Abstract
Background and Purpose
Current methods to estimate risk of radiation-induced lung toxicity (RILT) rely on dosimetric parameters. We aimed to improve prognostication by incorporating clinical and cytokine data, and to investigate how these factors may interact with the effect of mean lung dose (MLD) on RILT.
Materials and Methods
Data from 125 patients treated from 2004 to 2013 with definitive radiotherapy for stages I–III NSCLC on four prospective clinical trials were analyzed. Plasma levels of 30 cytokines were measured pretreatment, and at 2 and 4 weeks midtreatment. Penalized logistic regression models based on combinations of MLD, clinical factors, and cytokine levels were developed. Cross-validated estimates of log-likelihood and area under the receiver operating characteristic curve (AUC) were used to assess accuracy.
Results
In prognosticating grade 3 or greater RILT by MLD alone, cross-validated log-likelihood and AUC were −28.2 and 0.637, respectively. Incorporating clinical features and baseline cytokine levels increased log-likelihood to −27.6 and AUC to 0.669. Midtreatment cytokine data did not further increase log-likelihood or AUC. Of the 30 cytokines measured, higher levels of 13 decreased the effect of MLD on RILT, corresponding to a lower odds ratio for RILT per Gy MLD, while higher levels of 4 increased the association.
Conclusions
Although the added prognostic benefit from cytokine data in our model was modest, understanding how clinical and biologic factors interact with the MLD-RILT relationship represents a novel framework for understanding and investigating the multiple factors contributing to radiation-induced toxicity.
Introduction
Despite advances in conformal techniques, radiation-induced lung toxicity (RILT) remains a significant source of morbidity and mortality, occurring in 5–50% of patients treated with radiotherapy for lung cancer [1, 2]. Current methods to assess the risk of RILT are based on dose-volume parameters, such as mean lung dose (MLD) and the volume of lung receiving at least 20 Gy (V20) [3–7]. Such methods, however, are suboptimal for predicting risk in individual patients.
In addition to dosimetric variables, several patient-specific risk factors for RILT have been identified, including older age, coexisting lung disease, and specific chemotherapeutic agents [8–11]. While such factors are important to consider when assessing risk for RILT, a standardized, multi-factorial model is lacking.
Radiation-induced lung toxicity is in part regulated by inflammatory and fibrotic cytokines [12–15]. The most well-studied of these is transforming growth factor beta 1 (TGFβ1), which was first found to be associated with pneumonitis after autologous bone marrow transplantation for advanced breast cancer [16]. Subsequent studies showed radiation-induced elevation of plasma levels of TGFβ1 to be associated with increased risk of radiation pneumonitis [17–23]. While other studies failed to demonstrate an independent predictive value of TGFβ1, this may have been due to improper sample handling [24–26]. In a meta-analysis of 7 studies, a ratio of posttreatment to pretreatment TGFβ1 >=1 was a significant predictor of RILT [27]. Studies combining cytokine and dosimetric data have also been promising, with two studies showing improved RILT prediction by combining posttreatment TGFβ1 levels with dosimetric variables [19, 28]. Attempts have been made to use TGFβ1 levels to direct treatment planning, and some success has been reported in the setting of dose escalation [29].
In addition to TGFβ1, low plasma levels of interleukin (IL) 8 [20, 26], and high levels of IL6 [30, 31] and IL10 [30] have been found to correlate with lung toxicity, although these findings have not been consistent in all studies [20, 23]. Other cytokines, including tumor necrosis factor (TNF) α and IL1β, have failed to show prognostic value [20, 23].
The studies described above have primarily investigated the effect of clinical factors and cytokine levels on the risk of RILT directly. An alternative strategy would be to instead model multiple variables as statistical interactions with the MLD-RILT association. Such an approach could provide a unique avenue for investigating the functions of various risk factors and could prove useful for understanding how different variables influence a patient’s tolerability of high-dose radiotherapy. Herein we describe development and evaluation of such a model.
Methods and Materials
Study Population
This work analyzed data from 4 prospective Institutional Review Board (IRB) approved lung-cancer studies: (1) a phase 1/2 study of radiation dose escalation with concurrent chemotherapy, (2–3) 2 consecutive studies using functional imaging and biomarkers to assess patient outcome, and (4) a study using midtreatment PET to guide individualized dose escalation. Included in this analysis were patients with stage I–III NSCLC treated with standard fractionation, i.e. not stereotactic body radiotherapy (SBRT). Details of these studies are shown in Supplemental Table 1. As part of these clinical trials, patients consented to have blood samples collected and analyzed for proteins for investigation of potential factors correlating with toxicity and treatment response. Specimen collection and analysis was approved by the IRB committee. All clinical data were prospectively collected. Smoking status was missing for 11 patients, which was handled via single imputation. Patients consented to receive treatment per these investigational protocols, as well as
Treatment Regimen
All patients were treated with definitive RT with or without sequential or concurrent chemotherapy. In cases of sequential treatment, chemotherapy was administered following radiotherapy. Radiation was delivered using three-dimensional conformal radiotherapy (3DCRT) as previously described [32]. Gross tumor volume included the primary tumor and any involved hilar or mediastinal lymph nodes, as determined by tissue diagnosis and/or positron emission tomography (PET)-CT. Uninvolved lymph node regions were not included in the clinical target volume. Lung dose volume histograms (DVHs) were generated using both lungs with exclusion of gross tumor volume [33]. For the dose-escalation trial, we constrained the lung normal tissue complication probability (NTCP) to < 15%, and for the adaptive trial we constrained NTCP to < 17.5% [34]. Tissue inhomogeneity corrections were applied for all plans.
