Summary
Antigen-independent tonic signaling by chimeric antigen receptors (CARs) can increase differentiation and exhaustion of T cells, limiting their potency. Incorporating 4-1BB costimulation in CARs may enable T cells to resist this functional exhaustion; however, the potential ramifications of tonic 4-1BB signaling in CAR T cells remain unclear. Here, we found that tonic CAR-derived 4-1BB signaling can produce toxicity in T cells via continuous TRAF2-dependent activation of the NF-kB pathway and augmented Fas-dependent cell death. This mechanism was amplified in a non-self-inactivating gammaretroviral vector through positive feedback on the LTR promoter, further enhancing CAR expression and tonic signaling. Attenuating CAR expression by substitution with a self-inactivating lentiviral vector minimized tonic signaling and improved T-cell expansion and anti-tumor function. These studies illuminate the interaction between tonic CAR signaling and the chosen expression platform and identify inhibitory properties of the 4-1BB costimulatory domain that have direct implications for rational CAR design.
Keywords: Chimeric antigen receptor, costimulation, 4-1BB, T cells, adoptive T cell therapy
eTOC blurb
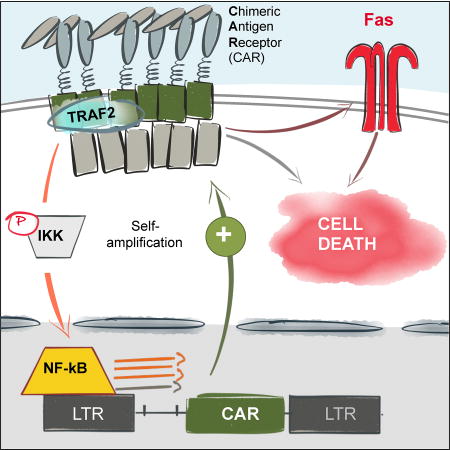
Gomes-Silva et al. model tonic signaling from chimeric antigen receptors (CARs) harboring the 4-1BB endodomain and describe a mechanism through which this signaling produces toxicity in T cells. CAR-driven tonic 4-1BB signaling activates the LTR promoter in gammaretroviral vectors, thus further amplifying the toxicity and undermining CAR T-cell anti-tumor activity.
Introduction
Ligation of chimeric antigen receptors (CARs) in T cells by surface tumorassociated antigens mimics the natural engagement of T cell receptors (TCRs), leading to activation and degranulation of transgenic T cells. Incorporation of costimulatory endodomains in second-generation CARs has increase proliferation and survival of CAR T cells by providing an additional Signal 2 (Brentjens et al., 2013; Lee et al., 2015; Maude et al., 2014; Savoldo et al., 2011). The most widely used costimulatory endodomains are derived from CD28 or 4-1BB genes. Despite stark differences in T cell kinetics, correlating with the distinct signaling cascades triggered by these endodomains, CARs incorporating either 4-1BB or CD28 have lead to dramatic CAR T cell expansion in patients with B-cell malignancies and the complete elimination of otherwise refractory tumors (Brentjens et al., 2013; Lee et al., 2015; Maude et al., 2014; Porter et al., 2011).
In addition to antigen-driven stimulation, CARs frequently produce antigen-independent tonic signaling in T cells. This constitutive signaling is commonly enhanced by the high surface density and self-aggregating properties of CARs, although the contribution of this signaling to regulating persistence and function of transgenic T cells has been debated. Tonic CAR stimulation could maintain T-cell expansion by mimicking signals that promote peripheral expansion of memory T cells specific for persistent pathogens (Klenerman and Oxenius, 2016). However, recent independent studies using c-Met and GD2-specific CARs expressed from lenti- and gammaretroviral vectors, respectively, indicated that, while tonic signaling from CARs harboring the CD28 costimulatory endodomains indeed promoted antigen-independent expansion of T cells in vitro, the expanded CAR T cells had inferior anti-tumor properties and limited persistence in vivo (Frigault et al., 2015; Long et al., 2015). Reducing surface levels of CD28.zeta CD19 CAR by expressing it from the endogenous TCR alpha (TRAC) gene locus prevented in vivo exhaustion and improved the anti-tumor function of CAR T cells (Eyquem et al., 2017). Moreover, replacing CD28 with 4-1BB costimulation reversed exhaustion in GD2 CAR T cells (Long et al., 2015). However, whether and under which circumstances tonic 4-1BB signaling can have similar adverse ramifications in T cells has not yet been studied.
Here, we model tonic CAR-derived 4-1BB signaling in T cells and demonstrate a mechanism by which it impairs CAR T cell expansion and cytotoxic function. We show that tonic 4-1BB signaling is amplified in gammaretroviral vectors, and attenuating CAR expression in alternative expression systems decreases tonic signaling-associated toxicities and augments anti-tumor activity. These results highlight potential inhibitory properties of 4-1BB costimulation and have direct implications for adoptive T cell therapy.
