Abstract
As the global population ages, older decision makers will be required to take greater responsibility for their own physical, psychological and financial well-being. With this in mind, researchers have begun to examine the effects of ageing on decision making and associated neural circuits. A new “affect, integration, motivation” (or AIM) framework may help clarify how affective and motivational circuits support decision making. Recent research has shed light on whether and how ageing influences these circuits, providing an interdisciplinary account of how ageing can alter decision making.
The global population is rapidly ageing. Projections suggest that the proportion of older individuals (those over 65) will double from 2000 to 2050 1, increasing the relative number of older decision makers in society, particularly in developed countries 2. Alongside these demographic shifts, governments and businesses have begun to implement policy changes that will increase older individuals’ responsibility for maintaining their own health and financial welfare.
Although research has begun to suggest that ageing might alter decision making, little is known about the trajectory or causes of these changes. An emerging interdisciplinary field that combines methods and theories from neuroscience, psychology and economics could bridge these gaps in knowledge, and so inform scientific theory as well as speed the translation of research findings into applications.
Both neurally and psychologically, multiple factors contribute to decision making. By connecting sensory input to motor output, both cognitive and affective capacities play critical roles in decision making, especially when individuals must weigh potential benefits against costs. In terms of cognition, accumulated evidence suggests that although ageing can compromise some cognitive capacities, others are preserved. Specifically, fluid cognitive abilities (such as working memory, attention, and executive control) steadily decline with ageing, whereas crystallized cognitive abilities (such as domain-specific knowledge) remain conserved 3. Thus older people may fare worse when making decisions that require fluid cognitive abilities (that is, choices that require multiple attributes and/or options to be simultaneously considered and compared).
More recent evidence has also revealed different influences of ageing on affect and motivation. Adults report reduced levels of negative affective experience as they age but preserved levels of positive affective experience 4, trends that have been linked to decreased attention to and memory for negative versus positive material 5. These changes may result either from shifts in motivational goals (which may stem from changing perceptions of remaining time, for example) 5, or from independent physiological changes in neural function, or both. Overall, this evidence implies that older adults might weigh costs and benefits differently than their younger counterparts 6.
Although researchers have made significant progress over the past few decades toward characterizing the impact of healthy ageing on the brain (BOX 1), only recently have they begun to examine how age-related changes in neural structure, chemistry and function influence decision making. Decision making has the potential to recruit almost any brain capacity and thus presents a broad and challenging research target. Different types of decisions may also recruit distinct circuits, either sequentially or in parallel. In this article, we primarily focus on decisions that involve affective and motivational processing 7–12, rather than those that rely upon sensorimotor processing (in which deficits should obviously compromise decisions) or cognitive processing (which has been reviewed elsewhere) 3. After describing a framework that can link neural activity in circuits implicated in affect and motivation to decision making, we review emerging neuroscientific findings that shed light on age-related changes in value-based decision making13.
Box 1. Age-related changes in brain structure and function.
Neuroimaging studies have documented numerous changes in the structure and function of the ageing brain 41,42,118. Generally, both cross-sectional and longitudinal studies of brain volume reveal linear and curvilinear changes in grey matter volume, as well as curvilinear changes in white matter volume over the life span. These volumes increase until adulthood and then steadily decrease with senescence 118–120. More recent studies using diffusion tensor imaging (DTI) have revealed age-related decreases in the connectivity of the major white matter tracts, with the most pronounced declines occurring in anterior and superior cortical regions 121,122. Studies of neurochemical changes across the life span have focused primarily on changes in the neurons releasing biogenic amine neurotransmitters (such as dopamine and serotonin), which emanate from focal nuclei in the midbrain to project broadly throughout the subcortex and cortex. Positron emission tomography (PET) studies in humans have revealed relatively linear declines across adulthood in serotonin receptors in cortex, in dopamine receptors (both D1-like and D2-like) in prefrontal cortex and striatum, and in dopamine transporters in striatum, but have yielded more mixed evidence for age-related changes in the presynaptic neurotransmitter availability (related to synthesis capacity and vesicular storage) 123,124. These PET imaging findings were mostly obtained in cross-sectional samples and so are limited by a lack of longitudinal data. Even less research has examined age-related changes in the availability of basic amino acid neurotransmitters (such as glutamate and GABA) that, although present throughout the brain, support more local and targeted circuit functions 125–127. Overall, research that links and integrates age-related changes in brain function, structure, and chemistry (such as multimodal neuroimaging studies 128) could help neuroscientists to pinpoint the circuits most compromised by ageing.
Neural circuits that promote choice
Beyond merely arising in reaction to the outcomes of decisions, mounting evidence suggests that affect can also proactively influence decision making 14,15. Throughout the twentieth century, affect was indirectly inferred from measures of self-reported experience, physiology, or nonverbal behaviour. Consistent with the predictions of pioneering psychologist Wilhelm Wundt 16, psychometric research revealed that emotional experience can be described using two independent dimensions – subjective valence and subjective arousal 17. Positive arousal might increase the motivation to approach opportunities, whereas negative arousal might increase the motivation to avoid threats 18,19. If positive and negative arousal reflect ongoing activity of independent mechanisms, additional mechanisms might integrate their influences to promote the next appropriate behavioural response. These elements – affect, integration, and motivation – form core components of the skeletal framework for neural circuits that promote choice proposed below.
Functional, chemical, and structural circuit characteristics
Neuroimaging methods with enhanced spatial, temporal, and depth resolution (such as FMRI) have enabled investigators to track activity in neural circuits that support affect and motivation. Resulting evidence suggested that positive subjective arousal correlates with nucleus accumbens (NAcc) activity, whereas negative subjective arousal correlates with anterior insula (AI; and possibly reduced NAcc) activity 20. When affect motivates behaviour, these findings imply that during consideration of choices, NAcc activity should promote approach behaviour, whereas AI activity should promote avoidance behaviour 21–23. Indeed, these predictions hold across diverse choice scenarios 24. Activity in these circuits also precedes social approach and avoidance: NAcc activity predicts cooperation whereas AI activity predicts defection in dyadic interactions with strangers 25.
Although approach and avoidance responses might suffice to drive simple choices, more complex value assessments require integration of these basic tendencies with other considerations (such as the potential likelihood, length of waiting time, or effort required to obtain something). Researchers found evidence that the medial prefrontal cortex (MPFC) played a prominent role in value integration in situations in which attributes both within and across choice options must be integrated 21,26–29. Currently, several meta-analyses of functional neuroimaging studies have implicated NAcc, AI, and MPFC activity in affect and choice 24,30–33. To motivate behaviour, these components must then activate circuits that prepare motor output, including the dorsal striatum and premotor cortical regions 34. FMRI evidence indicates that activity in all of these circuits can both precede and predict choice. These combined components therefore represent promising candidates as neural circuits that promote choice (FIG. 1).
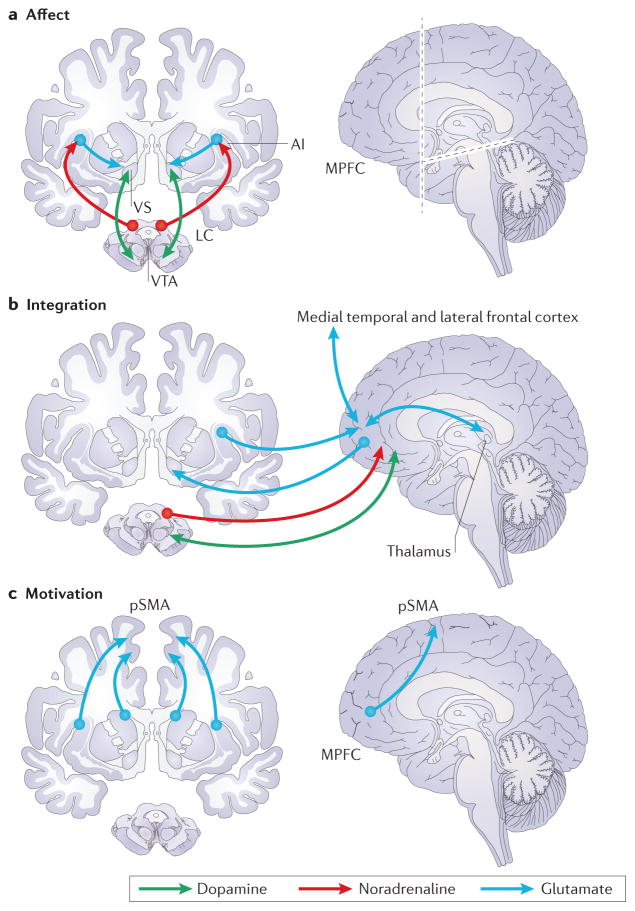
Figure 1. Critical components of the Affect-Integration-Motivation (AIM) framework.
