Abstract
We examined the impact of crosstalk between the insulin receptor (INSR) and G protein-coupled receptor (GPCR) signaling pathways on the regulation of Yes-associated Protein (YAP) localization, phosphorylation and transcriptional activity in the context of human pancreatic ductal adenocarcinoma (PDAC). Stimulation of PANC-1 or MiaPaCa-2 cells with insulin and neurotensin, a potent mitogenic combination of agonists for these cells, promoted striking YAP nuclear localization and decreased YAP phosphorylation at Ser127 and Ser397. Challenging PDAC cells with either insulin or neurotensin alone modestly induced the expression of YAP/TEAD-regulated genes, including Connective Tissue Growth Factor (CTGF), Cysteine-rich angiogenic inducer 61 (CYR61) and CXCL5 whereas the combination of neurotensin and insulin induced a marked increase in the level of expression of these genes. In addition, siRNA-mediated knockdown of YAP/TAZ prevented the increase in the expression of these genes. A small-molecule inhibitor (A66), selective for the p110α subunit of PI3K, abrogated the increase in phosphatidylinositol 3,4,5-trisphosphate (PIP3) production and the expression of CTGF, CYR61 and CXCL5 induced by neurotensin and insulin. Furthermore, treatment of PDAC cells with protein kinase D (PKD) family inhibitors (CRT0066101 or kb NB 142-70) or with siRNAs targeting the PKD family prevented the increase of CTGF, CYR61 and CXCL5 mRNA levels in response to insulin and neurotensin stimulation. Thus, PI3K and PKD mediate YAP activation in response to insulin and neurotensin in pancreatic cancer cells.
Keywords: Neurotensin, Hippo pathway, YAP phosphorylation, TAZ, PANC-1 cells, MiaPaCa-2 cells, PKD family inhibitors, PI3K inhibitor A66, CTGF, CYR61, CXCL5
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a devastating disease, with overall 5-year survival rate of only 7%. The incidence of this disease in the United States is estimated to increase to 53,070 new cases in 2016. It is anticipated that PDAC deaths will surpass those caused by breast cancer, moving the disease from the 4th to the 3rd leading cause of cancer-related deaths in the USA (1). As the current therapies offer very limited survival benefits (2), novel strategies are urgently required to prevent and treat this aggressive disease.
G protein–coupled receptors (GPCRs) and their cognate agonists are increasingly implicated in growth stimulation of multiple solid tumors, including small cell lung cancer, colon, prostate, breast and pancreas (3, 4). We showed that pancreatic cancer cell lines express multiple GPCRs (5) and a variety of GPCR agonists, including neurotensin, angiotensin II and bradykinin, stimulate DNA synthesis in these cells (5–8). Furthermore, a broad-spectrum GPCR antagonist (9, 10), inhibited the growth of pancreatic cancer cells either in vitro or xenografted into nu/nu mice (11). Other studies demonstrated increased expression of GPCRs, including those for neurotensin and angiotensin II in pancreatic cancer tissues (12). A recent characterization of cancer genomes demonstrated frequent mutations in GPCRs and G proteins (13). GPCRs and their cognate agonists also dramatically synergize with insulin/IGF-1 in inducing mitogenic signaling (14). Accordingly, we identified positive crosstalk between insulin/IGFI receptors and GPCR signaling systems in pancreatic cancer cells, leading to mTORC1 and ERK activation, and synergistic stimulation of DNA synthesis and cell proliferation (15–17). These findings assume an added importance in view of the large number of epidemiological studies linking long standing type-2 diabetes mellitus (T2DM), obesity and metabolic syndrome, characterized by peripheral insulin resistance and compensatory overproduction of insulin, with increased risk for developing pancreatic cancer (18, 19). Furthermore, myofibroblasts and macrophages in the tumor microenvironment release IGF-1 that also stimulates insulin/IGF-1 receptors in PDAC cells (20). Neurotensin, a GPCR agonist that acts as a potent mitogen for PDAC cells in combination with insulin (5–8), has been identified as an important gastrointestinal peptide in the pathogenesis of obesity in mice and humans (21). All these findings reinforce the notion that crosstalk between insulin/IGF-1 receptor and GPCR signaling pathways is a major driver of PDAC proliferation (16). Consequently, the identification of key downstream effectors in the signaling network that mediates crosstalk in PDAC cells is of importance for identifying novel targets for prevention and/or treatment of this devastating disease.
The transcriptional co-activators Yes-Associated Protein (YAP) and WW-domain-containing Transcriptional co-Activator with PDZ-binding motif (TAZ) are major downstream effectors of the Hippo pathway and novel sensors of insulin, GPCR and Ras signaling (22–25). The YAP/TAZ pathway, originally identified in Drosophila, is attracting intense attention as a key regulator of development, organ-size, tissue regeneration and tumorigenesis. It is increasingly accepted that YAP/TAZ acts as a context-specific oncogene and several studies indicate that YAP and TAZ are overactive in PDAC patient tumor samples (26–28). Here, we examined the impact of crosstalk between the insulin receptor and GPCR signaling pathways on the regulation of YAP localization, phosphorylation and transcriptional activity in PDAC cells. Based on the results presented here, we propose that YAP activation is a central node of transcriptional convergence in the crosstalk between insulin receptor and GPCR signaling systems and of critical importance in the proliferative response induced by these agonists in PDAC cells.
Material and Methods
Cell culture
The human pancreatic cancer cell lines PANC-1 and MiaPaCa-2 were obtained from the American Type Culture Collection (ATCC, Manassas, VA). These cell lines were chosen because they harbor mutations typical of human pancreatic cancer, including mutations in KRAS and TP53 (encoding the p53 protein) and deletion of CDKN2A (also known as p16 or p16INK4a). These cell lines, authenticated by ATCC by short-tandem repeat analysis, were used within 15 passages and cultured for less than 6 months after recovery from frozen stocks (no authentication was done by the authors). Cells were grown in Dulbecco's modified Eagle Medium (DMEM) with 2 mM glutamine, 1 mM Na-pyruvate, 100 units/mL penicillin, and 100 µg/mL streptomycin and 10% fetal bovine serum (FBS) at 37°C in a humidified atmosphere containing 10% CO2.
Western blot analysis
Confluent cultures of PANC-1 or MiaPaCa-2 cells, grown on 35 mm tissue culture dishes, were washed twice with DMEM and incubated in serum-free medium for 4 h and then treated as described in individual experiments. The cultures were then directly lysed in 2 × SDS-PAGE sample buffer [200 mM Tris-HCl (pH 6.8), 2 mM EDTA, 0.1 M Na3VO4, 6% SDS, 10% glycerol, and 4% 2-mercaptoethanol], followed by SDS-PAGE on 4–15% gels and transfer to Immobilon-P membranes (Millipore, Billerica, MA). For detection of proteins, membranes were blocked using 5% nonfat dried milk in PBS, pH 7.2, and then incubated overnight with the desired antibodies diluted in PBS containing 0.1% Tween. Primary antibodies bound to immunoreactive bands were visualized by enhanced chemiluminescence (ECL) detection with horseradish peroxidase-conjugated anti-mouse, anti-rabbit antibody and a FUJI LAS-4000 mini luminescent image analyzer. Quantification of the bands was performed using the FUJI Multi Gauge V3.0 analysis program.
Immunofluorescence
Immunofluorescence of PANC-1 and MiaPaCa-2 cells was performed by fixing the cultures with 4% paraformaldehyde followed by permeabilization with 0.4% Triton X-100. After extensive PBS washing, fixed cells were incubated for 2h at 25°C in blocking buffer (BB), consisting of PBS supplemented with 5% bovine serum albumin and then stained at 4°C overnight with a YAP mouse mAb (1:200) diluted in BB. Subsequently, the cells were washed with PBS at 25°C and stained at 25°C for 60 min with Alexafluor 488 - conjugated goat-anti mouse diluted in BB (1:100) and washed again with PBS. Nuclei were stained using a Hoechst 33342 stain (1:10,000).
Transfection of GFP-AKT-PH
PANC-1 and MiaPaCa-2 cells were transfected with the plasmid containing a cDNA encoding a green fluorescent protein (GFP) tagged-AKT pleckstrin homology domain (pcDNA3-AKT-PH-GFP was a gift from Craig Montell, Addgene plasmid # 18836) by using Lipofectamine 3000 as suggested by the manufacturer. Analysis of the cells were performed 24 h after transfection.
Microscopy and Image Analysis
Immunolabeled and GFP tagged AKT-PH samples were imaged and captured with an epifluorescence Zeiss Axioskop and a Zeiss (Achroplan 40/.75W objective). The selected cells displayed in the appropriate figures were representative of 90% of the population. For YAP localization the average fluorescence intensity in the nucleus and just outside the nucleus (cytoplasm) was measured to determine the nuclear/cytoplasmic ratios. To determine membrane/cytoplasmic ratios of GFP tagged AKT-PH, the average fluorescence intensity in the membrane and inside the cytoplasm was measured. All Image analysis was performed using ImageJ software.
Knockdown of YAP and PKD levels via siRNA transfection
Silencer Select siRNAs was purchased from Life Technologies and designed to target human YAP (#4392420, sirna_id: s20367), TAZ (#4390771, sirna_id: n269384) and PKD1(#4392420, sirna_id: s1111), PKD2 (#4390824, sirna_id: s24645) and PKD3 (#4390824, sirna_id: s233). Cells were transfected using the reverse transfection method. Either Silencer Select non-targeting negative control (10 nM) or a target siRNA (10 nM) was mixed with Lipofectamine RNAi MAX according to the manufacturer’s protocol and added to 35mm tissue culture plates. PANC-1 and MiaPaCa-2 cells were then plated on top of the siRNA/ Lipofectamine RNAiMAX complex at a density of 105 cells/well in DMEM containing 10% FBS. Three to four days after transfection, cells were used for experiments.
