Abstract
Presenilin-1 (PS1) or presenilin-2 (PS2), nicastrin (NCT), anterior pharynx-defective 1 (Aph-1), and presenilin enhancer-2 (Pen-2) have been considered the minimal essential subunits required to form an active γ-secretase complex. Besides PS, which has been widely believed to function as the catalytic subunit of the complex, the functional roles of the other subunits in the γ-secretase complex remain debatable. In the current study, we set out to determine the role of Pen-2 in γ-secretase activity. To this end, using knockout cells in combination with siRNA and immunoprecipitation approaches, our results revealed that Pen-2 together with presenilin are sufficient to form a functionally active enzyme to process Notch. Specifically, our data demonstrated that Pen-2 plays a crucial role in substrate binding, a mechanism by which Pen-2 contributes directly to the catalytic mechanism of γ-secretase activity. Our data also suggested that there may be different requirements for components to process ABPP and Notch. These information would be important for therapeutic strategy aimed at inhibition or modulation of γ-secretase activity.
Keywords: Alzheimer's Disease, γ-secretase, Pen-2, nicastrin, Aph-1, presenilin, AβPP, Notch
Introduction
The Alzheimer's disease (AD)-associated γ-secretase is a complex composed of Presenilin-1 (PS1) or presenilin-2 (PS2), nicastrin (NCT), anterior pharynx-defective 1 (Aph-1), and presenilin enhancer-2 (Pen-2) [1]. PS1 and PS2 proteins share high homology and are believed to function as the catalytic subunit in γ-secretase [2]. NCT has been reported to function as a substrate receptor [3]. Aph-1 was shown to be essential for both assembly and maturation of the γ-secretase complex [4, 5]. Pen-2 was recognized as an important component for PS endoproteolysis to generate the N-terminal and C-terminal fragments of presenilin (PSN and PSC), which is a critical step in γ-secretase complex maturation [6, 7]. Knockout of Pen-2 results in embryonic lethality, a phenotype similar to that of a PS1/PS2 double knockout, further validating the essential nature of Pen-2 [8]. However, recent studies have shown that Pen-2 is not absolutely required for endoproteolysis of PS1 and the generation of PS1N and PS1C [9, 10]. These findings prompted us to determine the role of Pen-2 in γ-secretase activity beyond formation and stabilization of the presenilin endoproteolysis products. In the current study, we report that Pen-2 together with presenilin can form a functional enzyme to catalyze Notch processing. Specifically, Pen-2 is crucial for substrate binding to γ-secretase complex.
Experimental Procedures
Cell culture–Mouse embryonic fibroblast (MEF) knockout cells Aph-1-/- [11], NCT-/- [12] were provided by Dr. Tong Li from John Hopkins University. Pen-2-/- [8], PS1-/- [13], PS2-/- [14], PS1/2-/- [15] and wild type (WT) MEF cells were provided by Dr. Bart De Strooper from Center of Human Genetics (Belgium). Wt-7 cells, which express both human PS1 and Swedish mutantant AβPP, was provoded by Drs. Sangram S.Sisodia and Seong-Hun Kim from University of Chicago. All cells were cultured in Dulbecco's modified Eagle's medium (DMEM, Lonza, Walkersville, MA, USA) supplemented with 10% fetal bovine serum, 2 mM L-glutamine (Lonza), 100 units/mL penicillin (Lonza), and 100 μg/mL streptomycin (Lonza).
Inhibitors and reagents–γ-secretase inhibitors Compound E and L685 were purchased from EMD Millipore (Billerica, MA, USA). Complete protease inhibitor cocktail tablets were purchased from Roche AβPPlied Science (Indianapolis, IN, USA). Lipofectamine LTX and plus reagent and Lipofectamine RNAiMAX reagent were purchased from Invitrogen (Carlsbad, CA, USA).
Antibodies–Anti-PS1C antibodies (#5643 and #3622) and NICD-specific antibody (#4147) were purchased from Cell Signaling (Danvers, MA). Polycolonal antibody C15 was raised against the last 15 amino acids at the C terminal of AβPP [16]. Monoclonal antibody 6E10 and Polyclonal antibody anti-PEN-2N were from Covance (Emeryville, CA). Anti-myc antibody (9E10) was purchased from Santa Cruz (Dallas, TX, USA). Anti-GAPDH (glyceraldehyde 3-phosphate dehydrogenase) was from EMD Millipore (Billerica, MA). Polycolonal NCT antibody N1660 was purchased from Sigma-Aldrich (St.Louis, MO). Anti-PS1N was raised against a peptide corresponding to residues 27–50 of human PS1 [17].
