Abstract
Antimalarials are used to treat dermatomyositis (DM) and cutaneous lupus erythematosus (CLE). Although hydroxychloroquine (HCQ) is frequently used, addition of quinacrine (QC) has shown additional clinical effects when combined with HCQ. To quantify the effects of HCQ versus QC in suppressing secretion of tumor necrosis factor-α (TNF-α) and IFN-α from the peripheral blood mononuclear cells of DM and CLE patients, lipopolysaccharide-stimulated and control peripheral blood mononuclear cells from DM and CLE patients and control subjects were analyzed for the effect of HCQ and QC on TNF-α and IFN-α production using ELISA testing. Flow cytometry showed the effects of these therapies on intracellular TNF-α in myeloid dendritic cells and monocytes of DM patients and control subjects. QC significantly suppressed TNF-α relative to HCQ from unstimulated and lipopolysaccharide-stimulated peripheral blood mononuclear cells of DM and CLE patients (P < 0.0001). It suppressed IFN-α as significantly as HCQ from cytosine phosphodiester guanine—stimulated peripheral blood mononuclear cells of DM and CLE patients (P < 0.0001). Flow cytometry showed that QC significantly suppressed intracellular expression of TNF-α from the lipopolysaccharide-stimulated myeloid dendritic cells and monocytes of DM patients (P-values≤0.0008). In conclusion, QC likely has a different mechanism of action than HCQ, given the broader inhibition of proinflammatory cytokines, including both TNF-α and IFN-α.
INTRODUCTION
Antimalarials are frequently used as first-line therapy to treat the skin manifestations of connective tissue diseases, including cutaneous lupus erythematosus (CLE) and dermatomyositis (DM) (Ang and Werth, 2005; Cavazzana et al., 2009; Chang et al., 2011). Several case series suggest that quinacrine (QC) confers significant additional therapeutic effects not seen with hydroxychloroquine (HCQ) alone in cases of recalcitrant CLE (Chang et al., 2011; Toubi et al., 2000). Although approximately 50% of CLE patients respond to HCQ, potentially two thirds of the remaining patients respond when QC therapy is added (Chang et al., 2011). However, QC is now available only as a compounded medication, making understanding potential distinguishing features of its mechanism of action imperative, particularly because there is risk of losing access to this medication (Benoit and Goebeler, 2015). African Americans frequently have generalized discoid lupus erythematosus, a form of CLE that is more refractory to therapy (Bonilla-Martinez et al., 2008; Moghadam-Kia et al., 2009).
Antimalarials have been used for decades because of their excellent risk-to-benefit ratio. Some of QC’s more frequent adverse effects mirror those of HCQ and chloroquine (CQ) and include gastrointestinal effects in the form of nausea, vomiting, and diarrhea and the development of gray-blue skin pigmentation, both of which are dose-dependent and reversible upon discontinuation (Ruiz-Irastorza et al., 2010). QC, unlike HCQ and CQ, fails to induce retinopathy, but may induce yellowing of the skin in some patients and carries a higher risk of hematologic adverse effects including aplastic anemia at doses higher than those recommended (Jover et al., 2012).
Antimalarial drugs exhibit a mechanism of action that has yet to be fully elucidated; however, they have been shown to inhibit the secretion of key proinflammatory cytokines such as tumor necrosis factor-α (TNF-α) and IFN-α from peripheral blood mononuclear cells (PBMCs) (Kalia and Dutz, 2007; Sacre et al., 2012). Given that both TNF-α and IFN-α have been implicated in the pathogenesis of DM and CLE, expanding understanding of the differential effects of antimalarials on proinflammatory cytokines may offer insight into why therapeutic responses vary (Braunstein et al., 2012; Nabatian et al., 2012; Wenzel et al., 2005; Wong et al., 2012). We examined the PBMCs of DM and CLE patients to quantify the ability of QC to inhibit secretion of TNF-α and IFN-α versus HCQ and controls.
RESULTS
QC, but not HCQ, suppresses TNF-α protein production by PBMCs
QC was found to significantly suppress secretion of TNF-α compared with HCQ from both the unstimulated and lipopolysaccharide (LPS)-stimulated PBMCs of DM and CLE patients and control subjects (Table 1). Linear mixed modeling found a statistically significant difference between the means for QC and QC plus HCQ versus sham (P < 0.0001, Table 1). The subgroup analyses within each patient group gave similar results (Figure 1). At 18 hours, 99.3% of sham-treated cells were viable, as assessed by Trypan Blue exclusion. 98.1% of cells treated with 1 µg/ml QC and LPS and 97.6% of cells treated with 1 µg/ml HCQ with LPS were viable.
Table 1.
