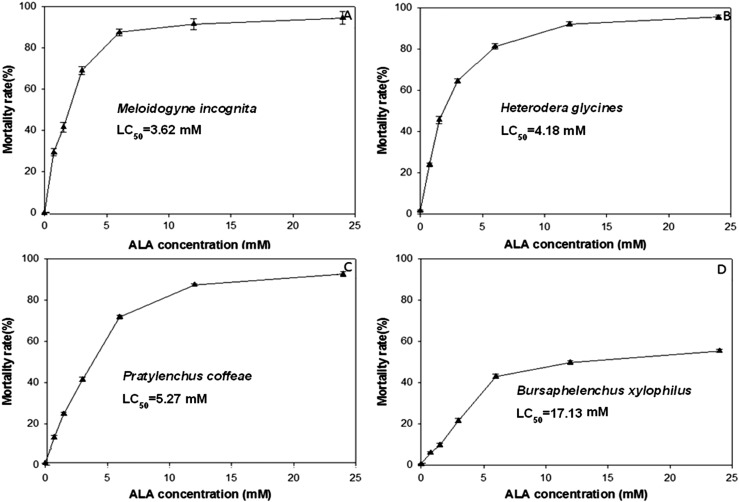
The mortality rates of M. incognita, H. glycines, and P. coffeae increase logarithmically as concentration of ALA increased, and reach 90% mortality at ≤12 mM (Fig. 1A–C). However, the toxicity of ALA on B. xylophilus was significantly less than that observed for the other three nematodes, and the mortality rate of B. xylophilus reached only slightly above 40% at 12.0 mM ALA application (Fig. 1D). The results from these in vitro assays showed that the LC50 of ALA against M. incognita, H. glycines, P. coffeae, and B. xylophilus at 48 hr after treatment were 3.62, 4.18, 5.27 and 17.13 mM, respectively (Fig. 1).
Fig. 1.
Toxicity of 5-aminolevulinic acid (ALA) on four nematode species at 48 hr after treatment. Mortality rates of (A) Meloidogyne incognita; (B) Heterodera glycines; (C) Pratylenchus coffeae; and (D) Bursaphelenchus xylophilus treated with various concentrations of ALA or water. Each data point is the mean of 12 replications along with its standard error bar.
Effect of ALA on hatching:
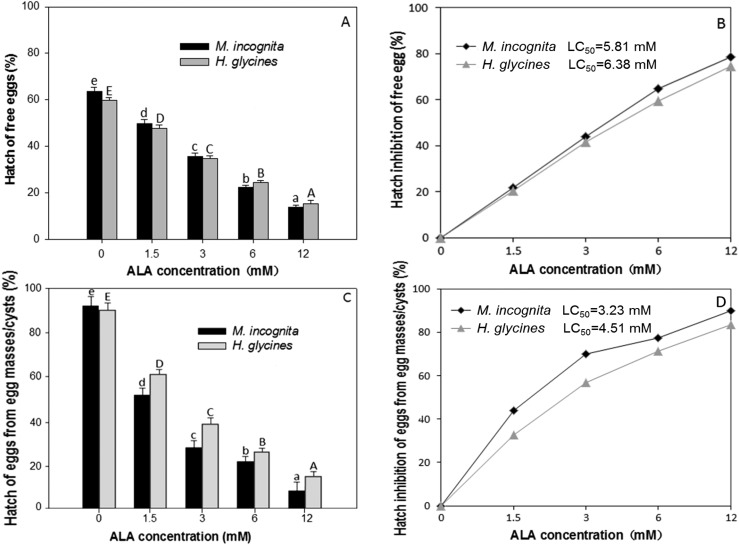
All ALA concentration tested inhibited hatching free eggs and egg masses/brown cysts of M. incognita and H. glycines (P ≤ 0.05, Fig. 2A–D). At 12.0 mM, ALA inhibited hatching of free eggs of M. incognita and H. glycines by 78.4% and 74.5%, respectively (P ≤ 0.05, Fig. 2A), and the LC50 of M. incognita and H. glycines was 5.81 and 6.38 mM, respectively (P ≤ 0.05, Fig. 2B). The hatch inhibition of egg masses of M. incognita and brown cysts of H. glycines was 90.1% and 83.4% at 12.0 mM of ALA, respectively (P ≤ 0.05, Fig. 2C) and the LC50 of M. incognita and H. glycines was 3.23 and 4.51 mM, respectively (P ≤ 0.05, Fig. 2D).
