Abstract
Protogamasellus mica was extracted from a sugarcane field in Australia and cultured on bacterial-feeding nematodes. Studies with various nematodes in laboratory arenas showed that one mite and its progeny reduced nematode numbers by between 26 and 50 nematodes/day. A bacterivore (Mesorhabditis sp.), a fungivore (Aphelenchus avenae), and two plant parasites (root-knot nematode, Meloidogyne javanica and root-lesion nematode, Pratylenchus zeae) were all reduced at much the same rate despite the fact that the nematodes are quite different in size and motility and belong to different trophic groups. When sugarcane was grown in the greenhouse for 8 wk, stunt nematode (Tylenchorhynchus annulatus), a plant parasite that feeds ectoparasitically on roots, was almost eliminated from pots inoculated with the mite, and numbers of microbivores and root-lesion nematode were markedly reduced. Huge reductions in nematode populations were also observed when mites were added to microcosms containing small quantities of defaunated soil. These results show that P. mica multiplies rapidly when nematodes are available as a food source and has the capacity to play a role in regulating populations of both plant-parasitic and free-living nematodes. Future research should focus on understanding the crop and soil management practices required to enable this mite and other predatory species to thrive.
Keywords: biological control, free-living nematodes, mite, nematophagous, Pratylenchus zeae, predator, Protogamasellus mica, regulatory, root-lesion nematode, stunt nematode, suppression, Tylenchorhynchus annulatus
Nematodes are an important component of the biological community in agricultural soils. Plant-parasitic nematodes have been widely studied because they damage root systems and reduce the yield of most crops (Evans et al., 1993; Luc et al., 2005), but from an ecological perspective, free-living nematodes are probably more important. These nematodes feed on bacteria, fungi, and other soil organisms, and during that process, they regulate populations of their prey and increase the availability of nutrients required by plants (Ingham et al., 1985; Yeates and Wardle, 1996; Ferris et al., 1998; Chen and Ferris, 1999; Stirling, 2014).
Plant-parasitic nematodes were considered an intractable problem in agriculture until the middle of the 20th century when broad-spectrum soil fumigants and a range of organophosphate and carbamate nematicides were found to provide a high level of control. However, the environmental risks associated with these chemicals were eventually recognized (Thomason, 1987), and efforts were then made to add biological control to the range of tools available to manage nematode pests. Most of the early work on the natural enemies of nematodes focused on fungal parasites and predators, bacterial parasites such as Pasteuria, and predatory nematodes (Stirling, 1991).
In the search for antagonists of nematodes, the animals that inhabit the air-filled passages in soil were largely ignored. Plant pathologists and soil zoologists knew that nematodes were sometimes eaten by mites and other microarthropods but considered these observations more a curiosity than of any practical value. However, as scientific interest turned to analyzing belowground food webs, it eventually became obvious that a wide range of microarthropods feed on nematodes and sometimes specialize on nematode prey (Moore et al., 1988; Walter and Ikonen, 1989). Preeminent among these nematophages are mites in the suborder Mesostigmata. Most of them have characteristics that could contribute to their effectiveness in nematode suppression, including high vagility, short generation times, and female-biased sex ratios (i.e., their reproductive output favors the larger and more voracious females).
A recent survey of sugarcane soils in Australia disclosed a diversity of nematophagous mesostigmatans worthy of evaluation for their ability to suppress nematode pests (D. E. Walter, unpublished). For this study, we chose P. mica (Athias-Henriot), one of the smallest known mesostigmatans. Full grown adult females of this species are less than 250 µm long and have a dry weight of 0.70 μg (Walter and Ikonen, 1989). P. mica has a semicosmopolitan distribution, and although it can feed on fungi and small Collembola prey, its preferred food source appears to be nematodes (Walter and Lindquist, 1989). Previous studies have shown that this mite is able to penetrate deeply into mineral soil (at least 8 m along roots) and is readily cultured on nematodes, whereas high populations have been associated with both nematode pest problems and with the decline of citrus nematode in pot cultures (Walter and Kaplan, 1990, Walter et al., 1993). P. mica has a generation time of about 10 d at 25°C, reproduction is by all-female parthenogenesis (thelytoky), and in spite of its small size, adults are able to consume more than their body mass of nematodes daily (Walter and Ikonen, 1989). As nothing is known about the capacity of these animals to prey on nematodes in a soil environment, this study aimed to determine whether they consumed enough nematodes to play a role in regulating populations of nematode pests.
