Abstract
Experiments on an inner ear sensory cell revealed that it converts electrical energy directly into mechanical energy at acoustic frequencies.
Introduction
Thirty-five years ago, the members of the Physiological and Psychological Acoustics Technical Committee of the Acoustical Society of America were at a pivotal juncture in understanding hearing. The original description of the ability of the ears to produce sound had been published in The Journal of the Acoustical Society of America a few years earlier. Remarkable differences in neural circuitry and structural specializations in the hearing organ (Figures 1–4) were being described. It was a propitious time to take a close look at the outer hair cell (OHC), which was then the most structurally and functionally mysterious cell in the ear. We discovered that OHCs undergo rapid changes of cell shape in response to electrical stimulation, something we now call OHC electromotility (http://acousticstoday.org/OHCEM1).1 OHC electromotility revolutionized the hearing sciences by revealing the active process responsible for the sounds coming from the ear. The history behind the discovery, the subsequent biophysical investigations, and the role of electromotility in hearing are the topics of this narrative.
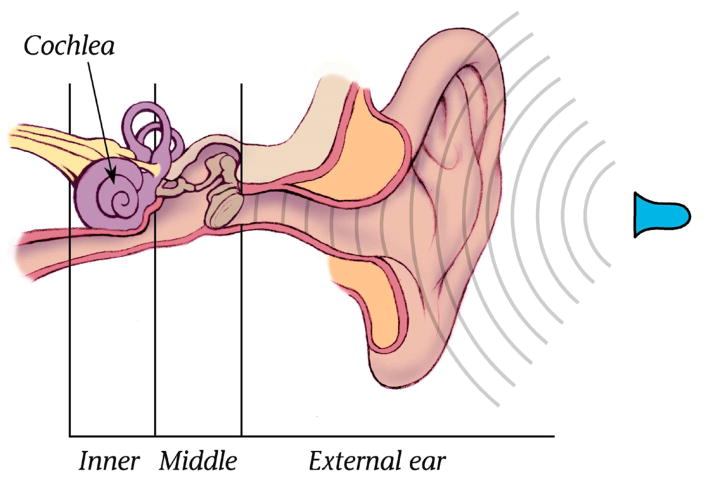
Figure 1.
Location of the spiral-shaped cochlea in the human head. It is found in a cavity that forms deep in the skull during development, behind and below the level of the eyes. The cavity also contains the sensory organs of balance (vestibular organs) that have their own sensory hair cells and communicate to the brain via a bundle of nerve fibers that terminate in different parts of the brain than do the auditory nerve fibers that emerge from the cochlea. The left and right cochleas are mirror images of one another. They spiral around their central axes in opposite directions. The direction of the spiral of the right cochlea is the same as for a right-handed screw while the spiral of the left cochlea is left-handed. The drawing shows the left ear.
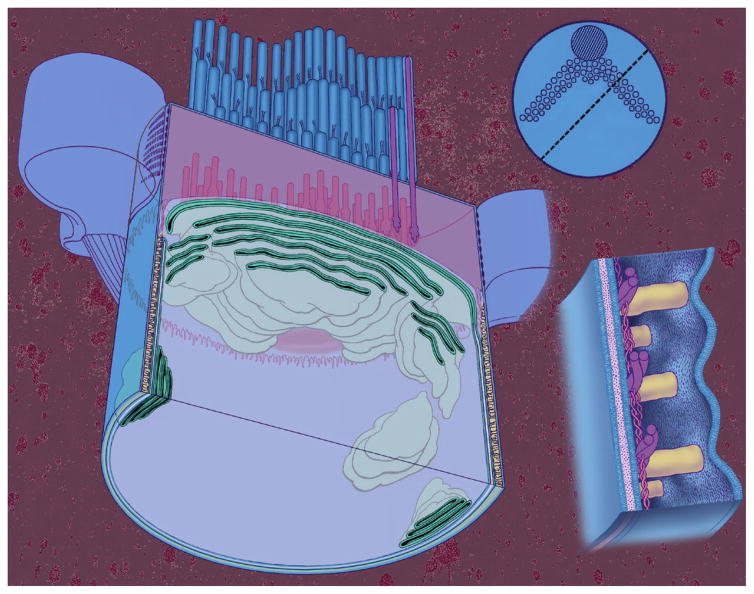
Figure 4.
The apical end of an OHC showing organization of the membranes and cytoskeletal structures. Left: a cylindrical OHC has been sliced parallel to its long axis along a plane defined by the dashed line in the insert at the top right. Top right: a view of the OHC looking down on the stereocilia bundle that has a typical W shape. Each small circle represents a single stereocilium, while the large circle represents a structure that disappears during development. Bottom right: high-power view of the lateral wall showing its three layers. The outermost layer is the plasma membrane and the innermost layer is made up of the subsurface cisterna. Sandwiched in between is the cortical lattice composed of circumferentially oriented actin filaments and axially oriented spectrin (spectrin filaments are the thinner filaments). The plasma membrane and cortical lattice form nanoscale motor elements that generate force at acoustic frequencies, resulting in electromotility.