Fig. 2.
Effect of of 5-aminolevulinic acid (ALA) on hatching rates (A and C) and inhibition rates (B and D) of Meloidogyne incognita and Heterodera glycine when their eggs were in free eggs form (A and B) or egg masses of M. incognita and cysts of H. glycine (C and D), as compared to that incubated in the water control. Mean for each column is an average of 12 replications. Error bars indicate standard error.
Effect of ALA against infection of M. incognita on greenhouse tomato:
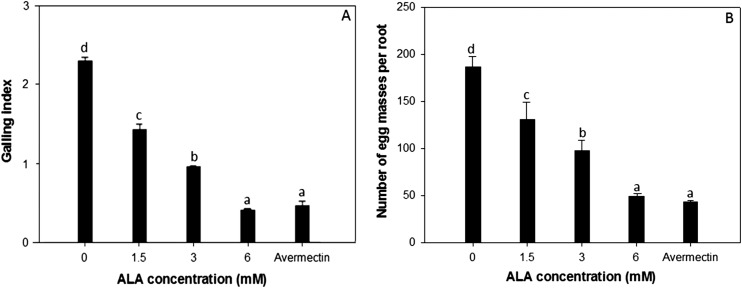
The root-gall index (Fig. 3A) and number of egg masses per root (Fig. 3B) were lower in all the ALA-treated tomato compared with the water-treated control (P ≤ 0.05). The reduction of galling index and number of egg masses per root were significantly enhanced with the increase of treatment concentrations. ALA at 6.0 mM suppressed root-gall formation as efficient as the commercial standard nematicide, avermectin, with 81.7% reduction of RGI compared with the water control. The egg masses per root showed a similar trend to root galls. At the same times, we found that the treatment of ALA at 1.5 to 3.0 mM concentrations could improve the growth of tomato significantly, and there was no phytotoxicity observed on tomato at 6.0 mM concentration of ALA.
Fig. 3.
Effect of 5-aminolevulinic acid (ALA) on (A) root-gall index (n = 60) and (B) egg mass (n = 12) formation on roots of tomato plants inoculated with Meloidogyne incognita during greenhouse studies. Error bars indicate the standard error.
Microscopic examination and TEM:
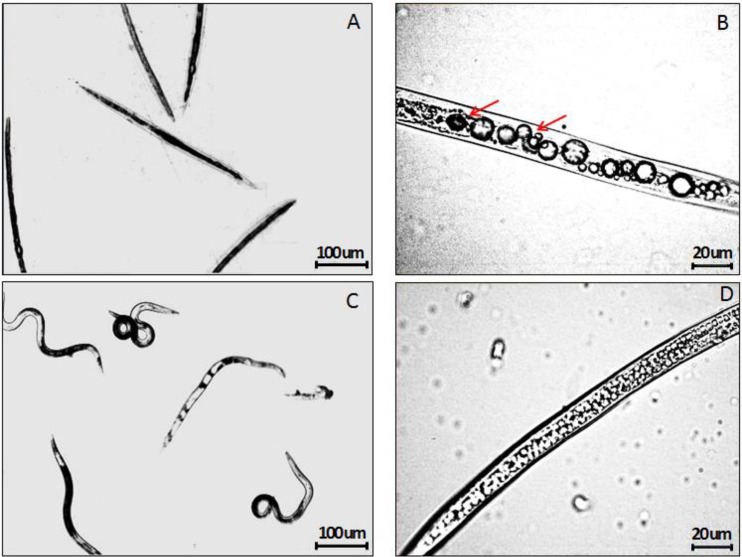
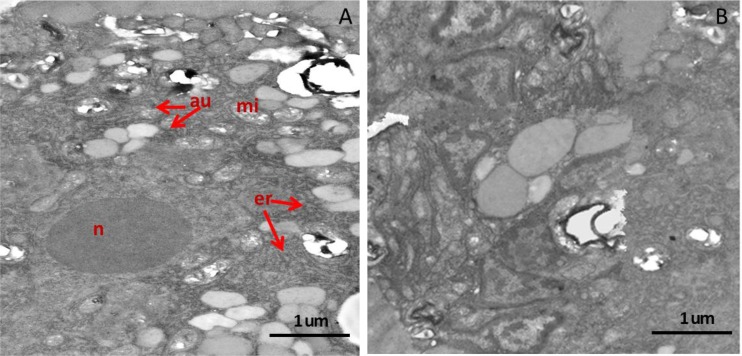
After treatment of M. incognita J2s with 3.0 mM ALA for 2 hr, the J2s became very agitated compared with the J2s treated with sterile water. The abnormal movement of ALA-treated J2s continued and then slowed down 6 hr after treatment. Twelve hours after ALA treatment, the bodies of ALA-treated J2s became rigid and straight whereas the J2s treated with water remained flexible (Fig. 4A,C). Under the microscope, the internal organ of dead M. incognita J2s shrank and separated from the body cuticle, and numerous large vesicles appeared inside the dead J2 (Fig. 4B). Examination of thin sections of the ALA-treated and water-treated J2s by TEM showed that the endoplasmic reticulum (er) in the ALA-treated J2s was clearly dilated compared with the controls (Fig. 5). In addition, a large number of autophagosomes which is excess or damaged organelles were found in sections from the ALA-treated M. incognita J2s.
