Abstract
Few pharmacotherapies are currently available to treat castration resistant prostate cancer (CRPC), with low impact on patient survival. Transforming growth factor-β (TGF-β) is a multi-functional peptide with opposite roles in prostate tumorigenesis as an inhibitor in normal growth and early stage disease and a promoter in advanced prostate cancer. Dysregulated TGF-β signaling leads to a cascade of events contributing to oncogenesis, including up-regulated proliferation, decreased apoptosis, epithelial-to-mesenchymal transition (EMT) and evasion of immune surveillance. TGF-β signaling pathway presents an appropriate venue for establishing a therapeutic targeting platform in CRPC. Exploitation of TGF-β effectors and their cross talk with the androgen axis pathway will provide new insights into mechanisms of resistance of the current antiandrogen therapeutic strategies and lead to generation of new effective treatment modalities for CRPC. Points of functional convergence of TGF-β with key oncogenic pathways, including mitogen-activated protein kinase (MAPK) and androgen receptor (AR), are discussed as navigated within the EMT landscape in the tumor microenvironment. In this context the emerging anti-TGF-β pharmacotherapies for prostate cancer treatment are considered. Targeting the functional cross-talk between the TGF-β signaling effectors with the androgen axis supports the development of novel therapeutic strategies for treating CRPC with high specificity and efficacy in a personalized-medicine approach.
Keywords: Transforming growth factor-β, Prostate tumors, Androgen receptor, Epithelial-mesenchymal transition, Therapeutic value
1. Introduction
1.1. Challenge of castration-resistant-prostate cancer
Prostate cancer is the second most frequently diagnosed cancer in men, following lung cancer, with a total of 238,590 new cases and 29,720 deaths in 2013 in the United States. Prostate cancer is responsible for 28% of the cancer diagnose and 10% of cancer deaths in men [1]. In addition, prostate cancer is most frequently diagnosed in aging male. Histological evidence of prostate cancer were found in almost 30% of men over the age of 50 years [2]. The increasing aged population in the US will make prostate cancer a greater health burden. By 2030, population of 65-year-plus age group is predicted to reach 72 million and concomitant increase in prostate cancer incidence will be inevitable [3]. Improvements in diagnostic, surgical and radiation techniques, and using of androgen-deprivation therapy (ADT) slow the disease progression and decrease the death rate of prostate cancer patients [4]. Although the consequence of ADT remains controversial, medical or surgical castration remains the standard of care for patients with advanced disease [5]. Following such ADT however patients eventually develop castration-resistant prostate cancer (CRPC) after 1–3 years [6]. Clinical progression of CRPC is manifested as biochemical progression (elevated prostate specific antigen [PSA]), radiographic progression (metastatic CRPC), or symptomatic progression [4]. Until 2010, the microtubule-targeting agent, docetaxel was the only first chemotherapy with survival benefit (2–3 months) in CRPC. More recently another taxane family member, cabazitaxel, a second-line chemotherapeutic, in combination with bevacizumab, thalidomide, and prednisone, was reported to increase patient survival, but only at a modest degree [7]. The second-line antiandrogens (agents targeting the androgen signaling axis), abiraterone and enzalutamide, led to increased survival by 5 months. In addition, the immunotherapeutic, sipuleucel-T, increased survival of CRPC patients by 4 months. The radiopharmaceutical radium-223 increased patient survival by 3 months [7].
1.2. Signaling of transforming growth factor-β (TGF-β)
Transforming growth factor-β (TGF-β) is a multifunctional peptide belonging to a superfamily cytokines [8]. TGF-β regulates cell proliferation, apoptosis, cell differentiation, and cell migration in multiple types of cells [9]. TGF-β constitutes three isoforms that share high homology, but unique heterologous motifs exist in each isoform. TGF-β-knockout is lethal to mice with survival up to 3–5 weeks [10], [11], [12]. Pro-TGF-βs, 75-kDa homodimers, are initially synthesized and then cleaved in Golgi to produce mature 25-kDa TGF-β homodimers. The TGF-β homodimers bind latency-associated proteins, generating small latent complex. A single latent TGF-β binding protein interacts with the TGF-β homodimer via a disulfide bond, generating a large latent complex in the endoplasmic reticulum. The large latent complexes are exported to the extracellular matrix to interact with fibronectin fibrils and heparin sulfate proteoglycans on the cell membrane and are stored in fibrillin-rich microfibrils in extracellular matrix. Latent TGF-β is activated and released from the latent complex by proteases, reactive oxygen species, integrins and thrombospondin 1 to initiate signaling [13].
TGF-β signaling is mediated through SMAD and non-SMAD pathways. In the SMAD dependent pathway, TGF-β binds to a type II receptor, TGF-βRII, followed by recruitment and phosphorylation of a type I receptor, TGF-βRI, at the serine and threonine residues. TGF-βRI serine/threonine kinase is activated by phosphorylation and the activated type I receptor recruits and phosphorylates downstream receptor regulated SMAD (R-SMAD), including R-SMAD2 and R-SMAD3. Subsequently, R-SMAD2 and R-SMAD3 form complexes with the cytosolic SMAD4, which are translocated to the nucleus to regulate target gene expression [9]. TGF-β inhibits proliferation and induces apoptosis in normal prostate epithelial cells and early stages of prostate cancer cells. Activated SMAD cascade leads to G1 arrest accompanied by up-regulation of cyclin-dependent kinase inhibitors [14] and down-regulation of c-Myc oncogene [15]. In addition, TGF-β1 signaling promotes apoptosis by inducing death associated protein kinase in a SMAD-dependent manner in the liver [16]. SMAD4 induces transcriptional activation of the translation-inhibiting protein 4E-BP1 (eukaryotic translation initiation factor 4E-binding protein 1) and catalytic inactivation of the translation initiation factor eEF1A1 (eukaryotic elongation factor 1A1), establishing the translational regulating effect [17], [18]. In contrast to the agonistic SMADs, inhibitory SMADs, SMAD6/7, negatively regulate R-SMAD activity and nuclear translocation [6]. Upon TGF-β activation, SMAD7 is recruited to TGF-βRI to block SMAD2/3 phosphorylation. In addition, SMAD7 induces proteasomal degradation of TGF-βRI, TGF-βRII, and other SMAD transcription factors to inhibit TGF-β signaling [19]. In addition to the SMAD dependent pathway, non-SMAD pathways, including MAPK, c-Src, m-TOR, RhoA, RAS, PI3K/Akt, protein phosphatase 2A/p70s6K [6], [20], as well as the actin cytoskeleton effector cofilin [21], [22] are regulated by TGF-β.
