Abstract
Recent anecdotal reports from VA audiology clinics as well as a few published studies have identified a sub-population of Service Members seeking treatment for problems communicating in everyday, noisy listening environments despite having normal to near-normal hearing thresholds. Because of their increased risk of exposure to dangerous levels of prolonged noise and transient explosive blast events, communication problems in these soldiers could be due to either hearing loss (traditional or “hidden”) in the auditory sensory periphery or from blast-induced injury to cortical networks associated with attention. We found that out of the 14 blast-exposed Service Members recruited for this study, 12 had hearing thresholds in the normal to near-normal range. A majority of these participants reported having problems specifically related to failures with selective attention. Envelope following responses (EFRs) measuring neural coding fidelity of the auditory brainstem to suprathreshold sounds were similar between blast-exposed and non-blast controls. Blast-exposed subjects performed substantially worse than non-blast controls in an auditory selective attention task in which listeners classified the melodic contour (rising, falling, or “zig-zagging”) of one of three simultaneous, competing tone sequences. Salient pitch and spatial differences made for easy segregation of the three concurrent melodies. Poor performance in the blast-exposed subjects was associated with weaker evoked response potentials (ERPs) in frontal EEG channels, as well as a failure of attention to enhance the neural responses evoked by a sequence when it was the target compared to when it was a distractor. These results suggest that communication problems in these listeners cannot be explained by compromised sensory representations in the auditory periphery, but rather point to lingering blast-induced damage to cortical networks implicated in the control of attention. Because all study participants also suffered from post-traumatic disorder (PTSD), follow-up studies are required to tease apart the contributions of PTSD and blast-induced injury on cognitive performance.
Keywords: Blast-induced traumatic brain injury, Selective attention, Envelope following response, Cochlear neuropathy, Hidden hearing loss
1. Introduction
Anecdotal reports as well as formal studies show that Veterans often have difficulty with certain auditory tasks, especially those that require understanding speech in the presence of competing sounds (Gallun et al., 2012; Lew, 2007; Oleksiak et al., 2012; Saunders et al., 2015). Since nearly every social setting, including a business meeting, a restaurant, a sporting event, or a party, involves simultaneous sounds, such communication difficulties can have enormous impact on everyday function. Veterans suffering from this kind of communication challenge may avoid situations that make them feel overwhelmed, leading to social isolation at home and reduced productivity at work. Two potential risk factors, both of which are common amongst Veterans, may contribute to problems communicating in crowded settings: hearing damage associated with noise exposure and cognitive problems associated with blast exposure.
Over $1 billion is spent annually on auditory dysfunction in Veterans, often associated with noise exposure as well as blast; hearing loss has become the most prevalent service-connected disability (Fausti et al., 2009). However, in addition to this, since 2000, there have been over 347,000 clinically confirmed cases of traumatic brain injury (TBI) across all branches of the military, 82% falling under the category of mild TBI (Defense and Veterans Brain Injury Center DVBIC, 2016). Both hearing damage and TBI are forms of the “invisible wounds of war” that are difficult to identify, but nonetheless devastating (Tanielian and Jaycox, 2008). Importantly, being able to understand speech in social settings places demands both on basic hearing function and central cognitive function, which may be damaged with TBI. Given how ubiquitous both hearing damage and TBI are amongst Veterans, it is critical to tease apart how both contribute to auditory dysfunction in Veterans. This is the goal of the current study.
1.1. “Traditional” and “hidden” hearing loss
Hearing loss is traditionally diagnosed when listeners have elevated hearing thresholds. Such loss is typically the result of irreversible damage to the hair cells of the cochlea. Damage to the outer hair cells in the cochlea, which are responsible for actively amplifying cochlear motion, compromises cochlear mechanical function. Sounds that are typically audible but quiet are no longer amplified, and may be inaudible, leading to elevated hearing thresholds. This form of hearing damage is what is diagnosed by current audiological screenings.
Listeners are said to have “normal hearing” as long as they have normal hearing thresholds. However, recent animal work has been exploring a subtler (but important) form of hearing loss known formally as “cochlear synaptopathy.” In mice and guinea pigs, cochlear synapses can be damaged after only moderate levels of noise exposure. The damage is thought be the result of excitotoxicity, which leads to destruction of synapses. Over time, this synaptic loss results in the degeneration and death of the spiral ganglia (ascending auditory nerve fibers or ANFs) normally enervated by the missing synapses (Kujawa and Liberman, 2009). Nerve fibers with low spontaneous firing rates appear to be most susceptible to such damage (Furman et al., 2013).
At hearing threshold, the low-spontaneous rate (LSR) fibers that are most vulnerable to noise damage do not contribute to neural responses. However, importantly, these LSR ANFs are critical for encoding amplitude modulation of supra-threshold sounds (short-term fluctuations in the level of clearly audible sounds). Such supra-threshold modulation is critical for conveying meaning in ongoing sound, especially in non-stationary signals like speech. Interestingly, at exposure levels that can lead to such synaptopathy, cochlear function can remain intact, and the ANFs encoding threshold-level sounds may respond normally. As a result, standard pure-tone audiograms do not detect this type of hearing loss (Lobarinas et al., 2013), which is colloquially referred to as “hidden hearing loss” (Schaette and McAlpine, 2011).
Service Members routinely suffer noise exposure, both during training and deployment. Noise exposure can lead not only to clinically recognized hearing deficits in the form of elevated thresholds, but also to cochlear synaptopathy, which is currently undiagnosed (and “hidden”). Thus, a good number of Service Members who do not have “impaired hearing” as defined by current clinical practice may nonetheless have damaged hearing in the form of “hidden hearing loss.”
Currently, the only direct evidence of cochlear synaptopathy and subsequent neuropathy in humans is from post-mortem immunohistochemical and electron microscopy analysis from temporal bones of five subjects without significant hair cell loss or any history of otologic disease (Viana et al., 2015). Indirect, non-invasive estimates of hidden hearing loss are being developed; these efforts focus on measurements that are sensitive to supra-threshold neural responses, such as the middle ear muscle reflex, auditory brainstem response, and envelope following response (EFR) (Bharadwaj et al., 2015; Mehraei et al., 2016; Shaheen et al., 2015; Valero et al., 2016).
The EFR, a measure of the fidelity with which the brainstem can follow periodic oscillations in sound inputs, has recently been shown to index hidden hearing loss, both in animals (Shaheen et al., 2015), and in humans (Bharadwaj et al., 2015; Ruggles et al., 2011). This measure thus provides a way of assessing supra-threshold hearing fidelity in listeners with normal hearing thresholds.
1.2. Blast exposure and TBI
For combat troops, the predominant vehicle for injury is exposure to explosive blast ordinance (IEDs, mortar rounds). Such exposure frequently results in blast-induced TBI (Terrio et al., 2009). While the most common symptom associated with TBI is non-headache and headache pain, Service Members with a history of TBI often seek treatment for other symptoms that can include sleep disorders, memory loss, cognitive dysfunction, and hearing problems (Bergemalm and Lyxell, 2009; Farmer et al., 2016; Hoover et al., 2015; Krause et al., 2014; Lew, 2007; Munjal et al., 2010; Oleksiak et al., 2012). These symptoms may resolve within weeks to months after injury (Kwok et al., 2009; Levin et al., 1987); however, in a subset of patients these problems persist and worsen into debilitating post-concussion syndrome (McKee and Robinson, 2014). In the most severe cases, patients go on to develop chronic traumatic encephalopathy (CTE), a condition whose symptoms includes problems with impulse control, paranoia, and severe depression; the disease can progress to early-onset dementia and ultimately death (McKee and Robinson, 2014; Mez et al., 2013).
Diagnosing mild TBI (mTBI) is complicated and far from an exact science. Because evidence of injury is often undetectable using neural imaging techniques (Hoge et al., 2008; Mac Donald et al., 2011), clinicians and combat medical personnel base their diagnoses, in part, on interviews that focus on whether the patient experienced a loss of or some form of altered consciousness (Management of Concussion/ mTBI Working Group, 2009). Depending on the circumstances surrounding the injury as well as whether or not trained medical staff were present at or near the time of injury, this information may be incomplete or inaccurate. In the military, it is thought that many such events go unreported (Chapman and Diaz-Arrastia, 2014; Schwab et al., 2007; Tanielian and Jaycox, 2008). In particular, once initial symptoms subside, soldiers are eager to return to duty, and fear the possibility of disqualification for medical reasons if they admit to TBI symptoms. Recent work suggests that CTE is a cumulative effect of multiple sub-concussive and concussive events (Baugh et al., 2012), which highlights both the need to understand how TBI affects the brain and the need to educate Service Members about the consequences of TBI.
1.3. Blast exposure and selective auditory attention
The ability to follow a conversation in crowded and noisy listening environments depends critically on the listener's ability to focus and sustain attention on a speaker of interest while simultaneously ignoring the interfering sounds that may be present in the room. This scenario is commonly referred to as the “cocktail party problem” (Cherry, 1953). Successful communication in everyday social settings typically strikes a balance between selective attention, where listeners maintain their attention on a single sound source, and divided attention, where listeners switch their attention from source to source (talker to talker or talker to TV, for example). In both cases, in order to ignore unwanted sounds and attend information from an important sound in a scene, a listener must be able to “segregate” or perceptually separate the sounds making up the mixture. Segregation can fail if the listener does not have an accurate and detailed sensory representation of the auditory scene (Shinn-Cunningham, 2008). If a listener cannot segregate the desired sound, he or she will struggle when trying to selectively direct their attention (Shinn-Cunningham and Best, 2008). This would certainly be the case for Service Members with evidence of “traditional” cochlear hearing loss. However, some Service Members who have audiograms that fall in the normal to near-normal range (H-1 hearing profile) nonetheless suffer from communication difficulties. In such cases, it is possible that blast-induced synaptopathy (explained previously) might contribute to degraded neural representations of suprathreshold sounds; however, blast exposure may also have damaged cortical regions implicated in auditory processing.
Oscillatory synchronization in the gamma band range (40–80 Hz) is generally thought to be essential in organizing activity within local ensembles of neurons, and has been implicated in the perceptual binding of sensory stimuli (Fries, 2009) as well as other higher cognitive functions including speech processing (Giraud and Poeppel, 2012) and attention (Engel et al., 2001). This oscillatory activity is driven by excitatory and inhibitory post-synaptic interactions between pyramidal cells and GABAergic interneurons (Hasenstaub et al., 2005). Animal models of TBI show these GABAergic neurons are particularly vulnerable to traumatic brain injury (Almeida-Suhett et al., 2015; Lowenstein et al., 1992).
Blast TBI may damage not only local regions of the brain, but also long-range neural connections (Taber et al., 2015). In particular, because the head contains not just brain tissue, but also air, bone, and fluid, it is inhomogeneous. The blast wave that passes through the head therefore does not travel at a uniform speed, but instead passes through different parts of the head at different speeds (Bauman et al., 2009; Sosa et al., 2013). The resulting shearing forces are thought to disrupt the long-range neural pathways that are critical in cognitive control networks responsible for focusing attention on task-relevant features in a complex scene and modulating sensory inputs based on current behavioral goals.