Mean lung doses are reported in terms of equivalent to 2-Gy daily dosing (EQD2), as calculated via the linear quadratic model using an alpha/beta of 2.5. All doses were recomputed using modern dose calculations (AAA in Eclipse, Varian Medical Systems, Palo Alto, CA).
Cytokine Analysis
Cytokine measurements were performed in platelet-poor plasma samples at 3 time points: at baseline (within 2 weeks before the start of RT) and at 2 and 4 weeks during RT. Plasma samples were collected and prepared as previously described [21].
Plasma concentrations of 30 cytokines were measured: epidermal growth factor (EGF), eotaxin, fractalkine, granulocyte colony stimulating factor (GCSF), granulocyte macrophage colony stimulating factor (GMCSF), interferon α (IFNα), IL1α, IL1β, IL2, IL4, IL5, IL6, IL7, IL8, IL10, IL12 subunit 40 (IL12p40), IL12 p35 and p40 heterodimer (IL12p70), IL13, IL15, IL17, IL1 receptor antagonist (IL1RA), monocyte chemoattractant protein 1 (MCP1), macrophage inflammatory protein (MIP) 1α, MIP1β, soluble CD40 ligand (SCD40L), TGFα, TGFβ1, TNFα, and vascular endothelial growth factor (VEGF).
TGFβ1 levels were measured using enzyme-linked immunosorbent assay (ELISA) as previously described [20], while the other 29 cytokines were measured using luminex multiplex assay (xMAP plasma assay; Luminx, St. Charles, MO). All sample tests were run in duplicate. Some cytokine measurements fell below a lower limit of detection. We used an ad hoc methodology to detect and account for these censored measurements, which is described in the supplement.
Follow Up and Toxicity Evaluation
Patients were evaluated weekly during radiotherapy, with follow-up evaluation at 1 month after completion of RT, then every 3 months for 1 year, followed by every 6 months for 1 additional year. At each follow-up, patients underwent a history and physical examination as well as chest computed tomography (CT). Radiation-induced lung toxicity, including radiation pneumonitis and clinical fibrosis, was evaluated and graded as previously described [4]. Grade 3 radiation pneumonitis or clinical fibrosis was defined as severe cough, dyspnea at rest, or oxygen requirement in the setting of radiographic evidence of pneumonitis or radiation fibrosis.
Statistical Modeling
We modeled the association between RILT and patient factors using logistic regression. Cytokine measurements were log transformed and all covariates were standardized to have mean zero and unit standard deviation prior to modeling. Baseline and change in cytokine measurements were assumed to modify the dose-RILT association as statistical interactions: changes in cytokines increased or decreased the association between dose and RILT. We considered patient factors in a nested fashion, beginning with MLD only and sequentially adding clinical characteristics, baseline cytokines, and two- or four-week midtreatment cytokines. Clinical factors investigated included administration of concurrent chemotherapy, former smoking status, current smoking status, age, sex, simple TNM stage, and Karnofsky performance status (KPS). For model-selection purposes, we used data from the same 95 patients for which complete data was available, to ensure valid between-model comparisons (all available patient data was used after model selection).
To account for overfitting and large variability in parameter estimates due to a small ratio of sample size to number of predictors, we penalized coefficient estimates, similar to the LASSO method [35]. The extent of penalization was selected by maximizing the cross-validated log-likelihood, which measures overall agreement between model-predicted risks and actual prevalence of RILT in our data. We averaged over 250 cross-validations to mitigate sensitivity to specific partitions of the data. A larger log-likelihood corresponds to explaining more variation in risk of RILT between patients, and cross-validating this estimate ensures that observed increases are not due to overfitting. Thus, following other penalized regression approaches, variables were retained in a model only if their inclusion increased the cross-validated log-likelihood. We determined the maximum cross-validated log-likelihood within each of the nested models and selected the overall best-performing model.
As with any agnostic model-building process, it would be misleading to report statistical significance or confidence of this ‘final’ model using standard Wald-based p-values (e.g. Section 3.3, [36]). Rather, by virtue of the cross-validated framework, a particular variable’s inclusion (or exclusion) from the model reflects significance, or a lack thereof. We also report a cross-validated area under the ROC curve (AUC), a measure between 0.5 and 1 that quantifies the ability of a model to discriminate the risk of RILT between patients with and without RILT. All analyses were conducted in R [37, 38]. Additional details are given in the Supplement.
Results
We identified 173 patients who had undergone definitive radiation on four prospective institutional protocols. Of these, 26 patients who had been treated with SBRT were excluded. Of the remaining 147, complete dose and clinical covariates were known for 125. Of these, complete cytokine information pretreatment (Cyt0) and at 2 weeks midtreatment (Δ2Cyt) was known for 109, and cytokine data at 4 weeks midtreatment (Δ4Cyt) were known for 95. Demographic and clinical characteristics of analyzed patients are shown in Table 1. Median MLD was 14.1 Gy (interquartile range 11.6 – 17.2 Gy).