Origins: Mammalian High-Frequency Hearing and the Outer Hair Cell
The ability to detect and analyze high-frequency sounds that other animals could not hear is likely to have provided early mammals a strong survival advantage (Allman, 1999). The mammalian cochlea (Figures 1 and 2) houses a mechanosensitive sensory hair cell that is structurally and functionally different from the sensory hair cells found in other vertebrates. The defining characteristic of the newly evolved OHCs is their electromotility. The role of OHC electromotility is linked to the nature of sound that Pythagoras and his fellow Greeks, inspired by stringed musical instruments, reasoned was a vibration. They introduced the concept of the octave that eventually led to quantification and a physical understanding of tone. The study of acoustics progressed from these early beginnings, but exploration on the biological basis of hearing lagged. Two millennia passed before the structural organization of the mammalian inner ear began to emerge. Its structure provided clues as to how the ear works, particularly after it was realized that the inner ear is fluid filled.
Figure 2.
Pathway of sound to the cochlea in humans. The external ear collects sound and directs it to the eardrum. Sound vibrations are conveyed across the middle ear by three tiny middle ear bones that match the acoustic impedance of air with the impedance of the fluids that fill the inner ear. The three middle ear bones and the outer hair cells (OHCs) are found in mammals. The bones and the OHCs are both required for high-frequency hearing.
Finding the Organ of Corti: Hair Cells in a Fluid-Filled Cavity
The inner ear is difficult to study because it is small and encased in bone. Gabriello Fallopio described the openings between the middle ear and the cochlea in a 1561 publication. Two centuries later, Domenico Cotugno, a Neapolitan surgeon and humanist, published an anatomical dissertation (Cotugno 1775)2 based on his scientific observations. He expanded on the findings of two 17th-century inner ear anatomists, Antonio Maria Valsalva and Guichard Joseph Duverney, correcting speculation by the latter about where high and low frequencies were processed in the cochlea. Cotugno found fluid in the inner ear of freshly harvested skulls, in contrast to Fallopio and earlier anatomists who examined desiccated cadaver skulls and assumed that the inner ear was filled with air. The fluid environment is relevant to understanding the role of OHC electromotility in hearing. But first, a greater understanding about the cellular organization of the inner ear and identification of its hair cells was needed.
Improvements in microscopic methods during the 19th century led to Alfonso Corti’s detailed description of the cellular organization of the mammalian hearing organ. Albert Kölliker had pioneered techniques to harden, section, and stain body tissue before looking at it through the microscope. Corti used these techniques in Kölliker’s laboratory to examine tissue he dissected from the inner ear of humans and other mammals (Corti, 1851). He described the sensory epithelium in the cochlea, which was soon named after him (the organ of Corti).3 His drawings depicted the sensory cells that became known as “hair cells“ because each had a tuft of enlarged microvilli at one end (see Figure 4 for greater detail of the stereociliary tuft). More than a century later, the function of the tuft was identified, and it was demonstrated that bending the bundle of stereocilia modulates the flow of ions into the cell (Wersäll et al., 1965; Harris et al., 1970). This, in turn, regulates the release of neurotransmitters that activate nerve fibers making contact at synapses located at the opposite end of the hair cell.
At the beginning of the 20th century, it had been established that the organ of Corti contained two types of hair cells. They were named based on their location relative to the central axis of the cochlear spiral. Inner hair cells (IHCs) make up a single spiraling row of mechanoreceptor cells located over the thin bony plate to which the axial margin of the basilar membrane is attached (Figure 3). Three rows of OHCs are located further away from the central axis. OHCs have a distinctly cylindrical shape and are not in intimate contact with supporting cells for most of their length. The large fluid spaces surrounding the lateral walls of the OHCs are biologically unusual. Cells in most organs (heart, brain, kidney, muscle) are within 10–30 nm of one another. OHCs, in contrast, are separated from adjacent cells by as much as 1 μm. The elegant colonnade appearance of the organ of Corti is due to the large and unique spacing between OHCs, but the reason for the large extracellular spaces was puzzling.
