Abstract
Childhood lead poisoning is a multi-faceted, complex condition, which affects not only the child’s health and well-being, but also the family’s housing security, economic status, job security, and stress level. This review updates the emergency department clinician on the management of childhood lead poisoning. Infants and children are at higher risk than adults for lead exposure due to their smaller size and proportionately larger dose of ingested toxins, their proximity to ground dirt and indoor dust, their energy and curiosity, their oral exploratory and pica behaviors, their proportionately larger daily water and milk intake, and dietary preferences that differ markedly from those of adults. Pediatric health care providers working in the emergency department can provide medical management, as well as preventive counseling and guidance, to parents of children presenting with evidence of acute or chronic lead poisoning.
Keywords: lead, lead poisoning, plumbism, chelation, metals, heavy metals, environmental toxins
Children’s exposure to sources of lead contamination continues to be an important public health concern. Lead has no biological role in the body, and any detectable lead level is abnormal. There is indisputable scientific evidence that blood lead levels (BLL) below 10 µg/dL are associated with adverse effects in infants and children.1–3 In response, in 2012, the Centers for Disease Control and Prevention (CDC) lowered the reference value BLL to 5 µg/dL.4 An estimated 3.6 million American homes with at least one child have significant lead paint hazards.5,6 As many as 500,000 US children (2.5%) under 6 years have BLLs ≥5 µg/dL. Each lead-exposed child costs an estimated $5600 in medical and special educational services.7 Lead exposure-related cognitive impairments cost an estimated $50.9 billion annually in lost US economic productivity.6
Nationally, US poison control centers (PCC) received 2241 single exposure calls about possible lead exposures in 2014.8 Lead exposure is the most frequent inquiry directed toward the professionals staffing the nation’s Pediatric Environmental Health Specialty Units (PEHSUs).9 Childhood lead poisoning is also a concern for clinicians working in pediatric emergency departments. Using discharge data for lead poisoning from the Agency for Healthcare Research and Quality, Healthcare Cost and Utilization Project from 2006–2014, we found that an average 1558 US emergency department (ED) visits occurred annually for assessment of possible lead exposure;10,11 55% of these ED visits involved patients less than 18 years of age and approximately 35% were admitted to the hospital.11 Although much of the management of children at risk of lead poisoning is nonclinical, clinicians working in EDs commonly find themselves directing the immediate care needs of lead-poisoned children.12
ROUTES AND SOURCES OF EXPOSURE
Most children with elevated BLLs today are contaminated through exposure to lead laden dust and paint chips from deteriorating lead paint on interior surfaces. Their developmentally appropriate hand-to-mouth exploratory behaviors make them susceptible in an environment that is contaminated with lead dust, even without frank pica.13 Contaminated soil from ‘legacy’ sources of lead (eg, leaded-gasoline, deteriorating lead-based exterior paint) can re-contaminate remediated houses.14,15 Residual lead in soil deposited there from airborne emissions during nearby industrial operations, such as around smelters, remains a hazard even decades after closure.16 Children may also inhale lead fumes or respirable dust particles resulting from unsafe remediation practices such as sanding or heating old paint, burning lead-painted wood indoors, burning automobile batteries for heat, or melting lead for use in a hobby or craft.
Other sources of lead hazards to be considered are included in Table 1. Imported cookware, cosmetics, ethnic remedies, dietary supplements, contaminated tap water, and imported foodstuffs are among the diverse sources of potential lead exposure in a home environment. Some toy jewelry is made of lead; a child who ingested a lead charm died of lead poisoning in 2006.17–19 Antique toys were sometimes painted with lead-based paint, and some plastic toys and vinyl have lead added as a softener.19,20 Since 2008, the US Consumer Product Safety Commission (CPSC) has set requirements to reduce the number of non-complying products entering the market.21 Novel sources of exposure include foreign-purchased cosmetics;22,23 Southeast Asian spices24,25 and herbs;26 dietary supplements;27 religious powders;25 ayurvedic28 or ethnic remedies;22,25 occupational take home exposures;29–31 and vocational exposures such as youth firearms marksmanship.32,33
TABLE 1.
Sources of lead exposure.
| Home Environment Sources | Other Sources |
|---|---|
| Interior or exterior paint, old putty, interior plaster, exterior decorative infrastructure (eg, ‘faux pewter’ fencing) | Folk remedies (examples include Ayurvedic medicines; Greta and Azarcon, Hispanic traditional medicines; Ghasard, an Indian folk medicine; ‘pay-loo-ah’; ‘litarigio’; ‘bali bali’; ‘Babaw-saw’, a Chinese herbal remedy; reuda; liga; coral; alkohl) |
| Household lead-laden dust | Foodstuffs: Some garden plants grown in contaminated soil (eg, leafy or root vegetables) |
| Soil | Herbs and dietary supplements: imported herbal products; dietary supplements (eg, calcium); imported spices (eg, turmeric); candy from Mexico (the ingredient ‘tamarind’ may contain lead) |
| Drinking water; household lead plumbing, standpipes, water mains, faucets, lead-soldered pipes | Cosmetics and religious powders (eg, ‘Swad’ brand Sindoor, a cosmetic product used in Hinduism); ‘Tiro’ eye cosmetic from Nigeria; ‘Kohl’ or ‘Surma’ eye cosmetics from Africa, Middle East or Asia); lead acetate hair dyes |
| Parental occupations ‘Take-Home’ Lead (examples include construction, renovation, and demolition work, lead-paint abatement, pipe fitting and plumbing, battery manufacturing, mining, ship building or other marine work, e-scrap recycling) | Hobbies (examples include hobbies involving soldering such as stained glass, making fishing lures, jewelry making, pottery glazes, some artists’ paints, fabricating bullets, lead solder, marksmanship at firing ranges, finishing sinkers) |
| Old ceramic, pewter, or antique cookware, old pots, pans, urns/ kettles, decorative pottery from Mexico; ceramics from China, or other imported cookware | Marine lead sources: marine paints, lead weights |