The AIM framework implies that three hierarchical and sequential processes can occur prior to and promote choice. a| Affect, in which ventral tegmental area (VTA) dopamine neurons project to the ventral striatum (VS, including the NAcc), locus coeruleus (LC) norepinephrine neurons project to the AI (AI), and AI glutamatergic neurons (blue) project to the VS, potentiating anticipation of gain and loss. b| Integration, in which VTA dopamine neurons and LC norepinephrine neurons modulate medial prefrontal cortex (MPFC) activity, VS indirectly projects to the MPFC via GABA connections in the pallidum (not depicted) and glutamate projections from the thalamus, AI projects to the MPFC, and MPFC glutamatergic neurons directly project back to the VS, facilitating integration of value and modulatory signals (for instance, from the medial temporal and lateral frontal cortical regions). c| Motivation, in which dorsal striatal and insular glutamatergic neurons project to the pre-supplementary motor area (pSMA), potentiating motor action. Healthy ageing is predicted to degrade glutamatergic projections from the prefrontal cortex to the striatum, thus diminishing value integration (b), and compromising choice optimality.
Chemically, both dopaminergic and noradrenergic neurons broadly but differentially innervate these regions, and can rapidly shift their firing rates in response to environmental opportunities and challenges. Specifically, the NAcc receives dense dopaminergic projections from the ventral tegmental area (VTA), but not noradrenergic projections from the locus coeruleus (LC). The MPFC and AI receive both dopaminergic projections from the VTA and noradrenergic projections from the LC. Dopamine reuptake mechanisms, however, primarily reside in the striatum (including the NAcc), enhancing both the release and clearance of synaptic dopamine 35.
Structurally, both primate and human studies of these circuits suggest evolutionarily conserved patterns of connectivity. VTA dopaminergic neurons project through the medial forebrain bundle to the NAcc, which then projects via GABAergic neurons indirectly through the globus pallidus to the medial thalamus. From there, glutamatergic neurons project to the MPFC and then back down to the ventral striatum (including the NAcc and adjacent ventral putamen and medial caudate). The glutamatergic projections from the MPFC to the NAcc are notably unidirectional. This indirect “looping” connectivity of striatal to frontal regions and back continues in an upward spiralling pattern that progresses through the medial caudate and the anterior cingulate to the dorsal caudate and premotor cortex, 36 and is thought to facilitate the conversion of motivation to action 34. Although structural connections between the AI and these regions have not received extensive characterization in primates, the AI does send unidirectional glutamatergic projections to the NAcc 37, and also projects forward to lateral aspects of the prefrontal cortex 37,38, potentially allowing the AI to directly influence NAcc and indirectly influence MPFC activity (FIG. 1).
The Affect-Integration-Motivation (AIM) framework
The findings described above thus converge upon a framework in which affective neural components first anticipate gains (via dopaminergic projections to the NAcc) and losses (via noradrenergic and dopaminergic projections to the AI) that are then integrated with further evaluative considerations (via glutamatergic projections to the MPFC) before feeding into a motivational component that promotes subsequent actions (via glutamatergic projections to the dorsal striatum and supplementary motor area; FIG. 1). When there are multiple attributes or options, additional integration (in the MPFC) or comparison (in the dorsolateral prefrontal cortex (DLPFC)) may be required.
This proposed “Affect-Integration-Motivation” or AIM framework builds upon previous findings and models that have associated some of these components with valuation 26,32,33 by assigning each component different (but connected) functions that begin with affect and end with motivation. Notably, the AIM framework is sequential and hierarchical. Sequentially, activity occurs first in connections among affective components and propagates over time to motivational components. Hierarchically, affective components can operate with variable input from motivational components, but motivational components cannot operate without some input from affective components. The framework specifies a set of minimal and necessary components that precede and predict choice, but retains flexibility to account for choice through different combinations of those components, and also remains open to input from additional components. For instance, for the affective components, information about salient options might enter through an amygdalar-orbitofrontal circuit 39, whereas for the integrative component, information about past memories or rule-based knowledge might enter through a medial temporal lobe-DLPFC circuit.
Although the AIM framework applies generally to decision making, it may also help scaffold specific predictions about how healthy ageing influences decision making. Ageing might globally compromise neural structure and function, which should cause general decline in all of the components and their associated functions. However, behavioural research suggests that ageing does not uniformly change cognition and affect. In terms of affect, if ageing decreases anticipation of losses but not gains, components associated with loss anticipation may show functional and structural declines. Similarly, if ageing compromises integration of value, components associated with value integration may show functional and structural declines. These age-related changes could exert specific effects on choice biases or optimality. In terms of cognition, if ageing compromises fluid but not crystallized cognition (BOX 1 and BOX 2) 40–42, it might diminish the function and structure of the prefrontal cortex and medial temporal lobes, which could impair value integration in complex choice tasks that require more attention and memory.
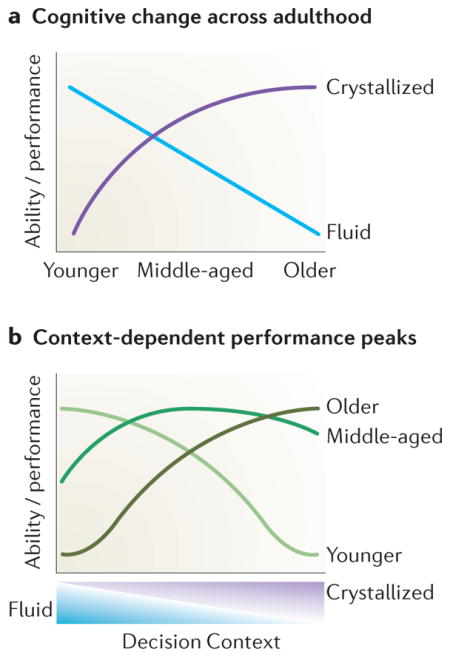
Box 2. Age-related differences in decision performance depend on cognitive demands.
Age-dependent differences in performance in decision making tasks depend heavily on the extent to which decisions make cognitive demands or provide opportunities to draw on prior knowledge 129. Recently, theorists have built upon classic observations of adult age differences in fluid and crystallized cognitive abilities 130 to explain age-related differences in decision performance 97,131,132. Although fluid cognitive ability decreases linearly across adulthood, crystallized ability increases non-linearly and begins to level off in late middle age 131 (see figure, panel a). These changes imply context-dependent differences in decision performance (see figure, panel b). For example, when a decision requires high fluid ability and low crystallized ability, younger adults should outperform middle-aged and older adults. When a decision instead requires low fluid ability and high crystallized ability, however, older adults should outperform middle-aged and younger adults.
This cognitive account predicts that middle-aged adults may make the most optimal decisions across a broad range of contexts, consistent with a recent suggestion that financial reasoning peaks in middle-age 131. Even in contexts in which decisions can be made based almost completely on crystallized cognitive abilities, older adults enjoy only a slight advantage over middle-aged adults. Although initial findings reviewed here and elsewhere 97 are consistent with these accounts, and specifically implicate input during value integration from medial temporal lobe and dorsolateral prefrontal cortex, they require further generalization across different decision contexts. When combined with this cognitive account the AIM framework implies that ageing may compromise performance in scenarios requiring integration of new value information but may enhance or preserve performance in situations that allow greater reliance on previously established value representations.
Changes in decision making with age
A simple but useful theory of optimal decision making states that individuals assess the expected value of options (or the magnitude of each option’s value multiplied by its probability of its occurrence) prior to choice 43,44. By separately considering gain and loss, expected value can be deconstructed as an option’s magnitude multiplied by the probability of potential gain minus the magnitude multiplied by the probability of potential loss. Expected value can further be modified by other factors (including how long one must wait before receiving an option and the effort required to obtain that option). Expected value can then recommend the best choice among a set of options, and expected value theory can even specify necessary criteria for optimal choice (such as choice consistency within a set of ordered preferences). Expected value theory inspired recent neuroimaging research in which investigators correlated activity in some AIM framework components with different aspects of expected value 45,46. For instance, NAcc activity was associated with gain magnitude; AI activity with loss as well as gain magnitude; and MFPC activity with value and probability integration (reviewed in 20).
However, although people often choose options consistent with the predictions of expected value theory, this is not always the case, creating opportunities to experimentally measure and account for suboptimal choices. For example, people often make suboptimal choices (or fail to maximize expected value) when evaluating risks and delays, or when learning about changes in value 47. An important question is therefore whether older adults make more or less optimal decisions in these scenarios than younger adults and, when differences exist, which underlying neural mechanisms can account for those differences?
Changes in value assessment
As described above, although older adults report similar levels of positive affective experience to younger adults, they report less negative affective experience 4. Consistent with this age-related asymmetry, experimental research indicates that older adults show reduced attention to and memory for negative material 5. However, these findings do not specify whether older adults show less negative affect during anticipation of events that have not yet occurred (which could implicate expected value), or in response to events that have already occurred, or both.
In behavioural research in younger adults, anticipation of uncertain monetary gains robustly and reliably elicits positive arousal, whereas anticipation of uncertain monetary losses elicits negative arousal 24. By contrast, although older adults also report increased positive arousal when anticipating monetary gains, they do not report increased negative arousal when anticipating losses 48,49. Younger and older adults do, however, report similarly positive affective responses to gain outcomes and negative affective responses to loss outcomes.
