Abstract
Background
Some studies have shown that protein-enriched diets can lead to greater weight loss and improvements in biomarkers of metabolic syndrome (MeS) than standard protein diets. Therefore, the aim of this study was to determine the effect of increased protein intake on weight loss in Mexican adults with MeS.
Methods
Randomized controlled trial in 118 adults aged 47.4 ± 11.5 years and meeting the established criteria for MeS were randomized to prescribed hypocaloric diets (500 kcal less than resting metabolic rate) providing either 0.8 g/kg body weight (standard protein diet (SPD)) or 1.34 g/kg body weight (higher protein diet (HPD)) for 6 months. Body weight, waist circumference, percent body fat by bioimpedance analysis, fasting blood glucose, fasting insulin, hemoglobin A1c, total cholesterol, high-density lipoprotein (HDL) cholesterol, very-low-density lipoprotein (VLDL) cholesterol, triglycerides, C-reactive protein, creatinine, blood urea nitrogen, alanine aminotransferase, aspartate aminotransferase, and gamma-glutamyl transferase were measured at baseline, 3 months and at 6 months.
Results
There were 105 subjects (51 for SPD and 54 for HPD) who completed the trial. Overall weight loss was 5.1 ± 3.6 kg in the SPD group compared to 7.0 ± 3.7 kg in the in HPD group. Both groups lost a significant percent of centimeters of waist circumference (SPD −6.5 ± 2.6 cm and HPD −8.8 ± 2.6 cm). There was no statistical difference Except for the varying weight losses the two groups did not show any further differences overall. However in the subgroup judged to be adherent more than 75% of the time with the prescribed diets, there was a significant difference in mean weight loss (SPD −5.8% vs. HPD −9.5%) after adjusting for baseline BMI. Both groups demonstrated significant decreases in waist circumference, glucose, insulin, triglycerides, and VLDL cholesterol, but there were no differences between the groups. There were no changes in blood tests for liver or renal function.
Conclusions
There were no significant differences in weight loss and biomarkers of MeS when the overall group was examined, but the participants with more adherence rate in the HPD group lost significantly more weight than adherent participants in the SPD group.
Keywords: Diet, Metabolic syndrome, Weight loss, Protein intake
Introduction
The prevalence of metabolic syndrome (MeS) is approximately 25% in the worldwide adult population [1] and 49.8% in the Mexican population [2]. MeS is defined as a cluster of metabolic disorders (central adiposity and any two of the following four factors: reduced high-density lipoprotein (HDL) cholesterol, increased triglycerides, high blood pressure, and hyperglycemia), associated with the increasing prevalence of obesity [3]. In Mexico, 25.2% of adults with MeS have a diagnosis of obesity, and this metabolic disorder is recognized as the main risk factor for MeS [4].
Moderate weight loss contributes to reducing the risk of MeS [5]. For weight loss, numerous diet regimens are available, including, but not limited to, restricting different proportions of fats and carbohydrates as well as consuming fewer calories [6]. However, some diets do not produce significant weight loss, causing a high percentage of adults to end the diet after a short period of time and return to their original weight [7].
There is a debate on which type of diet and what proportion of macronutrients is more effective to produce a greater weight loss [6]. Some studies have shown that a diet with a high protein content is the most effective way to result in a weight loss; however, other studies did not found any differences [8, 9]. Some of the mechanisms explaining the weight loss is that increasing the amount of dietary protein and reducing the proportion of carbohydrates promotes the oxidation of free fatty acids, increased satiety, and consequently lowers the energy intake in addition to a greater dietary thermogenesis [10, 11, 12, 13]. The amount of protein that enhances weight loss is controversial; nonetheless, diets with a higher proportion of protein and less carbohydrates have proven to be effective for weight loss in obese adults and lead to improvements of biomarkers of MeS [14].
The group of Flechtner [15, 16] demonstrated that protein-rich meal replacements were successful as a weight loss strategy in obese adults with MeS, as they simplified food preparation and achieved greater adherence to the diet. To our knowledge, the use of protein-rich meal replacements for weight loss has not yet been investigated in Mexican adults to date. Therefore, the aim of this study was to determine the effect of increased protein intake via partial diet substitution with meal replacements on weight loss in Mexican adults with MeS. We hypothesized that adults with MeS on a hypocaloric diet with increased protein and partial meal replacements would lose more weight than those on a hypocaloric conventional diet with standard protein content.
Material and Methods
Study Design
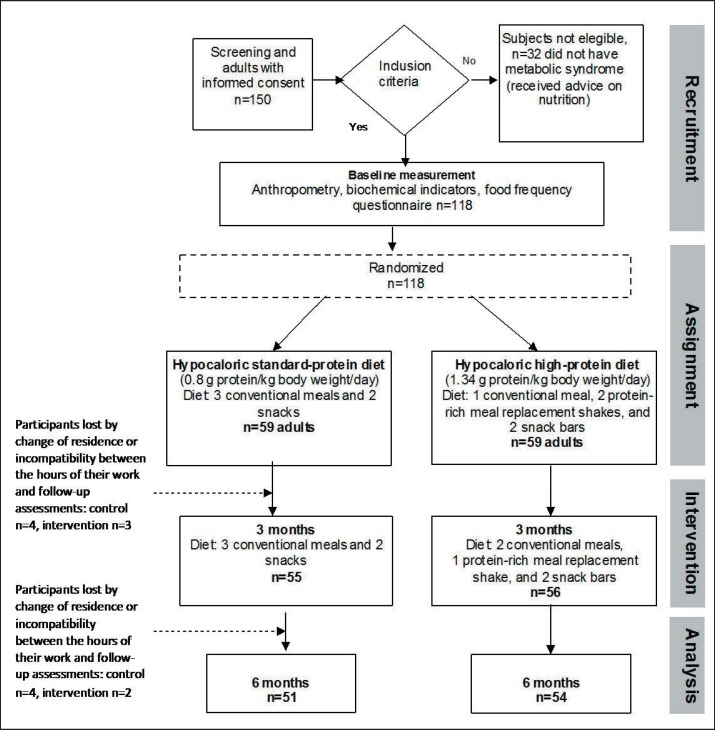
Our study was a randomized controlled clinical trial of parallel design with a 6-month intervention in free-living generally healthy Mexican adult men and women (aged 20–60 years) with MeS. Individuals were invited to participate by posters and social network advertising. The study was conducted and data were collected at the Obesity and Metabolic Disease Research Clinics (Mexico DF. and Cuernavaca Morelos) from January to August 2014. The sample size was calculated using body weight change as the primary endpoint (-3 kg of initial body weight and assuming a variance of 4 kg between groups) and a formula that compares two means, an alpha of 0.05, and a power of 95%: n = 2 (Zα + Zβ)2 S2/d2. Based on these calculations and assuming losses of 20% in the follow-up, we established a total baseline sample of at least 96 adults (n = 118 including losses), to allow the randomization of 59 participants per group. The study protocol was approved by the Ethics, Research, and Biosafety Committees of the Mexican National Institute of Public Health (MNIPH).