Plasmids–Plasmid expressing the ectodomain truncated and myc-tagged Notch molecule (NotchΔE) containing the murine Notch-1 leader peptide (1-23 aa) [18] was kindly provided by Raphael Kopan (Washington University) and Dr. Masayasu Okochi (Osaka University, Japan). The plasmid AβPPsw, which expresses a C-terminal myc-tagged Swedish mutant AβPP (AβPPsw) [19], was kindly provided by Dr Gopal Thinakaran (University of Chicago). The plasmids which expressing the PS1N terminal (1-292aa), PS1C terminals: PS1C293 (293-467aa), PS1C296 (296-467aa), PS1C299 (299-467aa), PS1C334(334-467aa), PS1C346 (346-467aa) were constructed in our laboratory and confirmed by sequencing. PS1 mutants PS1D257A, PS1D385A, PS1D257,385A expressing plasmids were constructed as previously described [20].
siRNA treatment–NCT specific siRNAs and control siRNA were purchased from Qiagen (Valencia, CA, USA). Cells were treated with siRNA twice every two days using Lipofectamine RNAiMAX reagent following manufacturer's instructions. After four days treatment and confirmation of NCT-knockdown, cells were transfected with NotchΔE or lacZ for another 24 h followed by Western blotting analysis.
Immunoprecipitation (IP) and Western blot analysis–Twenty-four hours after transfection, cells were collected with 1% Chapso buffer (20mM Tris pH 8.0, 150mM NaCl, 5mM EDTA and cocktail). The collected samples were then immunoprecipitated with AβPPropriate antibodies and Protein A conjugated beads overnight at 4 °C followed by Western blot analysis as described previously [9]. Protein A sepharose was purchased from Pharmacia Biotech (Piscataway, NJ). Chapso was phurchased from Amresco (Solon, OH).
Results
Pen-2 is required for the processing of both AβPP and Notch
To determine the roles of each components of γ-secretase complex in the γ-secretase activity, knockout cells with deletion of one of these components were cultured in the presence or absence of γ-secretase inhibitor L685-458 (L685). After 24 hours of culture, cell lysates were probed with C15, which is specific to the C-terminal fragment of AβPP [16]. As shown in Fig. 1A, in the absence of inhibitor, the level of CTFα, which is produced by α-secretase from AβPP, dramatically reduced in Aph-1 knockout cells (Aph-1-/-) (lane 1), PS1-/- cells (lane 7), PS2-/- cells (lane 9), as well as in wild type cells (lanes 13). When these cells were cultured in the presence of inhibitor, significant amount of CTFα was accumulated (lanes 2, 8, 10, and 14). However, regardless of the presence or absence of inhibitor, a high level accumulation of CTFα was detected in nicastrin knockout (NCT-/-), Pen-2 knockout (Pen-2-/-), and PS1 and PS2 double knockout (PS1/2-/-) cells (lanes 3, 4, 5, 6, 11, and 12). These results indicate that NCT and Pen-2 are essential for γ-secretase catalyzed CTFα turnover, but Aph-1 is dispensable. As shown in the top panel of Fig. 1A, relatively uniform expression levels of full-length AβPP (fAβPP) were detected through all of the cell lines. It was noted that the level of fAβPP in NCT-/- cells is slightly lower than those in other cells, suggesting a relatively quick turnover of fAβPP in NCT-deficient cells either by random degradation or by α- and/or β-secretase, a question awaiting further investigation. Next, we examined the effect of knockout of these components on the processing of Notch. Cells were transfected with plasmid expressing NotchΔE, a ectodomain truncated form of Notch. As shown in Fig. 1B, in the absence of inhibitor, a significant amount of NICD, which is produced from NotchΔE by γ-secretase activity, was detected in Aph-1-/- cells (lane 1), NCT-/- cells (lane 3), PS1-/- cells (lane 7), PS2-/- cells (lane 9) as well as in wild type cells (lane 13). When these cells were cultured in the presence of inhibitor, the NICD was reduced with a concomitant increase in the level of unprocessed NotchΔE (lanes 2, 4, 8, 10, and 14). Interestingly, in the Pen-2-/- cells (lanes 5 and 6) as well as PS1/2-/- cells (lanes 11 and 12) no NICD was detected regardless of the presence or absence of inhibitor. These results suggest that, unlike Aph-1 and NCT, Pen-2 is crucial for γ-secretase catalyzed processing of Notch. It was noted that, in the presence of the inhibitor, the levels of unprocessed NotchΔE accumulated in Aph-1-/-, PS2-/-, and PS1-/- cells (lanes 2, 8, and 10) are lower than those expected and this may be the result of random degradation of the unprocessed NotchΔE in these cells deficient in these γ-secretase components.
Figure 1.