Results
High expression of 4-1BB.zeta CARs impairs T-cell expansion
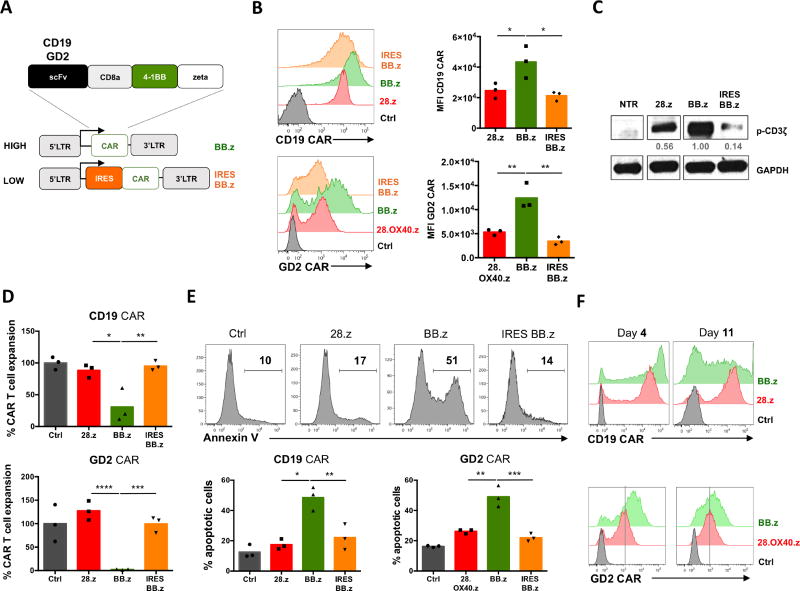
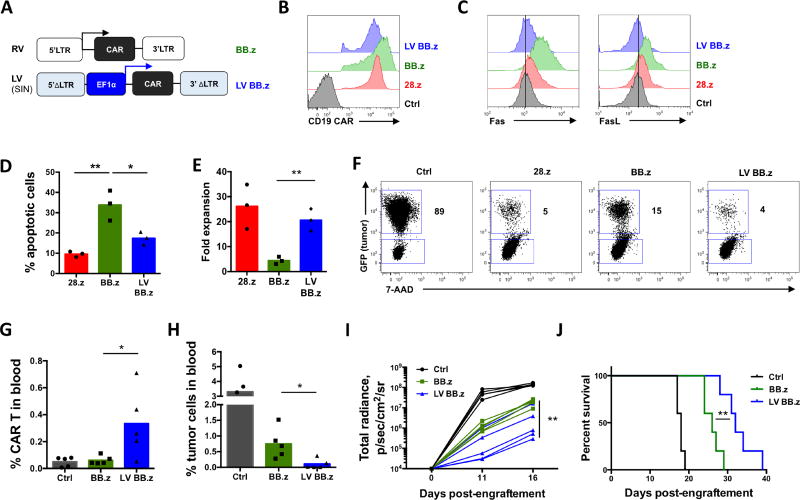
High CAR expression on the cell surface, driven by strong promoters in viral vectors, can result in spontaneous ligand-independent clustering of CAR molecules and produce tonic signaling (Frigault et al., 2015; Long et al., 2015). To assess whether the 4-1BB costimulatory endodomain produces this effect in CAR T cells, we used 2nd generation CD19 CAR containing a CD8a stalk and 4-1BB (BB.z), a construct that has proven successful in clinical studies (Maude et al., 2014; Porter et al., 2011). In addition, we created a GD2-specific CAR utilizing the same backbone (Figure 1A). To modulate the expression level of CARs in T cells – and hence the magnitude of tonic CAR signaling – we inserted the CAR cassettes in gammaretroviral vectors in which CAR expression was driven either directly by the retroviral LTR promoter (BB.z) or attenuated by an upstream IRES element (IRES BB.z) (Figure 1A). As controls we used clinically validated CARs with CD28 endodomains (28.z CD19 CAR (Savoldo et al., 2011) and 28.OX40.z GD2 CAR (Louis et al., 2011; Pule et al., 2008)). We found that the expression of BB.z CD19-and GD2-specific CARs was higher than control CD28-containing CARs, while incorporating an IRES reduced CAR expression (Figure 1B). As expected, decreasing CAR expression reduced tonic signaling and spontaneous phosphorylation of the CAR-derived CD3 zeta chain in transduced T cells (Figure 1C). Notably, T cells expressing high levels of BB.z CARs expanded significantly less following transduction than control CAR T cells (Figure 1D). Impaired expansion of BB.z CAR T cells over time was associated with dramatically increased apoptosis, as reflected by higher frequency of Annexin V+ cells 7 days post-transduction (Figure 1E). Moreover, the surviving BB.z CARs showed progressive downregulation of transgene expression from high initial levels (Figure 1F). We observed a similar effect in T cells transduced with BB.z kappa light chain-specific CAR (Figure S1), suggesting that high expression of BB.z CARs is toxic for T cells and that reduced CAR expression attenuates this ligand-independent signaling (Frigault et al., 2015).
Figure 1. High expression of BB.z CARs in T cells enhances apoptosis.
(A) Schematic representation of the expression systems utilized to express BB.z CARs in T cells. (B) Expression of CD19- and GD2-specific CARs on the cell surface of T cells 4 days post-transduction; mean fluorescence intensities are plotted on the bar graphs. (C) Relative magnitude of tonic signaling in BB.z and IRES BB.z CAR T cells 7 days post-transduction measured by western blot analysis. Numbers indicate relative signal intensity normalized to GAPDH. (D) Overall expansion of T cells retrovirally transduced with CD19- and GD2-specific CARs relative to that of control mock-transduced T cells. (E) Representative histograms showing Annexin V staining of CD19 CAR T cells 7 days post-transduction. Bar graphs show summarized data for both CARs from 3 donors. (F) Representative histograms showing expression of CD19-and GD2-specific CARs in expanding T cells 4 and 11 days post-transduction. Data represent two to five independent experiments. *, P<0.05; **, P<0.01; ***, P<0.001 by one-way ANOVA.
High expression of BB.z CAR alters the phenotype and reduces the anti-tumor activity of T cells
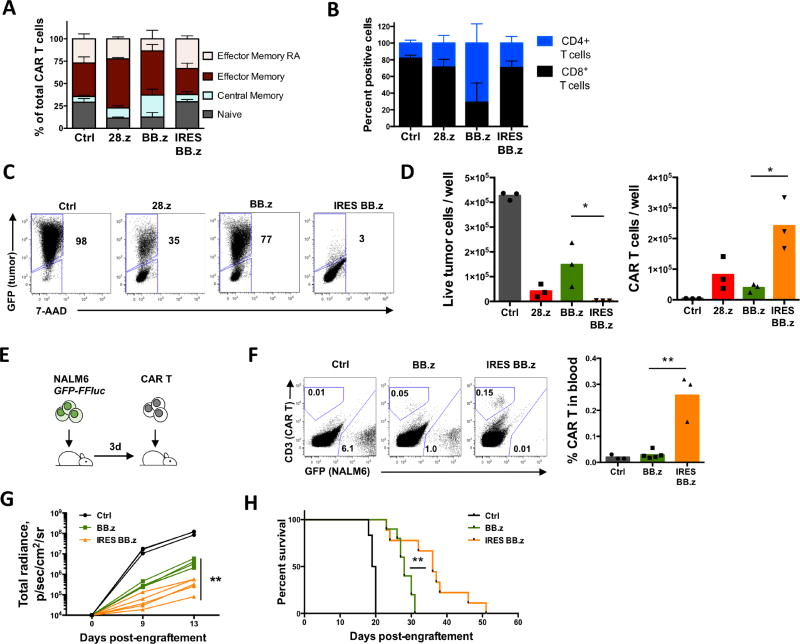
Next, we sought to determine how CAR-derived tonic 4-1BB costimulation affected the anti-tumor function of BB.z CAR T cells. We observed increased frequency of central memory (CCR7+ CD45RA−) cells among BB.z CD19 CAR T cells (Figure 2A), consistent with the role of 4-1BB in promoting central memory differentiation (Kawalekar et al., 2016), and an increased CD4:CD8 T cell ratio (Figure 2B). Attenuated expression in IRES BB.z CAR T cells produced a T cell phenotype similar to that of non-transduced T cells and reverted the CD4:CD8 ratio (Figure 2A, B). We observed similar phenotypic changes in T cells expressing GD2- and kappa light chain-specific CARs, while reducing CAR expression normalized T cell phenotype (Figure S1).