The clinical significance of targeting TGF-β signaling is built on a strong body of evidence demonstrating that loss of TGF-βRII and/or TGF-βRI receptors is associated with decreased survival rate of colon cancer, breast cancer, and prostate cancer patients [23], [24], [25]. At the cellular level, up-regulation of TGF-βRII promotes the pro-apoptotic function of TGF-β1, while receptor inactivation induces malignant transformation [26], [27]. Restoration of TGF-β1 signaling by overexpressing TGF-βRII suppresses prostate tumorigenic growth in vivo via a caspase-1-mediated mechanism [26]. More recent evidence indicated an association between reduced expression of TGF-βRII mRNA with higher Gleason score and elevated risk of relapse after ADT in prostate cancer patients, supporting the significance of TGF-β1 pathway in CRPC [28]. TGF-β type III receptor (TGF-βRIII or betaglycan) has also been defined as critical effector of TGF-β signaling, involved in prostate cancer progression. In general, TGF-βRIII facilitates signaling by increasing the affinity of TGF-β, especially TGF-β2, for its receptor [29]. TGF-βRIII binds GAIP (G alpha interacting protein) at the cell membrane to enhance TGF-β signaling, as well as minor effect on migration and invasion [29]. In contrast, soluble TGF-βRIII binds TGF-β to sequester the ligand and impair TGF-β signaling [29]. Meanwhile, interaction of TGF-βRIII with β-arrestin2 leads to co-internalization of TGF-βRIII with TGF-βRI/II, which further down-regulates TGF-β signaling [29]. A significant reduction or complete loss of TGF-βRIII is frequently associated with prostate cancer progression [30]. Further TGF-βRIII downregulation is detected in metastases relative to primary tumors [29]. Function loss of TGF-βRIII leads to increase of cells exhibiting stem cell characteristics, and alteration of genes commonly associated with prostate cancer progression [30]. Restoration of TGF-βRIII expression in human prostate cancer cells leads to inhibition of migration and invasion independent of the ligand TGF-β [31]. In a human prostate cancer xenograft model, restoring TGF-βRIII function decreases tumor growth, suggesting its tumor suppressor role [32].
In benign epithelial cells, TGF-β inhibits epithelial proliferation and promotes apoptosis via SMAD control over c-Myc and cyclin-dependent kinase inhibitors [14], [15]. In advanced prostate cancer, the functional distribution of action between SMAD-dependent and -independent signaling is apparently disrupted. Thus TGF-β switches to a tumor promoter enabling prostate cancer progression to metastasis, by dysregulation of several oncogenic factors and bypassing the classic pathway of TGF-β signaling activation (Fig. 1) [20]. Differential activation of MAP kinases ERK between benign and malignant cells is reported to control TGF-β synthesis in a homeostatic feedback manner [33]. TGF-β-induced ERK activation promotes TGF-β synthesis in prostate cancer cells, while it is repressed by benign prostate cells. In a mechanistic twist, TGF-β-induced ERK activation cannot be inhibited in advanced prostate cancer and thus the negative feedback loop is interrupted [33]. Constitutive ERK activation independent of TGF-β contributes to continuous production of TGF-β in prostate cancer cells [33]. In addition, control of receptors TGF-βRI and TGF-βRII gene methylation proceeds via ERK activated DNA methyltransferase (DNMT) function [34]. DNMT expression is up-regulated by TGF-β and this consequentially results in hypermethylation of TGF-βR promoters and down-regulation of their expression [34]. TGF-β and/or DNMTs overexpression have been correlated with aggressive prostate tumor phenotypes and more significantly as predictors of disease recurrence [34]. Another posttranslational modification of TGF-βRI involves its ubiquitination by an ubiquitin ligase tumor necrosis factor receptor associated factor (TRAF6) and subsequent cleavage by ADAM metallopeptidase domain 17 (ADAM17), ultimately inhibiting TGF-β signaling [35]. Cleavage of TGF-βRI generates an intracellular domain (ICD) that is translocated to the nucleus, leading to up-regulation of other oncogenic factors [35]. Cofilin is an F-actin-severing protein involved in cytoskeleton reorganization and filopodia formation. Cofilin binding and severing of F-actin contributes to cell migration during invasion and metastasis of prostate cancer [36]. Recent studies from this laboratory established that constitutively active cofilin (Fig. 1) facilitated filopodia formation and cell migration induced as mediated by TGF-β, and coordinated responses to TGF-β required for invasive cancer migration and metastasis [21].
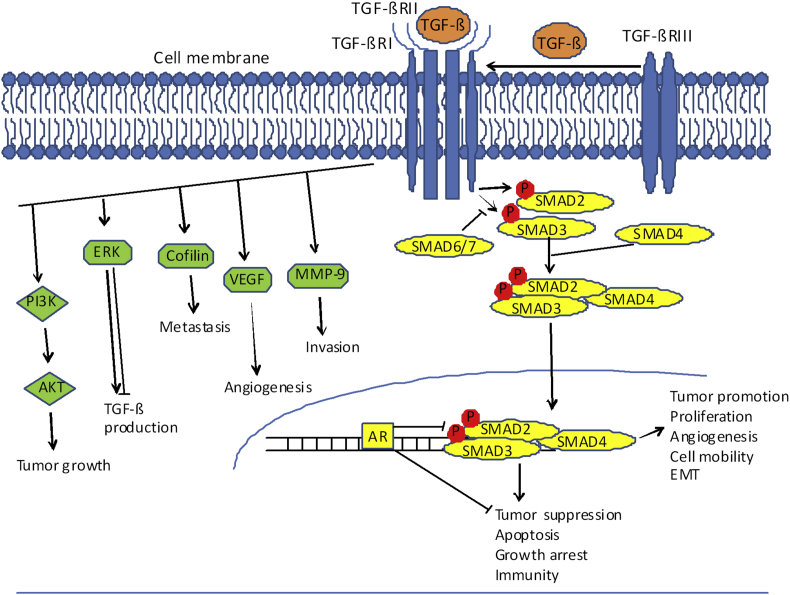
Figure 1.