Participants
Eligibility criteria included male and female adults (20–60 years of age) with a BMI between 25 and 45 kg/m2 and the presence of MeS. For the diagnosis of MeS, we used the classification from the International Diabetes Federation [17], which is the one that best characterizes this syndrome in Mexicans. Thus participants were required to have central obesity, which is defined as waist circumference ≥ 90 cm in men and ≥ 80 cm in women, and two or more of the following criteria: a) triglycerides ≥150 mg/dl, b) HDL cholesterol) < 40 mg/dl in men and < 50 mg/dl in women, c) blood pressure ≥ 130/85 mm Hg; or d) fasting glucose ≥100 mg/dl. Exclusion criteria included background of bariatric surgery, treatment for addiction to smoking and/or alcohol, drug abuse, psychiatric disorder, use of anti-obesity medication, soy allergy, women not using an appropriate birth control method or who were pregnant or lactating, and body weight gain or loss greater than 2% during the 3 months prior to the start of the study. To achieve the baseline sample of 118 adults, 150 patients were screened, and 32 were excluded because they did not meet the inclusion and exclusion criteria.
During screening, individuals interested in participating received an explanation of the study design and were given sufficient time to consider inclusion in the clinical trial and complete the form for informed consent. Written informed consent was obtained from all participants for screening measurements and just prior to baseline and study commencement for consent to participate in the trial. Confidentiality of data was maintained in accordance with the ethical standards of Good Clinical Practice and Research Development Regulations in Mexico. On the same day as consent, doctors recorded participants' medical history, nutritionists conducted and recorded anthropometry measurements (i.e., weight, height, and waist circumference), and nurses measured blood pressure and withdrew a fasting blood sample for determination of glucose, triglyceride, total cholesterol, and HDL cholesterol levels.
After the medical and anthropometric evaluations, 4 meal replacements enriched with soy protein were provided to all participants to be consumed during the following 2 days to evaluate tolerance to the high protein meal replacement. At the end of the evaluation of tolerance, no participant turned out to be intolerant to the meal replacement. Each clinic supervisor enrolled participants, and the study coordinator conducted the randomization sequencing and assigned participants to interventions. Randomization was achieved using a list of random numbers in Excel.
Diets and Intervention
After being randomly assigned to one of two groups, the control group was allocated to receive a diet regimen with a standard protein content (standard protein diet (SPD); providing 0.8 g/kg body weight/day), and the intervention group was allocated to receive a diet regimen with a higher protein content (higher protein diet (HPD); providing 1.34 g/kg body weight/day). The diet regimens had an equal amount of calories in both groups and a caloric restriction of 500 kcal less than the resting metabolic rate based on the Harris-Benedict predictive formula [18]. The composition of the diet regimen was estimated in order to ensure adequate intakes of carbohydrates and fat recommended by the US Institute of Medicine according to age and gender [19]. After randomization (week 0), participants in both groups received specific diet instructions. Each month, sample menus were provided that contained 15 different meal options (i.e., a 5-day cycle of 3 meal options and 2 snacks per day).
For the intervention group (HPD), meal replacements (as shakes) and protein bars (as snacks) made with soy protein were provided to participants monthly, along with individualized menus, which allowed for a more accurate provision of the total amount of protein consumed daily. For the control group (SPD), also monthly menus were provided that included conventional foods like chicken, fish, yogurt, cheese, beans, fruits, and vegetables in season.
Both groups received instructions for diet and exercise (e.g., walking, biking, or jogging at least 30 min/day, 5 days per week). The overall study design is shown in figure 1.
Fig. 1.
Study design.
In the HPD group, participants consumed two protein-enriched shakes each day, along with one conventional meal and two snacks to achieve a daily protein intake of 1.34 g protein/kg body weight. Used as a meal replacement, powder for the protein-enriched shakes was provided to participants each month in the two Obesity and Metabolic Disease Research Clinics, and the shakes were prepared daily at the time of consumption with 240 ml of either skimmed milk or water. The nutritional contents of the protein-enriched shake with milk comprised 189 kcal, 18.3 g protein, 1 g fat, and 27 g carbohydrates per serving. For the shakes prepared with water, the nutritional contents of the protein-enriched shake comprised 110 kcal, 10.3 g protein, 1 g fat, and 15 g carbohydrates per serving. The diet regimens were adjusted in the amount of energy and nutrients according to the choice of consuming the protein-enriched shake with water or milk. The snack bars each provided 160 kcal, 12 g protein, 6 g fat, and 17 g carbohydrates. The shakes and bars provided vitamins and minerals in the range of approximately 10–25% of the identified recommended daily value for each micronutrient. In the control group, participants ate three meals and two snacks per day following the recommended menu to achieve a daily intake of 0.8 g protein/kg body weight. For both groups, the caloric density of the diet was adjusted for the baseline metabolic rate of each participant with a reduction of 500 kcal/day from their calculated daily caloric needs.
Measurements
The primary outcome measure was change in weight, which was measured at screening and monthly thereafter. Secondary outcome measures included changes in parameters associated with MeS (i.e., waist circumference, HDL cholesterol, triglycerides, and fasting plasma glucose). Previously trained on standard operating procedures for data collection, nutritionists compiled monthly documentation of food frequency questionnaires (FFQ) for assessing participants' total caloric intake and grams of protein consumed per day. Each month the nutritionist provided the participants with a FFQ and did a 24-hour dietary recall interview. Upon completion of the questionnaires, the nutritionist asked the participant if they had forgotten some food. If there was any omission, then it was corrected in the record. The nutritionists also conducted anthropometry measurements (body weight and waist circumference) at baseline and at 3 and 6 months using internationally recognized techniques [20]. The weight and body composition was measured using an Avis 333 Body Composition Analyzer (Tradekorea, Seoul, South Korea) under the following conditions: people should not eat or drink for at least 30 min, exercise for at least 12 h and drink alcohol for at least 48 h, and they should urinate 30 min before the test. The waist circumference was measured with a tape measure using the line between the lower costal border and the iliac crest as reference points. At baseline, height was measured with a wall-mounted stadiometer (SECA Model 222; Seca, Birmingham, UK) with an accuracy of 0.1 cm. BMI was calculated as weight (kg)/height (m2).