Table 1.
Baseline demographic, clinical, and treatment characteristics of patients analyzed.
| Characteristic | Value | |
|---|---|---|
|
| ||
| Sex | ||
| Female | 30 (24.0%) | |
| Male | 95 (76.0%) | |
|
| ||
| Age | ||
| Mean | 65.9 years | |
| Median | 65.6 years | |
| Range | 39.6–85.2 years | |
|
| ||
| Race/Ethnicity | ||
| Caucasian | 120 (96.0%) | |
| African American | 3 (2.4%) | |
| Asian | 1 (0.8%) | |
| Not reported | 1 (0.8%) | |
|
| ||
| KPS | ||
| >= 70 | 122 (97.6%) | |
| < 70 | 3 (2.4%) | |
|
| ||
| Smoking Status | ||
| Former smoker | 61 (48.8%) | |
| Current smoker | 53 (42.4%) | |
| Never smoker | 7 (5.6%) | |
| Not reported | 4 (3.2%) | |
|
| ||
| Group Stage | ||
| I | 11 (8.8%) | |
| II | 13 (10.4%) | |
| III | 100 (80.0%) | |
| IV | 1 (0.8%) | |
|
| ||
| T Stage | ||
| T1 | 21 (16.8%) | |
| T2 | 29 (23.2%) | |
| T3 | 36 (28.8%) | |
| T4 | 38 (30.4%) | |
| Tx | 1 (0.8%) | |
|
| ||
| N Stage | ||
| N0 | 28 (22.4%) | |
| N1 | 15 (12.0%) | |
| N2 | 55 (44.0%) | |
| N3 | 27 (21.6%) | |
|
| ||
| Target Radiation Dose (BED) | ||
| Mean | 70.2 Gy (87.3 Gy) | |
| Median | 70.0 Gy (84.0 Gy) | |
| Range | 34.0 – 87.9 Gy (40.8 – 115.5 Gy) | |
|
| ||
| Mean Lung Dose | ||
| Mean | 14.1 Gy | |
| Interquartile range | 11.6 – 17.2 Gy | |
| Range | 3.0 – 25.8 Gy | |
|
| ||
| Concurrent Chemotherapy | ||
| No | 20 (16.0%) | |
| Yes | 105 (84.0%) | |
BED = Biologic equivalent dose. KPS = Karnofsky performance status. Gy = Gray.
Among the 125 patients with complete dose and clinical information, the incidence of grade >= 3 RILT was 8.8% (n=11). Two cases of grade 5 toxicity were recorded. The incidence of RILT among all analyzed patients is shown in Supplemental Figure 1. This incidence was similar in the 109 patients with known baseline and 2 week midtreatment cytokine levels, and in the 95 patients with additional known 4 week midtreatment cytokine levels (Supplemental Table 2).
In estimating grade >= 3 RILT by MLD alone, cross-validated log-likelihood and AUC were −28.2 and 0.637, respectively (Table 2). For this model, the change in log odds of grade >= 3 RILT per Gy MLD was 0.096, corresponding to an odds ratio (OR) of 1.101. In other words, for each increase in MLD by 1 Gy, the risk of grade >= 3 RILT increased with an OR of 1.101.
Table 2.
Prediction of grade >=3 RILT by MLD and four nested models: Pr(Tox) ~ Dose + Clinical + Cyt0 × Dose + ΔtCyt × dose. Models for this analysis were based on the 95 patients with full data available.
| AUC | Log Likelihood | |
|---|---|---|
| MLD Only | 0.637 | −28.2 |
| MLD + Clinical | 0.633 | −28.3 |
| MLD + Clinical + Cyt0 | 0.669 | −27.6 |
| MLD + Clinical + Cyt0 + Δ2Cyt | 0.661 | −27.9 |
| MLD + Clinical + Cyt0 + Δ4Cyt | 0.668 | −27.8 |
MLD = mean lung dose. Cyt0 = base line cytokine levels. Δ2Cyt = change in cytokine levels at 2 weeks midtreatment. Δ4Cyt = change in cytokine levels at 4 weeks midtreatment.
We then fit four nested multivariable models, sequentially incorporating clinical variables alone and then adding pretreatment or midtreatment plasma cytokine levels. Augmenting the MLD-only model with clinical factors only yielded a decreased cross-validated log-likelihood of −28.3 (Table 2).
We then further augmented the MLD-only model with both clinical factors and baseline cytokine measurements. This yielded a larger cross-validated log-likelihood of −27.6 and a cross-validated AUC of 0.669 (Table 2). Of note, each of the 250 log-likelihoods from each individual cross-validation exceeded that of the MLD-only model. Although clinical factors did not improve model fit when considered alone, when combined with baseline cytokine measurements, concurrent chemotherapy, former smoking status, and age were retained in this larger model (Supplemental Table 3). Adding changes in cytokine levels at two or four weeks midtreatment did not further improve predictive ability, with log-likelihoods of −27.9 and −27.8, respectively. Thus, our selected multivariable model incorporated clinical factors and baseline, but not midtreatment, cytokine measurements.