Quantitative reverse transcription PCR (qRT-PCR)
Relative transcript expression levels of CTGF, CYR61 and CXCL5 were determined by qRT-PCR using a TaqMan Gene Expression Assay. Briefly, total RNA was extracted from cells by using a PureLink RNA Mini Kit. Reverse transcription was performed with the High-Capacity cDNA Reverse Transcription Kit using 1µg of total input RNA. The synthesized cDNA samples were used as templates for the real-time (RT) PCR analysis. All reactions were performed using the Applied Biosystems StepOne system and the amplifications were done using the TaqMan Fast Advanced Master Mix. Gene-specific Homo sapiens oligonucleotide primers for CTGF (Assay ID: Hs01026927_g1), CYR61 (Assay ID: Hs00998500_g1), CXCL5 (Assay ID: Hs01099660_g1) and 18s (Assay ID: Hs03928990_g1) were from Life Technologies, Carlsbad, CA.
[3H]-Thymidine Incorporation into DNA
PANC-1 cells were transfected using the reverse transfection method with either 10nM non-targeted siRNA or a mixture of 5 nM YAP and 5 nM TAZ siRNA. and grown in 3.5 cm tissue culture plates for 5 days in DMEM and 10% FBS. The cultures were then washed twice with DMEM and stimulated with either neurotensin, insulin or a combination of both in serum free DMEM. After 18 h of incubation, the cultures were pulse-labeled for 6 h with [3H]-thymidine (0.25 Ci/ml), fixed with 5% trichloroacetic acid and washed twice with ethanol. Acid-insoluble pools corresponding to DNA were solubilized in 0.1 N NaOH with 1% SDS and counted in a liquid scintillation counter.
Assay of cell proliferation
Cells (4 × 104) were plated on 35 mm tissue culture dishes in DMEM containing 10% FBS. After 24 h of incubation at 37°C, cultures were incubated with DMEM containing 1% FBS in the absence or presence of insulin and neurotensin or the combination of both agonists. After 4 days, cell count was determined from a minimum of 3 dishes per condition using a Coulter counter, after cell clumps were disaggregated by passing the cell suspension 10 times through a 19-gauge, and subsequently, a 21-gauge needle.
For cell colony formation, 103 MiaPaCa-2 or PANC-1 cells were plated into 60 mm tissue culture dishes in DMEM containing 10% FBS. After 24 h of incubation at 37°C, cultures were incubated with DMEM containing 1% FBS either in the absence or presence of insulin, neurotensin or the combination of both drugs. A colony consisted of at least 50 cells. Cell colony numbers from 3 dishes per condition were determined after 8 days of incubation.
Statistical analysis
The one-way ANOVA followed by a Bonferroni t-test (SigmaPlot software) was used to analyze our data. Data were considered significant if P < 0.05 and were presented as average ± SEM.
Materials
DMEM (#11995-073), FBS, goat anti-mouse IgG secondary antibody conjugated to Alexa Fluor 488, Silencer Select siRNAs and all RT-qPCR reagents were obtained from Invitrogen (Carlsbad, CA). Neurotensin and insulin were purchased from Sigma Chemical (St. Louis, MO). The kinase inhibitors A66, CRT0066101, kb NB 142-70 and KU 0063794 were purchased from R&D Systems (Minneapolis, MN). Primary antibodies used were: YAP (63.7): sc-101199 final dilution 1:200 for immunofluorescence, 1:400 for western blotting) and GAPDH (sc-365062; final dilution 1:400) from Santa Cruz Biotechnology, Inc. Dallas, Texas), phospho-YAP Ser127 ((D9W2I #13008; final dilution 1:1000) and phospho-YAP Ser397 (D1E7Y #13619; final dilution 1:1000), phosphor-PKD Ser910 (#2051; final dilution 1:1000), PKD (#2052; final dilution 1:1000) and PKD3 (#5655; final dilution 1:1000) were all from Cell Signaling Technology (Danvers, MA). Horseradish peroxidase–conjugated anti-rabbit IgG and anti-mouse IgG were from GE Healthcare Bio-Sciences Corp (Piscataway, NJ). All other reagents were of the highest grade available.
RESULTS
Insulin and neurotensin promote nuclear localization and dephosphorylation of YAP in PDAC cells
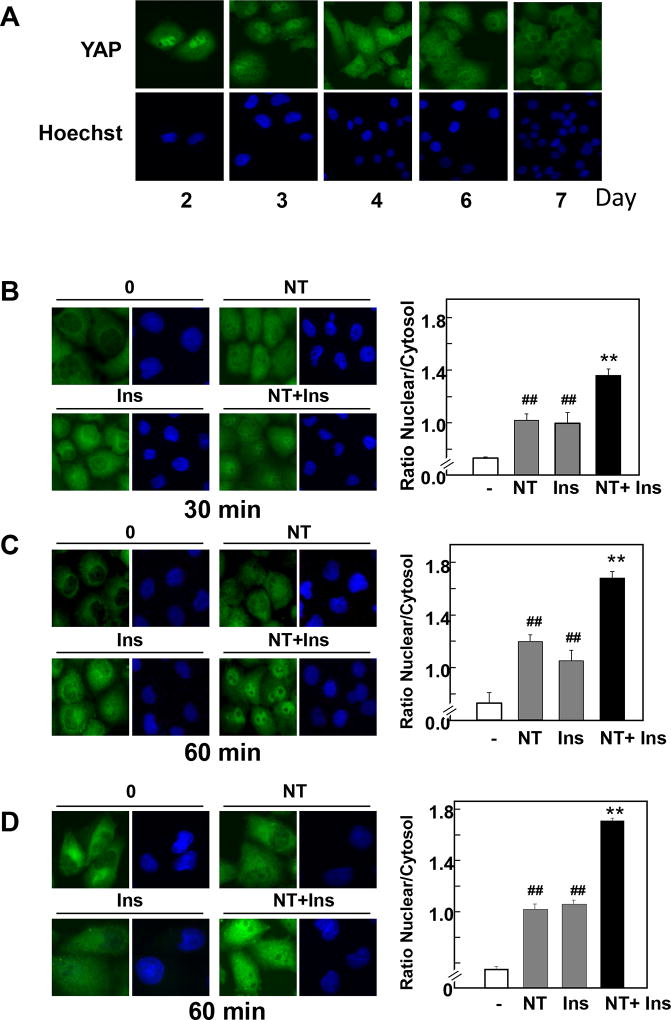
Because cell density regulates YAP localization through the Hippo pathway in a variety of cell types, we initially examined the localization of endogenous YAP in PANC-1 and MiaPaCa-2 cells after various times of plating. At low cell density, (2–4 days after plating) YAP was predominantly localized in the nuclei of these PDAC cells (Fig. 1 A). In contrast, YAP was confined to the cytoplasm (therefore inactive) in confluent cultures of PDAC cells, i.e. 6–7 days after plating.
Figure 1. A combination of neurotensin and insulin promotes YAP nuclear localization in confluent PDAC cells.
A, PANC-1 cells were fixed with 4% paraformaldehyde at various times after plating, as indicated. B and C, Confluent PANC-1 cells were stimulated without (−) or with 5 nM neurotensin (NT), 10 ng/ml insulin (Ins) or their combination (NT+Ins) for 30 min (B) or 60 min (C). D, Confluent MiaPaCa-2 cells were stimulated without (−) or with 5 nM neurotensin (NT), 10 ng/ml insulin (Ins) or NT+Ins for 60 min. In all cases the cultures were then washed, fixed with 4% paraformaldehyde and stained with an antibody that detects total YAP and with Hoechst 33342 to visualize the cell nuclei. Bars represent the ratio of nuclear/cytoplasm (180 to 240 cells) determined as described in Material and Methods. NT + Ins significantly increased nuclear localization of YAP as compared with untreated controls (**P < 0.01) or with either NT or Ins (##P < 0.01) as shown by one-way ANOVA with Bonferroni t-test.
In subsequent experiments, we used confluent PDAC cells to examine whether crosstalk between insulin receptor and GPCR signaling systems reactivates YAP by regulating its localization, phosphorylation and transcriptional co-activator activity. Cultures of PANC-1 cells were transferred to serum-free medium for 16 h and then stimulated with 10 ng/ml insulin, the GPCR agonist neurotensin at 5 nM or their combination to elicit positive crosstalk. In unstimulated cells and in line with the results shown in Fig. 1 A, YAP was detected mainly in the cytoplasm of PANC-1 cells plated at high cell density. Stimulation of these cells with either insulin or neurotensin induced a significant translocation of YAP to the nucleus. Challenging with a combination of insulin and neurotensin promoted striking YAP nuclear localization (Fig. 1 B, C). Quantification of multiple individual cells corroborated that exposure of PANC-1 cells to the combination of insulin and neurotensin was more effective in promoting YAP nuclear localization than each of the agonists added singly (Fig. 1 B, C; right panels). Similar results were obtained when YAP localization was determined in MiaPaCa-2 cells exposed to insulin, neurotensin or their combination (Fig. 1 D).