Reports hint that these communication difficulties are a result of damage to cortical networks associated with attentional control (Gallun et al., 2012; Lew, 2007). Frontal brain regions, including regions that are part of the cognitive attentional control network, are vulnerable to primary, secondary, and tertiary blast injury (Taber and Hurley, 2007; Taber et al., 2006). Either localized damage to grey matter and/or damage to neural connections that make up the cortical attentional network could impair selective attentional control (for review see Wolf and Koch, 2016). This may help explain the pattern of hearing dysfunction experienced by blast-exposed personnel. Interestingly, it is this kind of scenario that often reveals communication difficulties amongst Veterans with blast injury.
Importantly, a slightly degraded sensory representation may not cause communication problems if the listening conditions are simple, without any competing sound. Yet in the presence of competing sounds this degraded signal representation may be too impoverished to allow listeners to communicate effectively. Indeed, hidden hearing loss often seems to produce this combination of symptoms: an ability to understand a talker in quiet, but problems understanding a talker when there are multiple sources in an auditory scene (Ruggles et al., 2012; Ruggles and Shinn-Cunningham, 2011). This kind of problem is illustrated by visual analogy in Fig. 1.
Fig. 1.

Visual analogy illustrating the effects of a poor peripheral representation on the ability to process sources in a crowded setting. A normal-hearing listener has no problem understanding a male speaker either in quiet, or when there is a competing female talker (left). A listener with hidden hearing loss may still understand a male speaker in quiet even though the representation is somewhat degraded, yet will face real communication difficulties when there is a competing talker (right).
Still, focusing selective auditory attention depends not just on a good sensory representation of an auditory scene, but also on precise control of neural responses from cognitive networks in the brain (Choi et al., 2014; Michalka et al., 2015). When selective attention is focused on a particular sound source, feedback in the brain modulates the actual representation of the sound mixture in cortex. The brain filters out “distracting” sources and lets through whatever source is the focus of attention (Choi et al., 2014; Hillyard, 1976; Mesgarani and Chang, 2012; Picton and Hillyard, 1974). This allows a listener to process, in detail, the features of the desired sound source without interference from other unimportant signals. For selective auditory attention to operate effectively, the long-range connections within the cortical attentional network must be intact and functional.
1.4. Rationale for the current study
In the current study, we test Veterans with blast exposure on a task that has very low memory demands and does not require language processing, but that requires listeners to focus selective auditory attention in order to perform the task. We directly measure both behavioral ability and cortical responses to the sound mixture. Cortical responses are measured using electroencephalography (EEG), which allows us to quantify how strongly neural responses to sounds in the mixture are modulated by attentional focus. Specifically, we compare responses to identical sound mixtures when listeners are attending a sound stream compared to when they are ignoring that same stream. We separately measure sensory coding fidelity both using traditional hearing thresholds and our objective physiological measure of subcortical coding (the EFR).
We find that blast-exposed Service Members perform consistently worse than non-blast controls in our selective attention task. Furthermore, EEG responses to the sound mixture are degraded and show weak attentional modulation compared to controls. These findings are consistent with self-perceived difficulties communicating in cocktail-party-like situations. Importantly, the EFR of all of the tested Veterans falls within the normal range for our control listeners, suggesting that sensory differences cannot account for abnormal performance and cortical responses.
2. Methods
2.1. Subjects
Fourteen blast-exposed Veterans (13 male, 26–49 years, mean age = 33.5) were referred to the Auditory Neuroscience Laboratory at Boston University from the Neurorehabilitation Lab at the VA Boston Healthcare System, Jamaica Plain Campus (there, the Veterans were part of an investigational protocol for the treatment of post-traumatic stress disorder, or PTSD). All 14 study participants reported being within 100 m of at least one explosive blast during their time in service. Of the 14 volunteers, 12 participated in the EEG portion of the study. Six reported having 5 or fewer blast exposures, while the remaining six subjects reported having 10 or more exposures. When possible, the VA Boston Healthcare System provided additional TBI-related data including in-service and pre-deployment TBI diagnoses, loss of consciousness (LOC) duration, post-traumatic amnesia (PTA) duration, and number of blast exposures. Data reported for LOC and PTA pertain to the most severe blast event. Individual subject summaries are provided in Table 1. All subjects provided written informed consent as approved by the Boston University Institutional Review Board. Subjects were compensated at a base hourly rate with an additional performance bonus ($0.02 for each correct response, $7.20 maximum).
Table 1.
Summary of TBI and blast exposure by subject.
| Subject# | Total TBI | Past TBI | LOC | PTA | # Blast Exposures |
|---|---|---|---|---|---|
| 001 | N/A | 0 | None | 5–60 min | 31 |
| 003 | 2 | 6 | None | <2 min | 18 |
| 004 | N/A | 0 | None | None | 20 |
| 005 | 2 | 0 | None | 1–4 h | 31 |
| 006 | N/A | 0 | None | None | 3 |
| 007 | N/A | 0 | None | None | 1 |
| 008 | N/A | 0 | <2 min | 5–60 min | 18 |
| 009 | 2 | 12 | None | Dazed/Confused | 2 |
| 010 | N/A | N/A | None | Dazed/Confused | 3 |
| 011 | N/A | 0 | <2 min | 1–4 h | 10 |
| 114 | 0 | 0 | None | Dazed/Confused | 5 |
| 115 | N/A | N/A | None | None | 3 |
N/A: data not available, LOC = loss of consciousness, PTA = post-traumatic amnesia.
Total TBI = number of confirmed in-service TBI diagnoses.
Past TBI = number of reported pre-deployment TBI.
Seventeen subjects (6 male, 20–35 years, mean age = 32.5) were selected from a previous study (Choi et al., 2014) to serve as the control group for the auditory selective attention experiment. These subjects were recruited from advertisements posted at Boston University, and screened for normal hearing (pure tone thresholds less than 25 dB HL). Subjects were presumed to have no history of blast exposure or PTSD.
2.2. Objective hearing thresholds
Air conduction thresholds were measured in all participants for pulsed pure tones at frequencies of 500, 1 k, 2 k, 3 k, 4 k, and 8 kHz in both ears. Control subjects whose thresholds exceeded 25 dB HL were excluded from the study. Blast-exposed subjects whose 4-kHz thresholds exceeded 25 dB HL in any one ear were exempt from the EEG portion of the study. All of the Veterans remaining in the study were classified as having H-1 hearing profiles (Smetana, 1999). This is defined as an average hearing loss for each ear of no more than 25 dB HL at 500, 1 k, and 2 kHz with no individual level greater than 30 dB HL, and no hearing loss greater than 45 dB HL at 4 kHz.
2.3. Envelope following response
2.3.1. Stimuli and procedures
EFRs were measured in a roughly 40-min session using Biosemi ActiveTwo system hardware and its accompanying ActiveView data acquisition software. Scalp potentials were recorded from 32 electrodes (standard 10/20 montage) plus two reference electrodes placed on the left and right mastoids. Two vertical electro-oculogram (EOG) electrodes were also included to capture eye blink events. Timing of critical experimental events was marked with 5-V TTL pulses sent from the TDT hardware and recorded to an additional data channel alongside the EEG data.
Stimuli were sinusoidally amplitude-modulated pure tones, with different modulation depths. The tones were constructed from 4-kHz pure tones modulated by a half-wave rectified, lowpass filtered 100-Hz sinusoid at four different modulation index values equaling 1, 0.63, 0.40, and 0.25 (Bernstein and Trahiotis, 2002). The peak-to-peak values were equal across the four modulation depths, and presented at a level that corresponded to 75 dB SPL for the fully modulated stimuli. The stimuli were 400 ms in duration with 5-ms onset/offset ramps, and were presented in opposing polarities; by averaging together these responses, the neural response envelope is measured, while responses that follow temporal fine structure are canceled (Goblick and Pfeiffer, 1969; Skoe and Kraus, 2010; Zhu et al., 2013).
The various modulation depth/polarity combinations were presented in random order, with a total of 500 presentations per condition. The 4000 stimulus tokens were presented diotically roughly every 700 ms with a 100 ms jitter (to ensure that no repetition artifact was present in the responses).
During the recording session, subjects were allowed to watch a movie of their choosing with the subtitles enabled and the sound muted to help pass the time. We were able to obtain reliable data for 10 of the 12 participating blast-exposed Veteran participants, and compared it to data from 18 normal-hearing (non-blast) controls from a previous study (Bharadwaj et al., 2015).
2.3.2. Analysis
Data were sampled at 4096 Hz, re-referenced to the average of the two mastoid electrodes, and highpass filtered at 70 Hz using the eeg-filtfft( ) brick-wall filter function in the EEGLab toolbox (Delorme and Makeig, 2004). Epochs were extracted from −100 ms to 450 ms relative to stimulus onset. Epochs where the signal dynamic range exceeded 150 μV in any of the 32 scalp channels were excluded to reject eyeblink and other artifacts. After epoch rejection, for each subject and each modulation depth, 820 trials (410 per polarity) were selected randomly from the remaining epochs. Subjects who lacked the requisite number of trials were excluded from the final analysis, leaving a total of 8 blast-exposed and 12 non-blast control subjects.
Estimates of the phase-locking value (PLV) (Lachaux et al., 1999), which is a normalized measure of across-trial phase synchrony, were calculated by combining data across the 32 electrodes to improve the signal to noise ratio (Bharadwaj et al., 2014). From the PLVs of the 100-Hz envelope fundamental frequency for each of the four modulation depths, we also computed the slope describing how the PLV strength decreased with decreasing modulation depth. By cancelling out inter-subject differences due to differences in head geometry, electrode impedance, and other nuisance factors that contribute to the overall strength of the evoked response, this metric provides a better estimate of brainstem neural encoding fidelity in response to changes in modulation envelope depth (Bharadwaj et al., 2015). Subjects with shallower slopes display more robust encoding of the acoustic periodicity in the brainstem response, even as the available low frequency envelope information is reduced with the reduction in modulation depth. Conversely, steep slopes are associated with poor brainstem encoding of the suprathreshold stimulus envelope.
2.4. Subjective self-assessment of hearing
There are anecdotal reports of Service Members with H-1 hearing profiles seeking assistance at local VA hospitals for problems communicating in crowded, noisy environments (Oleksiak et al., 2012; Saunders et al., 2015; Saunders and Echt, 2012). A few published studies have confirmed these reports (Gallun et al., 2012; Lew, 2007). To verify whether we would observe similar findings, we administered the short form Speech, Spatial, and Qualities of Hearing questionnaire (SSQ12) to all study participants (Noble et al., 2013) and compared results for our listeners with those from a cohort of 103 normal-hearing subjects between the ages of 18–25 years of age (Demeester et al., 2012). This survey instrument consists of 12 ques tions designed to return a subjective measure of how well a person hears under several different real-world situations. It evaluates how well a person can 1) follow speech in the presence of noise and competing talkers, and 2) localize and identify sounds, and provides a way of summarizing a listener's subjective assessment of their own hearing abilities. Questions are scored on a Likert scale from 0 to 10.