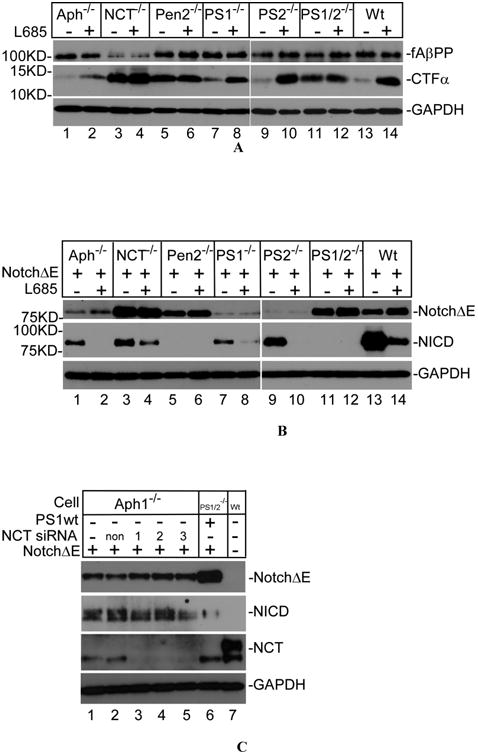
Pen-2 together with presenilin could form a functionally active γ-secretase. (A) Upper panel, cell lysates were probed with antibody C15, which specifically recognize the last 15 amino acid of AβPP. Lower panel, immunoblot was probed with anti-GAPDH antibody as loading control. (B) Top panel, cell lysates were probed with anti-Myc antibody to detect the expression of NotchΔE. Middle panel, immunoblot was probed with anti-NICD (#4147), which specifically recognizes the N-terminus of NICD generated by γ-secretase activity. Bottom panel, immunoblot probed with anti-GAPDH antibody as loading control. (C) Knockdown of NCT in Aph-1-/- cell does not affect the Notch processing. Top panel, immunoblot was probed with anti-Myc antibody to detect the expression of NotchΔE. Second panel, immunoblot was probed with anti-NICD (#4147) to detect the formation of NICD. Third panel, immunoblot was probed with anti-NCT antibody to confirm the knockdown of NCT. Bottom panel, immunoblot probed with anti-GAPDH antibody as loading control.
Pen-2 together with presenilin can form a functionally active γ-secretase for the processing of Notch
As reported in our previous study [21], data presented in Fig. 1A and B revealed very interesting findings that Aph-1 is dispensable for processing of both AβPP and Notch, that NCT is required for AβPP processing but not for Notch processing, and that Pen-2 is absolutely required for the processing of both AβPP and Notch. These findings suggest that there is a different requirement for NCT in processing of Notch and AβPP. Specifically, the finding that neither Aph-1 nor NCT was absolutely required for Notch processing when these genes were individually inactivated prompted us to determine the effect of inactivation of both Aph-1 and NCT genes simultaneously on the processing of Notch. As shown Fig. 1C, all three NCT-specific siRNAs efficiently blocked the expression of NCT (third panel, lanes 3-5) in Aph-1-/- cells. Intriguingly, the level of NICD was not changed upon knockdown of NCT in Aph-1-/- cells (compare lanes 3-5 with lanes 1-2). These data strongly indicate that Pen-2 is absolutely essential and sufficient for supporting presenilin-catalyzed Notch processing.
Ectopically expressed PS1N and PS1C are stable, but unable to restore γ-secretase activity in Pen-2-/-cells
Previous studies have revealed that Pen-2 plays roles in efficient proteolytic processing of presenilin and stabilization of the resulting N-terminal and C-terminal fragments of presenilin [9, 10]. Thus, to determine whether these roles account for the absolutely requirement of Pen-2 for presenilin-catalyzed Notch processing, we first examined the stability and the enzymatic activity of the N-terminal fragment (PS1N) and the C-terminal fragment (PS1C) of PS1 in Pen-2 knockout cells (Pen-2-/-). Since PS1 undergoes heterogeneous endoproteolysis between Thr291 and Ala299 and can also be processed by caspase activity to produce PS1Cs with different length [22-25], we constructed a series of plasmids (namely PS1C293, PS1C296, PS1C299, PS1C334 and PS1C346) expressing different PS1Cs starting from amino acid 293, 296, 299, 334, and 346 to the very end of C terminal and a plasmid PS1N expressing the N-terminal fragment of PS1 from amino acid 1 to 292. Pen-2-/- cells were co-transfected with NotchΔE along with a combination of PS1N and one the PS1Cs of different lengths. As shown in Fig. 2A, all recombinant proteins were expressed at significant levels. Specifically, the high levels of PS1N and PS1C detected in these cells indicate that the ectopically expressed PS1N and PS1C are stable in Pen-2-/- cells. It was noted that in the PS1wt-expressing cells the PS1N and PS1C produced by normal endoproteolytic processing were detected at relatively low levels and the most abundant PS1C detected is the one equivalent to the PS1C produced by caspase activity (top panel, lane 4). This may be due to the inefficient endoproteolytic processing of PS1 and the instability of the resulting PS1N and PS1C in the absence of Pen-2 as reported by previous study [9]. As a control, Wt-7 cells were also transfected with NotchΔE. As shown in fourth panel, in Wt-7 cells, a significant amount of NICD was detected (lane 10). However, no NICD was detected in Pen-2-/- cells co-transfected either with a combination of PS1N and PS1C (third panel, lanes 5-9) or with PS1wt (lane 4). The fact that NICD was not detected in Pen-2-/- cells expressing significant amounts of PS1C and PS1N strongly suggest that in addition to enhancing PS1 endoproteolytic processing and stabilization of the resulting PS1N and PS1C fragments, Pen-2 must play a more crucial role in in catalytic activity of γ-secretase.
Figure 2.