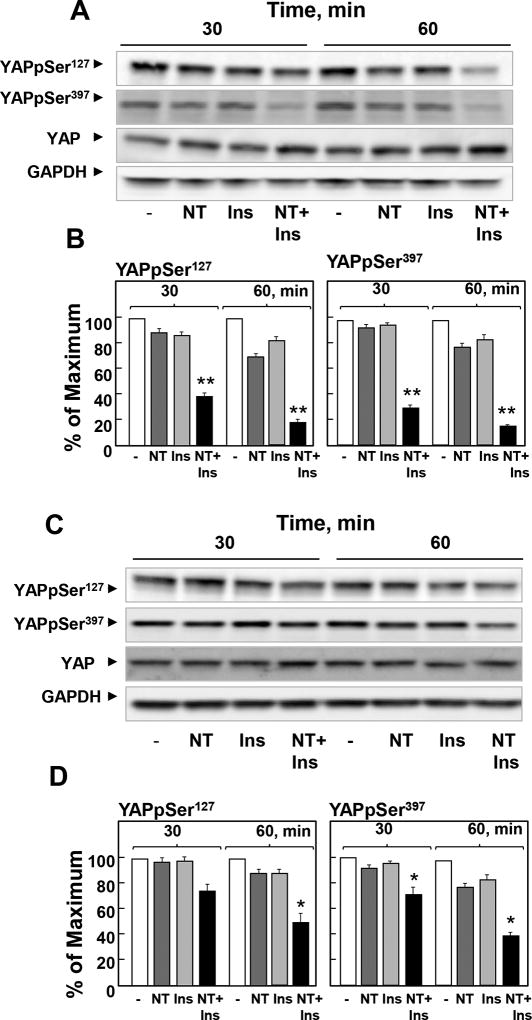
The phosphorylation of YAP at Ser127 plays a major role in restricting its cellular localization to the cytoplasm. Consequently, we determined whether insulin and neurotensin regulate YAP phosphorylation at Ser127 and Ser397, highly conserved residues located within a consensus sequence phosphorylated by the Hippo kinases Lats1/2 (HXRXXS). PANC-1 cells were stimulated with neurotensin, insulin or a combination of these agonists for 30 or 60 min and lysed. Cell lysates were analyzed by Western blotting with antibodies that detect the phosphorylated state of YAP at these residues. As shown in Fig. 2A and Fig. 2B, the combination of neurotensin and insulin induced a rapid decrease in YAP phosphorylation at Ser127 and Ser397 in PANC-1 cells. Similar results were obtained when we used MiaPaCa-2 cells instead of PANC-1 cells (Fig 2C, D). The results in Figs. 1 and 2 indicate that crosstalk between insulin and GPCR signaling pathways rapidly regulates YAP localization and phosphorylation in PDAC cells.
Figure 2. A combination of neurotensin and insulin potently decreases YAP phosphorylation at Ser127 and Ser397 in PDAC cells.
Confluent PANC-1 cells (A and B) or MiaPaca-2 cells (C and D) were stimulated either without (−) or with 5 nM neurotensin (NT), 10 ng/ml insulin (Ins) or their combination (NT+Ins) for 30 or 60 min, as indicated. Cultures were then lysed with 2×SDS–PAGE sample buffer and analyzed by immunoblotting with antibodies that detect YAP phosphorylated at Ser127 and Ser397. Immunoblotting for total YAP and GADPH were also included. B and D, Quantification of total YAP phosphorylated at Ser127 and Ser397 was performed using Multi Gauge V3.0. The results represent the mean ± S.E.; n = 4 (independent experiments) and are expressed as percentage of the maximal level of YAP phosphorylated at Ser127 and Ser397.
NT + Ins significantly decreased YAP phosphorylation at Ser127 and Ser397 as compared with untreated controls: **P < 0.01; *P < 0.05 (one-way ANOVA with Bonferroni t-test).
Insulin and neurotensin stimulate YAP/TEAD-regulated gene expression in PDAC cells
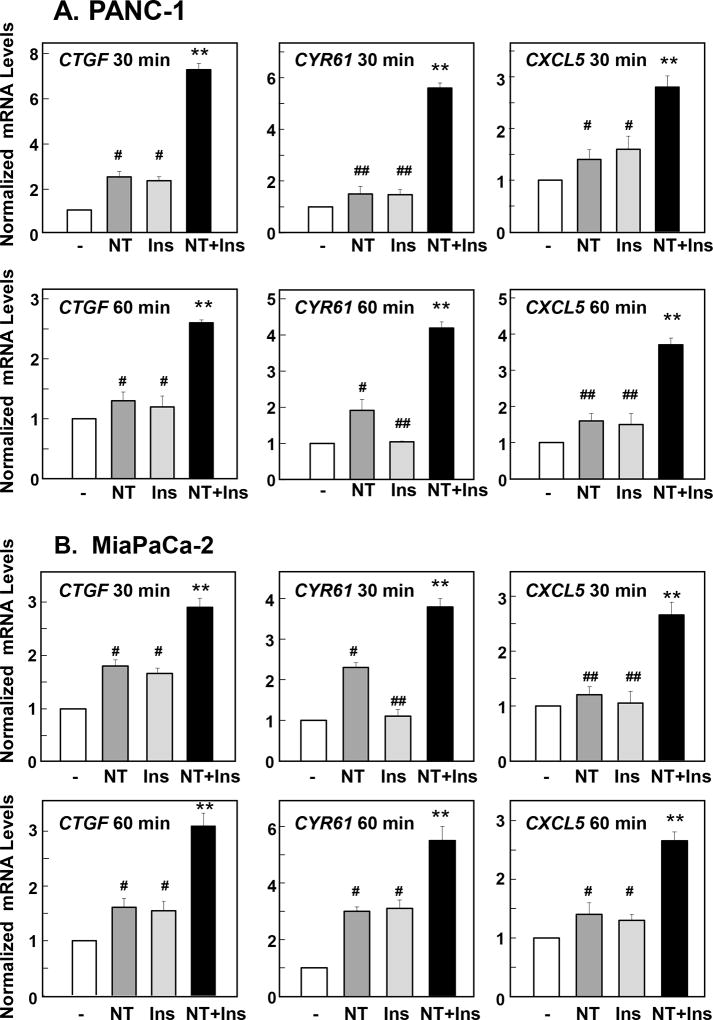
We next determined whether the rapid nuclear import and dephosphorylation of YAP in response to crosstalk between insulin receptor and GPCR signaling systems promotes its coupling to the transcription factors of the TEA domain–containing transcription factors (TEAD1–4) and thereby stimulate the expression of YAP/TEAD-regulated genes, including Connective Tissue Growth Factor (CTGF), and Cysteine-rich angiogenic inducer 61 (CYR61). Indeed, CTGF is one of the best-characterized direct target gene of YAP that contains three putative YAP-TEAD binding sites (GGAATG) in its promoter region. As shown in Fig. 3 A B, stimulation of either PANC-1 or MiaPaCa-2 cells for 30 min or 60 min with a combination of neurotensin and insulin induced a marked increase in the levels of CTGF and CYR61 transcripts, as determined by qRT-PCR. The combination of insulin and neurotensin was significantly more effective in promoting CTGF and CYR61 expression than each of the agonists added singly (Fig. 3 A B).
Figure 3. Neurotensin and Insulin stimulate YAP/TEAD-regulated gene expression in PDAC.
Confluent PANC-1 cells (A) or MiaPaca-2 cells (B) were stimulated either without (−) or with 5 nM neurotensin (NT), 10 ng/ml insulin (Ins) or their combination (NT+Ins) for 30 or 60 min as indicated. RNA was isolated and the relative levels (n=3) of CTGF, CYR61 or CXCL5 mRNA compared with 18s mRNA was measured by RT-qPCR. Data are presented as mean ± SEM. Similar results were obtained in 3 independent experiments. NT + Ins significantly increased relative mRNA levels of CTGF, CYR61 or CXCL5 as compared with untreated controls (**P < 0.01) or with either NT or Ins (##P < 0.01; #P<0.05) as shown by one-way ANOVA with Bonferroni t-test.
CXCL5, a chemokine produced by pancreatic cancer cells (29), has been proposed to promote infiltration by polymorphonuclear myeloid-derived suppressor cells (MDSC) into cancer tissues. Recently, CXCL5 has been identified as a novel YAP/TEAD-regulated gene in prostate cancer cells (30). We determined whether CXCL5 mRNA levels are also rapidly enhanced by insulin and neurotensin in PDAC cells. As shown in Fig. 3 A, B, stimulation of PANC-1 or MiaPaCa-2 cells with a combination of neurotensin and insulin markedly increased the levels of CXCL5 mRNA in these cells.
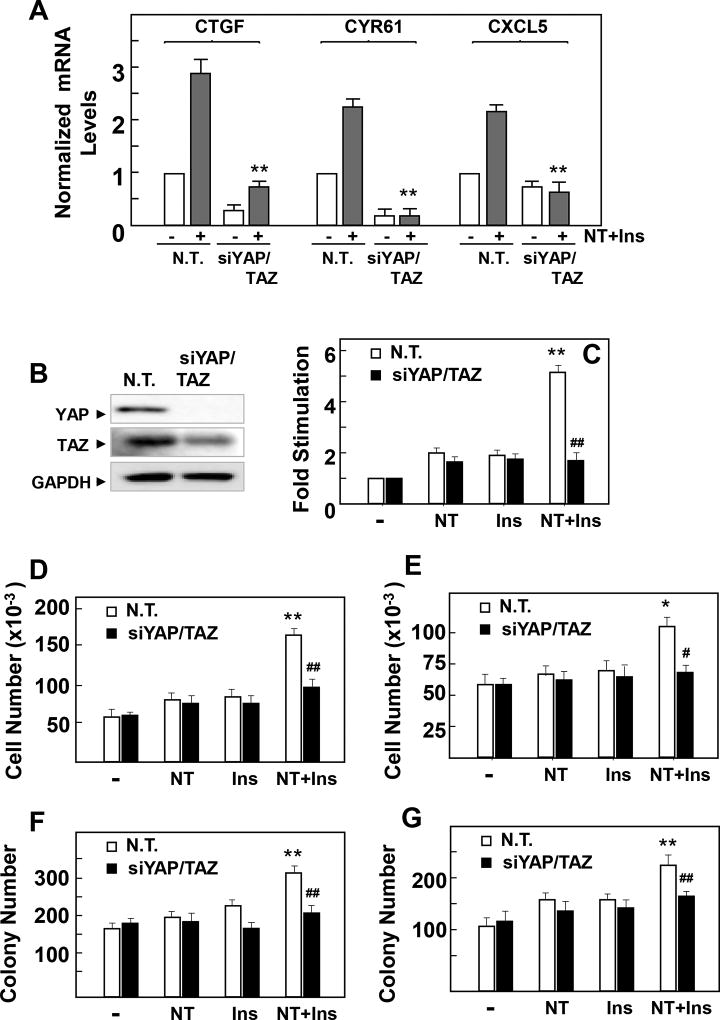
In order to verify that the increase in the expression of these early response genes is mediated by stimulation of YAP/TAZ co-activator activity, we determined whether siRNA-mediated knockdown of YAP/TAZ prevents the increase in the expression of CTGF, CYR61 and CXCL5. Transient transfection of PANC-1 cells with siRNAs targeting YAP and TAZ prevented the increase in CTGF, CYR61 and CXCL5 mRNA levels induced by challenging these cells with insulin and neurotensin (Fig. 4 A) and virtually extinguished the detection of YAP protein (Fig. 4 B). Collectively, these results indicate that YAP/TAZ activation is a novel early point of transcriptional convergence in the crosstalk between GPCR and insulin signaling pathways in PDAC cells.