TGF-β signaling pathway in prostate cancer cells. The ligand TGF-β binds to cell transmembrane receptor TGF-βRII (serine threonine kinase), subsequently recruiting TGF-βRI, to form receptor–ligand complex. This process can be promoted by the TGF-βRIII transmembrane receptor. The activated receptor–ligand complex leads to phosphorylation of SMAD2 and SMAD3 in the cytoplasm (receptor activated SMADs) and subsequent formation and nuclear translocation of SMAD2/3 and SMAD4 complex. Once in the nucleus activated Smad4 induces gene transcription for TGF-β target genes regulating proliferation, apoptosis, angiogenesis and EMT. SMAD6/7 negatively regulates R-SMAD activity and nuclear translocation. AR inhibits the TGF-β1/SMAD transcriptional activity, and ultimately TGF-β1-induced growth inhibition and apoptosis. Non-SMAD pathways, including ERK and PI3K/AKT are regulated by TGF-β to promote tumor growth and invasion. In addition, TGF-β promotes tumor growth and metastasis by VEGF-regulated angiogenesis and MMP-9-induced cell invasion. Cofilin coordinates responses to TGF-β required for migratory, invasive and metastatic properties. P, phosphorylation.
2. Merging pathways of TGF-β signaling and androgen axis in prostate cancer
Androgenic hormones, testosterone and its metabolite dihydrotestosterone (DHT), bind AR in the cytoplasm to induce nuclear translocation and transcriptional activation of target genes. Androgens promote prostate epithelial cell proliferation in the absence of stroma fibroblasts by engaging different cytokines, although in the presence of surrounding prostate stroma this effect is biphasic [37]. TGF-β signaling is mediated through SMAD and non-SMAD pathways towards apoptosis induction and inhibition of proliferation in early tumor development, while it promotes progression to metastasis in advanced disease [6], [20]. TGF-β treatment and physiological levels of DHT in LNCaP TGF-βRII cells enhances cell cycle arrest and apoptosis compared to TGF-β treatment alone [38]. Such combined TGF-β and DHT-induced apoptosis is inhibited by caspase-1 inhibition and Bcl-2 overexpression in prostate cancer cells [38], [39]. AR inhibits TGF β1-induced transcriptional activity in prostate cancer cells in the presence of an AR co-activator, AR-associated protein 55 (ARA55). Mechanistically overexpression of ARA55 inhibits TGF-β-mediated up-regulation of SMAD transcriptional activity via an interaction between C terminus of ARA55 and the MH2 domain of AR [40]. Direct interaction of TGF-β1 transcription with androgen and AR complex has been reported in human hepatoma cells [41]. In human prostate cancer cell lines, PC-3 and LNCaP, SMAD4 alone or the SMAD3/4 complex, interact with the AR transcriptional activation domain, regulating DHT-induced AR transcriptional activity [37], [42]. In the androgen-independent PC-3 cells, forced expression of AR inhibits the TGF-β1/SMAD transcriptional activity, as well as TGF-β1-induced growth inhibition and apoptosis [37]. Mechanistic evidence indicates that AR-induced transcriptional suppression of SMAD3 is responsible for the inhibition of tumor suppressor function of TGF-β in prostate cancer [42], [43]. AR suppresses expression of TGF-β1 through a negative androgen-response region containing multiple negative androgen-response elements in the TGF-β1 promoter in androgen-independent and androgen-sensitive human prostate cancer cells [44]. AR knockdown in carcinoma-associated fibroblasts (CAFs) leads to decreased expression of TGF-β2, indicating that AR by regulating TGF-β promotes prostate cancer epithelial growth and invasion [45]. In addition, AR and miR-21 increase each other's expression and promote tumor growth by attenuating TGF-βRII expression and TGF-β-induced SMAD2/3 activation [46]. Investigation of this dynamic interaction between TGF-β and AR signaling mechanism will potentially lead to new platforms for therapeutic management of advanced metastatic prostate cancer.
3. Landscape design by TGF-β: epithelial–mesenchymal transition (EMT)
TGF-β induces epithelial–mesenchymal transition (EMT) to facilitate tumor progression and metastasis [6]. EMT phenotype is characterized by loss of E-cadherin and expression of mesenchymal proteins, including N-cadherin, vimentin, and fibronectin. Transcriptional repression of E-cadherin and induction of mesenchymal phenotype can be facilitated by TGF-β in cancer cells [6]. In prostate cancer, TGF-β1 induces EMT in prostate tumor epithelial cells and in a mouse model of tumorigenesis with a targeted deletion of SMAD3, supporting a contributing role for TGF-β signaling to EMT and prostatic cancer metastasis [47], [48]. TGF-β induces EMT in prostate tumor through constitutively active Akt that inhibits translocation of SMAD3 and p21 to the nucleus [47]. Inhibition of vimentin, a mesenchymal type III intermediate filament, is sufficient to partially reverse EMT in prostate cancer cell lines, pointing to a therapeutic targeting value for vimentin [49]. In PC-3 prostate cancer cells, blockade of NF-κB signaling leads to decreased vimentin expression and inhibition of their invasive capability, indicating functional involvement of NF-κB in mediating TGF-β-induced EMT [50]. Other transcription factors, such as SNAI2/Slug that control E-cadherin expression (epithelial cell marker), are causally involved in TGF-β1-induced EMT in non-transformed prostate cells, conferring loss of polarity at the invading front [51]. In benign epithelial cells, EMT transcriptional inducers, Snail and Twist, contribute to the appearance of CD44Hi/CD24low cancer stem cells phenotype, implicating EMT as the process driving acquisition of stemlike characteristics in cancer cells [52].