With respect to the AIM framework, these findings imply that older adults might show decreased activity in loss anticipation circuits during value assessment (or possibly, increased activity in gain anticipation circuits). FMRI studies in younger adults typically show that anticipation of uncertain monetary gains increases NAcc, AI, and dorsomedial caudate activity, but that anticipation of uncertain monetary losses increases only AI and dorsomedial caudate activity 20,50. Although older adults show similar increases in NAcc activity during anticipation of monetary gains 49,51 (but see 52), they do not show the same increases in AI activity during anticipation of monetary losses (FIG. 2a) 49,51. Interestingly, younger and older adults show similar neural responses in both the MPFC and ventral striatum in response to gain and loss outcomes 49,52–55 (see also 56). Thus, both affective and neural responses during anticipation of gains are preserved in older adults, whereas affective and neural responses during anticipation of losses are reduced.
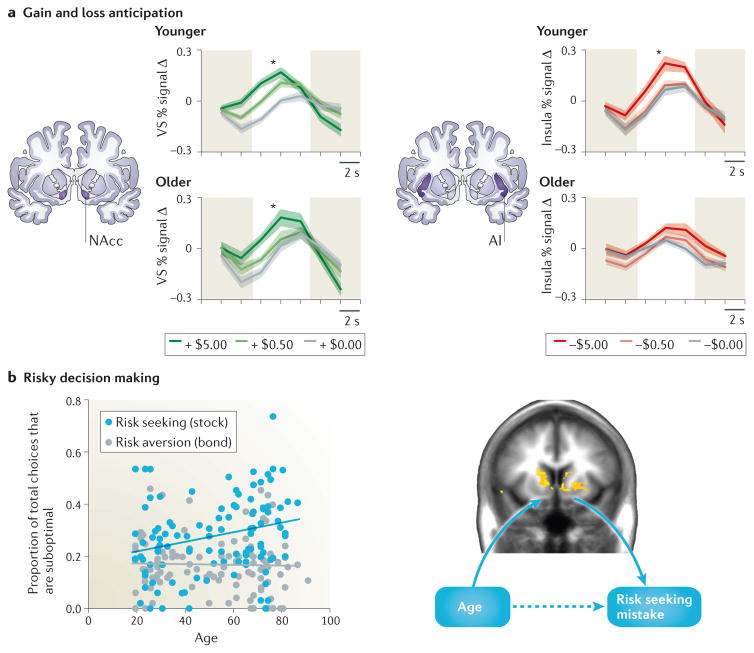
Figure 2. Age-related differences in incentive anticipation and risky decision making.
a| In an incentive anticipation task in which subjects saw cues signalling the potential gain or loss of varying amounts of money, gain anticipation increased nucleus accumbens (NAcc) activity in both younger (ages 19–27) and older (ages 65–81) adults (left panels). Loss anticipation, however, increased AI activity in younger, but not older, adults (right panels). Shaded error bars indicate standard error of the group mean. Y-axis represents percentage FMRI activity change in the ventral striatum (VS, including the NAcc).b| In a financially risky choice task, older adults make more mistakes than younger adults when seeking risk (selection of stocks) but not when avoiding risk (selection of bonds) (left panel). Age-related differences in behaviour (ages 19–85) were associated with age-related increased variability in striatal activity, including the NAcc (right panel; coloured areas overlaid on the brain are voxels for which a statistical test of the linear effect of age exceeded p <.0001, uncorrected). This increased variability mediated the association between increased age and risky stock (RS) mistakes. Figures in part a adapted from 49. Figures in part b adapted from 54.
The diminished responses of older adults to anticipated losses 5 may impose costs as well as conferring benefits. Specifically, although reduced loss anticipation could enhance well-being, it may also increase susceptibility to threats. For instance, one study found that older adults rated conventionally untrustworthy faces as more trustworthy and responded to these faces with less insula activity than did younger adults 57. In another study that used a socially incentivized game, older adults responded to unfair offers to divide a financial windfall with less insula activity than did younger adults 58. Despite this neural difference, however, older adults rejected slightly more unfair offers than younger adults. Although negative arousal correlated with rejection of unfair offers in younger adults, it did not in older adults, suggesting that other, more cognitive, mechanisms might have driven elders’ rejections. These findings broadly suggest that age-related changes in value assessment may sometimes directly influence decisions 57, but need not always alter choice 49,58.
Considered in light of the AIM framework, this evidence suggests that older adults show preserved positive affect and NAcc activity while anticipating gains, but reduced negative affect and insular activity while anticipating losses. Although age-related changes in these responses may influence choice, most of these experiments were designed only to elicit affect and brain activity. In addition, many of these experiments did not require value integration (either across potential gains and losses, or with respect to probability, delay, effort, and other factors). Thus, the impact of age-related affective changes on choice must be assessed rather than assumed, and choices requiring greater cognitive capacity incur the greatest age-related compromise.
Changes in risky decision making
Risky decision making requires, at a minimum, assessing uncertain future gains versus losses. Theorists have historically defined risk in different ways. Financially, expected value can be defined as the mean return of an option and risk as the mean variance of the option 59. Financial theories further posit that expected value attracts investors, whereas risk repels them. Younger adults generally prefer to avoid financial risk, which can lead to suboptimal decision making 60. Financial risk preferences show substantial individual differences, however, and also vary as a function of situational factors 61.
Perhaps because older adults generally avoid physical risks, people often assume that they will show even greater financial risk aversion than younger adults. These suspected differences, however, do not consistently emerge in well-controlled behavioural tasks 62. In fact, a recent meta-analysis showed no evidence of systematic age-related differences in risk taking 63. Rather, in tasks in which risk taking increased earnings, older adults avoided more risk, but in tasks in which risk taking decreased earnings, older adults sought more risk — suggesting that older adults made more mistakes overall. Furthermore, older adults performed more poorly than younger adults in tasks that required them to learn from recent experience, but not in tasks that did not require learning 63. Finally, older and younger adults did not differ in their tendency to take risks when choices were framed as gains versus losses. Together, this evidence is consistent with an account in which older adults evince cognitive limitations rather than different risk preferences 64.
With respect to the AIM framework, these findings suggest that although older adults show reduced loss anticipation during value assessment this may not necessarily translate into biased financial risk-taking. Instead, cognitive limitations and associated compromises in value integration might more prominently influence older adults’ financial risk taking. FMRI studies of younger adults indicate that anticipation of expected value is associated with NAcc and MPFC activity, whereas anticipation of risk is associated with AI activity 65. Furthermore, while NAcc activity and MPFC activity predicts financial risk seeking, AI activity predicts financial risk aversion 66.
Currently, only a few FMRI studies have compared financial risk taking in younger and older adults. Older adults showed greater AI activity and more risk averse choices than younger adults during a gambling task 67, in addition to greater prefrontal activity during a slot machine task 68, consistent with research showing increased prefrontal recruitment in older adults during risky decision making 69 and across a range of cognitive tasks 69–71. The small number of older adults in some of these studies (Ns~10), however, makes it difficult to generalise from these findings. A study with a larger sample examined age-related differences in risky choice during an investing task designed to elicit both high- and low-risk choices from each subject 54. The investing task also allows investigators to model the choices of an “optimal actor” and thereby quantify each subject’s “mistakes,” or deviations from optimal choice 72. While results showed no age-related differences in risk-averse choices, they did reveal that older adults made more risk-seeking mistakes (FIG 2b) 54. This behavioural pattern was subsequently replicated in two additional samples 54,73. Furthermore, older adults showed more random variation over time in NAcc activity, which could statistically account for increased risk-seeking mistakes 54,74. An independent study using a different task without financial incentives also found increased age-related variability in NAcc and midbrain activity 75. Although most FMRI studies focus on mean activity, variability may provide an important yet overlooked measure that can also clarify how ageing influences choice 76,77.
Together, these findings suggest that the variability of neural activity during expected value assessment may increase with age – particularly when previous value associations must be adjusted or relearned. Consistent with this account, older adults have more difficulty estimating expected value during reward learning 78. Apparent age-related differences in risk preferences may therefore result from increased neural variability during the estimation of expected value. The source of this variability may not merely reside in NAcc activity, but could also reflect more variable dopaminergic input from the midbrain, or more variable glutamatergic input from the MPFC. Consistent with this latter possibility, a study of older adults found that individuals with lower earnings in a gambling task with a learning component showed reduced MPFC recruitment 79. Considered in light of the AIM framework, these early findings suggest that greater variability in a value integration signal conveyed by MPFC projections to the NAcc may destabilize expected value estimates, as well as associated risky choices (a theme that will recur in the value learning section).
Changes in temporal decision making
People commonly devalue (or “discount”) potential rewards as a function of the time they must wait to obtain them. The rate at which young adults temporally discount gains is often steeper than the rate at which those gains actually decrease in value over time 80. This nonlinear devaluation of future gains (called “delay discounting”) can provoke suboptimal choices 81. As with risk preferences, individuals reliably differ in their tendency to discount future gains, and situational factors such as incentive type can also exacerbate delay discounting 82. Accumulating behavioural research suggests that older adults discount future rewards less steeply than younger adults 83 (a phenomenon also demonstrated in some animal models 84,85), and consequently the choices of older adults better approximate future rewards’ market value 86.
