Abstract
The bacterium Pasteuria penetrans is a parasite of root-knot nematodes (Meloidogyne spp.). Endospores of P. penetrans attach to the cuticle of second-stage juveniles (J2) and subsequently sterilize infected females. When encumbered by large numbers of spores, juveniles are less mobile and their ability to infect roots is reduced. This study looked at different factors that influence spore attachment of P. penetrans to the root-knot nematode Meloidogyne arenaria. Pretreatment of J2 with root exudates of eggplant (Solanum melongena cv. Black beauty) reduced spore attachment compared with pretreatment with phosphate-buffered saline (PBS), suggesting that the nematode surface coat was altered or the spore recognition domains on the nematode surface were blocked. Spore attachment was equally reduced following exposure to root exudates from both host and nonhost plants for M. arenaria, indicating a common signal that affects spore attachment. Although phytohormones have been shown to influence the lipophilicity of the nematode surface coat, auxins and kinetins did not affect spore attachment compared with PBS. Root exudates reduced spore attachment more in sterilized soil than in natural soil. Sterilization may have eliminated microbes that consume root exudates, or altered the chemical components of the soil solution or root exudates. Root exudates caused a greater decrease in spore attachment in loamy sand than in a sandy loam soil. The sandy loam had higher clay content than the loamy sand, which may have resulted in more adsorption of compounds in the root exudates that affect spore attachment. The components of the root exudates could have also been modified by soil type. The results of this study demonstrate that root exudates can decrease the attachment of P. penetrans endospores to root-knot nematodes, indicating that when these nematodes enter the root zone their susceptibility to spore attachment may decrease.
Keywords: Meloidogyne arenaria, Pasteuria penetrans, root exudates, root-knot nematode, spore attachment
Pasteuria penetrans is a widely-distributed, endospore-forming bacterium, which is a parasite of root-knot nematodes, Meloidogyne spp. (Sayre and Starr, 1985). Suppression of root-knot nematodes by P. penetrans has been evaluated on numerous crops, demonstrating the potential of this bacterium as a biological control organism (Stirling, 1984; Chen et al., 1996; Trudgill et al., 2000). The initial stage of infection occurs when endospores of P. penetrans attach to the cuticle of the second-stage juveniles (J2) of Meloidogyne spp. as they migrate through soil. The spores usually germinate within 4 to 10 d after J2 enters a host plant root and begins to feed. The bacterium goes through sporogenesis and eventually dominates the nematode body. When a small number of P. penetrans spores adhere to the J2, the infected female produces few to no eggs; when encumbered by a large number of spores (5 to 15), juveniles are less mobile and their ability to enter roots is reduced (Stirling, 1984; Davies et al., 1988). Attachment of endospores to J2 is a fundamental step in the infection process, without which the infection will not occur (Chen and Dickson, 1998).
The surface coat of nematodes is an amorphous layer that overlays the cuticle regions and is predominantly made up of glycosylated proteins (Davies and Curtis, 2011). The replacement of the nematode surface coat is a dynamic process reflected by continuous shedding and replacement of surface-associated antigens (Blaxter et al., 1992). The composition of the surface coat changes rapidly in response to environmental signals. When J2 of Meloidogyne javanica were treated with detergents, their ability to bind red blood cells was reduced. However, the binding property was completely renewed after 24 hr, suggesting that the surface coat was replaced (Spiegel et al., 1997). Nematodes can switch surface composition in response to environmental signals, which may be important in host-parasite interactions (Olsen et al., 2007). The surface coat is the outmost layer as well as the first to interface with host plants and parasitic organisms. Using the fluorescent lipid probe AF18 (5-N-[octodecanoyl] aminofluorescein), rapid changes in surface lipophilicity were observed after incubating Globodera rostochiensis with potato root exudates (Akhkha et al., 2002). Phytohormones, such as auxin and cytokinins, decreased AF18 uptake by G. rostochiensis, whereas they had the opposite effect on Meloidogyne incognita (Akhkha et al., 2002).
There is strong evidence that P. penetrans spores adhere to the nematode surface coat. When J2 were pretreated with detergents that removed the surface coat, fewer spores were attached compared with untreated J2 (Spiegel et al., 1996). When spores were pretreated with an extract of the surface coat, spore attachment was also reduced. Furthermore, when J2 were pretreated with lectin or carbohydrates that can bind to the nematode surface coat, fewer spores attached to the J2 (Spiegel et al., 1996), suggesting a carbohydrate-protein mechanism for spore attachment. Davies and Danks (1993) demonstrated that the N-acetylglucosamine residues on the spore surface of P. penetrans can recognize the carbohydrate-recognition domains (CRD) on the nematode surface that are involved in spore attachment.
Root exudates have been shown to alter the surface coat of plant-parasitic nematodes (Curtis, 2008); however, no studies have evaluated the effect of exudates on attachment of P. penetrans spores to the surface coat of root-knot nematodes. Root exudates contain substances, including ions, free oxygen, enzymes, mucilages, and a diverse array of primary and secondary metabolites (Bais et al., 2006). They are also important sources of organic carbon used by soil microbes. Through the exudation of a wide variety of compounds, roots impact the soil microbial community, alter the chemical and physical properties of the soil, and influence the microbe-nematode interactions (Bertin et al., 2003; Bais et al., 2006). Therefore, the objective of this study was to investigate the influence of root exudates on the attachment of P. penetrans to root-knot nematodes.