At baseline and monthly, nurses measured participants' blood pressure with a digital sphygmomanometer (OMRON HEM-907; OMRON Healthcare Inc., Bannockburn, IL, USA) after 10 min of rest, following the recommendations of the American Heart Association [21]. At screening and at 3 and 6 months, blood samples were drawn after a 9- to 12-hour fasting period and processed as soon as it was taken from the participant. HOMA index was calculated according to the formula: fasting insulin (µU/ml) × fasting glucose (mmol/l)/22.5. Laboratory testing included results for parameters of MeS (HDL cholesterol, triglycerides, and fasting plasma glucose), as well as insulin, glycated hemoglobin (HbA1c), C-reactive protein, direct bilirubin, indirect bilirubin, alanine aminotransferase; aspartate aminotransferase, gamma-glutamyl transferase, blood urea nitrogen, and creatinine. All analytical measurements were performed at the Nutrition Laboratory of the MNIPH.
To assess adherence to treatment, we used a questionnaire, which has been previously validated in Mexican adults with overweight or obesity [22]. Eight questions explored four recommendations: compliance with caloric prescription, compliance with macronutrient prescription (particularly protein intake), compliance to physical activity, and compliance for actions to avoid sedentarism. A 5-level Likert scale was utilized (ranging from no compliance = 0% over 25%, 50% and 75% to total compliance = 100). A participant was considered adherent to the recommendations when at least a 75% level of compliance was noted on each of the eight questions at the 6-month follow-up.
Statistical Analysis
The results are shown for the 6-month comparison to baseline measurements as means ± standard deviation for all parameters adjusted for age and sex. Only to describe the difference in weight loss between the groups, we report the difference in means and its confidence interval adjusting for baseline BMI, age, and sex. We used the analysis of variance (ANOVA) for means and chi-square test for percentage of weight loss in the comparison between the two groups over time. We analyzed the data of all participants who completed the study after 6 months. Additionally, the analysis of mean weight loss between baseline and 6 months was adjusted for adherence to study recommendations and baseline BMI. A subgroup analysis was performed only for the participants who adhered to a minimum of 75% of the study design recommendations. Statistical calculations were performed using the STATA Program Version 13 TX (StataCorp LLC, College Station, TX, USA).
Results
Before the trial, we estimated the usual protein intake in participants. Baseline protein intake was not different among participants randomly assigned to the control and intervention groups (SPD 0.9 vs. HPD 0.94 g kg/body weight; p = 0.67). In the group assigned to a protein intake of 0.8 g kg/body weight (SDP group), the protein intake was reduced by an average of 12.5%. In the group assigned to a protein intake of 1.3 g kg/body weight (HPD group), protein intake increased on average b< 38.3% when compared with the previously estimated protein intake.
Of 118 participants who were randomized, 105 (88.9%) completed the 6-month study. In the first 3 months, 3 participants were lost in the HPD group and 4 in the SPD group with cited reasons of change of residence or incompatibility between the hours of their work and follow-up assessments. Between the 4th and 6th month of the study, 2 participants dropped out in the HPD group and 4 in the SPD group. The causes of drop-out were not associated to the treatment (moving to other address = 6, new work changes in their working conditions = 7). There was no significant difference (p > 0.05) in body weight of those who dropped out the study (89.1 ± 19.1 kg) and those who completed (88.3 ± 20.2 kg) the study. The participants consuming either diet reported no side effects. The liver and renal functions measured at baseline and after 6 months were determined to be within normal ranges for both groups.
There were no statistically significant differences (p > 0.05) between groups for baseline characteristics of age, weight, height, waist circumference, systolic blood pressure, diastolic blood pressure, fasting blood glucose, insulin, triglycerides, cholesterol, HDL cholesterol (table 1).
Table 1.
Baseline characteristics of study participants who entered into the 6-month study by group and gendera
| HPD |
SPD |
|||||
|---|---|---|---|---|---|---|
| all (n = 59) | males (n = 26) | females (n = 33) | all (= 59) | males (n = 18) | females (n = 41) | |
| Age, years | 47.4 ± 11.5 | 44.8 ± 10.0 | 48.4 ± 12.1 | 41.1 ± 10.6 | 42.0 ± 9.8 | 40.4 ± 11.3 |
| Height, cm | 161.0 ± 8.6 | 169.0 ± 8.5 | 157.8 ± 6.3 | 164.0 ± 8.4 | 169.2 ± 8.5 | 159.9 ± 5.8 |
| Weight, kg | 85.4 ± 22.4 | 93.9 ± 25.1 | 82.0 ± 20.6 | 92.4 ± 19.2 | 99.2 ± 21.0 | 87.1 ± 16.1 |
| BMI, kg/m2 | 31.5 ± 4.7 | 31.5 ± 5.0 | 31.5 ± 4.6 | 33.3 ± 5.0 | 33.9 ± 5.9 | 32.8 ± 4.3 |
| Waist circumference, cm | 102.9 ± 14.5 | 107.8 ± 14.7 | 101.0 ± 14.1 | 107.6 ± 14.4 | 113.0 ± 16.0 | 103.7 ± 11.8 |
| Systolic blood pressure, mm Hg | 128.3 ± 16.8 | 130.0 ± 14.3 | 127.3 ± 18.7 | 129.6 ± 17.0 | 131.8 ± 10.6 | 128.7 ± 18.9 |
| Diastolic blood pressure, mm Hg | 82.1 ± 9.5 | 82.3 ± 8.9 | 81.8 ± 10.0 | 78.1 ± 8.6 | 82.6 ± 7.0 | 76.3 ± 8.6 |
| Fasting blood glucose, mg/dl | 82.4 ± 14.6 | 83.2 ± 10.9 | 81.8 ± 17.1 | 86.2 ± 17.1 | 92.6 ± 21.2 | 83.7 ± 14.7 |
| Insulin, μU/ml | 13.8 ± 6.7 | 14.6 ± 6.7 | 13.2 ± 6.7 | 14.5 ± 7.1 | 15.7 ± 7.7 | 14.1 ± 6.9 |
| Triglycerides, mg/dl | 186.2 ± 101.3 | 205 ± 118.4 | 172.4 ± 86.2 | 201.7 ± 88.3 | 233.5 ± 106.5 | 189.9 ± 78.6 |
| Total cholesterol, mg/dl | 174.4 ± 43.3 | 173.9 ± 45.3 | 174.9 ± 42.5 | 175.9 ± 40.9 | 177 ± 40.2 | 175.5 ± 41.6 |
| HDL cholesterol, mg/dl | 35.8 ± 9 | 33.7 ± 7.2 | 37.3 ± 10.0 | 36.5 ± 9.0 | 34.5 ± 9.2 | 37.2 ± 9.0 |
For all baseline characteristics there were no statistically significant between-group differences (Kruskal-Wallis test).