Neuroimaging studies have implicated NAcc and MPFC activity in younger adults’ tendency to weight immediate over future rewards (both sensory and financial) 87–90. Although NAcc activity also has a lesser role in the value assessment of future rewards 90, converging evidence suggests that activity in prefrontal cortex (including the DLPFC and possibly MPFC) has a more prominent role in the ability to imagine and extend value to future rewards 87,91–93. From the standpoint of the AIM framework, these findings suggest at least three possible accounts of older adults’ ability to more optimally balance future and present rewards. Specifically, older adults’ reduced delay discounting may result either from reduced anticipation of gain from immediate rewards, increased anticipation of gain from future rewards, or increased integration of future rewards into an overall value assessment.
Only two FMRI studies have directly compared neural responses during temporal valuation tasks in younger and older adults 94,95. In both studies, younger adults showed more ventral striatal activity in response to immediate rewards than future rewards, whereas older adults showed comparable ventral striatal activity in response to both immediate and future rewards (FIG. 3). Additionally, in adults of all ages ventral striatal activity in response to future rewards predicted individual differences in relative preferences for future rewards 93. Neither study, however, found differences in prefrontal activity of older versus younger adults during consideration of future rewards.
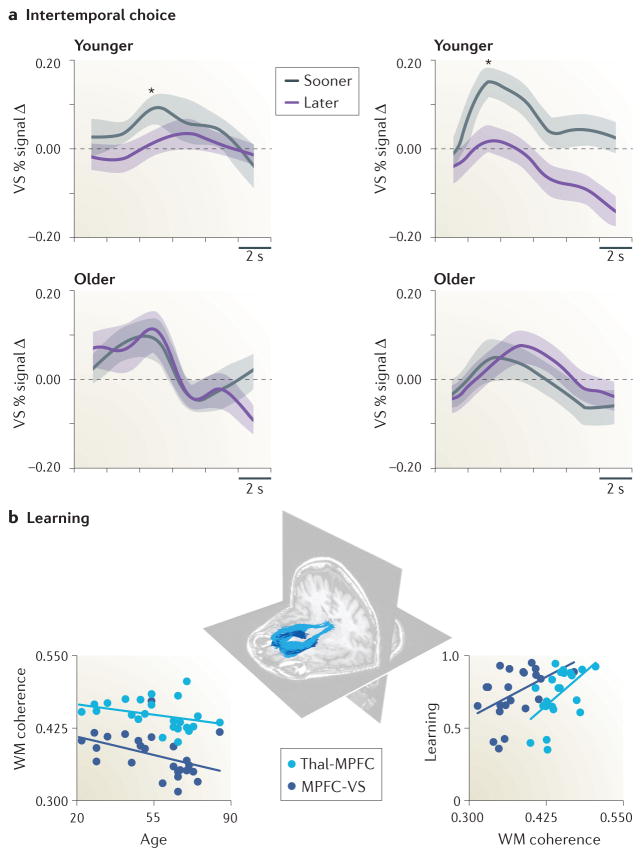
Figure 3. Age-related differences in temporal decision making and value learning.
a| Younger adults show reduced NAcc activity for rewards available at longer (grey line) versus shorter (orange line) time delays, but older adults show comparable activity for both short and long delays. Shaded error bars indicate standard error of group means. Y-axis represents percentage FMRI activity change in the ventral striatum (VS, including the NAcc). b| Structural coherence along a frontostriatal axonal tract extending from the dorsomedial nucleus of the thalamus (Thal) to the medial prefrontal cortex (MPFC; light blue) and from the MPFC to the ventral striatum (dark blue) was reduced in older age but associated with better learning. White matter coherence was indexed by measuring fractional anisotropy. Individual differences in learning were calculated as the percentage of choices of the higher expected value option. Left panels in part a adapted from 95. Right panels in part a adapted from 94. Figure in part b adapted from 108.
These neuroimaging results are consistent with behavioural findings indicating that although younger adults value immediate rewards more than future rewards, older adults value the two more similarly. With respect to the AIM framework, these findings either support the notion that immediate rewards evoke less gain anticipation, or future rewards elicit more gain anticipation in older adults, but do not implicate compromised value integration. Reduced responsiveness to immediate rewards might imply that ageing could compromise midbrain dopaminergic input to the ventral striatum 94, but pharmacological data call this account into question 96. Enhanced responsiveness to future rewards instead might imply that ageing preserves midbrain dopaminergic input or MPFC glutamatergic input to the ventral striatum during contemplation of future gains, thereby optimizing temporal choice 95,97. Future research using causal affective manipulations could compare the plausibility of these accounts, and might also test whether older adults’ more optimal temporal choices result from physiological changes, experience, or both 98.
Changes in value learning
Traditional theories of valuation often do not consider the source of those values. Although some values may have innate origins, most are learned, and may require continual adjustment to accommodate changing environmental circumstances. Value learning can be dynamic and complex, potentially recruiting and recalibrating any of the valuation mechanisms described above. Even in the case of simple probabilistic learning, although people can eventually learn that one option is more likely to yield gains than another, they often show suboptimal patterns of choice in the process. As with other types of valuation, individuals reliably differ in learning performance, and situational influences may speed or slow learning.
Possibly due to variable task demands, behavioural comparisons of value learning in older and younger adults have produced mixed results. For instance, although some studies suggest decreased responsiveness to gains during learning in older versus younger adults 99,100, others suggest decreased responsiveness to losses 101–103. When studies find decreased sensitivity to gains in older adults, it tends to appear in the very old 103,104, which may reflect a slight decrease in positive affect at the end of life 4. Across studies, however, researchers have generally noted slower learning about both gains and losses in older adults 10, consistent with general age-related decrements in value learning.
With respect to the AIM framework, these findings suggest that ageing may compromise either gain anticipation or value integration during probabilistic learning. In FMRI studies of younger adults, gain learning tasks typically elicit correlated activity in the NAcc and MPFC, whereas loss learning tasks more often elicit correlated activity in the AI 22,23. Consistent with decreased learning performance, FMRI studies have shown reduced NAcc activity during learning in older adults 105. Moreover, electroencephalographic studies have also shown decreased frontal potentials during learning in older adults 101,106. Recent research has explored whether these neural differences reflect diminished updating of reward prediction, as modelled by reward prediction errors55,102,107. Two FMRI studies of incentive learning specifically showed reduced neural activity associated with reward prediction errors in the MPFC and NAcc of older adults relative to younger adults 55,102. Furthermore, in a combined FMRI and pharmacological study, administration of a drug that increased dopamine availability improved learning and restored NAcc activity associated with reward prediction errors in underperforming older adults 107.
Age-related decreases in NAcc recruitment during reward learning might seem inconsistent with preserved NAcc activity during value assessment (described above). To directly compare value assessment and learning, an FMRI study examined older adults’ neural responses to gains in tasks with and without probabilistic learning demands. As in previous learning studies, older adults showed reduced MPFC and NAcc activity associated with reward prediction errors in the context of probabilistic learning, but preserved NAcc responses to monetary gains in a value assessment task 55,107. Thus, age-related decreases in NAcc activity during value learning seem not to stem from a lack of physiological responsiveness to reward, but rather from a slowness to alter existing reward predictions based on feedback. From the standpoint of the AIM framework, older adults may not suffer from reduced gain anticipation associated with NAcc activity, but rather from a lack of responsiveness to new information that corrects gain predictions, possibly conveyed by MPFC glutamatergic projections to the NAcc.
To specifically test whether frontostriatal connections could account for age-related decrements in reward learning, a diffusion tensor imaging (DTI) study assessed the structural coherence of mesolimbic white matter pathways in a community adult life-span sample. Measures of tract coherence were tested for associations with performance on a probabilistic learning task. Not only did ageing diminish the coherence of frontostriatal pathways (specifically, tracts that connect the thalamus to the MPFC, and the MPFC to the NAcc), but decreased coherence of these pathways could fully account for ageing’s influence on learning 108. With respect to the AIM framework, these findings highlight the importance of considering connections between components, and specifically imply that age-related decreases in frontostriatal signalling might account for decreased reward learning. Thus, an age-related compromise of value integration might account for deficits in reward learning – and may extend to any tasks that require reward learning (including dynamic risk taking). Age-related changes in implicit reward learning, however, must be distinguished from age-related changes in explicit memory, with respect to both relevant neural circuits and psychological processes 73.
Conclusions and implications
Growing interdisciplinary research has begun to link age-related changes in decision making to shifts in affective and motivational brain circuits. Specifically, emerging evidence suggests that, in older adults, gain anticipation and correlated NAcc activity are preserved but loss anticipation and correlated AI activity are relatively reduced. Older adults show more variable risky choices, which are related to more variable NAcc activity as they contemplate risky options. Older adults may place greater value on future rewards, which may be associated with relatively increased NAcc activity in response to future prospects. Finally, older adults show reduced reward learning, which may be related to diminished NAcc responsiveness to violated reward expectations, as well as degraded structural connectivity from the MPFC to the NAcc.