Figure 2. High expression of BB.z CD19 CAR alters the phenotype and undermines the anti-tumor activity of CAR T cells.
(A) Relative proportion of naïve-phenotype (TN), central memory (TCM), effector memory (TEM) and terminally differentiated effector cells (TEMRA) CD19 CAR T cells 14 days post-transduction defined by CCR7 and CD45RA expression. (B) Stacked bar graph showing relative proportion of CD4+ and CD8+ cells among CD19 CAR T cells 14d post-transduction. (C) CD19 CAR T cells were cocultured with GFP+ Raji cells (CD19+) at a 1:2 ratio for 72h. Representative dot plots show the frequency of residual live tumor cells at the end of coculture. Numbers indicate percent of tumor cells among total live cells. (D) Absolute cell counts of Raji and CAR T cells at the end of coculture determined by flow cytometry using counting beads. Data from individual donors are shown on each bar graph. (E) Schematic diagram of the experiment. NSG mice were intravenously engrafted with 1×106 GFP+ FFluc+ NALM-6 cells followed by a single intravenous injection of 1.0×106 CD19 CAR T cells 3d later. (F) Representative staining of peripheral blood of tumor-bearing mice 17 days after tumor engraftment with relative frequency of CD19 CAR T cells and GFP+ NALM-6 cells. Relative frequencies of CD19 CAR T cells in peripheral blood 17 days post-engraftment are shown on the bar graph. (G) Kinetics of NALM-6 luminescence in mice measured by IVIS. (H) Kaplan-Meier curves showing advantage in overall survival of mice receiving IRES BB.z CD19 CAR T cells compared to mice receiving BB.z T cells (P=0.0036 by Mantel-Cox Log-rank test). Data represent 2–3 independent experiments. *, P<0.05; **, P<0.01 by Student’s t test.
We have shown that toxicity from high BB.z CAR expression can limit T cell expansion and alter their phenotype, but whether it affects CAR T cell anti-tumor function is unclear. We therefore measured the cytotoxicity of BB.z CD19 CAR T cells upon coculture with fluorescently labeled CD19+ cell line Raji. We observed a significant increase in residual live tumor cells after coculture with BB.z CD19 CAR T cells (Figure 2C, D) and reduced expansion of CAR T cells at the end of coculture compared to T cells transduced with IRES BB.z CD19 CAR (Figure 2D). These results indicate that high expression of BB.z CD19 CAR from a gammaretroviral vector actually inhibits the anti-tumor function of T cells and that lower CAR expression in IRES BB.z cells still provides sufficient avidity to produce cytotoxicity. To verify the impact of CAR expression on anti-tumor activity in vivo, we tested the ability of BB.z CD19 CAR T cells to suppress progression of leukemia in a xenograft mouse model of pre-B ALL (NALM-6). We chose an aggressive systemic model in which most mice succumb to the disease within 3 weeks if left untreated. A single low dose of CAR T cells was administered intravenously 3 days after tumor delivery (Figure 2E). We observed significantly increased expansion of IRES BB.z CAR T cells in peripheral blood (Figure 2F) compared to BB.z CAR T cells. Overall, the IRES BB.z CAR T cells demonstrated more potent suppression of leukemia progression (Figure 2G), leading to a significant extension of survival compared to BB.z cells (Figure 2H). Therefore, toxicity associated with high expression of BB.z CAR in T cells impedes their expansion and anti-tumor function and can be reversed by attenuating CAR expression.
Disrupting TRAF2 signaling in the 4-1BB endodomain reverses toxicity and normalizes CAR expression
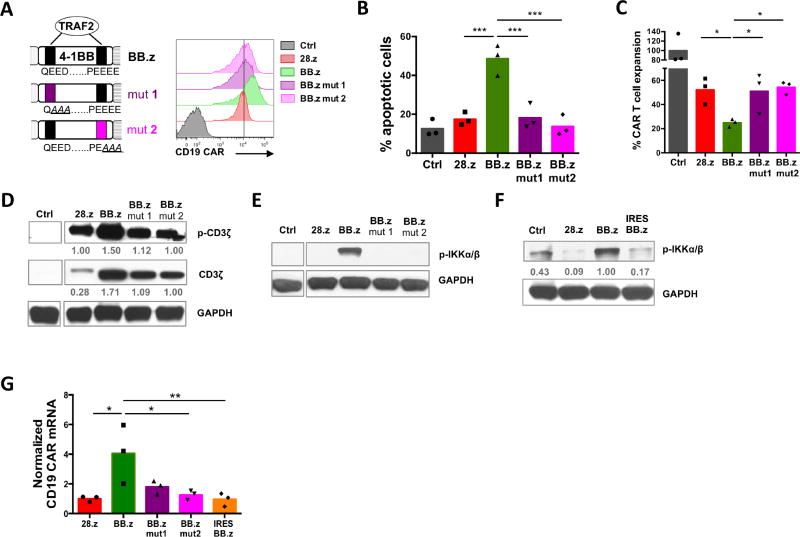
Because high expression of BB.z CARs led to cell toxicity regardless of CAR specificity, we hypothesized the enhanced apoptosis of BB.z CAR T cells was a generalizable phenomenon attributable to tonic signaling from the 4-1BB endodomain. We therefore substituted key amino acid residues in both TRAF-binding motifs of the 4-1BB endodomain; disruption of the N-terminal motif (mut1QEED->QAAA) specifically abrogates TRAF2 binding, while mutation in the C-terminal motif (mut2PEEEE->PEAAA) prevents binding with TRAF1, TRAF2 and TRAF3 (Jang et al., 1998) (Figure 3A). Altering either of these motifs normalized expression of the BB.z CAR on the cell surface to levels comparable to 28.z CAR (Figure 3A). Importantly, disruption of a single TRAF2 binding site was sufficient to minimize BB.z CAR T cell apoptosis (Figure 3B and Figure S1) and restore normal expansion (Figure 3C and Figure S1), indicating that tonic TRAF2 signaling indeed produces toxicity in BB.z CAR T cells.