| Hazardous neighborhoods: homes located near lead-smelters, mining, nearby homes undergoing demolition, toxic waste sites, homes under bridges, homes near incinerators, battery recycling facilities | Moonshine alcoholic beverages |
| Secondary home environments: family daycare, grandparents’ homes, homes of other family members where children spend substantial time | Fishing sinkers, curtain weights, automobile wheel balancing weights, ammunition (including pellets), lead tools |
| Home renovations | Novelty jewelry, charms, medallions |
| Burning painted wood indoors | Some imported toys, crayons, pewter figurines |
| Antique cribs or furniture | Aviation gasoline (‘Avgas’ for small piston engine planes) |
Data from American Academy of Pediatrics Council of Environmental Health, Pediatric Environmental Health, 3rd Edition.76
VULNERABLE POPULATIONS
Not only are young children more likely than older children, adolescents, and adults to have an elevated BLL secondary to differences in absorption from the gastrointestinal tract and exploration of one’s environment, they are also more susceptible to toxic effects than are adults because of direct entry of lead into a developing nervous system. Studies of children with higher BLLs have consistently demonstrated lower IQ scores,1,34,35 more language difficulties,36 learning disorders, attention problems,37 and behavioral issues.38,39
While BLLs have decreased in all children over the past 30 years, disparities in who has elevated BLLs persist, disproportionately impacting vulnerable groups, such as immigrant children, low-income families, and young children from ethnic and racial minorities, based on age, socioeconomic, occupational, developmental and cultural risk factors.40–44 Children living at or below the poverty line who live in older housing are at greatest risk of lead poisoning.7 Additionally, children of low socioeconomic status are at increased risk of nutritional problems such as iron deficiency, which has been associated with a 4- to 5-fold increase in baseline risk of lead poisoning due to increased absorption of lead by the divalent metal transporter in the gastrointestinal tract.45,46
Children with developmental conditions such as autism spectrum disorder and other neurological syndromes, who have persistent pica behaviors and/ or poor cognitive discriminatory recognition, are at increased risk of lead contamination.47–52 Their increased risk may persist into school age and adolescence, beyond when children are routinely screened for elevated BLLs. Another vulnerable group may be children living in foster care,53 whose lead poisoning risk may be related to other neurodevelopmental comorbidities in this population as well as increased residential mobility (especially in regions with older housing stock).
‘Take-home’ lead from the job is a common problem. The National Institute for Occupational Safety and Health (NIOSH) found common jobs with lead exposure include but are not limited to: painting, building renovation, demolition, shooting range work, metal scrap cutting and recycling, plumbing, and other industrial fields.54 Pediatric emergency physicians should ask about parents’ occupations and hobbies that might involve lead during evaluation of lead poisoned children.47–49
Clinical Diagnosis
Symptomatic childhood lead toxicity should be treated as an emergency. Children who present to the emergency department with unexplained symptoms and signs, especially those who are sluggish or comatose, who have persistent gastrointestinal symptoms (such as constipation or obstipation, abdominal pain, vomiting, recent anorexia, weight loss), or who have unexplained neurological or behavioral changes (eg, headaches, withdrawn, confusion, fatigue, lethargy, irritability, hyperactivity) or whose skin has a distinct pallor from severe anemia, should be suspected of suffering from acute lead poisoning. The differential diagnosis can include other causes of poisoning such as opioid ingestion or carbon monoxide poisoning, iron deficiency, thalassemia, Wilson’s Disease, acute intermittent porphyria, an acute surgical abdomen, encephalitis, and other causes of encephalopathy. Table 2 gives symptoms and signs of lead poisoning based on blood lead levels.
TABLE 2.
Summary of children’s health effects by blood lead level.
| Blood Lead Level |
Sufficient Evidence or Causal Determination of Children’s Health Effects |
|---|---|
| Below 5 µg/dL | Nervous System Effects: |
| Cognitive function: decreases in IQ, academic achievement, specific cognitive measures | |
| Externalizing behaviors: Increased incidence of attention-related and problem behaviors | |
| 5–10 µg/dL | Effects listed above plus |
| Nervous System Effects: decreased auditory function | |
| Reproductive and Developmental Effects: reduced postnatal growth, delayed puberty for girls and boys | |
| 10–44 µg/dL | Effects listed above plus |
| Nervous System Effects: slower nerve conduction | |
| Hematologic Effects: decreased hemoglobin, anemia | |
| 45–69 µg/dL | Effects listed above plus |
| Gastrointestinal Effects: abdominal pain, constipation, colic, anorexia and vomiting | |
| Above 70 µg/dL | Effects listed above plus |
| Nervous System Effects: severe neural effects including convulsions, coma, loss of voluntary muscle control, and death |
Data from President’s Task Force on Environmental Health Risks and Safety Risks to Children, Key Federal Programs to Reduce Childhood Lead Exposures and Eliminate Associated Health Impacts Report.21
Keep in mind that children with significant underlying lead poisoning can be relatively asymptomatic. Table 2 links clinical findings with the BLL. Some children with BLLs >45 µg/dL may complain of headaches, abdominal pain, loss of appetite, or constipation or they may be completely asymptomatic. Children displaying clumsiness, agitation, or decreased activity and somnolence are presenting with premonitory symptoms of central nervous system (CNS) involvement that may rapidly proceed to vomiting, stupor, and convulsions.55 Clinicians must have a high index of suspicion for a child who presents with a recent history of symptoms and/or signs presented in Table 2. Significant lead exposure in early childhood has been linked to a numerous adverse health outcomes later in childhood, adolescence and adulthood, which are also listed in Table 2.