Figure 4. Neurotensin and Insulin induce increases in CTGF, CYR61 and CXCL5 gene expression and stimulate cell proliferation and colony via YAP/TAZ in PDAC cells.
A and B, Cultures of PANC-1 cells were transfected with non-targeting siRNA (N. T.) or with siRNAs targeting YAP and TAZ (siYAP/TAZ). A, Cultures were then stimulated either without (−) or with NT+Ins (+) for 60 min. RNA was isolated and the relative levels (n=3) of CTGF, CYR61 or CXCL5 mRNA compared with 18s mRNA was measured by RT-qPCR. Data are presented as mean ± SEM. Similar results were obtained in 3 independent experiments. **P < 0.01, NT+I si RNA YAP/TAZ versus non-targeting siRNA (one-way ANOVA with Bonferroni t-test). B, Cultures were lysed with 2×SDS–PAGE sample buffer and analyzed by immunoblotting with antibodies that detect total YAP, TAZ and GAPDH. C, Cultures of PANC-1 cells were transfected with non-targeting siRNA (N. T.) or with siRNAs targeting YAP and TAZ (siYAP/TAZ) for 4 days. Then, the cultures were stimulated with 5 nM neurotensin (NT), 10 ng/ml insulin (Ins) or NT+Ins for 18 h and pulse-labeled with [3H]-thymidine (0.25 µCi/ml) for 6 h. The radioactivity incorporated into acid-insoluble pools was measured in a scintillation counter. Similar results were obtained in 3 independent experiments. **P < 0.01, NT+I vs control, NT or Ins; ## P< 0.001 NT+Ins YAP/TAZ siRNA versus NT+Ins non-targeting siRNA (one-way ANOVA with Bonferroni t-test). D and E, PANC-1 (D) MiaPaCa-2 (E) cells ransfected with non-targeting siRNA (N. T.) or with siRNAs targeting YAP and TAZ (siYAP/TAZ) were plated at a density of 4 × 104 cells per dish. After 24 hours, the cultures were shifted to media containing 1 % FBS either without (−) or with 5 nM neurotensin (NT), 10 ng/ml insulin (Ins) or their combination (NT+Ins). After 4 days, cell numbers were determined from 3 plates per condition. Results are presented as mean ± SEM n=3, Similar results were obtained in 2 separate independent experiments. **P < 0.01, * P < 0.05, NT+I vs control, NT or Ins; ## P< 0.001, # P < 0.05, NT+Ins YAP/TAZ siRNA versus NT+Ins non-targeting siRNA (one-way ANOVA with Bonferroni t-test). F and G, Colony formation by PANC-1 (F) or MiaPaCa-2 (G) cells transfected with non-targeting siRNA (N. T.) or with siRNAs targeting YAP and TAZ (siYAP/TAZ) was performed as described in the Materials and Methods section. Results are presented as mean ± SEM n=3, **P < 0.01, NT+I vs control, NT or Ins; ## P< 0.001, NT+Ins YAP/TAZ siRNA vs NT+Ins non-targeting siRNA (one-way ANOVA with Bonferroni t-test). Similar results were obtained in 2 separate independent experiments
Subsequently, we determined whether the increase in the activity of YAP/TAZ is required for the stimulation of DNA synthesis and cell proliferation induced by insulin and neurotensin in PDAC cells. As shown in Fig. 4 C, stimulation with insulin and neurotensin of control PANC-1 cells (either untreated cells or cells transiently transfected with non-targeting oligonucleotides) induced a marked increase in 3H-thymidine incorporation into DNA. Knockdown of YAP/TAZ sharply reduced the increase in DNA synthesis induced by the combination of insulin and neurotensin (from ~ 5-fold to 1.8-fold). In order to substantiate the results obtained with 3H-thymidine incorporation, we next examined whether YAP plays a role in the proliferation of PDAC cells in response to long-term exposure to insulin and neurotensin. The combination of these stimuli promoted a marked increase in the number of PANC-1 (Fig. 4 D) and MiaPaCa-2 (Fig. 4 E) cells. Similarly, insulin and neurotensin enhanced the ability to form colonies by these cells (Fig. 4 F, G). Knockdown of YAP/TAZ averted the increase in cell number (Fig. 4 D, E) and colony formation (Fig. 4 F, G) induced by insulin and neurotensin in these PDAC cells. Collectively, these results indicate that YAP/TAZ activity plays a critical role in mediating the growth-promoting crosstalk between insulin receptor and GPCR signaling pathways in PDAC cells.
Role of PI3K/mTOR in YAP activation by insulin and neurotensin in PDAC cells
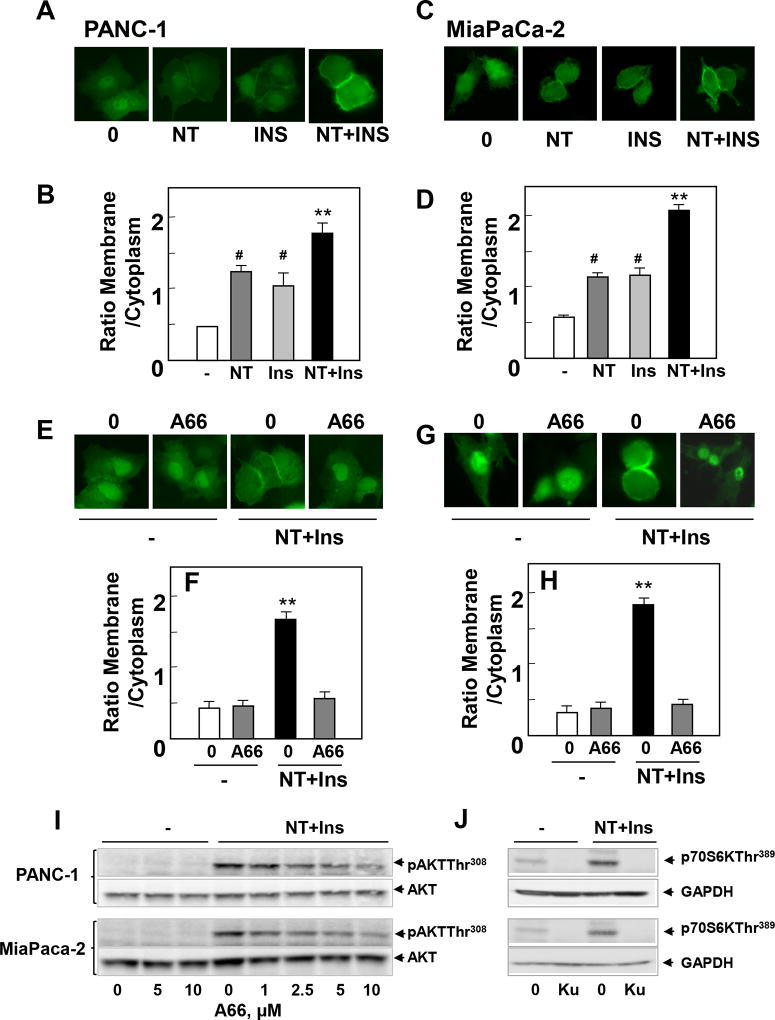
We next explored the mechanisms by which crosstalk between insulin receptor and GPCR signaling stimulate YAP/TEAD activity in PDAC cells. A key pathway in insulin receptor signaling is phosphatidylinositol 3-kinase (PI3K) leading to AKT/mTOR activation but it is not known whether PI3Ks are a point of convergence in the crosstalk between insulin/IGR-1R and GPCR signaling systems in PDAC cells. In order to examine the effect of crosstalk between insulin receptor and GPCR signaling systems on PI3K activity in single cells, PANC-1 cells were transiently transfected with a plasmid encoding a fusion protein between GFP and the PH domain of AKT (AKT-PH-GFP), an in vivo reporter of phosphatidylinositol 3,4,5-trisphosphate (PIP3) in the plasma membrane of individual PDAC cells. In most unstimulated PANC-1 and MiaPaCa-2 cells, the PIP3 sensor displays little localization at the plasma membrane. Stimulation with insulin or neurotensin markedly stimulated PI3K activity, as judged by the increase in the translocation of the PIP3 sensor to the plasma membrane of PANC-1 (Fig 5 A; quantification in Fig. 5 B) and MiaPaCa-2 cells (Fig 5 C ; quantification in Fig. 5 D).
Figure 5. The selective PI3K inhibitor A66 suppresses PIP3 accumulation, AKT phosphorylation at Thr308 and YAP/TAZ-regulated gene expression in PDAC cells.