Mean and SEM of TNF-α secretion from the unstimulated and LPS-stimulated PBMCs of DM and CLE patients and control subjects given treatment with HCQ, QC, combination QC + HCQ, and sham1
| Treatment | Mean | SEM | P-Value2 | Treatment | Mean | SEM | P-Value1 |
|---|---|---|---|---|---|---|---|
| Sham (reference group) | 2.08 | 0.62 | — | LPS (reference group) | 6.73 | 0.45 | — |
| HCQ | 1.63 | 0.62 | 0.0523 | LPS + HCQ | 6.86 | 0.45 | 0.3577 |
| QC | 0.54 | 0.62 | <0.00013 | LPS + QC | 5.70 | 0.45 | <0.00013 |
| QC + HCQ | 0.51 | 0.62 | <0.00013 | LPS + QC + HCQ | 5.54 | 0.45 | <0.00013 |
Abbreviations: CLE, cutaneous lupus erythematosus; DM, dermatomyositis; HCQ, hydroxychloroquine; LPS, lipopolysaccharide; PBMC, peripheral blood mononuclear cell; QC, quinacrine; SEM, standard error of the mean; TNF-α, tumor necrosis factor-α.
The values have been natural log transformed to account for a right-skewed distribution of the raw original values, and units are in ln(pg/ml).
Comparison with the reference group.
P < 0.0001 for QC versus sham, QC + HCQ versus sham, LPS + QC versus LPS, and LPS + QC + HCQ versus LPS.
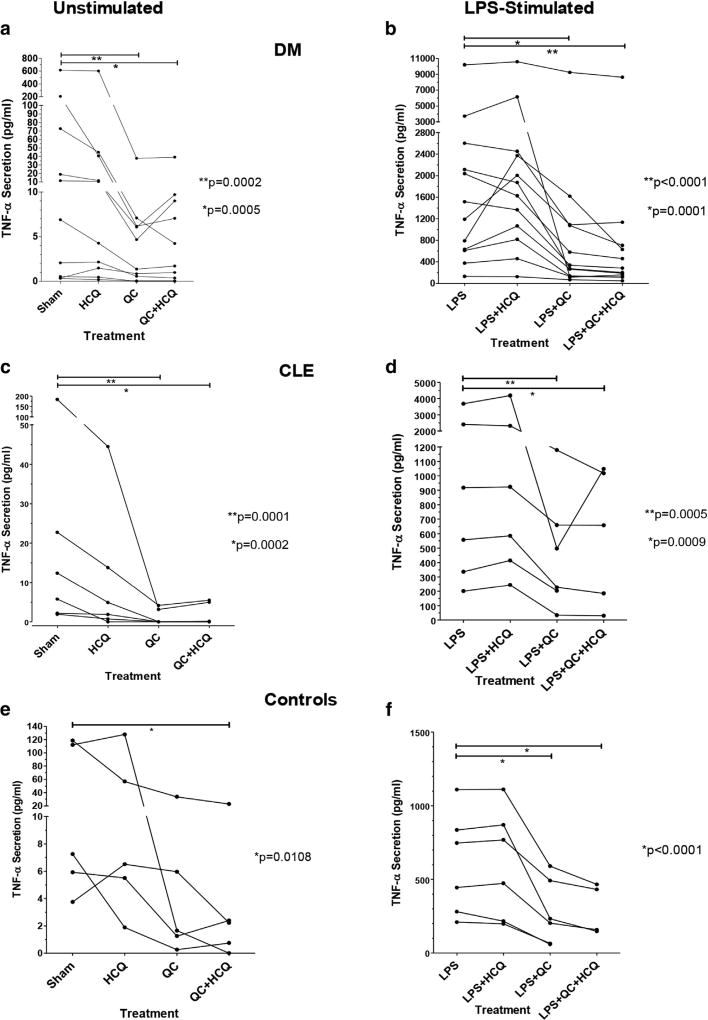
Figure 1. QC, but not HCQ, decreases TNF-α production from PBMCs.
Effects of HCQ, QC and combination QC and HCQ versus sham on secretion of TNF-α from the unstimulated and LPS-stimulated PBMCs of (a) DM patients, unstimulated (**P = 0.0002 for QC vs. sham and *P = 0.0005 for QC + HCQ vs. sham); (b) DM patients, stimulated (*P = 0.0001 for LPS + QC vs. LPS and **P < 0.0001 for LPS + QC þ HCQ vs. LPS); (c) CLE patients, unstimulated (**P = 0.0001 for QC vs. sham and *P = 0.0002 for QC + HCQ vs. sham); (d) CLE patients, stimulated (**P = 0.0005 for LPS + QC vs. LPS and *P = 0.0009 for LPS + QC + HCQ vs. LPS); (e) control subjects, unstimulated (*P = 0.0108 for QC + HCQ vs. sham); (f) control subjects, stimulated (*P < 0.0001 for both LPS + QC and LPS + QC + HCQ vs. LPS). The panels depict raw, ELISA-generated TNF-α concentration values and are connected with lines to show the drug effects on each patient. CLE, cutaneous lupus erythematosus; DM, dermatomyositis; HCQ, hydroxychloroquine; LPS, lipopolysaccharide; PBMC, peripheral blood mononuclear cell; QC, quinacrine; TNF-α, tumor necrosis factor-α.