2.5. Selective auditory attention task
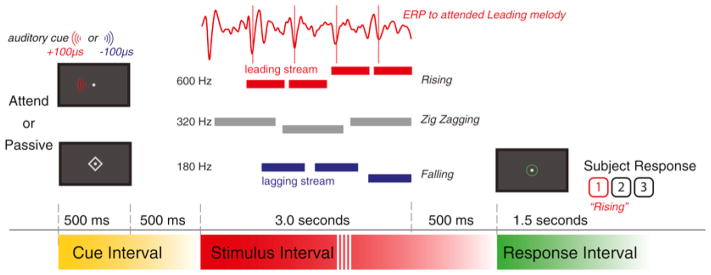
The selective auditory attention task used in this study (Fig. 2) was identical to the one described in Choi et al. (2014), and was performed in a separate experimental session from the EFR measurement. Briefly, each trial consisted of three simultaneous melodies, each simulated from a different lateral angle. Subjects were instructed as to what melody to attend using an informative auditory cue before the start of each trial. They were tasked with identifying the contour of the attended melody, which was rising, falling, or zig-zagging. Responses were registered using the numeric keypad on a computer keyboard during a prescribed response period.
Fig. 2.
Auditory Selective Attention Melody Detection Task. Subjects were provided with an informative 500-ms auditory cue 1 s before the 3-s three-melody stimulus. Subjects were given 1.2 s to enter their response. This example demonstrates an “attend leading” trial where the leading melody had a rising melodic contour. Passive trials were cued visually with a diamond centered over the central fixation point.
2.5.1. Equipment
Experimental stimuli were created in MATLAB (The Mathworks, Natick, MA). The experiment was controlled using the Psychtool-box 3 extension (Brainard, 1997) and TDT Active X Controls from Tucker-Davis Technologies (TDT, Alachua, FL). Auditory and stimulus event signals were presented via the System 3 RP 2.1 Realtime Processor with an HB7 Headphone Amplifier through ER-1 insert earphones (Etymotic, Elk Grove Village, IL). The stimulus sound level was fixed at 70 dB SPL (root-mean-squared). All experimental sessions were conducted in a 1.7 × 2.0 × 2.0 m sound-attentuated booth (Model C-14, Eckel Noise Control Technologies, Morrisburg, Ontario, CANADA).
2.5.2. Auditory stimuli and task
Each of the melodies was isochronous, with rhythmically regular onsets between successive notes, but with rates that differed across melodies. The staggered onsets of the notes in the competing melodies allowed us to temporally isolate the neural evoked response potentials (ERPs) in the EEG responses, which were evoked by the onsets of the notes in each melody. The envelope of each note had a slowly decaying exponential window (100 ms time constant) bookended by cosine-squared onset (10 ms duration) and offset (100 ms) ramps.
The melodies were lateralized to come from three different directions using interaural time differences (ITDs) of −100, 0, and +100 μs. The center melody (0 ITD) was a “Distractor,” and consisted of three notes, 1 s in duration; it was always to be ignored. The remaining two lateralized melodies were classified as either “Leading” or “Lagging.” The Leading melody consisted of four notes, 600 ms in duration, and started 600 ms after the start of the center melody. The Lagging melody consisted of three notes, 750 ms in duration, and started 150 ms after the leading melody. The lateral locations of the Leading and Lagging melodies were assigned randomly and separately on each trial (one at −100 μs, the other at +100 μs). We previously showed that the onsets of the Distractor and Leading melody draw attention exogenously, and that the ERP strength does not vary with attentional focus; in contrast, the Lagging melody, which begins very shortly after the first note of the Leading melody, shows top-down attention effects (Choi et al., 2014). All subsequent notes from both the Leading and Lagging streams also evoke ERPs whose magnitudes are modulated by attentional focus (Choi et al., 2014).
Each melody was constructed from only two pitches, a high and a low note, with pitches that differed between melodies. Each note contained six harmonics added in cosine phase—the fundamental frequency and the subsequent 5 harmonics. The magnitudes of the harmonics were inversely proportional to the harmonic number. Subjects were presented with an easier “different pitch” condition in which the fundamental frequencies of the notes of Leading, Distractor, and Lagging melodies occupied three non-overlapping frequency ranges (600–726 Hz, 320–387 Hz, and 180–218 Hz, respectively), and a difficult “same pitch” condition in which the fundamental frequencies of all three melodies were drawn from the same 320–387 Hz range. Because the blast-exposed Veterans were unable to successfully negotiate the difficult “same pitch” task, essentially performing at chance level, data from this condition were not included in any of our subsequent analyses.
On each trial, each of the streams was randomly chosen to have a melody contour that was rising, falling, or zig-zagging, with equal likelihood (1/3 each). The contours of the three melodies were chosen independently within each trial. If the contour of a given stream was rising, it started with a low (L) note; if it was falling, it started with a high (H) note, and if it was zig-zagging, it could start with either an L or an H note (with equal likelihood). For all sequences, the melody changed from its starting value to the other value (H or L, respectively) at some random point later in the sequence. For rising and falling sequences, this value was repeated in all subsequent notes (e.g., valid four-note ascending sequences were LHHH, LLHH, and LLLH). In order to ensure that listeners had to maintain attention on the target stream throughout the sequence, zig-zagging melodies always changed back to the original note value only for the final note of the melody (e.g., valid four-note zig-zagging sequences were LHHL, LLHL, HLLH, and HHLH).
At the start of each trial, subjects were instructed to fix their gaze on a dot located in the center of the computer monitor; they were instructed to maintain their gaze to the fixation dot throughout each trial. Depending on the trial, subjects were tasked with identifying the melodic contour of one of the lateralized melodies, or to withhold responses entirely. Prior to the start of the melodies, a cue directed subjects as to what the task was in the upcoming trial. For “Attend” trials, the cue was an auditory tone, 500 ms in duration, whose F0, timbre, and location matched that of the upcoming melody to be attended. In “Passive” trials, the cue was visual: a diamond (‘◇’) appeared around the fixation dot for 500 ms. One second after the cue, the 3-s long, three-melody stimulus was presented. 500 ms after the end of the stimulus, a green circle was presented around the central fixation dot to signify the 1.2-s response period. Subjects had to either respond (on Attend trials) or withhold any button presses (on Passive trials) during the response period. Visual feedback was provided at the end of each trial. Listeners were rewarded with a $0.02 bonus for each correct response entered in the response period (correct melody contour for Attend trials, no response for Passive trials).
2.5.3. Procedure
Experimental sessions were divided into 12 blocks of 30 trials. Within each experimental block, subjects were asked to identify the contours of 12 Leading and 12 Lagging melodies, divided evenly between different- and same-pitch conditions. The remaining 6 trials were Passive (no response) trials. The presentation order of the five different experimental conditions (Attend Leading/different pitch, Attend Lagging/different pitch, Attend Leading/same pitch, Attend Lagging/same pitch, Passive) was randomized within each block separately for each subject.
Subjects were screened with a short 12-trial training session that presented a single melody, without any competing melodies. The training session familiarized subjects with the pacing of the trials and the keyboard response method, but also was used to ensure that subjects could perform the melody classification when a target was presented in isolation: subjects had to score 10 out of 12 correct classifications (83.3%) on the single-trial training session within 3 training runs to be included in the main study. Two Veteran Service Members could not successfully complete the single-melody training task, and were excluded on this basis.
2.5.4. Behavioral scoring
Proportion correct scores were calculated for the Attention trials. Performance on the Passive trials was used to verify that the subjects were performing the task as instructed. Inhibition error rate (IE) was quantified as the proportion of Passive trials in which subjects incorrectly entered a response. High rates of inhibition errors were interpreted as evidence of problems with impulse control or hypervigilance, a symptom commonly associated with PTSD and TBI (Lagarde et al., 2014; Rosenfeld and Ford, 2010). Finally, the proportion of no-response trials (NR) was calculated as the percentage of Attend Leading and Attend Lagging trials in which subjects failed to register a valid response within the provided response period. Lack of responsiveness during a particular trial or block of trials was interpreted as a sign of task disengagement, e.g., due to momentary lapses (missing the pre-stimulus period cue, for example) or to longer-term changes in listener state (becoming drowsy or falling asleep during the experiment).
2.5.5. EEG acquisition and data analysis
Cortical EEG data were recorded using the same Biosemi ActiveTwo system hardware setup used for EFR measurements. Data were sampled at 2048 Hz, re-referenced to the average of the two mastoid electrodes, and bandpass filtered from 2 to 20 Hz using a 2048-point zero-phase FIR filter. Eye blink artifacts were removed using signal-space projection techniques (Uusitalo and Ilmoniemi, 1997). Trial epochs were extracted from −500 ms to 3000 ms relative the start of the three-melody stimulus, and sorted based on the experimental trial type. Any epoch with voltages exceeding ±100 μV from any of the 32 scalp electrodes was discarded to remove other recording artifacts. Attention trials were classified as either “Attend Leading” or “Attend Lagging,” collapsing across target direction. Because behavioral scores from the blast-exposed subject cohort was low, all valid EEG epochs were included in analysis, not just those from trials in which subjects responded correctly. After preprocessing and epoch rejection, a minimum of 49 out of a possible 72 trials remained for analysis for each subject and condition. To ensure a statistically fair comparison across subjects and conditions, all final analyses were done by randomly selecting 49 epochs from amongst all valid epochs for each subject and condition.
Previous work shows that attention-driven modulation of neural responses in this selective auditory attention task is maximal in a montage of five frontal electrodes: AF3, AF4, F3, F4, and Fz (see Fig. 3A from Choi et al., 2014). Therefore, we averaged these responses across trials for each subject and condition to find the final ERPs.
Fig. 3.
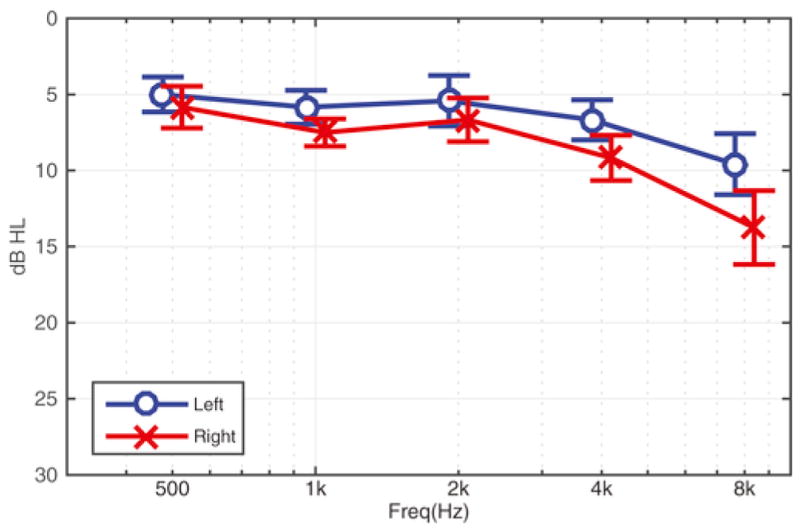
Average audiogram of H-1 blast-exposed Service Members (n = 12, mean ± SEM).