Figure 3. Enhanced apoptosis and increased expression of BB.z CAR in T cells requires 4-1BB-derived TRAF2 signaling.
(A) A schematic drawing of TRAF2 binding motifs in the 4-1BB domain and corresponding alterations in the protein sequence to disrupt either the N-terminal (mut 1) or the C-terminal (mut 2) motif. Histogram on the right shows relative expression of CD19 CARs with mutated TRAF2 motifs. (B) Frequency of apoptotic cells among CD19 CAR T cells with intact or mutated costimulatory domains 7 days post-transduction. (C) Overall expansion of CD19 CAR T cells on day 7 post-transduction. (D) Tonic CD19 CAR signaling and total CAR expression in T cells 7 days post-transduction was measured with anti-pTyr (CD3ζ) and total anti-CD3ζ antibodies by western blot. Numbers indicate relative signal intensity normalized to GAPDH. (E, F) Phosphorylation of IKKα/β in CD19 CAR T cells 7 days post-transduction by a western blot analysis. (G) Relative abundance of CD19 CAR transcripts was measured by calculating relative expression of retroviral mRNA normalized to the copy number of CD19 CAR-containing proviruses per 100ng of genomic DNA by qPCR in T cells 7 days post-transduction. Data represent 2–3 independent experiments with n=3 donors in each. *, P<0.05; **, P<0.01; ***, P<0.001 by one-way ANOVA.
To investigate whether 4-1BB affects tonic CAR signaling in T cells, we measured spontaneous phosphorylation of ITAM motifs of the CAR-embedded zeta chain by western blot. We observed increased ITAM phosphorylation (per cell) in BB.z CD19 CAR compared to the 28.z CAR control, while disrupting TRAF binding sites normalized spontaneous signaling (Figure 3D). The elevated tonic signaling of BB.z CD19 CAR was consistently associated with increased total CAR protein levels in T cells (Figure 3D). Of note, 28.z CAR produced more signaling per CAR molecule, corroborating the enhanced propensity for spontaneous signaling of 28.z CARs as previously demonstrated (Long et al., 2015). We therefore sought to determine the mechanism of enhanced CAR production in BB.z CAR-transduced T cells.
In non-self-inactivating gammaretroviral vectors, transgene expression is driven by the LTR promoter, which is positively regulated by the host transcription factor NF-kB (Hiscott et al., 2001; Roulston et al., 1995). In HIV-infected T cells, signaling from 4-1BB and other TNFR genes can activate the LTR promoter and promote viral replication (Herbein and Khan, 2008; Hiscott et al., 2001). We therefore checked whether tonic 4-1BB-derived TRAF2 signaling similarly increased CAR expression from gammaretroviral vectors by enhancing LTR promoter activity. TRAF2 signaling from 4-1BB activates NF-kB by interacting with and activating the IkB kinase complex (IKKα/β), a positive regulator of NF-kB (Jang et al., 1998; Pomerantz and Baltimore, 1999). Thus, we could block phosphorylation of IKKα/β in BB.z CAR T cells by disrupting TRAF2 signaling or replacing 4-1BB with CD28 (Figure 3E). Similarly, reducing the level of CAR expression in IRES BB.z CAR T cells attenuated tonic CAR signaling and decreased NF-kB pathway activation (Figure 3F). To investigate whether this signaling pathway modulated LTR promoter activity, we measured the levels of CD19 CAR mRNA in T cells and normalized to the number of provirus integrations into the genomic DNA. We found significant upregulation of CD19 CAR mRNA per genomic copy of the provirus (Figure 3G) in BB.z CAR T cells, suggesting that tonic 4-1BB signaling forms a positive feedback loop by enhancing activity of the gammaretroviral LTR promoter and amplifying CAR production in T cells.
Tonic 4-1BB signaling increases Fas-dependent apoptosis of CAR T cells
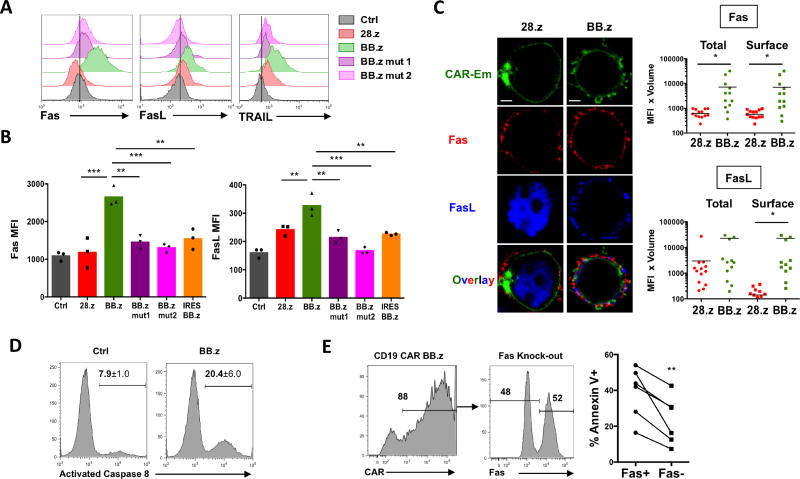
Overstimulation of T cells can provoke apoptosis via the activation-induced cell death (AICD) mechanism, which is predominantly mediated by the Fas/FasL interaction and subsequent activation of caspase 8 (Alderson et al., 1995; Green et al., 2003; Künkele et al., 2015; Muzio et al., 1996). Because both Fas and FasL genes can be directly activated by the NF-kB pathway (Chan et al., 1999; Lin et al., 1999; Singh et al., 2007), we investigated whether this mechanism causes apoptosis of BB.z CAR T cells. We observed an increase in surface expression of the proapoptotic genes Fas, FasL and TRAIL in BB.z CAR T cells, and reducing CAR expression or disrupting TRAF2 signaling reversed it (Figure 4A, B). Furthermore, levels of Fas and FasL on the cell surface positively correlated with CD19 CAR expression and negatively correlated with T cell expansion (Figure S2), suggesting that the upregulation of proapoptotic molecules is directly proportional to the magnitude of tonic signaling in T cells. Analysis of CAR T cells by confocal microscopy revealed that while FasL was mainly localized intracellularly in 28.z CAR T cells, Fas and FasL became co-localized on the cell surface in BB.z CD19 CAR T cells (Figure 4C and Figure S2). This co-localization triggers Fas signaling and subsequent AICD (Green et al., 2003). In addition, we observed an increased aggregation of BB.z CD19 CAR T cells that correlated with an upregulation of intercellular adhesion molecule 1 (ICAM-1) (Figure S3), which is directly activated by NF-kB in various cell types (Melotti et al., 2001; Roebuck and Finnegan, 1999). ICAM-1 expression in T cells facilitates their clustering (Zumwalde et al., 2013) and