QC and HCQ suppress IFN-α protein production by PMBCs
QC was found to suppress secretion of IFN-α as significantly as HCQ from the cytosine phosphodiester guanine (CpG)-stimulated PBMCs of DM and CLE patients and control subjects (Table 2). Drug effects on unstimulated PBMCs are not shown because of minute levels of secreted IFN-α that went largely undetected by ELISA. The CpG-stimulated PBMCs of seven DM patients, five CLE patients, and five control subjects showed a statistically significant difference between the means for HCQ (P = 0.0001), QC (P = 0.0001), and QC plus HCQ (P < 0.0001) versus sham. CpG was unable to stimulate IFN-α secretion from the PBMCs of patients receiving antimalarials at the time their blood was drawn because of suppression of IFN-α to levels undetectable by ELISA. This study therefore required that PBMC donors not be receiving antimalarial therapy. The results shown in Table 2 are for all patients groups tested. The subgroup analyses within each patient group gave comparable results (Figure 2).
Table 2.
Mean and SEM of IFN-α secretion from the CpG-stimulated PBMCs of DM and CLE patients and control subjects given treatment with HCQ, QC, a combination of QC and HCQ, and sham1
| Treatment | Mean | SEM | P-Value2 |
|---|---|---|---|
| CpG (reference group) | 4.87 | 0.24 | — |
| CpG + HCQ | 0.09 | 0.24 | 0.00013 |
| CpG + QC | –0.31 | 0.24 | 0.00013 |
| CpG + QC + HCQ | –0.22 | 0.24 | <0.00014 |
Abbreviations: CLE, cutaneous lupus erythematosus; CpG, cytosine phosphodiester guanine; DM, dermatomyositis; HCQ, hydroxychloroquine; PBMC, peripheral blood mononuclear cell; QC, quinacrine; SEM, standard error of the mean.
The values have been natural log transformed to account for a right-skewed distribution of the raw original values, and units are in ln(pg/ml).
Comparison with the reference group.
P = 0.0001 for CpG + HCQ versus CpG and CpG + QC versus CpG.
P < 0.0001 for CpG + QC + HCQ versus CpG.
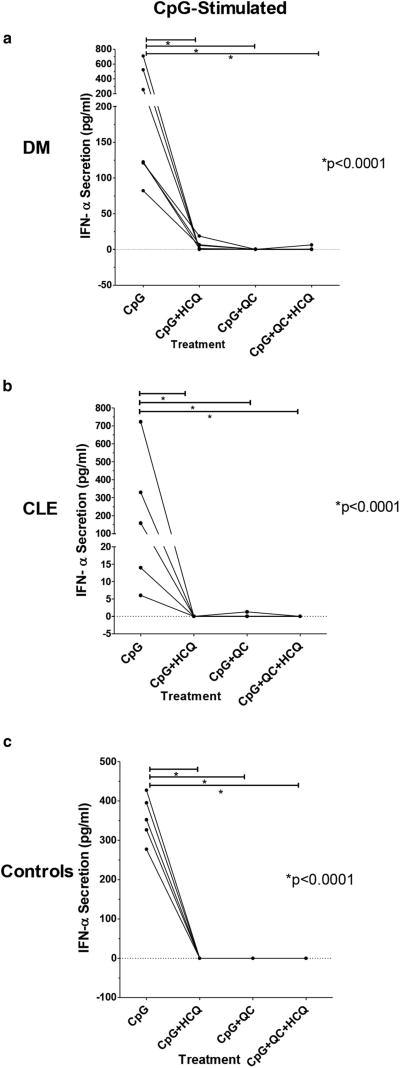
Figure 2. HCQ and QC decrease IFN-α production from PBMCs.
Effects of HCQ, QC, and a combination of QC and HCQ versus sham on secretion of IFN-α from the CpG-stimulated PBMCs of (a) DM patients (*P < 0.0001 for CpG + HCQ, CpG + QC, and CpG + QC + HCQ vs. CpG), (b) CLE patients (*P < 0.0001 for CpG + HCQ, CpG + QC, and CpG + QC + HCQ vs. CpG), and (c) control subjects (*P < 0.0001 for CpG + HCQ, CpG + QC, and CpG + QC + HCQ vs. CpG). The panels depict raw, ELISA-generated IFN-α concentration values and are connected with lines to show the drug effects on each patient. CLE, cutaneous lupus erythematosus; CpG, cytosine phosphodiester guanine; DM, dermatomyositis; HCQ, hydroxychloroquine; PBMC, peripheral blood mononuclear cell; QC, quinacrine.