3. Results
3.1. Objective hearing thresholds were near normal for included blast-exposed subjects
Twelve (12) of the 14 blast-exposed Military Service Members were classified as having H-1 profiles (Smetana, 1999). The two Service Members that had hearing loss greater than the H-1 criteria were excluded from the EEG portion of the study. Thus, hearing thresholds were near normal in all of the Veterans tested (see Fig. 3).
3.2. EFRs fall within the normal range in the blast-exposed listeners
Fig. 4 analyzes the phase locking value (PLV) (Lachaux et al., 1999) to the 100-Hz envelope (a way to quantify the strength of the EFR from the brainstem) as a function of stimulus modulation depth (decreasing from left to right). The top panel of the figure shows the PLV as a function of envelope modulation depth, while the bottom panel shows the slope describing how the PLV changes with modulation depth (derived from the data in the upper panel). The plots compare results from the blast-exposed Veterans (shown in black) to those from normal-hearing controls (shown in green).
Fig. 4.
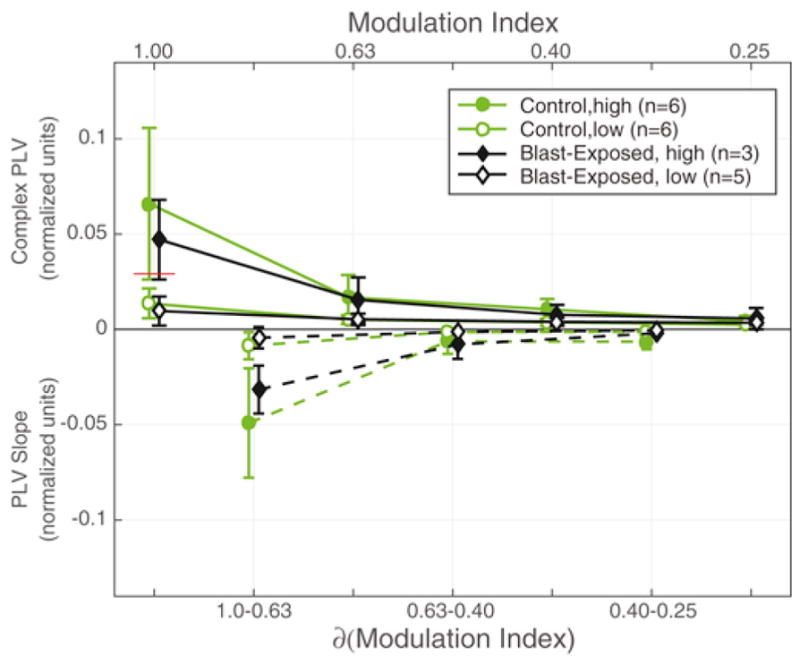
Phase Locking Values (PLVs) to 100-Hz modulated 4-kHz sinusoids. Solid lines in upper portion of the figure are average PLVs as a function of modulation index (upper axis label). Dashed lines in lower portion are the PLV slope estimates as a function of change in modulation index (lower axis label). Both groups were divided into high and low based on the median split (red line) of the PLVs from the non-blast controls of the fully modulated stimulus (modulation index = 1.00). Data expressed as mean ± 95% c.i.
Because these metrics vary significantly across subjects (Bharadwaj et al., 2015; Ruggles et al., 2011), when plotting the results, we divided each of the groups in half, based on the strength of the PLVs, defining a “high” group (filled symbols) and a “low” group (open symbols). For each group, we calculated the across-subject means and standard deviations. This allowed us to better demonstrate how great the inter-subject variation is in the PLV strength in both of the groups, consistent with published reports demonstrating that supra-threshold coding fidelity varies significantly in listeners with normal hearing thresholds. Importantly, while there is a lot of variation within both the blast-exposed and control groups, the across-group differences are small, especially when compared to the within-group differences.
Overall, the PLV decreases monotonically as the modulation depth of the stimulus envelope decreases (top panel), with the largest decline between modulation index values of 1.00 and 0.63. At a modulation index value of 1, PLVs for both the blast and non-blast controls were similar [Wilcoxon rank sum: U = 118, p = 0.1019, z = −1.2706]. Similarly, the PLV slopes were similar in the blast-exposed and control groups, including the slope calculated from modulation indices of 1.00 to 0.63, where individual differences are greatest [Wilcoxon rank sum: U = 99, p = 0.1316, z = 1.1187].
These results suggest that the blast-exposed listeners have supra-threshold hearing fidelity that overlaps substantially with that of normal-hearing controls. Thus, if there are differences in perceptual ability between the two groups, it is unlikely to arise due to differences in sensory coding fidelity.
3.3. Blast-exposed subjects report having trouble in everyday listening tasks
The SSQ12 questionnaire summarizes subjective self-assessments of hearing ability in understanding speech (questions 1–5), spatial perception (questions 6–8), and sound quality (questions 9–12). Fig. 5 shows box plots (white) of the numeric responses for our subjects and for 103 normal-hearing control subjects (green) from a previous study (Demeester et al., 2012). From the control data, we derived 95% confidence intervals, and then evaluated the number of our blast-exposed Veterans who fell outside this normal range (see numbers below box plots in Fig. 5).
Fig. 5.
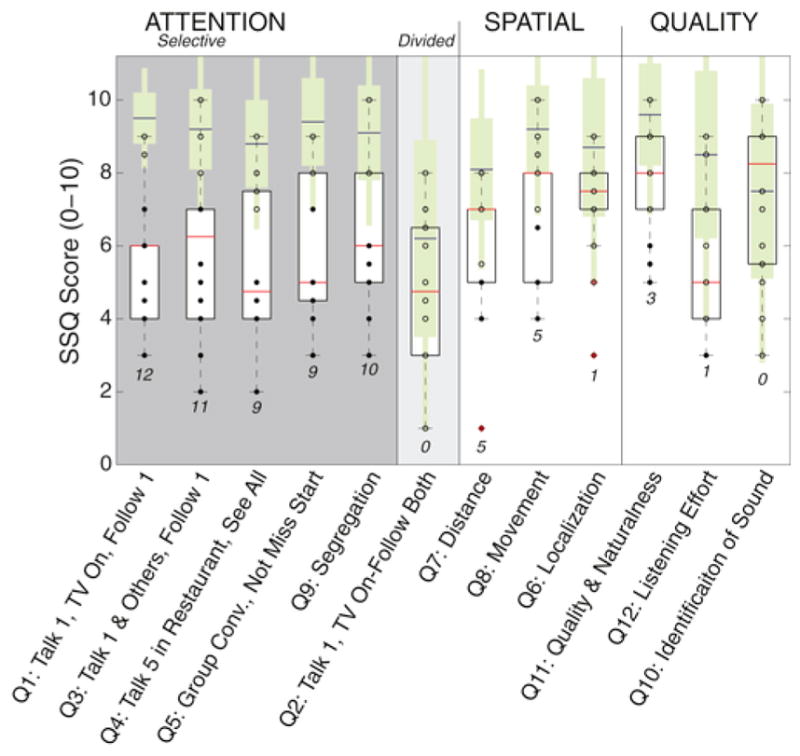
Short form SSQ results (white box plots) compared against published results from Demeester et al. mean ± standard deviation (green bars with blue mean lines) and extrapolated 95% confidence intervals (thin green bars). Numbers in below represent the number of subjects out of 14 that fell outside the 95% confidence intervals derived from published normal-hearing control data.
As Fig. 5 shows, the blast-exposed subjects tended to have lower (worse) scores on many questions, with a large percentage of the 12 listeners falling outside the 95% confidence intervals for normal-hearing listeners. These deficits were especially pronounced when listeners were assessing their ability to follow speech in the presence of interfering sound sources, such as a TV show, competing speech, or in a group setting: for questions 1, 3, 4, and 5, at least 75% of the blast-exposed Veterans fell outside the normal range. On the speech question related to dividing attention between two sources (question 2: single talker and TV on—can you follow both?), blast-exposed subjects were no better or worse than young normal hearing subjects, a result that may reflect the fact that even the control subjects varied in this self-assessment, with many control subjects reporting quite low scores.
Results are further summarized in Table 2, which gives the means and standard deviations of the scores on each question for the control and the blast-exposed groups, but with the questions organized according to the task that is being assessed. A majority of the blast-exposed of subjects (75% or more) fell outside the normal range for the four questions assessing understanding speech in a noisy setting (questions 1, 3, 4, and 5). For the question evaluating the ability to segregate simultaneous sources (question 9), 10 out of the 14 blast-exposed listeners fell outside the normal range. However, for the other categories, related to spatial hearing and overall quality of listening experience, the number of blast-exposed subjects reporting scores outside the 95% confidence interval for normal-hearing subjects was much smaller (36% or less).
Table 2.
Comparison results for normal-hearing control subjects (n = 103) and blast-exposed subjects (n = 14). The final column reports the number of blast-exposed subjects whose scores fall outside the calculated 95% confidence intervals for the scores from normal-hearing 18–25 year olds. Numbers in bold highlight that a majority of the blast-exposed subjects reported scores below the derived 95% confidence interval of the normal-hearing controls.
| SSQ 12 Item (SSQ 49 index) | Normal Hearinga mean ± SD | Blast Exposed mean ± SD | Blast Exposed n<95%c.i. | |
|---|---|---|---|---|
| Selective Attention | Q1: Speech in noise (1.1) | 9.5 ± 0.7 | 5.6 ± 1.7 | 12/14 |
| Q3: Speech in speech (1.11) | 9.2 ± 1.1 | 5.8 ± 2.5 | 11/14 | |
| Q4: Speech in noise (1.4) | 8.8 ± 1.2 | 5.4 ± 2.1 | 9/14 | |
| Q5: Multiple speech streams (1.12) | 9.4 ± 1.2 | 6.0 ± 2.2 | 9/14 | |
| Q9: Segregation (3.2) | 9.1 ± 1.3 | 6.1 ± 2.2 | 10/14 | |
| Divided Attention | Q2: Multiple speech streams (1.10) “single talker, TV on--follow both” | 6.2 ± 2.7 | 4.8 ± 2.0 | 0/14 |
| Spatial | Q7: Distance and movement (2.9) | 8.1 ± 1.4 | 6.0 ± 1.9 | 5/14 |
| Q8: Distance and movement (2.13) | 9.2 ± 1.2 | 7.1 ± 1.9 | 5/14 | |
| Q6: Localization (2.6) | 8.7 ± 1.9 | 7.1 ± 1.6 | 1/14 | |
| Quality | Q11: Quality and naturalness (3.9) | 9.6 ± 1.4 | 7.8 ± 1.5 | 3/14 |
| Q12: Listening effort (3.14) | 8.5 ± 2.3 | 5.7 ± 2.1 | 1/14 | |
| Q10: Identification of sound (3.7) | 7.5 ± 2.4 | 7.3 ± 2.2 | 0/14 |
From Demeester et al. (2012).