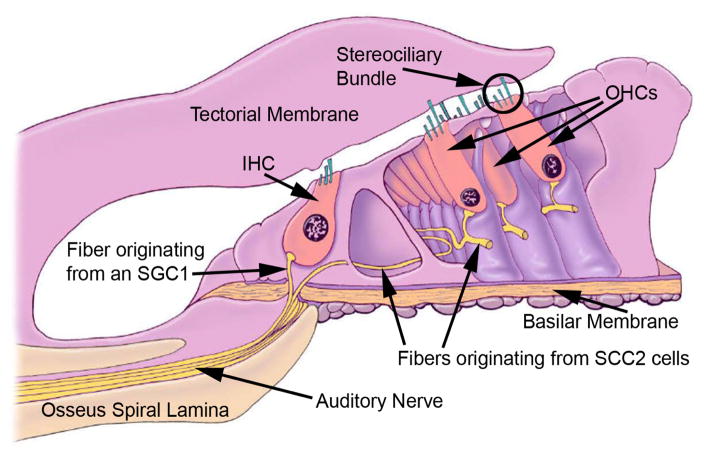
Figure 3.
A cross section of the organ of Corti showing inner hair cells (IHCs) and OHCs with their stereociliary bundles. An OHC ste-reociliary bundle is circled. The central axis of the cochlear spiral is to the left of the drawing. An IHC is located over the bony (osseus) spiral lamina and is tightly enveloped with supporting cells. Sound-evoked vibrations will be reduced at this location relative to those occurring under the OHCs located nearer the middle of the compliant basilar membrane. The mottled dark circle in the hair cells is the cell nucleus. Note the large fluid spaces around the OHCs created by the absence of adjacent supporting cells that surround the IHCs. Auditory nerve fibers (eighth cranial nerve) contact hair cells at the end opposite their mechanosensory stereociliary bundle. More than 15 fibers contact the base of each IHC while a few course laterally and each contacts more than 15 OHCs. The fibers innervating the IHCs come from type 1 spiral ganglion cells (SGC1), whereas those innervating the OHCs come from type 2 SGCs (SGC2).
Structural Evidence for Differences Between Inner and Outer Hair Cells
The invention of the electron microscope (EM) in the early 20th century provided a powerful new tool to examine the fine structure of cells because it could observe objects that were smaller than the wavelength of light. By midcentury, EM investigations had revealed structural similarities between all hair cells and revealed unique specializations in OHCs. One of these was the presence of flattened membrane-bound organelles near the OHC cell membrane at the same location where the lateral wall was exposed to the large extracellular spaces (Figure 3). The membranes of these “subsurface cisternae“ (Figure 4) invariably appeared crisp and flat, whereas the nearby OHC cell membrane was diaphanous and rippled.
In the late 1960s, Heinrich Hans Spoendlin (Figure 5) completed a laborious EM study of the auditory nerve fiber innervation pattern at the base of the IHCs and OHCs (Spoendlin, 1966). The results provided the first compelling evidence that the OHCs might be doing something entirely different than the IHCs. He meticulously reconstructed the auditory nerve fiber innervation pattern at the base of both the IHCs and OHCs by examining hundreds of ultrathin serial-section electromicrographs per hair cell. Each auditory nerve fiber that enters the organ of Corti arises from a single cell located in what is called the spiral ganglion (located closer to the spiral axis of the cochlea). He found that 90–95% of the fibers came from a population of spiral ganglion cells (SGCs) that he called type 1 SGCs (SGC1). The remainder were smaller fibers arising from type 2 SGCs (SGC2). SGC1 fibers were connected exclusively to the IHCs (about 15 fibers per IHC), whereas each SGC2 fiber passed radially near the basilar membrane, then turned basely and proceeded more than 500 μm before making contact with up to 20 OHCs. Before Spoendlin’s observations, both IHCs and OHCs were assumed to carry out similar roles of mechanotransduction. The innervation pattern led to the suggestion, however, that most, if not all, hearing information was coming from the IHCs. The big question raised by this discovery was what were the OHCs doing?
Figure 5.

Photograph of the Swiss physician-scientist and head of otolaryngology at the University of Innsbruck Heinrich Hans Spoendlin (1927–1991). Spoendlin provided the first structural evidence that most, if not all, neural acoustic information from the ear came via IHCs. His discovery set the stage for accepting OHCs as the cochlear amplifier. Photograph provided by Professor Joseph B. Nadol.
Passive Hearing Is Not Consistent with Auditory Nerve Fiber Tuning
Investigations on cochlear mechanics during the late 19th and first half of the 20th century by scientists such as Hermann von Helmholtz and Georg von Békésy led to a widely accepted model for how the vibrations of sound were analyzed by the cochlea. von Békésy used a light microscope and stroboscopic illumination to observe acoustically driven patterns of movement in the basilar membrane of cadavers (von Békésy, 1960). He confirmed the tonotopic organization of high- to low-frequency vibrations going from the base to the apex of the cochlea. He also described the traveling wave that resulted from hydraulic coupling. von Békésy was awarded the Nobel Prize for his work and helped establish a view of the cochlea as an elegant, but essentially passive, device for converting the mechanical energy of sound into electrical signals to the brain.