QC suppresses intracellular expression of TNF-α from LPS-stimulated myeloid dendritic cells and monocytes
Flow cytometric analysis was used to determine the percentage of CD11c+ myeloid dendritic cells (mDCs) and CD14+ monocytes expressing intracellular TNF-α in unstimulated and LPS-stimulated DM patients (mDCs, unstimulated and LPS-stimulated, n = 4; monocytes, unstimulated and LPS-stimulated, n = 5) and control subjects (mDCs, unstimulated and LPS-stimulated, n = 5; monocytes, unstimulated and LPS-stimulated, n = 5) (Table 3). QC significantly suppressed intracellular expression of TNF-α from the LPS-stimulated mDCs and monocytes of DM patients and control subjects compared with LPS alone (P = 0.0002 and P = 0.0008 for mDCs in DM patients and control subjects, respectively; P = 0.0004 and P < 0.0001 for monocytes in DM patients and control subjects, respectively) (Figure 3). Two CLE patient samples evaluated showed a similar result to DM and control samples (Figure 3).
Table 3.
Mean and SEM1 of the percentage of unstimulated and LPS-stimulated CD11c+ mDCs and CD14+ monocytes from DM patients and control subjects expressing TNF-α given treatment with HCQ, QC, and sham CD11c+ mDCs
| CD11c+mDCs | |||||||
| Treatment | Mean | SEM | P-Value2 | Treatment | Mean | SEM | P-Value2 |
| Sham (reference group) | 5.07 | 2.43 | — | LPS (reference group) | 43.78 | 4.40 | — |
| HCQ | 2.92 | 2.43 | 0.4528 | LPS + HCQ | 39.27 | 4.40 | 0.2968 |
| QC | 1.95 | 2.43 | 0.2791 | LPS + QC | 10.69 | 4.40 | <0.00013 |
| CD14+monocytes | |||||||
| Treatment | Mean | SEM | P-Value1 | Treatment | Mean | SEM | P-Value1 |
| Sham (reference group) | 5.37 | 2.74 | — | LPS (reference group) | 79.08 | 7.39 | — |
| HCQ | 2.59 | 2.74 | 0.2826 | LPS + HCQ | 77.87 | 7.39 | 0.7262 |
| QC | 1.31 | 2.74 | 0.1231 | LPS + QC | 50.08 | 7.39 | <0.00013 |
Abbreviations: DM, dermatomyositis; HCQ, hydroxychloroquine; LPS, lipopolysaccharide; mDC, myeloid dendritic cell; QC, quinacrine; SEM, standard error of the mean; TNF-α, tumor necrosis factor-α.
The units are pg/ml.
Comparison with the reference group.
P < 0.0001 for LPS + QC versus LPS.
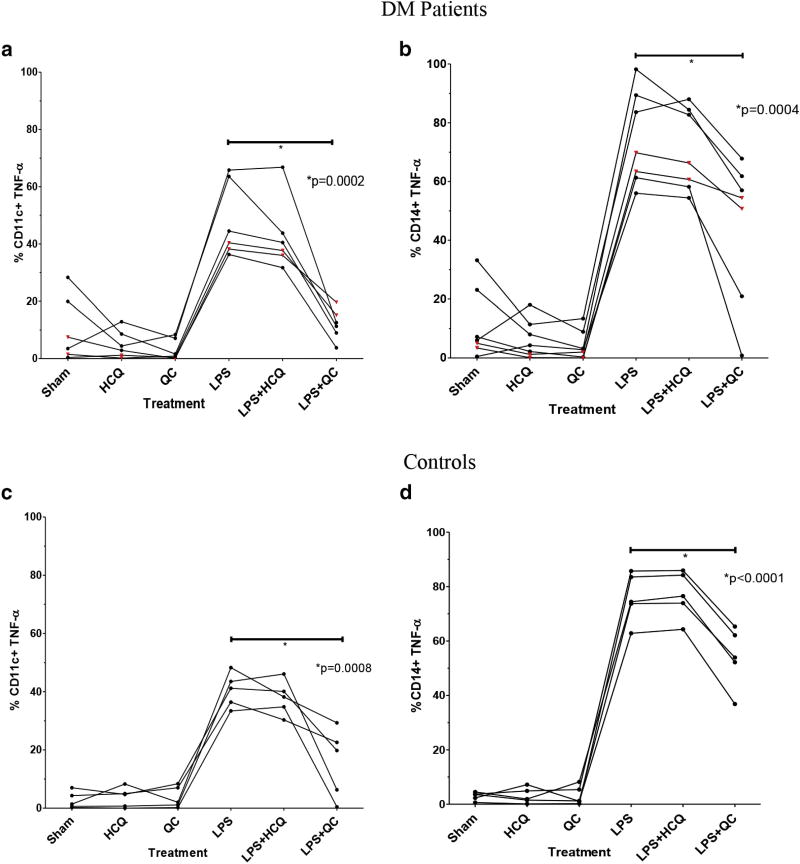
Figure 3. QC, but not HCQ, decreases intracellular expression of TNF-α by mDCs and monocytes.