Materials and Methods
Culture of the mite:
P. mica was obtained from a sugarcane field in Bundaberg, Queensland, Australia and cultured on an unidentified species of Mesorhabditis. Nematodes and their naturally co-occurring bacteria were transferred to petri dishes containing 1/4-strength corn meal agar or rolled oats agar, and 1 to 3 wk later the nematode progeny were washed from the culture media with water. The nematode suspension was allowed to settle and then drops containing thousands of nematodes were added to a 15-mm layer of Plaster of Paris and activated charcoal (7:1 v/v) that had previously been dispensed into screw-capped plastic vials 55 mm high and 45 mm diameter. A few mites were then transferred to the vial using a damp brush, and provided they were fed every 5 to 7 d, large numbers were soon available for use in experiments.
Feeding studies in laboratory arenas:
Initial feeding studies were done with four nematodes: Mesorhabditis sp. (cultured with its associated bacteria on 1/4-strength corn meal agar), A. avenae (cultured on Rhizoctonia solani), M. javanica (cultured on tomato in the greenhouse) and P. zeae (cultured on sterile carrot tissue in the laboratory [Moody et al., 1973]). For each experiment, a suspension of one of the nematodes was prepared, and between 3,000 and 4,000 nematodes were added to the Plaster of Paris/charcoal mix in either 10 or 12 screw-capped vials. Ten P. mica were then added to half the vials, and after an incubation period of 5 d at ambient temperatures of 22 to 26°C, water was added and the vials were shaken vigorously to ensure that mites and nematodes were removed from cracks and crevices in the Plaster of Paris. The animals were then washed into a container and counted. For each experiment, the number of nematodes consumed or otherwise destroyed by one mite and its progeny/day was calculated by determining the difference in nematode numbers between the mite and no-mite treatments and dividing that number by 50 (10 mites × 5 d).
Effect of P. mica on nematodes in pots:
In 2015, an experiment was set up in 400-mL pots containing pasteurized coarse sand to which peat (4% by volume) had been added to improve water retention. Mill mud, a by-product of the sugar milling process, was also mixed with the sand (0.5 g mill mud/kg sand or the equivalent of incorporating 1 t/ha organic matter to a depth of 15 cm) to provide a food source for the bacteria and fungi required by microbivorous nematodes. Pots were filled with this soil, a tissue-cultured plantlet of sugarcane (Q249) was planted and then each pot was inoculated with 300 stunt nematodes (T. annulatus) and 150 root-lesion nematodes (P. zeae). Because the stunt nematodes were obtained from greenhouse cultures that also contained free-living nematodes, each pot also received about 300 fungal-feeding and bacterial-feeding nematodes (predominantly nematodes in the families Rhabditidae, Cephalobidae, and Aphelenchidae). P. mica was then inoculated into half the pots (20 mites/pot) to establish the following experiment: 2 P. mica treatments (mites and no mites) × 2 sampling times (4 and 8 wk) × 10 replicates.
Pots were maintained in a greenhouse at temperatures that generally ranged from 22 to 34°C. At each harvest time, shoots were cut from all the plants, dried, and weighed. The soil and roots from 5 of the 10 replicate pots were then placed in a Tullgren funnel, and mites were retrieved in 70% EtOH. Soil and roots from the other replicates were spread on two trays, and nematodes were recovered after 2 d by sieving twice on a 38-µm sieve (a modification of Whitehead and Hemming, 1965).
The experiment was repeated under similar conditions in 2016 except that there were a few minor changes. The soil used to fill the pots was the same except that peat was omitted; a different sugarcane variety (Q124) was planted; only 40 T. annulatus and 40 P. zeae, together with associated microbivorous nematodes, were added per pot; and an additional sampling time was included (2, 4, and 8 wk). Also, only five replicate pots were included because when pots were harvested, the soil and roots were mixed gently and half was spread on trays to recover nematodes while the other half was placed on a Tullgren funnel to retrieve mites. Numbers of nematodes and mites/pot were determined by multiplying the numbers recovered by 2.