Materials and Methods
General methods:
Three single egg mass (SEM) lines of M. arenaria race 1 were obtained from a field population in Tifton, GA, and maintained on eggplant (S. melongena) in a greenhouse at 22 to 30°C. Eggs were separated from egg masses using 0.85% NaOCl (Hussey and Barker, 1973). Second-stage juveniles (J2) were hatched on a tissue paper supported by a screen placed on a hatching dish. Tap water was added to the hatching dish to just submerge the tissue paper. The hatched J2 migrated through the tissue paper into the water and they were collected every 2 d. After the final collection, J2 were left in tap water for 24 hr to develop their surface coat.
Two single spore (SS) lines of a population of P. penetrans originating from Florida were obtained from the University of Florida. To propagate the spore lines, J2 of M. arenaria were incubated in PBS, pH 7.2 (PBS) with 105 endospores from each SS line for 4 hr, and 30 J2 were examined for spore attachment at ×400 magnification. Juveniles with an average of 2 to 5 spores/J2 were inoculated onto 4-wk-old eggplant seedlings (cv. Black Beauty) to produce spores. The plants were grown in a greenhouse for 4 mon before root harvest. Harvested roots were washed with running tap water and placed in a beaker containing 100 ml of 1:10 aqueous solution of Lallzyme EX-V (Lallemand, Montreal, Canada) to digest for 1 d on a shaker (100 rpm). Opaque infected females were freed from roots and hand-picked into glass dishes containing deionized water (dH2O) with the aid of a dissecting microscope. Females were crushed with a dissecting needle to free endospores into dH2O, and the eggs were vacuum filtered (8 to 12 µm) to remove the female cuticle. The spores that passed through the filter were enumerated on a hemocytometer at ×1,000 magnification. Spore concentration was adjusted to 106 spores/ml for each SS line and kept frozen.
For the endospore attachment bioassay, 200 infective J2 were incubated in 4 ml of 1× PBS in a small (150 mm × 25 mm) glass Petri dish with 0.1 ml of 106 spores/ml of P. penetrans. Dishes were shaken horizontally with a shaker at 100 rpm for 6 hr at room temperature (24 to 26°C). The number of spores attached to 30 randomly selected J2 was determined using ×400 magnification. Three SEM lines (SEM8, SEM14, and SEM40) of M. arenaria susceptible to the two SS lines (SS16, SS17) of P. penetrans were used in the experiments.
Root exudates of eggplant, tomato (Solanum lycopersicum), corn (Zea mays), and cotton (Gossypium hirsutum) were obtained from plant seedlings grown in vermiculite. After approximately 4 wk after planting, seedlings with root volumes equivalent to 5 ml (measured by water displacement) were submerged in 30 ml tap water in foil covered glass jars with the stem and leaves exposed to the air. Seedlings were incubated in the jars in a greenhouse for 24 hr, and the root exudates were used immediately for experiments.
Effect of root exudates on spore attachment:
To determine the effect of root exudates from eggplant cv. Black beauty and sodium dodecyl sulfate (SDS) detergent on the attachment of P. penetrans endospores to M. arenaria, the J2 were exposed to the following treatments: root exudates for 6 hr, root exudates for 6 hr followed by a 24 hr recovery, 1% SDS, and a PBS control. For the root-exudate treatments, J2 were incubated with 4 ml of eggplant root exudates for 6 hr and rinsed with PBS three times before conducting the spore attachment bioassay. To test if the surface coat would recover after incubating with root exudates, the J2 were rinsed and left in PBS for 24 hr before conducting the endospore attachment assay. For the SDS treatment, J2 were incubated for 30 min in PBS containing 1% SDS and rinsed three times with PBS to remove the detergent. For the control, J2 were incubated in PBS for 6 hr. Incubation in exudates, SDS, and PBS was done in 150 mm × 25 mm foil covered glass Petri dishes with 200 J2 on a horizontally shaker (100 rpm) at room temperature. Two SEM lines (SEM 8, 14) and two SS lines (SS 16, 17) were used in this experiment, and it was conducted twice.
Effect of phytohormones on spore attachment:
To determine the effect of phytohormones on attachment of P. penetrans to M. arenaria, J2 of SEM 40 were incubated in kinetin (6-furfurylaminopurine) with cytokinin activity and 1-naphthaleneacetic acid with auxin activity in foil covered glass dishes for 6 hr. A concentration of 10 μM of each phytohormones was prepared as described by Akhkha et al. (2002). As a control, J2 were incubated in PBS without phytohormones. After incubation, J2 were rinsed with PBS three times followed by an endospore attachment bioassay. SEM lines 40 and SS 16 were used. There were three replicates for each treatment, and the experiment was conducted twice.
Effect of root exudates from nonhost and host plants:
To determine whether root exudates from nonhosts of M. arenaria have the same effect on attachment of P. penetrans spores as host plants, exudates from eggplant cv. Black beauty, tomato cv. Rutgers, corn cv. Agra Tech, and cotton cv. DP 0935 were tested. Eggplant and tomato are hosts, corn is a poor host, and cotton is a nonhost to M. arenaria. Root exudates were obtained as described earlier. Two hundred infective J2 of SEM 8 were incubated in 4 ml of each root exudate as treatments. As a control, the J2 were incubated in 4 ml of PBS. The J2 were incubated as described earlier before rinsing three times with PBS. The endospore attachment bioassay was conducted after exposure to root exudates with SS16. There were four replicate dishes set up for each treatment, and the experiment was conducted twice.