These findings suggest that ageing does not uniformly degrade decision performance, and under some circumstances may improve it. Optimal criteria for decision making are difficult to define, but according to expected value theory, people should prefer options that return greater value, and should consistently choose in a way that matches their preferences. Consistent with optimal choice, older adults seem to anticipate gains (but not losses) to the same extent as younger adults. Older adults also tend to show more optimal choices in temporal valuation. Inconsistent with optimal choice, however, when value assessment dynamically varies or requires integration across many attributes or options, as in the case of risky choice or probabilistic learning, older adults appear to choose less optimally than younger adults.
The findings also paint a varied picture of how ageing influences neural activity preceding choice, which does not neatly fit a profile of global age-related neural decline. As an alternative, we introduce the AIM framework, which delineates different critical neural components and connections that can together promote optimal decision making. This hierarchical and componential framework might provide a richer and more accurate view of the diverse effects of ageing on decision performance. For instance, if gain anticipation circuits including the NAcc are relatively preserved with ageing, but value integration associated with the MFPC and its connections to the NAcc degrade, gain anticipation might be preserved, but dynamic updating in the context of risky choice and probabilistic learning might be compromised 55,109.
The AIM framework thus fills a theoretical gap and complements existing neural accounts of age-related changes in attention, memory, and cognitive control 71,110–114. Many of these cognitive contributions to decision making may enter the AIM framework at the value integration phase, deranging this component’s contribution to decision making while leaving other types of input intact 64,93,115,116. For instance, a recent study using a purchasing task found that older adults made similarly optimal choices to younger adults with respect to simple choices, but not complex choices requiring working memory. Older adults who showed increased MPFC activity during complex choices, however, were able to match younger adults’ performance 117. An exciting future research agenda involves connecting the AIM framework to existing cognitive models of the influence of ageing on decision making.
Beyond organizing previous findings and generating future research questions, knowledge of neural components could inspire more specific ideas for applications. Complex decision tasks probably engage more components of the AIM framework, making performance failures more challenging to diagnose. Although different components may produce similar choices, understanding the underlying mechanisms could help investigators identify targeted interventions with the best chance of optimizing function. Thus, an important goal of neurally deconstructing decision making is to use this knowledge to inform the design of interventions that can enhance performance across the life span (BOX 3). However, before innovating interventions, it will be critical to verify that laboratory behaviour connects to decision making in the real world (BOX 4).
Box 3. Innovating targeted decision aids.
When older adults’ decision performance trails behind that of younger adults, research might identify opportunities for intervention. Building from the finding that age-related changes in risky choice may result from impairments in updating expected value, recent work demonstrated that providing graphical representations of expected value information could improve financial risk taking 73. With these targeted decision aids, older adults chose as optimally as younger adults without decision aids (see also 133).
Although promising, this early research is still several steps away from real-world implementation. The complexity and changing nature of many significant financial choices often resists easy graphical or numerical depiction. Furthermore, providing helpful information does not necessarily guarantee implementation. Adopting new strategies requires motivation as well as information. For instance, a recent study that trained individuals to use an expected value based decision strategy was less effective in older than younger adults 134, since older adults drifted from the suggested strategy within minutes. Research has yet to clarify whether elders’ shift occurred due to memory decay or active doubts about the usefulness of recommended strategies. Novel decision-making strategies may prove difficult to substitute after a lifetime of using other effective or familiar strategies.
Precise value calculations, however, may not be necessary for solving most decision problems outside the laboratory. In fact, some theorists suggest that rapidly grasping the gist rather than exact verbatim details of most decision problems provides critical leverage for success in the real world 135,136. In many situations, older individuals might play to their strengths without sacrificing decision quality by choosing simpler strategies 137,138.
In contrast to a general degradation account of neurobiological function, a componential approach like the AIM framework naturally implies targeted interventions for specific age-related changes in decision making 139,140. Through the lens of the AIM framework, the studies reviewed imply that decision aids for older adults should strive to focus on gains rather than losses, leverage emphasis on long-term gains, build on rather than changing existing value associations, and present simple and limited sets of options. Combined with an appreciation of cognitive limitations, decision neuroscience may eventually yield more effective decision aids for people of all ages.
Box 4. Generalizing from the laboratory to the real world.
The field of decision neuroscience is both interdisciplinary and young, and researchers have yet to link most laboratory measures of decision performance to significant real-world outcomes. Nevertheless, some links have begun to emerge. Individuals who make more optimal choices (or fewer “mistakes”) in a laboratory financial investment task also report having accumulated more real-world assets 54. In probabilistic value learning tasks, individuals who learn to acquire gains more rapidly also report having accumulated more real-world assets, whereas individuals who learn to avoid losses more rapidly report having less financial debt 141. Some of these measures have been validated not only with self-report but also with independent financial records (for instance, credit reports) 132,141.
The possibility of associating a laboratory measure with real-world behaviour is limited by the reliability of both the measure and the behaviour, which could present significant challenges for some traditional measures of economic choice. Fortunately, interdisciplinary collaboration can encourage innovation of measures with improved reliability when traditional measures do not suffice. Furthermore, the extent to which laboratory findings can be generalized to the real world may be limited by varying choice conditions. For instance, older individuals may not have the opportunity to avoid choices in the laboratory that they might in everyday life 62.
Beyond providing evidence for the validity of laboratory-based tasks, improving the predictive power of laboratory assessments could also help researchers to identify vulnerable individuals who are prone to making suboptimal choices in the real world 142. The elderly may be disproportionately targeted by fraudulent financial appeals, although evidence does not strongly suggest that they are more susceptible 143. Researchers are currently studying older individuals at heightened risk for making financial mistakes (based on prior victimization, for example) to understand how potential vulnerability to financial fraud relates to physiological, psychological, and behavioural variables across the adult life span. Future research should attempt to link laboratory measures to real-world decisions 131,144 so as to ensure that findings are consequential and have the best chance of improving the wealth and health of individuals, as well as the broader society they inhabit.
The application of neuroscience methods to address age-related changes in decision making has just begun, and raises more questions than it answers. For instance, in which decision scenarios is diminished loss anticipation helpful versus harmful? To what extent are age-related changes in decision performance due to physiological changes versus psychological strategies for coping with those changes? Although some evidence suggests age-related diminutions in dopamine activity 107, other evidence points towards broader age-related neurochemical changes (in noradrenergic and glutamatergic activity) 108, all of which could influence decision performance. Furthermore, to what extent do structural changes influence communication between critical components, which connections are central to decision making, and can they be targeted and modified with interventions? Finally, how can neuroscience improve the design and assessment of decision aids (BOX 3)?
As scientific research on ageing and decision making grows, so will societal interest in using this research to inform policy. Findings may inform the development of more targeted behavioural and neural interventions that leverage strengths and minimize weaknesses of the ageing brain. As increasing numbers of elders strive to make more optimal decisions, this new information may offer the best hope for improving their aim.
Online Summary.
Research has begun to explore how age-related changes in brain systems implicated in affect and motivation influence decision making.
Older and younger adults show similar affective and neural sensitivity to anticipated financial gains during value assessment, as well as to gain and loss outcomes. Older adults, however, show reduced affective and neural sensitivity to anticipated financial losses.
Older adults make more suboptimal choices during financial risk taking, which appear to be related not to shifts in risk preference, but rather to increased variability in NAcc activity.
Older adults make more optimal choices during delay discounting by assigning higher value to future gains, which appears to be related to increased NAcc activity during consideration of future rewards.
Older adults make more suboptimal choices when engaging in probabilistic reward learning, which appear to be related to decreased NAcc activity associated with reward prediction errors (but not necessarily reward predictions), which may result from reduced MPFC input into striatal circuits.
Understanding how ageing variably influences brain function and structure may better inform targeted interventions designed to improve decision performance in individuals of all ages.
Acknowledgments
Much of the research and ideas mentioned were supported by grants from the U.S. National Institute on Aging (R21AG030778 to BK and F31AG032804, F32AG039131, and R00AG042596 to GRS-L) and the FINRA Investor Education Foundation. We thank Ben Eppinger, Katherine Whalley, and three anonymous reviewers for comments on earlier drafts of the manuscript. Both authors contributed equally to the manuscript.
Glossary Terms
- Fluid cognitive ability
The ability to flexibly generate, transform, and manipulate new information.
- Crystallized cognitive ability
The ability to invoke previously stored information drawn from experience or accumulated knowledge.
- Cross-sectional studies
Studies that compare individuals (e.g., of different ages) at one simultaneous time point.
- Longitudinal studies
Studies that compare the same individuals (e.g., of different ages) repeatedly over multiple time points to assess change.
- Diffusion tensor imaging
A neuroimaging technique that uses the restricted diffusion of water around neural membranes and myelinated fibres to map anatomical connectivity between brain areas.
- Positron emission tomography (PET)
A nuclear imaging technique that produces three-dimensional images of brain activity by detecting photons that are emitted by a positron-emitting radionuclide tracer.
- Affect
A combination of subjective valence and arousal that is sometimes depicted in two-dimensional space.
