Abstract
INTRODUCTION
We previously demonstrated that atorvastatin up-regulates proangiogenic proteins and increases arteriolar density in ischemic myocardium. Despite this, there was a lack of collateral-dependent perfusion, possibly related to apoptosis. We utilized a swine model of metabolic syndrome and chronic myocardial ischemia to investigate the effects of atorvastatin on apoptosis.
MATERIALS AND METHODS
Sixteen Ossabaw miniswine were fed a high-cholesterol diet for fourteen weeks then underwent surgical placement of an ameroid constrictor to their circumflex artery inducing chronic ischemia. Eight pigs received supplemental Atorvastatin (1.5 mg/kg daily). Myocardium was harvested six months later for western blotting and TUNEL staining.
RESULTS
Animals supplemented with atorvastatin had significant increases in markers associated with apoptosis including p-38, BAX, and caspase 3 (p< 0.05). Atorvastatin supplementation also resulted in significant increases in expression of cell survival proteins Bcl-2 and P-ERK and an overall decrease in apoptosis demonstrated by TUNEL staining (p< 0.05).
CONCLUSIONS
Atorvastatin acts on multiple pathways and its effects on angiogenesis remain unclear. Although there is increased expression in several markers of apoptosis, key anti-apoptotic proteins were also up-regulated with an overall decrease in apoptosis. Further investigation of these pathways may provide insight into the role of statins on myocardial protection after ischemia.
Keywords: Statins, Apoptosis, Myocardial ischemia, Metabolic syndrome, Angiogenesis
Introduction
3-Hydroxyl-3-methylglutarl coenzyme A reductase inhibitors (statins) are commonly used medications in patients with coronary artery disease (CAD). Numerous studies have demonstrated that statin therapy prior to high-risk procedures reduces the risk of post-operative myocardial infarction and mortality (Guay & Ochroch, 2013, Lazar, 2003 #41; Kulik & Ruel, 2011). These medications are highly effective at reducing low-density lipoprotein (LDL) cholesterol, though it has become increasingly evident that the cardioprotective effects of statins are not fully explained by this benefit alone {Fonarow, 2005 #33;Wang, 2008 #3, Beckman, 2006 #4}. The proposed non-lipid pleotropic effects of statins are vast and include decreased inflammation, regulation of platelet function, and improved angiogenesis (Kinlay et al., 2003; Wang, Liu, & Liao, 2008). Though not fully understood, statin-mediated angiogenesis is likely related to improved endothelial cell survival by increasing proangiogenic proteins and inhibiting apoptosis (Wood, Igbavboa, Muller, & Eckert, 2013) (Sata, 2002; Zaitone & Abo-Gresha, 2012). We previously demonstrated that atorvastatin supplementation increases proangiogenic proteins and increased capillary and arteriolar density in ischemic myocardium in a swine model with metabolic syndrome. Despite this there was a lack of collateral-dependent perfusion and evidence of increased oxidative stress (Elmadhun et al., 2012). We hypothesized that in addition to increasing oxidative stress, atorvastatin supplementation would increase apoptosis in the ischemic myocardium and that this may contribute to the lack of improvement in myocardial perfusion. We utilized a clinically relevant swine model of metabolic syndrome and chronic myocardial ischemia to further investigate the effects of atorvastatin on apoptosis and cell survival.