LABORATORY AND IMAGING STUDIES
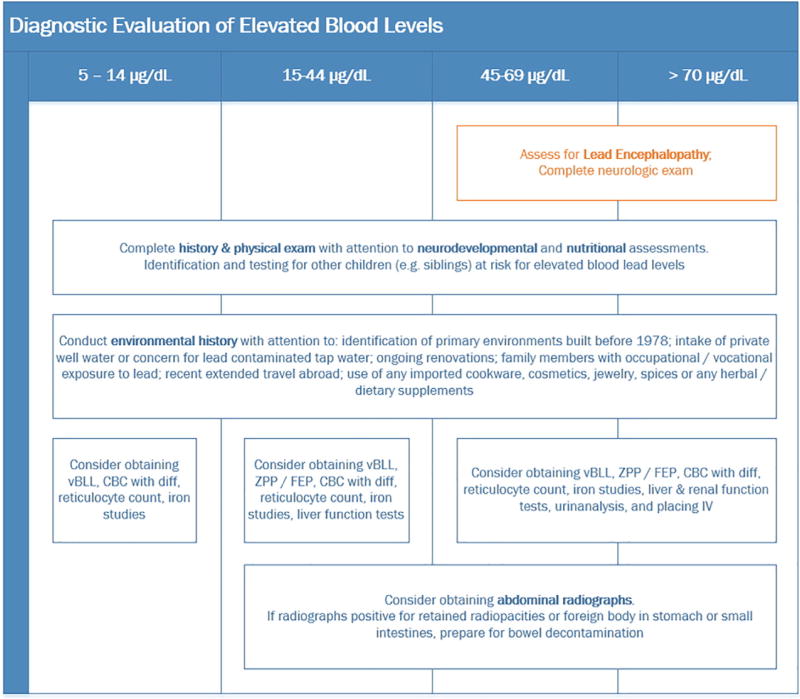
The emergency department evaluation of a lead poisoned child often includes blood testing and radiographic studies (Table 3).
Table 3.
Diagnostic evaluation of elevated blood lead levels. 56
Blood Lead Level (BLL)
Measurement of a venous blood lead level (vBLL) is key to the diagnosis of lead poisoning. For screening, a finger-stick sample (fsBLL) can be used if care is taken to avoid contamination. An elevated fsBLL (≥5 µg/dL) should be confirmed with a timely vBLL.12,56,57 Hair or urine lead levels give little useful additional information.58
Zinc-Chelated Protoporphyrin (ZPP)
Lead interferes with heme synthesis beginning at BLLs of approximately 25 µg/dL and after 50–70 days or more of exposure.59 Both D-aminolevulinate dehydratase, an early-step enzyme, and ferrochelatase, which closes the heme ring, are inhibited. Ferrochelatase inhibition is the basis of a supplemental test for lead poisoning that measures in blood the quantity of zinc-chelated protoporphyrin (ZPP) and free erythrocyte protoporphyrin (FEP), the immediate heme precursor. These markers are insensitive to lower BLL and are not specific since they are also elevated in the presence of iron deficiency, a common comorbidity in children with elevated BLLs. ZPP or FEP can give insight into the chronicity of ongoing exposure and can be used during management, since an unexpected rise in these markers during patient monitoring over a period of weeks or months may indicate re-exposure and the need to reassess the environment.
Iron Status
Many young children with elevated BLLs will have iron insufficiency or iron deficiency anemia. Since lead and iron both use the same GI tract transporter, located in the small intestine, lead absorption is enhanced in children with iron deficits. Thus iron deficiency is an important comorbidity of lead toxicity; pica behavior has sometimes been associated with iron-deficient status. Therefore, markers of iron deficiency such as low ferritin or serum iron levels, even in the absence of anemia, low mean corpuscular volume (MCV), or elevated red cell distribution width (RDW) or low reticulocyte hemoglobin should be treated with therapeutic doses of iron as indicated.
Complete Blood Count
In addition to screening for comorbid anemia or iron deficiency, a complete blood count (CBC) with differential should be obtained before starting chelation, since chelants can cause depression of any or all three cell lines. Basophilic stippling may be seen at higher BLL. Basophilic stippling refers to small blue granules (ie, ribosomes) located inside of the cytoplasm when the smear is stained with Wright’s stain.60
Liver and Renal Function Tests
Baseline liver and renal function tests, serum electrolytes, and glucose are also indicated in the child with suspected moderate–severe lead poisoning, since chelants commonly used in the medical management can have liver and/or renal toxicity or cause metabolic derangements. Periodic monitoring of the CBC, electrolytes, and liver and kidney function throughout the course of chelation therapy is recommended.
Radiographs
With lead-containing foreign body ingestions, BLLs rise rapidly (within hours to days) and can continue to rise during bowel transit of the object. Once the object has been excreted, the BLL falls to a new body equilibrium over the next month. In the emergency department, an abdominal radiograph to determine the presence of lead-containing substances may be indicated if a child’s BLL is ≥15 µg/dL or, regardless of the BLL, if a parent has witnessed or suspects that the child has recently ingested paint chips or a foreign body.12,56 If the radiograph is positive for metal-density opacities in the stomach or small intestine, then hospitalization and gut decontamination with a polyethylene glycol solution (‘whole bowel irrigation’) may be beneficial. Radiographs of long bones to assess “lead lines” (ie, densemetaphyseal lines of growth arrest) are no longer necessary or recommended.
TREATMENT
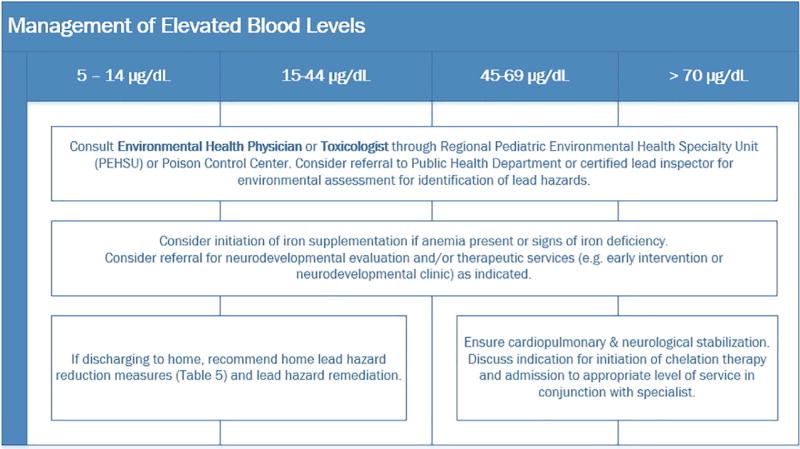
Multipronged management should be provided to all children with BLLs above the CDC reference value, as of time of manuscript preparation BLL ≥ 5 µg/dL. 4,12,61 Tables 3 and 4 give details of diagnostic evaluation and management strategies to consider based on a child’s BLL. Management includes finding and eliminating the source of the lead, instruction in proper hygienic measures (personal and household), optimizing the child’s diet and nutritional status, and close follow-up. Many children with higher BLLs live in or visit regularly a home with deteriorating lead paint. Successful therapy depends on eliminating the child’s exposure; case management should address and control environmental sources of lead. Families of children with elevated BLLs should be referred to local public health officials and/or a certified lead inspector for an inspection and assessment of the child’s residence(s) for lead hazards. Clinicians as a first step often will start children identified as having an elevated BLL on supplemental iron therapy (3–6 mg/kg per day of free iron) to repair any iron deficiency.