Effects of HCQ and QC on intracellular expression of TNF-α in unstimulated and LPS-stimulated (a) CD11c+ mDCs of DM patients (*P = 0.0002 for LPS + QC vs. LPS), (b) CD14+ monocytes of DM patients (*P = 0.0004 for LPS + QC vs. LPS), (c) CD11c+ mDCs of control subjects (*P = 0.0008 for LPS + QC vs. LPS), and (d) CD14+ monocytes of control subjects (*P < 0.0001 for LPS + QC0 vs. LPS). The black circles depict the results of flow cytometric analyses showing the percentage of unstimulated and LPS-stimulated CD11c+ mDCs and CD14+ monocytes with intracellular expression of TNF-α for DM patients and control subjects given treatment with HCQ, QC, and sham. The red triangles depict the results of two CLE patients who were tested. Raw percentage values are shown and connected with lines to show the drug effects on each patient. CLE, cutaneous lupus erythematosus; DM, dermatomyositis; HCQ, hydroxychloroquine; LPS, lipopolysaccharide; mDC, myeloid dendritic cell; QC, quinacrine; TNF-α, tumor necrosis factor-α.
The results shown in Figure 3 are supported by Supplementary Figure S1 online, indicating the significant effect of QC in reducing the percentage of mDCs and monocytes exhibiting intracellular expression of TNF-α in a representative DM patient. A total of 65.1% of LPS-stimulated mDCs expressed intracellular TNF-α before treatment withQC (mean fluorescence intensity (MFI) = 63,734 arbitrary units), but only 20.7% of them expressed it after treatment (MFI = 7,734 arbitrary units). Moreover, we found that 85.0% of monocytes expressed intracellular TNF-α before treatment with QC (MFI = 39,612 arbitrary units), but only 59.1% expressed it after treatment (MFI = 11,309 arbitrary units). Although not shown, a similar result was obtained for control subjects.
DISCUSSION
Antimalarial drugs have been used for decades to treat DM and CLE, despite a dearth of conclusive information about how they work. Studies suggest that HCQ does not interfere with protease function through a pH increase (Kuznik et al., 2011). Recent studies show that HCQ acts as an antagonist of endosomal toll-like receptors (TLRs), and HCQ inhibits activation of TLRs by directly binding to nucleic acids (TLR ligands) (Kuznik et al., 2011; Kyburz et al., 2006). Presentation to CD4+ T lymphocytes is subsequently averted, and the secretion of proinflammatory cytokines leading to the inflammatory response seen in DM and CLE is inhibited (Kalia and Dutz, 2007).
TLRs are critical pathogen molecule pattern-recognition receptors present on antigen-producing cells (APCs) and play a crucial role in innate and adaptive immune responses linked to the pathogenesis of autoimmune diseases such as DM and CLE (Aringer et al., 2013; Takeda et al., 2003). Antimalarials inhibit TLRs and thus impede the hyperactive immune response associated with these two diseases (Willis et al., 2012). Inhibition of intracellular TLRs 7 and 9 by QC and HCQ to varying degrees is generally thought to result in decreased secretion of proinflammatory cytokines and antigen presentation by PBMCs and thus lead to disease improvement in DM and CLE patients (Sun et al., 2007; Willis et al., 2012).
Two proinflammatory cytokines implicated in the pathogenesis of DM and CLE are TNF-α and IFN-α (Baechler et al., 2003; Braunstein et al., 2012; Moran and Mastaglia, 2014; Wong et al., 2012). Our study compares the effectiveness of two antimalarial drugs, QC and HCQ, at suppressing secretion of TNF-α and IFN-α from the PBMCs of DM and CLE patients and control subjects. We found that QC suppresses the secretion of TNF-α significantly more than HCQ in both unstimulated and LPS-stimulated DM and CLE patients and control subjects. Moreover, QC suppresses secretion of IFN-α as significantly as HCQ in CpG-stimulated DM and CLE patients and control subjects. Flow cytometric analyses corroborated our findings by showing that QC suppresses intracellular expression of TNF-α from both the unstimulated and LPS-stimulated CD14+ monocytes and CD11c+ mDCs of DM patients and control subjects significantly more than HCQ. Monocytes and mDCs have been established as major producers of TNF-α in DM and CLE, particularly of the discoid lupus erythematosus subtype (Nabatian et al., 2012). Although antimalarials are generally thought to work by inhibiting activation of TLRs 7 and 9, our results suggest that QC may have significant effects on other intracellular TLRs and thus confirm previous findings (Kuznik et al., 2011). This conclusion is supported by the fact that human mDCs, unlike plasmacytoid dendritic cells, do not express TLRs 7 and 9 yet were affected by QC more profoundly than monocytes that do express TLRs 7 and 9 (Cardoso et al., 2013; Monrad and Kaplan, 2007).