According to this multivariable model, when baseline cytokine measurements were at their average values, the change in log-odds of grade >= 3 RILT per Gy MLD was 0.118, corresponding to an OR of 1.125. In other words, for a patient in which the plasma level of each cytokine was at its respective average, the risk of grade >= 3 RILT increased with an OR of 1.125 for each increase in MLD by 1 Gy.
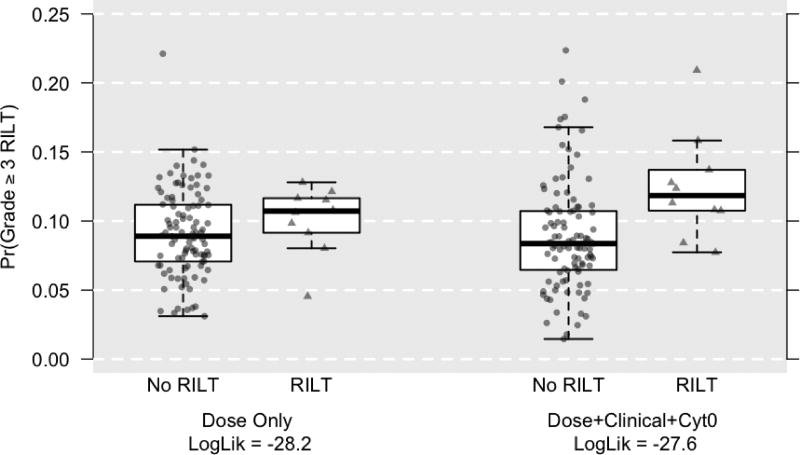
Figure 1 compares the estimated cross-validated probabilities of grade >= 3 RILT for the MLD-only model against the multivariable model. According to the MLD-only model, the median probabilities were 0.089 and 0.107, respectively, for patients who, in truth, did not and did have grade >= 3 RILT. For the multivariable model, these median probabilities were 0.084 and 0.118, respectively, indicating a gain in explained variability. With an increase in AUC from 0.637 to 0.669, we expect that our model would be able to correctly distinguish between a high-risk and low-risk patient approximately 3.2% more frequently than a MLD-only model.
Figure 1.
Estimated probabilities of grade >= 3 lung toxicity by MLD-only versus multivariable prediction models. Circles represent cases without RILT, while triangles represent cases with RILT. The multivariable model demonstrates improved prediction compared to the MLD-only model as demonstrated by greater separation of the ‘No RILT’ versus ‘RILT’ populations on the Y axis.
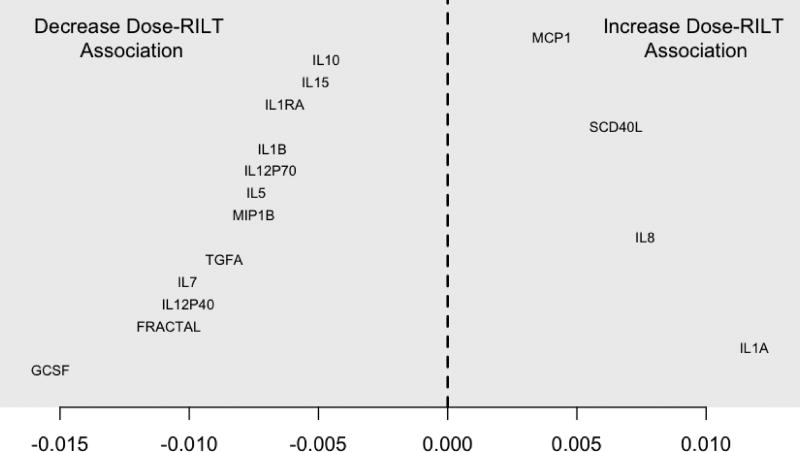
Among the 30 cytokines measured, 17 were included in the multivariable model on the basis of modifying the dose-RILT association. Thirteen of these decreased the dose-RILT association, with GCSF doing so by the largest amount. A one standard deviation increase in baseline GCSF levels above the average decreased the log-odds for grade >= 3 RILT per Gy MLD from 0.118 to 0.102. This corresponded to a decreased OR of 1.107 from 1.125. In other words, when GCSF was elevated one standard deviation above the mean, and all other cytokines remained at their averages, each increase in MLD by 1 Gy increased the risk for >= 3 RILT not with an OR of 1.125, but of 1.107.
Four cytokines increased the dose-RILT association, with IL1A doing so by the largest amount. A one standard deviation increase in baseline IL1A level above the average increased the log-odds per Gy from 0.118 to 0.130. This corresponded to an increased OR of 1.139 compared to 1.125. The modifying effects of all 17 cytokines are plotted in Figure 2. The predictive model combines the effect of each cytokine to generate a composite value that describes the relative sensitivity to developing grade >= 3 RILT as a function of MLD.
Figure 2.
Effect on dose-RILT association by increase in cytokine level one standard deviation above mean, as expressed in Log OR per Gy. The zero point of the x axis corresponds to no modification of the overall dose-RILT relationship, at which state the Log OR of grade >= 3 RILT increases by 0.118 for each 1 Gy increase in MLD. For these 17 cytokines, when the pretreatment level of a given cytokine was elevated 1 standard deviation above its mean value, the increase in Log OR for grade >= 3 RILT per 1 Gy MLD was adjusted by the value on the x axis corresponding to that cytokine’s position.