Effect of soil microorganisms on root exudate activity:
To test if soil microorganisms can modify the effect of root exudates on attachment of P. penetrans to M. arenaria, soil types were used in this experiment. A Tifton loamy sand soil (fine-loamy, siliceous, thermic Plinthic Kandiudult; 85% sand, 11% silt, 4% clay, pH 6.1) was collected from the root zone of peanut at the Gibbs Farm, Tifton, GA, and a Greenville sandy loam soil (Fine, kaolinitic, thermic Rhodic Kandiudults; 69% sand, 15% silt, 16% clay; pH 5.6) was collected from the root zone of cotton from the Southwest Georgia Research and Education Center, Plains, GA. Soils were autoclaved for 30 min at 121°C, cooled for 24 hr, and autoclaved for another 30 min to kill microorganisms in soils. Natural and autoclaved soils were bagged and kept at 4°C for a month before use. The following treatments were used for both soils: natural soil with a plant, sterilized soil with a plant, and natural soil without a plant. Soils were added to small pots (120 cm3). Both PBS and root exudates from eggplant seedlings incubated in foil covered bottles were used as controls. For treatments with plants, three 1-mon-old eggplant seedlings were planted in each pot. All treatments, except the PBS, were left in the greenhouse for 3 d. To extract the soil solution, soil was saturated with water and vacuum filtered (Whatman #1) using a Buchner Funnel at 72 kPa pressure. For treatments with the loamy sand, soil was vacuum filtered for 3 min and for treatments with sandy loam, soil was vacuum filtered for 4 min. The soil solutions were tested for pH and 5 ml was used to incubate J2 of SEM40 for 6 hr. Following incubation, the J2 were rinsed with PBS three times before conducting the endospore attachment bioassay with SS17. There were three replicates per treatment, and the experiment was conducted twice.
Statistical analysis:
Data were analyzed using the PROC GLIMMIX (generalized linear models) with a negative binomial distribution in SAS (v. 9.3). For the first experiment with root exudates, the spores per J2 for 30 individuals were used as data points. SEM line, SS line, Treatment, and Trial were included in the model as well as the fully factorial interaction terms. Later, we found that there was a variation in spore acquisition among replicate dishes in the attachment bioassay. To account for this variation, the mean spore per J2 for the 30 individuals in a replicate was used as data points in all future experiments where only one SEM line and SS line was used. Treatment, Trial, and Treatment Trial and Reps were used to construct model effects. For all experiments, Tukey’s HSD test was used to test pairwise comparison of model effects. For the experiment to test the effect of soil microorganisms on root exudate activity, contrasts were used to compare the effect of soil sterilization, root exudates, and different soil types on spore attachment.
Results
Effect of root exudates on spore attachment:
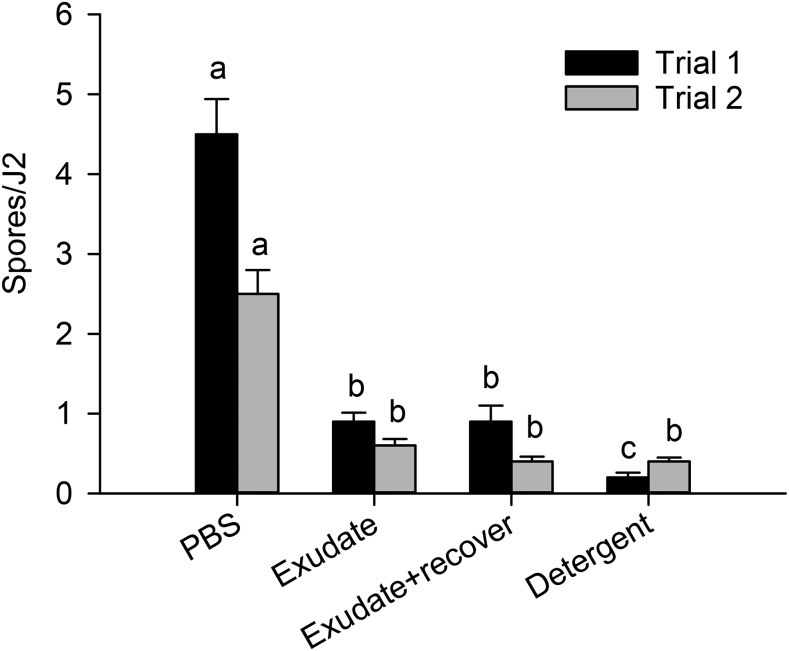
The effect of root exudates on spore attachment was consistent among SEM lines, SS lines, and trials (no three- or four-way interactions). However, the effect of the root-exudate treatments on spore attachment differed between the trials (Treatment * Trial interaction, P = 0.004). In both trials, the J2 treated with root exudates and the detergent had lower (P < 0.0001) spore attachment than those treated with PBS (Fig. 1). In Trial 1, J2 pretreated with detergent had fewer attached spores than those pretreated with exudates, but this result was not confirmed in Trial 2. Incubating J2 in root exudates or detergent reduced spore attachment by 80% to 95% in Trial 1, and 76% to 84% in Trial 2 compared with J2 incubated in PBS. Even after 24 hr of recovery, the J2 incubated in root exudates still had 80% lower spore attachment in Trial 1 and 84% lower spore attachment in Trial 2 than J2 incubated in PBS (Fig. 1).