After 6 months of intervention, the percentage of weight loss was higher in participants consuming the HPD than in those consuming the SPD (7.0% vs. 5.1%; p < 0.05) as shown in table 2. In both groups, waist circumference was reduced over time; however, there were no statistically significant differences between groups (p > 0.05). There were also no statistically significant differences in the percentages of abdominal fat and systolic blood pressure reduction between the two groups but participants in the HPD experienced significant reduction after 6 months of follow-up. The means of weight loss, adjusted for baseline BMI, age and sex, were not statistically significant between groups (p = 0.42).
Table 2.
Absolute changes in weight, BMI, percentage of weight loss, waist circumference, percentage of body fat and percentage of abdominal fat in the completers of both diet groups from baselinea
| Baseline mean ± SD | 6 months mean ± SD | Absolute change |
Value between groups |
|||
|---|---|---|---|---|---|---|
| mean ± SD | p value | mean ± SD | p value | |||
| Weight, kg | ||||||
| HPD | 90.5 ± 2.6 | 83.5 ± 2.6 | –7.0 ± 3.7 | 0.046* | –2.0 ± 5.1 | 0.601 |
| SPD | 87.1 ± 2.6 | 82.1 ± 2.6 | –5.1 ± 3.6 | 0.157 | ||
| BMI, kg/m2 | ||||||
| HPD | 33.2 ± 0.7 | 31.8 ± 0.7 | –1.4 ± 1.0 | 0.175 | –0.6 ± 1.4 | 0.67 |
| SPD | 31.7 ± 0.7 | 30.8 ± 0.6 | –0.6 ± 0.9 | 0.417 | ||
| Percentage of weight loss, % | ||||||
| HPD | – | 7.6 ± 0.7 | – | – | 2.0 ± 0.9 | 0.03** |
| SPD | – | 5.5 ± 0.6 | – | – | ||
| Waist circumference, cm | ||||||
| HPD | 107.3 ± 1.9 | 98.5 ± 1.9 | –8.8 ± 2.6 | 0.001* | –2.2 ± 3.7 | 0.54 |
| SPD | 103.4 ± 1.8 | 96.8 ± 1.8 | –6.5 ± 2.6 | 0.011* | ||
| Percentage of body fat, % | ||||||
| HPD | 39.3 ± 1.4 | 36.9 ± 1.4 | –2.4 ± 1.9 | 0.212 | 0.1 ± 2.6 | 0.955 |
| SPD | 38.3 ± 1.2 | 35.8 ± 1.3 | –2.6 ± 1.7 | 0.136 | ||
| Percentage of abdominal fat, F%b | ||||||
| HPD | 59.0 ± 4.5 | 45.3 ± 4.7 | –13.7 ± 6.4 | 0.034* | –4.1 ± 8.6 | 0.634 |
| SPD | 48.8 ± 4.0 | 39.2 ± 4.2 | –9.6 ± 5.7 | 0.092 | ||
| Percentage of fat free mass, F%b | ||||||
| HPD | 55.4 ± 1.5 | 53.9 ± 1.6 | –1.47 ± 2.2 | 0.532 | –0.23 ± 2.9 | 0.938 |
| SPD | 52.4 ± 1.4 | 51.2 ± 1.4 | –1.24 ± 2.0 | 0.510 | ||
| Systolic blood pressure, mm Hg | ||||||
| HPD | 129.6 ± 2.0 | 122.5 ± 2.0 | –7.1 ± 2.9 | 0.014* | 1.46 ± 3.99 | 0.715 |
| SPD | 128.6 ± 2.0 | 120.0 ± 2.0 | –8.6 ± 2.8 | 0.002* | ||
| Diastolic blood pressure, mm Hg | ||||||
| HPD | 82.1 ± 1.24 | 78.8 ± 1.24 | –3.3 ± 1.7 | 0.059 | –0.38 ± 2.4 | 0.877 |
| SPD | 78.2 ± 1.21 | 75.3 ± 1.21 | –2.9 ± 1.7 | 0.086 | ||
| Baseline mean (IC 95%) | 6 months mean (IC 95%) | Mean difference | Value between groups | |||
| mean (IC 95%) | p value | mean (IC 95%) | p value | |||
| Weight, kg* | ||||||
| HPD | 90.5 (88.1, 93.5) | 83.7 (81.3, 86.8) | −6.8 (−10.6, −2.9) | 0.001 | −2.1 (−7.5, 3.2) | 0.427 |
| SPD | 87.1 (84.4, 89.7) | 82.5 (79.8, 85.o) | −4.6 (−8.3, −0.93) | 0.014 | ||
All results were adjusted for age and sex. Standard-protein diet (n = 51) and high-protein diet (n = 54).
F% is based on the percentage of total fat.
Mean difference in weight was adjusted by baseline BMI, age and sex.
As presented in table 3, each group demonstrated a significant decrease (p < 0.05) in fasting blood glucose, insulin, HOMA index, triglyceride, total cholesterol and VLDL cholesterol levels; however, in the comparison between groups, there were no statistically significant differences for any of these parameters (p > 0.05).
Table 3.