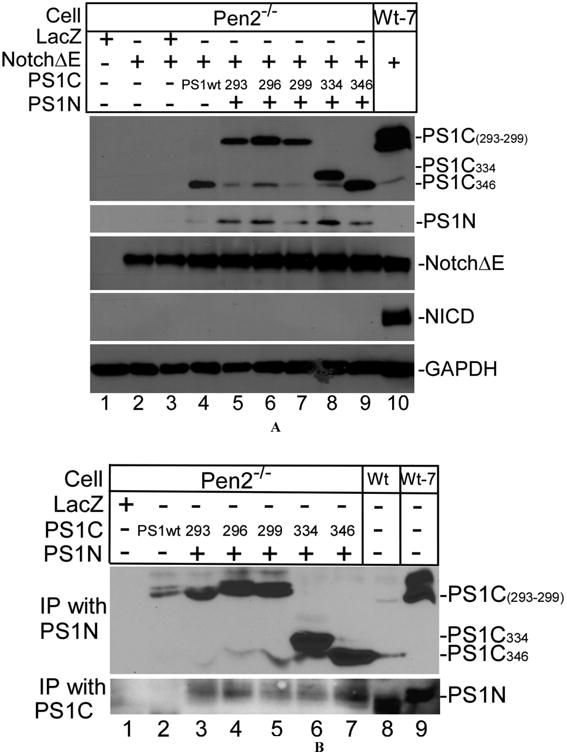
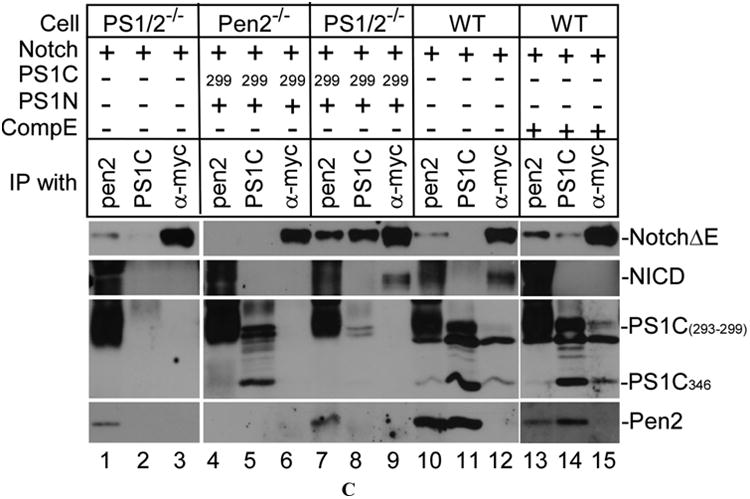
(A) Overexpression of PS1N and PS1Cs did not restore γ-secretase activity in Pen-2-/- cells. All cells were transfected with NotchΔE. In Pen-2-/- cells, in addition to NotchΔE, PS1N and PS1C were also co-expressed. Expression of PS1C was detected by PS1C-specific antibody #5643 (top panel). PS1N was detected by anti-PS1N antibody (second panel). Expression of NotchΔE was detected by anti-Myc antibody (third panel). NICD was detected by antibody #4147 (fourth panel). The immunoblot in the fourth panel was also probed with anti-GAPDH to indicate relative loading of samples (bottom panel). (B) The association of PS1N and PS1C was not disturbed by the deletion of Pen-2. Pen-2-/- cells were transfected with PS1N along with one of the PS1C variants (PS1C293, PS1C296, PS1C299, PS1C334, PS1C346). Cell lysates were immunoprecipitated with anti-PS1N and anti-PS1C antibodies. Up panel, immunoprecipitates were probed with anti-PS1C antibody #5643. Low panel, immunoblots were probed with anti-PS1N. WT cell and Wt-7 cell were used as controls. (C) Pen-2 plays a crucial role in Notch binding to PS1. All cells were transfected with NotchΔE in combination with PS1wt or both PS1N and PS1C as indicated. After cultured in the presence or absence of γ-secretase inhibitor compound E (CompE) for 24 hrs, cell lysates were subjected to immunoprecipitation using antibodies against Pen-2, PS1C, and Myc, respectively as indicated. First panel, immunoblots were probed with anti-Myc to determine the full-length NotchΔE. Second panel, immunoprecipitates were probed with anti-NICD to detect NICD. Third panel, immunoblots were probed with anti-PS1C #5643 antibody to detect all PS1Cs of different lengths; Bottom panel, immunoblots were probed with anti-Pen-2 antibody. Note that anti-Pen2 immunoprecipitates (second panel, lanes 1, 4, 7, 10, and 13) non-specifically react with antibody #4147, which is specific to NICD.
Pen-2 is not required for the heterodimer formation and stabilization of PS1N and PS1C
The presenilin NTF/CTF heterodimers are considered to constitute the active site in the γ-secretase complex [26-28]. A study has also suggested that Pen-2 may be required for the stabilization of PS1N/PS1C heterodimer [29]. Thus, we examined the effect of knockout of Pen-2 on the formation of the PS1N/PS1C heterodimer. As shown in up panel of Fig. 2B, when the cells were transfected with PS1wt, a band corresponding to PS1C293 was co-immunoprecipitated with PS1N by anti-PS1N antibody (lane 2). Similarly, significant amount of PS1Cs were co-immunoprecipitated by anti-PS1N antibody from cells expressing PS1N and various PS1Cs including the PS1Cs produced by caspase activity (lanes 3-7). The similar co-immunoprecipitation results were also observed in wild type MEF cells (lane 8) and Wt-7 cells (Lane 9). These results indicate that Pen-2 is not required for the formation and stabilization of PS1N/PS1C heterodimer.