Fig. 1.
Effect of root exudates on attachment of Pasteuria penetrans endospores to Meloidogyne arenaria second-stage juveniles (J2). PBS = phosphate-buffered saline. Within a trial, means followed by the same letter are not significantly different (P > 0.05). Each bar represents the mean spores per 30 J2.
Effect of host plant and phytohormones on spore attachment:
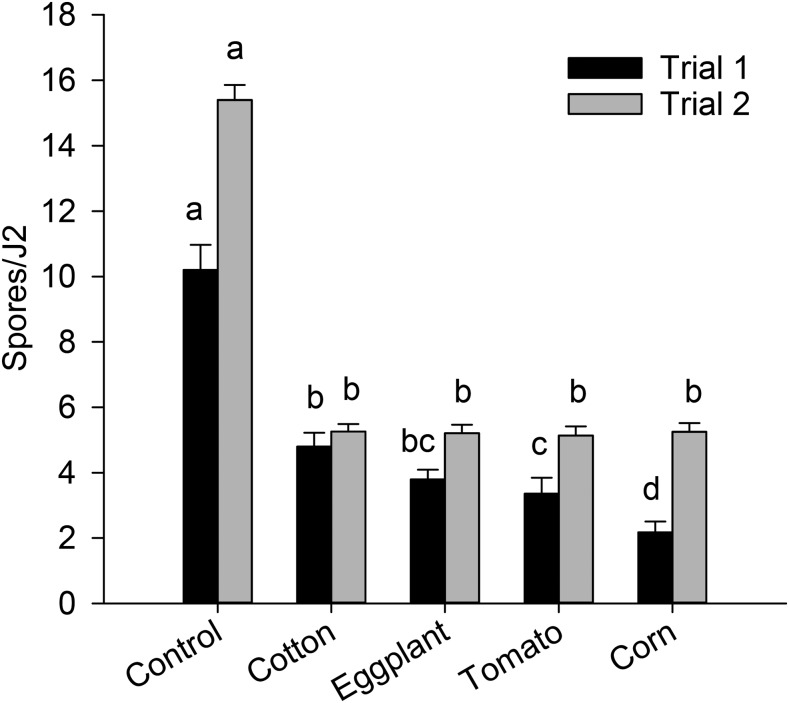
The effect of root exudates from various plants on spore attachment differed between the two trials (Treatment * Trial interaction, P < 0.0001). In both trials, all root exudates reduced (P < 0.0001) spore attachment compared with the PBS control (Fig. 2). Among the root exudates, pretreatment of J2 with exudates from corn resulted in the lowest spore attachment, whereas pretreatment with exudates from cotton resulted in the highest spore attachment in Trial 1; the root exudates did not vary in their effect on spore attachment in Trial 2. Preincubating J2 in phytohormones, including auxin and cytokinins, did not influence attachment of P. penetrans spores to M. arenaria J2 (data not shown).
Fig. 2.
Effect of root exudates from host (corn, eggplant, tomato) and nonhost (cotton) plants on attachment of Pasteuria penetrans to second-stage juveniles (J2) of Meloidogyne arenaria. Within a trial, means followed by the same letter are not significantly different (P > 0.05). Each bar represents the mean of four replicates.
Effect of soil microorganisms on root exudate activity:
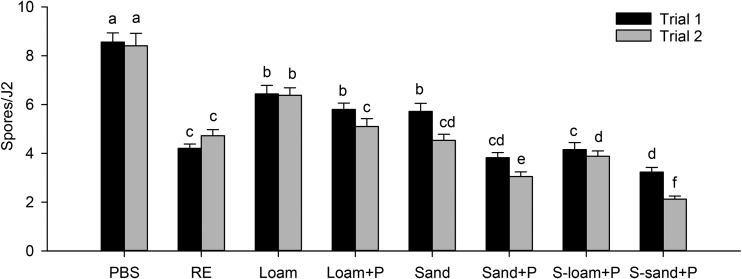
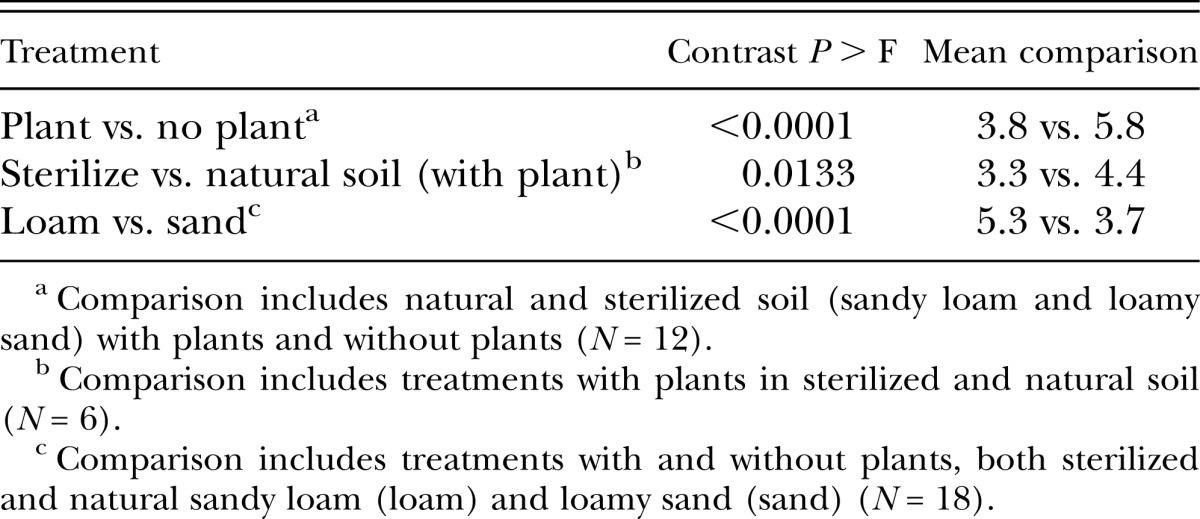
The effect of the soil treatments on spore attachment was not consistent between the two trials (Treatment * Trial interaction, P = 0.001). For both trials, pretreatment of J2 with either soil solutions (with and without plants) and root exudates reduced spore attachment compared with pretreatment with PBS (Fig. 3). The presence of plant roots in soil decreased (P < 0.0001) spore attachment compared with soil without plants (Table 1). However, in Trial 1, there was no difference between the sandy loam with and without plants (Fig. 3). The effect of root exudates on spore attachment tended to be greater in the loamy sand than in sandy loam. Root exudates reduced spore attachment more (P = 0.013) in sterilized soil than in natural soil (Table 1, Fig. 3). However, in Trial 1, there was no difference between natural and sterilized loamy sand with plants. Across all treatments, spore attachment was greater (P < 0.0001) in the sandy loam than in the loamy sand (Table 1). The pH of extracts from the natural soils without plants was 6.8 to 6.9, from natural soils with plants was 7.0 to 7.1, and from autoclaved soil with plants was 7.2 to 7.3. The pH of the root exudates (without soil) and the PBS was 7.4 and 7.2, respectively.
Fig. 3.
Effect of root exudates and soil type on attachment of Pasteuria penetrans to Meloidogyne arenaria. PBS: phosphate-buffered saline. RE: root exudates. Loam: natural sandy loam. Loam + P: natural sandy loam with plant. Sand: natural loamy sand. Sand + P: natural loamy sand with plant. S-loam + P: sterilized sandy loam with plant. S-sand + P: sterilized loamy sand with plant. Within a trial, means followed by the same letter are not significantly different (P > 0.05). Each bar represents the mean of three replicates.
Table 1.
Contrast comparisons between treatment groups on attachment of Pasteuria penetrans endospores to Meloidogyne arenaria second-stage juveniles.
Discussion