thus may enable trans-engagement of Fas in CAR T cells by neighboring FasL-expressing cells. Indeed, we detected enhanced activation of caspase 8 in BB.z CD19 CAR T cells (Figure 4D). In order to verify the role of Fas upregulation in BB.z CAR T cell death, we disrupted the Fas gene in BB.z CAR T cells using the CRISPR/Cas9 complex with a Fas-specific sgRNA. We observed a moderate decrease in the frequency of Annexin V+ cells among Fas-negative CAR T cells (Figure 4E), indicating that Fas-mediated AICD contributes to the cell death of BB.z CAR T cells. Disruption of Fas in non-transduced T cells did not significantly decrease their apoptosis (Figure S4). Thus, tonic 4-1BB signaling activates pro-apoptotic pathways, and this toxicity is augmented in CARs expressed from a gammaretroviral vector via the formation of a positive feedback loop.
Figure 4. Tonic 4-1BB signaling promotes Fas-mediated apoptosis in CAR T cells.
(A) Representative staining for surface Fas, FasL and TRAIL by flow cytometry in CD19 CAR T cells 8 days post-transduction. (B) MFI of Fas and FasL on the surface of CD19 CAR on day 8 post-transduction. **, P<0.01; ***, P<0.001 by one-way ANOVA. (C) Representative images showing cellular localization of CD19 CAR fused with Emerald fluorescent protein, Fas and FasL. Bottom panel shows overlay of all three signals. Scale bar = 5 um. Bar graphs show quantification of Fas (top) and FasL (bottom) total and surface signal by confocal microscopy. *, P<0.05; by Student’s t test. (D) Staining for activated caspase 8 in CD19 CAR T cells using a specific fluorescent inhibitor FAMLETD-FMK. Numbers indicate mean ± s.d. percentage of cells with activated caspase 8. (E) BB.z CD19 CAR T cells were electroporated with Cas9 protein and a Fas-specific sgRNA 24h post-transduction and allowed to expand for 7 days. Histograms show expression of CD19 CAR (left) and surface expression of Fas (center) on electroporated T cells. Corresponding changes in the frequency of Annexin V cells gated on Fas+ and Fas- populations are shown on the right with lines indicating cells from the same donor. **, P<0.01 by paired two-tailed Student’s t test. Experiments were replicated twice with n=3 donors in each.
Expressing BB.z CARs from a self-inactivating lentiviral vector reduces toxicity and improves the anti-tumor function of CAR T cells
As our results indicate that expression of BB.z CARs from gammaretroviral LTR promoters amplify toxic 4-1BB signaling, we attenuated the NF-kB feedback loop by replacing the LTR promoter in a viral vector with an alternative promoter and measured effects on toxicity. We assessed the level of CD19 CAR expression from a clinically validated self-inactivating lentiviral vector (Maude et al., 2014; Milone et al., 2009) in which transgene expression is driven by a non-LTR promoter, EF-1a (LV BB.z, Figure 5A), that produces lower initial CAR expression level on the T cell surface (Figure 5B). Indeed, this reduction normalized the expression of Fas and FasL on the cell surface (Figure 5C), resembling attenuation of CAR expression via IRES. The expression of BB.z CD19 and GD2 CARs from the lentiviral vector also significantly reduced cell death and restored overall expansion of CAR T cells in vitro (Figure 5D, E and Figure S1). Lentiviral CAR expression promoted more efficient clearance of tumor cells during in vitro coculture (Figure 5F). Similar to IRES BB.z CD19 CAR T cells, lentivirally transduced BB.z CD19 CAR T cells were more protective in the systemic mouse xenograft model of B-ALL, resulting in increased expansion of CAR T cells in peripheral blood (Figure 5G) and lower frequencies of tumor cells (Figure 5H). Overall, LV BB.z CD19 CAR T cells achieved more potent suppression of leukemia progression (Figure 5I), leading to a significant extension of survival compared to BB.z control (Figure 5J).
Figure 5. Expression of BB.z CD19 CAR from a lentiviral vector reduces toxicity and improves the anti-tumor function of CAR T cells.
(A) Schematic representation of the gammaretroviral (BB.z) and self-inactivating lentiviral (LV BB.z) vectors. Arrows indicate promoter activity in each expression system. (B) Representative histograms showing CD19 CAR expression in T cells 7 days after transduction BB.z or LV BB.z vectors. (C) Representative histograms showing surface expression of Fas and FasL in CD19 CAR T cells. (D) Frequency of apoptotic cells in CD19 CAR T cells 7 days post-transduction measured by Annexin V staining. (E) Expansion of CD19 CAR T cells over 7 days. (F) CD19 CAR T cells were cocultured with GFP+ CD19+Raji cells at a 1:1 ratio for 72h. Representative dot plots show the frequency of residual live tumor cells at the end of coculture. Numbers indicate percent of tumor cells among total live cells. (G) Expansion of LV BB.z CD19 CAR T cells in peripheral blood of NSG mice engrafted with 1×106 GFP+ FFluc+ NALM-6 cells 15 days prior followed by a single intravenous injection of 8×105 CD19 CAR T cells 3 days later. (H) Frequency of GFP+ tumor cells in peripheral blood of mice 15 days post-engraftment. (I) Tumor burden in mice measured by IVIS imaging at indicated time points. (J) Survival of tumor-bearing mice receiving either BB.z or LV BB.z CD19 CAR T cells (P=0.0062 by Mantel-Cox Log-rank test). Experiments were replicated three times for (A–F) and twice for (G–J). *, P<0.05; **, P<0.01 by Student’s t test or one-way ANOVA.