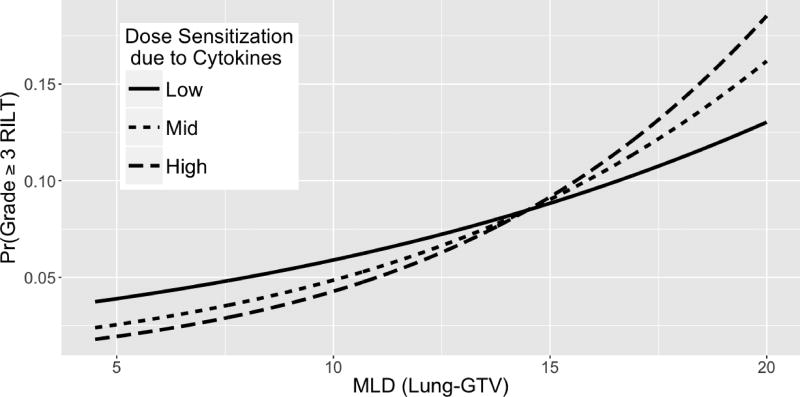
The effect of pretreatment cytokine levels on the dose-RILT relationship is further illustrated in Figure 3, which plots the predicted risk of grade >= 3 RILT as a function of MLD. Patients with baseline cytokine profiles corresponding to increased sensitivity to MLD demonstrate a steeper slope, representing a greater influence of MLD lung toxicity. Conversely, patients with profiles corresponding to decreased sensitivity demonstrate a flatter slope. The curves cross because the model describes the effect of cytokines on the dose-RILT relationship only and not on the risk of RILT directly. As such, cytokine levels influence only the slopes of the lines, which necessitates a point of intersection. This point was determined by the best fit of these curves as predicted by the model.
Figure 3.
Three model fitted dose-RILT curves for predicting grade 3 or greater RILT. The ‘low’, ‘mid’, and ‘high’ curves represent the first, second, and third quartiles of dose-RILT slopes due to cytokine modification.
Discussion
In the work described here, we report development a multivariable model combining pretreatment plasma cytokine levels, clinical factors, and MLD which demonstrated improved prognostication of grade 3 or greater RILT compared to MLD alone. An important feature of our approach was that we did not correlate clinical and cytokine data with RILT directly, but instead described the effect of these factors on the dose-RILT relationship. In other words, the model correlated an individual patient’s pretreatment cytokine and clinical profile with a relative sensitivity to developing RILT as a function of MLD.
The improvement in RILT prognostication from the multivariable model was relatively modest. Potential reasons for this include measurement of an inadequate number of cytokines, suboptimal detection methods, and an inherent limitation of plasma cytokine levels to prognosticate RILT. It is possible that plasma cytokine levels do not fully represent changes in the local inflammatory milieu, which may influence RILT more directly. Performance of this model was likely further limited by the conservative nature of penalized regressions in the presence of limited sample sizes. These limitations notwithstanding, our findings indicate that the improved prognostication, while modest, was not a statistical artifact, as in each of the 250 unique partitions of the data that were used for cross-validation, the multivariable model was able to explain more variation in risk of grade >= 3 RILT between patients than the MLD-only model.
Although this multivariable model may have limited clinical applicability in its current form, there are important lessons to be taken from this approach. Describing the mechanisms by which various patient-specific factors affect the dose-RILT relationship could inform decisions regarding radiotherapy planning and dose escalation. With the identification of additional factors that interact with the effect of dose on lung or other toxicities, it could be possible to identify patients who demonstrate better tolerance of high-dose radiotherapy. In such patients, the relative increase in risk from higher radiation dose may be less significant than in patients with profiles corresponding to increased sensitivity to dose. In addition, understanding how cytokines and other factors affect the dose-toxicity relationship could provide a unique framework for investigating biologic function.
The response to radiation-induced injury in the lung-tumor microenvironment is regulated by complex networks of inflammatory and fibrotic cytokines [13, 39]. As reviewed in the introduction, variations in levels of multiple cytokines have been shown to directly affect the risk of RILT. In comparison to those studies, we, by modeling cytokines as modifiers of the dose-toxicity relationship, identified a different subset of cytokines that influence RILT. For example, while elevated plasma levels of TGFβ1 have been described to correlate with risk of RILT, TGFβ1 was not identified in our model as a modifier of the effect of dose on RILT. This suggests that the effect of TGFβ1 may be independent of radiation dose, while the cytokines identified in our analysis affect risk by a separate, dose-dependent mechanism.
Of the 17 cytokines that showed prognostic utility in our model, high levels of 13 were associated with a decreased effect of dose on risk, while elevation of the remaining 4 was associated with an increased effect. The varying effect of these cytokines is not readily explained by their respective, currently understood biologic activity. For instance, we found some proinflammatory cytokines, including MCP1, SCD40L, IL8, and IL1α, to increase the dose-RILT relationship, while others, such as IL1β, IL7, IL12, IL15, and TGFα, decreased the effect of dose on risk [39–41]. While these findings do not describe the mechanisms by which these cytokines interact with radiation to influence the risk of RILT, they do suggest novel functions which should be the focus of future investigation.