Our results demonstrate that exposure of J2 to root exudates reduced attachment of P. penetrans spores. The level of suppression of spore attachment was similar when SDS was used to remove the nematode surface coat. It is noteworthy that even given a 24-hr recovery time, the spore attachment was still lower than the PBS control, indicating that this is a nonreversible process. Many factors have been shown to affect attachment of P. penetrans to M. arenaria, including temperature, pH, ion concentration, and electrostatic and hydrophobic interactions (Ahmed and Gowen, 1991; Hatz and Dickson, 1992; Afolabi et al., 1995; Mateille et al., 1995). In our study, we pre-exposed J2 to root exudates and soil solutions before conducting the attachment assay in PBS to exclude the influence of these factors. The compounds in root exudates that influence the surface coat are unknown. Root exudates from both host and nonhost plants of M. arenaria reduced spore attachment to J2 suggesting that root exudates from diverse families share a common signal that affects spore attachment.
Nematodes can alter their surface composition in response to environmental signals, and evidence for this switching has been observed in changes in surface antigens, as well as changes in surface lipophilicity (Proudfoot et al., 1993; Olsen et al., 2007). Caenorhabditis elegans, widely studied as a model for environmental induction of surface changes, was found to switch its surface antigens based on environmental cues, and the switch involved chemosensory neurons (Grenache et al., 1996; Olsen et al., 2007). Changes in the lipophilicity of G. rostochiensis and M. incognita were observed after exposure to phytohormones (auxin and kinetin) and root exudates (de Mendoza et al., 2000; Akhkha et al., 2002). Phytohormones decreased the uptake of the fluorescent surface probe AF18 in G. rostochiensis, whereas they increased the uptake of AF18 in M. incognita. Root exudates increased AF18 uptake by G. rostochiensis, but had no effect on M. incognita. Although phytohormones are constituents of root exudates (Martinez-Toledo et al., 1988; Muhammad and Frankenberger, 1998), our results showed that they had no effect on attachment of spores to J2. It is likely that the changes in lipophilicity observed previously are not involved in attachment of P. penetrans spores to the surface coat of M. arenaria.
The nematode surface coat can be shed upon exposure to host signals (Maizels et al., 1993; Modha et al., 1995), and this may explain the reduced spore attachment following exposure to root exudates. The level of reduction in spore attachment following pretreatment with root exudates was similar to pretreatment with SDS, which has been shown to remove the surface coat. However, if the surface coat was shed, replacement would have been rapid. Studies with monoclonal antibodies raised against the surface coat of Globodera pallida and M. javanica, showed a rapid replacement of the nematode surface (Fioretti et al., 2002). For instance, antibodies recognizing the nematode surface impaired nematode movement, but movement fully recovered after 1 to 6 hr indicating the rapid replacement of antigens on the surface. Human blood cells also have been used in studying nematode surface properties because of their ability to bind to the surface of several plant-parasitic nematode species (Spiegel and McClure, 1995). Spiegel et al. (1997) showed that binding of human blood cells to M. javanica decreased after treating J2 with detergents, but binding was completely renewed after 24 hr. In studies with animal-parasitic nematodes, changes in the nematode surface coat may be an important process for evading the immune response of hosts. The surface coat of Romanomermis culicivorax, a parasite of mosquito larvae, is shed continuously to avoid the mosquito immune system (Shamseldean et al., 2007). The shedding of the surface coat can effectively remove host immune products from the surface coat.