Table 4.
Management of elevated blood lead levels. 56
Hospitalization
Hospitalization may be necessary for symptomatic children and for those with BLL ≥ 45 µg/dL. Hospital admission is also determined by several considerations:
Is the child symptomatic?
Are there unabsorbed lead-containing foreign bodies in the stomach or small intestine?
Are there parental or other external factors making a safe discharge and timely follow-up difficult?
Is the home unsafe with respect to sources of lead contamination readily accessible to the child?
Discharge Planning
Although the main concern for the pediatric emergency physician regarding disposition is admission or discharge from the emergency department, the following hospital discharge criteria are important to consider when arranging a discharge plan. After inpatient management (eg. whole bowel irrigation, course of parenteral chelation), hospital discharge planning should determine:
Sources of lead exposure hazard have been identified and remediated
Parents or guardians understand dosing of oral chelants and there is a strong likelihood of adherence to medical instructions
The BLL has dropped adequately during inpatient therapy
Discharge counseling should include referral to public health officials for environmental assessment, temporary abatement recommendations to minimize ongoing exposure (eg, taping up chipping interior paint using contact paper or duct/masking tape), frequent hand washing, frequent dusting/wet mopping of the home (several times per week), leaving shoes at the threshold, and dietary recommendations (Table 5).
TABLE 5.
Home lead hazard reduction measures.
| Specific Recommendations |
|---|
Residential Sources of Lead Exposure
|
Water sources
|
Guidance for lead hazard remediation
|
Outdoor exposures
|
Data from American Academy of Pediatrics Council of Environmental Health, Pediatric Environmental Health, 3rd Edition.76
Chelation and Management of Elevated BLLs
Chelants are chemicals whose structures include side-groups that can bind to lead and facilitate its excretion in urine. They are indicated emergently in cases of moderate–severe and life-threatening childhood lead poisoning. Chelation therapy for children with venous BLLs of 20 to 44 µg/dL can be expected to lower BLLs but has not been shown to reverse or diminish cognitive impairment or other behavioral or neuropsychological effects of lead.62 If the venous BLL is ≥45 µg/dL and the exposure has been identified and controlled, chelation treatment should always be considered. A pediatrician experienced in managing children with lead poisoning should be consulted—these can be found through the PEHSUs,56 PCCs or through lead programs at state health departments. (See Appendix A) There are 4 chelants currently recognized as having efficacy in lead poisoning: dimercaprol (British Anti-Lewisite [BAL]), calcium disodium edetate (ethylenediaminetetraacetate; Versenate), dimercapto succinic acid (DMSA; Succimer; Chemet), and d-penicillamine (Cupramine, Depen). See Table 6 for educational purposes for details surrounding the administration of each. Treatment decisions are the responsibility of the treating clinician and should always be tailored to individual clinical circumstances.
TABLE 6.
Summary of common chelants used in lead poisoning.
| Chelant | Notes |
|---|---|
| BAL (British Anti-Lewisite) [2,3-dimercapto propanol] [Dimercaprol] |
|
| CaNa2EDTA [Calcium disodium ethyleneaminetetraacetate] [Edetate disodium calcium, Versenate] |
|
| DMSA (Dimercaptosuccinic acid) [Succimer] [Chemet] |
|
| d-penicillamine (3-mercapto-d-valine) [Depen, Cupramine] |
|
Data from American Academy of Pediatrics Council of Environmental Health, Pediatric Environmental Health, 3rd Edition.76
Dimercaprol promotes the renal excretion through the formation of stable, nontoxic, soluble lead chelates. Dissolved in peanut oil for deep intramuscular injection, dimercaprol is associated with a high incidence of adverse effects, including fever, rashes, and pain at the injection site. It is contraindicated in persons with a peanut allergy or underlying hepatic insufficiency and may cause hemolysis in individuals who have glucose-6-phosphatase deficiency. Iron therapy needs to be discontinued because dimercaprol and iron form a complex that causes vomiting. NOTE: adequate patient hydration and good urine flow during chelation therapy with dimercaprol are of paramount importance, given its risk of renal toxicity.
Calcium disodium ethylenediaminetetraacetate (CaNa2EDTA) increases the urinary excretion of lead 20- to 50-fold through the formation of nonionizing salts. CaNa2EDTA removes lead only extracellularly; it does not enter cells and thus does not cross the blood brain barrier. WARNING: Some hospitals still stock the incorrect disodium EDTA salt. It is crucial that the calcium disodium salt be used, because the disodium EDTA salt alone avidly binds calcium and can cause severe, life-threatening hypocalcemia.63,64 CaNa2EDTA is given intravenously usually for 5 day cycles. Side effects include local reaction at the injection site, fever, calcium abnormalities, renal dysfunction, and excretion of essential minerals. NOTE: maintaining adequate patient hydration and good urine flow during CaNa2EDTA chelation therapy is important.
Meso-2,3-dimercaptosuccinic acid (DMSA) is a water-soluble analogue of dimercaprol that was approved for oral administration by the US Food and Drug Administration in 1991 for chelating children who have BLL ≥45 µg/dL. DMSA is given orally, has less toxicity than CaNa2 EDTA, and causes less urinary loss of essential minerals. Side effects include abdominal distress, transient rash, elevated liver transaminase enzymes, and neutropenia. The 100-mg gelatin capsules have a strong sulfur (“rotten egg”) odor.
d-Penicillamine is an oral chelating agent used to treat Wilson’s disease (hepatolenticular degeneration). It has also been used by some clinicians for treating lead poisoning.75 When used for chelation of lead in young children, low doses are recommended, with close monitoring of the CBC and renal function. Allergic rashes, marrow suppression, nephrotoxicity, and anaphylaxis are possible adverse effects.