Understanding the differential effect of QC on innate immune cells, including mDCs and macrophages, may help account for some of the heterogeneity of responses seen therapeutically. Future studies are needed to dissect the exact mechanism for the effects seen here, in addition to other arms of the immune system that play a role in CLE.
It is imperative that patients with DM and CLE have access to the best possible treatment given how remarkably these conditions affect patient quality of life. Underscoring this necessity, quality of life as measured by the Skindex-29 and Short Form-36 Health Survey assessments was worse for DM and CLE patients than for those with many other skin diseases (Goreshi et al., 2011; Klein et al., 2011).
HCQ has for decades been the antimalarial drug of choice for treating patients with DM and CLE, with little or no consideration given to the potential benefit of comparably safe alternative therapies such as QC. The results of our study showing the superior overall effectiveness of QC versus HCQ at suppressing both TNF-α and IFN-α in PBMCs from DM and CLE, together with limited but suggestive clinical therapeutic response data, establishes the likelihood that patients who are responsive to QC and not HCQ may have different pathways or cells activated than those responding to HCQ. Further study in this field is required to differentiate a mechanism by which each drug works.
MATERIALS AND METHODS
Patient recruitment and isolation of PBMCs
Thirteen DM patients, six CLE patients, and six control subjects without DM or CLE were recruited from the Department of Dermatology at the Hospital of the University of Pennsylvania in accordance with an approved institutional review board protocol. Information regarding patients’ demographics and disease activity assessment according to the Cutaneous Dermatomyositis Area and Severity Index for DM patients and the Cutaneous Lupus Erythematosus Disease Area and Severity Index for CLE patients was also obtained for each patient (see Supplementary Table S1 online) (Albrecht et al., 2005; Yassaee et al., 2010). The experimental protocol was approved by the Ethics Committee of the University of Pennsylvania and conformed to the principles outlined in the Declaration of Helsinki. A written informed consent was given by every subject involved in the study.
Treatment of PBMCs and cell culture
PBMCs were isolated from the samples using Ficoll-Paque PLUS (GE Healthcare, Uppsala, Sweden). Isolated PBMCs were plated into cell culture plates containing 1.5 ml of RPMI 1640 (1×) [+] l-glutamine [−] phenol red medium (Gibco Life Technologies, Grand Island, NY) conditioned with 10% fetal bovine serum and 1% penicillin-streptomycin in the amount of 2.5 × 106 PBMCs per well. The PBMCs were subsequently treated with 3 µmol/L concentrations of HCQ (Sigma-Aldrich, St. Louis, MO), QC dihydrochloride (Sigma-Aldrich), and a combination QC and HCQ in the appropriate wells and allowed to incubate for 18 hours at 37 °C and5%CO2. To also test the effect of these drugs on stimulated versus unstimulated PBMCs, 3-µmol/L concentrations of Escherichia coli-derived LPS (Sigma-Aldrich) and CpG oligonucleotides (InvivoGen, San Diego, CA) were used to stimulate secretion of TNF-α and IFN-α, respectively.
Collection of PBMC supernatants and ELISA
After 18 hours of incubation, cell-free supernatants were collected. The concentrations of TNF-α and IFN-α secreted into the supernatants were quantified in triplicate via ELISA (BD OptEIA Human TNFα ELISA Set, BD Biosciences, San Diego, CA; VeriKine Human IFNα ELISA, PBL Assay Science, Piscataway, NJ).
Flow cytometric analyses
Flow cytometric analyses were conducted to supplement the quantification of the effects of HCQ and QC on TNF-α obtained through ELISA. PBMCs were isolated and treated with HCQ and QC, but not a combination of both, according to the procedure delineated. They were subsequently incubated for 3.5 hours at 37 °C and 5% CO2. BD GolgiPlug protein transport inhibitor containing brefeldin A (BD Biosciences) was added to the LPS-stimulated PBMCs 40 minutes after stimulation. To detect intracellular expression of TNF-α in mDCs and monocytes, PBMCs were stained with FITC lineage 1 cocktail (containing anti-human antibodies against CD3, CD16, CD19, CD20, and CD56) (BioLegend, San Diego, CA), APC/Cy7 HLA-DR (BioLegend), PE anti-human CD11c (BioLegend), and PerCP anti-human CD14 (BioLegend) before being set on ice for 25 minutes, treated with fixation and permeabilization solution (BD Cytofix/Cytoperm Plus, BD Biosciences) for 20 minutes, washed (BD PermWash, BD Biosciences), and stained with allophycocyanin mouse anti-human TNF-α (BD Biosciences) for an additional 20 minutes. Cells were analyzed with an LSRII flow cytometer (Becton Dickinson, San Jose, CA) and FloJo software (Tree Star, Ashland, OR) at the Flow Cytometry and Cell Sorting Core of the Abramson Cancer Center of the University of Pennsylvania. A total of 150,000 events of live cells were acquired.