Effect of P. mica on nematodes in screw-capped vials:
The soil used for these experiments was a mixture of washed sand and peat (25:1 vol/vol) amended with a 50:50 mixture (w/w) of mill mud and finely chopped sugarcane residue. The amendment was passed through a 1-mm sieve and was then mixed with the sand and peat at a rate of 0.25 g/kg (i.e., the equivalent of incorporating 500 kg of organic matter/ha to a depth of 15 cm). After the amended soil was defaunated with heat (80°C for 6 hr) the equivalent of 60 g dry soil was added to 135-mL screw-capped vials (108 mm high, 45 mm diam.), and a tissue-cultured sugarcane plantlet (variety Q124) was planted in each vial. To replace the microorganisms that may have been affected by the defaunation process, a healthy garden soil was mixed with water, and a suspension containing the bacteria and fungi isolated by dilution plating was added to the soil in each vial.
Because mites had to be prevented from migrating into or out of the vials, and also to minimize condensation, provide aeration, and enable light to enter, a 15-mm diameter hole was cut in the screw-cap lid, and a piece of 10-µm plastic mesh was glued on the lid to cover the hole. The vials were then transferred to an incubator containing a fluorescent light and maintained at a temperature of 26 to 28°C (Fig. 1). Every few days, the vials were weighed, and water was added to maintain the soil moisture content between 7% and 10%. Aquasol (a commercial fertilizer containing macro- and micronutrients) was added to the water every 10 to 14 d to fertilize the plants.
Fig. 1.

Meshed, screw-capped vials containing tissue-cultured sugarcane plantlets that had been transferred to the vials 14 d previously.
The plant-parasitic nematodes inoculated onto the plants were P. zeae and T. annulatus. These nematodes were retrieved from greenhouse cultures, but because these cultures also contained a range of bacterial and fungal-feeding nematodes, these nematodes were added to the vials with the plant parasites.
P. mica was cultured using methods described previously, and experiments were established by adding these mites to vials containing tissue-cultured sugarcane. At the end of each experiment, the shoots of plants were dried and weighed and about 400 mL water was used to wash the soil and roots into a 2-L beaker. The suspension was then agitated and decanted over a 75-µm sieve. The mites retained on the sieve were washed into a beaker, while the water passing through the sieve was collected, as it contained the nematodes. More water was then added to the beaker containing the soil and roots, and the whole sieving process was repeated two more times. The water washed from the 75-µm sieve was then poured (to a depth of about 3 mm) into plastic petri dish with parallel lines marked on the base. Because mites are hydrophobic and float on the water, they were counted under a microscope by focusing on the surface of the water.
The nematodes in the suspension which passed through the 75-µm sieve were retrieved by sieving twice on a 38-µm sieve. However, because the material retained on the sieve was very dirty due to the presence of peat fragments and organic matter, it was poured onto a nematode extraction tray together with the decanted soil and the roots retained in the 2-L beaker. After 24 hr, the water in the trays was sieved twice on a 38-µm sieve, and the nematodes recovered were counted.
In the first experiment, 5 P. zeae and 10 T. annulatus were handpicked from suspensions obtained from greenhouse cultures and added to sugarcane plantlets that had been growing in vials for 5 wk. About 50 bacterial and fungal-feeding nematodes from the same cultures were also inoculated into the vials. One week later, four adult Protogamasellus were transferred to 12 vials, and another 12 vials received no mites. Because six replicates of each mite treatment were to be taken down at different times after the mites were added, the experiment consisted of 2 mite treatments (mites, no mites) × 2 incubation times (15 and 30 d) × 6 replicates.
Experiment 2 was similar to the previous experiment except that vials were inoculated with 5 P. zeae, 5 T. annulatus, and 50 free-living nematodes, the nematodes were inoculated 3 wk after the sugarcane was planted, P. mica was added 2 wk after the nematodes, and vials were incubated for 15 and 40 d.
Statistical analyses:
Data were analyzed by one- or two-way analysis of variance using Genstat 8. Nematode counts from pot experiments were transformed (log10 no. nematodes +1) before analysis.
Results
Feeding studies in laboratory arenas:
Results of four similar experiments with different nematodes are presented in Table 1, and they show that the number of nematodes was markedly reduced when P. mica was present, regardless of the nematode species used as a food source. Mites were never recovered from the no mite treatment but P. mica multiplied in the vials into which it had been added. Numbers increased on all the nematodes, with the number recovered on day 5 more than double the number originally inoculated. Some eggs were also present at the end of the experiment, with numbers possibly higher when the food source was Mesorhabditis. Calculations of nematode consumption rates indicated that on average, one mite and its progeny consumed between 26 and 50 nematodes/day.