Pen-2 is required for Notch binding to PS1
Next, we determined the effect of knockout of Pen-2 on the binding of Notch to γ-secretase. Cells were transfected with NotchΔE alone with either non-related protein LacZ or plasmids expressing PS1 variants. As shown in Fig. 2C, when the cell lysates were immunoprecipitated with anti-Myc antibody, high levels of NotchΔE was detected in all transfectants (top panel, lanes 3, 6, 9, 12, and 15). When the cell lysates were subjected to co-immunoprecipitation using anti-Pen-2 antibody, as shown in lane 10 of top panel, NotchΔE was readily co-immunoprecipitated with Pen-2 in wild type cells. However, anti-PS1C antibody pulled down hardly any NotchΔE in the absence of γ-secretase inhibitor (lane 11). This is likely due to the activity of γ-secretase that processes NotchΔE into NICD, which is no longer associated with PS1. Indeed, NotchΔE was pulled down by anti-PS1C antibody in wild type cells in the presence of γ-secretase inhibitor compound E (lane 14, top panel). It was also noted that the, in the presence of compound E, the level of NotchΔE pulled down by anti-Pen-2 antibody was also increased and higher than that pulled down by anti-PS1C antibody (compare lane 13 with lane 10 and lane 13 with 14), suggesting that the association of Notch with Pen- 2 is stronger than that with PS1. This notion was strongly supported by the results that NotchΔE was readily co-immunoprecipitated with Pen-2 in PS1/2-/- cells (lane 1, top panel), indicating that Pen-2 is capable of interacting with NotchΔE in the absence of presenilin. However, in Pen-2-/- cells, anti-PS1C antibody did not bring down any NotchΔE (lane 5, top panel), even in the presence of ectopically expressed PS1N and PS1C. It was also noted that when the PS1/2-/- cells were transfected with PS1N and PS1C, not only anti-PS1C antibody but also anti-Pen-2 antibody brought down significantly more NotchΔE than that in cells without overexpression of PS1 (compare lane 8 with lane 11 and lane 7 with lane 10). These results suggest that PS1 also enhances the interaction between Pen-2 and NotchΔE. In addition, this set of experiments further confirmed that, similar to PS1/2-/- cells, Notch is not processed in the absence of Pen-2—even in the presence of overexpressed PS1 (lane 6, second panel). It was also noted that NICD was immunoprecipitated by anti-Myc antibody (lanes 9 and 12, second panel), but not by anti-Pen-2 antibody (lanes 7 and 10, second panel) nor by anti-PS1C antibody (lanes 8 and 11), suggesting that, upon formation, NICD was released from γ-secretase complex. In addition, in wild type cells, PS1C was co-immunoprecipitated with Pen-2 by anti-Pen-2 antibody (lanes 10 and 13, third and fourth panels) vice versa (lanes 11 and 14, third and fourth panels). Note that the bands detected in lanes 1, 4, 7, 10, and 13 in second and third panels are non-specific bands corresponding to heavy chain and light chain of anti-Pen-2 antibody, respectively.
Discussion
In studying the functional roles of NCT, Aph-1α, and Pen-2, the three reportedly essential components of γ-secretase, a previous study has proposed that NCT functions as a substrate receptor for γ-secretase [3]. However, this notion remains controversial. Subsequent studies showed that NCT was important for maturation and stability but was not essential for the activity of γ-secretase complex [30, 31]. Our recent study showed that, Pen-2 is absolutely required for both AβPP and Notch processing, while Aph-1a is dispensable for the processing of both AβPP and Notch, and NCT is essential for AβPP but not for Notch processing, [21]. These findings were further confirmed by current study and suggest that there may be different component requirements for γ-secretase to catalyze the processing of AβPP and Notch. This finding also raises the question as to what the minimal requirements for γ-secretase are to catalyze Notch processing. To address this question, in the current study, the first and the most interesting finding is that knockdown of NCT in Aph-1-/- cells had no effect on the processing of Notch under our experimental conditions. This finding strongly suggests a novel notion that presenilin together with Pen-2 are sufficient to form a functionally active γ-secretase that is capable of catalyzing the processing of Notch.