In our study, spore attachment did not increase after 24 hr suggesting that the J2 surface coat was either shed permanently or was replaced with a new surface coat resistant to spore attachment. Similarly, Modha et al. (1999) observed shedding of the surface coat in Trichinella spiralis after exposure to host cues. When juveniles are activated by exposure to host cues, the probe PKH26, which is bound to the surface coat is lost, and the nematode cannot be relabeled. However, when an unactivated juvenile (not exposed to host cues) sheds its surface coat, it can be relabeled with PKH26, suggesting that the host cues are triggering a prolonged or irreversible change in the surface of the nematode. In our study, we hypothesize that the surface coat was shed upon exposure to root exudates and replaced with an altered surface coat.
Carbohydrate, lipid, and protein constituents of the nematode surface have been characterized (Robertson et al., 1989). A CRD on the nematode surface coat recognizing N-acetylglucosamine residues on the P. penetrans spore surface has been reported to be involved in the endospore attachment to root-knot nematodes (Davies and Danks, 1992). Lectins concanavalin A and wheat germ agglutinin containing N-acetylglucosamine residues were found to bind to the surface of M. javanica inhibiting attachment by P. penetrans spores (Bird et al., 1989; Sharon and Spiegel, 1996). Carbohydrate residues, such as mannose or glucose, which are also found on the spore surface, can also be candidates for binding to nematode surface CRD. Pretreatment of M. javanica with the carbohydrates fucose or α-methyl mannoside decreased P. penetrans spore attachment (Spiegel et al., 1996). Lectins and carbohydrates are common components of root exudates (Badri and Vivanco, 2009; De Hoff et al., 2009), and they may have bound to CRDs on the nematode surface, thus preempting attachment of P. penetrans spores. However, this blockage should be transient with the continual replacement of the surface coat.
Prior exposure of M. arenaria juveniles to soil extracts in the absence of plants reduced attachment of P. penetrans spores to the nematode surface, and attachment was lower in extracts from loamy sand than from sandy loam soil. It is not clear whether this reduction in spore attachment is due to an altered surface coat or blockage of CRD domains on the nematode surface. However, the presence of root exudates in the soil extract further reduced spore attachment compared with extracts without root exudates confirming the results obtained with root exudates collected in tap water.
Root exudates reduced spore attachment more in sterilized soil than in natural soil. With sterilization, microbes that consume root exudates were eliminated, presumably resulting in greater availability of root exudates than in natural soil. Sterilization can also alter the components of soil solution, including the physicochemical characteristics (Egli et al., 2006; Dao, 2014; Mahmood et al., 2014). Studies showed that autoclaving can increase soluble organic matter as well as a small increase in soil pH (Berns et al., 2008; Liegel, 1986). These physicochemical differences can have an impact on the plant rhizosphere, which could influence root exudates in sterilized and nonsterilized soil.
We observed an interaction between soil type and root exudates; spore attachment decreased more in the loamy sand than in sandy loam soil, indicating that root exudates had a greater effect on attachment in the sandier soil. The higher percentage of clay in the sandy loam compared with the loamy sand (16% vs. 4%) may have reduced the bioavailability of some components of root exudates due to adsorption onto the clay particles (Jones and Edwards, 1998). The active component of the root exudates which triggers changes in the surface coat may have been bound to the clay particles resulting in lower concentrations in the soil solution from the sandy loam compared with the loamy sand soil. Furthermore, the constituents of root exudates can differ among soils (Huang et al., 2014; Neumann et al., 2014). A study with lettuce cultivated in three different types of soil showed that there were large quantitative differences, particularly for sugars and amino acids, among the three soil types (Neumann et al., 2014). This difference in root exudate profile may, in part, explains the interaction between soil type and root exudates on spore attachment.
In conclusion, the attachment of P. penetrans endospore to root-knot nematodes is a complex interaction with many factors influencing the outcome, including exposure to root exudates. In the field, the first-generation nematodes that migrate to the roots are likely to be more susceptible to Pasteuria spore attachment because of lower exposure to root exudates than future generations that will be hatching around the roots. Understanding the interaction among root exudates, the nematode surface coat, and spore attachment is important to help develop P. penetrans as a biological control agent of root-knot nematodes.
