Highlights
-
•
Laser therapy for fetal reduction could be associate with Aplasia Cutis Congenita.
-
•
Despite of size of lesion conservative treatment could be an effective option.
-
•
Extreme prematurity did not affected degree of spontaneous epithelization.
-
•
Patient was followed up to 5 years and no complications were detected.
Abbreviations: ACC, Aplasia Cutis Congenita; GA, gestational age; NICU, neonatal intensive care unit; MC, monochorionic; SCARE, surgical case report; TTS, twin-to twin transfusion
Keywords: Case report, Extreme prematurity, Interstitial laser therapy, Fetal reduction, Aplasia Cutis Congenita, Outcome
Abstract
Monochorionic (MC) twin pregnancies are known to carry a high risk of twin-to-twin transfusion syndrome (TTTS) that could lead to miscarriage and perinatal death. Demise of one fetus is frequently associated with co-fetal death. Fetal reduction by interstitial laser therapy is an effective procedure to prevent this outcome, but it may be associated with significant risks for both mother and fetus. Aplasia Cutis Congenita (ACC) may occur in up to 8% cases of fetal reduction by laser therapy. We report ACC in a preterm infant, a survivor of interstitial laser therapy for fetal reduction in MC pregnancy. Despite of massive skin lesions we were able to manage this case conservatively. Follow-up at 5 years of age revealed minimal scarring and no motor function limitations.
1. Background
Aplasia Cutis Congenita (ACC) is not a common condition. It occurs with an incidence rate of 1–3 cases per 10 000 live births, regardless of sex or race. Most of the lesions present on the scalp, and congenital skin aplasia on the lower limb is a rare finding. The cause of ACC lesions is heterogeneous. Cases of familial ACC have been described, as well as association with genetic disorders such as Adams-Oliver syndrome or Bart syndrome. The common mechanisms of ACC include vascular disruption, trauma, teratogens, or certain maternal medications during pregnancy (methimazole, azathioprine, valproic acid).
Monochorionic (MC) twin pregnancies are known to carry a high risk of twin-to-twin transfusion syndrome (TTTS) that could lead to miscarriage and perinatal death. Demise of one fetus is associated with co-fetal death in 12–25% of cases [1]. Fetal reduction is the only procedure that could prevent these outcomes. Interstitial laser therapy is commonly used, and considered an effective and safe procedure but it still carries risks for both mother and fetus [2]. O'Donoghue et al. reported that interstitial laser therapy may be associated with Aplasia Cutis Congenita (ACC) in up to 8% of fetuses [3].
We report ACC in a preterm infant, a survivor of interstitial laser therapy for fetal reduction in MC pregnancy. This work has been reported in accordance with the SCARE guidelines criteria [4].
2. Case report
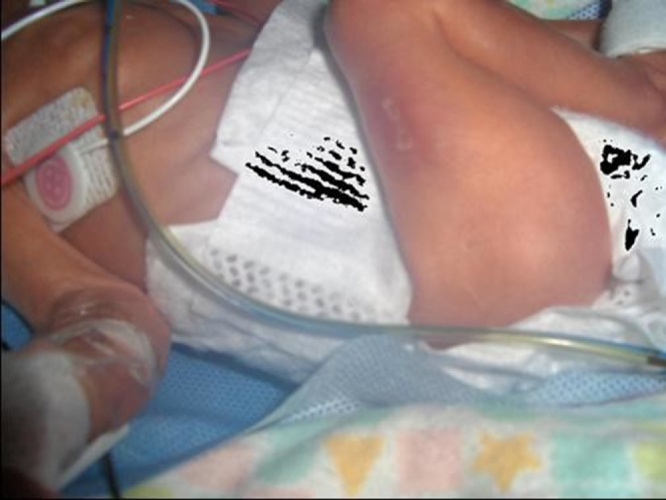
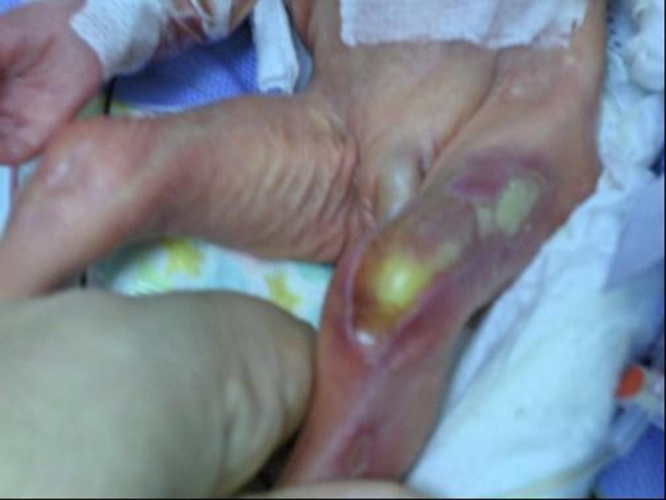
A healthy 27-year-old primigravida mother underwent intra-uterine laser ablation at 26 weeks for demise of a twin due to severe IUGR, bladder agenesis and reversed diastolic flow secondary to Twin–Twin Transfusion Syndrome (TTTS). Follow up ultrasound and Doppler studies of the surviving twin revealed no abnormalities. Upon birth at 27 weeks of GA, two small 0.5 mm lesions were found on the right thigh of infant (Fig. 1). A large (2 cm by 7 cm) lesion covered with granulated tissue, along with a 1 cm lesion were seen on the left leg (Fig. 2).