- Valence
The subjectively positive or negative feeling evoked by an experience.
- Arousal
The subjective level of alertness, activation, or energy elicited by an experience.
- Functional magnetic resonance imaging (FMRI)
A functional imaging technique that uses a magnetic field and radio waves to measure the blood-oxygenation-level-dependent signal, which indexes regional brain activity.
- Probabilistic learning
Learning in which individuals use recent feedback to guide future choices among options of uncertain value.
- Reward prediction error
A quantity denoting the difference between the received versus expected reward.
- Reward prediction
A quantity denoting the expected reward.
Biographies
Gregory Samanez-Larkin is an Assistant Professor of Psychology, Cognitive Science, and Neuroscience at Yale University. He received a BA in psychology from the University of Michigan, a PhD in psychology from Stanford University, and completed a post-doctoral fellowship at Vanderbilt University. His research examines how individual and age differences in motivation and cognition influence decision making across the life span with an emphasis on changes in neural systems across adulthood.
Brian Knutson is an Associate Professor of Psychology and Neuroscience at Stanford University. He received BAs in psychology and comparative religion from Trinity University, a PhD in psychology from Stanford University, and completed post-doctoral fellowships at the University of California, San Francisco and the U.S. National Institutes of Health. His research combines psychological theory and neuroscience methods to investigate neural mechanisms that support emotion, and has explored implications of resulting discoveries for decision making and mental health.
References
- 1.World Population Prospects: The 2006 Revision. The United Nations; 2007. [Google Scholar]
- 2.Hayutin AM. Global Demographic Shifts Create Challenges and Opportunities. PREA Quarterly. 2007:47–53.
- 3.Cognitive aging: A primer. Psychology Press; 2000. [Google Scholar]
- 4.Carstensen LL, et al. Emotional experience improves with age: Evidence based on over 10 years of experience sampling. Psychol Aging. 2011;26:21–33. doi: 10.1037/a0021285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carstensen LL. The influence of a sense of time on human development. Science. 2006;312:1913–1915. doi: 10.1126/science.1127488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samanez-Larkin GR, Carstensen LL. In: The Handbook of Social Neuroscience. Decety J, Cacioppo JT, editors. Oxford University Press; 2011. pp. 507–521. [Google Scholar]
- 7.Mohr PNC, Li SC, Heekeren HR. Neuroeconomics and aging: neuromodulation of economic decision making in old age. Neuroscience and Biobehavioral Reviews. 2010;34:678–688. doi: 10.1016/j.neubiorev.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Brown SBRE, Ridderinkhof KR. Aging and the neuroeconomics of decision making: A review. Cognitive, Affective, & Behavioral Neuroscience. 2009;9:365–379. doi: 10.3758/CABN.9.4.365. [DOI] [PubMed] [Google Scholar]
- 9.Weierich MR, et al. Older and wiser? An affective science perspective on age-related challenges in financial decision making. Social Cognitive and Affective Neuroscience. 2011;6:195–206. doi: 10.1093/scan/nsq056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eppinger B, Hämmerer D, Li SC. Neuromodulation of reward-based learning and decision making in human aging. Ann N Y Acad Sci. 2011;1235:1–17. doi: 10.1111/j.1749-6632.2011.06230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nielsen L, Mather M. Emerging perspectives in social neuroscience and neuroeconomics of aging. Social Cognitive and Affective Neuroscience. 2011;6:149–164. doi: 10.1093/scan/nsr019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsu M, Lin H, Mcnamara P. Neuroeconomics of decision making in the aging brain: the example of long-term care. Advances in Health Economics and Health Services Research. 2008;20:203–225. [PubMed] [Google Scholar]
- 13.Braver TS, et al. Mechanisms of motivation-cognition interaction: challenges and opportunities. Cognitive, Affective, & Behavioral Neuroscience. 2014;14:443–472. doi: 10.3758/s13415-014-0300-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loewenstein G, Lerner JS. Handbook of Affective Science. Handbook of …; 2003. pp. 619–642. [Google Scholar]
- 15.Naqvi N, Shiv B, Bechara A. The Role of Emotion in Decision Making A Cognitive Neuroscience Perspective. Current Directions in Psychological Science. 2006;15:260–264. [Google Scholar]
- 16.Wundt W. Outlines of psychology. York University; 1897. [DOI] [Google Scholar]
- 17.Russell JA. A circumplex model of affect. Journal of Personality and Social Psychology. 1980;39:1161–1178. [Google Scholar]
- 18.Bradley MM. In: Handbook of Psychophysiology. Cacioppo JT, Tassinary LG, Berntson GG, editors. 2000. pp. 602–642. [Google Scholar]
- 19.Watson D, Wiese D, Vaidya J, Tellegen A. The two general activation systems of affect: Structural findings, evolutionary considerations, and psychobiological evidence. Journal of Personality and Social Psychology. 1999;76:820–838. [Google Scholar]
- 20.Knutson B, Katovich K, Suri G. Inferring affect from fMRI data. Trends Cogn Sci. 2014 doi: 10.1016/j.tics.2014.04.006. [DOI] [PubMed]
- 21.Knutson B, Rick S, Wimmer GE, Prelec D, Loewenstein G. Neural predictors of purchases. Neuron. 2007;53:147–156. doi: 10.1016/j.neuron.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pessiglione M, Seymour B, Flandin G, Dolan RJ, Frith CD. Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature. 2006;442:1042–1045. doi: 10.1038/nature05051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palminteri S, et al. Critical Roles for Anterior Insula and Dorsal Striatum in Punishment-Based Avoidance Learning. Neuron. 2012;76:998–1009. doi: 10.1016/j.neuron.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 24.Knutson B, Greer SM. Anticipatory affect: neural correlates and consequences for choice. Philos Trans R Soc Lond, B, Biol Sci. 2008;363:3771–3786. doi: 10.1098/rstb.2008.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanfey AG. Social decision-making: insights from game theory and neuroscience. Science. 2007;318:598–602. doi: 10.1126/science.1142996. [DOI] [PubMed] [Google Scholar]
- 26.Levy DJ, Glimcher PW. The root of all value: a neural common currency for choice. Curr Opin Neurobiol. 2012;22:1027–1038. doi: 10.1016/j.conb.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rangel A, Camerer CF, Montague PR. A framework for studying the neurobiology of value-based decision making. Nature Reviews Neuroscience. 2008;9:545–556. doi: 10.1038/nrn2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alexander WH, Brown JW. Medial prefrontal cortex as an action-outcome predictor. Nat Neurosci. 2011;14:1338–1344. doi: 10.1038/nn.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plassmann H, O’doherty JP, Shiv B, Rangel A. Marketing actions can modulate neural representations of experienced pleasantness. Proc Natl Acad Sci USA. 2008;105:1050–1054. doi: 10.1073/pnas.0706929105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu X, Hairston J, Schrier M, Fan J. Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews. 2011;35:1219–1236. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diekhof EK, Kaps L, Falkai P, Gruber O. The role of the human ventral striatum and the medial orbitofrontal cortex in the representation of reward magnitude - an activation likelihood estimation meta-analysis of neuroimaging studies of passive reward expectancy and outcome processing. Neuropsychologia. 2012;50:1252–1266. doi: 10.1016/j.neuropsychologia.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 32.Bartra O, McGuire JT, Kable JW. The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. NeuroImage. 2013;76:412–427. doi: 10.1016/j.neuroimage.2013.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clithero JA, Rangel A. Informatic parcellation of the network involved in the computation of subjective value. Social Cognitive and Affective Neuroscience. 2013:nst106. doi: 10.1093/scan/nst106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- 35.Jones SR, et al. Profound neuronal plasticity in response to inactivation of the dopamine transporter. PNAS. 1998;95:4029–4034. doi: 10.1073/pnas.95.7.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. Outlines evolutionarily-conserved neural circuits implicated in reward processing, motivation, and choice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chikama M, McFarland NR, Amaral DG, Haber SN. Insular cortical projections to functional regions of the striatum correlate with cortical cytoarchitectonic organization in the primate. J Neurosci. 1997;17:9686–9705. doi: 10.1523/JNEUROSCI.17-24-09686.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mesulam MM, Mufson EJ. Association and Auditory Cortices. Vol. 4. Springer; US: 1985. pp. 179–226. [Google Scholar]
- 39.Phelps EA, Lempert KM, Sokol-Hessner P. Emotion and decision making: multiple modulatory neural circuits. Annu Rev Neurosci. 2014;37:263–287. doi: 10.1146/annurev-neuro-071013-014119. [DOI] [PubMed] [Google Scholar]
- 40.Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44:195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 41.Hedden T, Gabrieli JDE. Insights into the ageing mind: a view from cognitive neuroscience. Nature Reviews Neuroscience. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- 42.Grady C. The cognitive neuroscience of ageing. Nature Reviews Neuroscience. 2012;13:491–505. doi: 10.1038/nrn3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neumann von J, Morgenstern O. Theory of Games and Economic Behavior. Princeton University Press; 1953. [Google Scholar]