Statistical analyses
The raw TNF-α and IFN-α concentration values had right-skewed distributions and were thus natural log transformed before the analyses. Because each sample was subjected to four different treatment conditions (i.e., HCQ, QC, combination QC + HCQ, and sham), the concentration values of TNF-α and IFN-α among these four conditions were compared using a linear mixed model, controlled for each patient group (i.e., DM, CLE, and controls) and correlations within each individual patient. The differences between the three treatment condition comparison groups (HCQ, QC, and QC + HCQ) and the sham group were reflected in the regression coefficients, which were estimated using the maximum likelihood approach and tested by the Wald tests. A significance level of 0.017 was considered to be statistically significant after adjusting for the three comparisons. In addition, the raw TNF-α and IFN-α concentration values were plotted for each individual patient along the four different treatment conditions and compared using a linear mixed model within each patient group.
The flow cytometric data were normally distributed; therefore, no natural log transformation was performed, and raw original values were used in the analyses. All analyses followed the same method as described above. A significance level of 0.025 was considered statistically significant after adjusting for two comparisons.
Supplementary Material
Acknowledgments
Publication of this article was supported by the National Institutes of Health.
This work was supported by the United States Department of Veterans Affairs (Veterans Health Administration, Office of Research and Development and Biomedical Laboratory Research and Development) Merit Review 5 I01 BX000706-04 (Principle Investigator: Werth) and the National Institutes of Health NIH K24-AR 02207 and NIH R21 AR066286 to VPW.
Abbreviations
- CLE
cutaneous lupus erythematosus
- CpG
cytosine phosphodiester guanine
- CQ
chloroquine
- DM
dermatomyositis
- HCQ
hydroxychloroquine
- LPS
lipopolysaccharide
- mDC
myeloid dendritic cell
- MFI
mean fluorescence intensity
- PBMC
peripheral blood mononuclear cell
- QC
quinacrine
- TLR
toll-like receptor
- TNF-α
tumor necrosis factor-α
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
Supplementary material is linked to the online version of the paper at www.jidsponline.org, and at http://dx.doi.org/10.1016/j.jisp.2016.11.001.
References
- 1.Albrecht J, Taylor L, Berlin JA, Dulay S, Ang G, Fakhazardeh S, et al. The CLASI (Cutaneous LE Disease Area and Severity Index): an outcome instrument for cutaneous lupus erythematosus. J Invest Dermatol. 2005;125:889–94. doi: 10.1111/j.0022-202X.2005.23889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ang GC, Werth VP. Combination antimalarials in the treatment of cutaneous dermatomyositis: a retrospective study. Arch Dermatol. 2005;141:855–9. doi: 10.1001/archderm.141.7.855. [DOI] [PubMed] [Google Scholar]
- 3.Aringer M, Gunther C, Lee-Kirsch MA. Innate immune processes in lupus erythematosus. Clin Immunol. 2013;147:216–22. doi: 10.1016/j.clim.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci USA. 2003;100:2610. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benoit S, Goebeler M. Mepacrine in recalcitrant cutaneous lupus erythematosus: old-fashioned or still useful? Acta Derm Venereol. 2015;95:596–9. doi: 10.2340/00015555-2031. [DOI] [PubMed] [Google Scholar]
- 6.Bonilla-Martinez ZL, Albrecht J, Troxel AB, Taylor L, Okawa J, Dulay S, et al. The cutaneous lupus erythematosus disease area and severity index: a responsive instrument to measure activity and damage in patients with cutaneous lupus erythematosus. Arch Dermatol. 2008;144:173–80. doi: 10.1001/archderm.144.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braunstein I, Klein R, Okawa J, Werth VP. The IFN-regulated gene signature is elevated in SCLE and DLE and correlates with CLASI score. Br J Dermatol. 2012;166:971–5. doi: 10.1111/j.1365-2133.2012.10825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardoso EC, Pereira NZ, Mitsunari GE, Oliveira LM, Ruocco RM, Francisco RP, et al. TLR7/TLR8 activation restores defective cytokine secretion by myeloid dendritic cells but not by plasmacytoid dendritic cells in HIV-infected pregnant women and newborns. PLoS One. 2013;8(6):e67036. doi: 10.1371/journal.pone.0067036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavazzana I, Sala R, Bazzani C, Ceribelli A, Zane C, Cattaneo R, et al. Treatment of lupus skin involvement with quinacrine and hydroxychloroquine. Lupus. 2009;18:735–9. doi: 10.1177/0961203308101714. [DOI] [PubMed] [Google Scholar]
