Abstract
We examined the pre-clinical activity of pan- histone deacetylase inhibitor LBH589 in combination with mTORC1 inhibitor RAD001 and observed that the drug combination strongly synergized in inducing cytotoxicity in multiple myeloma (MM) cells. LBH589 caused an increase in acetylated histones and RAD001 inhibited mTORC1 activity. RAD001 caused potent G0/G1 arrest while LBH589 induced pronounced apoptosis, both of which were enhanced when the drugs were used in combination. LBH589/RAD001 combination led to down regulation of pStat3, cyclins, CDKs and XIAP and up regulation of pro-apoptotic Bcl-2 family proteins. A clinical trial is underway using LBH589/RAD001 combination in relapsed MM patients.
Keywords: Multiple Myeloma, Histone Deacetylase inhibitor, mTOR inhibitor, apoptosis, proliferation
1. Introduction
Histone acetylation is a process regulated by two groups of enzymes with opposite functions, namely histone acetyl transferase [HAT] and histone deacetylase [HDAC][1]. In the nucleus, genomic DNA is tightly wrapped around histone octamers called nucleosomes[2]. Histone acetylation by HAT neutralizes the positive charge of lysine residues on histones thereby weakening the DNA-histone interaction and increasing transcription whereas histone deacetylation catalyzed by HDAC reverses this effect leading to a global decrease in histone acetylation and transcription[3, 4]. HDAC inhibitors (HDACi) have shown potent anti-cancer effects in hematological tumor systems including MM [5]. In MM, HDACi have been shown to up regulate p21, p53, pro-apoptotic members of the Bcl2 family and dephosphorylated Rb [6-8]. In addition, it is now known that HDAC inhibitors can also cause acetylation of non-histone proteins thus modulating the expression of an even wider array of proteins[9]. Thus, the mechanism of action of HDAC inhibition is unclear and anti-tumor effects elicited by HDACi could differ greatly. Despite encouraging preclinical activity demonstrated by HDAC inhibitors, the clinical activity of these compounds as single agents has been limited in a MM setting[10-12]. Clinical trials are currently underway testing HDACi in combination with existing therapies and initial responses are promising when HDACi is used in combination with bortezomib alone or bortezomib plus dexamethasone[13].
The mammalian target of rapamycin (mTOR) protein, a member of the PI3K-related kinase family physically interacts with multiple proteins to form two different complexes called mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). Out of the two, mTORC1 is the well studied rapamycin sensitive complex. In addition to being activated through the PI3K/Akt pathway, mTORC1 can be regulated through several other signals including the Mek/Erk pathway, cellular energy levels, amino acids and DNA damage[14-18]. Activated mTORC1 promotes cap dependent translation, lipid synthesis and inhibits autophagy, all leading to increased tumor growth and survival[19-21]. Aberrant activation of the PI3K/Akt/mTOR pathway is a common feature in MM and is a contributing factor in promoting tumor cell proliferation and apoptosis resistance to existing therapies[22-25]. In addition, the Mek/Erk pathway is also activated in MM through either activating Ras or Raf mutations or up regulation of tumor promoting cytokines including IGF, IL6 and VEGF[23, 24, 26-28]. Both these pathways are therapeutically relevant with inhibitors targeting either of these pathways alone or in combination showing encouraging preclinical activity in MM[25, 29-31]. Rapamycin and rapalogs that block mTORC1 activity can induce a cytostatic effect on MM cells in vitro[32]. However, mTORC1 inhibitors fail to induce significant cytotoxicity in MM cells and have shown poor activity in the clinic as a single agent when tested on patients with MM[33].
Since LBH589 and RAD001 have demonstrated direct anti-MM effects through non-overlapping mechanisms as well as anti-angiogenic properties[34, 35] in vitro, we wanted to investigate potential activity of a combination of the two drugs against myeloma cells and patient samples. Here, we show the efficacy of LBH589/RAD001 combination in inhibiting MM cell proliferation, inducing apoptosis and inhibiting angiogenesis in vitro on myeloma cell lines both alone and in combination with bone marrow stromal cells and tumor promoting cytokines. Though LBH/RAD was synergistic in their action in killing all MM cell lines examined, we present evidence indicating that the drug combination could elicit their effects through different mechanisms in different cell lines and patient cells. A clinical trial is currently ongoing examining this combination in relapsed/refractory MM patients.
2. Materials and Methods
2.1. Multiple myeloma cell lines, patient cells and bone marrow stromal cells
Dexamethasone sensitive (MM1.S) and resistant (MM1.R) human MM cell lines; doxorubicin resistant (DOX 40), and melphalan resistant (LR5) RPMI 8226 human MM cell lines and sensitive RPMI 8226 cell line, OPM-2, NCI-H929 and U266 cell lines were used for the current study. All the cell lines were cultured in RPMI 1640 media (Sigma Chemical, St. Louis, MO) that contained 10% fetal bovine serum, 2 mM L-glutamine (GIBCO, Grand Island, NY), 100U/mL penicillin, and 100μg/mL streptomycin. All cell lines were obtained as indicated earlier[25]. Freshly obtained BM aspirates from patients were collected with informed consent and were processed to obtain patient samples or stromal cells as previously mentioned[31, 36]. Patient cells were cultured in RPMI 1640 media that contained 20% fetal bovine serum, 2mM L-glutamine (GIBCO), 100U/ml penicillin and 100μg/ml streptomycin.
2.2. LBH589 and RAD001
LBH589 and RAD001 were synthesized and provided by Novartis (Basel, Switzerland) under a Material Transfer Agreement (MTA).
2.3. Cell viability and proliferation assays
Cell viability and proliferation assays were performed using MTT and tritiated thymidine uptake assays respectively as described earlier[25, 31, 36, 37]. In experiments with BMSC, 5000 cells (BMSCs) were plated onto 96 well plates and allowed to adhere overnight before adding myeloma cells. For cytokine experiments, cells were treated with IL6 (25ng/ml) or VEGF (50ng/ml) along with either LBH589 or RAD001 or both for 48hrs. All experiments were performed in triplicate.