Table 1.
Numbers of nematodes in vials with and without 10 Protogamasellus mica for 5 d, numbers of mites at the end of the experiment, and nematode consumption rates when different nematodes were available as a food source.
Effect of P. mica on nematodes in pots:
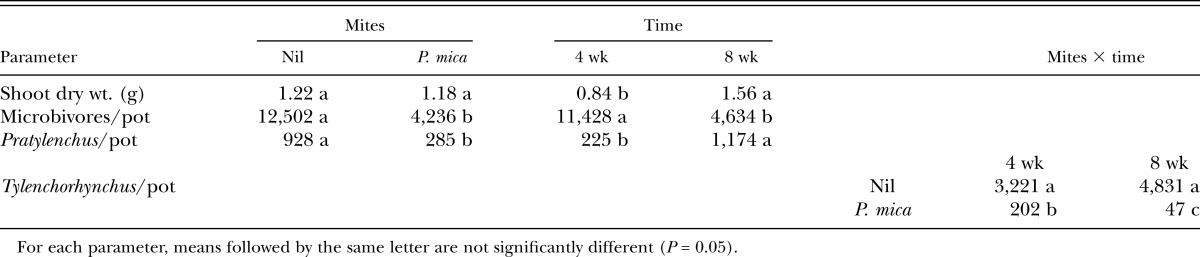
In the 2015 experiment, shoot dry weight was significantly greater at the second harvest than the first, but the presence of P. mica had no effect on plant growth (Table 2). Analyses of the nematode data (Table 2) showed that populations of microbivorous nematodes were highest at 4 wk and then declined whereas populations of plant-parasitic nematodes increased with time. Numbers of P. zeae were relatively low at 4 wk and then increased but populations of T. annulatus were high at both sampling times.
Table 2.
Main effects of mites and time (and mite × time interactions, where significant) on shoot biomass and numbers of nematodes recovered from pots of sugarcane 4 and 8 wk after all pots were inoculated with nematodes and 20 Protogamasellus mica were added to half the pots.
Inoculation of pots with P. mica had a highly significant effect (P < 0.001) on all groups of nematodes (Table 2). Numbers of microbivores, P. zeae and T. annulatus in pots with P. mica were respectively 70%, 70%, and 99% lower at 8 wk than in pots without the mite. The effects on T. annulatus were also significant at 4 wk, with numbers 94% lower in the P. mica treatment.
Counts of the microarthropods in the other five replicate pots showed that P. mica was present in inoculated pots at both harvests (84 ± 23 and 61 ± 24 P. mica/pot at 4 and 8 wk, respectively). However, P. mica was also found in the noninoculated pots (6 ± 3 and 60 ± 25 P. mica/pot at 4 and 8 wk, respectively), with more than 100 P. mica being recovered from some pots at 8 wk. Regardless of treatment, isotomid springtails and a few other microarthropods (mostly fungivores in the mite family Eupodidae) were also detected in the pots.
One observation made when the nematodes were being counted was that T. annulatus tended to adhere to each other. In many cases, the nematodes would aggregate in groups of 10 to 15 nematodes, and because numbers were very high in the non-mite treatment, it was difficult to obtain an accurate count.
In the 2016 experiment, shoot dry weight increased significantly with time but the presence of P. mica did not affect plant growth. P. mica significantly reduced numbers of microbivorous nematodes and T. annulatus at both 4 and 8 wk, with numbers of both nematodes reduced by more than 98% at 8 wk (Table 3). Populations of P. zeae were relatively low at all harvest times, but P. mica reduced numbers by 72% at 8 wk.
Table 3.
Interaction table showing the effects of Protogamasellus mica on numbers of nematodes recovered from pots of sugarcane 2, 4 and 8 wk after all pots were inoculated with nematodes and 20 P. mica were added to half the pots.