Discussion
Clinical studies of BB.z CD19 CAR T cells demonstrated complete remissions in patients with B cell malignancies associated with increased persistence of transgenic T cells (Maude et al., 2014; Porter et al., 2011; Turtle et al., 2016). BB.z CAR T cells additionally have been shown to resist the functional exhaustion associated with constant CAR signaling, leading to the suggestion that 4-1BB costimulation may be universally beneficial for CAR T cells (Long et al., 2015). 4-1BB signaling in T cells is primarily mediated by TRAF adaptor proteins that can activate JNK and p38 as well as NF-kB via both canonical and non-canonical pathways (McPherson et al., 2012). Acute stimulation of 4-1BB in human T cells protected CD28− tumor-specific cells against AICD upon restimulation (Hernandez-Chacon et al., 2011), promoted mitochondrial biogenesis and enhanced the development of central memory cells (Kawalekar et al., 2016). These studies indicate 4-1BB can have a protective role in T cell persistence.
To study the effects of continuous 4-1BB costimulation in CAR T cells, we overexpressed BB.z CARs to induce their tonic antigen-independent signaling. In our studies, we did see an increase in the central memory population among cells expressing tonically signaling BB.z CARs. However, constant BB.z CAR signaling resulted in augmented T cell apoptosis, illustrating that in different conditions 4-1BB stimulation can have vastly different effects on T cells. In fact, NF-kB can play a pro-apoptotic role in T cells by directly activating expression of TRAIL (Baetu et al., 2001). We found that upregulation in BB.z CAR T cells of Fas and FasL — another pair of NF-kB target genes — contributed to the enhanced apoptosis. Our results suggest that by stable upregulation of pro-apoptotic target genes of NF-kB, tonic 4-1BB signaling may undermine the beneficial effects of BB.z sequences in the CARs expressed by T cells. Therefore, 4-1BB stimulation of T cells may not be universally beneficial; rather, the overall outcome of 4-1BB signaling may depend on its intensity and duration.
While T cells expressing CD19 CARs with either the CD28 or 4-1BB costimulatory endodomain produced complete responses in patients with advanced CD19+ tumors (Brentjens et al., 2013; Lee et al., 2015; Maude et al., 2014; Turtle et al., 2016), these studies used different expression systems. CD28.z CARs expression was driven by gammaretrovirus, whereas BB.z CARs were expressed from self-inactivating lentiviral vectors. Our results show that when BB.z CD19 CAR is expressed from a gammaretroviral system it can produce sufficient spontaneous activation to precipitate apoptosis in T cells, impairing their expansion and thereby potentially diminishing their anti-tumor activity in patients. This mechanism of toxicity from tonic signaling is not restricted to the CD19 CAR, but rather represents a more general mechanism, since we show the same effects for two other clinically implemented CARs.
Our data demonstrate the value of two alternative approaches to overcome the problem of excessive tonic signaling associated with a given CAR and its expression system. The first is to reduce CAR expression, since high CAR expression is not always necessary for T cell activation or efficient recognition of target cells. While insufficient avidity of the CAR-antigen interaction may impact formation of a productive immunologic synapse, for some antigens the optimal range of CAR expression may be much lower than that produced by a given expression system. Our results corroborated previous studies where lowering expression of 28.z c-Met and CD19 CARs by modulating the promoter reduced spontaneous activation of CAR T cells and augmented their anti-tumor function (Eyquem et al., 2017; Frigault et al., 2015). Secondly, the level of activity of a given costimulatory molecule, and thus its capacity to induce tonic signaling, is additionally determined by the non-translated portions of the expression system.
Thus, while inclusion of the costimulatory 4-1BB endodomain in CAR constructs has improved their clinical success, we show that it can cause toxic tonic signaling even in CD19 CAR T cells. This signaling is not only a function of extracellular CAR domains, but can also be amplified by the interaction between costimulatory signaling and the expression system. Disrupting the positive feedback loop between tonic costimulation and a CAR expression system by utilizing alternative promoters may help reduce tonic signaling in T cells and thus mitigate its consequences. Taken together, these studies underscore the potential toxicity of tonic 4-1BB signaling CARs and thus will contribute to the rational design of CAR platforms for optimal clinical performance.
Experimental Procedures
Chimeric antigen receptor (CAR) constructs
To model tonic 4-1BB signaling, we used a CAR backbone containing 4-1BB and CD3zeta endodomains and the transmembrane and stalk region of CD8a. We used anti-CD19 (FMC63), anti-GD2 (14G2a) and anti-kappa light chain single-chain variable fragments (scFv) to create 2nd generation CARs harboring either 4-1BB or CD28 costimulatory domains. A CAR containing CD28 and OX40 costimulation was used as control for the GD2 CAR studies. CAR constructs were subcloned into SFG gammaretroviral vectors. An EMCV-derived internal ribosomal entry site (IRES) was cloned immediately upstream of the CAR in IRES BB.z constructs. A lentiviral vector REL was created by replacing the PGK-GFP cassette in the pRRLSIN.cPPT.PGK-GFP. WPRE vector (a gift from Didier Trono, Addgene plasmid #12252) with the full human EF-1a promoter containing intron 1. For the confocal microscopy studies, we generated CD19 CARs fused on the C-terminus with Emerald GFP via a flexible linker. We verified the functionality of CD19 CAR-Emerald in cytotoxicity assays to ensure intact CAR signaling. Site-directed mutagenesis of the TRAF2 sites in the 4-1BB endodomain was performed using the InFusion cloning kit (Clontech). Final constructs were verified by Sanger DNA sequencing.