2.4. Isobologram analysis
The interaction between LBH589 and RAD001 was analyzed using the CalcuSyn™ software program (Biosoft, Ferguson, MO). This program is based upon the Chou-Talalay method, which calculates a combination index (CI), and analysis is performed based on the following equation: CI = (D)1/(Dx)1 + (D)2/(Dx)2 + (D)1(D)2/(Dx)1(Dx)2, where (D)1 and (D)2 are the doses of drug 1 and drug 2 that have x effect when used in combination, and (Dx)1 and (Dx)2 are the doses of drug 1 and drug 2 that have the same x effect when used alone[38]. Data from the MTT viability assay was expressed as the fraction of cells killed by the individual drug or the combination in drug-treated cells compared with untreated cells. A CI of 1.0 indicates an additive effect, whereas CI values below 1.0 indicate synergistic effects.
2.5. Apoptosis measurement
Apoptosis of MM cell lines was examined using the Annexin/PI assay as described before[25, 31, 36, 37]. Briefly, cells were washed twice with annexin binding buffer (ABB) (10mM HEPES pH 7.4, 140mM NaCl, 2.5mM CaCl2). 100μl cells were treated with 3μl of annexin V-FITC (Caltag, Burlingame, CA, USA) for 15mins at room temperature. Cells were washed again with ABB and resuspended in 500μl of ABB containing 5μl of 1mg/ml propidium iodide (Sigma). Samples were then run on a Canto flow cytometer (BD Biosciences, San Jose, CA, USA).
For patient cells, apoptosis was examined using the Apo2.7 assay as described earlier[31, 37]. Briefly, fresh bone marrow cells were ACK lysed, washed and resuspended in culture media and incubated with the drugs for the indicated time points. The cultures were harvested, washed once in PBS and resuspended in 1ml PBS/3%BSA. 100μl of cells were then stained with Apo 2.7 PE (Beckman Coulter, Miami, FL, USA) or isotype control for 15minutes. Cells were washed again in PBS and resuspended in 1% paraformaldehyde and stored in the dark at 4°C until run on the Canto Flow cytometer (BD Biosciences)
2.6. Mitocapture and Cytochrome c assays
The MitoCapture Apoptosis Detection Kit and cytochrome c release assay kit (both from Calbiochem, San Diego, CA, USA) were used as per the manufacturer's recommendations and as described in a previous study[39]. The mitocapture assay was done using the MitoCapture apoptosis detection kit (Calbiochem, San Diego, CA, USA). Cells were spun and resuspended in 0.5ml of freshly prepared MitoCapture reagent (1μl of MitoCapture reagent to 1ml of MitoCapture buffer) and incubated for 1hr in a 37°C incubator. Cells were spun and resuspended in 0.5ml of MitoCapture buffer. For cytochrome c assay, we used the cytochrome c release assay kit (Calbiochem). Cells were spun and resuspended and incubated in 100μl of permeabilization buffer for 10 minutes on ice. Following this, cells were fixed using 200 μl of 8% paraformaldehyde for 20 minutes at room temperature. Cells were then spun and washed thrice in PBS. Cells were then incubated in blocking buffer for 1hr at room temperature. Cells were then washed once with wash buffer and incubated with either anti- cytochrome c antibody or mouse IgG for 1hr at room temperature. After washing cells once with wash buffer, cells were incubated with anti-IgG FITC for 1hr at room temperature. Cells were then washed once in wash buffer and resuspended in 0.5ml of 1% paraformaldehyde and run on a Canto flow cytometer.
2.7. BrdU assay
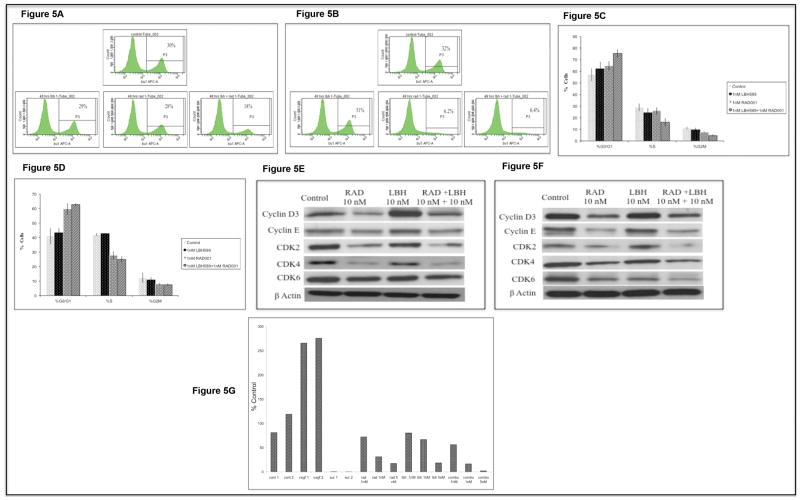
After treating cells with the drug(s), cells were harvested, washed once in staining buffer (SB, 1% BSA/PBS) and resuspended in 1 ml of culture media plus 10μl of BrdU (APC BrdU Flow Kit Cat# 552598, BD Biosciences) for a one hour incubation. After harvest, the cells were processed and stained as per the manufacturer's instructions. The numbers indicated in figures 5A and B indicate % cells positive for BrdU.
Figure 5.
2.8. Cell cycle analysis
Cells were incubated with the drug(s) as indicated. Cells were harvested, counted, and washed with PBS. While vortexing, 2ml of cold 85% ETOH was slowly added to the dry pellet. The tubes were capped and left at 4 degrees overnight. The tubes were spun and the pellets washed twice with PBS. The pellet was resuspended in 0.1 ml RNase (5ug/ml cat# R7884, Sigma, St.Louis, MO.) and incubated at 37 degrees. 0.9 ml PBS was added to each tube and mixed. 10μl of PI (1mg/ml) was added to each tube, mixed and held at 4 degrees until run on the Canto flow cytometer. Cell cycle statistics were calculated using the cell cycle platform analysis, FlowJo software (Tree Star, Inc. Ashland, OR).