A wealth of evidence has established the ability of TGF-β to up-regulate matrix metalloproteinase-9 (MMP-9) via translocation of TGF-βRI-ICD to the nucleus, promoting tumor invasion [35], [53]. Recombinant soluble betaglycan inhibits DU145 human prostate cancer cell xenograft growth, tumor blood volume and microvessel density, and an elevation of apoptosis by inhibiting TGF-β-induced MMP-9 activity and expression [32]. Anethole, an aromatic compound with antitumor activities, impairs prostate cancer metastasis by decreasing expression of MMP-9 and up-regulating the cell adherens junction mediator, E-cadherin [54]. Such metastasis-blocking effects are reversed by TGF-β-induced EMT, indicating a crosstalk between EMT effectors and MMP-9 in prostate tumor progression to metastasis. Moreover, clinically used first and second generation anti-androgens, Casodex or MDV3100 (enzalutamide), suppress prostate cancer cell growth yet promote prostate cancer cell invasion by activating the TGF-β1/SMAD3/MMP9 pathway [55]. A newly developed anti-AR compound, ASC-J9, produces anti-invasion effects against prostate cancer by down-regulating MMP9 expression [55]. Endothelial cell-induced prostate cancer invasion can be functionally prevented by blocking the IL-6/AR/TGF-β/MMP-9 signaling pathway [56]. In addition to prostate cancer, TGF-β1 up-regulates MMP-9 expression and activity in human skin, corneal epithelial cells, and brain astrocytes, indicating potential therapeutic benefits in multiple clinical settings [56]. Recently identified additional molecular effectors of TGF-β signaling have been directly implicated in prostate cancer cell progression and metastasis [57], [58]. NEDD9, one of the Crk-associated substrate family proteins, is shown to mechanistically drive TGF-β-induced EMT in prostate cancer epithelial cells, ultimately emerging as a potential marker, as well as a target for metastatic disease [57]. Clusterin is a pleiotropic molecular chaperone that has recently emerged as an essential effector of TGF-β-induced EMT, with a promise for therapeutic targeting potential in prostate cancer progression [58].
4. Dictations by TGF-β in the prostate tumor microenvironment
The prostate microenvironment plays an important role in the development and progression of prostate cancer [6]. Diverse cell types including carcinoma-associated stromal cells (CAFs), endothelial cells, lymphocytes and cancer epithelial cells comprise the dynamic tumor microenvironment, under the regulatory control of TGF-β to promote tumor growth and progression [59]. While normal fibroblasts are responsible for maintaining tissue homeostasis, myofibroblasts and CAFs facilitate tumor progression via their repair-centric system and pro-survival biology, promoting new growth and vascularity. Establishment of vascular supply and angiogenesis allow for exchange of nutrient and waste, further facilitating prostate cancer progression and metastasis [59]. Generation of myofibroblasts in the prostate tumor microenvironment is dynamically controlled by TGF-β signaling [60]. Targeted overexpression of TGF-β1 in the mouse prostate gland leads to increased frequency of unique fibrotic collagenous micronodules, associated with clinical prostate cancer initiation [61]. Silibinin, an extract of the milk thistle seeds, inhibits prostate cancer cell-mediated differentiation of naïve fibroblasts into CAFs via inhibition of TGF-β2 expression in PC-3 cells, further supporting a role of TGF-β in fibroblast differentiation [60]. Another forum via which TGF-β1 exerts its control over the tumor microenvironment dynamics, is via its stimulatory effect on vascular endothelial growth factor (VEGF), the critical regulator in angiogenesis, in human prostate cancer PC-3-M and LNCaP C4–2B cells [62]. Upregulation of VEGF expression proceeds via SMAD complex regulated transcription, and the Src/FAK/Akt signaling pathways [62]. Apigenin, a natural product belonging to the flavone, decreases VEGF expression by selectively inhibiting TGF-β1-induced phosphorylation of SMAD2 and SMAD3, further supporting the functional involvement of TGF-β in angiogenesis [62]. TGF-β exerts its regulatory role in angiogenesis, invasion and migration and EMT via navigating an array of interactions between tumor epithelial cells and myofibroblasts/CAFs maintaining a reactive tumor microenvironment, crucial to prostate cancer metastatic spread [21], [59], [63].
5. TGF-β in control of immune surveillance
TGF-β, also serves as a role of a repressor of the immune system via its ability to regulate T-cells. Full activation of T-cells is inhibited by constitutive active receptor TGF-βRI [64]. TGF-β1 knockout in mice specific and ablation of TGF-βRII in T-cells lead to autoimmunity [65]. T-cell and tumor-induced TGF-β inhibit surrounding host immune cells, resulting in cancer cell evasion of the host immune surveillance, towards tumor progression [66]. In addition to suppressing the effector T-cell function, TGF-β induces regulatory T-cells to repress effector T cells-induced cytotoxicity and inflammation [67]. Forkhead family transcription factor, Foxp3, regulates development and function of regulatory T-cells [67]. TGF-β induces transcription factor Foxp3 to convert CD4+CD25− naive T cells to CD4+CD25+ regulatory T-cells and maintains Foxp3 expression [68]. Interestingly enough, tumor-reactive TGF-β-insensitive CD8+ T-cells infiltrate into the tumor parenchyma and induce tumor cell apoptosis, indicating the ability of TGF-β to navigate evasion of the immune system by tumor cells [69].
6. Targeting value of TGF-β mechanisms in prostate tumor progression
Several transgenic mouse models have been generated and characterized to interrogate the molecular events that drive prostate cancer initiation, progression, and metastasis. The Pten knockout, the Nkx3.1 knockout, the transgenic adenocarcinoma of the mouse prostate (TRAMP) mouse model and probasin-large T-antigen transgenic mouse (LADY) model have been established and effectively exploited [70]. Work from this laboratory demonstrated that dominant-negative mutant TGF-βRII receptor accelerates prostate tumorigenesis in the TRAMP mouse model by enhancing EMT and disrupting the growth kinetics within the tumor microenvironment [27]. In vivo evidence gathered from different investigations provides solid support for this action: First, conditional knockout of TGF-βRII receptor in stromal fibroblasts leads to prostatic intraepithelial neoplasia and adenocarcinoma, and ultimately bone metastatic growth [63], [71]. Furthermore, in a constitutively active TGF-β1 transgenic mouse model, inflammation of nerve ganglia and fibroplasia occurs in an age-dependent manner in the prostate stroma [59]. Development of poorly differentiated prostate adenocarcinoma in Ras and Myc-driven mouse models, is associated with elevated TGF-β1 [72]. Loss of SMAD4 function (nuclear effector of TGF-β) leads to invasive and metastatic prostate cancer with 100% penetrance in the Pten-null mouse prostate, further supporting the significance of TGF-β signaling in prostate cancer progression to lethal disease [73].