Fig. 4.
Morphology of second stage juveniles of Meloidogyne incognita treated with 3.0 mM 5-aminolevulinic acid (ALA). Arrows indicated the separation of internal organ with cuticle. All images were taken under an inverted optical microscope at 48 hr post treatment.
Fig. 5.
Effect of 5-aminolevulinic acid (ALA) on morphology of second stage juveniles of Meloidogyne incognita observed under a transmission electron microscope. Thin sections were taken from an ALA-treated J2 (A) or from a water-treated J2 (B). Au: autophagy; mi: mitochondria; n: nucleus; rer: endoplasmic reticulum.
Effect of ALA on the metabolism of M. incognita J2:
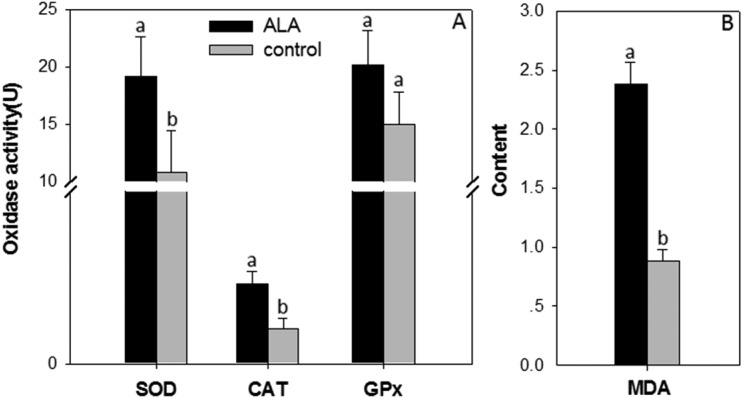
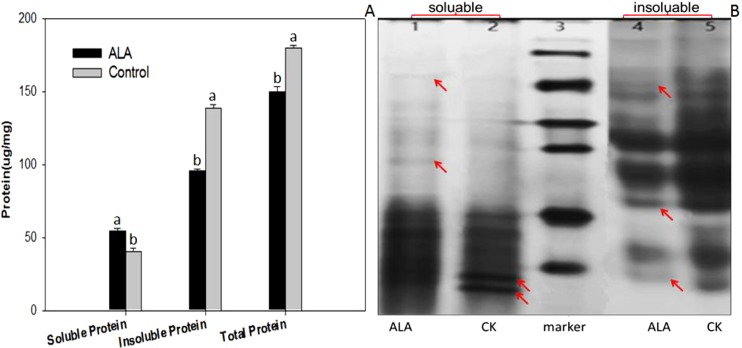
The activities of SOD and CAT were increased in the ALA-treated M. incognita J2s compared with the control (P ≤ 0.05, Fig. 6). The levels of SOD and CAT activity in the ALA-treated J2s were 1.78- and 2.32-fold higher than that observed for the water-treated control, respectively (Fig. 6A). In addition, the level of MDA in the ALA-treated J2s was about 2.71-fold higher than that in the control (Fig. 6B). The soluble protein level in the ALA-treated J2s was about 33.58% higher than that in the control. However, the insoluble and total protein levels in the ALA-treated J2s was about 31.10% and 16.42% lower than that in the control, respectively (Fig. 7A). SDS-PAGE analysis showed that there were significant changes in the soluble and insoluble protein band patterns between the ALA-treated and water-treated control samples (Fig. 7B). It is noteworthy that the activity of AChE in the ALA-treated J2s was not significantly changed compared with the controls (P > 0.05). The mean AChE activity of the ALA-treated M. incognita J2 group was 0.39 , whereas the mean AChE activity of the control group was 0.39 μmol mg−1 min−1, whereas the mean AChE activity of the control group was 0.33 μmol mg−1 min−1.