CRISPR/Cas9-mediated disruption of the Fas gene
A Fas-directed sgRNA with zero predicted off-target sites was selected using CRISPRScan and COSMID (Cradick et al., 2014) algorithms, synthesized using HiScribe T7 High Yield RNA Synthesis kit (NEB) and purified with MegaClear Kit (Ambion). Purified RNA was mixed with recombinant Cas9 protein (PNA Bio) at a 0.35:1 (m/m) ratio and electroporated into T cells using Neon Transfection System (Invitrogen) as previously described (Gomes-Silva et al., 2017). Cells were subsequently transduced with CD19 CAR the following day and expanded for 7 days.
Mouse xenograft model
8-10wk old female NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice (The Jackson Laboratory) were inoculated intravenously with 1×106 NALM-6 cells engineered to express FFluc-GFP fusion protein. Three days later, mice received a single intravenous injection of 0.8–1.0×106 CD19 CAR T cells. Tumor burden was monitored by recording luminescence with an IVIS Imaging system (Caliper Life Sciences). Mice were euthanized after the tumor burden reached luminescence level of 108 photons/sec or after displaying signs of high tumor burden. Peripheral blood was collected by tail vein bleeding. All animal experiments were conducted in compliance with the Baylor College of Medicine IACUC.
Cell culture, generation of gammaretroviral and lentiviral vectors and T cell transduction
Detailed procedures are described in the Supplemental Experimental Procedures
Cytotoxicity assays and flow cytometry
Detailed procedures are described in the Supplemental Experimental Procedures
Western blot, Real-Time PCR and fluorescent microscopy analyses
Detailed procedures are described in the Supplemental Experimental Procedures
Statistics
Unpaired 2-tailed Student’s t-test or one-way ANOVA with Bonferroni post-test correction were used to determine statistical significance, with P values below 0.05 considered statistically significant. Data points from individual donors are shown unless indicated otherwise. Analysis of the Kaplan-Meier survival curves was done using log rank (Mantel-Cox) test. All statistical analyses were performed in GraphPad Prism 6.
Supplementary Material
Highlights.
Tonic 4-1BB signaling in CARs causes T cell apoptosis, impeding antitumor activity.
Tonic 4-1BB signaling enhances CAR expression from gammaretroviral LTR promoters.
Reducing CAR expression in retro- or SIN lentiviral vectors attenuates this toxicity.
Acknowledgments
The authors acknowledge Stephen Gottschalk and Gianpietro Dotti for providing CD19+ cell lines and Laurence Cooper for providing anti-CD19 CAR antibodies. The authors thank Catherine Gillespie for the editing of the manuscript and Leonid S. Metelitsa for the critical review. This study was supported by a grant from the National Institutes of Health (NIH), National Cancer Institute (NCI) (P50 CA126752) and by the ASH Scholar Award (to MM). DGS acknowledges Fundação para a Ciência e Tecnologia (FCT, Portugal) for financial support (SFRH/BD/52479/2014). The authors appreciate the support of shared resources in the Cancer Center (support grant NCI P30 CA125123). The imaging work was supported by R01 AI067946 (to JSO).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
MM and MKB conceived and directed the study, designed experiments, analyzed data and wrote the manuscript; MM, DGS, MMu, MS, GK, OD, YZ, LD performed the experiments and analyzed data; YZ and CMR performed studies on GD2 CAR T cells; JMSC supported the study, MMu and JSO performed imaging analysis; all authors edited the manuscript.
References
- Alderson MR, Tough TW, Davis-Smith T, Braddy S, Falk B, Schooley KA, Goodwin RG, Smith CA, Ramsdell F, Lynch DH. Fas ligand mediates activation-induced cell death in human T lymphocytes. J. Exp. Med. 1995;181:71–77. doi: 10.1084/jem.181.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baetu TM, Kwon H, Sharma S, Grandvaux N, Hiscott J. Disruption of NF-κB Signaling Reveals a Novel Role for NF-κB in the Regulation of TNF-Related Apoptosis-Inducing Ligand Expression. J. Immunol. 2001;167:3164–3173. doi: 10.4049/jimmunol.167.6.3164. [DOI] [PubMed] [Google Scholar]
- Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, Bartido S, Stefanski J, Taylor C, Olszewska M, et al. CD19-Targeted T Cells Rapidly Induce Molecular Remissions in Adults with Chemotherapy-Refractory Acute Lymphoblastic Leukemia. Sci. Transl. Med. 2013;5:177ra38–177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan H, Bartos DP, Owen-Schaub LB. Activation-Dependent Transcriptional Regulation of the Human fas Promoter Requires NF-κB p50–p65 Recruitment. Mol. Cell. Biol. 1999;19:2098–2108. doi: 10.1128/mcb.19.3.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cradick TJ, Qiu P, Lee CM, Fine EJ, Bao G. COSMID: A Web-based Tool for Identifying and Validating CRISPR/Cas Off-target Sites. Mol. Ther. — Nucleic Acids. 2014;3:e214. doi: 10.1038/mtna.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyquem J, Mansilla-Soto J, Giavridis T, van der Stegen SJC, Hamieh M, Cunanan KM, Odak A, Gönen M, Sadelain M. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature. 2017;543:113–117. doi: 10.1038/nature21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigault MJ, Lee J, Basil MC, Carpenito C, Motohashi S, Scholler J, Kawalekar OU, Guedan S, McGettigan SE, Posey AD, et al. Identification of chimeric antigen receptors that mediate constitutive or inducible proliferation of T cells. Cancer Immunol. Res. 2015;3:356–367. doi: 10.1158/2326-6066.CIR-14-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes-Silva D, Srinivasan M, Sharma S, Lee CM, Wagner DL, Davis TH, Rouce RH, Bao G, Brenner MK, Mamonkin M. CD7-edited T cells expressing a CD7-specific CAR for the therapy of T-cell malignancies. Blood. 2017;130:285–296. doi: 10.1182/blood-2017-01-761320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DR, Droin N, Pinkoski M. Activation-induced cell death in T cells. Immunol. Rev. 2003;193:70–81. doi: 10.1034/j.1600-065x.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- Herbein G, Khan KA. Is HIV infection a TNF receptor signalling-driven disease? Trends Immunol. 