Literature Cited
- Afolabi P, O’Shea PS, Davies KG. The electrostatic nature of the spore of Pasteuria penetrans, the bacterial parasite of root-knot nematodes. Journal of Applied Bacteriology. 1995;79:244–249. [Google Scholar]
- Ahmed R, Gowen SR. Studies on the infection of Meloidogyne spp. with isolates of Pasteuria penetrans. Nematologia Mediterranea. 1991;19:229–233. [Google Scholar]
- Akhkha A, Kusel J, Kennedy M, Curtis R. Effects of phytohormones on the surfaces of plant-parasitic nematodes. Parasitology. 2002;125:165–175. doi: 10.1017/s0031182002001956. [DOI] [PubMed] [Google Scholar]
- Badri DV, Vivanco JM. Regulation and function of root exudates. Plant, Cell and Environment. 2009;32:666–681. doi: 10.1111/j.1365-3040.2008.01926.x. [DOI] [PubMed] [Google Scholar]
- Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM. The role of root exudates in rhizosphere interactions with plants and other organisms. Annual Review of Plant Biology. 2006;57:233–266. doi: 10.1146/annurev.arplant.57.032905.105159. [DOI] [PubMed] [Google Scholar]
- Berns AE, Philipp H, Narres HD, Burauel P, Vereecken H, Tappe W. Effect of gamma-sterilization and autoclaving on soil organic matter structure as studied by solid state NMR, UV and fluorescence spectroscopy. European Journal of Soil Science. 2008;59:540–550. [Google Scholar]
- Bertin C, Yang X, Weston LA. The role of root exudates and allelochemicals in the rhizosphere. Plant and Soil. 2003;256:67–83. [Google Scholar]
- Bird AF, Bonig I, Bacic A. Factors affecting the adhesion of micro-organisms to the surfaces of plant-parasitic nematodes. Parasitology. 1989;98:155–164. [Google Scholar]
- Blaxter ML, Page AP, Rudin W, Maizels RM. Nematode surface coats - Actively evading immunity. Parasitology Today. 1992;8:243–247. doi: 10.1016/0169-4758(92)90126-m. [DOI] [PubMed] [Google Scholar]
- Chen ZX, Dickson DW. Review of Pasteuria penetrans: Biology, ecology, and biological control potential. Journal of Nematology. 1998;30:313–340. [PMC free article] [PubMed] [Google Scholar]
- Chen ZX, Dickson DW, McSorley R, Mitchell DJ, Hewlett TE. Suppression of Meloidogyne arenaria race 1 by soil application of endospores of Pasteuria penetrans. Journal of Nematology. 1996;28:159–168. [PMC free article] [PubMed] [Google Scholar]
- Curtis RHC. Plant-nematode interactions: Environmental signals detected by the nematode’s chemosensory organs control changes in the surface cuticle and behaviour. Parasite. 2008;15:310–316. doi: 10.1051/parasite/2008153310. [DOI] [PubMed] [Google Scholar]
- Dao T. Influence of sterilization methods on selected soil microbiological, physical, and chemical properties. Journal of Environmental Quality. 2014;18:39–44. [Google Scholar]
- Davies KG, Curtis RHC. Cuticle surface coat of plant-parasitic nematodes. Annual Review of Phytopathology. 2011;49:135–156. doi: 10.1146/annurev-phyto-121310-111406. [DOI] [PubMed] [Google Scholar]
- Davies KG, Danks C. Interspecific differences in the nematode surface coat between Meloidogyne incognita and M. arenaria related to the adhesion of the bacterium Pasteuria penetrans. Parasitology. 1992;105:475–480. [Google Scholar]
- Davies KG, Danks C. Carbohydrate/protein interactions between the cuticle of infective juveniles of Meloidogyne incognita and spores of the obligate hyperparasite Pasteuria penetrans. Nematologica. 1993;39:53–64. [Google Scholar]
- Davies KG, Flynn CA, Kerry BR. 1988. The life cycle and pathology of the root-knot nematode parasite Pasteuria penetrans. Brighton Crop Protection Conference. London: British Crop Protection Council.
- De Hoff PL, Brill LM, Hirsch AM. Plant lectins: The ties that bind in root symbiosis and plant defense. Molecular Gnetics and Genomics. 2009;282:1–15. doi: 10.1007/s00438-009-0460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mendoza MEL, Modha J, Roberts MC, Curtis R, Kusel JR. Changes in the lipophilicity of the surfaces of Meloidogyne incognita and Haemonchus contortus during exposure to host signals. Parasitology. 2000;120:203–209. doi: 10.1017/s0031182099005326. [DOI] [PubMed] [Google Scholar]
- Egli M, Mirabella A, Kaegi B, Tomasone R, Colorio G. Influence of steam sterilisation on soil chemical characteristics, trace metals and clay mineralogy. Geoderma. 2006;131:123–142. [Google Scholar]
- Fioretti L, Porter A, Haydock PJ, Curtis R. Monoclonal antibodies reactive with secreted–excreted products from the amphids and the cuticle surface of Globodera pallida affect nematode movement and delay invasion of potato roots. International Journal for Parasitology. 2002;32:1709–1718. doi: 10.1016/s0020-7519(02)00178-9. [DOI] [PubMed] [Google Scholar]
- Grenache DG, Caldicott I, Albert PS, Riddle DL, Politz SM. Environmental induction and genetic control of surface antigen switching in the nematode Caenorhabditis elegans. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:12388–12393. doi: 10.1073/pnas.93.22.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatz B, Dickson DW. Effect of temperature on attachment, development, and interactions of Pasteuria penetrans on Meloidogyne arenaria. Journal of Nematology. 1992;24:512–521. [PMC free article] [PubMed] [Google Scholar]