P. mica was recovered from all inoculated pots and numbers increased with time (5 ± 1; 18 ± 2 and 97 ± 21 P. mica/pot at 2, 4 and 8 wk, respectively). P. mica was not observed in the noninoculated pots at 2 and 4 wk, but at 8 wk, it was observed in three of the eight replicate pots (1, 1 and 4 individuals in the subsample that was processed). Isotomid springtails were found in a few pots at 4 wk, and numbers were sometimes greater than 200 individuals/pot at 8 wk. A mesostigmatid mite (Lasioseus subterraneus) was also present in most pots at 8 wk, generally at population densities of 10 to 40 individuals/pot. A purple springtail (Sminthuridae) and another mite (Eupodes sp.) were observed in a few pots, but numbers were always relatively low.
Effect of P. mica on nematodes in screw-capped vials:
Results of the two experiments are presented in Tables 4 and 5, and they show that the biomass of sugarcane shoots was not affected by the presence of mites or harvest time. In the first experiment, P. mica multiplied in the vials to which it was inoculated, with its population at 30 d about five times greater than the number inoculated. The mite significantly affected populations of the dominant nematodes, with numbers of microbivores and T. annulatus at 30 d 86% and 97% lower than the no-mite treatment, respectively. Populations of P. zeae were low at both harvest times and were not affected by the presence of mites (Table 4).
Table 4.
Main effects of mites and time, and mite × time interactions, on shoot biomass and numbers of nematodes recovered from vials containing tissue-cultured sugarcane plants 15 and 30 d after all vials were inoculated with nematodes and four Protogamasellus mica were added to half the vials.
Table 5.
Main effects of mites and time, and mite × time interactions, on shoot biomass and numbers of nematodes recovered from vials containing tissue-cultured sugarcane plants 15 and 40 d after all vials were inoculated with nematodes and four Protogamasellus mica were added to half the vials.
In the second experiment, the population of P. mica at 15 and 40 d had increased 4.7 and 8.7 times, respectively. In the presence of the mite, populations of all nematode groups were reduced significantly, with numbers of microbivores, T. annulatus and P. zeae at 40 d reduced by 86%, 96%, and 67%, respectively, compared with the no-mite treatment (Table 5).
Discussion
Our studies in laboratory arenas clearly show that P. mica has the capacity to kill or damage large numbers of nematodes. Walter and Ikonen (1989) previously found that this mite consumed an average of 3.7 nematodes (Acrobeloides sp.)/adult/day but the level of predation in our experiments appeared to be much higher, presumably because the mites were in reproductive mode, and food supplies were plentiful. However, some superfluous killing may also have occurred (Sunderland, 1999). If it is assumed that the ten mites originally added to each vial were still alive at the end of the 5-d feeding period and that the other mites present were juveniles that had been feeding for an average of 2 d, then the data in Table 1 suggest that the number of nematodes damaged or killed by P. mica ranged from 18 to 33 nematodes/mite/day. This is similar to the levels of consumption and/or damage observed in laboratory studies with other mites. For example, Oliveira et al. (2007) estimated that a single oribatid mite (Pergalumna sp.) consumed 18 juveniles of M. javanica and 42 adults and juveniles of Pratylenchus coffeae per day whereas a species of Sancassania killed about 40 entomopathogenic nematodes in a day (Karagoz et al., 2007).
Our observations in the laboratory also suggested that P. mica has the capacity to use a wide range of nematodes as a food source. The nematodes included in our study were all consumed at much the same rate despite the fact that they were quite different in size and motility and belonged to different trophic groups. Feeding studies with other mesostigmatids also suggest that they generally consume a wide range of nematodes (Walter and Ikonen, 1989).
The results of our pot experiments indicated that P. mica had a marked effect on the nematode community in a soil system that mimicked to some extent the conditions that occur in natural soils. The mite almost eliminated T. annulatus, had a similar effect on microbivorous nematodes in one of the experiments, and reduced numbers of P. zeae by about 70%. However, as has been found previously with greenhouse experiments (Walter and Kaplan, 1990), our pots were invaded by other mites, and P. mica also moved into pots that were not inoculated. Although most of the microarthropods that invaded the pots were fungivores and detritivores, predators such as L. subterraneus were also observed. This mite may have consumed some nematodes, but as its numbers were similar in both treatments, it could not have been responsible for the differences in nematode numbers between inoculated and noninoculated pots. Also, P. mica was mainly seen in noninoculated pots at the end of the experiment rather than at earlier sampling times, again suggesting that the reductions in nematodes observed in the inoculated pots were largely due to this mite.