Materials and Methods
Animal Model and Surgical Interventions
Ossabaw miniswine (Purdue Ossabaw Facility, Indiana University, Indianapolis, IN) were divided into two groups based on daily diet fed over a fourteen-week period. The high cholesterol control group ([OHC], n=8; initial average weight: 23.1 ± 4.5 kg) were given daily feedings of 500 g of a high cholesterol diet consisting of 75 % regular chow, 4% cholesterol, 17.2 % coconut oil, 2.3% corn oil, 1.5% sodium cholate (Sinclair Research, Columbia, MO). Animals in the experimental group were fed the same diet as the OHC animals but were also supplemented with daily oral Atorvastatain (1.5 mg/kg/d; [OHCS], n=8; initial average weight: 21.2 ± 3.5 kg) (Pfizer Inc., New York, NY). Feedings were observed to confirm complete consumption of chow and supplement. After fourteen weeks animals underwent surgical placement of a titanium ameroid constrictor (Research Instruments SW, Escondito, CA) on the proximal left circumflex coronary artery (LCx). Six months after ameroid placement the animals were euthanized and their hearts were harvested. The timeline for giving the high calorie, high cholesterol diet was based on studies from our group and others which have demonstrated that Ossabaw miniwine given this diet for six months reliably develop metabolic syndrome (Lee et al., 2009) (Lassaletta et al., 2012). Tissue samples from the non-ischemic and chronically ischemic myocardium (LCx territory) were collected and rapidly frozen in liquid nitrogen. The methods for anesthesia and surgical interventions have been previously described (Elmadhun et al., 2012). All experiments were approved by the Institutional Animal Care and Use Committee of the Rhode Island Hospital. Animals were cared for in compliance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals.
Myocardial Perfusion and Microvessel Reactivity
The methods for myocardial perfusion and microvessel reactivity have been previously described (Elmadhun et al., 2012).
Protein Expression
Methods for protein lysate preparation, western blotting, image capture and band quantification have been previously described (Sabe, Elmadhun, Robich, Dalal, & Sellke, 2013). Protein lysates transferred to polyyvinylidene fluoride membranes were incubated overnight in primary antibodies at dilutions recommend by the manufacturer. Primary antibodies used were heat shock protein 90 (HSP 90), apoptosis inducing factor (AIF), tumor necrosis factor alpha (TNF alpha), extra cellular signal-regulated kinase (ERK), phosphorylated ERK (P-ERK), p38 mitogen-activated protein kinase (p38), phosphorylated p38 (P-p38), phosphorylated nuclear factor kappa-light-chain-enhancer of activated B cells (P-NFK Beta), B-cell lymphoma 2 (Bcl-2), Bcl-2-associated X (BAX), Bcl-2-associated death promotor (BAD), caspase 3, caspase 9, and caspase 12 (all from Cell Signaling, Danvers, MA). All membranes were probed with glyceraldehyde-3-phophate dehydrogenase (Cell Signaling) to correct for loading error.
TUNEL Assay
Frozen sections of myocardium were fixed in formalin and DNA breaks were identified according to the manufacturer’s specifications using the ApoTag ISOL immunofluorescence kit (EMD Millipore, Billerica, MA) for Terminal deoxycnucleotidyl transferase dUTP nick end labeling (TUNEL). Sections were mounted in Vectashield (Vector Laboratories, Inc., Burlingame, CA) with 4′,6-diamidino-2-phenylindole (DAPI). Three 10× images from each section were taken in sequence and TUNEL stained nuclei were counted. The results of the three images were averaged and expressed as nuclei per high-powered field.
Data Analysis
All results are expressed as fold change ± standard deviation, or standard error of the mean, as compared to OHC. Probability values (P-values) are reported from a t-test and were considered significant if less than 0.05.
Results
Animal Model
Animals included for analysis survived to completion of the study. As previously described in animals supplemented with atorvastatin there was a significant increase in capillary density (p= 0.02) and arteriolar density (p= 0.003) (Elmadhun et al., 2012). Despite this there were no significant differences in myocardial perfusion or coronary microvessel reactivity at rest or with demand pacing (Elmadhun et al., 2012).
Western Blotting
In chronically ischemic myocardium atorvastatin supplementation resulted in significant up-regulation in several markers associated with apoptotic cell death including p-38, BAX, and caspase 3 (p< 0.05; Table 1, Figure 1). Interestingly, atorvastatin supplementation also resulted in a significant increase in expression of cell survival associated proteins BCL-2 and P-ERK (p< 0.05; Table 1, Figure 1). In the non-ischemic myocardium atorvastatin supplementation resulted in a significant up-regulation in cell survival protein BCL-2 with a significant down-regulation in TNF alpha (Table 2).
Table 1.