Interestingly, only pretreatment, and not midtreatment, plasma cytokine levels were useful in our model. This may reflect a baseline state of relative sensitivity to RILT as a function of MLD. Alternatively, as lung toxicity often develops on a more chronic timeline, it is possible that postreatment levels could provide additional information. However, data obtained after completion of radiotherapy would be inherently less useful, as they would not be available to guide treatment planning.
As it is well-appreciated that radiation-induced toxicity is influenced by multiple dosimetric, clinical, and biologic factors, multivariable prognostic models may better describe these risks than single-factor approaches. There are multiple valid methods to correlate prognostic variables with an outcome, including the parametric linear model described in this work. Another approach is Bayesian network analysis, which explores probabilistic relationships among multiple variables by representing their interactions and dependences on a directed acyclic graph [42]. Recently, Bayesian network analysis has shown promise as a method to estimate the risk of radiation pneumonitis (RP) [43, 44].
Of these, Luo et al. reported an analysis of a cohort of patients that significantly overlaps with the patients from this work. There are several important distinctions between that study and ours, including use of different primary outcomes (grade 3 or higher RP or clinical fibrosis in this analysis versus grade 2 or higher RP by Luo et al.). The statistical modeling approaches are also distinct. We utilized penalized logistic regression to model the association between the outcomes and covariates. Luo et al. utilized Bayesian network analysis. These two approaches differ in several respects. First, the former uses covariates (e.g. dose and cytokines) continuously while the latter dichotomizes continuous covariates as greater than or less than a threshold. Secondly, the former utilizes penalized regression while the latter does not. Imposing a penalty in parameter estimation can improve prediction by limiting overfitting. Finally, in addition to modeling covariate-outcome relationships, Bayesian network analysis also describes associations between the prognosticators themselves. Our paper does not consider associations between covariates.
In the manuscript by Luo et al., the only pretreatment cytokine found to be associated with RP was IL15. By comparison, our analysis identified 17 cytokines that interacted with the dose-RILT relationship, including IL15, which we observed to decrease the effect of MLD on RILT. The fact that these two methods identified different cytokines highlights the importance of using multiple approaches to investigate risk factors for radiation-induced toxicity, as all analytical tools have their benefits and drawbacks.
In conclusion, while identifying factors that directly correlate with radiation-induced toxicity remains relevant, our work demonstrates that understanding how such factors modify the effects of radiation may be particularly useful for selecting patients for dose-escalation and informing the investigation of biologic macromolecules. Different approaches for multivariable modeling of RILT risk should be employed to improve prognostication and uncover topics for further investigation.
Supplementary Material
Acknowledgments
Grant or financial support
This work was supported in part by R01 CA142840 (Kong) and P01 CA059827 (Ten Haken and Lawrence).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no conflict of interest regarding the topic under consideration.
References
- 1.Mehta V. Radiation pneumonitis and pulmonary fibrosis in non-small-cell lung cancer: pulmonary function, prediction, and prevention. International journal of radiation oncology, biology, physics. 2005;63:5–24. doi: 10.1016/j.ijrobp.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 2.Marks LB, Yu X, Vujaskovic Z, Small W, Jr, Folz R, Anscher MS. Radiation-induced lung injury. Seminars in radiation oncology. 2003;13:333–45. doi: 10.1016/S1053-4296(03)00034-1. [DOI] [PubMed] [Google Scholar]
- 3.Graham MV, Purdy JA, Emami B, Harms W, Bosch W, Lockett MA, et al. Clinical dose-volume histogram analysis for pneumonitis after 3D treatment for non-small cell lung cancer (NSCLC) International journal of radiation oncology, biology, physics. 1999;45:323–9. doi: 10.1016/s0360-3016(99)00183-2. [DOI] [PubMed] [Google Scholar]
- 4.Kong FM, Hayman JA, Griffith KA, Kalemkerian GP, Arenberg D, Lyons S, et al. Final toxicity results of a radiation-dose escalation study in patients with non-small-cell lung cancer (NSCLC): predictors for radiation pneumonitis and fibrosis. International journal of radiation oncology, biology, physics. 2006;65:1075–86. doi: 10.1016/j.ijrobp.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 5.Marks LB, Bentzen SM, Deasy JO, Kong FM, Bradley JD, Vogelius IS, et al. Radiation dose-volume effects in the lung. International journal of radiation oncology, biology, physics. 2010;76:S70–6. doi: 10.1016/j.ijrobp.2009.06.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodrigues G, Lock M, D'Souza D, Yu E, Van Dyk J. Prediction of radiation pneumonitis by dose - volume histogram parameters in lung cancer--a systematic review. Radiother Oncol. 