The complexity of the mechanisms navigating the dual function of TGF-β as an inhibitor of prostate tumor growth in early stage cancer, and a promoter of progression to metastasis in advanced stages, requires exhaustive understanding of its signaling interactions with critical cell survival pathways, primarily the androgen axis [37]. Thus, it is clear that timing of anti-TGF-β directed therapies is of paramount significance in effectively targeting the functional contribution of TGF-β to impair progression to metastasis. Small molecules inhibiting the kinase activity of TGF-β receptors such as LY2157299, a kinase inhibitor targeting ATP-binding site of TGF-βRI, are in Phase I clinical trial, with good safety profile in patients with prostate cancer [74], as well as colon and breast cancer [75]. Another kinase inhibitor, LY573636, is currently investigated in a Phase II clinical trial in patients with advanced stage melanoma [74].
The therapeutic value of targeting TGF-β in the context of immunotherapy approach has also been pursued. FC1008, a neutralizing antibody that bind all isoforms of TGF-β, is undergoing a Phase I/II clinical trial to treat breast cancer and pleural mesothelioma [76]. Antisense inhibition of oncogene expression provides a potential therapeutic platform for treatment of several malignancies [77]. Specifically for prostate cancer, an antisense oligonucleotide targeting TGF-β1, AP11014, decreases TGF-β1 secretion by prostate cancer cells [74]. AP 12009, an antisense oligonucleotide targeting TGF-β2, is safe and well-tolerated in patients with high-grade glioma, and a Phase I/II clinical trial in pancreatic carcinoma and malignant melanoma is currently under investigation [74]. In breast cancer, soluble TGF-βRII receptor can sequester TGF-β from the cellular receptors, to inhibit cell survival, migration, and metastases [78], and in vivo prostate cancer growth [32]. ASC-J9, a recently developed anti-AR compound, prevents prostate cancer cell growth as well as metastasis by down-regulating MMP9 activity and expression [55].
7. Conclusion
TGF-β signaling pathway has been extensively studied as a potential therapeutic target to treat prostate cancer [6]. TGF-β receptor inhibitors have been evaluated in pre-clinical in vivo models, as well as in the clinical setting of prostate cancer patients [6]. Considering the dual function of TGF-β in prostate tumorigenesis, as an inhibitor of prostate tumor growth in early stage cancer and a promoter of progression to metastasis in advanced stages, as well as its role in repression of the immune system, therapeutics only inhibiting the tumor-promoting action of TGF-β could be challenging in eliciting a therapeutic effect. Consequently, treatment strategies based on the use of TGF-β signaling inhibitors must clinically be considered with caution and in the context of personalized therapy approach for targeting individual patients with CRPC based on their molecular signature profile. Exploitation platforms must include points of the multi-cytokine convergence of TGF-β signaling, the androgen-AR axis and the EMT landscape in the prostate tumor microenvironment, presenting therapeutic optimizing options for personal combination therapeutic strategies.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgment
This work was supported by an NIH grant (RO1 DK 083761).
Footnotes
Peer review under responsibility of Chinese Urological Association and SMMU.
References
- 1.Siegel R., Naishadham D., Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Scardino P.T. Early detection of prostate cancer. Urol Clin North Am. 1989;16:635–655. [PubMed] [Google Scholar]
- 3.HHS . 2011. U.S. Department of health and human services (HHS), a profile of older Americans. [Google Scholar]
- 4.Sridhar S.S., Freedland S.J., Gleave M.E., Higano C., Mulders P., Parker C. Castration-resistant prostate cancer: from new pathophysiology to new treatment. Eur Urol. 2014;65:289–299. doi: 10.1016/j.eururo.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Kageyama Y., Hyochi N., Kihara K., Sugiyama H. The androgen receptor as putative therapeutic target in hormone-refractory prostate cancer. Recent Pat Anticancer Drug Discov. 2007;2:203–211. doi: 10.2174/157489207782497172. [DOI] [PubMed] [Google Scholar]
- 6.Jones E., Pu H., Kyprianou N. Targeting TGF-beta in prostate cancer: therapeutic possibilities during tumor progression. Expert Opin Ther Targets. 2009;13:227–234. doi: 10.1517/14728220802705696. [DOI] [PubMed] [Google Scholar]
- 7.Lorente D., De Bono J.S. Molecular alterations and emerging targets in castration resistant prostate cancer. Eur J Cancer. 2014;50:753–764. doi: 10.1016/j.ejca.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Herpin A., Lelong C., Favrel P. Transforming growth factor-beta-related proteins: an ancestral and widespread superfamily of cytokines in metazoans. Dev Comp Immunol. 2004;28:461–485. doi: 10.1016/j.dci.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Massague J. TGFbeta signalling in context. Nat Rev Mol Cell Biol. 2012;13:616–630. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kulkarni A.B., Karlsson S. Transforming growth factor-beta 1 knockout mice. A mutation in one cytokine gene causes a dramatic inflammatory disease. Am J Pathol. 1993;143:3–9. [PMC free article] [PubMed] [Google Scholar]
- 11.Proetzel G., Pawlowski S.A., Wiles M.V., Yin M., Boivin G.P., Howles P.N. Transforming growth factor-beta 3 is required for secondary palate fusion. Nat Genet. 1995;11:409–414. doi: 10.1038/ng1295-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanford L.P., Ormsby I., Gittenberger-de Groot A.C., Sariola H., Friedman R., Boivin G.P. TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development. 1997;124:2659–2670. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Annes J.P., Munger J.S., Rifkin D.B. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 14.Guo Y., Kyprianou N. Overexpression of transforming growth factor (TGF) beta1 type II receptor restores TGF-beta1 sensitivity and signaling in human prostate cancer cells. Cell Growth Differ. 1998;9:185–193. [PubMed] [Google Scholar]