Fig. 6.
Effect of 5-aminolevulinic acid (ALA) on expression of metabolites (A) superoxide-dismutase (SOD), catalase (CAT), and glutathione-peroxidase (GPx) and (B) malondialdehyde (MDA) in second stage juveniles of Meloidogyne incognita as compared to the water control. Means are average of six replications. Error bars indicate the standard error.
Fig. 7.
Effect of 5-aminolevulinic acid (ALA) treatment on (A) accumulation of soluble, insoluble, and total proteins in the second stage juvenile (J2) of Meloidogyne incognita; and (B) SDS-PAGE profiles of soluble (lane 1 and 2) and insoluble (lane 4 and 5) proteins isolated from the ALA-treated or water-treated control. Protein marker is loaded in lane 3. Arrows indicated the upregulated or downregulated protein bands.
Discussion
Results from this research partially supported the findings from the previous report that ALA was an effective nematicide against M. incognita, H. glycines, P. coffeae, and B. xylophilus in China (Cheng et al., 2008, 2014). The LC50 at 48 hr after ALA treatment determined in the current study for M. incognita, H. glycines, and P. coffeae were relatively low but not for B. xylophilus. ALA is also effective in inhibiting hatch of from eggs of M. incognita and H. glycines. The ability to inhibit egg hatch is advantageous because it can suppress initial population of the nematodes before nematode infection. The in vitro studies conducted were consistent with the greenhouse experiments indicating that 6.0 mM ALA reduced RGI of M. incognita on tomato by 81.7%, similar to that observed for the avermectin-treated tomato plants. For egg masses formation, treatment of plants with 6.0 mM ALA gave about 73.8% reduction of egg masses formation per root, slightly lower than that observed for the avermectin-treated control plants. The increased frequency of avermectin application had resulted in selection of root-knot nematode resistance population in China. Taken together, our results showed that ALA is a promising nematicide to be rotated with avermectin to mitigate selection pressure for nematode population with nematicide resistant properties.
The increase in activities of oxidases (i.e., SOD and CAT) in M. incognita J2s after exposure to 3 mM of ALA is consistent with the findings of over production of oxygen superoxide and hydroxyl-free radicals on ALA-treated insects (Rocha et al., 2003). The increased activities of oxidases in the ALA-treated J2s suggested an increase accumulation of reactive oxygen species (ROS). Because the increase in ROS in the J2s might not be sufficiently scavenged by cytochrome C oxidase, additional antioxidant responses would be activated through activating the antioxidase system. High levels of ROS accumulation might lead to the lipid peroxidation. Oxidation of lipoprotein of cell membrane was previously reported to cause insect death (Amor et al., 2000). MDA is a product of lipid peroxidation in vivo and can be used as an important indicator for levels of oxidative damages (Esterbauer and Cheeseman, 1990). The results presented in this article indicated that the MDA content in the ALA-treated J2s was significantly increased compared with the control J2s. The increased MDA content suggested that the levels of ROS in the ALA-treated J2s might exceed the scavenging capacity of the antioxidase system and thus caused lipid peroxidation. Monteiro et al. (1989) suggested that ALA could cause ROS production via enolization, which the substrate changed to the enolic form of ALA in an aerobic or alkalescent environment, leading to lipid peroxidation. Oteiza and Bechara’s (1993) also showed that treatment with 0.1 to 3.0 mM ALA could induce lipid peroxidation in cardiolipin-rich liposomes. ROS accumulation is also known to influence protein metabolism and to change the normal architecture of cell membranes, mitochondria, and lysosomes, leading to tissue injury and damage of the cellular components (Kurien and Scofield, 2003). The results presented also showed that the total protein content in the ALA-treated M. incognita J2s was significantly altered compared with that observed in the control J2s. This leads to speculation that ALA increases activities of oxidases and decreases functional lipoproteins in the J2s. Furthermore, a large amount of autophagosomes was found in the tissue TEM sections from the ALA-treated J2s. The changes of MDA content, protein content and the forming of autophagosomes indicated that ALA injuried and damaged the cellular components. This damage could have impacted the nematode development and survival capacity.