2004;71:127–38. doi: 10.1016/j.radonc.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 7.Shi A, Zhu G, Wu H, Yu R, Li F, Xu B. Analysis of clinical and dosimetric factors associated with severe acute radiation pneumonitis in patients with locally advanced non-small cell lung cancer treated with concurrent chemotherapy and intensity-modulated radiotherapy. Radiat Oncol. 2010;5:35. doi: 10.1186/1748-717X-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vogelius IR, Bentzen SM. A literature-based meta-analysis of clinical risk factors for development of radiation induced pneumonitis. Acta Oncol. 2012;51:975–83. doi: 10.3109/0284186X.2012.718093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palma DA, Senan S, Tsujino K, Barriger RB, Rengan R, Moreno M, et al. Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: an international individual patient data meta-analysis. International journal of radiation oncology, biology, physics. 2013;85:444–50. doi: 10.1016/j.ijrobp.2012.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rancati T, Ceresoli GL, Gagliardi G, Schipani S, Cattaneo GM. Factors predicting radiation pneumonitis in lung cancer patients: a retrospective study. Radiother Oncol. 2003;67:275–83. doi: 10.1016/s0167-8140(03)00119-1. [DOI] [PubMed] [Google Scholar]
- 11.Claude L, Perol D, Ginestet C, Falchero L, Arpin D, Vincent M, et al. A prospective study on radiation pneumonitis following conformal radiation therapy in non-small-cell lung cancer: clinical and dosimetric factors analysis. Radiother Oncol. 2004;71:175–81. doi: 10.1016/j.radonc.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Fleckenstein K, Gauter-Fleckenstein B, Jackson IL, Rabbani Z, Anscher M, Vujaskovic Z. Using biological markers to predict risk of radiation injury. Seminars in radiation oncology. 2007;17:89–98. doi: 10.1016/j.semradonc.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Kong FM, Ten Haken R, Eisbruch A, Lawrence TS. Non-small cell lung cancer therapy-related pulmonary toxicity: an update on radiation pneumonitis and fibrosis. Seminars in oncology. 2005;32:S42–54. doi: 10.1053/j.seminoncol.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Kong FM, Wang S. Nondosimetric risk factors for radiation-induced lung toxicity. Seminars in radiation oncology. 2015;25:100–9. doi: 10.1016/j.semradonc.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodemann HP, Bamberg M. Cellular basis of radiation-induced fibrosis. Radiother Oncol. 1995;35:83–90. doi: 10.1016/0167-8140(95)01540-w. [DOI] [PubMed] [Google Scholar]
- 16.Anscher MS, Peters WP, Reisenbichler H, Petros WP, Jirtle RL. Transforming growth factor beta as a predictor of liver and lung fibrosis after autologous bone marrow transplantation for advanced breast cancer. The New England journal of medicine. 1993;328:1592–8. doi: 10.1056/NEJM199306033282203. [DOI] [PubMed] [Google Scholar]
- 17.Anscher MS, Kong FM, Andrews K, Clough R, Marks LB, Bentel G, et al. Plasma transforming growth factor beta1 as a predictor of radiation pneumonitis. International journal of radiation oncology, biology, physics. 1998;41:1029–35. doi: 10.1016/s0360-3016(98)00154-0. [DOI] [PubMed] [Google Scholar]
- 18.Anscher MS, Kong FM, Marks LB, Bentel GC, Jirtle RL. Changes in plasma transforming growth factor beta during radiotherapy and the risk of symptomatic radiation-induced pneumonitis. International journal of radiation oncology, biology, physics. 1997;37:253–8. doi: 10.1016/s0360-3016(96)00529-9. [DOI] [PubMed] [Google Scholar]
- 19.Zhao L, Sheldon K, Chen M, Yin MS, Hayman JA, Kalemkerian GP, et al. The predictive role of plasma TGF-beta1 during radiation therapy for radiation-induced lung toxicity deserves further study in patients with non-small cell lung cancer. Lung cancer. 2008;59:232–9. doi: 10.1016/j.lungcan.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 20.Stenmark MH, Cai XW, Shedden K, Hayman JA, Yuan S, Ritter T, et al. Combining physical and biologic parameters to predict radiation-induced lung toxicity in patients with non-small-cell lung cancer treated with definitive radiation therapy. International journal of radiation oncology, biology, physics. 2012;84:e217–22. doi: 10.1016/j.ijrobp.2012.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao L, Wang L, Ji W, Wang X, Zhu X, Hayman JA, et al. Elevation of plasma TGF-beta1 during radiation therapy predicts radiation-induced lung toxicity in patients with non-small-cell lung cancer: a combined analysis from Beijing and Michigan. International journal of radiation oncology, biology, physics. 2009;74:1385–90. doi: 10.1016/j.ijrobp.2008.10.065. [DOI] [PubMed] [Google Scholar]
- 22.Novakova-Jiresova A, Van Gameren MM, Coppes RP, Kampinga HH, Groen HJ. Transforming growth factor-beta plasma dynamics and post-irradiation lung injury in lung cancer patients. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2004;71:183–9. doi: 10.1016/j.radonc.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 23.Kim JY, Kim YS, Kim YK, Park HJ, Kim SJ, Kang JH, et al. The TGF-beta1 dynamics during radiation therapy and its correlation to symptomatic radiation pneumonitis in lung cancer patients. Radiation oncology. 2009;4:59. doi: 10.1186/1748-717X-4-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Jaeger K, Seppenwoolde Y, Kampinga HH, Boersma LJ, Belderbos JS, Lebesque JV. Significance of plasma transforming growth factor-beta levels in radiotherapy for non-small-cell lung cancer. International journal of radiation oncology, biology, physics. 2004;58:1378–87. doi: 10.1016/j.ijrobp.2003.09.078. [DOI] [PubMed] [Google Scholar]