- 15.Massague J., Blain S.W., Lo R.S. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell. 2000;103:295–309. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- 16.Jang C.W., Chen C.H., Chen C.C., Chen J.Y., Su Y.H., Chen R.H. TGF-beta induces apoptosis through smad-mediated expression of DAP-kinase. Nat Cell Biol. 2002;4:51–58. doi: 10.1038/ncb731. [DOI] [PubMed] [Google Scholar]
- 17.Azar R., Alard A., Susini C., Bousquet C., Pyronnet S. 4E-BP1 is a target of Smad4 essential for TGFbeta-mediated inhibition of cell proliferation. EMBO J. 2009;28:3514–3522. doi: 10.1038/emboj.2009.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hussey G.S., Chaudhury A., Dawson A.E., Lindner D.J., Knudsen C.R., Wilce M.C. Identification of an mRNP complex regulating tumorigenesis at the translational elongation step. Mol Cell. 2011;41:419–431. doi: 10.1016/j.molcel.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kavsak P., Rasmussen R.K., Causing C.G., Bonni S., Zhu H., Thomsen G.H. Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGF beta receptor for degradation. Mol Cell. 2000;6:1365–1375. doi: 10.1016/s1097-2765(00)00134-9. [DOI] [PubMed] [Google Scholar]
- 20.Zhu B., Kyprianou N. Transforming growth factor beta and prostate cancer. Cancer Treat Res. 2005;126:157–173. doi: 10.1007/0-387-24361-5_7. [DOI] [PubMed] [Google Scholar]
- 21.Collazo J., Zhu B., Larkin S., Martin S.K., Pu H., Horbinski C. Cofilin drives cell-invasive and metastatic responses to TGF-beta in prostate cancer. Cancer Res. 2014;74:2362–2373. doi: 10.1158/0008-5472.CAN-13-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu B., Fukada K., Zhu H., Kyprianou N. Prohibitin and cofilin are intracellular effectors of transforming growth factor beta signaling in human prostate cancer cells. Cancer Res. 2006;66:8640–8647. doi: 10.1158/0008-5472.CAN-06-1443. [DOI] [PubMed] [Google Scholar]
- 23.Bacman D., Merkel S., Croner R., Papadopoulos T., Brueckl W., Dimmler A. TGF-beta receptor 2 downregulation in tumour-associated stroma worsens prognosis and high-grade tumours show more tumour-associated macrophages and lower TGF-beta1 expression in colon carcinoma: a retrospective study. BMC Cancer. 2007;7:156. doi: 10.1186/1471-2407-7-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong M., How T., Kirkbride K.C., Gordon K.J., Lee J.D., Hempel N. The type III TGF-beta receptor suppresses breast cancer progression. J Clin Invest. 2007;117:206–217. doi: 10.1172/JCI29293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo Y., Jacobs S.C., Kyprianou N. Down-regulation of protein and mRNA expression for transforming growth factor-beta (TGF-beta1) type I and type II receptors in human prostate cancer. Int J Cancer. 1997;71:573–579. doi: 10.1002/(sici)1097-0215(19970516)71:4<573::aid-ijc11>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 26.Guo Y., Kyprianou N. Restoration of transforming growth factor beta signaling pathway in human prostate cancer cells suppresses tumorigenicity via induction of caspase-1-mediated apoptosis. Cancer Res. 1999;59:1366–1371. [PubMed] [Google Scholar]
- 27.Pu H., Collazo J., Jones E., Gayheart D., Sakamoto S., Vogt A. Dysfunctional transforming growth factor-beta receptor II accelerates prostate tumorigenesis in the TRAMP mouse model. Cancer Res. 2009;69:7366–7374. doi: 10.1158/0008-5472.CAN-09-0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teixeira A.L., Gomes M., Nogueira A., Azevedo A.S., Assis J., Dias F. Improvement of a predictive model of castration-resistant prostate cancer: functional genetic variants in TGFbeta1 signaling pathway modulation. PLoS One. 2013;8:e72419. doi: 10.1371/journal.pone.0072419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gatza C.E., Oh S.Y., Blobe G.C. Roles for the type III TGF-beta receptor in human cancer. Cell Signal. 2010;22:1163–1174. doi: 10.1016/j.cellsig.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharifi N., Hurt E.M., Kawasaki B.T., Farrar W.L. TGFBR3 loss and consequences in prostate cancer. Prostate. 2007;67:301–311. doi: 10.1002/pros.20526. [DOI] [PubMed] [Google Scholar]
- 31.Turley R.S., Finger E.C., Hempel N., How T., Fields T.A., Blobe G.C. The type III transforming growth factor-beta receptor as a novel tumor suppressor gene in prostate cancer. Cancer Res. 2007;67:1090–1098. doi: 10.1158/0008-5472.CAN-06-3117. [DOI] [PubMed] [Google Scholar]
- 32.Bandyopadhyay A., Wang L., López-Casillas F., Mendoza V., Yeh I.T., Sun L. Systemic administration of a soluble betaglycan suppresses tumor growth, angiogenesis, and matrix metalloproteinase-9 expression in a human xenograft model of prostate cancer. Prostate. 2005;63:81–90. doi: 10.1002/pros.20166. [DOI] [PubMed] [Google Scholar]