AChE is an enzyme found in the synaptic cleft and maintains the levels of neurotransmitter acetylcholine by catalyzing the hydrolysis of acetylcholine to thiocholine. Many nematicides, including organophosphate and carbamate, can inhibit AChE activity and paralyze the nematodes (Rich et al., 2004). Yin et al. (2008) reported that treatment of locust with 1,000 mM ALA could cause 52% reduction of AChE activity in females and 43% in males compared with the control treatments. A separate report by Xie et al. (2007) indicated that the activity of AChE in the 10 mM ALA-treated locust was increased by 1.78 fold compared with the controls. However, this study did not show significant changes in the activity of AChE in the ALA-treated M. incognita J2s at 3.0 mM concentration. Mode of action of ALA against AChE may not be the same as that of other organophosphate or carbamate nematicides. Nonetheless, this research strongly supported that ALA is a promising biologically based nematicide and can be used to control different species of plant-parasitic nematode with a very different mode of action than the organophosphate or carbamate nematicides.
Literature Cited
- Abawi GS, Widmer TL. Impact of soil health management practices on soilborne pathogens, nematodes and root diseases of vegetable crops. Applied Soil Ecology. 2000;15:37–47. [Google Scholar]
- Aebi H. 1974. Catalase. Pp. 674–684 in H. U. Bergmeyer, ed. Methods of enzymatic analysis. New York: Academic Press.
- Amor BT, Bortolotto L, Jori G. Porphyrins and related compounds as photoactivatable insecticides: 3. Laboratory and field studies. Photochemistry and Photobiology. 2000;71:124–128. doi: 10.1562/0031-8655(2000)071<0124:sippar>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Baldwin JG, Hirschmann H. Body wall fine structure of the anterior region of Meloidogyne incognita and Heterodera glycines males. Journal of Nematology. 1975;7:175–193. [PMC free article] [PubMed] [Google Scholar]
- Barker KR. 1978. Determining nematode population responses to control agents. Pp. 114–125 in E. I. Zehr, ed. Methods for evaluating plant fungicides, nematicides and bactericides. St. Paul, MN: American Phytopathological Society Press.
- Beeley JA, Newman F, Wilson PHR, Shimmin IC. Sodium dodecyl sulphate-polyacrylamide gel electrophoresis of human parotid salivary proteins: Comparison of dansylation, coomassie blue R-250 and silver detection methods. Electrophoresis. 1996;17:505–506. doi: 10.1002/elps.1150170314. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cheng F, Zhang D, Liu Y, Wang J, Cheng J, Zhang Z. The nematicial effects of 5-aminolevulinic acid on Meloidogyne incognita. Journal of Nematology. 2014;46:144. [Google Scholar]
- Cheng FX, Zhang DY, He MY, Liu Y. Species identification and occurrence investigation of root-knot nematodes on vegetables in Hunan Province. Plant Protection. 2010;36:128–132. [Google Scholar]
- Cheng XY, Cheng FX, Xu RM, Xie BY. Genetic variation in the invasive process of Bursaphelenchus xylophilus (Aphelenchida: Aphelenchoididae) and its possible spread routes in China. Heredity (Edinb) 2008;100:356–365. doi: 10.1038/sj.hdy.6801082. [DOI] [PubMed] [Google Scholar]
- Chitwood DJ. Research on plant-parasitic nematode biology conducted by the United States Department of Agriculture-Agricultural Research Service. Pest Management Science. 2003;59:748–753. doi: 10.1002/ps.684. [DOI] [PubMed] [Google Scholar]
- Draper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods in Enzymology. 1990;186:421–431. doi: 10.1016/0076-6879(90)86135-i. [DOI] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical Pharmacology. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Cheeseman KH. 1990. Determination of aldehydiclipid peroxidation products: Malonaldehyde and hydroxynonenal. Pp. 407–421 in L. Parcker and A. N. Glazer, eds. Methods in enzymology, vol. 186. Part B. New York: Academic Press.