A–D. PANC-1 (A and B) and MiaPaCa-2 cells (C and D) were transiently transfected with a plasmid encoding a fusion protein between GFP and the PH domain of Akt (Akt-PH-GFP). The cultures were then stimulated with 5 nM neurotensin (NT), 10 ng/ml insulin (Ins) or NT+Ins for 5 min. The intracellular distribution of Akt-PH-GFP was monitored under a fluorescence microscope. The cells shown in A and C were representative of 90% of GFP-positive cells. B and D, Bars represent the ratio of membrane/cytoplasm (40 to 60 cells) determined as described in Material and Methods. **P<0.001, NT+I vs control; # P< 0.05, NT+Ins vs NT or Ins (one-way ANOVA with Bonferroni t-test). E–H. PANC-1 (E and F) and MiaPaCa-2 cells (G and H), transiently transfected with Akt-PH-GFP (as above), were incubated in the absence or in the presence of A66 at 10µM in DMEM for 1h prior to stimulation with NT+Ins for 5 min. The intracellular distribution of Akt-PH-GFP was monitored under a fluorescence microscope. The selected cells shown in E and G were representative of 95% of the population of GFP-positive cells. F and H, Bars represent the ratio of membrane/cytoplasm (40 to 60 cells) determined as described in Material and Methods **P<0.001, NT+I vs NT+Ins + A66 (one-way ANOVA with Bonferroni t-test). I Confluent cultures of PANC-1 or MiaPaCa-2 cells were incubated in the absence (0) or presence of increasing concentrations of A66 for 1 h in DMEM prior to stimulation with NT+Ins for 10 min. Cultures were then lysed with 2×SDS–PAGE sample buffer and analyzed by immunoblotting with antibodies that detect AKT phosphorylated at Thr308 and total AKT. Similar results were obtained in 2 independent experiments. J Confluent cultures of PANC-1 or MiaPaCa-2 cells were incubated in the absence (0) or presence of 5 µM KU63794 for 1 h in DMEM prior to stimulation with NT+Ins for 10 min. Cultures were then lysed with 2×SDS–PAGE sample buffer and analyzed by immunoblotting with antibodies that detect p70S6K phosphorylated at Thr389 and GADPH. Similar results were obtained in 2 independent experiments. K and L, Confluent cultures of PANC-1 (K) and MiaPaCa-2 cells (L) were incubated in the absence or in the presence of A66 at 10µM in DMEM for 1h prior to stimulation with 5 nM neurotensin (NT), 10 ng/ml Insulin (Ins) or NT+Ins for 60 min. RNA was isolated and the relative levels (n=3) of CTGF, CYR61 or CXCL5 mRNA compared with 18s mRNA was measured by RT-qPCR. Data are presented as mean ± SEM n=3. Similar results were obtained in 3 independent experiments. **P<0.001, NT+I vs NT+Ins + A66 (one-way ANOVA with Bonferroni t-test).
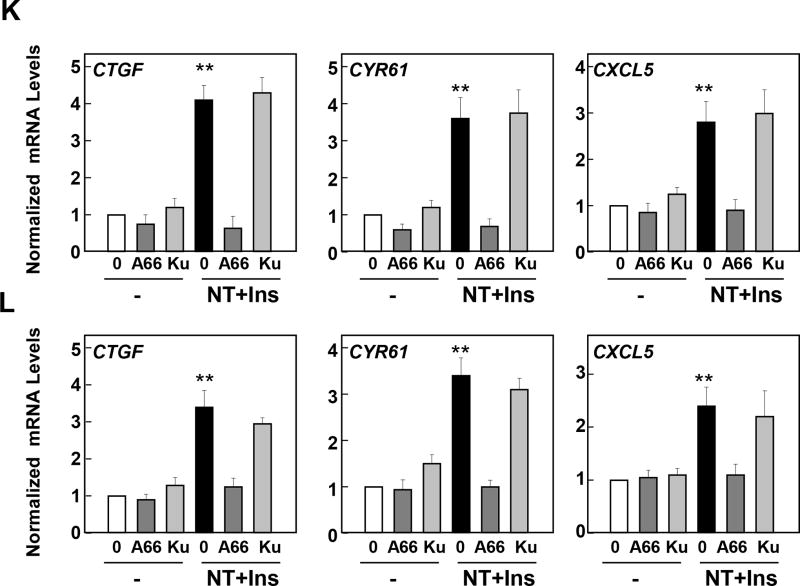
Subsequently, we determined the contribution of PI3K to the synergistic stimulation of YAP/TEAD-regulated genes CTGF, CYR61 and CXCL5 induced by insulin and neurotensin in PANC-1 and MiaPaCa-2 cells. We used A66, a highly selective inhibitor of the catalytic activity of the PI3Ks with preferential inhibitory activity against the p110α catalytic subunit of PI3K (31). Indeed, in cell-free assays, A66 at 10 µM only inhibited PI3K isoforms when tested against a panel of 318 protein kinases (31). Treatment of intact PANC-1 with 10 µM A66 prevented the increase in the localization of the PIP3 sensor to the plasma membrane (Fig 5 E; quantification in Fig. 5 F) or MiaPaCa-2 (Fig 5 G; quantification in Fig. 5 H) cells induced by insulin and neurotensin. A66 impeded AKT-PH-GFP localization to the plasma membrane in a dose-dependent manner with inhibitory effects at 2.5 µM and 5 µM (Supplemental Fig. S1). Accordingly, treatment of PANC-1 or MiaPaCa-2 cells with increasing concentrations of A66 prior to stimulation with insulin and neurotensin greatly attenuated AKT phosphorylation at Thr308, a site targeted by phosphoinositide-dependent protein kinase 1 (PDK1), a kinase activated by PIP3 (Fig 5 I). Maximal inhibitory effect of A66 on AKT phosphorylation at Thr308 was achieved at 10 µM in intact PANC-1 and MiaPaCa-2 cells (84 % and 87% inhibition, respectively). Importantly, treatment with A66 at 10 µM also blocked the increase in the expression of the YAP/TEAD-regulated genes CTGF, CYR61 and CXCL5 induced by insulin and neurotensin in PANC-1 (Fig 5 K) or MiaPaCa-2 (Fig 5 L). Inhibition of CTGF, CYR61 and CXCL5 expression was also observed when A66 was added at 2.5 µM or 5 µM with maximal effect achieved at 10 µM (Supplemental Fig. S1). These results imply that PI3K isoforms contribute to YAP activation in response to crosstalk between insulin/IGF1R and GPCR signaling pathways in PDAC cells.
Since PI3K leads to mTORC1 and mTORC2 activation in PDAC cells, we determined whether mTOR plays a role in mediating the increase in the expression of CTGF, CYR61 and CXCL5. Treatment with the active-site mTOR inhibitor KU63794 (32), did not interfere with the increase in the expression of CTGF, CYR61 and CXCL5 in PANC-1 or MiaPaCa-2 cells (Fig. 5 K, L). We verified that KU63794, at the concentration used, suppressed mTOR activation by insulin and neurotensin in PANC-1 and MiaPaCa-2 cells, as scored by inhibition of p70S6K phosphorylation at Thr389, a direct target of mTORC1 (Fig. 5 J). In agreement with results in other cell types (33, 34), we conclude that a PI3K-dependent pathway acting upstream of mTOR leads to YAP/TEAD activation in response to insulin and neurotensin in PDAC cells.
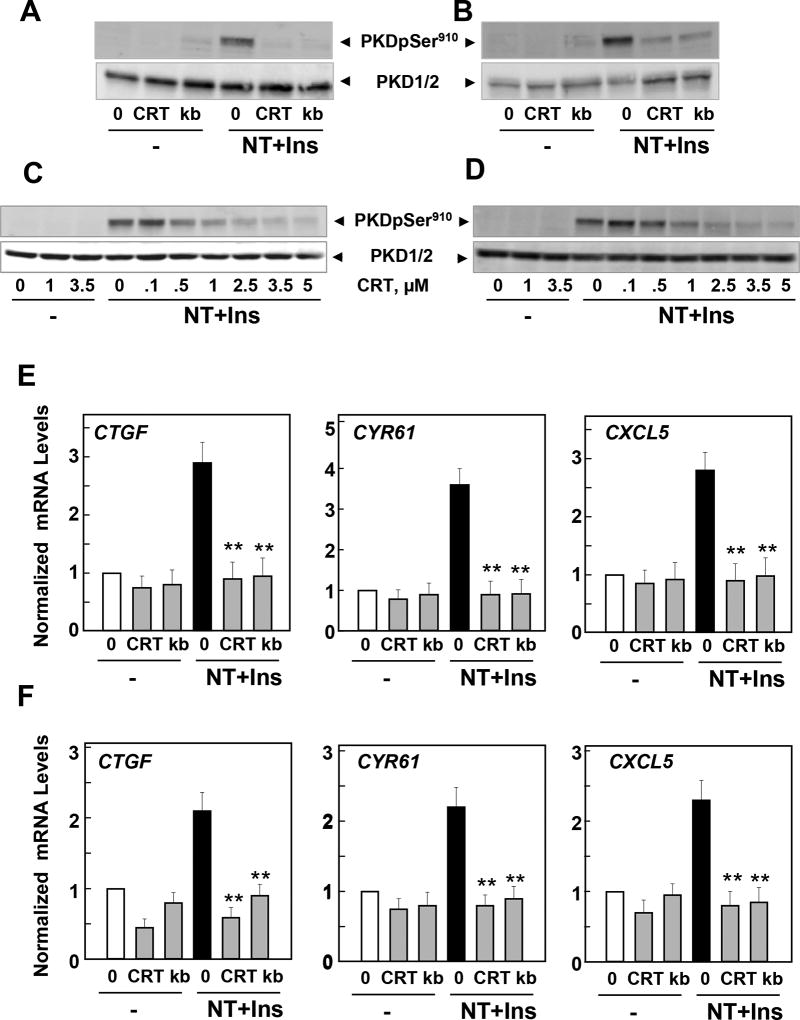
Role of the PKD family in mediating YAP activation in response to neurotensin and insulin in PDAC cells
We previously demonstrated that neurotensin increases PKD family (PKD1, PKD2 and PKD3) activity in PDAC cells (6, 35, 36) and induces proliferation of these cells in a PKD-dependent manner (37, 38). Here, we show that the combination of neurotensin and insulin potently stimulated PKD activity in PANC-1 (Fig. 6 A) and MiaPaCa-2 (Fig.6 B), as scored by the increase in its autophosphorylation at Ser910. To determine the role of PKD in PDAC cell signaling we used two different PKD family inhibitors with different structure, to minimize possible off-target effects. Treatment of intact PANC-1 and MiaPaCa-2 cells with the PKD inhibitors CRT0066101 (2.5 µM) or kb NB 142-70 (3.5µM) prevented PKD activation (scored by autophosphorylation at Ser910) in these cells in response to challenge with insulin and neurotensin (Fig. 6 A, B). Further studies showed that treatment with CRT0066101 counteracted PKD activation in a dose-dependent manner in PANC-1 and MiaPaCa-2 cells stimulated with insulin and neurotensin. Maximal inhibition was achieved at 2.5 µM in these cells (Fig. 6 C, D). Recently, we implicated the PKD family in the regulation of YAP/TEAD activity in intestinal epithelial cells (25) but the role of the PKDs in YAP activation in response to growth-promoting factors in PDAC cells has not been examined. Consequently, we next determined whether the PKDs contribute to mediate YAP activation in PDAC cells challenged with neurotensin and insulin. Exposure of intact PDAC cells to the structurally unrelated PKD family inhibitors CRT0066101 and kb NB 142-70 prevented the increase of CTGF, CYR61 and CXCL5 mRNA levels induced by insulin and neurotensin in PANC1 (Fig. 6E) and MiaPaCa-2 cells (Fig. 6F). These results imply that the PKDs contribute to the stimulation of YAP/TEAD transcriptional activity in response to crosstalk signaling in PDAC cells.