Neurophysiological investigations of sensory coding by the auditory nerve began with the advent of vacuum tube amplifiers and kymograph recordings. The introduction of oscilloscopes and digital recording techniques facilitated neurophysiological research. A research monograph on the response characteristics of single auditory nerve fibers was published by Nelson Kiang and colleagues in the mid-sixties (Kiang et al., 1965). It was a compendium of sensory coding in the 8th nerve using well-controlled stimuli and provided a convenient summary of work in the field up to that point. The tuning of the fibers was consistent across the entire sample population. There were no surprising, broadly tuned fibers that may have originated from Spoendlin’s SGC2s. It was also noted that the auditory nerve fiber response was more highly tuned than the traveling wave patterns that von Békésy had described. The neural tuning was closer to what was needed to explain the frequency selectivity of mammalian hearing.
Active Hearing: Early Speculations and Evidence
An active process in the cochlea had been hypothesized by Thomas Gold (1948), a Viennese-born English polymath (Figure 6). He realized that viscous damping in the fluid environment of the cochlea was incompatible with the exquisite frequency-resolving powers of human hearing. He postulated that an active, essentially piezoelectric mechanism that converted electrical to mechanical energy could provide feedback to counteract viscous damping from cochlear fluids. His hypothesis was largely ignored, in part, because of opposition by von Békésy.
Figure 6.

Photograph of Thomas Gold (1920–2004) who, over a long active career, studied biophysics, astronomy, aerospace engineering, and geophysics. Gold was born in Austria and moved with his family to England where he studied at Cambridge. He contributed to the British war effort by working on improvements to radar. While working with R. J. Pumphrey after the war, Gold concluded that an electromechanically active mechanism was necessary to counteract viscous damping by cochlear fluids. Photograph provided by Professor David Kemp.
Evidence that the cochlea was active began to mount in the 1970s. Brian John-stone in Perth, WA, Australia, and Bill Rhode in Madison, WI, pioneered the use of the Mössbauer effect to measure the movement of a gamma radiation source placed on the basilar membrane in living animals (Johnstone and Boyle, 1967; Rhode, 1971). By the mid-1970s, they had established that, in the best preparations, mechanical tuning approached that of single auditory nerve fibers. When the animal died, the motion rapidly deteriorated to the passive tuning that von Békésy had measured in cadaver ears. Shyam Khanna in New York City soon confirmed these observations using laser interferometry (Khanna and Leonard, 1982).
The most direct demonstration of a mechanism in the cochlea that could generate mechanical energy was the discovery of otoacoustic emissions by David Kemp in London late in the 1970s. He initially reported the presence of sound coming from the cochlea, now called otoacoustic emissions, after stimulation with a brief sound (Kemp, 1978). He then showed that some ears produce sound without being stimulated (for historical details, see Kemp, 2008). This was direct evidence of Gold’s active process. Measuring otoacoustic emissions has become an important tool for assessing hearing loss, even in newborns and unresponsive patients. Steve Neely, Duck Kim, Egbert de Boer, David Mountain, and others began to include a source of mechanical energy in their theoretical models of cochlear mechanics. In 1983, Hallowell Davis, a senior hearing scientist at the Central Institute for the Deaf in St. Louis, MO, coined the term “cochlear amplifier“ for the active process. The cellular basis of the cochlear amplifier was a puzzle that was soon resolved.
The First Observation of Outer Hair Cell Electromotility
The discovery of OHC electromotility took place late in the morning on December 3, 1982, in the medical school laboratories of Daniel Bertrand and Charles Bader at the University of Geneva where I was taking a sabbatical from the University of Florida. Charlie and Daniel had pioneered techniques to dissect, isolate, and record photoreceptors to characterize ionic movement across their membranes. We had met at a photoreceptor meeting in Sicily a year earlier. I attended the meeting because of the many structural and functional similarities between vertebrate photoreceptors and hair cells. I had described these similarities and proposed isolated hair cell studies in a review paper that was published the same year as the discovery (Brownell, 1982). Several months were required to optimize the primary cell culture procedures for hair cells. OHCs proved easier to isolate, most likely because of the absence of supporting cells on their lateral walls. We began voltage-clamp attempts in October. The homemade optics, amplifiers, and data-collection programs required a team effort to pull off the experiments. We were using micropipettes to make intracellular recordings (whole cell patch-clamp techniques were just being developed). The standard routine was for Charlie to advance the electrode while looking at it and the cell through a microscope. Daniel was poised over the electronics looking for the tell-tale voltage shift that would indicate we were inside the cell. I was responsible for dissecting the cochlea and isolating the cells, after which I would sit behind the others and record the results in the lab notebook.