Protein Expression in Ischemic Myocardium
| Targets | OHC | OHCS | p value |
|---|---|---|---|
| HSP 90 | 1 ± 0.156 | 1.19 ± 0.141 | 0.37 |
| AIF | 1 ± 0.092 | 0.98 ± 0.117 | 0.90 |
| TNF alpha | 1 ± 0.134 | 1.09 ± 0.303 | 0.78 |
| p38 | 1 ± 0.082 | 1.99 ± 0.282 | 0.0045* |
| P-p38 | 1 ± 0.036 | 0.85 ± 0.110 | 0.19 |
| ERK | 1 ± 0.103 | 2.15 ± 0.515 | 0.061 |
| P-ERK | 1 ± 0.174 | 2.45 ± 0.343 | 0.0018* |
| P-NFK Beta | 1 ± 0.206 | 0.88 ± 0.136 | 0.62 |
| BCL 2 | 1 ± 0.042 | 1.30 ± 0.086 | 0.0078* |
| BAX | 1 ± 0.133 | 3.45 ± 0.333 | <0.0001* |
| BAD | 1 ± 0.091 | 1.27 ± 0.106 | 0.075 |
| Caspase 3 | 1 ± 0.046 | 1.29 ± 0.053 | 0.0011* |
| Caspase 9 | 1 ± 0.145 | 1.53 ± 0.239 | 0.091 |
| Caspase 12 | 1 ± 0.127 | 1.42 ± 0.152 | 0.050 |
Protein expression listed as fold change ± SEM compared to OC.
indicates significant difference between OHC and OHCS group (P value < 0.05). P values determined from a t-test.
Figure 1. Protein Expression in Ischemic Myocardium.
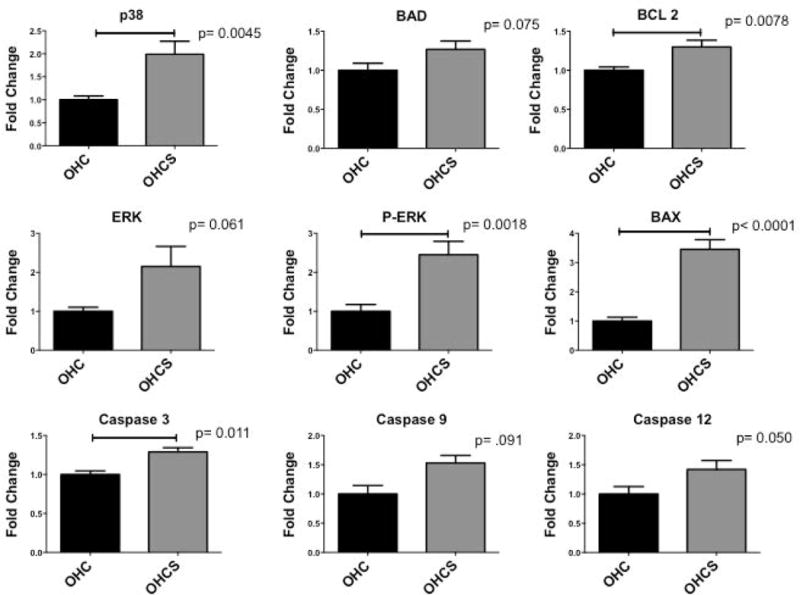
Fold change ± SEM compared to OHC. P values determined from a t-test.
Table 2.