These results confirm that the conditions in which the blast-exposed listeners feel that they have real difficulty are those in which there are simultaneous sources. They report having trouble both when trying to understand the content of speech when there are competing sounds and when trying to perceptually separate competing sounds.
3.4. Blast-exposed service members perform poorly in the selective auditory attention task
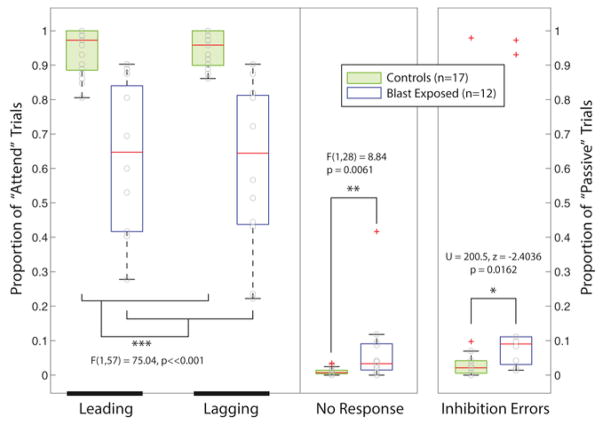
Blast-exposed Service Members performed substantially worse than the healthy, non-blast controls in the selective auditory attention melody classification task. On average, the blast-exposed group was equally bad at classifying Leading and Lagging melodies, with correct responses on only 65% of the Attend trials. In contrast, control subjects performed over 95% correct on Attend trials, on average. In the blast-exposed group, individual subject performance varied greatly, from scores that were not significantly different from chance (1/3) up to a maximum of about 90%. The arcsin transformed percent correct scores were compared using a 2-way ANOVA with factors of Group (Blast Exposed vs. Control) and Melody Type (Leading vs. Lagging). The main effect of Group was significant [F(1,57) = 75.04, p ≪ 0.001]; however, neither the main effect of Melody Type nor the interaction was significant.
Compared to control subjects, blast-exposed subjects also exhibited both a larger Inhibition Error rate (failure to withhold a response) on Passive trials [Wilcoxon rank sum: U = 200.5, p = 0.0162, z = −2.4036] and a larger No Response rate on Attention trials [ANOVA: F(1,28) = 5.19, p = 0.0308] (see middle and right portions of panels in Fig. 6).
Fig. 6.
Behavioral scores for the selective auditory attention task for both blast-exposed and control subjects. Left panel: percent correct responses for Leading and Lagging melody identification on Attend trials. Middle panel: No Response rates for Attend trials, where subjects failed to respond within the allotted response period. Right panel: Inhibition Error rates for Passive trials, where listeners are supposed to withhold responses.
Individual differences in performance were large and consistent across Attend Leading and Attend Lagging trials. This is illustrated by Fig. 7, which gives a scatter plot of the scores on the two types of trials for each subject. This plot emphasizes that the range of scores was very large for the blast-exposed subjects (30% to about 90%) compared to the control subjects (ranging from about 90% to 100%). Furthermore, only the two best performers in the blast-exposed group had scores that overlapped with the range of scores from the control subjects, and these scores fell just at the bottom edge of the range from all control subjects (around 90%). However, the inter-subject differences are consistent in both groups (even the control subjects, where the range of scores is small): correlations between Attend Leading and Attend Lagging trials reaches a value of τ16 = 0.78 (p < 10−5) for controls and τ11 = 0.88 (p < 10−4) for the blast-exposed subjects.
Fig. 7.
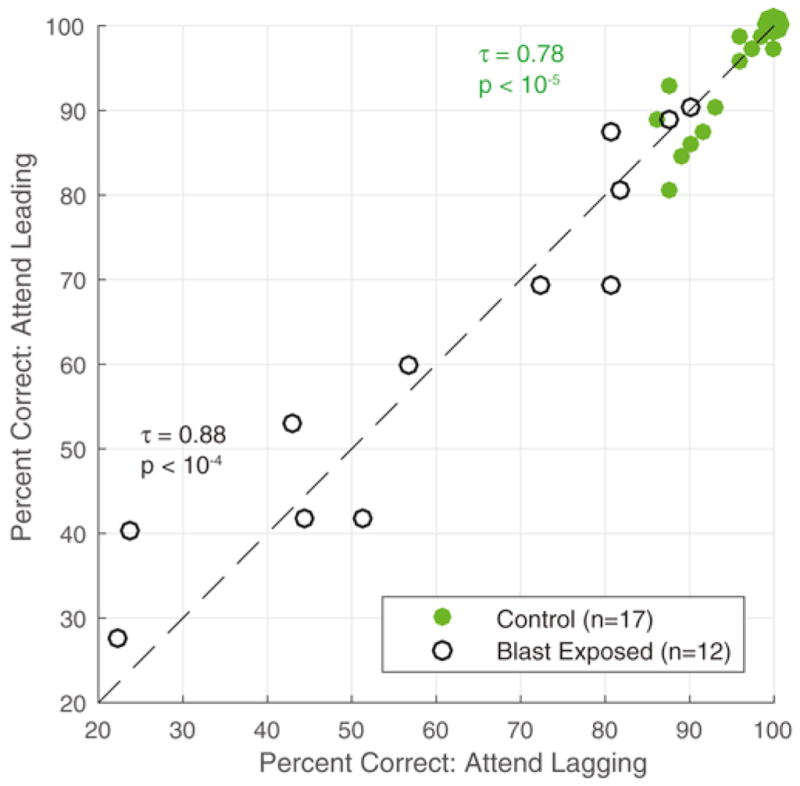
Attend Leading versus Attend Lagging performance for Controls (n = 17, solid green) and Blast Exposed (n = 12, open black).
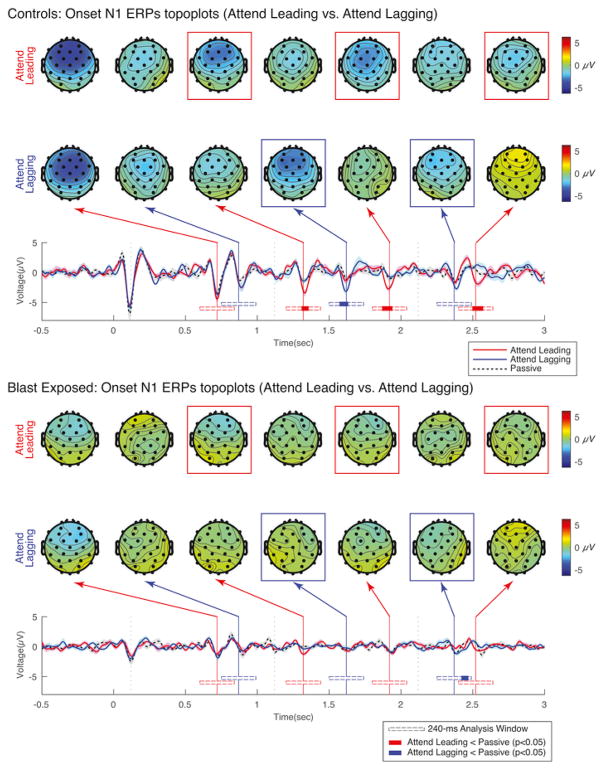
3.5. Attentional modulation of ERPs is weak in blast-exposed listeners
Fig. 8 shows EEG responses to identical sound mixtures for both control subjects (top panel) and blast-exposed subjects (bottom panel). The top of each panel shows the average scalp distribution taken at key times that correspond to the expected times of N1 peaks in response to different notes in the sound mixture (120 ms after the onset of the notes in Leading and Lagging melodies), separately for Attend Leading (top row), Attend Lagging (bottom row) trials. The bottom of each panel shows the across-subject average ERP (averaged across frontal-central electrodes AF3, AF4, F3, F4, and Fz), separately for Attend Leading (solid red), Attend Lagging (solid blue), and Passive (dashed black) trials. The vertical lines in the ERP plots denote the expected N1 times, colored according to whether the corresponding note onset was in the leading melody (red) or the lagging melody (blue) in the mixture.
Fig. 8.
Evoked response potentials to onsets of notes in the same sound mixtures when listeners are attending and when they are ignoring Leading and Lagging streams. Top panel shows results for control subjects while bottom panel shows results for blast-exposed subjects. The bottom of each panel plots the average ERP from frontal electrodes as a function of time. Vertical lines in these plots show the expected times of N1 ERPs to notes in the Leading (red) and Lagging (blue) melodies. Scalp distributions for these time points are shown at the top of each panel for Attend Leading trials (top row) and Attend Lagging trials (bottom row). Beneath the ERPs are shown analysis windows for the cluster-based analysis (open horizontal bars). Times in which there are significantly stronger responses to an attended note than to that same note when it is ignored are shown in color (red for the leading note onsets, blue for the lagging note onsets).
As reported in the original control-subject study (Choi et al., 2014), for the control subjects, the N1 peaks evoked by particular notes in the mixture are relatively large when the corresponding stream is being attended, and relatively small when the same stream is being ignored. In other words, for the top panel, we see larger peaks in the red traces at the times marked by the vertical red lines and larger peaks in the blue traces at the times marked by the vertical blue lines. The scalp distributions for the control subjects demonstrate that the N1 peaks are strongest over the fronto-central electrodes, as mentioned previously. Here, we reanalyzed the original ERP data to contrast Attend Leading and Passive trials and Attend Lagging and Passive trials using a previously developed non-parametric cluster-level analysis method (Maris and Oostenveld, 2007). Specifically, we analyzed samples within a 240-ms time window starting at the onset of each note, shown below the average ERP data as a horizontal bar. The solid areas within each of these analysis bars denote time periods when the N1 response is significantly larger when subjects are asked to attend to the corresponding stream than in the Passive condition.
As previously reported, the N1 responses to the first note of each of the Leading melodic streams were strong in all three listening conditions (Attend Leading, Attend Lagging, and Passive), and did not differ in strength across listening condition (analysis bars are open for the initial onsets). This result is thought to be the result of robust stimulus-driven exogenous attention (Choi et al., 2014). In the selected control subjects, the N1 response to the first note in the Lagging stream did not differ significantly across conditions; however, the N1 peaks to the onsets of all subsequent notes in both of the attended melodies tended to be stronger than the same N1 peaks in the Passive trials (p < 0.05). In particular, for all three subsequent notes in the leading melody and the second note onset in the lagging melody, significant modulation of the N1 was found with attentional focus. For the third note in the lagging melody, the difference went in the expected direction (the N1 was larger in Attend Lagging than Passive trials); however, this difference failed to reach statistical significance.