A common technique at the time was to “buzz“ the electrode when its tip was positioned on the cell membrane. The buzz was achieved by throwing the amplifier headstage into electrical oscillation. For reasons that are still not clear, the buzz would push the tip of the electrode through the membrane. The pivotal moment occurred when Daniel buzzed and I could see Charlie’s back and shoulders jerk in a classic startle response to what he had seen through the microscope. Still looking though the microscope, Charlie called for another buzz, and his body responded with another somewhat smaller response. At that point, Charlie turned around and said “We have a problem. “ Daniel and I then took turns looking at OHC electromotility as Charlie buzzed. Proving that the electrically evoked length change was not an artifact was the “problem“ to which Charlie was referring.
The remainder of my sabbatical was given over to OHC electromotility. We eliminated potential artifacts and further characterized the phenomenon. It was only after I established that hyperpolarization caused elongation and depolarization caused shortening was I confident that OHC electromotility was not an artifact. For several months, we had no equipment for recording the conspicuous movements and visiting scientists were asked to sign our lab book acknowledging that they saw the movements. I eventually captured the movements using video microscopy (see http://acousticstoday.org/OHCEM1), eliminating the need for affidavits, and we published our findings (Brownell 1983; Brownell et al., 1985).
The OHC was quickly identified as Gold’s piezoelectric-like energy source. The modelers now had a cellular locus for the cochlear amplifier. The fact that OHC axial length changes would lose energy if they contacted adjacent cells explained the large extracellular spaces around the OHCs. I had speculated about an electromechanical mechanism in my review paper (Brownell, 1982) that was based on the synaptic activity in the base of the OHC. Looking through the microscope and later examining the videotapes revealed that the active process was in the lateral wall, thereby suggesting a function for the structurally unique organization of the lateral wall (Figure 4). Other work going on at the same time identified the battery that powers the process.
What Powers Outer Hair Cell Electromotility? - The Silent Current
My laboratory in Gainesville, FL, had completed an in vivo current-density analysis of the cochlea before I left for Geneva. Paul Manis, Michael Zidanic, George Spirou, and I measured evoked currents in the fluid spaces of the cochlea (Brownell et al., 1983). We adapted techniques and analytic tools that Paul had developed for his study of neural organization in the auditory brainstem (Manis and Brownell, 1983). Our examination of the inner ear revealed that acoustic stimuli that moved the organ of Corti downward toward the scala tympani (Figure 7) resulted in decreased ion flow, whereas upward movements toward the scala media resulted in an increased ion flow. The modulation in ion flow through an OHC in either direction was about 500 pA and represents one of the largest known cell currents. It had previously been proposed that the electroanatomy of the cochlea could result in a steady current across the organ of Corti that we called the “silent current, “ in analogy to a current in the retina called the dark current. We interpreted our initial findings as modulation of the silent current resulting from deflection of the OHC stereocilia. Because our recordings were AC coupled, we had not measured the actual steady-state silent current. Michael took on the challenge and several years later reported his DC-coupled recordings (Zidanic and Brownell, 1990) confirming that the silent current was similar to what we had estimated in 1982. The silent current is the power source for cochlear transduction (Figure 7). It is the source of a steady flow of ions through the OHC even in silence. The changes in membrane potential generated in OHCs when the current is modulated drive electromotility.
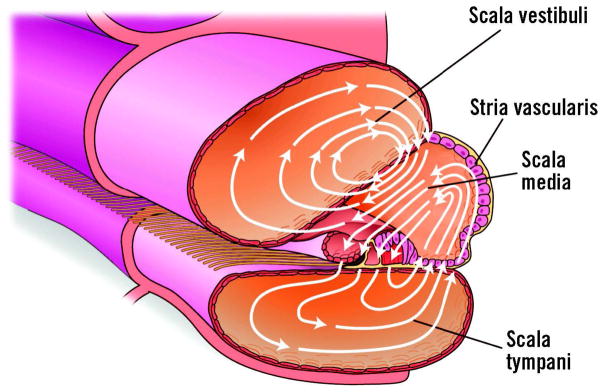
Figure 7.
Cross section of the cochlea showing the flow of ions making up the silent current. There are three fluid-filled chambers. The scala vestibuli and scala tympani contain a conventional extracellular fluid called perilymph, whereas the scala media contains endolymph that is high in potassium and low in sodium. The stria vascularis maintains an endolymphatic potential and electrochemical gradient that drives the silent current (arrows). The metabolically active stria vascularis pumps potassium into the middle cochlear compartment. The potassium passes through the hair cells into the scala tympani and then back to the stria vascularis. There is also a passive route for potassium through the cells, forming the boundary between the scala media and scala vestibuli before returning to the stria vascularis through the perilymph. The silent current is the power source for OHC electromotility.