Protein Expression in Non-Ischemic Myocardium
| Targets | OHC | OHCS | p value |
|---|---|---|---|
| HSP 90 | 1 ± 0.107 | 1.32 ± 0.121 | 0.07 |
| AIF | 1 ± 0.104 | 1.10 ± 0.140 | 0.56 |
| TNF alpha | 1 ± 0.099 | 0.33 ± 0.103 | 0.0004* |
| p38 | 1 ± 0.216 | 0.947 ± 0.172 | 0.86 |
| P-p38 | 1 ± 0.147 | 0.78 ± 0.121 | 0.26 |
| ERK | 1 ± 0.060 | 1.19 ± 0.081 | 0.079 |
| P-ERK | 1 ± 0.238 | 0.59 ± 0.106 | 0.14 |
| P-NFK Beta | ND | ND | ND |
| BCL 2 | 1 ± 0.138 | 1.80 ± 0.236 | 0.02* |
| BAX | 1 ± 0.073 | 1.25 ± 0.118 | 0.10 |
| BAD | 1 ± 0.084 | 1.36 ± 0.204 | 0.41 |
| Caspase 3 | ND | ND | ND |
| Caspase 9 | ND | ND | ND |
| Caspase 12 | 1 ± 0.182 | 0.723 ± 0.123 | 0.28 |
Protein expression listed as fold change ± SEM compared to OC.
indicates significant difference between OHC and OHCS group (P value < 0.05). P values determined from a t-test. ND: Not Detected - indicates protein levels not detected with western blotting using similar assay as performed in ischemic myocardium.
Apoptosis
In ischemic myocardium, apoptosis as assessed by TUNEL staining was significantly decreased in the OHCS group compared with the OHC group (p= 0.01; Figure 2).
Figure 2.
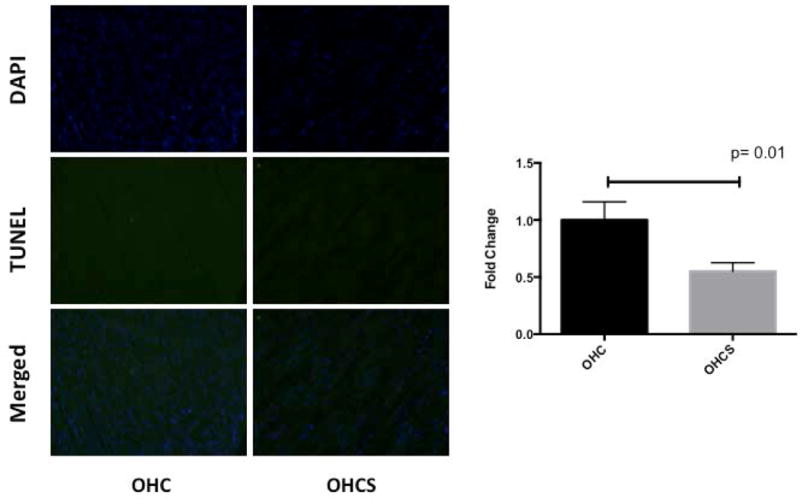
TUNEL staining in chronically ischemic myocardium
Discussion
In a swine model with metabolic syndrome and chronic myocardial ischemia we demonstrate that atorvastatin significantly regulates apoptosis. We had hypothesized that atorvastatin supplementation would increase apoptosis and may be one mechanism to explain the lack of improvement in collateral myocardial perfusion. In the ischemic myocardium, though atorvastatin supplementation significantly increased the expression of several protein markers associated with apoptotic cell death, there was a concomitant increase in markers associated with cell survival and overall there was actually a trend towards decreased apoptosis as demonstrated by TUNEL staining. These findings suggest that atorvastatin results in complex regulation of apoptosis, ultimately down-regulating this cellular process in the setting of chronic ischemia and metabolic syndrome.
Inhibition of apoptosis has been shown to facilitate angiogenesis, though the underlying mechanism remains under investigation. Postulated mechanisms include inhibition of pro-apoptotic proteins versus increasing proteins associated with cell survival, or more likely a combination of the two. The effects of statins on angiogenesis appear to be biphasic and dose dependent. At a low dose statins have been shown to promote angiogenesis and endothelial cell survival by increasing pro-angiogenic proteins and inhibiting apoptosis, whereas at higher doses these effects are often reversed. Through inhibition of HMG-coA reductase, statins decrease mevalonate metabolism resulting in an increase in LDL receptors and removal of LDL from the blood. However, mevalonate also regulates numerous other cellular processes. Mevalonate acts as a precursor for two isoprenoids, farnesyl pyrophosphate (FFP) and geranylgeranyl pyrophosphate (GPP). The anti-angiogenic effect of high-dose statins is thought to involve the reduction of these isopreniods and results in prenylation, or post-translational modification, of multiple proteins including Rho and Ras. Rho and Ras dysfunction have been shown to increase apoptosis. Our group and other have attempted to better understand the differential effects of statins on these pathways (Boodhwani, Mieno, et al., 2006; Boodhwani, Nakai, et al., 2006; Boodhwani et al., 2008; Elmadhun et al., 2012; Sabe et al., 2014, Beckman, 2006 #4).