Figure 6. Neurotensin and Insulin induce CTGF, CYR61 or CXCL5 expression through PKDs in PDAC cells.
A and B, Confluent cultures of PANC-1 (A) or MiaPaCa-2 cells (B) were incubated in the absence (0) or presence of either 2.5 µM CRT0066101 (CRT) or 3.5 µM kb NB 142–70 (kB) for 1 h in DMEM prior to stimulation with 5 nM neurotensin (NT) and 10 ng/ml Insulin (Ins) for 10 min. Cultures were then lysed with 2×SDS–PAGE sample buffer and analyzed by immunoblotting with antibodies that detect PKD1 phosphorylated at Ser910 and total PKD1/2. Similar results were obtained in 3 independent experiments. C and D, Confluent cultures of PANC-1 (C) or MiaPaCa-2 cells (D) were incubated in the absence (0) or presence of increasing concentrations of CRT0066101 (CRT) for 1 h in DMEM prior to stimulation with NT+Ins for 10 min. PKD1 phosphorylation was determined as above. E and F, Confluent cultures of PANC-1 (E) and MiaPaCa-2 cells (F) were incubated in the absence (0) or presence of either 2.5 µM CRT0066101 (CRT) or 3.5 µM kb NB 142–70 (kB) for 1 h in DMEM prior to stimulation with NT+Ins for 60 min. RNA was isolated and the relative levels (n=3) of CTGF, CYR61 or CXCL5 mRNA compared with 18s mRNA was measured by RT-qPCR. Data are presented as mean ± SEM. Similar results were obtained independent experiments. Treatment with CRT0066101 or kb NB 142–70 significantly decreased relative mRNA levels of CTGF, CYR61 or CXCL5 as compared with untreated NT+Ins, **P < 0.01 as shown by one-way ANOVA with Bonferroni t-test.
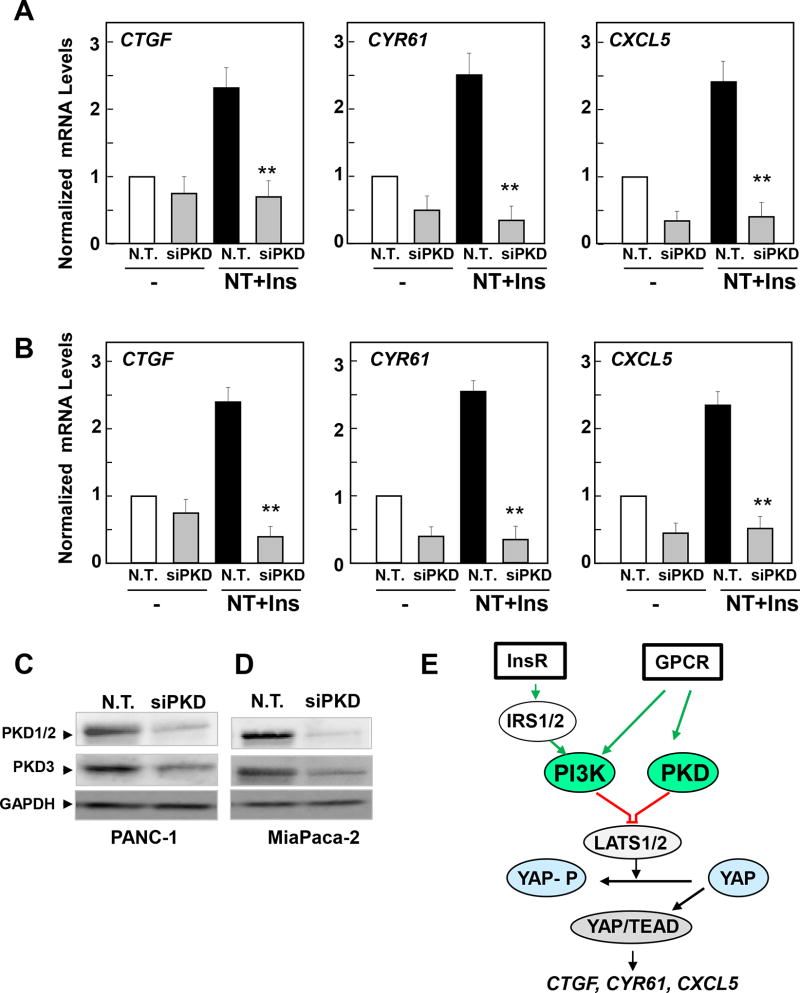
In consonance with the results obtained with chemical inhibitors in Fig. 6, transfection of a mixture of siRNAs targeting PKD1, PKD2 and PKD3 abrogated the increase in CTGF, CYR61 and CXCL5 expression in response to insulin and neurotensin stimulation in PANC1 (Fig. 7A) and MiaPaCa-2 cells (Fig. 7B). We verified that the mixture of siRNAs markedly decreased the expression of the PKDs in these cells (Fig. 7 C, D). Collectively, our findings identify a novel function for PKDs in mediating YAP/TEAD transcriptional activity in response to insulin and neurotensin in PDAC cells (Fig. 7 E).
Figure 7. Knockdown of PKD family expression prevents neurotensin and insulin induced increases in CTGF, CYR61 or CXCL5 in PDAC cells.
Cultures of PANC-1 (A) and MiaPaCa-2 cells (B) were transfected with non-targeting siRNA (N. T.) or with a mixture of siRNAs targeting PKD1, PKD2 and PKD3 (siPKD). The cultures were then stimulated without (−) or with 5 nM neurotensin (NT), 10 ng/ml insulin (Ins) or NT+Ins for 60 min. RNA was isolated and the relative levels (n=3) of CTGF, CYR61 or CXCL5 mRNA compared with 18s mRNA was measured by RT-qPCR. Data are presented as mean ± SEM. Similar results were obtained in 5 independent experiments for PANC-1 cells and 3 independent experiments for MiaPaCa-2 cells. siRNA transfection of PKD significantly decreased relative mRNA levels of CTGF, CYR61 or CXCL5 as compared with non-targeted controls, **P < 0.01 (one-way ANOVA with Bonferroni t-test). Cultures of PANC-1 (C) and MiaPaCa-2 (D) cells were transfected with non-targeting siRNA (N. T.) or with a mixture of siRNAs targeting PKD1, PKD2 and PKD3 (siPKD) for 4 days. Cells were then lysed with 2×SDS–PAGE sample buffer and analyzed by immunoblotting with antibodies that detect total PKD1/2, PKD3 and GAPDH. E, Scheme representing a model of YAP/TEAD activation in response to PI3K and PKD activation induced by crosstalk between insulin receptor and GPCR signaling systems in PDAC cells.
DISCUSSION
Many studies have indicated that crosstalk between the insulin/IGF-1 receptor and GPCR signaling systems plays a critical role in the regulation of a variety of normal and abnormal processes, including cardiovascular and renal pathologies in obesity, metabolic syndrome and T2DM. GPCR agonists also mediate autocrine/paracrine growth stimulation in a variety of cancer cells and dramatically synergize with insulin/IGF-1 in inducing growth-promoting signaling. Accordingly, our previous studies identified crosstalk mechanisms between insulin/IGF-1 receptor and GPCR signaling systems that stimulate DNA synthesis and cell proliferation in human PDAC cells (15, 17, 39). Consequently, we hypothesized that crosstalk between insulin/IGF-1 receptor and GPCR signaling systems is a mechanism for promoting the development of PDAC (16), the 3rd leading cause of cancer-related mortality in the USA (1). However, a major gap in our current understanding is the identity of the key downstream transcriptional effectors in signaling crosstalk that drive PDAC development.
The highly conserved YAP/TAZ pathway is attracting intense interest as a key regulator in cancer cell biology. Preclinical studies indicated that YAP is a key downstream target of K-Ras signaling required for acinar-to-ductal metaplasia (ADM) and subsequent PanIN progression into PDAC (40, 41). Furthermore, amplification and overexpression of Yap can substitute for mutant K-Ras expression in PDAC (26). These studies indicate that Yap acts downstream of K-Ras and that its hyper-activation can compensate for the need of K-Ras mutant expression in PDAC (42). Several studies indicate that YAP and TAZ are active in PDAC patient tumor samples (26–28). However, the impact of crosstalk between insulin/IGF-1R and GPCR signaling pathways on YAP/TAZ activity had not been previously examined in PDAC cells or in any other cell type.