Ultrasonic Force Generation, Turgor Pressure, Cortical Lattice, and Membrane-Based Motor
Video was too slow to capture OHC length changes faster than the 30 per second frame rate. A succession of techniques revealed that OHC electromechanical force production occurred at rates consistent with hearing. Eventually, Tony Gummer’s lab in Tübingen, Germany, developed a way to measure isometric force production with a calibrated probe while electrically stimulating and showed that the OHC generates force at frequencies approaching 100 kHz (Frank et al., 1999). Before that, Joe Santos-Sacchi at the Medical College of New Jersey used a variety of ion-channel toxins and manipulated the electrochemical gradients across the cell membrane to show that electromotility was based on the voltage across and not the current through the membrane (Santos-Sacchi and Dilger, 1988). This and the speed of the electromechanical transduction indicated that the energy was produced by a membrane-based motor.
The OHC is able to undergo rapid shape changes because it does not have the typical arrangement of rigid cytoskeletal proteins that maintains the shape in other types of cells. It is, instead, a cellular hydrostat composed of an elastic outer shell enclosing a modestly pressurized core (approximately 1–2 kPa). Most of the other cells in the body have no pressure. The cytosolic turgor pressure and the tensile properties of the lateral wall together form a hydraulic skeleton that facilitates shape changes and hydraulic force transmission at high frequencies. OHC electromotility diminishes and vanishes if the cell becomes flaccid (Brownell 1983, 1990; Brownell et al., 1985; Santos-Sacchi, 1991; Shehata et al., 1991). Most cells burst when their internal pressure is increased by even a small amount. The reinforcement provided by the lateral wall (Figure 4) and more specifically the cortical lattice (Oghalai et al., 1998) prevents this from happening in OHCs.
Displacement Currents and the Motor Gets a Protein
Passive OHC length changes resulting from mechanical stretch or electrically evoked length changes produce a “displacement“ current. The electrically evoked current is capacitive in nature because it is out of phase with the stimulus voltage. This reactive (nonohmic) current is vulnerable to manipulations such as aspirin or loss of turgor pressure that modify electromotility (Santos-Sacchi, 1991; Shehata et al., 1991). The magnitude of the displacement current varies with the membrane potential in a manner similar to the relationship between length change and membrane potential. The displacement current was found to require a membrane protein.
Peter Dallos and his colleagues found a protein important for electromotility at the the turn of the century (Zheng et al., 2000). The protein was discovered using a differential expression assay that showed that a protein was strongly expressed in OHCs. When they introduced the protein into other cell types, the large displacement currents characteristic of OHCs were also found. The investigators called the protein “prestin“ from the Italian word “presto, “ which indicates a rapid tempo in a muscial composition. Genetic manipulations that removed the protein altogether resulted in severe hearing loss and decreased otoacoustic emisisons (Liberman et al., 2002). These observations demonstrated that prestin plays an important role in the OHC electromechanical motor. The hearing science community was ecstatic with the protein.
The ability to insert prestin into the membranes of other cell types and to manipulate its molecular structure provided further evidence for the strength of the membrane protein interactions that underlie the OHC electromechanical motor mechanism (Rajagopalan et al., 2006, 2007; Zhang et al., 2007). The precise role of the protein and its interaction with the motor elements in the OHC lateral wall remain to be identified. Tremendous strides have been made in characterizing the structural and molecular components that make up the motor elements, and we are getting closer to a complete biophysical understanding of OHC electromotility. New tools have been introduced that allow direct measurement of the length changes at acoustic frequencies in living animals (Gao et al., 2014; Ren et al., 2016). Analysis of these organ-level results will help clarify the cellular role of OHC electromotility. Gold (1948) had proposed a piezoelectric-like energy source that was necessary to compensate for viscous damping resulting from the fluid environment that Cotugno (1775) had originally discovered. Spoendlin (1966) provided an important clue by showing that the OHCs did something other than convey information to the brain. OHC electromotility proved to be the piezoelectric-like source of mechanical energy that acts as the cochlear amplifier to compensate for viscous damping in the fluid-filled cochlea. The end result is greater sensitivity and sharper tuning. Although we now know with reasonable certainty how the OHC contributes to hearing, we still don’t know the role of SGC2 fibers that could, in principle, convey information to the brain directly from the OHCs. And so, as is usually true in science, puzzles remain, and their resolution will bring even more puzzles.
Acknowledgments
The discovery of OHC electromotility was made possible by sabbatical funding from the University of Florida and research funding from Hoffman LaRoche. My subsequent investigations on the mechanisms involved and the writing of this narrative have been funded by the citizens of the United States in the form of Research Grants R01-DC-00354 and R01-DC-02775 from the National Institute on Deafness and Other Communication Disorders, National Institutes of Health. My wife Nancy and editor Arthur Popper made many contributions to the style and delivery of the story.