Absolute and relative changes in biochemical indicators in the completers of both groups from baseline
| Baseline | 6 months | Absolute change |
Value between groups |
|||
|---|---|---|---|---|---|---|
| mean ± SD | p value | mean ± SD | p value | |||
| C-reactive protein, mg/l | ||||||
| HPD | 2.9 ± 0.3 | 2.8 ± 0.3 | –0.1 ± 0.5 | 0.852 | 0.6 ± 0.7 | 0.345 |
| SPD | 3.1 ± 0.3 | 2.4 ± 0.3 | –0.7 ± 0.5 | 0.125 | ||
| Fasting blood glucose, mg/dl | ||||||
| HPD | 82.1 ± 2.7 | 69.1 ± 2.7 | –13.0 ± 3.8 | 0.001 | 6.7 ± 5.2 | 0.205 |
| SPD | 86 ± 2.6 | 66.4 ± 2.6 | –19.6 ± 3.6 | 0.001 | ||
| Insulin, μU/ml* | ||||||
| HPD | 13.6 ± 0.8 | 9.4 ± 0.8 | –4.3 ± 1.2 | 0.001 | 0.5 ± 1.6 | 0.753 |
| SPD | 14.6 ± 0.8 | 9.8 ± 0.8 | –4.8 ± 1.1 | 0.001 | ||
| HOMA Index | ||||||
| HPD | 2.7 ± 0.2 | 1.6 ± 0.2 | –1.0 ± 0.2 | 0.001 | 0.5 ± 0.3 | 0.189 |
| SPD | 3.1 ± 0.1 | 1.5 ± 0.1 | –1.5 ± 0.2 | 0.001 | ||
| HbA1c, % | ||||||
| HPD | 6.2 ± 0.2 | 6.2 ± 0.2 | 0.0 ± 0.2 | 0.949 | –0.2 ± 0.3 | 0.472 |
| SPD | 6.4 ± 0.2 | 6.6 ± 0.2 | 0.2 ± 0.2 | 0.339 | ||
| Triglycerides, mg/dl | ||||||
| HPD | 182.5 ± 12.5 | 119.0 ± 12.9 | –63.5 ± 17.6 | 0.001 | 5.3 ± 24.5 | 0.220 |
| SPD | 204.7 ± 11.9 | 135.8 ± 12.4 | –68.8 ± 17.0 | 0.001 | ||
| Total cholesterol, mg/dl | ||||||
| HPD | 173.4 ± 6.3 | 148.3 ± 6.5 | –25.1 ± 8.9 | 0.005 | 11.9 ± 12.6 | 0.950 |
| SPD | 176.6 ± 6.2 | 139.6 ± 6.5 | –37.0 ± 8.9 | 0.001 | ||
| HDL cholesterol, mg/dl | ||||||
| HPD | 36.0 ± 1.5 | 34.6 ± 1.5 | –1.4 ± 2.1 | 0.517 | 5.7 ± 3.0 | 0.057 |
| SPD | 36.3 ± 1.5 | 29.2 ± 1.5 | –7.1 ± 2.1 | 0.001 | ||
| VLDL cholesterol, mg/dl | ||||||
| HPD | 44.6 ± 4.1 | 28.6 ± 4.1 | –16.0 ± 5.7 | 0.006 | 3.4 ± 8.0 | 0.666 |
| SPD | 42.8 ± 3.9 | 23.3 ± 4.0 | –19.5 ± 5.5 | 0.001 | ||
| Direct bilirubin, mg/dl | ||||||
| HPD | 0.2 ± 0.1 | 0.2 ± 0.1 | 0 ± 0 | 0.857 | 0.048 ± 0.03 | 0.088 |
| SPD | 0.3 ± 0.1 | 0.2 ± 0.1 | 0 ± 0 | 0.025 | ||
| Indirect bilirubin, mg/dl | ||||||
| HPD | 0.5 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.088 | 0.14 ± 0.1 | 0.073 |
| SPD | 0.7 ± 0.1 | 0.6 ± 0.1 | 0 ± 0.1 | 0.410 | ||
| AST, UI/l | ||||||
| HPD | 27.5 ± 1.5 | 23.6 ± 1.6 | –3.9 ± 2.2 | 0.080 | –1.05 ± 3.0 | 0.729 |
| SPD | 23.4 ± 1.5 | 20.6 ± 1.6 | –2.8 ± 2.1 | 0.185 | ||
| ALT, UI/l | ||||||
| HPD | 21.1 ± 1.2 | 17.3 ± 1.3 | –3.8 ± 1.8 | 0.032 | –1.9 ± 2.4 | 0.433 |
| SPD | 19.5 1.2 | 17.6 ± 1.2 | –1.9 ± 1.7 | 0.267 | ||
| GGT, Ul/l | ||||||
| HPD | 38.6 ± 3.1 | 30.9 ± 3.3 | –7.7 ± 4.5 | 0.087 | –3.4 ± 6.24 | 0.583 |
| SPD | 28.0 ± 3.0 | 23.7 ± 3.2 | –4.3 ± 4.3 | 0.321 | ||
| BUN, mg/dl | ||||||
| HPD | 14.6 ± 0.5 | 15.6 ± 0.6 | 0.9 ± 0.8 | 0.212 | 0.83 ± 1.04 | 0.422 |
| SPD | 14.1 ± 0.5 | 14.2 ± 0.5 | 0.1 ± 0.7 | 0.887 | ||
| Creatinine, mg/dl | ||||||
| HPD | 0.8 ± 0.1 | 0.8 ± 0.1 | 0 ± 0 | 0.601 | 0.03 ± 0.038 | 0.433 |
| SPD | 0.8 ± 0.1 | 0.8 ± 0.1 | 0 ± 0 | 0.557 | ||
HbA1c = Glycated hemoglobin;; cALT = alanine aminotransferase; AST = aspartate aminotransferase; GGT = gamma-glutamyl transferase; BUN = blood urea nitrogen.
All results were adjusted for age and sex.
Statistically significant from baseline (p<0.05).
**Statistically significant between groups (p<0.05).
Standard-protein diet (n = 51) and high-protein diet (n = 54).
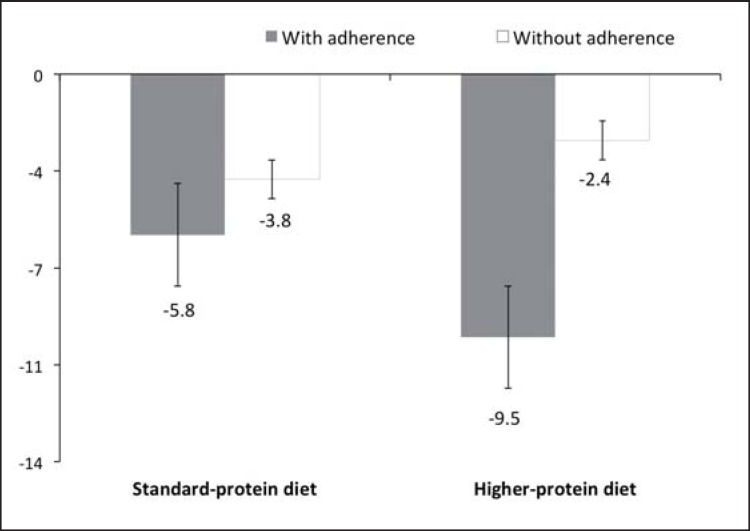
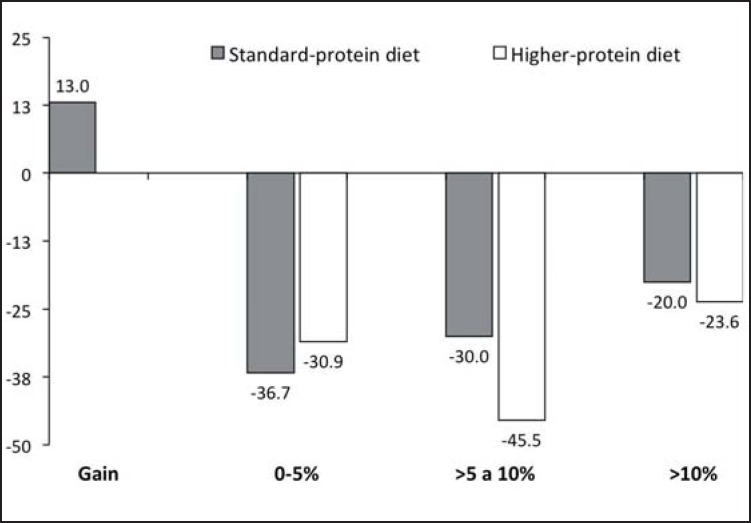
For the participants who adhered to a minimum of 75% of the study design recommendations, mean weight loss between baseline and 6 months in the HPD group was −3.7 kg (fig. 2). As a percentage of their baseline weight, 15.5% more subjects in the HPD group than in the SDP group had a percentage of weight loss between 5 to 10% (fig. 3). The number of participants who were judged to be adherent was 92.5% in the HPD group (n = 50) and 88.2% in the SDP group (n = 45).