- 44.Social Learning Theory. 1971. [Google Scholar]
- 45.Knutson B, Taylor J, Kaufman M, Peterson R, Glover G. Distributed neural representation of expected value. J Neurosci. 2005;25:4806–4812. doi: 10.1523/JNEUROSCI.0642-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yacubian J, et al. Dissociable systems for gain- and loss-related value predictions and errors of prediction in the human brain. J Neurosci. 2006;26:9530–9537. doi: 10.1523/JNEUROSCI.2915-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loewenstein G, Rick S, Cohen JD. Neuroeconomics. Annu Rev Psychol. 2008;59:647–672. doi: 10.1146/annurev.psych.59.103006.093710. [DOI] [PubMed] [Google Scholar]
- 48.Nielsen L, Knutson B, Carstensen LL. Affect dynamics, affective forecasting, and aging. Emotion. 2008;8:318–330. doi: 10.1037/1528-3542.8.3.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Samanez-Larkin GR, et al. Anticipation of monetary gain but not loss in healthy older adults. Nat Neurosci. 2007;10:787–791. doi: 10.1038/nn1894. Demonstrates an asymmetry in which older adults show less neural sensitivity to anticipated losses than younger adults, but not anticipated gains or outcomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Curr Opin Neurol. 2005;18:411–417. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- 51.Wu CC, Samanez-Larkin GR, Katovich K, Knutson B. Affective traits link to reliable neural markers of incentive anticipation. NeuroImage. 2014;84:279–289. doi: 10.1016/j.neuroimage.2013.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schott BH, et al. Ageing and early-stage Parkinson’s disease affect separable neural mechanisms of mesolimbic reward processing. Brain. 2007;130:2412–2424. doi: 10.1093/brain/awm147. Clarifies differences between healthy aging and Parkinson’s disease in neural activity and functional connectivity during the anticipation and receipt of monetary rewards. [DOI] [PubMed] [Google Scholar]
- 53.Cox KM, Aizenstein HJ, Fiez JA. Striatal outcome processing in healthy aging. Cognitive, Affective, & Behavioral Neuroscience. 2008;8:304–317. doi: 10.3758/cabn.8.3.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Samanez-Larkin GR, Kuhnen CM, Yoo DJ, Knutson B. Variability in nucleus accumbens activity mediates age-related suboptimal financial risk taking. J Neurosci. 2010;30:1426–1434. doi: 10.1523/JNEUROSCI.4902-09.2010. Identifies a novel measure of neural signal variability that mediates age differences in risky choice during a financial investment task. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Samanez-Larkin GR, Worthy DA, Mata R, McClure SM, Knutson B. Adult age differences in frontostriatal representation of prediction error but not reward outcome. Cognitive, Affective, & Behavioral Neuroscience. 2014;14:672–682. doi: 10.3758/s13415-014-0297-4. Shows that age differences in neural representations of reward prediction error do not result from more basic age differences in reward sensitivity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spaniol J, Bowen HJ, Wegier P, Grady C. Neural responses to monetary incentives in younger and older adults. Brain Res. 2014 doi: 10.1016/j.brainres.2014.09.063. [DOI] [PubMed] [Google Scholar]
- 57.Castle E, et al. Neural and behavioral bases of age differences in perceptions of trust. PNAS. 2012;109:20848–20852. doi: 10.1073/pnas.1218518109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harlé KM, Sanfey AG. Social economic decision-making across the lifespan: An fMRI investigation. Neuropsychologia. 2012;50:1416–1424. doi: 10.1016/j.neuropsychologia.2012.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Markowitz H. Portfolio selection. Journal of Finance. 1952;7:77–91. [Google Scholar]
- 60.Kahneman D, Tversky A. Prospect Theory: An Analysis of Decision under Risk. Econometrica. 1979;47:263–291. [Google Scholar]
- 61.Weber EU, Blais AR, Betz NE. A domain-specific risk-attitude scale: measuring risk perceptions and risk behaviors. J Behav Decis Making. 2002;15:263–290. [Google Scholar]
- 62.Mather M. A Review of Decision-Making Processes: Weighing the Risks and Benefits of Aging. When I’m. 2006;64:145–173. [Google Scholar]
- 63.Mata R, Josef AK, Samanez-Larkin GR, Hertwig R. Age differences in risky choice: a meta-analysis. Ann N Y Acad Sci. 2011;1235:18–29. doi: 10.1111/j.1749-6632.2011.06200.x. Quantitative meta-analysis implies age-related performance decrements in learning-dependent compared to non-learning-dependent tasks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Henninger DE, Madden DJ, Huettel SA. Processing speed and memory mediate age-related differences in decision making. Psychol Aging. 2010;25:262–270. doi: 10.1037/a0019096. Highlights the role of fluid cognitive abilities on decision making across adulthood. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Knutson B, Bossaerts P. Neural antecedents of financial decisions. J Neurosci. 2007;27:8174–8177. doi: 10.1523/JNEUROSCI.1564-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu CC, Sacchet MD, Knutson B. Toward an affective neuroscience account of financial risk taking. Front Neurosci. 2012;6:159. doi: 10.3389/fnins.2012.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee TMC, Leung AWS, Fox PT, Gao JH, Chan CCH. Age-related differences in neural activities during risk taking as revealed by functional MRI. Social Cognitive and Affective Neuroscience. 2008;3:7–15. doi: 10.1093/scan/nsm033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McCarrey AC, et al. Age differences in neural activity during slot machine gambling: an fMRI study. PLoS ONE. 2012;7:e49787. doi: 10.1371/journal.pone.0049787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hosseini SMH, et al. Aging and decision making under uncertainty: behavioral and neural evidence for the preservation of decision making in the absence of learning in old age. NeuroImage. 2010;52:1514–1520. doi: 10.1016/j.neuroimage.2010.05.008. Demonstrates similar functional neural recruitment across age in risky decisions that do not require recent learning. [DOI] [PubMed] [Google Scholar]
- 70.Cabeza R. Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychol Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- 71.Reuter-Lorenz PA, Cappell KA. Neurocognitive Aging and the Compensation Hypothesis. Curr Dir Psychol Sci. 2008;17:177–182. [Google Scholar]
- 72.Kuhnen CM, Knutson B. The neural basis of financial risk taking. Neuron. 2005;47:763–770. doi: 10.1016/j.neuron.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 73.Samanez-Larkin GR, Wagner AD, Knutson B. Expected value information improves financial risk taking across the adult life span. Social Cognitive and Affective Neuroscience. 2011;6:207–217. doi: 10.1093/scan/nsq043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li Lindenberger U, Sikström S. Aging cognition: from neuromodulation to representation. Trends Cogn Sci. 2001;5:479–486. doi: 10.1016/s1364-6613(00)01769-1. [DOI] [PubMed] [Google Scholar]
- 75.Garrett DD, Kovacevic N, McIntosh AR, Grady CL. Blood oxygen level-dependent signal variability is more than just noise. J Neurosci. 2010;30:4914–4921. doi: 10.1523/JNEUROSCI.5166-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Garrett DD, et al. Moment-to-moment brain signal variability: A next frontier in human brain mapping? Neuroscience and Biobehavioral Reviews. 2013;37:610–624. doi: 10.1016/j.neubiorev.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Garrett DD, McIntosh AR, Grady CL. Moment-to-moment signal variability in the human brain can inform models of stochastic facilitation now. Nature Reviews Neuroscience. 2011;12:612. doi: 10.1038/nrn3061-c1. author reply 612. [DOI] [PubMed] [Google Scholar]
- 78.Eppinger B, Kray J. To choose or to avoid: age differences in learning from positive and negative feedback. J Cogn Neurosci. 2011;23:41–52. doi: 10.1162/jocn.2009.21364. [DOI] [PubMed] [Google Scholar]
- 79.Rogalsky C, Vidal C, Li X, Damasio H. Risky decision-making in older adults without cognitive deficits: an fMRI study of VMPFC using the Iowa Gambling Task. Social Neuroscience. 2012;7:178–190. doi: 10.1080/17470919.2011.588340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Frederick S, Loewenstein G, O’Donoghue T. Time Discounting and Time Preference: A Critical Review. Journal of Economic Literature. 2002;40:351–401. [Google Scholar]
- 81.Berns GS, Laibson D, Loewenstein G. Intertemporal choice--toward an integrative framework. Trends Cogn Sci. 2007;11:482–488. doi: 10.1016/j.tics.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 82.Peters J, Büchel C. The neural mechanisms of inter-temporal decision-making: understanding variability. Trends Cogn Sci. 2011;15:227–239. doi: 10.1016/j.tics.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 83.Löckenhoff CE. Age, time, and decision making: from processing speed to global time horizons. Ann N Y Acad Sci. 2011;1235:44–56. doi: 10.1111/j.1749-6632.2011.06209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Simon N, et al. Good things come to those who wait: Attenuated discounting of delayed rewards in aged Fischer 344 rats. Neurobiol Aging. 2010;31:853–862. doi: 10.1016/j.neurobiolaging.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Roesch MR, Bryden DW, Cerri DH, Haney ZR, Schoenbaum G. Willingness to Wait and Altered Encoding of Time-Discounted Reward in the Orbitofrontal Cortex with Normal Aging. J Neurosci. 2012;32:5525–5533. doi: 10.1523/JNEUROSCI.0586-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Löckenhoff CE, O’Donoghue T, Dunning D. Age differences in temporal discounting: The role of dispositional affect and anticipated emotions. Psychol Aging. 2011;26:274–284. doi: 10.1037/a0023280. [DOI] [PubMed] [Google Scholar]