With our current model, we previously reported that atorvastatin supplementation at 1.5 mg/kg/d increased proangiogenic markers, including VEGF, and resulted in increased capillary cell and arteriolar density without improvement in collateral perfusion or myocardial fibrosis (Elmadhun et al., 2012). In our current study, statin administration resulted in a significant upregulation of Bcl-2 in ischemic and non-ischemic myocardium. There is an overall decrease in apoptosis with atorvastatin supplementation as demonstrated by TUNEL staining, despite the increase in proapoptotic proteins caspase 3, caspase 9, and BAX in the ischemic myocardium. This may be partially explained by the upregulation of anti-apoptotic proteins like Bcl-2 and phosphorylated ERK, as these proteins have been shown to halt the progression and culmination of caspase-induced apoptosis (Nikoletopoulou, Markaki, Palikaras, & Tavernarakis, 2013; Wood et al., 2013). In a similar swine model with metabolic syndrome Boodhwani et al demonstrated that atorvastatin supplemented at a higher dose (3 mg/kg/d) also resulted in improvements in endothelial dysfunction without enhancing angiogenesis. However, at this higher dose of atorvastatin there was a down-regulation in the activated form of the anti-apoptotic protein Bcl-2 (Boodhwani, Nakai, et al., 2006). As higher doses of statins are thought to be anti-angiogenic, this differential regulation of Bcl-2 provides interesting mechanistic insights into the varying effects of statins. More specifically, the differential effects of statins on Bcl-2 may represent a key regulatory point in preventing caspase-induced apoptosis in myocardium.
Interestingly, our currents study also demonstrated that in the non-ischemic myocardium in addition to an up-regulation of Bcl-2, there was a significant down-regulation of TNF alpha with atorvastatin supplementation. Statins have been shown in preclinical and clinical studies to reduce markers of inflammation (Blanco-Colio, Tunon, Martin-Ventura, & Egido, 2003; Kinlay et al., 2003). Simvastatin and atorvastatin have been shown in vitro to reduce levels of TNF alpha and inhibit some of its downstream effects by regulating transcription factors downstream of mevalonate (Henrich, Seebach, Wilhelm, & Marzi, 2007; Methe, Kim, Kofler, Nabauer, & Weis, 2005). Alteration of these transcription factors has been also shown to prevent cytokine-mediated apoptosis(Matsumoto, Einhaus, Gold, & Aderem, 2004). In our study however, levels of TNF alpha in the ischemic myocardium were not significantly altered with atorvastatin supplementation. One hypothetical reason for this is that in the ischemic myocardium there may be an overwhelming increase of general inflammation and cytokine release, such that any regulation of TNF alpha by atorvastatin treatment is comparably insignificant.
Limitations
There are several important limitations to this study. Although atorvastatin is a commonly prescribed statin, several different statins are currently widely prescribed, each with different lipophilicity and bioavailabilty. Thus, the results of this study may differ depending on the statin given. Though we have performed similar studies using higher dose of statins, in this study the dose of statin was fixed for all animals. Again, this is particularly important to note given that our group and several others have demonstrated the differential effects of statins at various doses. It is also important to note that the tissue used for analysis in this study was harvested at a single time-point. This is an important limitation when studying dynamic pathways like apoptosis and cell survival. Indeed, further studies elucidating the short- and long-term effects of atorvastatin will be important in further understanding mechanisms of this drug. Also, it is important to mention the limitations of measuring apoptosis in the heart. Although TUNEL staining has been widely used as a direct measure of apoptosis, the specificity of this method has been called into question especially in the setting of acute ischemia and reperfusion injury. In the setting of acute ischemic injury TUNEL staining is not specific in distinguishing necrosis from apoptosis, though this should be less of an issue in our chronic model of ischemia. However, in chronic heart disease there is a paucity of apoptotic cells, which may limit TUNEL staining especially when false-positives occur. Therefore, TUNEL staining alone is not sufficient to evaluate the extent of apoptosis in cardiac tissue, instead it is important to also evaluate other markers of apoptosis and anti-apoptosis in combination with TUNEL staining, as we have done (Kang & Izumo, 2000).