Fig. 2.
Percentage of weight loss average between baseline and 6 months, by adherence. With adherence = fully followed all the recommendations at least 75% of the time. Without adherence = followed the recommendations less than 75% of the time. Means adjusted by baseline BMI. There was a significant interaction (p < 0.001) noted between mean weight loss and adherence.
Fig. 3.
Percentage of participants in the SPD and the HPD groups, classified by percentage of weight loss between baseline and 6 months.
Discussion
Mexico has one of the highest incidences of overweight and obesity in the world, a statistic which has become more serious in recent years and, according to the results of the Health and Nutrition National Survey 2012, comprises a combined percentage of overweight and obese adult men and women of more than 70% of the total Mexican population [23]. This prevalence of overweight and obesity is associated with an increased risk of noncommunicable diseases, such as diabetes, cardiovascular disease, and various types of cancer [24], and poses an estimated economic burden on the healthcare system in an amount of 2.5% of the Mexico's gross domestic product [25]. Safe and effective measures to calm the obesity epidemic in Mexico are much needed. Meal replacements can improve compliance to a diet by offering a simple, convenient, and healthy alternative meal option. Meal replacements may also help to instill regular eating patterns and make meal planning easier by increasing the accuracy of calorie and portion size estimation, thus supporting dietary self-monitoring. Future studies of 1- or 2-year duration are needed to determine the long-terms effect of using protein-enriched meal replacements as part of a HPD. At present, studies have only been performed with a follow-up of 3–6 months, demonstrating that higher protein intake improves adherence to the treatment and results in less weight regain [26].
The meal replacement formula used in this study has been supplemented with methionine and has a protein quality of 0.8 using the PDCAAS method (Dupont Nutrition and Health, Personal Communication). In some studies it has also shown that soy protein helps to lose weight without losing muscle mass [27, 28]. However, it has been described that vegetable protein has less potential than animal protein to preserve muscle mass [29].
To our knowledge, this is the first study conducted in Mexico that investigated the impact of partial diet replacement with protein-enriched meal in individuals with MeS, although similar studies in overweight and obese adults had been conducted in other countries. A single-blind randomized, prospective, controlled trial conducted at the University of California, Los Angeles, CA, enrolled 100 overweight and obese participants in order to examine the effects of two different levels of dietary protein on weight loss over a period of 12 weeks [30]. At the completion of the study (85 completers), both groups lost weight, and overall weight loss was the same in both groups, as would be expected when comparing isocaloric diets; however, the participants consuming more dietary protein/day (HPD group) lost more fat than the people consuming standard amounts of protein, and also maintained the same amount of lean body mass. As mentioned, in a study conducted in Germany at the University Obesity Center of the Department of Medicine at the University of Ulm, Flechtner-Mors et al. [16] enrolled 110 people with MeS and randomly assigned them to either a protein-rich group, consuming 30% of total daily calories from protein (providing approximately 1.34 g protein/kg body weight/day), or a conventional diet group, consuming a standard amount of protein which corresponded to approximately 15% of total daily calories from protein (providing approximately 0.8 g/kg body weight/day). At 3 and at 12 months, the participants in the protein-rich group lost significantly more body weight and body fat than those in the standard protein group. This study demonstrated that more weight and fat loss over 1 year can be achieved in participants with MeS when using protein-enriched meal replacements within a controlled diet instead of standard protein intake [16]. An increased intake of protein has been reported to have a greater satiety effect than intake of carbohydrates and so can help control hunger between meals [13]. Increased protein intake also helps to maintain lean body mass when combined with physical activity [28]. The recently completed DIOGENES study in Europe demonstrated that a high protein and low glycemic index diet maintained weight losses for 6 months after an 8-week weight loss regimen, while other combinations of high or low protein and low glycemic index diets led to weight regain [31].
The present study was conducted to determine the effect of consuming a HPD, facilitated by the use of protein-rich meal replacements, in adults with MeS in Mexico. The levels of dietary protein designed to be consumed by the two groups were equivalent to those provided by Flechtner-Mors et al. [16], although the participants in our study additionally were given advice on physical exercise in an effort to aid in weight loss and physical activity was monitored throughout the study. Unlike the data of Flechtner-Mors et al. [16], in the present study both diets resulted in a comparable amount of weight loss at 3 and 6 months. Although we did not found any significant differences in absolute weight loss between groups, there were differences in the means of weight loss adjusted for baseline BMI; it also should be noted that, when participants with an adherence rate to study protocol of >75% were analyzed, adherent subjects in the HPD group lost significantly more weight than adherent subjects in the SPD group (i.e., average of −9.5 vs. −5.8% weight loss after adjusting for baseline BMI). Moreover, more subjects in the HPD group lost >5% of their baseline body weight. This is of clinical significance as in terms of overall health status it is well recognized that losses of >5% body weight are associated with clinical benefits with regard to metabolic diseases such as diabetes, hypertension, and cardiovascular disorders.
Although this study had the limitation that we did not measure the clinical biomarker traditionally used to assess the intake of protein (urine nitrogen), the 24-hour dietary recall applied repeatedly can be used as an approximate measurement of the protein intake in the diet [32]. However, we recognize that the lack of a biomarker does not allow us to fully attribute the difference in weight to different protein intake. A limitation of the current study is that, although we used a validated questionnaire to measure adherence to practice physical activity, this instrument did not directly measure energy expenditure. In our view, the questionnaire identified those who followed the recommendations and justified the analysis conducted.