2.9. Western Blotting
MM cell lines MM1S or DOX40 were incubated with LBH589 or RAD001 or the combination for the indicated time points and lysed using RIPA buffer (50mM HEPES (pH 7.4), 150mM NaCl, 1% Triton X-100, 30mM sodium pyrophosphate, 5mM EDTA, 2mM Na3VO4, 5mM NaF, 1mM phenylmethyl-sulfonyl-fluoride (PMSF) and protease inhibitor cocktail). Protein concentrations in the lysate were measured by bicinchoninic protein assay (Pierce, Rockford, IL, USA). Equal amounts of protein were loaded onto a SDS-PAGE gel and transferred onto a nitrocellulose membrane. Membranes were probed with individual antibodies and antigen-antibody complexes were detected using enhanced chemiluminescence (Amersham, Arlington Heights, IL). All antibodies except those mentioned below were purchased from Cell Signaling Technologies (Daverns, MA). Puma antibody was purchased from ProSci Inc. (Poway, CA) and AcH3 and AcH4 antibodies were purchased from Millipore (Billerica, MA).
Angiogenesis assay
We performed an in vitro angiogenesis assay (Angiokit, TCS Cellworks, Buckinghamshire, UK) as described earlier[40].
3. Results
3.1. LBH589 and RAD001 are cytotoxic to myeloma cells
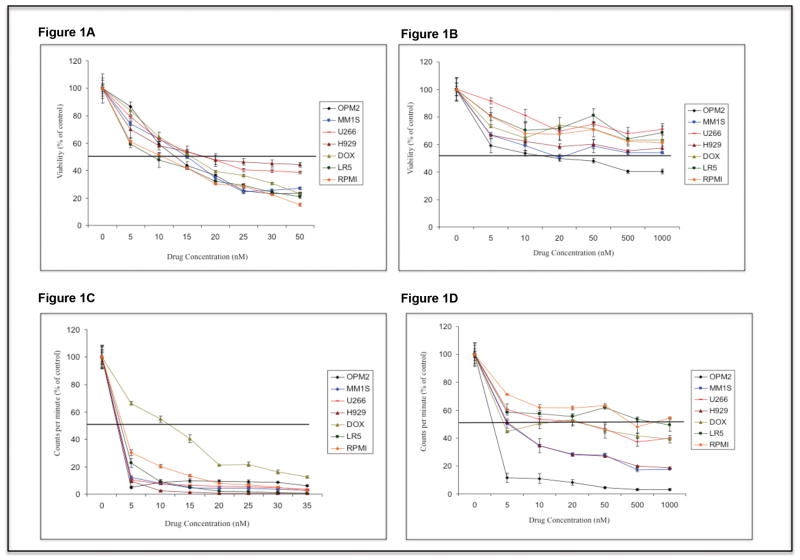
First, we examined the cytotoxic effects of LBH589 and RAD001 as single agents on MM cells. LBH589 demonstrated dose dependent cytotoxicity on all tested MM cells (Figure 1A). The IC50 ranged from 10-20nM. However, RAD001 as a single agent was able to induce significant dose dependent cytotoxicity only in OPM2 cells and not in the other MM cell lines examined clearly indicating resistance mechanisms activated by MM cells upon RAD001 treatment (Figure 1B). We next evaluated the capability of LBH589 and RAD001 to inhibit proliferation of MM cells in vitro. Both LBH589 and RAD001 demonstrated inhibition of proliferation of MM cells at doses lower than cytotoxic concentrations. The IC50 for LBH589 and RAD001 in proliferation assays were found to be 5-15nM and 5-1000nM respectively. (Figures 1C and D respectively).
Figure 1.
3.2. LBH589 and RAD001 demonstrate synergy as anti-MM agents
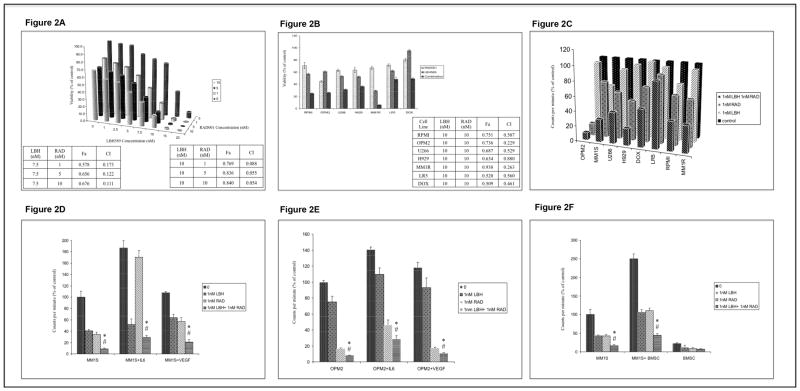
We tested LBH589/RAD001 combination first on MM1S cells, where the combination induced synergistic killing of MM1S cells after 48hours of drug treatment (Figure 2A). Next, we wanted to examine if this synergistic effect was observed in other MM cell lines. When we incubated the MM cell lines with 10nM of LBH589 and RAD001 in combination, we observed synergy as indicated by CI and Fa values (Figure 2B). The proliferation of all MM cell lines was inhibited to a significantly greater extent when LBH589 and RAD001 were used in combination than when used as single agents (Figure 2C). Such inhibitory effects were observed at doses as low as 1nM of either of the drugs.
Figure 2.
3.3. LBH589/RAD001 combination overcomes the protective effects of the tumor microenvironment
The tumor promoting cellular and non-cellular members of the microenvironment have been shown to protect MM cells to existing therapies. Hence, we wanted to examine if LBH589/RAD001 combination was able to overcome this protection when co-cultured with bone marrow stromal cells (BMSCs) or with the cytokines IL6 or VEGF. When MM1S or OPM2 cells were co-cultured with either IL6 (25ng/ml) or VEGF (50ng/ml) and incubated with LBH589 and RAD001 as single agents, we observed that both the drugs as single agents were able to inhibit the cytokine induced proliferation. However, the drugs when treated together were able to inhibit the cytokine-induced proliferation more efficiently than when treated as single agents (Figures 2D and E). Similar effects were observed when MM1S cells were co-cultured with BMSCs (Figure 2F).