For the blast-exposed subjects, the scalp potentials were substantially weaker than for the control group. Furthermore, we observed no enhancement of the neural representation of an attended melody. The same cluster analysis used to compare Attend and Passive conditions in the control group found no significant differences when comparing the three N1s evoked by notes in the leading melody for Attend Leading versus Passive trials, and found no difference in the N1 evoked by the second note in the lagging melody for Attend Lagging versus Passive trials. The cluster analysis did find one significant difference within the N1-evoked analysis window at the final note in the lagging melody for Attend Lagging versus Passive trials; however, given the latency of this difference, it is unlikely this difference is representative of a true N1 onset-evoked response.
4. Discussion
4.1. Peripheral hearing loss cannot account for poor selective attention ability
A majority of our blast-exposed participants complained of having trouble following conversations in situations with multiple talkers or interfering sounds, based on self-report from the short-form SSQ survey. The same survey also suggests that the blast-exposed subjects have problems with perceptually segregating multiple sound sources from one another. These findings are consistent with anecdotal reports coming from several VA audiology clinics (Saunders and Echt, 2012) and a few published studies (Gallun et al., 2012; Lew, 2007; Lew et al., 2007). The blast-exposed subjects also exhibited poor behavioral ability on our selective auditory attention task.
By the very nature of their duties, Military Service Members are at increased risk of exposure to dangerously high levels of sound, both from prolonged (e.g., engine rumble) and/or multiple transient events (e.g. small arms fire, blast). The effects of blast on sensory coding in the periphery of the auditory system are not yet understood, but could lead to additional damage, including both “ordinary” hearing loss as well as hidden hearing loss.
Both elevated hearing thresholds and hidden hearing loss are associated with noise exposure. It is therefore reasonable to wonder if one or both forms of sensory damage explain the poor hearing abilities in our blast-exposed subjects. Specifically, our subjects report problems with processing sound when there are simultaneous sources and performed poorly in our laboratory test of selective auditory attention.
We excluded listeners who had significant hearing loss, as measured by the audiogram, and specifically evaluated the fidelity of the brainstem responses from the remaining (H1 hearing profile) blast-exposed Service Members using the EFR. We found no significant difference between the supra-threshold coding fidelity in the blast-exposed subjects compared to a cohort of normal-hearing young adult listeners. Indeed, the strength of the brainstem EFR in the top half of our blast-exposed listeners was comparable to that of the top half of our control subjects—and exceeded the strength of the response from the bottom half of the control subject group. However, when comparing selective auditory attention ability, the best of our blast-exposed listeners barely reached the performance levels of the worst of our control subjects.
In other words, there are significant differences in supra-threshold hearing fidelity amongst the blast-exposed subjects we tested; however, the best of our blast-exposed listeners appear to have better supra-threshold hearing than the worst of our control listeners. Despite this, our best blast-exposed listeners perform equal to or worse than control listeners on our selective auditory attention task. Given this, we do not believe that differences in sensory coding can explain the poor hearing ability of our blast-exposed listeners.
4.2. Damage to cortical networks may explain poor selective auditory attention performance
Problems controlling selective attention are a common symptom associated with mild traumatic brain injury (Nuwer et al., 2005). Given the importance of communication within diverse brain regions that make up the network responsible for attentional control, it is possible that the difficulties our blast-exposed subjects experience in multi-source settings arises due to damage to these cortical networks, either from focal damage to computational areas important for attention, or from damage to white-matter tracks critical for conveying information from one region to another. Fronto-parietal regions are particularly vulnerable to subdural hemorrhage due to blast (Taber et al., 2006), and are also critical for executive control of attention (Corbetta and Shulman, 2002; Michalka et al., 2015). Another study looking at the effects of blast exposure in subjects with and without a diagnosis of TBI documented significantly lower fractional anisotrophy scores in the inferior fronto-occipital fasciculus, a fiber tract bundle connecting the ventromedial occipital lobe and the orbitofrontal cortex (Martino et al., 2010). This result suggests that blast disrupts long-range connections from fronto-occipital areas involved in attentional processing to sensory and parietal regions that help make up the spatial-attention network (Corbetta and Shulman, 2002; Michalka et al., 2015).
The auditory selective attention task in this study utilized stimuli that contained salient pitch differences as well as modest spatial differences that allowed normal-hearing control subjects to easily segregate, select, and analyze whatever stream was to be attended in the mixture of sounds. Normal-hearing non-blast control subjects performed at or near ceiling and exhibited enhanced neural representations of the onsets to the individual notes of the attended melody (see Fig. 8, top). In the original study, normal-hearing controls also performed reasonably well when the pitch cue was removed (same pitch condition), which made it harder to focus attention on the correct melody (Choi et al., 2014). In this harder version of the task, performance for the control subjects varied from perfect down to chance—comparable to how our blast-exposed subjects performed when the “redundant” pitch cue was available (and when control subjects performed at or better than 90% correct). Importantly, in the original study, the amount of attentional amplification of neural ERPs that an individual control subject exhibited in the easy, different-pitch condition correlated with performance in the same-pitch task. This result suggests that the efficacy of attentional control (as measured by the modulation of ERPs based on attentional focus) varies significantly across control listeners; when a task is sufficiently easy, all listeners may do well, regardless of how well they can control attention, but when a task is hard, these individual differences in attentional control determine performance.
Here, blast-exposed subjects performed substantially worse than normal hearing controls, as if they are particularly bad at controlling selective auditory attention. This poor performance was also reflected in weaker ERPs; moreover, there was no evidence of neural modulation of ERPs due to attentional focus in the blast-exposed listeners. This result is consistent with the idea that blast-exposure damages control networks that are critical in selective auditory attention tasks. Previous electrophysiological evidence suggests that TBI patients are impaired in their ability to filter out unwanted or irrelevant sensory information (Arciniegas et al., 2014), supporting this kind of explanation. Such impairments of executive function certainly could explain why more and more normal-hearing blast-exposed Service Members are seeking aid in VA-affiliated audiology clinics across the country.
4.3. Caveats
Problems with memory are also associated with TBI; thus, we cannot rule out the possibility that on some trials subjects either forgot the cue that described what stream to attend or whether to withhold a response. Similarly, even though the memory load on our task was low, it is possible that memory impairments prevented the blast-exposed listeners to hold the note-by-note sequence in memory over the course of the 3-s stimulus, and retain the representation long enough to determine how to answer in the response interval. These types of cognitive failures could explain the poor performance and weak neural responses in our blast-exposed listeners rather than damage to specific attentional networks. Regardless, such deficits are cognitive, rather than sensory, in nature.
Indeed, the behavioral deficits of our blast-exposed subjects are unlikely to be associated exclusively with damage to attentional networks. The blast-exposed group was more likely to fail to respond on Attend trials than were the controls. The blast-exposed group was also less likely to withhold a response on Passive trials than were the controls. These deficits, combined with the low percentage of correct responses, suggest that the blast-exposed listeners had general cognitive deficits that go beyond damage that is specific to control of selective attention. Cognitive function, in general, depends on communication between pre-frontal executive control regions with other brain structures. It is likely the case that cortical damage is present in a range of tasks, not just on selective auditory attention tasks.
It is well established that post-traumatic stress disorder (PTSD) is comorbid with traumatic brain injury (Hoge et al., 2008). All of the blast-exposed subjects recruited for this study were referred to Boston University through the VA Boston Healthcare System as part of an interventional study examining the efficacy of cognitive therapy on PTSD outcomes. Every study participant in the blast group had a PTSD diagnosis; however, not every participant had a confirmed mTBI diagnosis. Because TBI and PTSD have overlapping symptomology, it is possible that additional PTSD-specific symptoms contributed to the behavioral and electrophysiological outcomes of this study. This is a commonly encountered problem with studies involving blast injury in military populations. We are currently gathering data with active duty Service Members both with and without a PTSD diagnosis to tease apart how PTSD and blast may contribute to the deficits we observe here.
While it is possible that damage to cortical grey matter and/or white matter connections in attentional control networks explain the deficits exhibited by our blast-exposed subjects, more work is needed to rule other reasons for problems with cortical control. For instance, PTSD often leads to sleep disorders, drug and alcohol abuse, and other behaviors, which are known to impair cognitive abilities. Rather than physical damage to brain structures, the difficulties that the blast-exposed subjects have may be caused by short-term impairments that can be treated through effective behavioral modification. This is a possibility that needs further investigation.
Finally, it is worth reiterating that results from the normal-hearing controls were from historical data from two previously published studies from our group. Because subjects from these two studies were recruited through advertisements posted on the Boston University campus, we assumed control participants had no previous exposure to blast. Additionally, we cannot rule out possible effects due to differences in education level achieved or to musical experience, as we did not collect this information in our initial surveys of the blast-exposed Service Members and did not have such information about our control subjects. Our access to blast-exposed Military Service Members was only made possible through generous participant referrals by the Neurorehabilitation Lab at VA Boston Healthcare under the direction of Dr. Yelena Bogdanova. At the time this study was conducted, this arrangement did not permit us to directly recruit military personnel from the VA Boston Healthcare campus. Ideally, our control group would have been a better demographic match to the blast-exposed participants; however, we did not have access to such a pool of participants at that time. The above-mentioned study now underway, as well as other future studies, need to directly address this issue.
5. Conclusions
Despite the prevalence of noise exposure in the Veteran population, sensory damage alone cannot account for the behavioral and electrophysiological deficits we found. Blast-exposed subjects had near normal hearing thresholds; they also demonstrated normal supra-threshold sound coding fidelity. Despite this, their self-reports indicate great difficulties understanding speech in noise and segregating sounds appearing in a mixture of competing sounds. The blast-exposed Veterans also fail when asked to perform a low-memory load task that requires them to focus selective auditory attention—as well as on other cognitively demanding aspects of our experiment, such as withholding responses on certain trials, or making responses within a limited time period. While it is beyond the scope of this study to determine the cause of these deficits, we conclude that cognitive, rather than sensory, factors are likely to blame.
Most importantly, this work demonstrates that blast-exposed Veterans have difficulty understanding sound when there are competing, distracting sounds. Given this, blast-exposed military personnel are likely to have difficulty communicating in everyday social settings, which can lead to social isolation and depression. Further work to understand the root causes of these cognitive deficits is critical in order to determine how to treat such problems.
Acknowledgments
The authors thank Dr. Yelena Bogdanova, Sarah Kark, and Vivian Ho at VA Boston Healthcare System, Jamaica Plain Campus for their assistance with subject referrals. This project was supported by NIH RO1 DC009477, and by a National Security Science and Engineering Fellowship to BGSC. The authors declare no competing financial interests.