Conclusions
In ischemic myocardium, although there is evidence of increased expression in several markers of apoptotic cell death, key markers related to cell survival were also up-regulated. Further investigation of these pathways may provide insight into the role of statins on angiogenesis and myocardial protection after ischemia. These findings are especially relevant in cardiac surgery, and may offer additional insight into mechanisms underlying protective effects of statins in coronary revascularization. Specifically, the mechanisms by which statins regulate cell survival proteins, like Bcl-2, should be investigated not only as relevant to apoptosis, but also with respect to overlapping pathways. Perhaps the differential regulation of Bcl-2 by statins is critical in the prevention of apoptosis and the essential up regulation of capillaries and arterioles essential in the process of angiogenesis. Given the widespread use of statins, the future direction of these studies should ultimately be focused in a clinical setting. Future proteomic and genomic analysis can be employed to help delineate differential expression of proteins and genes in patients who take statins and those who do not, and may allow us to more specifically delineate how statins affect patient outcomes.
Acknowledgments
Funding: Funding for this research was provided by the National Heart, Lung, and Blood Institute (R01HL46716 and R01HL69024 Dr. Sellke), NIH Training grant 5T32-HL094300-03, (Dr. Sabe Dr. Elmadhun, and Dr. Chu).
Footnotes
All authors listed have contributed to the conception and design, analysis and interpretation, data collection, writing/critical revision of this manuscript.
Disclosures: Dr. Sellke is a consultant for CSL Behring
References
- Blanco-Colio LM, Tunon J, Martin-Ventura JL, et al. Anti-inflammatory and immunomodulatory effects of statins. Kidney international. 2003;63:12–23. doi: 10.1046/j.1523-1755.2003.00744.x. [DOI] [PubMed] [Google Scholar]
- Boodhwani M, Mieno S, Voisine P, et al. High-dose atorvastatin is associated with impaired myocardial angiogenesis in response to vascular endothelial growth factor in hypercholesterolemic swine. The Journal of thoracic and cardiovascular surgery. 2006;132:1299–1306. doi: 10.1016/j.jtcvs.2006.05.060. [DOI] [PubMed] [Google Scholar]
- Boodhwani M, Nakai Y, Voisine P, et al. High-dose atorvastatin improves hypercholesterolemic coronary endothelial dysfunction without improving the angiogenic response. Circulation. 2006;114:I402–408. doi: 10.1161/CIRCULATIONAHA.105.000356. [DOI] [PubMed] [Google Scholar]
- Boodhwani M, Mieno S, Feng J, et al. Atorvastatin impairs the myocardial angiogenic response to chronic ischemia in normocholesterolemic swine. The Journal of thoracic and cardiovascular surgery. 2008;135:117–122. doi: 10.1016/j.jtcvs.2007.04.021. [DOI] [PubMed] [Google Scholar]
- Elmadhun NY, Lassaletta AD, Chu LM, et al. Atorvastatin increases oxidative stress and modulates angiogenesis in Ossabaw swine with the metabolic syndrome. The Journal of thoracic and cardiovascular surgery. 2012;144:1486–1493. doi: 10.1016/j.jtcvs.2012.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guay J, Ochroch EA. Effects of Adding Statins Before Surgery on Mortality and Major Morbidity: A Meta-analysis. Journal of cardiothoracic and vascular anesthesia. 2013 doi: 10.1053/j.jvca.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Henrich D, Seebach C, Wilhelm K, et al. High dosage of simvastatin reduces TNF-alpha-induced apoptosis of endothelial progenitor cells but fails to prevent apoptosis induced by IL-1beta in vitro. The Journal of surgical research. 2007;142:13–19. doi: 10.1016/j.jss.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Kang PM, Izumo S. Apoptosis and heart failure: A critical review of the literature. Circulation research. 2000;86:1107–1113. doi: 10.1161/01.res.86.11.1107. [DOI] [PubMed] [Google Scholar]