We overcame the problem of microarthropods moving from pot to pot in the greenhouse by carrying out experiments in soil microcosms. Although the microcosms could only be used for short-term observations with small plants and small amounts of soil, they proved effective. Reductions in nematode populations were similar to the pot experiments, and P. mica or other microarthropods were never observed in noninoculated vials.
One unexpected result from our pot experiments was that P. mica consistently reduced populations of microbivorous nematodes, a group that have short life cycles and an enormous capacity to multiply. These bacterial and fungal feeding nematodes are always present in soil and are likely to be the primary food source for this mite. Their importance was shown by our results in soil microcosms (Tables 4 and 5). In the first 15 d, a period when populations of plant parasites were low, P. mica presumably used these nematodes as its main food source.
One surprising finding from this study was the effectiveness of P. mica against stunt nematode (T. annulatus). Regardless of the experimental system used, P. mica consistently reduced nematode numbers on sugarcane by more than 90%. There are two possible reasons why the mite may have been particularly effective against this nematode. First, T. annulatus is an obligate parasite of roots and so it aggregates in the rhizosphere. However, because the nematode is an ectoparasite, its head does not move while it is feeding, and its body is exposed to the soil. Thus, it is an easy target for a predator capable of moving around the root system. Second, individuals of T. annulatus tend to adhere to each other when their cuticles touch, a phenomenon that has been termed “swarming” (Hollis, 1958, 1962; Ibrahim and Hollis, 1973). Clumps of nematodes were observed when suspensions containing high numbers of T. annulatus were being counted, and if swarming also occurs in soil then this nematode is likely to be highly vulnerable to any predator in its vicinity.
P. mica also reduced numbers of root-lesion nematode, probably the most important nematode pest of sugarcane worldwide. However, it was not as effective as it was against T. annulatus, presumably because this nematode is endoparasitic and spends much of its life cycle within roots. It will only be vulnerable to predation by mites when it leaves a root to find another feeding site.
In conclusion, our results show that P. mica multiplies rapidly when nematodes are available as a food source and has the capacity to play a role in regulating populations of both plant-parasitic and free-living nematodes. However, we do not claim that the effects on nematode populations observed in our microcosm and pot studies would be obtained in more complex environments (e.g., the soils used for agriculture). Although the biological community in most agricultural soils has been depleted to some extent (Lehman et al., 2015; Stirling et al., 2016), P. mica would still face greater competition for food in such environments than it did in our studies. Also, it would be subject to predation from other soil organisms.
When experimental evidence is produced to show that a particular antagonist is an effective predator of nematodes, it is often argued that resources should be devoted to mass-producing the organism and introducing it into soil as a biocontrol agent. In this case, we do not believe that this approach is appropriate. P. mica is only one of many nematophagous microarthropods in agricultural soils and future research should focus on managing these soils in ways that enable this community of predators to thrive. This means understanding the preferred microhabitats of various members of the microarthropod community, knowing the pathways they use to move through soil, and determining the impact of soil physical and chemical properties (e.g., texture, bulk density and soil organic matter levels) and management practices (e.g., tillage, wheel traffic, nutrient inputs, and pesticide inputs) on their capacity to multiply and prey on nematodes.