Biography

Bill Brownell is a professor at the Baylor College of Medicine, Houston, TX, where he holds the Jake and Nina Kamin Chair of Otorhinolaryngology. Previously held appointments were at the University of Florida, Gainesville, FL, and the Johns Hopkins School of Medicine, Baltimore, MD. He spent two years (1965–1966) teaching science and mathematics in Nigeria with the United States Peace Corps between his undergraduate training in physics and graduate training in physiology at the University of Chicago, Chicago, IL. He is a Fellow of the Acoustical Society of America and a past president of the Association for Research in Otolaryngology. His main research focus is on the electromechanics of hearing.
Footnotes
This is the first video recording of outer hair cell electromotility. An explanation of the video and links to other videos are provided in the supplementary material at the end of the article.
Available online at https://goo.gl/PtvBFW.
Book available online at https://goo.gl/WAIsnS.
Supplementary Material
The first video recording of outer hair cell electromotility recorded on a VCR in April 1983 is presented at http://acousticstoday.org/OHCEM1. A glass pipette electrode has been positioned at the basal end of one of the OHCs in a cluster of OHCs joined together at their apex. The pipette is the sharply conical object pointing up from the bottom of the field of view. The audio indicates the presentation of an extracellular electrical pulse. After a few pulses the stimulated cell begins to shorten with sufficient force to move the entire cluster. There is a progressive cell shortening with repeated stimulation. The evoked movements are large enough to damage the cell and it undergoes a volume increase from water influx. Note the sudden lengthening about 2/3 of the way through the video. This is due to a volume decrease after which the cell fails to respond to electrical stimulation because of the loss of internal hydrostatic (turgor) pressure.
Two other videos of OHC electromotility in response to musical recordings are available on line at http://acousticstoday.org/OHCEM2 and http://acousticstoday.org/OHCEM3.
References
- Allman JM. Evolving Brains. Scientific American Library; New York: 1999. [Google Scholar]
- Brownell WE. Cochlear transduction: An integrative model and review. Hearing Research. 1982;6:335–360. doi: 10.1016/0378-5955(82)90064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell WE. Observation on a motile response in isolated hair cells. In: Webster WR, Aiken LM, editors. Mechanisms of Hearing. Monash University Press; Melbourne, VIC, Australia: 1983. pp. 5–10. [Google Scholar]
- Brownell WE. Outer hair cell electromotility and otoacoustic emissions. Ear and Hearing. 1990;11:82–92. doi: 10.1097/00003446-199004000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell WE, Manis PB, Zidanic M, Spirou GA. Acoustically evoked radial current densities in scala tympani. The Journal of the Acoustical Society of America. 1983;74:792–800. doi: 10.1121/1.389866. [DOI] [PubMed] [Google Scholar]
- Brownell WE, Bader CR, Bertrand D, de Ribaupierre Y. Evoked mechanical responses of isolated cochlear outer hair cells. Science. 1985;227:194–196. doi: 10.1126/science.3966153. [DOI] [PubMed] [Google Scholar]
- Corti A. Recherches sur l’organe de l’ouïe des mammifères. Zeitschrift für wissenschaftlicke Zoologie. 1851;3:109–169. [Google Scholar]
- Cotugno D. Typographia Sanctae Tomae Aquinatis, Neopoli et Bononiae. 1775. De Aquaeductibus Auris Humanae Internae Anatomica Dissertation. [Google Scholar]
- Frank G, Hemmert W, Gummer AW. Limiting dynamics of high-frequency electromechanical transduction of outer hair cells. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:4420–4425. doi: 10.1073/pnas.96.8.4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao SS, Wang R, Raphael PD, Moayedi Y, Groves AK, Zuo J, Applegate BE, Oghalai JS. Vibration of the organ of Corti within the cochlear apex in mice. Journal of Neurophysiology. 2014;112:1192–1204. doi: 10.1152/jn.00306.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold T. Hearing. II. The physical basis of the action of the cochlea. Proceedings of the Royal Society B Biological Sciences. 1948;135:492–498. [Google Scholar]
- Harris GG, Frishkopf LS, Flock A. Receptor potentials from hair cells of the lateral line. Science. 1970;167:76–79. doi: 10.1126/science.167.3914.76. [DOI] [PubMed] [Google Scholar]