Other Management
Nutrition
Treatment strategies in the pediatric emergency department setting include family counseling and education on dietary sources of iron, calcium, vitamins C and D, zinc and magnesium to attenuate increased absorption of lead in the setting of nutritional deficiencies.
Educational Enrichment
Another disposition recommended for young children discovered to have an elevated BLL is consideration of referral for neurodevelopmental evaluation and/or therapeutic services (eg, Early Intervention, Individualized Education Program (IEP) or other appropriate neurodevelopmental clinic or education enrichment program.65
PREVENTION OF EXPOSURE
The CDC and American Academy of Pediatrics (AAP) both emphasize that the best way to end childhood lead poisoning is to prevent, control and eliminate lead exposures.12 The focus is shifting from the care of symptomatic children toward a primary prevention approach targeting high-risk communities, as the most reliable and cost-effective strategy to protect children from lead toxicity.12,66 Table 5 presents some recommendations for families to insure that their home is hazard-free with respect to lead contamination. It is critical that the individuals conducting residential abatement, or the removal, enclosure, or encapsulation of lead-based paint or lead-contaminated dust or soil receive appropriate training, and pregnant women, infants and children are out of the home environment during remediation and renovations in order to minimize further exposure to lead.15,67,68 When done safely, paint stripping, covering over painted areas by sealing, encapsulation, or encasement, using high-efficiency particulate arrestance (HEPA) vacuums, HEPA air filters, and soil and dust removal, can be effective methods for lead abatement.
SUMMARY
Exposure of children to harmful lead-containing dust, paint, drinking water, and other sources in their environment continues to pose an enormous public health challenge, not only in the United States but around the world. Vulnerable groups include immigrant children, low-income families, children in transitional foster care, young children from ethnic and racial minorities and those with underlying autism or other developmental delays who have persistent pica behaviors. Clinicians working in the emergency department are advised to keep a high index of suspicion for lead poisoning among the possible diagnoses for children presenting with pallor and anemia, loss of appetite, irritability and behavioral changes, colicky abdominal pain, chronic constipation, or other symptoms and signs typical of lead poisoning. Management of children identified as having elevated blood lead levels is multi-faceted and includes attention to diet, mitigation of environmental lead hazards so as to decrease further exposure, referral to community-based agencies, and developmental specialists, and in severe cases, chelation therapy. Prevention of exposure, including the identification of community-based resources to assist families and landlords in lead-hazard abatement, is the most effective public health strategy, requiring the concerted efforts of health care providers, local, state and Federal public health officials, health policy makers, and relevant community-based services and advocacy groups.
Acknowledgments
ACKNOWLEDGMENTS& DISCLAIMERS
This publication was supported by the cooperative agreement award number 1U61TS000237-03 from the Agency for Toxic Substances and Disease Registry (ATSDR). Its contents are the responsibility of the authors and do not necessarily represent the official views of the Agency for Toxic Substances and Disease Registry (ATSDR).
The U.S. Environmental Protection Agency (EPA) supports the PEHSU by providing funds to ATSDR under Inter-Agency Agreement number DW-75-95877701. Neither EPA nor ATSDR endorse the purchase of any commercial products or services mentioned in PEHSU publications.
APPENDIX A. RESOURCES-GOVERNMENTAL AND NONGOVERNMENTAL ORGANIZATIONS
Alliance for Healthy Homes; www.afhh.org.htm; 202-543-1147; Provides additional information on residential lead contamination and how to safely remove it.
American Association of Poison Control Centers www.aapcc.org; 1-800-222-1212.
Coalition to End Childhood Lead Poisoning; www.leadsafe.org.htm; 800-370-5323; Provides information for parents regarding childhood lead poisoning and its treatment and prevention.
-
Centers for Disease Control and Prevention (CDC); www.cdc.gov/nceh/lead/grants/contacts/CLPPP%20Maphtm;
Provides state and local contacts for CDC funded childhood lead poisoning prevention programs.
Department of Housing and Human Development (HUD); www.hud.gov/offices/lead.htm Office of Healthy Homes and Lead Hazard Control provides ability to track HUD’s progress in the abatement of lead hazards in residences.
-
Environmental Protection Agency; www.epa.gov/lead.htm;
EPA Lead Awareness Program provides information on residential lead abatement. EPA Safe Drinking Water Hotline; 1-800-426-4791.
-
National Lead Information Center Hotline (1-800-LEAD-FYI) and Clearinghouse (1-800-424-LEAD): established by four Federal agencies (the EPA, CDC, HUD, and DOD) to provide the public and professional audiences with information in English or Spanish about lead poisoning and prevention.
National Lead Information Center.
1019 19th St, NW, Suite 401.
Washington, DC 20036.
US Department of Housing and Urban Development (HUD): 800-RID-LEAD.
National Lead Information Center - www.epa.gov/lead; (800) 424-5323; 800-LEAD-FYI.
Pediatric Environmental Health Subspecialty Units (PEHSU); www.pehsu.net (ATSDR and EPA-sponsored regional centers providing clinical evaluation and consultation regarding pediatric environmental health issues, including lead poisoning).
References
- 1.Lanphear BP, Hornung R, Khoury J, et al. Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environ Health Perspect. 2005;113(7):894–9. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nigg JT, Nikolas M, Mark Knottnerus G, et al. Confirmation and extension of association of blood lead with attention-deficit/hyperactivity disorder (ADHD) and ADHD symptom domains at population-typical exposure levels. J Child Psychol Psychiatry. 2010;51(1):58–65. doi: 10.1111/j.1469-7610.2009.02135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Canfield RL, Henderson CR, Cory-Slechta DA, et al. Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. N Engl J Med. 2003;348(16):1517–26. doi: 10.1056/NEJMoa022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC) CDC response to Advisory Committee on Childhood Lead Poisoning Prevention recommendations in “Low Level Lead Exposure Harms Children: A Renewed Call of Primary Prevention”. Atlanta, GA: Centers for Disease Control and Prevention; 2012. [Accessed November 1, 2016]. Available at: http://www.cdc.gov/nceh/lead/acclpp/cdc_response_lead_exposure_recs.pdf. [Google Scholar]
- 5.Jacobs DE, Clickner RP, Zhou JY, et al. The prevalence of lead-based paint hazards in U.S. housing. Environ Health Perspect. 2002;110(10):A599–606. doi: 10.1289/ehp.021100599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trasande L, Liu Y. Reducing the staggering costs of environmental disease in children, estimated at $76.6 billion in 2008. Health Aff (Millwood) 2011;30(5):863–70. doi: 10.1377/hlthaff.2010.1239. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention (CDC) [Accessed January 24, 2016];At-risk populations. 2015 Available at: http://www.cdc.gov/nceh/lead/tips/populations.htm.