Regarding the mechanism by which Pen-2 plays an essential role in γ-secretase activity, studies have suggested that Pen-2 plays a role in presenilin endoproteolysis and stabilization of the resulting N- and C-terminal fragments of presenilin [6, 7, 9, 10]. In this current study, our data demonstrated that the ectopically expressed PS1N and PS1C were stable in Pen-2-/- cells; however, the γ-secretase was inactive. This finding suggests that enhancing presenilin endoproteolytic processing and stabilization of the N- and C-terminal fragments of presenilin are not the key functions that make Pen-2 as an essential component of γ-secretase. Study has suggested that Pen-2 may be required for the stabilization of PS1N/PS1C heterodimer [29], which is considered to constitute the active site in of γ-secretase complex [26-28]. Thus, we examined the effect of knockout of Pen-2 on the formation of PS1N/PS1C heterodimer. As a result, our data clearly demonstrated that Pen-2 is not required for the formation and stabilization of the PS1N/PS1C heterodimer. Next, we investigated the possible role of Pen-2 in substrate biding. Very interestingly our results revealed that Pen-2 interacts with Notch in the absence of presenilins, but PS1 was virtually unable to interact with Notch in the absence of Pen-2. These findings strongly suggest that presenilin interaction with Notch is mediated or enhanced by Pen-2. Using a knockout mouse model, a recent study hypothesized that Pen-2 is more than just a structural component of the γ-secretase complex, and may contribute to the catalytic mechanism of the enzyme [8]. In this regard, our current study strongly suggests that one of the mechanisms by which Pen-2 directly contributes to the catalytic activity of γ-secretase is to function as a recruiter or receptor for substrate of γ-secretase.
In summary, using knockout cell lines in combination with siRNA and immunoprecipitation approaches, our data clearly demonstrated that the Pen-2 and PS1 are sufficient to form a functionally active γ-secretase that is capable of catalyzing the processing of Notch. This finding strongly suggests that Pen-2 is the most crucial component in γ-secretase complex in addition to presenilin that functions as the catalytic subunit. Our current study further suggests that Pen-2 directly contributes to the catalytic mechanism of γ-secretase by functioning as the substrate recruiter or receptor. In addition, our study revealed that there is different requirement for γ-secretase components in catalyzing Notch and AβPP processing, suggesting that processing of Notch through a mechanism that is slightly different that underlying AβPP processing. Thus, the information obtained in this study would be important for therapeutic strategies aimed at inhibition or modulation of γ-secretase activity, which is currently challenging under the assumption that the same γ-secretase activity governs both AβPP and Notch processing.
Acknowledgments
We thank Dr Bart De Strooper (Center for Human Genetics, K.U. Leuven, Herestraat 49, B 3000 Leuven, Belgium) for providing the presenilin-knockout and Pen-2-knockout cell lines. We thank Dr Tong Li (John Hopkins University, Baltimore, MD, USA) for providing the NCT-knockout and Aph-1-knockout cell lines. We also thank Derek Xu for his critical reading of this manuscript.
Footnotes
This work was supported by National Institutes of Health Grants NS095256 and R21AG039596 (to X.X.) and HL107466 (to M.-Z.C.). This work was also supported by an Alzheimer's Association grant and a grant from the American Health Assistance Foundation (to X.X.) and by the University of Tennessee Center of Excellence in Livestock Diseases and Human Health (to X.X.). The authors declare no conflict of interest.
The abbreviations used are: AD, Alzheimer's disease; PS1, Presenilin-1; Aph-1, anterior pharynx-defective 1; NCT, nicastrin; Pen-2, presenilin enhancer-2; LacZ, β-galactosidase; CTFα, C-terminal fragment of AβPP produced by α-secretase activity; NotchΔE, ectodomain-truncated Notch, NICD, Notch intracellular domain.
Conflict of interest: The authors declare that they have no conflicts of interest with the contents of this article.
Author contribution: CH performed and analyzed most of the experiments presented in this manuscript. JX performed the experiments showing in figure 2A. LZ performed and analyzed the experiment showing in figure 1b. TL contributed to figure 2B. MZC and XX designed the study, analyzed the data, and wrote the paper. All authors reviewed the results and AβPProved the final version of the manuscript.