Fig. 1.
Two small ACC lesions on the right thigh after intrauterine vascular ablation.
Fig. 2.
Large ACC lesions on the left leg after intrauterine vascular ablation.
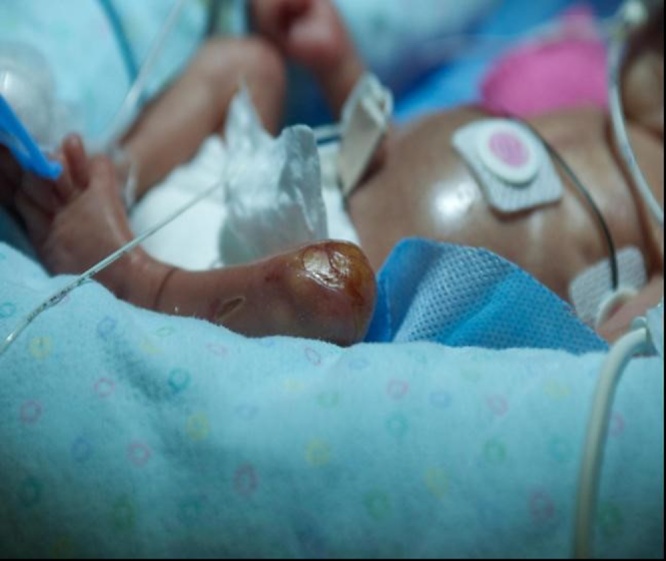
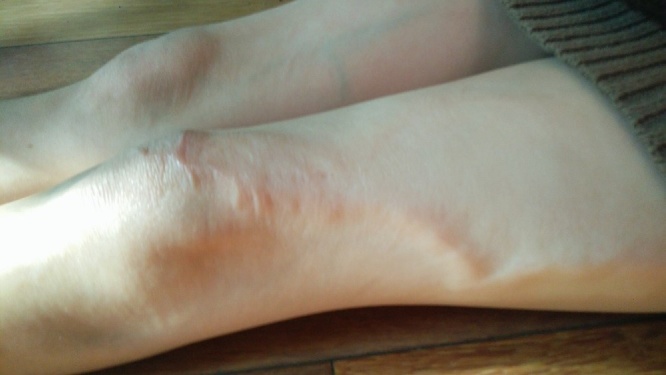
These lesions were diagnosed as Aplasia Cutis Congenita, a rare potentially serious skin defect involving absence of all skin layers. Dermatology and plastic surgery were consulted; they suggested complete emollient therapy to prevent desiccation of the defect. No other therapeutic interventions were used. Gradual epithelization was noted by the end of 6th week of life (Fig. 3). Even though ACC was found in extremely preterm infant, skin lesions healed with no ulcerations or erosions, with formation of a hairless, atrophic scar, which became less prominent with age (Fig. 4). No contractures, changes in pain, temperature and touch perception, or motor limitations were detected at the age of 5 years.
Fig. 3.
Day 35 of emollient therapy: epithelization of ACC lesions.
Fig. 4.
Atrophic scars at the age of 5 years.
3. Discussion
ACC is a result of abnormal ectodermal development that could be triggered by mechanical, vascular, teratogenic, or genetic factors. Etiologic mechanism of ACC in multiple gestational pregnancies is different, and usually related to embolic phenomena due to TTTS [1]. Lesions of the scalp are more common (70–90% of all cases), and usually associated with serious complications (hemorrhage, sagittal sinus thrombosis, central nervous system infection) [5]. Less commonly ACC present on trunk, extremities, or abdomen; frequently these lesions are bilateral and symmetric. Mortality depends on size and location of lesions, presence of associated defects, and degree of prematurity; for example, reported mortality for large ACC of the scalp in term infants ranging from 20% to 50%, and in preterm infants it could reach 95% [6], [7].
Management of ACC depends on extend of the defect. Treatment choices include use of non-adherent and adherent dressings, skin graft cultured allografts, autografts with acellular dermis, delayed flap reconstructions [6], [8]. Conservative approach with isolated emollient therapy is not common choice for management of large lesions [6], [9]. Available literature supports aggressive treatment of large defects to prevent development of hypertrophic scars and patchy contractures [6], [7], [9]. At the same time, emollient therapy reported to increase sub-epidermal inflammatory cells, decrease necrosis, and support regeneration of epidermis [10].