3.4. LBH589/RAD001 induces apoptosis in MM cells
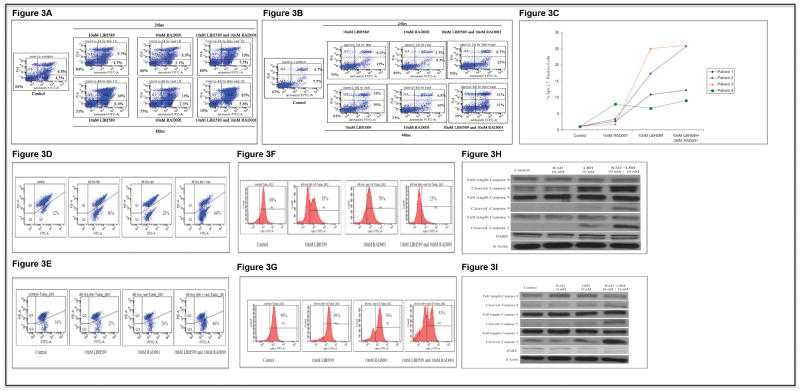
When we treated MM1S or OPM2 cells with indicated concentrations of the drugs for 24 or 48hours, we observed time dependent increase in cells undergoing apoptosis with LBH589 treatment. RAD001 was unable to induce potent apoptosis. More importantly, the drug combination was able to induce significantly more apoptosis in both cell lines tested (Figures 3A and B). Similar effects were also observed when we treated patient derived myeloma cells with either the drugs alone or in combination (Figure 3C).
Figure 3.
We next wanted to examine if the apoptosis induced by LBH589/RAD001 combination involved mitochondrial mechanisms. For this, we treated MM1S or OPM2 cells with indicated concentrations of the drugs for 48hrs and examined for mitochondrial injury by performing mitocapture (Figures 3D and E) and cytochrome c assays (Figures 3F and G). Clearly, LBH589 unlike RAD001 elicited increased mitochondrial damage and cytochrome c release in both MM1S and OPM2 cells, both clear indications of the involvement of the mitochondrial apoptotic pathway (Figures 3D-G). Here again, LBH589/RAD001 combination was more effective in inducing mitochondrial damage and cytochrome c release into cytosol in both cell lines examined. To further confirm apoptotic cell death, we examined levels of caspases and PARP. We observed increase in activated caspases 8, 9, 3 and cleaved PARP after LBH589 treatment and this effect was enhanced when LBH589 was used in combination with RAD001 (Figures 3H and I).
3.5. LBH589/RAD001 combination induces mitochondrial apoptosis through different signals in different MM cells
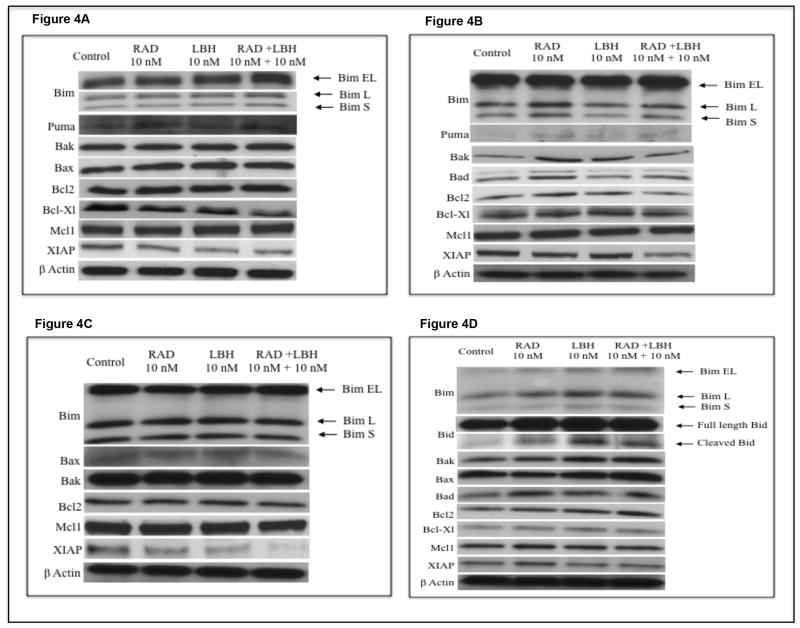
Given that LBH589/RAD001 combination induced potent apoptosis, we then wanted to examine the mechanisms mediating this effect. For this, we incubated MM1S and OPM2 cells with indicated concentrations of the drugs for 24hrs and monitored expression levels of activator BH3- only proteins Bim, Bid and Puma, sensitizer BH3-only protein Bad, the multi domain pro-apoptotic proteins Bax and Bak and the anti-apoptotic proteins Bcl2, Bcl-Xl, Mcl1 and XIAP. We observed up regulation of all three isoforms of Bim (Bim EL, Bim L and Bim S), Puma, Bax and Bak expression in MM1S cells and down regulation of Bcl-Xl and XIAP (Figure 4A). In OPM2 cells, we observed that LBH589/RAD001 combination induced expression levels of Puma, Bad and Bak and down regulation of XIAP (Figure 4B). Bid expression was not altered in both cell lines (data not shown). These differences in the ability of LBH589/RAD001 combination to modulate levels of pro and anti apoptotic proteins was also observed in MM patient cells with LBH589/RAD001 down regulating XIAP and Mcl1 in patient 1 (Figure 4C). There was no difference in the expression of other pro and anti apoptotic proteins. In patient 2, however, LBH589/RAD001 induced up regulation of all pro apoptotic proteins examined including Bim, Bid, Bad, Bak and Bax with minimal effects on the levels of anti apoptotic proteins examined (Figure 4D). Both patients did not demonstrate expression of Puma (data not shown). Thus, our data clearly indicated that multiple mechanisms that are specific for a cell line/patient cell could mediate the apoptosis induced by LBH589/RAD001 combination.
Figure 4.