- 25.Evans ES, Kocak Z, Zhou SM, Kahn DA, Huang H, Hollis DR, et al. Does transforming growth factorbeta1 predict for radiation-induced pneumonitis in patients treated for lung cancer? Cytokine. 2006;35:186–92. doi: 10.1016/j.cyto.2006.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hart JP, Broadwater G, Rabbani Z, Moeller BJ, Clough R, Huang D, et al. Cytokine profiling for prediction of symptomatic radiation-induced lung injury. International journal of radiation oncology, biology, physics. 2005;63:1448–54. doi: 10.1016/j.ijrobp.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 27.Zhang XJ, Sun JG, Sun J, Ming H, Wang XX, Wu L, et al. Prediction of radiation pneumonitis in lung cancer patients: a systematic review. J Cancer Res Clin Oncol. 2012;138:2103–16. doi: 10.1007/s00432-012-1284-1. [DOI] [PubMed] [Google Scholar]
- 28.Fu XL, Huang H, Bentel G, Clough R, Jirtle RL, Kong FM, et al. Predicting the risk of symptomatic radiation-induced lung injury using both the physical and biologic parameters V(30) and transforming growth factor beta. International journal of radiation oncology, biology, physics. 2001;50:899–908. doi: 10.1016/s0360-3016(01)01524-3. [DOI] [PubMed] [Google Scholar]
- 29.Anscher MS, Marks LB, Shafman TD, Clough R, Huang H, Tisch A, et al. Using plasma transforming growth factor beta-1 during radiotherapy to select patients for dose escalation. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001;19:3758–65. doi: 10.1200/JCO.2001.19.17.3758. [DOI] [PubMed] [Google Scholar]
- 30.Arpin D, Perol D, Blay JY, Falchero L, Claude L, Vuillermoz-Blas S, et al. Early variations of circulating interleukin-6 and interleukin-10 levels during thoracic radiotherapy are predictive for radiation pneumonitis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:8748–56. doi: 10.1200/JCO.2005.01.7145. [DOI] [PubMed] [Google Scholar]
- 31.Hartsell WF, Scott CB, Dundas GS, Mohiuddin M, Meredith RF, Rubin P, et al. Can serum markers be used to predict acute and late toxicity in patients with lung cancer? Analysis of RTOG 91-03. American journal of clinical oncology. 2007;30:368–76. doi: 10.1097/01.coc.0000260950.44761.74. [DOI] [PubMed] [Google Scholar]
- 32.Kong FM, Ten Haken RK, Schipper MJ, Sullivan MA, Chen M, Lopez C, et al. High-dose radiation improved local tumor control and overall survival in patients with inoperable/unresectable non-small-cell lung cancer: long-term results of a radiation dose escalation study. International journal of radiation oncology, biology, physics. 2005;63:324–33. doi: 10.1016/j.ijrobp.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 33.Wang W, Xu Y, Schipper M, Matuszak MM, Ritter T, Cao Y, et al. Effect of normal lung definition on lung dosimetry and lung toxicity prediction in radiation therapy treatment planning. International journal of radiation oncology, biology, physics. 2013;86:956–63. doi: 10.1016/j.ijrobp.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ten Haken RK, Martel MK, Kessler ML, Hazuka MB, Lawrence TS, Robertson JM, et al. Use of Veff and iso-NTCP in the implementation of dose escalation protocols. Int J Radiat Oncol Biol Phys. 1993;27:689–95. doi: 10.1016/0360-3016(93)90398-f. [DOI] [PubMed] [Google Scholar]
- 35.Tibshirani R. Regression shrinkage and selection via the lasso. Journal of the Royal Statistical Society Series B (Methodological) 1996:267–88. [Google Scholar]
- 36.Hastie T, Tibshirani R, Friedman JH. The elements of statistical learning : data mining, inference, and prediction. 2. New York, NY: Springer; 2009. [Google Scholar]
- 37.Wickham H. Ggplot2 : elegant graphics for data analysis. New York: Springer; 2009. [Google Scholar]
- 38.Team RC. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2016. [Google Scholar]
- 39.Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer. 2008;8:887–99. doi: 10.1038/nrc2507. [DOI] [PubMed] [Google Scholar]
- 40.Kelso A. Cytokines: principles and prospects. Immunol Cell Biol. 1998;76:300–17. doi: 10.1046/j.1440-1711.1998.00757.x. [DOI] [PubMed] [Google Scholar]
- 41.Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat Rev Cancer. 2004;4:11–22. doi: 10.1038/nrc1252. [DOI] [PubMed] [Google Scholar]
- 42.Pearl J. Probabilistic reasoning in intelligent systems : networks of plausible inference. San Mateo, Calif.: Morgan Kaufmann Publishers; 1988. [Google Scholar]
- 43.Luo Y, El Naqa I, McShan DL, Ray D, Lohse I, Matuszak MM, et al. Unraveling biophysical interactions of radiation pneumonitis in non-small-cell lung cancer via Bayesian network analysis. Radiother Oncol. 2017;123:85–92. doi: 10.1016/j.radonc.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee S, Ybarra N, Jeyaseelan K, Faria S, Kopek N, Brisebois P, et al. Bayesian network ensemble as a multivariate strategy to predict radiation pneumonitis risk. Med Phys. 2015;42:2421–30. doi: 10.1118/1.4915284. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.