References
- 1.De Strooper B, Iwatsubo T, Wolfe MS. Presenilins and γ-Secretase: Structure, Function, and Role in Alzheimer Disease. Cold Spring Harbor Perspectives in Medicine. 2012;2 doi: 10.1101/cshperspect.a006304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolfe MS. Therapeutic strategies for Alzheimer's disease. Nat Rev Drug Discov. 2002;1:859–866. doi: 10.1038/nrd938. [DOI] [PubMed] [Google Scholar]
- 3.Shah S, Lee SF, Tabuchi K, Hao YH, Yu C, LaPlant Q, Ball H, Dann CE, 3rd, Sudhof T, Yu G. Nicastrin functions as a gamma-secretase-substrate receptor. Cell. 2005;122:435–447. doi: 10.1016/j.cell.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 4.Francis R, McGrath G, Zhang J, Ruddy DA, Sym M, Apfeld J, Nicoll M, Maxwell M, Hai B, Ellis MC, Parks AL, Xu W, Li J, Gurney M, Myers RL, Himes CS, Hiebsch R, Ruble C, Nye JS, Curtis D. aph-1 and pen-2 are required for Notch pathway signaling, gamma-secretase cleavage of betaAPP, and presenilin protein accumulation. Dev Cell. 2002;3:85–97. doi: 10.1016/s1534-5807(02)00189-2. [DOI] [PubMed] [Google Scholar]
- 5.Lee SF, Shah S, Li H, Yu C, Han W, Yu G. Mammalian APH-1 interacts with presenilin and nicastrin and is required for intramembrane proteolysis of amyloid-beta precursor protein and Notch. J Biol Chem. 2002;277:45013–45019. doi: 10.1074/jbc.M208164200. [DOI] [PubMed] [Google Scholar]
- 6.Luo WJ, Wang H, Li H, Kim BS, Shah S, Lee HJ, Thinakaran G, Kim TW, Yu G, Xu H. PEN-2 and APH-1 coordinately regulate proteolytic processing of presenilin 1. J Biol Chem. 2003;278:7850–7854. doi: 10.1074/jbc.C200648200. [DOI] [PubMed] [Google Scholar]
- 7.Takasugi N, Tomita T, Hayashi I, Tsuruoka M, Niimura M, Takahashi Y, Thinakaran G, Iwatsubo T. The role of presenilin cofactors in the gamma-secretase complex. Nature. 2003;422:438–441. doi: 10.1038/nature01506. [DOI] [PubMed] [Google Scholar]
- 8.Bammens L, Chávez-Gutiérrez L, Tolia A, Zwijsen A, De Strooper B. Functional and Topological Analysis of Pen-2, the Fourth Subunit of the γ-Secretase Complex. J Biol Chem. 2011;286:12271–12282. doi: 10.1074/jbc.M110.216978. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Mao G, Cui MZ, Li T, Jin Y, Xu X. Pen-2 is dispensable for endoproteolysis of presenilin 1, and nicastrin-Aph subcomplex is important for both gamma-secretase assembly and substrate recruitment. J Neurochem. 2012;123:837–844. doi: 10.1111/jnc.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holmes O, Paturi S, Selkoe DJ, Wolfe MS. Pen-2 Is Essential for γ-Secretase Complex Stability and Trafficking but Partially Dispensable for Endoproteolysis. Biochemistry (Mosc) 2014;53:4393–4406. doi: 10.1021/bi500489j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiang PM, Fortna RR, Price DL, Li T, Wong PC. Specific domains in anterior pharynx-defective 1 determine its intramembrane interactions with nicastrin and presenilin. Neurobiol Aging. 2012;33:277–285. doi: 10.1016/j.neurobiolaging.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li T, Ma G, Cai H, Price DL, Wong PC. Nicastrin is required for assembly of presenilin/gamma-secretase complexes to mediate Notch signaling and for processing and trafficking of beta-amyloid precursor protein in mammals. J Neurosci. 2003;23:3272–3277. doi: 10.1523/JNEUROSCI.23-08-03272.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Strooper B, Saftig P, Craessaerts K, Vanderstichele H, Guhde G, Annaert W, Von Figura K, Van Leuven F. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature. 1998;391:387–390. doi: 10.1038/34910. [DOI] [PubMed] [Google Scholar]
- 14.Herreman A, Hartmann D, Annaert W, Saftig P, Craessaerts K, Serneels L, Umans L, Schrijvers V, Checler F, Vanderstichele H, Baekelandt V, Dressel R, Cupers P, Huylebroeck D, Zwijsen A, Van Leuven F, De Strooper B. Presenilin 2 deficiency causes a mild pulmonary phenotype and no changes in amyloid precursor protein processing but enhances the embryonic lethal phenotype of presenilin 1 deficiency. Proc Natl Acad Sci U S A. 1999;96:11872–11877. doi: 10.1073/pnas.96.21.11872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herreman A, Serneels L, Annaert W, Collen D, Schoonjans L, De Strooper B. Total inactivation of gamma-secretase activity in presenilin-deficient embryonic stem cells. Nat Cell Biol. 2000;2:461–462. doi: 10.1038/35017105. [DOI] [PubMed] [Google Scholar]
- 16.Zhao G, Mao G, Tan J, Dong Y, Cui MZ, Kim SH, Xu X. Identification of a New Presenilin-dependent z-Cleavage Site within the Transmembrane Domain of Amyloid Precursor Protein. J Biol Chem. 2004;279:50647–50650. doi: 10.1074/jbc.C400473200. [DOI] [PubMed] [Google Scholar]