- Johnstone BM, Boyle AJ. Basilar membrane vibration examined with the Mossbauer technique. Science. 1967;158:389–390. doi: 10.1126/science.158.3799.389. [DOI] [PubMed] [Google Scholar]
- Kemp DT. Stimulated acoustic emissions from within the human auditory system. The Journal of the Acoustical Society of America. 1978;64:1386–1391. doi: 10.1121/1.382104. [DOI] [PubMed] [Google Scholar]
- Kemp DT. Otoacoustic emissions: Concepts and origins. In: Manley GA, Popper AN, Fay RR, editors. Active Processes and Otoacoustic Emissions. Springer-Verlag; New York: 2008. pp. 1–38. [Google Scholar]
- Khanna SM, Leonard DG. Basilar membrane tuning in the cat cochlea. Science. 1982;215:305–306. doi: 10.1126/science.7053580. [DOI] [PubMed] [Google Scholar]
- Kiang NY-S, Watanabe T, Thomas EC, Clark LF. Discharge Patterns of Single Fibers in the Cat’s Auditory Nerve. IT Press; Cambridge, MA: 1965. [Google Scholar]
- Liberman MC, Gao J, He DZ, Wu X, Jia S, Zuo J. Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature. 2002;419:300–304. doi: 10.1038/nature01059. [DOI] [PubMed] [Google Scholar]
- Manis PB, Brownell WE. Synaptic organization of eighth nerve afferents to cat dorsal cochlear nucleus. Journal of Neurophysiology. 1983;50:1156–1181. doi: 10.1152/jn.1983.50.5.1156. [DOI] [PubMed] [Google Scholar]
- Oghalai JS, Patel AA, Nakagawa T, Brownell WE. Fluorescence-imaged microdeformation of the outer hair cell lateral wall. Journal of Neuroscience. 1998;18:48–58. doi: 10.1523/JNEUROSCI.18-01-00048.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan L, Patel N, Madabushi S, Goddard JA, Anjan V, Lin F, Shope C, Farrell B, Lichtarge O, Davidson AL, Brownell WE, Pereira FA. Essential helix interactions in the anion transporter domain of prestin revealed by evolutionary trace analysis. Journal of Neuroscience. 2006;26:12727–12734. doi: 10.1523/JNEUROSCI.2734-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan L, Greeson JN, Xia A, Liu H, Sturm A, Raphael RM, Davidson AL, Oghalai JS, Pereira FA, Brownell WE. Tuning of the outer hair cell motor by membrane cholesterol. Journal of Biological Chemistry. 2007;282:36659–36670. doi: 10.1074/jbc.M705078200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren T, He W, Kemp D. Reticular lamina and basilar membrane vibrations in living mouse cochleae. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:9910–9915. doi: 10.1073/pnas.1607428113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode WS. Observations of the vibration of the basilar membrane in squirrel monkeys using the Mossbauer technique. The Journal of the Acoustical Society of America. 1971;49:1218–1231. doi: 10.1121/1.1912485. [DOI] [PubMed] [Google Scholar]
- Santos-Sacchi J. Reversible inhibition of voltage-dependent outer hair cell motility and capacitance. Journal of Neuroscience. 1991;11:3096–3110. doi: 10.1523/JNEUROSCI.11-10-03096.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi J, Dilger JP. Whole cell currents and mechanical responses of isolated outer hair cells. Hearing Research. 1988;35:143–150. doi: 10.1016/0378-5955(88)90113-x. [DOI] [PubMed] [Google Scholar]
- Shehata WE, Brownell WE, Dieler R. Effects of salicylate on shape, electromotility and membrane characteristics of isolated outer hair cells from guinea pig cochlea. Acta Otolaryngology. 1991;111:707–718. doi: 10.3109/00016489109138403. [DOI] [PubMed] [Google Scholar]
- Spoendlin H. The organization of the cochlear receptor. Fortschr Hals Nasen Ohrenheilkd. 1966;13:1–227. [PubMed] [Google Scholar]
- von Békésy G. In: Experiments in Hearing. Wever EG, translator. McGraw-Hill Book Company; New York: 1960. [Google Scholar]
- Wersäll J, Flock A, Lundquist PG. Structural basis for directional sensitivity in cochlear and vestibular sensory receptors. Cold Spring Harbor Symposium on Quantitative Biology. 1965;30:115–132. doi: 10.1101/sqb.1965.030.01.015. [DOI] [PubMed] [Google Scholar]
- Zhang R, Qian F, Rajagopalan L, Pereira FA, Brownell WE, Anvari B. Prestin modulates mechanics and electromechanical force of the plasma membrane. Biophysical Journal. 2007;93:L07–L09. doi: 10.1529/biophysj.107.107573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Shen W, He DZ, Long KB, Madison LD, Dallos P. Prestin is the motor protein of cochlear outer hair cells. Nature. 2000;405:149–155. doi: 10.1038/35012009. [DOI] [PubMed] [Google Scholar]
- Zidanic M, Brownell WE. Fine structure of the intracochlear potential field. I. The silent current. Biophysical Journal. 1990;57:1253–1268. doi: 10.1016/S0006-3495(90)82644-8. [DOI] [PMC free article] [PubMed] [Google Scholar]