- Hussey RS, Barker KR. A comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Disease Reporter. 1973;57:1025–1028. [Google Scholar]
- Kurien BT, Scofield RH. Free radical mediated peroxidative damage in systemic lupus erythematosus. Life Science. 2003;73:1655–1666. doi: 10.1016/s0024-3205(03)00475-2. [DOI] [PubMed] [Google Scholar]
- Li WJ, Li Q, Hu XQ. Nematicidal activity and control efficiency of pyroligneous liquor on Meloidogyne spp. Scientia Agricultura Sinica. 2009;42:4120–4126. [Google Scholar]
- Li YM, Guo YP, Li Q, Zheng H, Ma E. Effect of porphyrin insecticides agent on Oxya chinensis and mechanisms. Journal Shanxi University (Natscied) 2005;28:196–201. [Google Scholar]
- Mamiya Y. Estimating population of nematodes inhabiting pine wood by the Baermann funnel method. Forest Pests. 1975;24:115–119. [Google Scholar]
- Monteiro HP, Abdalla DSP, Augusto O, Bechara EJH. Free radical generation during δ-aminolevulinic acid autoxidation: Induction by hemoglobin and connections with porphyrinpathies. Archives of Biochemistry and Biophysics. 1989;271:206–216. doi: 10.1016/0003-9861(89)90271-3. [DOI] [PubMed] [Google Scholar]
- Moody EH, Lownsbery BF, Ahmed JM. Culture of the root-lesion nematode Pratylenchus vulnus on carrot disks. Journal of Nematology. 1973;5:225–226. [PMC free article] [PubMed] [Google Scholar]
- Oka Y, Koltai H, Bareyal M, Mor M, Sharon E, Chet I, Spiegel Y. New strategies for the control of plant-parasitic nematodes. Pest Management Science. 2000;56:983–988. [Google Scholar]
- Oteiza PI, Bechara EJ. 5-aminolevulinic acid induces lipid peroxidation in cardiolipin-rich liposomes. Archives of Biochemistry and Biophysics. 1993;305:282–287. doi: 10.1006/abbi.1993.1424. [DOI] [PubMed] [Google Scholar]
- Rebeiz CA, Juvik JA, Rebeiz CC. Porphyric insecticides: 1. Concept and phenomenology. Pesticide Biochemistry and Physiology. 1988;30:11–27. [Google Scholar]
- Rebeiz CA, Montazer-Zouhoor A, Hopen HJ, Wu SM. Photodynamic herbicides: 1. Concept and phenomenology. Enzyme and Microbial Technology. 1984;6:390–401. [Google Scholar]
- Rich JR, Dunn RA, Noling JW. 2004. Nematicides: Past and present uses. Pp. 1179–1200 in Z. X. Chen, S. Y. Chen, and D. W. Dickson, eds. Nematology: Advances and perspectives, nematode management and utilization, vol. 2. Wallingford, UK: CABI Publishing.
- Rocha ME, Dutra F, Bandy B, Baldini RL, Gomes SL, Faljoni-Alário A, Liria CW, Miranda MT, Bechara EJ. Oxidative damage to ferritin by 5-aminolevulinic acid. Archives of Biochemistry and Biophysics. 2003;409:349–356. doi: 10.1016/s0003-9861(02)00633-1. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Watanabe M, Tanaka T. Biosynthesis, biotechnological production and applications of 5-aminolevulinic acid. Applied Microbiology and Biotechnology. 2002;58:23–29. doi: 10.1007/s00253-001-0858-7. [DOI] [PubMed] [Google Scholar]
- Sasikala C, Ramana CV, Rao RP. 5-aminolevulinic acid: A potential herbicide/insecticide from microorganisms. Biotechnology Progress. 1994;10:451–459. [Google Scholar]
- Schneider SM, Rosskopf EN, Leesch JG, Chellemi DO, Bull CT, Mazzola M. United States Department of Agriculture-Agricultural Research Service research on alternatives to methyl bromide: Pre-plant and post-harvest. Pest Management Science. 2003;59:814–826. doi: 10.1002/ps.728. [DOI] [PubMed] [Google Scholar]
- Xie CR, Zhang JZ, Yin K, Wu HH, Gou YP, Ma EB. 5-Aminolevulinic acid, exerts effective toxicity to Oxya chinensis (Orthoptera:Acridoidean) and effect on enzymes. Journal of Shanxi Agricultural University. 2007;30:103–107. [Google Scholar]
- Yin K, Ma EB, Xue CR, Wu HH, Guo YP, Zhang JZ. Insecticidal activities of 5-aminolevulinic acid on Oxya chinensis and effect on three kinds of enzymes. Scientia Agricultura Sinica. 2008;41:2003–2007. [Google Scholar]
- Zhang DY, Cheng FX, Cheng JE, Zhang ZH, Liu Y. Cloning and prokaryotic expression of Rhodoblastus acidophilus 5-aminolevlinate synthase gene. Acta Microbiologica Sinica. 2007;47:639–644. [PubMed] [Google Scholar]