- 33.Yu N., Kozlowski J.M., Park I.I., Chen L., Zhang Q., Xu D. Overexpression of transforming growth factor beta1 in malignant prostate cells is partly caused by a runaway of TGF-beta1 auto-induction mediated through a defective recruitment of protein phosphatase 2A by TGF-beta type I receptor. Urology. 2010;76:1519.e8–1519.e13. doi: 10.1016/j.urology.2010.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Q., Chen L., Helfand B.T., Jang T.L., Sharma V., Kozlowski J. TGF-beta regulates DNA methyltransferase expression in prostate cancer, correlates with aggressive capabilities, and predicts disease recurrence. PLoS One. 2011;6:e25168. doi: 10.1371/journal.pone.0025168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mu Y., Sundar R., Thakur N., Ekman M., Gudey S.K., Yakymovych M. TRAF6 ubiquitinates TGFbeta type I receptor to promote its cleavage and nuclear translocation in cancer. Nat Commun. 2011;2:330. doi: 10.1038/ncomms1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang W., Mouneimne G., Sidani M., Wyckoff J., Chen X., Makris A. The activity status of cofilin is directly related to invasion, intravasation, and metastasis of mammary tumors. J Cell Biol. 2006;173:395–404. doi: 10.1083/jcb.200510115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu M.L., Kyprianou N. Androgen receptor and growth factor signaling cross-talk in prostate cancer cells. Endocr Relat Cancer. 2008;15:841–849. doi: 10.1677/ERC-08-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bruckheimer E.M., Kyprianou N. Dihydrotestosterone enhances transforming growth factor-beta-induced apoptosis in hormone-sensitive prostate cancer cells. Endocrinology. 2001;142:2419–2426. doi: 10.1210/endo.142.6.8218. [DOI] [PubMed] [Google Scholar]
- 39.Bruckheimer E.M., Kyprianou N. Bcl-2 antagonizes the combined apoptotic effect of transforming growth factor-beta and dihydrotestosterone in prostate cancer cells. Prostate. 2002;53:133–142. doi: 10.1002/pros.10143. [DOI] [PubMed] [Google Scholar]
- 40.Wang H., Song K., Sponseller T.L., Danielpour D. Novel function of androgen receptor-associated protein 55/Hic-5 as a negative regulator of Smad3 signaling. J Biol Chem. 2005;280:5154–5162. doi: 10.1074/jbc.M411575200. [DOI] [PubMed] [Google Scholar]
- 41.Yoon G., Kim J.Y., Choi Y.K., Won Y.S., Lim I.K. Direct activation of TGF-beta1 transcription by androgen and androgen receptor complex in Huh7 human hepatoma cells and its tumor in nude mice. J Cell Biochem. 2006;97:393–411. doi: 10.1002/jcb.20638. [DOI] [PubMed] [Google Scholar]
- 42.Hayes S.A., Zarnegar M., Sharma M., Yang F., Peehl D.M., ten Dijke P. SMAD3 represses androgen receptor-mediated transcription. Cancer Res. 2001;61:2112–2118. [PubMed] [Google Scholar]
- 43.Song K., Wang H., Krebs T.L., Wang B., Kelley T.J., Danielpour D. DHT selectively reverses Smad3-mediated/TGF-beta-induced responses through transcriptional down-regulation of Smad3 in prostate epithelial cells. Mol Endocrinol. 2010;24:2019–2029. doi: 10.1210/me.2010-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qi W., Gao S., Chu J., Zhou L., Wang Z. Negative androgen-response elements mediate androgen-dependent transcriptional inhibition of TGF-beta1 and CDK2 promoters in the prostate gland. J Androl. 2012;33:27–36. doi: 10.2164/jandrol.110.011999. [DOI] [PubMed] [Google Scholar]
- 45.Yu S., Xia S., Yang D., Wang K., Yeh S., Gao Z. Androgen receptor in human prostate cancer-associated fibroblasts promotes prostate cancer epithelial cell growth and invasion. Med Oncol. 2013;30:674. doi: 10.1007/s12032-013-0674-9. [DOI] [PubMed] [Google Scholar]
- 46.Mishra S., Deng J.J., Gowda P.S., Rao M.K., Lin C.L., Chen C.L. Androgen receptor and microRNA-21 axis downregulates transforming growth factor beta receptor II (TGFBR2) expression in prostate cancer. Oncogene. 2014;33:4097–4106. doi: 10.1038/onc.2013.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ao M., Williams K., Bhowmick N.A., Hayward S.W. Transforming growth factor-beta promotes invasion in tumorigenic but not in nontumorigenic human prostatic epithelial cells. Cancer Res. 2006;66:8007–8016. doi: 10.1158/0008-5472.CAN-05-4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roberts A.B., Tian F., Byfield S.D., Stuelten C., Ooshima A., Saika S. Smad3 is key to TGF-beta-mediated epithelial-to-mesenchymal transition, fibrosis, tumor suppression and metastasis. Cytokine Growth Factor Rev. 2006;17(1–2):19–27. doi: 10.1016/j.cytogfr.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 49.Zhang X., Fournier M.V., Ware J.L., Bissell M.J., Yacoub A., Zehner Z.E. Inhibition of vimentin or beta1 integrin reverts morphology of prostate tumor cells grown in laminin-rich extracellular matrix gels and reduces tumor growth in vivo. Mol Cancer Ther. 2009;8:499–508. doi: 10.1158/1535-7163.MCT-08-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Q., Helfand B.T., Jang T.L., Zhu L.J., Chen L., Yang X.J. Nuclear factor-kappaB-mediated transforming growth factor-beta-induced expression of vimentin is an independent predictor of biochemical recurrence after radical prostatectomy. Clin Cancer Res. 2009;15:3557–3567. doi: 10.1158/1078-0432.CCR-08-1656. [DOI] [PubMed] [Google Scholar]
- 51.Slabáková E., Pernicová Z., Slavíčková E., Staršíchová A., Kozubík A., Souček K. TGF-beta1-induced EMT of non-transformed prostate hyperplasia cells is characterized by early induction of SNAI2/Slug. Prostate. 2011;71:1332–1343. doi: 10.1002/pros.21350. [DOI] [PubMed] [Google Scholar]
- 52.Mani S.A., Guo W., Liao M.J., Eaton E.N., Ayyanan A., Zhou A.Y. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Konrad L., Scheiber J.A., Schwarz L., Schrader A.J., Hofmann R. TGF-beta1 and TGF-beta2 strongly enhance the secretion of plasminogen activator inhibitor-1 and matrix metalloproteinase-9 of the human prostate cancer cell line PC-3. Regul Pept. 2009;155(1–3):28–32. doi: 10.1016/j.regpep.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 54.Ha B., Ko H., Kim B., Sohn E.J., Jung J.H., Kim J.S. Regulation of crosstalk between epithelial to mesenchymal transition molecules and MMP-9 mediates the antimetastatic activity of anethole in DU145 prostate cancer cells. J Nat Prod. 2014;77:63–69. doi: 10.1021/np4006376. [DOI] [PubMed] [Google Scholar]