- 17.Zhao G, Cui MZ, Mao G, Dong Y, Tan J, Sun L, Xu X. gamma-Cleavage is dependent on zeta-cleavage during the proteolytic processing of amyloid precursor protein within its transmembrane domain. J Biol Chem. 2005;280:37689–37697. doi: 10.1074/jbc.M507993200. [DOI] [PubMed] [Google Scholar]
- 18.Kopan R, Schroeter EH, Weintraub H, Nye JS. Signal transduction by activated mNotch: importance of proteolytic processing and its regulation by the extracellular domain. Proceedings of the National Academy of Sciences. 1996;93:1683–1688. doi: 10.1073/pnas.93.4.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thinakaran G, Teplow DB, Siman R, Greenberg B, Sisodia SS. Metabolism of the “Swedish” amyloid precursor protein variant in neuro2a (N2a) cells. Evidence that cleavage at the “beta-secretase” site occurs in the golgi apparatus. J Biol Chem. 1996;271:9390–9397. doi: 10.1074/jbc.271.16.9390. [DOI] [PubMed] [Google Scholar]
- 20.Xu X, Shi YC, Gao W, Mao G, Zhao G, Agrawal S, Chisolm GM, Sui D, Cui MZ. The novel presenilin-1-associated protein is a proapoptotic mitochondrial protein. J Biol Chem. 2002;277:48913–48922. doi: 10.1074/jbc.M209613200. [DOI] [PubMed] [Google Scholar]
- 21.Hu C, Zeng L, Li T, Meyer MA, Cui MZ, Xu X. Nicastrin is required for amyloid precursor protein (APP) but not Notch processing, while anterior pharynx-defective 1 is dispensable for processing of both APP and Notch. J Neurochem. 2016;136:1246–1258. doi: 10.1111/jnc.13518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim TW, Pettingell WH, Jung YK, Kovacs DM, Tanzi RE. Alternative Cleavage of Alzheimer-Associated Presenilins During Apoptosis by a Caspase-3 Family Protease. Science. 1997;277:373–376. doi: 10.1126/science.277.5324.373. [DOI] [PubMed] [Google Scholar]
- 23.Loetscher H, Deuschle U, Brockhaus M, Reinhardt D, Nelboeck P, Mous J, Grünberg J, Haass C, Jacobsen H. Presenilins Are Processed by Caspase-type Proteases. J Biol Chem. 1997;272:20655–20659. doi: 10.1074/jbc.272.33.20655. [DOI] [PubMed] [Google Scholar]
- 24.Podlisny MB, Citron M, Amarante P, Sherrington R, Xia W, Zhang J, Diehl T, Levesque G, Fraser P, Haass C, Koo EHM, Seubert P, St George-Hyslop P, Teplow DB, Selkoe DJ. Presenilin Proteins Undergo Heterogeneous Endoproteolysis between Thr291and Ala299and Occur as Stable N- and C-Terminal Fragments in Normal and Alzheimer Brain Tissue. Neurobiol Dis. 1997;3:325–337. doi: 10.1006/nbdi.1997.0129. [DOI] [PubMed] [Google Scholar]
- 25.Grunberg J, Walter J, Loetscher H, Deuschle U, Jacobsen H, Haass C. Alzheimer's disease associated presenilin-1 holoprotein and its 18-20 kDa C-terminal fragment are death substrates for proteases of the caspase family. Biochemistry (Mosc) 1998;37:2263–2270. doi: 10.1021/bi972106l. [DOI] [PubMed] [Google Scholar]
- 26.Thinakaran G, Borchelt DR, Lee MK, Slunt HH, Spitzer L, Kim G, Ratovitsky T, Davenport F, Nordstedt C, Seeger M, Hardy J, Levey AI, Gandy SE, Jenkins NA, Copeland NG, Price DL, Sisodia SS. Endoproteolysis of Presenilin 1 and Accumulation of Processed Derivatives In Vivo. Neuron. 1996;17:181–190. doi: 10.1016/s0896-6273(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 27.Esler WP, Kimberly WT, Ostaszewski BL, Diehl TS, Moore CL, Tsai JY, Rahmati T, Xia W, Selkoe DJ, Wolfe MS. Transition-state analogue inhibitors of gamma-secretase bind directly to presenilin-1. Nat Cell Biol. 2000;2:428–434. doi: 10.1038/35017062. [DOI] [PubMed] [Google Scholar]
- 28.Li YM, Xu M, Lai MT, Huang Q, Castro JL, DiMuzio-Mower J, Harrison T, Lellis C, Nadin A, Neduvelil JG, Register RB, Sardana MK, Shearman MS, Smith AL, Shi XP, Yin KC, Shafer JA, Gardell SJ. Photoactivated gamma-secretase inhibitors directed to the active site covalently label presenilin 1. Nature. 2000;405:689–694. doi: 10.1038/35015085. [DOI] [PubMed] [Google Scholar]
- 29.Prokop S, Shirotani K, Edbauer D, Haass C, Steiner H. Requirement of PEN-2 for Stabilization of the Presenilin N-/C-terminal Fragment Heterodimer within the γ-Secretase Complex. J Biol Chem. 2004;279:23255–23261. doi: 10.1074/jbc.M401789200. [DOI] [PubMed] [Google Scholar]
- 30.Chavez-Gutierrez L, Tolia A, Maes E, Li T, Wong PC, de Strooper B. Glu332 in the Nicastrin Ectodomain Is Essential for {gamma}-Secretase Complex Maturation but Not for Its Activity. J Biol Chem. 2008;283:20096–20105. doi: 10.1074/jbc.M803040200. [DOI] [PubMed] [Google Scholar]
- 31.Zhao G, Liu Z, Ilagan MX, Kopan R. Gamma-secretase composed of PS1/Pen2/Aph1a can cleave notch and amyloid precursor protein in the absence of nicastrin. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:1648–1656. doi: 10.1523/JNEUROSCI.3826-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]