The phosphorylation of YAP is thought to restrict its activity, cellular localization and stability. In the absence of phosphorylation, YAP localizes to the nucleus where it binds and activates the TEA-domain DNA-binding transcription factors (TEAD 1–4) thereby stimulating the expression of a variety of genes. Typical YAP/TEAD-regulated genes include CTGF, CYR61/CCN1 and the cytokine CXCL5. The products of these genes have a major impact on important cell processes, including proliferation, survival and differentiation as well as in shaping the micro-environment (CTGF), promoting angiogenesis (CYR61) and mediating communication between cancer and immune cells (CXCL5). The results presented here demonstrate that stimulation of confluent cultures of PDAC cells with a combination of insulin and the GPCR agonist neurotensin induces rapid YAP nuclear localization, decreases YAP phosphorylation at Ser127 and promotes expression of YAP/TEAD-regulated genes, including CTGF, CYR61 and CXCL5. We conclude that activation of YAP/TEAD is a novel early point of transcriptional convergence in the crosstalk between GPCR and insulin receptor signaling pathways in PDAC cells.
We next explored the mechanism(s) that mediate YAP activation in response to PDAC cell stimulation with insulin and neurotensin. Recently, p110α PI3K has been identified as a major signaling enzyme for PDAC development (43) and PI3K isoforms play a key role in insulin receptor signaling. Here, we show that neurotensin and insulin rapidly stimulated PI3K activity, as judged by the increase in the translocation of the PIP3 sensor GFP-PH-AKT to the plasma membrane of PDAC cells. A66, a selective inhibitor of PI3K with preferential activity against p110α PI3K (31), prevented the accumulation of the PIP3 sensor at the plasma membrane and AKT phosphorylation at Thr308 in a dose-dependent manner. At similar concentrations, A66 abrogated the increase in the expression of YAP/TEAD-regulated genes. We conclude that PI3K-mediated formation of PIP3 contributes to YAP activation in response to crosstalk between insulin receptor and GPCR signaling pathways in PDAC cells (Fig. 7E).
PKDs have been identified as a major downstream signaling node in GPCR signaling (4, 45). PKDs are rapidly activated by GPCR agonists in PDAC cells (6, 35, 36) and are over-expressed in PDAC tissues (46). Induced PKD over-expression in PDAC cells promotes cell proliferation (37), invasion (38) and acinar to ductal trans-differentiation (47). Here, we examined whether PKDs are implicated in the regulation of YAP activity in PDAC cells. Using structurally unrelated PKD family inhibitors and siRNA-mediated knockdown of the PKDs, we identify a novel role of the PKDs in the stimulation of YAP/TEAD transcriptional activity in response to crosstalk signaling in PDAC cells (Fig. 7E).
Our findings have potential translational implications. Obesity and T2DM, which are prominent in the western world, have been linked to increased risk and worse clinical outcomes for developing PDAC (16, 18, 19). These diet-related metabolic disorders are multifaceted but characterized by peripheral insulin resistance and compensatory overproduction of insulin. In turn, neurotensin has been implicated in the pathogenesis of obesity (21). Therefore, our results showing that crosstalk between insulin and neurotensin stimulates YAP/TEAD via PI3K and PKD not only reveal a potential mechanism linking obesity, T2DM and PDAC but also identify potential targets highly relevant in PDAC. A variety of pharmacologic inhibitors directed against the PI3K pathway are now available for clinical investigation (48) and orally active PKD inhibitors, including CRT0066101(46), are being identified. Our results imply that combinations of PI3K and PKD inhibitors could provide a novel strategy for targeting YAP and for disrupting growth-promoting crosstalk between insulin/IGF-1R and GPCR signaling in PDAC.
In conclusion, we show that stimulation of PDAC cells with insulin and the GPCR agonist neurotensin induces rapid nuclear import and dephosphorylation of YAP and marked increase in the expression of YAP/TEAD-regulated genes through PI3K and PKD dependent pathways (Fig. 7 E). We propose that YAP is a central node of transcriptional convergence in the crosstalk between insulin receptor and GPCR signaling systems and of major importance in the proliferative response induced by these growth-promoting agonists in PDAC cells.
Supplementary Material
Implications.
Inhibitors of PI3K or PKD disrupt crosstalk between insulin receptor and GPCR signaling systems by blocking YAP/TEAD-regulated gene expression in pancreatic cancer cells.
Acknowledgments
This work was supported by NIH Grants R01DK100405, P30DK41301 and P01CA163200 and by the Department of Veterans Affair Grant 1I01BX001473 (to ER). Additional funding came from the Ronald S. Hirshberg Endowed Chair of Pancreatic Cancer Research to ER. We also thank the Imaging and Stem Cell Biology Core of the CURE: Digestive Diseases Research Center (P30DK41301) for their assistance. Fang Hao and Qinhong Xu were recipients of grants from the China Scholarship Council.
Footnotes
Conflict of interest - The authors declare that they have no conflicts of interest with the contents of this article.
Author contributions - ER and JSS are responsible for conception and design of the research; FH, QX, JSS, JVS and SHY performed the experiments; FH, JSS and ER analyzed the data; ER and JSS interpreted the results of the experiments; JSS and FH prepared the figures; ER drafted the manuscript; JSS, SHY, and ER edited and revised the manuscript; ER approved the final version of the manuscript.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: A Cancer Journal for Clinicians. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Mosquera C, Maglic D, Zervos EE. Molecular targeted therapy for pancreatic adenocarcinoma: A review of completed and ongoing late phase clinical trials. Cancer Genet. 2016;209:567–81. doi: 10.1016/j.cancergen.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Rozengurt E, Walsh JH. Gastrin, CCK, signaling, and cancer. Annu Rev Physiol. 2001;63:49–76. doi: 10.1146/annurev.physiol.63.1.49. [DOI] [PubMed] [Google Scholar]
- 4.Heasley LE. Autocrine and paracrine signaling through neuropeptide receptors in human cancer. Oncogene. 2001;20:1563–9. doi: 10.1038/sj.onc.1204183. [DOI] [PubMed] [Google Scholar]
- 5.Ryder NM, Guha S, Hines OJ, Reber HA, Rozengurt E. G protein-coupled receptor signaling in human ductal pancreatic cancer cells: Neurotensin responsiveness and mitogenic stimulation. J Cell Physiol. 2001;186:53–64. doi: 10.1002/1097-4652(200101)186:1<53::AID-JCP1004>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 6.Guha S, Rey O, Rozengurt E. Neurotensin Induces Protein Kinase C-dependent Protein Kinase D Activation and DNA Synthesis in Human Pancreatic Carcinoma Cell Line PANC-1. Cancer Res. 2002;62:1632–40. [PubMed] [Google Scholar]
- 7.Guha S, Lunn JA, Santiskulvong C, Rozengurt E. Neurotensin Stimulates Protein Kinase C-dependent Mitogenic Signaling in Human Pancreatic Carcinoma Cell Line PANC-1. Cancer Res. 2003;63:2379–87. [PubMed] [Google Scholar]
- 8.Kisfalvi K, Guha S, Rozengurt E. Neurotensin and EGF induce synergistic stimulation of DNA synthesis by increasing the duration of ERK signaling in ductal pancreatic cancer cells. J Cell Physiol. 2005;202:880–90. doi: 10.1002/jcp.20187. [DOI] [PubMed] [Google Scholar]
- 9.Seckl MJ, Higgins T, Widmer F, Rozengurt E. [D-Arg1,D-Trp5,7,9,Leu11]substance P: a novel potent inhibitor of signal transduction and growth in vitro and in vivo in small cell lung cancer cells. Cancer Res. 1997;57:51–4. [PubMed] [Google Scholar]
- 10.Sinnett-Smith J, Santiskulvong C, Duque J, Rozengurt E. [D-Arg(1),D-Trp(5,7,9),Leu(11)]substance P inhibits bombesin-induced mitogenic signal transduction mediated by both G(q) and G(12) in Swiss 3T3 cells] J Biol Chem. 2000;275:30644–52. doi: 10.1074/jbc.M003702200. [DOI] [PubMed] [Google Scholar]
- 11.Guha S, Eibl G, Kisfalvi K, Fan RS, Burdick M, Reber H, et al. Broad-spectrum G protein-coupled receptor antagonist, [D-Arg1,D-Trp5,7,9,Leu11]SP: a dual inhibitor of growth and angiogenesis in pancreatic cancer. Cancer Res. 2005;65:2738–45. doi: 10.1158/0008-5472.CAN-04-3197. [DOI] [PubMed] [Google Scholar]
- 12.Wang L, Friess H, Zhu Z, Graber H, Zimmermann A, Korc M, et al. Neurotensin receptor-1 mRNA analysis in normal pancreas and pancreatic disease. Clin Cancer Res. 2000;6:566–71. [PubMed] [Google Scholar]
- 13.Kan Z, Jaiswal BS, Stinson J, Janakiraman V, Bhatt D, Stern HM, et al. Diverse somatic mutation patterns and pathway alterations in human cancers. Nature. 2010;466:869–73. doi: 10.1038/nature09208. [DOI] [PubMed] [Google Scholar]
- 14.Rozengurt E. Mitogenic signaling pathways induced by G protein-coupled receptors. J Cell Physiol. 2007;213:589–602. doi: 10.1002/jcp.21246. [DOI] [PubMed] [Google Scholar]