- 55.Lin T.H., Lee S.O., Niu Y., Xu D., Liang L., Li L. Differential androgen deprivation therapies with anti-androgens casodex/bicalutamide or MDV3100/Enzalutamide versus anti-androgen receptor ASC-J9(R) Lead to promotion versus suppression of prostate cancer metastasis. J Biol Chem. 2013;288:19359–19369. doi: 10.1074/jbc.M113.477216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang X., Lee S.O., Xia S., Jiang Q., Luo J., Li L. Endothelial cells enhance prostate cancer metastasis via IL-6-->androgen receptor-->TGF-beta-->MMP-9 signals. Mol Cancer Ther. 2013;12:1026–1037. doi: 10.1158/1535-7163.MCT-12-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morimoto K., Tanaka T., Nitta Y., Ohnishi K., Kawashima H., Nakatani T. NEDD9 crucially regulates TGF-beta-triggered epithelial-mesenchymal transition and cell invasion in prostate cancer cells: involvement in cancer progressiveness. Prostate. 2014;74:901–910. doi: 10.1002/pros.22809. [DOI] [PubMed] [Google Scholar]
- 58.Shiota M., Zardan A., Takeuchi A., Kumano M., Beraldi E., Naito S. Clusterin mediates TGF-beta-induced epithelial-mesenchymal transition and metastasis via twist1 in prostate cancer cells. Cancer Res. 2012;72:5261–5272. doi: 10.1158/0008-5472.CAN-12-0254. [DOI] [PubMed] [Google Scholar]
- 59.Barron D.A., Rowley D.R. The reactive stroma microenvironment and prostate cancer progression. Endocr Relat Cancer. 2012;19:R187–R204. doi: 10.1530/ERC-12-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ting H.J., Deep G., Jain A.K., Cimic A., Sirintrapun J., Romero L.M. Silibinin prevents prostate cancer cell-mediated differentiation of naive fibroblasts into cancer-associated fibroblast phenotype by targeting TGF beta2. Mol Carcinog. 2014 doi: 10.1002/mc.22135. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Epstein J.I. Diagnosis and reporting of limited adenocarcinoma of the prostate on needle biopsy. Mod Pathol. 2004;17:307–315. doi: 10.1038/modpathol.3800050. [DOI] [PubMed] [Google Scholar]
- 62.Mirzoeva S., Franzen C.A., Pelling J.C. Apigenin inhibits TGF-beta-induced VEGF expression in human prostate carcinoma cells via a Smad2/3- and Src-dependent mechanism. Mol Carcinog. 2014;53:598–609. doi: 10.1002/mc.22005. [DOI] [PubMed] [Google Scholar]
- 63.Bhowmick N.A., Chytil A., Plieth D., Gorska A.E., Dumont N., Shappell S. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–851. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- 64.Bartholin L., Cyprian F.S., Vincent D., Garcia C.N., Martel S., Horvat B. Generation of mice with conditionally activated transforming growth factor beta signaling through the TbetaRI/ALK5 receptor. Genesis. 2008;46:724–731. doi: 10.1002/dvg.20425. [DOI] [PubMed] [Google Scholar]
- 65.Marie J.C., Liggitt D., Rudensky A.Y. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-beta receptor. Immunity. 2006;25:441–454. doi: 10.1016/j.immuni.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 66.Donkor M.K., Sarkar A., Savage P.A., Franklin R.A., Johnson L.K., Jungbluth A.A. T cell surveillance of oncogene-induced prostate cancer is impeded by T cell-derived TGF-beta1 cytokine. Immunity. 2011;35:123–134. doi: 10.1016/j.immuni.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fontenot J.D., Gavin M.A., Rudensky A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 68.Chen W., Jin W., Hardegen N., Lei K.J., Li L., Marinos N. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang Q., Jang T.L., Yang X., Park I., Meyer R.E., Kundu S. Infiltration of tumor-reactive transforming growth factor-beta insensitive CD8+ T cells into the tumor parenchyma is associated with apoptosis and rejection of tumor cells. Prostate. 2006;66:235–247. doi: 10.1002/pros.20340. [DOI] [PubMed] [Google Scholar]
- 70.Hensley P.J., Kyprianou N. Modeling prostate cancer in mice: limitations and opportunities. J Androl. 2012;33:133–144. doi: 10.2164/jandrol.111.013987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li X., Sterling J.A., Fan K.H., Vessella R.L., Shyr Y., Hayward S.W. Loss of TGF-beta responsiveness in prostate stromal cells alters chemokine levels and facilitates the development of mixed osteoblastic/osteolytic bone lesions. Mol Cancer Res. 2012;10:494–503. doi: 10.1158/1541-7786.MCR-11-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thompson T.C., Truong L.D., Timme T.L., Kadmon D., McCune B.K., Flanders K.C. Transgenic models for the study of prostate cancer. Cancer. 1993;71(3 Suppl):1165–1171. doi: 10.1002/1097-0142(19930201)71:3+<1165::aid-cncr2820711440>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 73.Ding Z., Wu C.J., Chu G.C., Xiao Y., Ho D., Zhang J. SMAD4-dependent barrier constrains prostate cancer growth and metastatic progression. Nature. 2011;470:269–273. doi: 10.1038/nature09677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Flavell R.A., Sanjabi S., Wrzesinski S.H., Licona-Limón P. The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol. 2010;10:554–567. doi: 10.1038/nri2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Calve-Aller E. First human dose escalation study in patients with metastatic malignancies to determine safety and pharmacokinetics of LY2157299, a small molecule inhibitor of the transforming growth factor-β receptor I kinase. ASCO Annu Meet J Clin Oncol. 2008;26:14554. Abstr. [Google Scholar]
- 76.Trachtman H., Fervenza F.C., Gipson D.S., Heering P., Jayne D.R., Peters H. A phase 1, single-dose study of fresolimumab, an anti-TGF-beta antibody, in treatment-resistant primary focal segmental glomerulosclerosis. Kidney Int. 2011;79:1236–1243. doi: 10.1038/ki.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Katragadda L., Carter B.Z., Borthakur G. XIAP antisense therapy with AEG 35156 in acute myeloid leukemia. Expert Opin Investig Drugs. 2013;22:663–670. doi: 10.1517/13543784.2013.789498. [DOI] [PubMed] [Google Scholar]
- 78.Muraoka R.S., Dumont N., Ritter C.A., Dugger T.C., Brantley D.M., Chen J. Blockade of TGF-beta inhibits mammary tumor cell viability, migration, and metastases. J Clin Invest. 2002;109:1551–1559. doi: 10.1172/JCI15234. [DOI] [PMC free article] [PubMed] [Google Scholar]