We believe that ACC lesions in this patient are most likely caused by direct burns from the laser therapy. Typical appearance and asymmetric location of ACC lesions on lateral sides of legs support this theory. Extreme prematurity considered as a possible contraindication for aggressive treatment; thus, emollient therapy was used as a first line treatment. Despite of extensive size of lesions and extreme prematurity of patient conservative management of ACC was successful, and not associated with long term complications.
Conflicts of interest
No conflict of interest.
Funding
No funding.
Ethical approval
As it is case report, no ethical approval requested. Parental consent obtained.
Consent
Written informed consent was obtained from the patient’ parents for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request”.
Author contribution
-
1
Dr. Veronica Mugarab Samedi: direct patient care, data collection, manuscript preparation.
-
2
Dr. Lodha: direct patient care over the first week of life, collaboration with plastic surgeon, obtained first images of lesions.
-
3
Dr. ElSharkawy: direct patient care and follow up of patient up to 5 years of age.
-
4
Dr. Al Awad: direct patient care from day 8 of life till discharge, collaborate with plastic surgery and dermatology, proposed conservative approach for treatment, obtained consent for publication and images at 6 weeks of life.
Guarantor
Dr. Veronica Mugarab Samedi.
Dr. Essa Al Awad.
Grant information
Not applicable.
Contributor Information
Veronica Mugarab-Samedi, Email: Veronica.Samedi@albertahealthservices.ca.
Abhay Lodha, Email: Abhay.Lodha@ahs.ca.
Adel ElSharkawy, Email: Adel.elsharkawy@albertahealthservices.ca.
Essa Al Awad, Email: Essa.AlAwad@ahs.ca.
References
- 1.Ong S. Prognosis for the co-twin following single-twin death: a systematic review. BJOG. 2006;113:992–998. doi: 10.1111/j.1471-0528.2006.01027.x. [DOI] [PubMed] [Google Scholar]
- 2.Roman A. Selective reduction in complicated monochorionic pregnancies: radiofrequency ablation vs. bipolar cord coagulation. Ultrasound Obstet. Gynecol. 2010;36:37–41. doi: 10.1002/uog.7567. 2. [DOI] [PubMed] [Google Scholar]
- 3.O'Donoghue K. Interstitial laser therapy for fetal reduction in monochorionic multiple pregnancy: loss rate and association with aplasia cutis congenital. Prenat. Diagn. 2008;28(6):535–543. doi: 10.1002/pd.2025. [DOI] [PubMed] [Google Scholar]
- 4.Agha R.A., Fowler A.J., Saetta A., Barai I., Rajmohan S., Orgill D.P., The SCARE Group The SCARE statement: consensus-based surgical case report guidelines. Int. J. Surg. 2016;34:180–186. doi: 10.1016/j.ijsu.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 5.Frieden I.J. Aplasia cutis congenita: a clinical review and proposal for classification. J. Am. Acad. Dermatol. 1986;14:646Y660. doi: 10.1016/s0190-9622(86)70082-0. [DOI] [PubMed] [Google Scholar]
- 6.Bang R.L., Ghoneim I.E., Gang R.K. Treatment dilemma: conservative versus surgery in cutis aplasia congenita. Eur. J. Pediatr. Surg. 2003;13:125Y129. doi: 10.1055/s-2003-39562. [DOI] [PubMed] [Google Scholar]
- 7.Kantor J., Yan A.C., Hivnor C.M., Honig P.J., Kirschner R. Extensive aplasia cutis congenita and the risk of sagittal sinus thrombosis. Arch. Dermatol. 2005;141(May (5)):554–556. doi: 10.1001/archderm.141.5.554. [DOI] [PubMed] [Google Scholar]
- 8.Bui D., Ikeda C. Reconstruction of aplasia cutis congenital (group V) of the trunk in a newborn. Plast. Reconstr. Surg. 2003;111:2119Y2120. doi: 10.1097/01.PRS.0000057071.85921.3D. [DOI] [PubMed] [Google Scholar]
- 9.Ross D.A., Laurie S.W., Coombs C.J. Aplasia cutis congenita: failed conservative treatment. Plast. Reconstr. Surg. 1995;95:124Y129. doi: 10.1097/00006534-199501000-00020. [DOI] [PubMed] [Google Scholar]
- 10.Vogt P.M., Andree C., Breuing K. Dry, moist, and wet skin wound repair. Ann. Plast. Surg. 1995;34:493Y499. doi: 10.1097/00000637-199505000-00007. [DOI] [PubMed] [Google Scholar]