- 10.Chang AY, Piette EW, Foering KP, Tenhave RR, Okawa J, Werth VP. Response to antimalarials in cutaneous lupus erythematosus: a prospective analysis. Arch Dermatol. 2011;147:1261–7. doi: 10.1001/archdermatol.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goreshi R, Chock M, Foering K, Feng R, Okawa J, Rose M, et al. Quality of life in dermatomyositis. J Am Acad Dermatol. 2011;65:1107–16. doi: 10.1016/j.jaad.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jover JA, Leon L, Pato E, Loza E, Rosales Z, Matias MA, et al. Long-term use of antimalarial drugs in rheumatic diseases. Clin Exp Rheumatol. 2012;30:380–7. [PubMed] [Google Scholar]
- 13.Kalia S, Dutz JP. New concepts in antimalarial use and mode of action in dermatology. Dermatol Ther. 2007;20:160–74. doi: 10.1111/j.1529-8019.2007.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein RS, Moghadam-Kia S, Lomonico J, Chilek K, Gaines E, Okawa J, et al. Quality of life in cutaneous lupus erythematosus. J Am Acad Dermatol. 2011;64:649–58. doi: 10.1016/j.jaad.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuznik A, Bencina M, Svajger U, Jeras M, Rozman B, Jerala R. Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. J Immunol. 2011;186:4794–804. doi: 10.4049/jimmunol.1000702. [DOI] [PubMed] [Google Scholar]
- 16.Kyburz D, Brentano F, Gay S. Mode of action of hydroxychloroquine in RA-evidence of an inhibitory effect on toll-like receptor signaling. Nat Clin Pract Rheumatol. 2006;2:458–9. doi: 10.1038/ncprheum0292. [DOI] [PubMed] [Google Scholar]
- 17.Moghadam-Kia S, Chilek K, Gaines E, Costner M, Rose ME, Okawa J, et al. Cross-sectional analysis of a collaborative Web-based database for lupus erythematosus-associated skin lesions: prospective enrollment of 114 patients. Arch Dermatol. 2009;145:255–60. doi: 10.1001/archdermatol.2008.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monrad S, Kaplan MJ. Dendritic cells and the immunopathogenesis of systemic lupus erythematosus. Immunologic research. 2007;37:135–45. doi: 10.1007/BF02685895. [DOI] [PubMed] [Google Scholar]
- 19.Moran EM, Mastaglia FL. Cytokines in immune-mediated inflammatory myopathies: cellular sources, multiple actions and therapeutic implications. Clin Exp Immunol. 2014;178:405–15. doi: 10.1111/cei.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nabatian AS, Bashir MM, Wysocka M, Sharma M, Werth VP. TNFα release in peripheral blood mononuclear cells of cutaneous lupus and dermatomyositis patients. Arthritis Res Ther. 2012;14(1):R1. doi: 10.1186/ar3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, Khamashta MA. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Annal Rheum Dis. 2010;69:20–8. doi: 10.1136/ard.2008.101766. [DOI] [PubMed] [Google Scholar]
- 22.Sacre K, Criswell LA, McCune JM. Hydroxychloroquine is associated with impaired interferon-alpha and tumor necrosis factor-alpha production by plasmacytoid dendritic cells in systemic lupus erythematosus. Arthritis Res Ther. 2012;14(3):R155. doi: 10.1186/ar3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun S, Rao NL, Venable J, Thurmond R, Karlsson L. TLR7/9 antagonists as therapeutics for immune-mediated inflammatory disorders. Inflamm Allergy Drug Targets. 2007;6:223–35. doi: 10.2174/187152807783334300. [DOI] [PubMed] [Google Scholar]
- 24.Takeda K, Kaisho T, Akira S. Toll-like receptors. Ann Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 25.Toubi E, Rosner I, Rozenbaum M, Kessel A, Golan TD. The benefit of combining hydroxychloroquine with quinacrine in the treatment of SLE patients. Lupus. 2000;9:92–5. doi: 10.1191/096120300678828082. [DOI] [PubMed] [Google Scholar]
- 26.Wenzel J, Scheler M, Bieber T, Tuting T. Evidence for a role of type I interferons in the pathogenesis of dermatomyositis. Br J Dermatol. 2005;153:462–3. doi: 10.1111/j.1365-2133.2005.06786.x. [DOI] [PubMed] [Google Scholar]
- 27.Willis R, Seif AM, McGwin G, Jr, Martinez-Martinez LA, Gonzalez EB, Dang N, et al. Effect of hydroxychloroquine treatment on pro-inflammatory cytokines and disease activity in SLE patients: data from LUMINA (LXXV), a multiethnic US cohort. Lupus. 2012;21:830–5. doi: 10.1177/0961203312437270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong D, Kea B, Pesich R, Higgs BW, Zhu W, Brown P, et al. Interferon and biologic signatures in dermatomyositis skin: specificity and heterogeneity across diseases. PLoS One. 2012;7(1):e29161. doi: 10.1371/journal.pone.0029161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yassaee M, Fiorentino D, Okawa J, Taylor L, Coley C, Troxel AB, et al. Modification of the Cutaneous Dermatomyositis Disease Area and Severity Index, an outcome instrument. Br J Dermatol. 2010;162:669–73. doi: 10.1111/j.1365-2133.2009.09521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.