- 15.Kisfalvi K, Rey O, Young SH, Sinnett-Smith J, Rozengurt E. Insulin Potentiates Ca2+ Signaling and Phosphatidylinositol 4,5-Bisphosphate Hydrolysis Induced by Gq Protein-Coupled Receptor Agonists through an mTOR-Dependent Pathway. Endocrinology. 2007;148:3246–57. doi: 10.1210/en.2006-1711. [DOI] [PubMed] [Google Scholar]
- 16.Rozengurt E, Sinnett-Smith J, Kisfalvi K. Crosstalk between Insulin/Insulin-like Growth Factor-1 Receptors and G Protein-Coupled Receptor Signaling Systems: A Novel Target for the Antidiabetic Drug Metformin in Pancreatic Cancer. Clin Cancer Res. 2010;16:2505–11. doi: 10.1158/1078-0432.CCR-09-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Young SH, Rozengurt E. Crosstalk between insulin receptor and G protein-coupled receptor signaling systems leads to Ca2+ oscillations in pancreatic cancer PANC-1 cells. Biochem Biophys Res Commun. 2010;401:154–8. doi: 10.1016/j.bbrc.2010.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body Fatness and Cancer — Viewpoint of the IARC Working Group. New Engl J Med. 2016;375:794–8. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arslan AA, Helzlsouer KJ, Kooperberg C, Shu X-O, Steplowski E, Bueno-de-Mesquita HB, et al. Anthropometric Measures, Body Mass Index, and Pancreatic Cancer: A Pooled Analysis From the Pancreatic Cancer Cohort Consortium (PanScan) Arch Intern Med. 2010;170:791–802. doi: 10.1001/archinternmed.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ireland L, Santos A, Ahmed MS, Rainer C, Nielsen SR, Quaranta V, et al. Chemoresistance in Pancreatic Cancer Is Driven by Stroma-Derived Insulin-Like Growth Factors. Cancer Res. 2016;76:6851–63. doi: 10.1158/0008-5472.CAN-16-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J, Song J, Zaytseva YY, Liu Y, Rychahou P, Jiang K, et al. An obligatory role for neurotensin in high-fat-diet-induced obesity. Nature. 2016;533:411–5. doi: 10.1038/nature17662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu F-X, Guan K-L. The Hippo pathway: regulators and regulations. Genes Dev. 2013;27:355–71. doi: 10.1101/gad.210773.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moroishi T, Hansen CG, Guan K-L. The emerging roles of YAP and TAZ in cancer. Nat Rev Cancer. 2015;15:73–9. doi: 10.1038/nrc3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Straßburger K, Tiebe M, Pinna F, Breuhahn K, Teleman AA. Insulin/IGF signaling drives cell proliferation in part via Yorkie/YAP. Dev Biol. 2012;367:187–96. doi: 10.1016/j.ydbio.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 25.Wang J, Sinnett-Smith J, Stevens JV, Young SH, Rozengurt E. Biphasic Regulation of Yes-associated Protein (YAP) Cellular Localization, Phosphorylation, and Activity by G Protein-coupled Receptor Agonists in Intestinal Epithelial Cells: A NOVEL ROLE FOR PROTEIN KINASE D (PKD) J Biol Chem. 2016;291:17988–8005. doi: 10.1074/jbc.M115.711275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kapoor A, Yao W, Ying H, Hua S, Liewen A, Wang Q, et al. Yap1 Activation Enables Bypass of Oncogenic Kras Addiction in Pancreatic Cancer. Cell. 2014;158:185–97. doi: 10.1016/j.cell.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang S, Zhang L, Purohit V, Shukla SK, Chen X, Yu F, et al. Active YAP promotes pancreatic cancer cell motility, invasion and tumorigenesis in a mitotic phosphorylation-dependent manner through LPAR3. Oncotarget. 2015;6:36019–31. doi: 10.18632/oncotarget.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie D, Cui J, Xia T, Jia Z, Wang L, Wei W, et al. Hippo transducer TAZ promotes epithelial mesenchymal transition and supports pancreatic cancer progression. Oncotargert. 2015;6:35949–63. doi: 10.18632/oncotarget.5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li A, King J, Moro A, Sugi MD, Dawson DW, Kaplan J, et al. Overexpression of CXCL5 Is Associated With Poor Survival in Patients With Pancreatic Cancer. Am J Pathol. 2011;178:1340–9. doi: 10.1016/j.ajpath.2010.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang G, Lu X, Dey P, Deng P, Wu CC, Jiang S, et al. Targeting YAP-Dependent MDSC Infiltration Impairs Tumor Progression. Cancer Discov. 2016;6:80–95. doi: 10.1158/2159-8290.CD-15-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jamieson S, Flanagan JU, Kolekar S, Buchanan C, Kendall JD, Lee WJ, et al. A drug targeting only p110 alpha can block phosphoinositide 3-kinase signalling and tumour growth in certain cell types. Biochem J. 2011;438:53–62. doi: 10.1042/BJ20110502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soares HP, Ni Y, Kisfalvi K, Sinnett-Smith J, Rozengurt E. Different Patterns of Akt and ERK Feedback Activation in Response to Rapamycin, Active-Site mTOR Inhibitors and Metformin in Pancreatic Cancer Cells. PLoS One. 2013;8:e57289. doi: 10.1371/journal.pone.0057289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim N-G, Gumbiner BM. Adhesion to fibronectin regulates Hippo signaling via the FAK–Src–PI3K pathway. J Cell Biol. 2015;210:503–15. doi: 10.1083/jcb.201501025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan R, Kim N-G, Gumbiner BM. Regulation of Hippo pathway by mitogenic growth factors via phosphoinositide 3-kinase and phosphoinositide-dependent kinase-1. Proc Natl Acad Sci U S A. 2013;110:2569–74. doi: 10.1073/pnas.1216462110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rey O, Yuan J, Young SH, Rozengurt E. Protein kinase C nu/protein kinase D3 nuclear localization, catalytic activation, and intracellular redistribution in response to G protein-coupled receptor agonists. J Biol Chem. 2003;278:23773–85. doi: 10.1074/jbc.M300226200. [DOI] [PubMed] [Google Scholar]
- 36.Yuan J, Rozengurt E. PKD, PKD2, and p38 MAPK mediate Hsp27 serine-82 phosphorylation induced by neurotensin in pancreatic cancer PANC-1 cells. J Cell Biochem. 2008;103:648–62. doi: 10.1002/jcb.21439. [DOI] [PubMed] [Google Scholar]
- 37.Kisfalvi K, Hurd C, Guha S, Rozengurt E. Induced overexpression of protein kinase D1 stimulates mitogenic signaling in human pancreatic carcinoma PANC-1 cells. J Cell Physiol. 2010;223:309–16. doi: 10.1002/jcp.22036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ochi N, Tanasanvimon S, Matsuo Y, Tong Z, Sung B, Aggarwal BB, et al. Protein kinase D1 promotes anchorage-independent growth, invasion, and angiogenesis by human pancreatic cancer cells. J Cell Physiol. 2011;226:1074–81. doi: 10.1002/jcp.22421. [DOI] [PubMed] [Google Scholar]
- 39.Kisfalvi K, Eibl G, Sinnett-Smith J, Rozengurt E. Metformin disrupts crosstalk between G protein-coupled receptor and insulin receptor signaling systems and inhibits pancreatic cancer growth. Cancer Res. 2009;69:6539–45. doi: 10.1158/0008-5472.CAN-09-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang W, Nandakumar N, Shi Y, Manzano M, Smith A, Graham G, et al. Downstream of Mutant KRAS, the Transcription Regulator YAP Is Essential for Neoplastic Progression to Pancreatic Ductal Adenocarcinoma. Sci Signal. 2014;7:ra42-ra. doi: 10.1126/scisignal.2005049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gruber R, Panayiotou R, Nye E, Spencer-Dene B, Stamp G, Behrens A. YAP1 and TAZ Control Pancreatic Cancer Initiation in Mice by Direct Up-regulation of JAK–STAT3 Signaling. Gastroenterology. 2016;151:526–39. doi: 10.1053/j.gastro.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ying H, Dey P, Yao W, Kimmelman AC, Draetta GF, Maitra A, et al. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2016;30:355–85. doi: 10.1101/gad.275776.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baer R, Cintas C, Dufresne M, Cassant-Sourdy S, Schönhuber N, Planque L, et al. Pancreatic cell plasticity and cancer initiation induced by oncogenic Kras is completely dependent on wild-type PI 3-kinase p110α. Genes Dev. 2014;28:2621–35. doi: 10.1101/gad.249409.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rozengurt E, Rey O, Waldron RT. Protein Kinase D Signaling. J Biol Chem. 2005;280:13205–8. doi: 10.1074/jbc.R500002200. [DOI] [PubMed] [Google Scholar]
- 45.Rozengurt E. Protein Kinase D Signaling: Multiple Biological Functions in Health and Disease. Physiology. 2011;26:23–33. doi: 10.1152/physiol.00037.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harikumar KB, Kunnumakkara AB, Ochi N, Tong Z, Deorukhkar A, Sung B, et al. A Novel Small-Molecule Inhibitor of Protein Kinase D Blocks Pancreatic Cancer Growth In vitro and In vivo. Mol Cancer Ther. 2010;9:1136–46. doi: 10.1158/1535-7163.MCT-09-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liou G-Y, Döppler H, Braun UB, Panayiotou R, Scotti Buzhardt M, Radisky DC, et al. Protein kinase D1 drives pancreatic acinar cell reprogramming and progression to intraepithelial neoplasia. Nat Commun. 2015:6200. doi: 10.1038/ncomms7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Munster P, Aggarwal R, Hong D, Schellens JHM, van der Noll R, Specht J, et al. First-in-Human Phase I Study of GSK2126458, an Oral Pan-Class I Phosphatidylinositol-3-Kinase Inhibitor, in Patients with Advanced Solid Tumor Malignancies. Clin Cancer Res. 2016;22:1932–9. doi: 10.1158/1078-0432.CCR-15-1665. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.