3.6. LBH589/RAD001 combination inhibits MM cell proliferation, induces G0/G1 arrest and inhibits cyclins and cyclin dependent kinases
From figure 2D it was apparent that LBH589/RAD001 significantly inhibited proliferation at doses much lower than IC50 values. The results were confirmed by performing BrdU incorporation assays. After 48hrs of incubation with 1nM of LBH589/RAD001 combination, there was about a 50% reduction in cells expressing BrdU (Figure 5A) while the drugs, as single agents were unable to inhibit the proliferation significantly in MM1S cells. Treating OPM2 cells with 1nM of LBH589/RAD001 combination inhibited the proliferation by 80% as observed by the decrease in BrdU expressing cells (Figure 5B). Next, we performed cell cycle assays to identify the stage at which the cells were arrested by LBH589/RAD001. In both MM1S and OPM2, 1nM of the drug combination was able to arrest cells in the G0/G1 stage (Figures 5C and D). Unlike apoptosis induction, this effect was primarily driven by RAD001.
We then examined the expression levels of proteins implicated in G0/G1 progression to better understand the mechanism of LBH589/RAD001 induced cell cycle arrest. As shown in figures 5E and F there was a clear decrease in levels of cyclin D3, Cdk4, cyclin E, Cdk2 and Cdk6 in both cell lines. As expected, this decrease was most pronounced when cells were treated with RAD001 or the combination.
3.7. LBH589/RAD001 combination inhibits angiogenesis
We have shown that tumor angiogenesis increases with disease progression in MM patients[41, 42]. Both HDAC and mTOR inhibitors have been shown to inhibit angiogenesis[34, 35]. We therefore performed an in vitro angiogenesis assay to observe if LBH589/RAD001 combination inhibited tubule formation in vitro. We observed reduced angiogenesis when myeloma cells were treated with LBH589 or RAD001 with a greater reduction when treated in combination. Importantly, this inhibitory effect on tubule formation was observed at non-cytotoxic concentrations (Figure 5G).
3.8. LBH589/RAD001 modulates multiple signaling pathways in MM
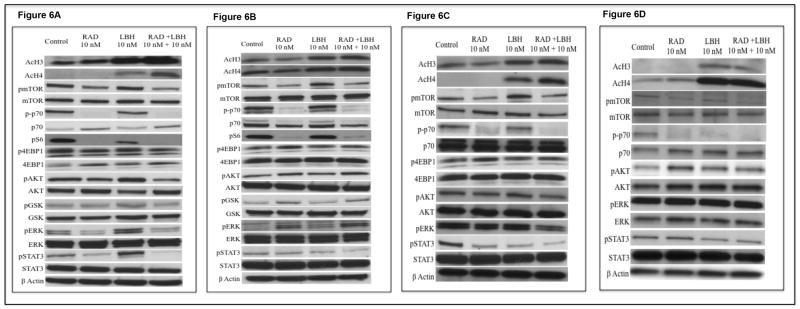
We then examined the expression levels of both acetylated histone H3 (AcH3) and acetylated histone H4 (AcH4) post LBH589 treatment as readout for the HDAC inhibitory activity of LBH589 in both MM cell lines and patient samples. We observed significant up regulation of both AcH3 and AcH4 when cells were treated with LBH589 or the drug combination (Figures 6A-D). Next, we examined mTOR activity levels post drug treatment. pmTOR, as expected, was potently inhibited by RAD001 and the drug combination in both MM cell lines and patient cells (Figures 6A-D). RAD001 was clearly able to inhibit rapamycin sensitive mTORC1 activity as shown by decrease in both p-p70S6K (Figures 6A-D) and pS6 (Figures 6A and B) while being unable to inhibit the rapamycin insensitive mTORC1 activity as shown by the lack of inhibition of p4EBP1 (The37/46) (Figures 6A-C).
Figure 6.
It is known that mTORC2 mediated activation of pAkt (Ser473) by rapamycin and rapalogs contributes towards resistance to these agents[43]. In some studies, LBH589 has been shown to be able to down regulate pAkt thereby sensitizing cells to mTORC1 inhibitors. In other studies, synergy between HDAC inhibitors with inhibitors of the PI3K/Akt pathway has been shown to be independent of pAkt down regulation. We therefore wanted to examine levels of pAkt after the drug treatment. In addition, we also examined how the drug combination modulates other important signaling pathways in MM, namely MEK/Erk and Jak2/Stat3 pathways. We observed down regulation of pAkt post RAD001 treatment in MM1S cells (Figure 6A). On the contrary, LBH589 caused an up regulation of pAkt and other members of the PI3K/Akt pathway including pGSK3β and pmTOR. However, LBH589/RAD001 combination effectively down regulated pAkt, pmTOR and p-p70S6K (Figure 6A). In OPM2 cells, we observed a slight up-regulation of pAkt post RAD001 treatment. However neither LBH589 nor the combination was able to down regulate this induction in pAkt levels (Figure 6B). When we examined pAkt pathway regulation in primary MM patient cells, we observed down regulation of pAkt when treated with LBH589/RAD001 combination in cells from patient 1 (Figure 6C). However, in cells from patient 2 we observed up regulation of pAkt post RAD001 treatment and this was not abolished by LBH589 treatment alone or in combination with RAD001 (Figure 6D). Thus, the observed synergy between LBH589/RAD001 in MM cells appears to be independent of pAkt down regulation. Similarly, MEK/Erk pathway inhibition also does not seem to contribute to the observed synergy as noted by pErk levels post drug treatment (Figures 6A-D). LBH589 was able to down regulate pStat3 in OPM2 and the two patient cells examined (Figures 6B-D). However, LBH589 caused an up regulation of pStat3 in MM1S cells (Figures 6A). RAD001 caused a decrease in pStat3 levels in MM1S and patient 1(Figures 6A and C) while not modulating the levels of pStat3 in OPM2 and patient 2 (Figures 6B and D). Importantly, pStat3 was down regulated when treated with LBH589/RAD001 combination in both MM cell lines and patient samples (Figures 6A-D). Thus, LBH589/RAD001 combination shows promising synergistic activity in MM cells with RAD001 causing more potent growth arrest and LBH589 inducing more pronounced apoptosis.