- 8.Mowry JB, Spyker DA, Brooks DE, et al. 2014 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 32nd annual report. Clin Toxicol. 2015;53(10):962–1147. doi: 10.3109/15563650.2015.1102927. [DOI] [PubMed] [Google Scholar]
- 9.Woolf AD, Sibrizzi C, Kirkland K. Pediatric environmental health specialty units: an analysis of operations. Acad Pediatr. 2016;16(1):25–33. doi: 10.1016/j.acap.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 10.HCUP Nationwide Emergency Department Sample (NEDS) Healthcare Cost and Utilization Project (HCUP) Rockville, MD: Agency for Healthcare Research and Quality; 2009. [Accessed February 27, 2017]. Available at: https://www.hcup-us.ahrq.gov/nedsoverview.jsp. [Google Scholar]
- 11.HCUPnet. Healthcare Cost and Utilization Project (HCUP). 2006–2014. Rockville, MD: Agency for Healthcare Research and Quality; 2015. [Accessed February 27, 2017]. Available at: http://hcupnet.ahrq.gov/ [PubMed] [Google Scholar]
- 12.American Academy of Pediatrics Council on Environmental Health. Prevention of childhood lead toxicity. [Accessed July 28, 2017. Epub 2016 Jun 20];Pediatrics. 2016 138(1) Available at: http://dx.doi.org/10.1542/peds.2016-1493. [Google Scholar]
- 13.Charney E, Kessler B, Farfel M, Jackson D. Childhood lead poisoning. A controlled trial of the effect of dust-control measures on blood lead levels. Med. 1983;309(18):1089–93. doi: 10.1056/NEJM198311033091804. [DOI] [PubMed] [Google Scholar]
- 14.Farfel MR, Chisolm JJ, Rohde CA. The longer-term effectiveness of residential lead paint abatement. Environ Res. 1994;66(2):217–21. doi: 10.1006/enrs.1994.1057. [DOI] [PubMed] [Google Scholar]
- 15.Lanphear BP, Matte TD, Rogers J, et al. The contribution of lead-contaminated house dust and residential soil to children’s blood lead levels. A pooled analysis of 12 epidemiologic studies. Environ Res. 1998;79(1):51–68. doi: 10.1006/enrs.1998.3859. [DOI] [PubMed] [Google Scholar]
- 16.von Lindern IH, Spalinger SM, Bero BN, et al. The influence of soil remediation on lead in house dust. Sci Total Environ. 2003;303(1–2):59–78. doi: 10.1016/s0048-9697(02)00356-x. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Death of a child after ingestion of a metallic charm-Minnesota, 2006. MMWR Morb Mortal Wkly Rep. 2006;55(12):340–1. [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. Lead poisoning of a child associated with use of a Cambodian Amulet—New York City, 2009. MMWR Morb Mortal Wkly Rep. 2011;60(3):69–71. [PubMed] [Google Scholar]
- 19.Weidenhamer JD, Clement ML. Widespread lead contamination of imported low-cost jewelry in the US. Chemosphere. 2007;67(5):961–5. doi: 10.1016/j.chemosphere.2006.10.071. [DOI] [PubMed] [Google Scholar]
- 20.Yost JL, Weidenhamer JD. Lead contamination of inexpensive plastic jewelry. Sci Total Environ. 2008;393(2–3):348–50. doi: 10.1016/j.scitotenv.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 21.President’s Task Force on Environmental Health Risks and Safety Risks to Children. [Accessed November 27, 2016];Key federal programs to reduce childhood lead exposures and eliminate associated health impacts. 2016 Available at: https://ptfceh.niehs.nih.gov/features/assets/files/key_federal_programs_to_reduce_childhood_lead_exposures_and_eliminate_associated_health_impactspresidents_508.pdf.