In 2010, the European Food Safety Authority (EFSA) issued an opinion on the substantiation of health claims related to meal replacements for weight control and reduction in body weight in which they concluded that ‘a cause and effect relationship has been established between the consumption of meal replacements in substitution of regular meals' and weight loss and weight management [33]. EFSA's opinion was based on two meta-analyses of numerous controlled intervention studies in overweight and obese participants that utilized commercial meal replacement products containing up to 250 kcal/serving for periods of 3–51 months. The agency recognized that greater weight loss was achieved in the meal replacement groups when compared to groups consuming conventional diets, and compliance was better for participants utilizing the meal replacements. In our study, it is not possible to conclude that the differences in percentage of weight loss between the groups were due to the use of meal replacements or to the protein content of the diet because the control group did not receive meal replacements. The control group was only instructed to follow a diet providing the same number of calories as prescribed to the intervention group, but with less protein content. One possible explanation for the effect described by the EFSA may be that the use of meal replacements improved compliance to the HPD as they simplified the diet [34]. In addition, there are some mechanisms that could support an additional role of a higher protein intake on weight loss such as increased satiety which could have lowered calorie intake as shown in previous studies [35].
The use of 1–2 meal replacements/day in the intervention group of the US National Institutes of Health LOOK Ahead study of weight management in overweight and obese individuals with type 2 diabetes mellitus was reported to be one of the factors of success in the intervention program and a strong correlate of weight loss at 1 year, along with physical activity and attendance at treatment sessions in which participants would present ongoing records of food intake [36]. The more meal replacements were consistently consumed by participants, the greater the weight loss that was observed both at 26 weeks and at 1 year.
At the beginning of the study we measured in participants in both groups the total protein intake/kg body weight (SPD 0.9 vs. HPD 0.94 g kg/body weight), and there were no statistically significant differences (p = 0.67). This shows that the SPD group did not reduce its protein intake during the intervention, and then had some effect by this change.
Conclusions
The current study utilized a hypocaloric diet with different protein content, and there were no significant differences in weight loss and biomarkers when the overall group was examined. When comparing the measurements between baseline and the 6th month of follow-up, both diets produced weight loss and improvement in biomarkers of MeS. The participants with an adherence rate to study protocol of >75% in the HPD group lost significantly more weight than adherent subjects in the SPD group. Meal replacements could have improved adherence to the diet by offering a simple and healthy alternative meal option. Thus, it is not possible to conclude that the differences in percentage of weight loss between the groups were due to the protein content of the diet or to the use of meal replacements, because the control group did not receive a meal replacement. Since MeS can lead to type 2 diabetes mellitus and other age-related chronic diseases with significant public health impacts, the use of a hypocaloric diet facilitated by the use of protein-rich meal replacements together with a healthy, active lifestyle might provide an important tool in public health strategies to combat obesity and obesity-associated diseases in Mexico.
Trial Registration
Authors’ Contributions
ICN and SB designed the research, supervised the study at the study site, and wrote the paper; LHB analyzed data. ICN, SB and LHB interpreted the data. Each author read and approved the contents of the submitted manuscript.
Disclosure Statement
During the development of the study ICN received a partial scholarship from the National Council of Science and Technology (CONACyT). ICN, LHB, and SB, no competing interests.
Acknowledgments
ICN would like to thank to the National Council of Science and Technology (CONACyT) for a partial scholarship during the development of the study.
References
- 1.Federation ID. The IDF consensus; in IDF Communications. Brussels: IDF Task Force on Epidemiology and Prevention; 2006. Metabolic syndrome. [Google Scholar]
- 2.Rojas-Martinez R, Aguilar-Salinas CA, Jimenez-Corona A, Villalpando S, Lazcano-Ponce E. Metabolic syndrome in Mexican adults. Results from the National Health and Nutrition Survey 2006. Salud Publica Mex. 2010;52(suppl 1):S11–S18. doi: 10.1590/s0036-36342010000700004. [DOI] [PubMed] [Google Scholar]
- 3.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 4.Rojas-Martinez R, Aguilar-Salinas CA, Jimenez-Corona A, Gomez-Perez FJ, Barquera S, Lazcano-Ponce E. Prevalence of obesity and metabolic syndrome components in Mexican adults without type 2 diabetes or hypertension. Salud Publica Mex. 2012;54:7–12. [PubMed] [Google Scholar]
- 5.Phelan S, Wadden TA, Berkowitz RI, Sarwer DB, Womble LG, Cato RK, Rothman R. Impact of weight loss on the metabolic syndrome. Int J Obes (Lond) 2007;31:1442–1448. doi: 10.1038/sj.ijo.0803606. [DOI] [PubMed] [Google Scholar]
- 6.Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, McManus K, Champagne CM, Bishop LM, Laranjo N, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360:859–873. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kruger J. Attempting to lose weight. Specific practices among U.S. adults. Am J Prev Med. 2014;26:402–406. doi: 10.1016/j.amepre.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Due A, Toubro S, Skov AR, Astrup A. Effect of normal-fat diets, either medium or high in protein, on body weight in overweight subjects: a randomised 1-year trial. Int J Obes Relat Metab Disord. 2004;28:1283–1290. doi: 10.1038/sj.ijo.0802767. [DOI] [PubMed] [Google Scholar]
- 9.Skov AR, Toubro S, Ronn B, Holm L, Astrup A. Randomized trial on protein vs carbohydrate in ad libitum fat reduced diet for the treatment of obesity. Int J Obes Relat Metab Disord. 1999;23:528–536. doi: 10.1038/sj.ijo.0800867. [DOI] [PubMed] [Google Scholar]