References
- Almeida-Suhett CP, Prager EM, Pidoplichko V, Figueiredo TH, Marini AM, Li Z, Eiden LE, Braga MFM. GABAergic interneuronal loss and reduced inhibitory synaptic transmission in the hippocampal CA1 region after mild traumatic brain injury. Exp Neurol. 2015;273:11–23. doi: 10.1016/j.expneurol.2015.07.028. http://dx.doi.org/10.1016/j.expneurol.2015.07.028. [DOI] [PubMed] [Google Scholar]
- Arciniegas D, Olincy A, Topkoff J, McRae K, Cawthra E, al E. Impaired auditory gating and P50 nonsuppression following traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2014;12:77–85. doi: 10.1176/jnp.12.1.77. [DOI] [PubMed] [Google Scholar]
- Baugh CM, Stamm JM, Riley DO, Gavett BE, Shenton ME, Lin A, Nowinski CJ, Cantu RC, McKee AC, Stern RA. Chronic traumatic en cephalopathy: neurodegeneration following repetitive concussive and subconcussive brain trauma. Brain Imaging Behav. 2012;6:244–254. doi: 10.1007/s11682-012-9164-5. http://dx.doi.org/10.1007/s11682-012-9164-5. [DOI] [PubMed] [Google Scholar]
- Bauman RA, Ling G, Tong L, Januszkiewicz A, Agoston D, Delanerolle N, Kim Y, Ritzel D, Bell R, Ecklund J, Armonda R, Bandak F, Parks S. An Introductory Characterization of a Combat-casualty-care Relevant Swine Model of Closed Head Injury Resulting from Exposure to Explosive Blast. 2009 doi: 10.1089/neu.2008.0898. http://dx.doi.org.ezproxy.bu.edu/10.1089/neu.2008.0898. [DOI] [PubMed]
- Bergemalm PO, Lyxell B. Appearances are deceptive? Long-term cognitive and central auditory sequelae from closed head injury Int J Audiol. 2009;44:39–49. doi: 10.1080/14992020400022546. http://dx.doi.org/10.1080/14992020400022546. [DOI] [PubMed] [Google Scholar]
- Bernstein LR, Trahiotis C. Enhancing sensitivity to interaural delays at high frequencies by using “transposed stimuli. J Acoust Soc Am. 2002;112:1026–1036. doi: 10.1121/1.1497620. http://dx.doi.org/10.1121/1.1497620. [DOI] [PubMed] [Google Scholar]
- Bharadwaj HM, Bharadwaj H, Shinn-Cunningham BG, Shinn-Cunningham B. Rapid acquisition of auditory subcortical steady state responses using multi-channel recordings. Clin Neurophysiol. 2014;125:1878–1888. doi: 10.1016/j.clinph.2014.01.011. http://dx.doi.org/10.1016/j.clinph.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj HM, Masud S, Mehraei G, Verhulst S, Shinn-Cunningham BG. Individual differences reveal correlates of hidden hearing deficits. J Neurosci. 2015;35:2161–2172. doi: 10.1523/JNEUROSCI.3915-14.2015. http://dx.doi.org/10.1523/JNEUROSCI.3915-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spatial Vision. 1997;10(4):433–436. [PubMed] [Google Scholar]
- Chapman JC, Diaz-Arrastia R. Military traumatic brain injury: a review. Alzheimer's Dementia. 2014;10:S97–S104. doi: 10.1016/j.jalz.2014.04.012. http://dx.doi.org/10.1016/j.jalz.2014.04.012. [DOI] [PubMed] [Google Scholar]
- Cherry EC. Some experiments on the recognition of speech, with one and with two ears. J Acoust Soc Am. 1953;25:975–979. http://dx.doi.org/10.1121/1.1907229. [Google Scholar]
- Choi I, Wang Le, Bharadwaj H, Shinn-Cunningham B. Hearing research. Hear Res. 2014;314:10–19. doi: 10.1016/j.heares.2014.04.008. http://dx.doi.org/10.1016/j.heares.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. http://dx.doi.org/10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Defense and Veterans Brain Injury Center DVBIC. DoD TBI Worldwide Numbers since 2000. 2016. [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Demeester K, Topsakal V, Hendrickx JJ, Fransen E, van Laer L, Van Camp G, Van de Heyning P, Van Wieringen A. Hearing disability measured by the Speech, Spatial, and Qualities of Hearing Scale in clinically normal-hearing and hearing-impaired middle-aged persons, and disability screening by means of a reduced SSQ (the SSQ5) Ear Hear. 2012;33:615–616. doi: 10.1097/AUD.0b013e31824e0ba7. [DOI] [PubMed] [Google Scholar]
- Engel A, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top–down processing. Nat Rev Neurosci. 2001;2(10):704–716. doi: 10.1038/35094565. http://doi.org/10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- Farmer CM, Krull H, Concannon TW, Simmons M. Characteristics and Treatment Patterns of Service Members with Mild Traumatic Brain Injury. RAND Corporation; 2016. [Google Scholar]
- Fausti SA, Wilmington DJ, Gallun FJ, Myers PJ, Henry JA. Auditory and vestibular dysfunction associated with blast-related traumatic brain injury. JRRD. 2009;46:797. doi: 10.1682/jrrd.2008.09.0118. http://dx.doi.org/10.1682/JRRD.2008.09.0118. [DOI] [PubMed] [Google Scholar]
- Fries P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu Rev Neurosci. 2009;32:209–224. doi: 10.1146/annurev.neuro.051508.135603. http://dx.doi.org/10.1146/annurev.neuro.051508.135603. [DOI] [PubMed] [Google Scholar]
- Furman AC, Kujawa SG, Liberman MC. Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. J Neurophysiol. 2013;110:577–586. doi: 10.1152/jn.00164.2013. http://dx.doi.org/10.1152/jn.00164.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallun FJ, Diedesch AC, Kubli LR, Walden TC, Folmer RL, Lewis MS, McDermott DJ, Fausti SA, Leek MR. Performance on tests of central auditory processing by individuals exposed to high-intensity blasts. J Rehabil Res Dev. 2012;49:1005–1025. doi: 10.1682/jrrd.2012.03.0038. [DOI] [PubMed] [Google Scholar]
- Giraud AL, Poeppel D. Cortical oscillations and speech processing: emerging computational principles and operations. Nat Neurosci. 2012;15:511–517. doi: 10.1038/nn.3063. http://dx.doi.org/10.1038/nn.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goblick TJ, Jr, Pfeiffer RR. Time-domain measurements of cochlear nonlinearities using combination click stimuli. J Acoust Soc Am. 1969;46:924–938. doi: 10.1121/1.1911812. [DOI] [PubMed] [Google Scholar]
- Hasenstaub A, Shu Y, Haider B, Kraushaar U, Duque A, McCormick DA. Inhibitory postsynaptic potentials carry synchronized frequency information in active cortical networks. Neuron. 2005;47:423–435. doi: 10.1016/j.neuron.2005.06.016. http://dx.doi.org/10.1016/j.neuron.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Hillyard S. Auditory evoked potentials during selective listening to dichotic speech messages. Percept Psychophys. 1976;20:236–242. [Google Scholar]
- Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in US soldiers returning from Iraq. N Engl J Med. 2008;358:453–463. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- Hoover EC, Souza PE, Gallun FJ. Competing views on abnormal auditory results after mild traumatic brain injury. Perspect Hear Hear Dis Res Diagn. 2015:19. http://dx.doi.org/10.1044/hhd19.1.12.12-10.
- Krause MO, Kennedy MRT, Nelson PB. Masking release, processing speed and listening effort in adults with traumatic brain injury. Brain Inj. 2014;28:1473–1484. doi: 10.3109/02699052.2014.920520. http://dx.doi.org/10.3109/02699052.2014.920520. [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009;29:14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. http://dx.doi.org/10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok FY, Lee TMC, Leung CHS, Poon WS. Changes of cognitive functioning following mild traumatic brain injury over a 3-month period. Brain Inj. 2009;22:740–751. doi: 10.1080/02699050802336989. http://dx.doi.org/10.1080/02699050802336989. [DOI] [PubMed] [Google Scholar]
- Lachaux J, Rodriguez E, Martinerie J, Varela F. Measuring phase synchrony in brain signals. Hum Brain Mapp. 1999;8:194–208. doi: 10.1002/(SICI)1097-0193(1999)8:4<194::AID-HBM4>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagarde E, Salmi L-R, Holm LW, Contrand B, Masson F, Ribéreau-Gayon R, Laborey M, Cassidy JD. Association of symptoms following mild traumatic brain injury with posttraumatic stress disorder vs postconcussion syndrome. JAMA Psychiatry. 2014 doi: 10.1001/jamapsychiatry.2014.666. http://dx.doi.org/10.1001/jamapsychiatry.2014.666. [DOI] [PubMed]
- Levin HS, Mattis S, Ruff RM, Eisenberg HM, Marshall LF, Tabaddor K, High WM, Frankowski RF. Neurobehavioral outcome following minor head injury: a three-center study. J Neurosurg. 1987;66:234–243. doi: 10.3171/jns.1987.66.2.0234. http://dx.doi.org/10.3171/jns.1987.66.2.0234. [DOI] [PubMed] [Google Scholar]
- Lew HL. Program development and defining characteristics of returning military in a VA Polytrauma Network Site. JRRD. 2007;44:1027–1034. http://dx.doi.org/10.1682/JRRD.2007.05.0073. [PubMed] [Google Scholar]
- Lew HL, Jerger JF, Guillory SB, Henry JA. Auditory dysfunction in traumatic brain injury. JRRD. 2007;44:921–928. doi: 10.1682/jrrd.2007.09.0140. http://dx.doi.org/10.1682/JRRD.2007.09.0140. [DOI] [PubMed] [Google Scholar]
- Lobarinas E, Salvi R, Ding D. Insensitivity of the audiogram to carboplatin induced inner hair cell loss in chinchillas. Hear Res. 2013;302:113–120. doi: 10.1016/j.heares.2013.03.012. http://dx.doi.org/10.1016/j.heares.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein DH, Thomas MJ, Smith DH, McIntosh TK. Selective vulnerability of dentate hilar neurons following traumatic brain injury: a potential mechanistic link between head trauma and disorders of the hippocampus. J Neurosci. 1992;12:4846–4853. doi: 10.1523/JNEUROSCI.12-12-04846.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Donald CL, Johnson AM, Cooper D, Nelson EC, Werner NJ, Shimony JS, Snyder AZ, Raichle ME, Witherow JR, Fang R, Flaherty SF, Brody DL. Detection of blast-related traumatic brain injury in U.S. Military personnel. N Engl J Med. 2011;364:2091–2100. doi: 10.1056/NEJMoa1008069. http://dx.doi.org/10.1056/nejmoa1008069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Management of Concussion/mTBI Working Group. VA/DoD clinical practice guideline for management of concussion/mild traumatic brain injury. J Rehabil Res Dev. 2009;46:CP1–68. [PubMed] [Google Scholar]
- Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007;164:177–190. doi: 10.1016/j.jneumeth.2007.03.024. http://dx.doi.org/10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Martino J, Brogna C, Robles SG, Vergani F, Duffau H. Anatomic dissection of the inferior fronto-occipital fasciculus revisited in the lights of brain stimulation data. Cortex. 2010;46:691–699. doi: 10.1016/j.cortex.2009.07.015. http://dx.doi.org/10.1016/j.cortex.2009.07.015. [DOI] [PubMed] [Google Scholar]
- McKee AC, Robinson ME. Military-related traumatic brain injury and neu-rodegeneration. Alzheimer's Dementia. 2014;10:S242–S253. doi: 10.1016/j.jalz.2014.04.003. http://dx.doi.org/10.1016/j.jalz.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehraei G, Hickox AE, Bharadwaj HM, Goldberg H, Verhulst S, Liberman MC, Shinn-Cunningham BG. Auditory brainstem response latency in noise as a marker of cochlear synaptopathy. J Neurosci. 2016;36:3755–3764. doi: 10.1523/JNEUROSCI.4460-15.2016. http://dx.doi.org/10.1523/JNEUROSCI.4460-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesgarani N, Chang EF. Selective cortical representation of attended speaker in multi-talker speech perception. Nature. 2012:1–5. doi: 10.1038/nature11020. http://dx.doi.org/10.1038/nature11020. [DOI] [PMC free article] [PubMed]
- Mez J, Stern RA, McKee AC. Chronic traumatic encephalopathy: where are we and where are we going? Curr Neurol Neurosci Rep. 2013;13:407–412. doi: 10.1007/s11910-013-0407-7. http://dx.doi.org/10.1007/s11910-013-0407-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalka SW, Kong L, Rosen ML, Shinn-Cunningham BG, Somers DC. Short-term memory for space and time flexibly recruit complementary sensory-biased frontal lobe attention networks. Neuron. 2015;87:882–892. doi: 10.1016/j.neuron.2015.07.028. http://dx.doi.org/10.1016/j.neuron.2015.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munjal SK, Panda NK, Pathak A. Relationship between severity of traumatic brain injury (TBI) and extent of auditory dysfunction. Brain Inj. 2010;24:525–532. doi: 10.3109/02699050903516872. http://dx.doi.org/10.3109/02699050903516872. [DOI] [PubMed] [Google Scholar]
- Noble W, Jensen NS, Naylor G, Bhullar N. A short form of the Speech, Spatial and Qualities of Hearing scale suitable for clinical use: the SSQ12. Int J Audiol. 2013:409–412. doi: 10.3109/14992027.2013.781278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuwer MR, Hovda DA, Schrader LM, Vespa PM. Routine and quantitative EEG in mild traumatic brain injury. Clin Neurophysiol. 2005;116:2001–2025. doi: 10.1016/j.clinph.2005.05.008. http://dx.doi.org/10.1016/j.clinph.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Oleksiak M, Smith BM, Andre JRS, Caughlan CM, Steiner M. Audiological issues and hearing loss among veterans with mild traumatic brain injury. JRRD. 2012;49:995–1010. doi: 10.1682/jrrd.2011.01.0001. http://dx.doi.org/10.1682/JRRD.2011.01.0001. [DOI] [PubMed] [Google Scholar]
- Picton TW, Hillyard SA. Human auditory evoked potentials. II: effects of attention Electroencephalogr Clin Neurophysiol. 1974;36:191–200. doi: 10.1016/0013-4694(74)90156-4. [DOI] [PubMed] [Google Scholar]
- Rosenfeld JV, Ford NL. Bomb blast, mild traumatic brain injury and psychiatric morbidity: a review. Injury. 2010;41:437–443. doi: 10.1016/j.injury.2009.11.018. http://dx.doi.org/10.1016/j.injury.2009.11.018. [DOI] [PubMed] [Google Scholar]
- Ruggles D, Bharadwaj H, Shinn-Cunningham BG. Why middle-aged listeners have trouble hearing in everyday settings. Curr Biol. 2012;22:1417–1422. doi: 10.1016/j.cub.2012.05.025. http://dx.doi.org/10.1016/j.cub.2012.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggles D, Bharadwaj H, Shinn-Cunningham BG. Normal hearing is not enough to guarantee robust encoding of suprathreshold features important in everyday communication. PNAS. 2011;108:15516–15521. doi: 10.1073/pnas.1108912108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggles D, Shinn-Cunningham B. Spatial selective auditory attention in the presence of reverberant energy: individual differences in normal-hearing listeners. JARO. 2011;12:395–405. doi: 10.1007/s10162-010-0254-z. http://dx.doi.org/10.1007/s10162-010-0254-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders GH, Echt KV. Blast exposure and dual sensory impairment: an evidence review and integrated rehabilitation approach. JRRD. 2012:49. doi: 10.1682/jrrd.2010.08.0157. [DOI] [PubMed]
- Saunders GH, Frederick MT, Arnold M, Silverman S, Chisolm TH, Myers P. Auditory difficulties in blast-exposed Veterans with clinically normal hearing. JRRD. 2015;52:343–360. doi: 10.1682/JRRD.2014.11.0275. http://dx.doi.org/10.1682/jrrd.2014.11.0275. [DOI] [PubMed] [Google Scholar]
- Schaette R, McAlpine D. Tinnitus with a normal audiogram: physiological evidence for hidden hearing loss and computational model. J Neu-rosci. 2011;31:13452–13457. doi: 10.1523/JNEUROSCI.2156-11.2011. http://dx.doi.org/10.1523/JNEUROSCI.2156-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab KA, Ivins B, Cramer G, Johnson W, Sluss-Tiller M, Kiley K, Lux W, Warden D. Screening for traumatic brain injury in troops returning from deployment in Afghanistan and Iraq: initial investigation of the usefulness of a short screening tool for traumatic brain injury. J Head Trauma Rehabil. 2007;22:377–389. doi: 10.1097/01.HTR.0000300233.98242.87. http://dx.doi.org/10.1097/01.HTR.0000300233.98242.87. [DOI] [PubMed] [Google Scholar]
- Shaheen LA, Valero MD, Liberman MC. Towards a diagnosis of cochlear neuropathy with envelope following responses. JARO. 2015 doi: 10.1007/s10162-015-0539-3. http://dx.doi.org/10.1007/s10162-015-0539-3. [DOI] [PMC free article] [PubMed]
- Shinn-Cunningham BG. Object-based auditory and visual attention. Trends Cogn Sci. 2008;12:182–186. doi: 10.1016/j.tics.2008.02.003. http://dx.doi.org/10.1016/j.tics.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinn-Cunningham BG, Best V. Selective attention in normal and impaired hearing. Trends Amplif. 2008;12:283–299. doi: 10.1177/1084713808325306. http://dx.doi.org/10.1177/1084713808325306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoe E, Kraus N. Auditory brain stem response to complex sounds: a tutorial. Ear Hear. 2010;31:302. doi: 10.1097/AUD.0b013e3181cdb272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smetana WR. Predicting Hearing Health Readiness as a Function of the Army Profile System Formula and Occupational Safety and Health Administration Reportable Hearing Loss 1999 [Google Scholar]
- Sosa MAG, De Gasperi R, Paulino AJ, Pricop PE, Shaughness MC, Maudlin-Jeronimo E, Hall AA, Janssen WGM, Yuk FJ, Dorr NP, Dickstein DL, McCarron RM, Chavko M, Hof PR, Ahlers ST, Elder GA. Blast overpressure induces shear-related injuries in the brain of rats exposed to a mild traumatic brain injury. Acta Neuropathol Commun. 2013;1:51. doi: 10.1186/2051-5960-1-51. http://dx.doi.org/10.1186/2051-5960-1-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber K, Hurley R. Traumatic axonal injury: atlas of major pathways. J Neuropsychiatry Clin Neurosci. 2007;19:iv–104. doi: 10.1176/jnp.2007.19.2.iv. [DOI] [PubMed] [Google Scholar]
- Taber K, Warden D, Hurley R. Blast-related traumatic brain injury: what is known? J Neuropsychiatry Clin Neurosci. 2006;18:141–145. doi: 10.1176/jnp.2006.18.2.141. [DOI] [PubMed] [Google Scholar]
- Taber KH, Hurley RA, Haswell CC, Rowland JA, Hurt SD, Lamar CD, Morey RA. White matter compromise in veterans exposed to primary blast forces. J Head Trauma Rehabil. 2015;30:E15–E25. doi: 10.1097/HTR.0000000000000030. http://dx.doi.org/10.1097/HTR.0000000000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanielian T, Jaycox LH. Invisible Wounds of War. RAND Corporation; 2008. [Google Scholar]
- Terrio H, Brenner LA, Ivins BJ, Cho JM, Helmick K, Schwab K, Scally K, Bretthauer R, Warden D. Traumatic brain injury screening: preliminary findings in a US Army Brigade Combat Team. J Head Trauma Reha-bil. 2009;24:14–23. doi: 10.1097/HTR.0b013e31819581d8. [DOI] [PubMed] [Google Scholar]
- Uusitalo MA, Ilmoniemi RJ. Signal-space projection method for separating MEG or EEG into components. Med Biol Eng Comput. 1997;35:135–140. doi: 10.1007/BF02534144. http://dx.doi.org/10.1007/BF02534144. [DOI] [PubMed] [Google Scholar]
- Valero MD, Hancock KE, Liberman MC. The middle ear muscle reflex in the diagnosis of cochlear neuropathy. Hear Res. 2016;332:29–38. doi: 10.1016/j.heares.2015.11.005. http://dx.doi.org/10.1016/j.heares.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana LM, O'Malley JT, Burgess BJ, Jones DD, Oliveira CACP, Santos F, Merchant SN, Liberman LD, Liberman MC. Cochlear neuropathy in human presbycusis: confocal analysis of hidden hearing loss in post-mortem tissue. Hear Res. 2015;327:78–88. doi: 10.1016/j.heares.2015.04.014. http://dx.doi.org/10.1016/j.heares.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf JA, Koch PF. Disruption of network synchrony and cognitive dysfunction after traumatic brain injury. Front Syst Neurosci. 2016;10:1629–1714. doi: 10.3389/fnsys.2016.00043. http://dx.doi.org/10.3389/fnsys.2016.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Bharadwaj H, Xia J, Shinn-Cunningham B. A comparison of spectral magnitude and phase-locking value analyses of the frequency-following response to complex tones. J Acoust Soc Am. 2013;134:384. doi: 10.1121/1.4807498. http://dx.doi.org/10.1121/1.4807498. [DOI] [PMC free article] [PubMed] [Google Scholar]