- 22.Woolf AD, Hussain J, McCullough L, et al. Infantile lead poisoning from an Asian tongue powder: a case report & subsequent public health inquiry. Clin Toxicol. 2008;46(9):841–4. doi: 10.1080/15563650801898536. [DOI] [PubMed] [Google Scholar]
- 23.Nasidi A, Karwowski M, Woolf AD, et al. Infant lead poisoning associated with use of Tiro, an eye cosmetic from Nigeria -Boston, Massachusetts; 2011. Morb Mortal Wkly Rep. 2012;61(30):574–6. [PubMed] [Google Scholar]
- 24.Woolf AD, Woolf NT. Childhood lead poisoning in 2 families associated with spices used in food preparation. Pediatrics. 2005;116(2):e314–8. doi: 10.1542/peds.2004-2884. [DOI] [PubMed] [Google Scholar]
- 25.Lin CG, Schaider LA, Brabander DJ, Woolf AD. Pediatric lead exposure from imported Indian spices and cultural powders. Pediatrics. 2010;125(4):e828–35. doi: 10.1542/peds.2009-1396. [DOI] [PubMed] [Google Scholar]
- 26.Harris ESJ, Cao S, Littlefield BA, et al. Heavy metal and pesticide content in commonly prescribed individual raw Chinese herbal medicines. Sci Total Environ. 2011;409:4297–305. doi: 10.1016/j.scitotenv.2011.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woolf AD, Gardiner P. Use of complementary and alternative therapies in children. Clin Pharmacol Ther. 2010;87:155–7. doi: 10.1038/clpt.2009.224. [DOI] [PubMed] [Google Scholar]
- 28.Meiman J, Thiboldeaux R, Anderson H. Lead poisoning and anemia associated with use of ayurvedic medications purchased on the internet-Wisconsin, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(32):883. doi: 10.15585/mmwr.mm6432a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newman N, Jones C, Page E, et al. Investigation of childhood lead poisoning from parental take-home exposure from an electronic scrap recycling facility - Ohio, 2012. MMWR Morb Mortal Wkly Rep. 2015;64(27):743–5. [PMC free article] [PubMed] [Google Scholar]
- 30.Roscoe RJ, Gittleman JL, Deddens JA, et al. Blood lead levels among children of lead-exposed workers: a meta-analysis. Ind Med. 1999;36(4):475–81. doi: 10.1002/(sici)1097-0274(199910)36:4<475::aid-ajim9>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention (CDC) Occupational and take-home lead poisoning associated with restoring chemically stripped furniture-California, 1998. MMWR Morb Mortal Wkly Rep. 2001;50(13):246–8. [PubMed] [Google Scholar]
- 32.Beaucham C, Page E, Alarcon WA, et al. Indoor firing ranges and elevated blood lead levels - United States, 2002–2013. MMWR Morb Mortal Wkly Rep. 2014;63(16):347–51. [PMC free article] [PubMed] [Google Scholar]
- 33.Shannon M. Lead poisoning in adolescents who are competitive marksmen. N Engl J Med. 1999;341(11):852. doi: 10.1056/NEJM199909093411118. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz J. Low-level lead exposure and children’s IQ: a meta-analysis and search for a threshold. Environ Res. 1994;65(1):42–55. doi: 10.1006/enrs.1994.1020. [DOI] [PubMed] [Google Scholar]
- 35.Pocock SJ, Smith M, Baghurst P. Environmental lead and children’s intelligence: a systematic review of the epidemiological evidence. BMJ. 1994;309(6963):1189–97. doi: 10.1136/bmj.309.6963.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Needleman HL, Gunnoe C, Leviton A, et al. Deficits in psychologic and classroom performance of children with elevated dentine lead levels. N Engl J Med. 1979;300(13):689–95. doi: 10.1056/NEJM197903293001301. [DOI] [PubMed] [Google Scholar]
- 37.Burke MG, Miller MD. Practical guidelines for evaluating lead exposure in children with mental health conditions: molecular effects and clinical implications. Postgrad Med. 2011;123(1):160–8. doi: 10.3810/pgm.2011.01.2256. [DOI] [PubMed] [Google Scholar]
- 38.Bellinger D, Needleman HL, Bromfield R, Mintz M. A follow up study of the academic attainment and classroom behavior of children with elevated dentine lead levels. Biol Trace Elem Res. 1984;6(3):207–23. doi: 10.1007/BF02917507. [DOI] [PubMed] [Google Scholar]
- 39.Chen A, Cai B, Dietrich KN, et al. Lead exposure, IQ, and behavior in urban 5- to 7-year-olds: does lead affect behavior only by lowering IQ? Pediatrics. 2007;119(3):e650–8. doi: 10.1542/peds.2006-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eisenberg KW, van Wijngaarden E, Fisher SG, et al. Blood lead levels of refugee children resettled in Massachusetts, 2000 to 2007. Publ Health. 2011;101(1):48–54. doi: 10.2105/AJPH.2009.184408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raymond JS, Kennedy C, Brown MJ. Blood lead level analysis among refugee children resettled in New Hampshire and Rhode Island. Publ Health Nurs. 2013;30(1):70–9. doi: 10.1111/phn.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmidt CW. Unsafe harbor? Elevated blood lead levels in refugee children. Environ Health Perspect. 2013;121(6):A190–5. doi: 10.1289/ehp.121-a190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Proue M, Jones-Webb R, Oberg C. Blood lead screening among newly arrived refugees in Minnesota. Minn Med. 2010;93(6):42–6. [PubMed] [Google Scholar]
- 44.Centers for Disease Control and Prevention. Elevated blood lead levels in refugee children-New Hampshire, 2003–2004. MMWR Morb Mortal Wkly Rep. 2005;54(2):42–6. [PubMed] [Google Scholar]
- 45.Wright RO, Shannon MW, Wright RJ, Hu H. Association between iron deficiency and low-level lead poisoning in an urban primary care clinic. Publ Health. 1999;89(7):1049–53. doi: 10.2105/ajph.89.7.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wright RO, Tsaih SW, Schwartz J, et al. Association between iron deficiency and blood lead level in a longitudinal analysis of children followed in an urban primary care clinic. J Pediatr. 2003;142(1):9–14. doi: 10.1067/mpd.2003.mpd0344. [DOI] [PubMed] [Google Scholar]
- 47.Accardo P, Whitman B, Caul J, Rolfe U. Autism and plumbism. A possible association. Clin Pediatr. 1988;27(1):41–4. doi: 10.1177/000992288802700108. [DOI] [PubMed] [Google Scholar]
- 48.Shannon M, Graef JW. Lead intoxication in children with pervasive developmental disorders. J Toxicol Clin Toxicol. 1996;34(2):177–81. doi: 10.3109/15563659609013767. [DOI] [PubMed] [Google Scholar]
- 49.Fitzpatrick M. Autism and environmental toxicity. Lancet Neurol. 2007;6(4):297. doi: 10.1016/S1474-4422(07)70066-2. [DOI] [PubMed] [Google Scholar]
- 50.George M, Heeney MM, Woolf AD. Encephalopathy from lead poisoning masquerading as a flu-like syndrome in an autistic child. Pediatr Emerg Care. 2010;26(5):370–3. doi: 10.1097/PEC.0b013e3181db2237. [DOI] [PubMed] [Google Scholar]
- 51.Zeager M, Heard T, Woolf AD. Lead poisoning in two children with Landau-Kleffner syndrome. Clin Toxicol. 2012;50(5):448. doi: 10.3109/15563650.2012.685523. [DOI] [PubMed] [Google Scholar]
- 52.Filipek PA, Accardo PJ, Ashwal S, et al. Practice parameter: screening and diagnosis of autism: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Child Neurology Society. Neurology. 2000;55(4):468–79. doi: 10.1212/wnl.55.4.468. [DOI] [PubMed] [Google Scholar]
- 53.Chung EK, Webb D, Clampet-Lundquist S, Campbell C. A comparison of elevated blood lead levels among children living in foster care, their siblings, and the general population. Pediatrics. 2001;107(5):E81. doi: 10.1542/peds.107.5.e81. [DOI] [PubMed] [Google Scholar]
- 54.Centers for Disease Control & Prevention. [Accessed November 1, 2016];Report to Congress on the workers’ home contamination study conducted under the Workers’ Family Protection Act (29 U.S.C. 671a) 1995 DHHS (NIOSH) publication no. 95–123. Available at: www.cdc.gov/niosh/docs/95-123.