4. Discussion
Novel therapies have improved the clinical outcomes in MM[44]. Despite improvements, MM still remains incurable and MM patients eventually relapse and become refractory to existing therapies. Significant efforts are underway to develop newer drugs based on a better understanding of the disease biology. It is now well appreciated that clonal heterogeneity is a common feature in MM[45]. This could be an important factor for why majority of newer targeted therapies fail to produce significant and prolonged responses in clinical trials and also for the relapse observed in patients treated with existing therapies. Hence, novel agents that can act on multiple targets or those that modulate pathways regulating multiple targets down stream have the potential to elicit significant anti-MM responses across a wider group of patients. LBH589 is an epigenetic drug with an increasing number of targets it can act on. Likewise, mTORC1 modulates various cellular processes causing cellular growth and proliferation. Here, we have examined the activity of LBH589 with the mTORC1 inhibitor RAD001 and observed potent synergy when used in combination across several MM cell lines and patient cells. The combination was able to induce apoptosis, inhibit proliferation and overcome tumor protective effects of the microenvironment at concentrations much lower than effective single agent doses.
We then examined expression levels of pro and anti apoptotic proteins, cell cycle regulatory proteins and signaling proteins to better understand the mechanism of LBH589/RAD001 induced synergy. Though our results were inconclusive in identifying a particular mechanism of action of this drug combination, we clearly demonstrated that LBH589/RAD001 could elicit its functions through multiple routes in different cells. Some possible explanations for this are: 1) LBH589 is an epigenetic drug with a growing list of effector molecules including non-histone proteins Hsp90, Stat3, p53, NF-κB and several other transcription factors[9], 2) LBH589 is a pan- HDACi and its functions therefore could depend on expression levels of individual HDACs and 3) LBH589, in addition, might carry out its functions by influencing and maintaining double stranded break (DSB) points and thereby promoting DNA instability[46]. Though all MM cell lines proliferate, differences in their proliferating rate could alter their sensitivity to the drug. 4) mTORC1 can regulate multiple processes including translation, lipogenesis, autophagy. In addition, mTORC1 can regulate activation states of pAkt, pStat3 and HIF1α. To complicate things further, all these effects by mTORC1 could considerably differ between cell lines even within the same disease[47].
There are reports indicating that RAD001 and other mTOR inhibitors up regulate pAkt (Ser 473). However, it is also known that mTORC1 inhibitors could cause either an increase or a decrease in the levels of pAkt (Ser 473) depending on the cell line by differentially modulating mTORC2 assembly and also the duration of exposure to the inhibitor[48]. In our hands, RAD001 treatment was not observed to stimulate increase in levels of pAkt (Ser 473) in MM1S cells. In order to check if the RAD001 induced down regulation of pAkt (Ser 473) was also observed in other cell lines, we examined levels of pAkt (Ser 473) post RAD001 treatment in MM1R, H929, U266, RPMI8226, DOX and LR5 cells. We observed that pAkt (Ser 473) was up regulated in H929, U266, RPMI8226, DOX and LR5 cells in addition to OPM2. pAkt (Ser 473) was down regulated in MM1S and MM1R cells (data not shown). We then examined pAkt (Ser 473) levels after treatment with 10nM rapamycin or 10nM RAD001 for 24hrs in three cell lines H929, MM1S and RPMI8226 (data not shown). Like RAD001, rapamycin down regulated pAkt (Ser 473) in MM1S cells and up-regulated pAkt in H929 and RPMI8226 cells. It was noted that RAD001 caused a greater down regulation of pAkt (Ser 473) in MM1S cells and a greater up regulation in H929 and RPMI8226 cells (data not shown). Thus, this differential regulation of pAkt (Ser 473) was not limited to RAD001 but was also found to occur with other mTORC1 inhibitors.
To conclude, we report the effectiveness of LBH589/RAD001 combination in killing myeloma cells at low nanomolar concentrations. Furthermore, LBH589/RAD001 could inhibit proliferation, induce G0/G1 arrest and induce apoptosis by modulating multiple signaling pathways and down stream effector proteins. While the studies do not pinpoint a single mechanism active in all cell lines and patient samples, it demonstrates pleiotropic mechanisms in different cell lines the cumulative effect of which appear to induce cell cycle arrest, tilting the balance between pro- and anti-apoptotic proteins towards apoptosis and down regulation of cell survival signaling pathways. This is particularly relevant in a cancer such as myeloma, which is very heterogeneous in terms of their underlying biology, in that it can overcome the effect of multiple overlapping and compensatory pathways for survival. Though much remains to be done in identifying precise mechanism of action, the data from our study clearly indicates the effectiveness of LBH589/RAD001 combination as anti-MM agents. In clinical trials, the immune status of patients should be closely monitored as LBH589 at similar doses used in our study has been shown to modulate the phenotype and impair the function of antigen presenting dendritic cells[49].
Acknowledgments
We would like to acknowledge Kimberly Henderson, Roberta DeGoey and Steven Zincke for their assistance with processing of tumor cells and all of the patients who provided us with the tumor samples.
Financial support: This study was supported in part by Hematological Malignancies Program (Mayo Clinic Cancer Center); and CA90628 (SK) from National Cancer Institute. LBH589 and RAD001 were synthesized and provided by Novartis (Basel, Switzerland) under a Material Transfer Agreement (MTA).
Footnotes
Conflict of Interest: SK: research support from Celgene, Millennium, Novartis, Merck, Cephalon, Genzyme and Bayer. SK: advisory board- Merck.
Contributions: VR, SVR and SK- designed the experiments, VR, TK, MT and JH- performed the experiments, VR, TK and SK- analyzed the data and VR, SVR and SK-wrote the manuscript and all authors edited the manuscript.