- 10.Paoli A, Rubini A, Volek JS, Grimaldi KA. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr. 2014;68:641. doi: 10.1038/ejcn.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weigle DS, Breen PA, Matthys CC, Callahan HS, Meeuws KE, Burden VR, Purnell JQ. A high-protein diet induces sustained reductions in appetite, ad libitum caloric intake, and body weight despite compensatory changes in diurnal plasma leptin and ghrelin concentrations. Am J Clin Nutr. 2005;82:41–48. doi: 10.1093/ajcn.82.1.41. [DOI] [PubMed] [Google Scholar]
- 12.Westerterp-Plantenga MS, Nieuwenhuizen A, Tomé D, Soenen S, Westerterp KR. Dietary protein, weight loss, and weight maintenance. Annu Rev Nutr. 2009;29:21–41. doi: 10.1146/annurev-nutr-080508-141056. [DOI] [PubMed] [Google Scholar]
- 13.Halton TL, Hu FB. The effects of high protein diets on thermogenesis, satiety and weight loss: a critical review. J Am Coll Nutr. 2004;23:373–385. doi: 10.1080/07315724.2004.10719381. [DOI] [PubMed] [Google Scholar]
- 14.Astrup A, Meinert T, Harper A. Atkins and other low-carbohydrate diets: hoax or an effective tool for weight loss? Lancet. 2004;364:897–899. doi: 10.1016/S0140-6736(04)16986-9. [DOI] [PubMed] [Google Scholar]
- 15.Ditschuneit HH, Flechtner-Mors M, Johnson TD, Adler G. Metabolic and weight-loss effects of a long-term dietary intervention in obese patients. Am J Clin Nutr. 1999;69:198–204. doi: 10.1093/ajcn/69.2.198. [DOI] [PubMed] [Google Scholar]
- 16.Flechtner-Mors M, Boehm BO, Wittmann R, Thoma U, Ditschuneit HH. Enhanced weight loss with protein-enriched meal replacements in subjects with the metabolic syndrome. Diabetes Metab Res Rev. 2010;26:393–405. doi: 10.1002/dmrr.1097. [DOI] [PubMed] [Google Scholar]
- 17.Grundy SM. Metabolic syndrome scientific statement by the American Heart Association and the National Heart, Lung, and Blood Institute. Arterioscler Thromb Vasc Biol. 2005;25:2243–2244. doi: 10.1161/01.ATV.0000189155.75833.c7. [DOI] [PubMed] [Google Scholar]
- 18.Cunningham JJ. Body composition as a determinant of energy expenditure: a synthetic review and a proposed general prediction equation. Am J Clin Nutr. 1991;54:963–969. doi: 10.1093/ajcn/54.6.963. [DOI] [PubMed] [Google Scholar]
- 19.Institute of Medicine . Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington: Institute of Medicine; 2005. [DOI] [PubMed] [Google Scholar]
- 20.Habicht J. Standardization of quantitative epidemiological methods in the field. Bol Oficina Sanit Panam. 1974;76:375–385. [PubMed] [Google Scholar]
- 21.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111:697–716. doi: 10.1161/01.CIR.0000154900.76284.F6. [DOI] [PubMed] [Google Scholar]
- 22.Barriguete A, Barquera S, Villalobos J, Campos I. Adherencia en enfermedades crónicas. In: BSaC, editor. Dislipidemias: epidemiología, evaluación, adherencia y tratamiento. 1st ed. Volume 1. Cuernavaca: Instituto Nacional de Salud Pública; 2010. pp. 120–135. [Google Scholar]
- 23.Barquera S, Campos-Nonato I, Hernández-Barrera L, Pedroza-Tobías A, Rivera-Dommarco J. Prevalence of obesity in Mexican adults, ENSANUT 2012. Salud Publica Mex. 2013;55((suppl 1)):S151–S160. [PubMed] [Google Scholar]
- 24.Kopelman P. Health risks associated with overweight and obesity. Obes Rev. 2007;8(suppl 1):13–17. doi: 10.1111/j.1467-789X.2007.00311.x. [DOI] [PubMed] [Google Scholar]
- 25.Rivera-Dommarco J, Campos-Nonato I, Barquera S, Gonzalez T. Epidemiología de la obesidad en méxico: magnitud, distribución, tendencias y factores de riesgo. In: Rivera-Dommarco J, Hernández-Ávila M, Aguilar-Salinas C, editors. Obesidad en México. Volume 1. Mexico City: UNAM; 2012. pp. 79–98. [Google Scholar]
- 26.Westerterp-Plantenga MS, Lejeune MP, Nijs I, van Ooijen M, Kovacs EM. High protein intake sustains weight maintenance after body weight loss in humans. Int J Obes Relat Metab Disord. 2004;28:57–64. doi: 10.1038/sj.ijo.0802461. [DOI] [PubMed] [Google Scholar]
- 27.Deibert P, Konig D, Schmidt-Trucksaess A, Zaenker KS, Frey I, Landmann U, Berg A. Weight loss without losing muscle mass in pre-obese and obese subjects induced by a high-soy-protein diet. Int J Obes Relat Metab Disord. 2004;28:1349–1352. doi: 10.1038/sj.ijo.0802765. [DOI] [PubMed] [Google Scholar]
- 28.Leidy HJ, Carnell NS, Mattes RD, Campbell WW. Higher protein intake preserves lean mass and satiety with weight loss in pre-obese and obese women. Obesity (Silver Spring) 2007;15:421–429. doi: 10.1038/oby.2007.531. [DOI] [PubMed] [Google Scholar]
- 29.van Vliet S, Burd NA, van Loon LJ. The skeletal muscle anabolic response to plant- versus animal-based protein consumption. J Nutr. 2015;145:1981–1991. doi: 10.3945/jn.114.204305. [DOI] [PubMed] [Google Scholar]
- 30.Treyzon L, Chen S, Hong K, Yan E, Carpenter CL, Thames G, Bowerman S, Wang HJ, Elashoff R, Li Z. A controlled trial of protein enrichment of meal replacements for weight reduction with retention of lean body mass. Nutr J. 2008;7:23. doi: 10.1186/1475-2891-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gogebakan O, Kohl A, Osterhoff MA, van Baak MA, Jebb SA, Papadaki A, Martinez JA, Handjieva-Darlenska T, Hlavaty P, Weickert MO, et al. Effects of weight loss and long-term weight maintenance with diets varying in protein and glycemic index on cardiovascular risk factors: the diet, obesity, and genes (DiOGenes) study: a randomized, controlled trial. Circulation. 2011;124:2829–2838. doi: 10.1161/CIRCULATIONAHA.111.033274. [DOI] [PubMed] [Google Scholar]
- 32.Bingham SA, Cummings JH. Urine nitrogen as an independent validatory measure of dietary intake: a study of nitrogen balance in individuals consuming their normal diet. Am J Clin Nutr. 1985;42:1276–1289. doi: 10.1093/ajcn/42.6.1276. [DOI] [PubMed] [Google Scholar]
- 33.EFSA Scientific opinion on the substantiation of health claims related to meal replacements for weight control (as defined in Directive 96/8/EC on energy restricted diets for weight loss) and reduction in body weight (ID 1417), and maintenance of body weight after weight loss (ID 1418) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2010;8:1466. [Google Scholar]
- 34.EFSA Statement on the conditions of use for health claims related to meal replacements for weight control. EFSA Panel on Dietetic Products, Nutrition and Allergies. EFSA J. 2015;13:1–12. [Google Scholar]
- 35.Leidy HJ, Clifton PM, Astrup A, Wycherley TP, Westerterp-Plantenga MS, Luscombe-Marsh ND, Woods SC, Mattes RD. The role of protein in weight loss and maintenance. Am J Clin Nutr. 2015;101:1320S–1329S. doi: 10.3945/ajcn.114.084038. [DOI] [PubMed] [Google Scholar]
- 36.Wadden TA, West DS, Neiberg RH, Wing RR, Ryan DH, Johnson KC, Foreyt JP, Hill JO, Trence DL, Vitolins MZ, Look ARG. One-year weight losses in the Look AHEAD study: factors associated with success. Obesity (Silver Spring) 2009;17:713–722. doi: 10.1038/oby.2008.637. [DOI] [PMC free article] [PubMed] [Google Scholar]