- Huang XF, Chaparro JM, Reardon KF, Zhang RF, Shen Q, Vivanco JM. Rhizosphere interactions: Root exudates, microbes, and microbial communities. Botany. 2014;92:267–275. [Google Scholar]
- Hussey RS, Barker KR. A comparison of methods of collecting inocula for Meloidogyne spp., including a new technique. Plant Disease Reporter. 1973;57:1025–1028. [Google Scholar]
- Jones DL, Edwards AC. Influence of sorption on the biological utilization of two simple carbon substrates. Soil Biology and Biochemistry. 1998;30:1895–1902. [Google Scholar]
- Liegel LH. Effects of sterilization procedures on the biological, chemical, and physical properties of soils: A review. Turrialba. 1986;36:11–19. [Google Scholar]
- Mahmood T, Mehnaz S, Fleischmann F, Ali R, Hashmi ZH, Iqbal Z. Soil sterilization effects on root growth and formation of rhizosheaths in wheat seedlings. Pedobiologia. 2014;57:123–130. [Google Scholar]
- Maizels RM, Blaxter ML, Selkirk ME. Forms and functions of nematode surfaces. Experimental Parasitology. 1993;77:380–384. doi: 10.1006/expr.1993.1096. [DOI] [PubMed] [Google Scholar]
- Martinez-Toledo MV, de la Rubia T, Moreno J, Gonzalez-Lopez J. Root exudates of Zea mays and production of auxins, gibberellins and cytokinins by Azotobacter chroococcum. Plant and soil. 1988;110:149–152. [Google Scholar]
- Mateille T, Duponnois R, Diop MT. Influence of abiotic soil factors and the host plant on the infection of photoparasitic nematodes of the genus Meloidogyne by the actinomycete parasitoid Pasteuria penetrans. Agronomie. 1995;15:581–591. [Google Scholar]
- Modha J, Kennedy MW, Kusel JR. A role for second messengers in the control of activation-associated modification of the surface of Trichinella spiralis infective larvae. Molecular and biochemical parasitology. 1995;72:141–148. doi: 10.1016/0166-6851(95)00080-k. [DOI] [PubMed] [Google Scholar]
- Modha J, Roberts MC, Robertson WM, Sweetman G, Powell KA, Kennedy MW, Kusel JR. The surface coat of infective larvae of Trichinella spiralis. Parasitology. 1999;118:509–522. doi: 10.1017/s0031182099004266. [DOI] [PubMed] [Google Scholar]
- Muhammad A, Frankenberger KT., Jr Plant growth-regulating substances in the rhizosphere: Microbial production and functions. Advances in Agronomy. 1998;62:145–151. [Google Scholar]
- Neumann G, Bott S, Ohler MA, Mock HP, Lippmann R, Grosch R, Smalla K. Root exudation and root development of lettuce (Lactuca sativa L. cv. Tizian) as affected by different soils. Frontiers in Microbiology. 2014;5:2. doi: 10.3389/fmicb.2014.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen DP, Phu D, Libby LJM, Cormier JA, Montez KM, Ryder EF, Politz SM. Chemosensory control of surface antigen switching in the nematode Caenorhabditis elegans. Genes, Brain and Behavior. 2007;6:240–252. doi: 10.1111/j.1601-183X.2006.00252.x. [DOI] [PubMed] [Google Scholar]
- Proudfoot L, Kusel JR, Smith HV, Harnett W, Worms MJ, Kennedy MW. Rapid changes in the surface of parasitic nematodes during transition from pre- to post-parasitic forms. Parasitology. 1993;107:107–117. doi: 10.1017/s0031182000079464. [DOI] [PubMed] [Google Scholar]
- Robertson WM, Spiegel Y, Jansson HB, Marbanmendoza N, Zuckerman BM. Surface carbohydrates of plant parasitic nematodes. Nematologica. 1989;35:180–186. [Google Scholar]
- Sayre RM, Starr MP. Pasteuria penetrans (ex Thorne, 1940) nom. rev., comb. n., sp. n. a mycelial and endospore-forming bacterium parasitic in plant-parasitic nematodes. Proceedings of the Helminthological Society of Washington. 1985;52:149–165. [Google Scholar]
- Shamseldean MM, Platzer EG, Gaugler R. Role of the surface coat of Romanomermis culicivorax in immune evasion. Nematology. 2007;9:17–19. [Google Scholar]
- Sharon E, Spiegel Y. Gold-conjugated reagents for the labelling of carbohydrate recognition domains and glycoconjugates on nematode surfaces. Journal of Nematology. 1996;28:124–127. [PMC free article] [PubMed] [Google Scholar]
- Spiegel Y, Kahane I, Cohen L, Sharon E. Meloidogyne javanica surface proteins: Characterization and lability. Parasitology. 1997;115:513–519. doi: 10.1017/s0031182097001637. [DOI] [PubMed] [Google Scholar]
- Spiegel Y, McClure MA. The surface-coat of plant-parasitic nematodes - chemical-composition, origin, and biological role - A review. Journal of Nematology. 1995;27:127–134. [PMC free article] [PubMed] [Google Scholar]
- Spiegel Y, Sharon E, Mor M. Attachment of Pasteuria penetrans endospores to the surface of Meloidogyne javanica second-stage juveniles. Journal of nematology. 1996;28:328–334. [PMC free article] [PubMed] [Google Scholar]
- Stirling GR. Biological control of Meloidogyne javanica and Bacillus penetrans. Phytopathology. 1984;74:55–60. [Google Scholar]
- Trudgill DL, Bala G, Blok VC, Daudi A, Davies KG, Gowen SR, Voyoukallou E. The importance of tropical root-knot nematodes (Meloidogyne spp.) and factors affecting the utility of Pasteuria penetrans as a biocontrol agent. Nematology. 2000;2:823–845. [Google Scholar]