References
- 1.Eberharter A, Becker PB. Histone acetylation: a switch between repressive and permissive chromatin. Second in review series on chromatin dynamics. EMBO Rep. 2002;3:224–9. doi: 10.1093/embo-reports/kvf053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayes JJ, Hansen JC. Nucleosomes and the chromatin fiber. Curr Opin Genet Dev. 2001;11:124–9. doi: 10.1016/s0959-437x(00)00168-4. [DOI] [PubMed] [Google Scholar]
- 3.Roth SY, Denu JM, Allis CD. Histone acetyltransferases. Annu Rev Biochem. 2001;70:81–120. doi: 10.1146/annurev.biochem.70.1.81. [DOI] [PubMed] [Google Scholar]
- 4.Thiagalingam S, Cheng KH, Lee HJ, Mineva N, Thiagalingam A, Ponte JF. Histone deacetylases: unique players in shaping the epigenetic histone code. Ann N Y Acad Sci. 2003;983:84–100. doi: 10.1111/j.1749-6632.2003.tb05964.x. [DOI] [PubMed] [Google Scholar]
- 5.West AC, Johnstone RW. New and emerging HDAC inhibitors for cancer treatment. J Clin Invest. 2014;124:30–9. doi: 10.1172/JCI69738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catley L, Weisberg E, Tai YT, Atadja P, Remiszewski S, Hideshima T, et al. NVP-LAQ824 is a potent novel histone deacetylase inhibitor with significant activity against multiple myeloma. Blood. 2003;102:2615–22. doi: 10.1182/blood-2003-01-0233. [DOI] [PubMed] [Google Scholar]
- 7.Maiso P, Carvajal-Vergara X, Ocio EM, Lopez-Perez R, Mateo G, Gutierrez N, et al. The histone deacetylase inhibitor LBH589 is a potent antimyeloma agent that overcomes drug resistance. Cancer Res. 2006;66:5781–9. doi: 10.1158/0008-5472.CAN-05-4186. [DOI] [PubMed] [Google Scholar]
- 8.Mitsiades CS, Mitsiades NS, McMullan CJ, Poulaki V, Shringarpure R, Hideshima T, et al. Transcriptional signature of histone deacetylase inhibition in multiple myeloma: biological and clinical implications. Proc Natl Acad Sci U S A. 2004;101:540–5. doi: 10.1073/pnas.2536759100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benedetti R, Conte M, Altucci L. Targeting HDACs in diseases: where are we? Antioxid Redox Signal. 2014 doi: 10.1089/ars.2013.5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richardson P, Mitsiades C, Colson K, Reilly E, McBride L, Chiao J, et al. Phase I trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) in patients with advanced multiple myeloma. Leuk Lymphoma. 2008;49:502–7. doi: 10.1080/10428190701817258. [DOI] [PubMed] [Google Scholar]
- 11.Niesvizky R, Ely S, Mark T, Aggarwal S, Gabrilove JL, Wright JJ, et al. Phase 2 trial of the histone deacetylase inhibitor romidepsin for the treatment of refractory multiple myeloma. Cancer. 2011;117:336–42. doi: 10.1002/cncr.25584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolf JL, Siegel D, Goldschmidt H, Hazell K, Bourquelot PM, Bengoudifa BR, et al. Phase II trial of the pan-deacetylase inhibitor panobinostat as a single agent in advanced relapsed/refractory multiple myeloma. Leuk Lymphoma. 2012;53:1820–3. doi: 10.3109/10428194.2012.661175. [DOI] [PubMed] [Google Scholar]
- 13.Hideshima T, Anderson KC. Histone deacetylase inhibitors in the treatment for multiple myeloma. Int J Hematol. 2013;97:324–32. doi: 10.1007/s12185-013-1290-3. [DOI] [PubMed] [Google Scholar]
- 14.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–57. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 15.Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell. 2005;121:179–93. doi: 10.1016/j.cell.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 16.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–90. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 17.Feng Z, Zhang H, Levine AJ, Jin S. The coordinate regulation of the p53 and mTOR pathways in cells. Proc Natl Acad Sci U S A. 2005;102:8204–9. doi: 10.1073/pnas.0502857102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, et al. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gingras AC, Raught B, Sonenberg N. mTOR signaling to translation. Curr Top Microbiol Immunol. 2004;279:169–97. doi: 10.1007/978-3-642-18930-2_11. [DOI] [PubMed] [Google Scholar]
- 20.Porstmann T, Santos CR, Griffiths B, Cully M, Wu M, Leevers S, et al. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 2008;8:224–36. doi: 10.1016/j.cmet.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jung CH, Ro SH, Cao J, Otto NM, Kim DH. mTOR regulation of autophagy. FEBS Lett. 2010;584:1287–95. doi: 10.1016/j.febslet.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zollinger A, Stuhmer T, Chatterjee M, Gattenlohner S, Haralambieva E, Muller-Hermelink HK, et al. Combined functional and molecular analysis of tumor cell signaling defines 2 distinct myeloma subgroups: Akt-dependent and Akt-independent multiple myeloma. Blood. 2008;112:3403–11. doi: 10.1182/blood-2007-11-119362. [DOI] [PubMed] [Google Scholar]
- 23.Hideshima T, Nakamura N, Chauhan D, Anderson KC. Biologic sequelae of interleukin-6 induced PI3-K/Akt signaling in multiple myeloma. Oncogene. 2001;20:5991–6000. doi: 10.1038/sj.onc.1204833. [DOI] [PubMed] [Google Scholar]
- 24.Mitsiades CS, Mitsiades N, Poulaki V, Schlossman R, Akiyama M, Chauhan D, et al. Activation of NF-kappaB and upregulation of intracellular anti-apoptotic proteins via the IGF-1/Akt signaling in human multiple myeloma cells: therapeutic implications. Oncogene. 2002;21:5673–83. doi: 10.1038/sj.onc.1205664. [DOI] [PubMed] [Google Scholar]