2008;29:61–67. doi: 10.1016/j.it.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Hernandez-Chacon JA, Li Y, Wu RC, Bernatchez C, Wang Y, Weber J, Hwu P, Radvanyi L. Co-stimulation through the CD137/4-1BB pathway protects human melanoma tumor-infiltrating lymphocytes from activation-induced cell death and enhances anti-tumor effector function. J. Immunother. Hagerstown Md 1997. 2011;34:236–250. doi: 10.1097/CJI.0b013e318209e7ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscott J, Kwon H, Génin P. Hostile takeovers: viral appropriation of the NF-κB pathway. J. Clin. Invest. 2001;107:143–151. doi: 10.1172/JCI11918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang IK, Lee ZH, Kim YJ, Kim SH, Kwon BS. Human 4-1BB (CD137) Signals Are Mediated by TRAF2 and Activate Nuclear Factor-κB. Biochem. Biophys. Res. Commun. 1998;242:613–620. doi: 10.1006/bbrc.1997.8016. [DOI] [PubMed] [Google Scholar]
- Kawalekar OU, O’Connor RS, Fraietta JA, Guo L, McGettigan SE, Posey AD, Patel PR, Guedan S, Scholler J, Keith B, et al. Distinct Signaling of Coreceptors Regulates Specific Metabolism Pathways and Impacts Memory Development in CAR T Cells. Immunity. 2016;44:380–390. doi: 10.1016/j.immuni.2016.01.021. [DOI] [PubMed] [Google Scholar]
- Klenerman P, Oxenius A. T cell responses to cytomegalovirus. Nat. Rev. Immunol. 2016;16:367–377. doi: 10.1038/nri.2016.38. [DOI] [PubMed] [Google Scholar]
- Künkele A, Johnson AJ, Rolczynski LS, Chang CA, Hoglund V, Kelly-Spratt KS, Jensen MC. Functional Tuning of CARs Reveals Signaling Threshold above Which CD8+ CTL Antitumor Potency Is Attenuated due to Cell Fas–FasLDependent AICD. Cancer Immunol. Res. 2015;3:368–379. doi: 10.1158/2326-6066.CIR-14-0200. [DOI] [PubMed] [Google Scholar]
- Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M, Shah NN, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. The Lancet. 2015;385:517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B, Williams-Skipp C, Tao Y, Schleicher MS, Cano LL, Duke RC, Scheinman RI. NF-kappaB functions as both a proapoptotic and antiapoptotic regulatory factor within a single cell type. Cell Death Differ. 1999;6:570–582. doi: 10.1038/sj.cdd.4400528. [DOI] [PubMed] [Google Scholar]
- Long AH, Haso WM, Shern JF, Wanhainen KM, Murgai M, Ingaramo M, Smith JP, Walker AJ, Kohler ME, Venkateshwara VR, et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat. Med. 2015;21:581–590. doi: 10.1038/nm.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD, Rossig C, Russell HV, Diouf O, Liu E, et al. Antitumor activity and long-term fate of chimeric antigen receptor–positive T cells in patients with neuroblastoma. Blood. 2011;118:6050–6056. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, et al. Chimeric Antigen Receptor T Cells for Sustained Remissions in Leukemia. N. Engl. J. Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson AJ, Snell LM, Mak TW, Watts TH. Opposing Roles for TRAF1 in the Alternative versus Classical NF-κB Pathway in T Cells. J. Biol. Chem. 2012;287:23010–23019. doi: 10.1074/jbc.M112.350538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melotti P, Nicolis E, Tamanini A, Rolfini R, Pavirani A, Cabrini G. Activation of NF-κB mediates ICAM-1 induction in respiratory cells exposed to an adenovirus-derived vector. Gene Ther. 2001;8:1436–1442. doi: 10.1038/sj.gt.3301533. [DOI] [PubMed] [Google Scholar]
- Milone MC, Fish JD, Carpenito C, Carroll RG, Binder GK, Teachey D, Samanta M, Lakhal M, Gloss B, Danet-Desnoyers G, et al. Chimeric Receptors Containing CD137 Signal Transduction Domains Mediate Enhanced Survival of T Cells and Increased Antileukemic Efficacy In Vivo. Mol. Ther. 2009;17:1453–1464. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzio M, Chinnaiyan AM, Kischkel FC, O’Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz JD, Zhang M, Gentz R, et al. FLICE, A Novel FADD-Homologous ICE/CED-3–like Protease, Is Recruited to the CD95 (Fas/APO-1) Death-Inducing Signaling Complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- Pomerantz JL, Baltimore D. NF-kappaB activation by a signaling complex containing TRAF2, TANK and TBK1, a novel IKK-related kinase. EMBO J. 1999;18:6694–6704. doi: 10.1093/emboj/18.23.6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric Antigen Receptor–Modified T Cells in Chronic Lymphoid Leukemia. N. Engl. J. Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, Huls MH, Liu E, Gee AP, Mei Z, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat. Med. 2008;14:1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roebuck KA, Finnegan A. Regulation of intercellular adhesion molecule-1 (CD54) gene expression. J. Leukoc. Biol. 1999;66:876–888. doi: 10.1002/jlb.66.6.876. [DOI] [PubMed] [Google Scholar]
- Roulston A, Lin R, Beauparlant P, Wainberg MA, Hiscott J. Regulation of human immunodeficiency virus type 1 and cytokine gene expression in myeloid cells by NF-kappa B/Rel transcription factors. Microbiol. Rev. 1995;59:481–505. doi: 10.1128/mr.59.3.481-505.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, Kamble RT, Bollard CM, Gee AP, Mei Z, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor–modified T cells in lymphoma patients. J. Clin. Invest. 2011;121:1822–1826. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NP, Nagarkatti M, Nagarkatti PS. Role of dioxin response element and nuclear factor-kappaB motifs in 2,3,7,8-tetrachlorodibenzo-p-dioxin-mediated regulation of Fas and Fas ligand expression. Mol. Pharmacol. 2007;71:145–157. doi: 10.1124/mol.106.028365. [DOI] [PubMed] [Google Scholar]
- Turtle CJ, Hanafi L-A, Berger C, Gooley TA, Cherian S, Hudecek M, Sommermeyer D, Melville K, Pender B, Budiarto TM, et al. CD19 CAR–T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J. Clin. Invest. 2016;126:2123–2138. doi: 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumwalde NA, Domae E, Mescher MF, Shimizu Y. ICAM-1– Dependent Homotypic Aggregates Regulate CD8 T Cell Effector Function and Differentiation during T Cell Activation. J. Immunol. 2013;191:3681–3693. doi: 10.4049/jimmunol.1201954. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.