- Kinlay S, Schwartz GG, Olsson AG, et al. High-dose atorvastatin enhances the decline in inflammatory markers in patients with acute coronary syndromes in the MIRACL study. Circulation. 2003;108:1560–1566. doi: 10.1161/01.CIR.0000091404.09558.AF. [DOI] [PubMed] [Google Scholar]
- Kulik A, Ruel M. Lipid-lowering therapy and coronary artery bypass graft surgery: what are the benefits? Current opinion in cardiology. 2011;26:508–517. doi: 10.1097/HCO.0b013e32834b9fb1. [DOI] [PubMed] [Google Scholar]
- Lassaletta AD, Chu LM, Robich MP, et al. Overfed Ossabaw swine with early stage metabolic syndrome have normal coronary collateral development in response to chronic ischemia. Basic research in cardiology. 2012;107:243. doi: 10.1007/s00395-012-0243-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L, Alloosh M, Saxena R, et al. Nutritional model of steatohepatitis and metabolic syndrome in the Ossabaw miniature swine. Hepatology. 2009;50:56–67. doi: 10.1002/hep.22904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Einhaus D, Gold ES, et al. Simvastatin augments lipopolysaccharide-induced proinflammatory responses in macrophages by differential regulation of the c-Fos and c-Jun transcription factors. Journal of immunology. 2004;172:7377–7384. doi: 10.4049/jimmunol.172.12.7377. [DOI] [PubMed] [Google Scholar]
- Methe H, Kim JO, Kofler S, et al. Statins decrease Toll-like receptor 4 expression and downstream signaling in human CD14+ monocytes. Arteriosclerosis, thrombosis, and vascular biology. 2005;25:1439–1445. doi: 10.1161/01.ATV.0000168410.44722.86. [DOI] [PubMed] [Google Scholar]
- Nikoletopoulou V, Markaki M, Palikaras K, et al. Crosstalk between apoptosis, necrosis and autophagy. Biochimica et biophysica acta. 2013;1833:3448–3459. doi: 10.1016/j.bbamcr.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Sabe AA, Elmadhun NY, Robich MP, et al. Does resveratrol improve insulin signaling in chronically ischemic myocardium? The Journal of surgical research. 2013;183:531–536. doi: 10.1016/j.jss.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabe AA, Elmadhun NY, Sadek AA, et al. Differential effects of atorvastatin on autophagy in ischemic and nonischemic myocardium in Ossabaw swine with metabolic syndrome. The Journal of thoracic and cardiovascular surgery. 2014 doi: 10.1016/j.jtcvs.2014.07.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sata M. Biphasic effects of statins on angiogenesis. Circulation. 2002;106:e47. doi: 10.1161/01.cir.0000030081.54465.2d. author reply e47. [DOI] [PubMed] [Google Scholar]
- Wang CY, Liu PY, Liao JK. Pleiotropic effects of statin therapy: molecular mechanisms and clinical results. Trends in molecular medicine. 2008;14:37–44. doi: 10.1016/j.molmed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood WG, Igbavboa U, Muller WE, et al. Statins, Bcl-2, and apoptosis: cell death or cell protection? Molecular neurobiology. 2013;48:308–314. doi: 10.1007/s12035-013-8496-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitone SA, Abo-Gresha NM. Rosuvastatin promotes angiogenesis and reverses isoproterenol-induced acute myocardial infarction in rats: role of iNOS and VEGF. European journal of pharmacology. 2012;691:134–142. doi: 10.1016/j.ejphar.2012.06.022. [DOI] [PubMed] [Google Scholar]