- 25.Ramakrishnan V, Kimlinger T, Haug J, Painuly U, Wellik L, Halling T, et al. Anti-myeloma activity of Akt inhibition is linked to the activation status of PI3K/Akt and MEK/ERK pathway. PloS one. 2012;7:e50005. doi: 10.1371/journal.pone.0050005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neri A, Murphy JP, Cro L, Ferrero D, Tarella C, Baldini L, et al. Ras oncogene mutation in multiple myeloma. The Journal of experimental medicine. 1989;170:1715–25. doi: 10.1084/jem.170.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chng WJ, Gonzalez-Paz N, Price-Troska T, Jacobus S, Rajkumar SV, Oken MM, et al. Clinical and biological significance of RAS mutations in multiple myeloma. Leukemia. 2008;22:2280–4. doi: 10.1038/leu.2008.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Podar K, Tai YT, Davies FE, Lentzsch S, Sattler M, Hideshima T, et al. Vascular endothelial growth factor triggers signaling cascades mediating multiple myeloma cell growth and migration. Blood. 2001;98:428–35. doi: 10.1182/blood.v98.2.428. [DOI] [PubMed] [Google Scholar]
- 29.Hideshima T, Catley L, Yasui H, Ishitsuka K, Raje N, Mitsiades C, et al. Perifosine, an oral bioactive novel alkylphospholipid, inhibits Akt and induces in vitro and in vivo cytotoxicity in human multiple myeloma cells. Blood. 2006;107:4053–62. doi: 10.1182/blood-2005-08-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tai YT, Fulciniti M, Hideshima T, Song W, Leiba M, Li XF, et al. Targeting MEK induces myeloma-cell cytotoxicity and inhibits osteoclastogenesis. Blood. 2007;110:1656–63. doi: 10.1182/blood-2007-03-081240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramakrishnan V, Timm M, Haug JL, Kimlinger TK, Wellik LE, Witzig TE, et al. Sorafenib, a dual Raf kinase/vascular endothelial growth factor receptor inhibitor has significant anti-myeloma activity and synergizes with common anti-myeloma drugs. Oncogene. 2010;29:1190–202. doi: 10.1038/onc.2009.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frost P, Moatamed F, Hoang B, Shi Y, Gera J, Yan H, et al. In vivo antitumor effects of the mTOR inhibitor CCI-779 against human multiple myeloma cells in a xenograft model. Blood. 2004;104:4181–7. doi: 10.1182/blood-2004-03-1153. [DOI] [PubMed] [Google Scholar]
- 33.Farag SS, Zhang S, Jansak BS, Wang X, Kraut E, Chan K, et al. Phase II trial of temsirolimus in patients with relapsed or refractory multiple myeloma. Leukemia research. 2009;33:1475–80. doi: 10.1016/j.leukres.2009.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ria R, Catacchio I, Berardi S, De Luisi A, Caivano A, Piccoli C, et al. HIF-1alpha of Bone Marrow Endothelial Cells Implies Relapse and Drug Resistance in Patients with Multiple Myeloma and May Act as a Therapeutic Target. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014 doi: 10.1158/1078-0432.CCR-13-1950. [DOI] [PubMed] [Google Scholar]
- 35.Frost P, Shi Y, Hoang B, Lichtenstein A. AKT activity regulates the ability of mTOR inhibitors to prevent angiogenesis and VEGF expression in multiple myeloma cells. Oncogene. 2007;26:2255–62. doi: 10.1038/sj.onc.1210019. [DOI] [PubMed] [Google Scholar]
- 36.Ramakrishnan V, Kimlinger T, Haug J, Timm M, Wellik L, Halling T, et al. TG101209, a novel JAK2 inhibitor, has significant in vitro activity in multiple myeloma and displays preferential cytotoxicity for CD45+ myeloma cells. American journal of hematology. 2010;85:675–86. doi: 10.1002/ajh.21785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramakrishnan V, Ansell S, Haug J, Grote D, Kimlinger T, Stenson M, et al. MRK003, a gamma-secretase inhibitor exhibits promising in vitro pre-clinical activity in multiple myeloma and non-Hodgkin's lymphoma. Leukemia. 2012;26:340–8. doi: 10.1038/leu.2011.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 39.Ramakrishnan V, Painuly U, Kimlinger T, Haug J, Rajkumar SV, Kumar S. Inhibitor of apoptosis proteins (IAPs) as therapeutic targets in multiple myeloma (MM) Leukemia. 2014 doi: 10.1038/leu.2014.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumar S, Witzig TE, Timm M, Haug J, Wellik L, Kimlinger TK, et al. Bone marrow angiogenic ability and expression of angiogenic cytokines in myeloma: evidence favoring loss of marrow angiogenesis inhibitory activity with disease progression. Blood. 2004;104:1159–65. doi: 10.1182/blood-2003-11-3811. [DOI] [PubMed] [Google Scholar]
- 41.Rajkumar SV, Mesa RA, Fonseca R, Schroeder G, Plevak MF, Dispenzieri A, et al. Bone marrow angiogenesis in 400 patients with monoclonal gammopathy of undetermined significance, multiple myeloma, and primary amyloidosis. Clinical cancer research : an official journal of the American Association for Cancer Research. 2002;8:2210–6. [PubMed] [Google Scholar]
- 42.Kumar S, Gertz MA, Dispenzieri A, Lacy MQ, Wellik LA, Fonseca R, et al. Prognostic value of bone marrow angiogenesis in patients with multiple myeloma undergoing high-dose therapy. Bone marrow transplantation. 2004;34:235–9. doi: 10.1038/sj.bmt.1704555. [DOI] [PubMed] [Google Scholar]
- 43.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 44.Kumar SK, Dispenzieri A, Lacy MQ, Gertz MA, Buadi FK, Pandey S, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2013 doi: 10.1038/leu.2013.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lohr JG, Stojanov P, Carter SL, Cruz-Gordillo P, Lawrence MS, Auclair D, et al. Widespread genetic heterogeneity in multiple myeloma: implications for targeted therapy. Cancer cell. 2014;25:91–101. doi: 10.1016/j.ccr.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eot-Houllier G, Fulcrand G, Magnaghi-Jaulin L, Jaulin C. Histone deacetylase inhibitors and genomic instability. Cancer Lett. 2009;274:169–76. doi: 10.1016/j.canlet.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 47.Laplante M, Sabatini DM. Regulation of mTORC1 and its impact on gene expression at a glance. Journal of cell science. 2013;126:1713–9. doi: 10.1242/jcs.125773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Molecular cell. 2006;22:159–68. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 49.Song W, Tai YT, Tian Z, Hideshima T, Chauhan D, Nanjappa P, et al. HDAC inhibition by LBH589 affects the phenotype and function of human myeloid dendritic cells. Leukemia. 2011;25:161–8. doi: 10.1038/leu.2010.244. [DOI] [PMC free article] [PubMed] [Google Scholar]