- 55.Woolf AD, Bellinger D, Goldman R. Clinical approach to childhood lead poisoning. Pediatr Clin North Am. 2007;54:271–94. doi: 10.1016/j.pcl.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 56.Newman N, Binns HJ, Karwowski M, Lowry J. Pediatric Environmental Health Specialty Unit (PEHSU) Lead Working Group. [Accessed March 21, 2017];Medical management of childhood lead exposure and poisoning. 2013 Available at: http://www.pehsu.net/_Childhood_Lead_Exposure.html.
- 57.Centers for Disease Control and Prevention. Advisory Committee on Childhood Lead Poisoning Prevention. low level lead exposure harms children: a renewed call for primary prevention. Atlanta, GA: Centers for Disease Control and Prevention; 2012. [Accessed November 1, 2016]. Available at: http://www.cdc.gov/nceh/lead/ACCLPP/Final_Document_030712.pdf. [Google Scholar]
- 58.Esteban E, Rubin CH, Jones RL, Noonan G. Hair and blood as substrates for screening children for lead poisoning. Arch Environ Health. 1999;54(6):436–40. doi: 10.1080/00039899909603376. [DOI] [PubMed] [Google Scholar]
- 59.McIntire MS, Wolf GL, Angle CR. Red cell lead and delta-amino levulinic acid dehydratase. Clin Toxicol. 1973;6(2):183–8. doi: 10.3109/15563657308990516. [DOI] [PubMed] [Google Scholar]
- 60.Walker HK, Hall WD, Hurst JW, editors. Clinical methods: the history, physical and laboratory examinations. 3. Boston, MA: Butterworths; 1990. [PubMed] [Google Scholar]
- 61.Binns HJ, Campbell C, Brown MJ. Centers for Disease Control and Prevention Advisory Committee on Childhood Lead Poisoning. Prevention Interpreting and managing blood lead levels of less than 10 microg/dL in children and reducing childhood exposure to lead: recommendations of the Centers for Disease Control and Prevention Advisory Committee on Childhood Lead Poisoning Prevention. Pediatrics. 2007;120(5):e1285–98. doi: 10.1542/peds.2005-1770. [DOI] [PubMed] [Google Scholar]
- 62.Dietrich KN, Ware JH, Salganik M, et al. Effect of chelation therapy on the neuropsychological and behavioral development of lead-exposed children after school entry. Pediatrics. 2004;114(1):19–26. doi: 10.1542/peds.114.1.19. [DOI] [PubMed] [Google Scholar]
- 63.Baxter AJ, Krenzelok EP. Pediatric fatality secondary to EDTA chelation. Clin Toxicol. 2008;46(10):1083–4. doi: 10.1080/15563650701261488. [DOI] [PubMed] [Google Scholar]
- 64.Centers for Disease Control and Prevention. Deaths associated with hypocalcemia from chelation therapy-Texas, Pennsylvania, and Oregon, 2003–2005. MMWR Morb Mortal Wkly Rep. 2006;55(8):204–7. [PubMed] [Google Scholar]
- 65.Centers for Disease Control & Prevention. Educational services for children affected by lead expert panel. Atlanta, GA: U.S. Department of Health and Human Services; 2015. [Accessed November 1, 2016]. Available at http://www.cdc.gov/nceh/lead/publications/Educational_Interventions_Children_Affected_by_Lead.pdf. [Google Scholar]
- 66.Nussbaumer-Streit B, Yeoh B, Griebler U, et al. Household interventions for preventing domestic lead exposure in children. Cochrane Database Syst Rev. 2016;10:CD006047. doi: 10.1002/14651858.CD006047.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aschengrau A, Beiser A, Bellinger D, et al. Residential lead-based-paint hazard remediation and soil lead abatement: their impact among children with mildly elevated blood lead levels. Am J Publ Health. 1997;87(10):1698–702. doi: 10.2105/ajph.87.10.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brown MJ, Gardner J, Sargent JD, et al. The effectiveness of housing policies in reducing children’s lead exposure. Am J Publ Health. 2001;91(4):621–4. doi: 10.2105/ajph.91.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Etzel RA, Balk SJ. Pediatric environmental health. 3. Elk Grove Village, IL: American Academy of Pediatrics; 2011. American Academy of Pediatrics Council of Environmental Health, editors. [Google Scholar]
- 70.Taylor Pharmaceuticals. [Accessed March 21, 2017];BAL in oil ampules. 2006 Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/005939s007lbl.pdf.
- 71.Hoffman RS, Howland MA, Lewin NA, et al., editors. Gold-frank’s toxicologic emergencies. 10. New York, NY: McGraw Hill Education; 2015. [Google Scholar]
- 72.Graceway Pharmaceuticals I. [Accessed March 21, 2017];Calcium Disodium Versenate (edetate calcium disodium injection, USP) Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/008922s016lbl.pdf.
- 73.Schwarz Pharma Mfg. Inc. [Accessed March 21, 2017];Chemet (Succimer) 2007 Jul; Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2007/019998s013lbl.pdf.
- 74.Shannon MW, Townsend MK. Adverse effects of reduced-dose D-penicillamine in children with mild-to-moderate lead poisoning. Ann Pharmacother. 2000;34(1):15–8. doi: 10.1345/aph.19084. [DOI] [PubMed] [Google Scholar]
- 75.Shannon M, Graef J, Lovejoy FH. Efficacy and toxicity of D-penicillamine in low-level lead poisoning. J Pediatr. 1988;112(5):799–804. doi: 10.1016/s0022-3476(88)83212-8. [DOI] [PubMed] [Google Scholar]
- 76.Merck & Co. I. [Accessed March 21, 2017];Cuprimine (Pencillamine) Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2004/19853s012,014lbl.pdf.