Literature Cited
- Chen J, Ferris H. The effects of nematode grazing on nitrogen mineralization during fungal decomposition of organic matter. Soil Biology and Biochemistry. 1999;31:1265–1279. [Google Scholar]
- Evans K, Trudgill DL, Webster JM, editors. 1993. Plant parasitic nematodes in temperate agriculture. Wallingford: CAB International. [Google Scholar]
- Ferris H, Venette RC, van der Meulen HR, Lau SS. Nitrogen mineralization by bacterial feeding nematodes: Verification and measurement. Plant and Soil. 1998;203:159–171. [Google Scholar]
- Hollis JP. Induced swarming of a nematode as a means of isolation. Nature. 1958;182:956–957. doi: 10.1038/182956b0. [DOI] [PubMed] [Google Scholar]
- Hollis JP. Nature of swarming in nematodes. Nature. 1962;193:798–799. [Google Scholar]
- Ibrahim IKA, Hollis JP. Electron microscope studies of the cuticle of swarming and nonswarming Tylenchorhynchus martini. Journal of Nematology. 1973;5:275–281. [PMC free article] [PubMed] [Google Scholar]
- Ingham RE, Trofymow JA, Ingham ER, Coleman DC. Interactions of bacteria, fungi, and their nematode grazers: Effects on nutrient cycling and plant growth. Ecological Monographs. 1985;55:119–140. [Google Scholar]
- Karagoz M, Gulcu B, Cakmak I, Kaya HK, Hazir S. Predation of entomopathogenic nematodes by Sancassania sp. (Acari: Acaridae) Experimental and Applied Acarology. 2007;43:85–95. doi: 10.1007/s10493-007-9105-y. [DOI] [PubMed] [Google Scholar]
- Lehman RM, Cambardella CA, Stott DE, Acosta-Martinez V, Manter DK, Buyer JS, Maul JE, Smith JL, Collins HP, Halvorson JJ, Kremer RJ, Lundgren JG, Ducey TF, Jin VL, Karlen DL. Understanding and enhancing soil biological health: The solution for reversing soil degradation. Sustainability. 2015;7:988–1027. [Google Scholar]
- Luc M, Sikora RA, Bridge J, editors. 2005. Plant parasitic nematodes in subtropical and tropical agriculture. Wallingford: CAB International. [Google Scholar]
- Moody EH, Lownsbery BF, Ahmed JM. Culture of the root-lesion nematode Pratylenchus vulnus on carrot disks. Journal of Nematology. 1973;5:225–226. [PMC free article] [PubMed] [Google Scholar]
- Moore JC, Walter DE, Hunt HW. Arthropod regulation of micro- and mesobiota in belowground detrital food webs. Annual Review of Entomology. 1988;33:419–439. [Google Scholar]
- Oliveira AR, de Moraes GJ, Ferraz LCCB. Consumption rate of phytonematodes by Pergalumna sp. (Acari: Oribatida: Galumnidae) under laboratory conditions determined by a new method. Experimental and Applied Acarology. 2007;41:183–189. doi: 10.1007/s10493-007-9062-5. [DOI] [PubMed] [Google Scholar]
- Stirling GR. 1991. Biological control of plant-parasitic nematodes. Wallingford: CAB International. [Google Scholar]
- Stirling GR. 2014. Biological control of plant parasitic nematodes: Soil ecosystem management in sustainable agriculture. 2nd ed. Wallingford: CAB International. [Google Scholar]
- Stirling GR, Hayden HL, Pattison AB, Stirling AM. 2016. Soil biology, soilborne diseases and sustainable agriculture: A guide. Melbourne: CSIRO Publishing. [Google Scholar]
- Sunderland K. Mechanisms underlying the effects of spiders on pest populations. The Journal of Arachnology. 1999;27:308–316. [Google Scholar]
- Thomason IJ. 1987. Challenges facing nematology: Environmental risks with nematicides and the need for new approaches. Pp. 469–476 in J. A. Veech and D. W. Dickson, eds. Vistas on nematology. Hyattsville, MD: Society of Nematologists. [Google Scholar]
- Walter DE, Ikonen EK. Species, guilds and functional groups: Taxonomy and behavior in nematophagous arthropods. Journal of Nematology. 1989;21:315–327. [PMC free article] [PubMed] [Google Scholar]
- Walter DE, Kaplan DT. A guild of thelytokous mites associated with citrus roots in Florida. Environmental Entomology. 1990;19:1338–1343. [Google Scholar]
- Walter DE, Kaplan DT, Davis EL. Colonization of greenhouse nematode cultures by nematophagous mites and fungi. Journal of Nematology. 1993;25:789–795. [PMC free article] [PubMed] [Google Scholar]
- Walter DE, Lindquist EE. Life history and behavior of mites in the genus Lasioseius (Acari: Mesostigmata: Ascidae) from grassland soils in Colorado, with taxonomic notes and description of a new species. Canadian Journal of Zoology. 1989;67:2797–2813. [Google Scholar]
- Whitehead AG, Hemming JR. A comparison of some quantitative methods of extracting small vermiform nematodes from soil. Annals of Applied Biology. 1965;55:25–38. [Google Scholar]
- Yeates GW, Wardle DA. Nematodes as predators and prey: Relationships to biological control and soil processes. Pedobiologia. 1996;40:43–50. [Google Scholar]