- 87.McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- 88.McClure SM, Ericson KM, Laibson DI, Loewenstein G, Cohen JD. Time discounting for primary rewards. J Neurosci. 2007;27:5796–5804. doi: 10.1523/JNEUROSCI.4246-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nat Neurosci. 2007;10:1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kable JW, Glimcher PW. An ‘as soon as possible’ effect in human intertemporal decision making: behavioral evidence and neural mechanisms. J Neurophysiol. 2010;103:2513–2531. doi: 10.1152/jn.00177.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ballard K, Knutson B. Dissociable neural representations of future reward magnitude and delay during temporal discounting. NeuroImage. 2009;45:143–150. doi: 10.1016/j.neuroimage.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Figner B, et al. Lateral prefrontal cortex and self-control in intertemporal choice. Nat Neurosci. 2010;13:538–539. doi: 10.1038/nn.2516. [DOI] [PubMed] [Google Scholar]
- 93.Peters J, Büchel C. Episodic future thinking reduces reward delay discounting through an enhancement of prefrontal-mediotemporal interactions. Neuron. 2010;66:138–148. doi: 10.1016/j.neuron.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 94.Eppinger B, Nystrom LE, Cohen JD. Reduced Sensitivity to Immediate Reward during Decision-Making in Older than Younger Adults. PLoS ONE. 2012;7:e36953. doi: 10.1371/journal.pone.0036953. Explains older adults’ relative patience for delayed rewards by showing a lack of delay-related reduction in neural activity in older age. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Samanez-Larkin GR, et al. Age Differences in Striatal Delay Sensitivity during Intertemporal Choice in Healthy Adults. Front Neurosci. 2011;5:126. doi: 10.3389/fnins.2011.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Phillips PEM, Walton ME, Jhou TC. Calculating utility: preclinical evidence for cost–benefit analysis by mesolimbic dopamine. Psychopharmacology. 2006;191:483–495. doi: 10.1007/s00213-006-0626-6. [DOI] [PubMed] [Google Scholar]
- 97.Li Y, Baldassi M, Johnson EJ, Weber EU. Complementary cognitive capabilities, economic decision making, and aging. Psychol Aging. 2013;28:595–613. doi: 10.1037/a0034172. Demonstrates that enhanced crystallized cognitive abilities can compensate for diminished fluid cognitive capacities in old age across a range of decision-making tasks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gilbert RJ, et al. Risk, reward, and decision-making in a rodent model of cognitive aging. Front Neurosci. 2011;5:144. doi: 10.3389/fnins.2011.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Denburg NL, Recknor EC, Bechara A, Tranel D. Psychophysiological anticipation of positive outcomes promotes advantageous decision-making in normal older persons. International Journal of Psychophysiology. 2006;61:19–25. doi: 10.1016/j.ijpsycho.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 100.Wood S, Busemeyer J, Koling A, Cox CR, Davis H. Older adults as adaptive decision makers: evidence from the Iowa Gambling Task. Psychol Aging. 2005;20:220–225. doi: 10.1037/0882-7974.20.2.220. [DOI] [PubMed] [Google Scholar]
- 101.Hämmerer D, Li SC, Müller V, Lindenberger U. Life span differences in electrophysiological correlates of monitoring gains and losses during probabilistic reinforcement learning. J Cogn Neurosci. 2011;23:579–592. doi: 10.1162/jocn.2010.21475. [DOI] [PubMed] [Google Scholar]
- 102.Eppinger B, Schuck NW, Nystrom LE, Cohen JD. Reduced Striatal Responses to Reward Prediction Errors in Older Compared with Younger Adults. J Neurosci. 2013;33:9905–9912. doi: 10.1523/JNEUROSCI.2942-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Frank MJ, Kong L. Learning to avoid in older age. Psychol Aging. 2008;23:392–398. doi: 10.1037/0882-7974.23.2.392. [DOI] [PubMed] [Google Scholar]
- 104.Simon JR, Howard JH, Howard DV. Adult Age Differences in Learning From Positive and Negative Probabilistic Feedback. Neuropsychology. 2010;24:534–541. doi: 10.1037/a0018652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mell T, et al. Altered function of ventral striatum during reward-based decision making in old age. Front Hum Neurosci. 2009;3:34. doi: 10.3389/neuro.09.034.2009. Demonstrates that age differences in frontostriatal function are most pronounced during early stages of learning and after reversal of incentive contingencies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Eppinger B, Kray J, Mock B, Mecklinger A. Better or worse than expected? Aging, learning, and the ERN Neuropsychologia. 2008;46:521–539. doi: 10.1016/j.neuropsychologia.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 107.Chowdhury R, et al. Dopamine restores reward prediction errors in old age. Nat Neurosci. 2013;16:648–653. doi: 10.1038/nn.3364. Uses a dopaminergic precursor (L-DOPA) to enhance reward prediction error-related neural activity in underperforming older adults. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Samanez-Larkin GR, Levens SM, Perry LM, Dougherty RF, Knutson B. Frontostriatal white matter integrity mediates adult age differences in probabilistic reward learning. J Neurosci. 2012;32:5333–5337. doi: 10.1523/JNEUROSCI.5756-11.2012. Shows that the structural connectivity of frontostriatal projections mediates age differences in reward learning. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fellows LK. Orbitofrontal contributions to value-based decision making: evidence from humans with frontal lobe damage. Ann N Y Acad Sci. 2011;1239:51–58. doi: 10.1111/j.1749-6632.2011.06229.x. [DOI] [PubMed] [Google Scholar]
- 110.Braver TS, Barch DM. A theory of cognitive control, aging cognition, and neuromodulation. Neuroscience and Biobehavioral Reviews. 2002;26:809–817. doi: 10.1016/s0149-7634(02)00067-2. [DOI] [PubMed] [Google Scholar]
- 111.Bäckman L, Nyberg L, Lindenberger U, Li SC, Farde L. The correlative triad among aging, dopamine, and cognition: current status and future prospects. Neuroscience and Biobehavioral Reviews. 2006;30:791–807. doi: 10.1016/j.neubiorev.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 112.Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The posterior-anterior shift in aging. Cereb Cortex. 2008;18:1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.West RL. An application of prefrontal cortex function theory to cognitive aging. Psychol Bull. 1996;120:272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- 114.Rubin DC. Frontal-Striatal Circuits in Cognitive Aging: Evidence for Caudate Involvement. Aging, Neuropsychology, and Cognition. 1999;6:241–259. Early cognitive aging paper which emphasizes the importance of examining frontostriatal circuits rather than the frontal cortex in isolation. [Google Scholar]
- 115.Benoit RG, Gilbert SJ, Burgess PW. A neural mechanism mediating the impact of episodic prospection on farsighted decisions. J Neurosci. 2011;31:6771–6779. doi: 10.1523/JNEUROSCI.6559-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kwan D, et al. Future decision-making without episodic mental time travel. Hippocampus. 2012;22:1215–1219. doi: 10.1002/hipo.20981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lighthall NR, Huettel SA, Cabeza R. Functional compensation in the ventromedial prefrontal cortex improves memory-dependent decisions in older adults. J Neurosci. 2014;34:15648–15657. doi: 10.1523/JNEUROSCI.2888-14.2014. Shows that increased medial prefrontal activity in older adults promotes improved decision making in cognitively demanding decision tasks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Raz N. In: Cognitive neuroscience of aging: Linking cognitive and cerebral aging. Cabeza R, Nyberg L, Park D, editors. Oxford University Press; New York, NY, US: 2005. pp. 19–57. [Google Scholar]
- 119.Fjell AM, et al. Critical ages in the life course of the adult brain: nonlinear subcortical aging. Neurobiol Aging. 2013;34:2239–2247. doi: 10.1016/j.neurobiolaging.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Raz N, Rodrigue KM. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neuroscience and Biobehavioral Reviews. 2006;30:730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bennett IJ, Madden DJ. Disconnected aging: Cerebral white matter integrity and age-related differences in cognition. Neuroscience. 2013 doi: 10.1016/j.neuroscience.2013.11.026. [DOI] [PMC free article] [PubMed]
- 122.Sullivan EV, Pfefferbaum A. Diffusion tensor imaging and aging. Neuroscience and Biobehavioral Reviews. 2006;30:749–761. doi: 10.1016/j.neubiorev.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 123.Bäckman L, Lindenberger U, Li SC, Nyberg L. Linking cognitive aging to alterations in dopamine neurotransmitter functioning: recent data and future avenues. Neuroscience and Biobehavioral Reviews. 2010;34:670–677. doi: 10.1016/j.neubiorev.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 124.Klostermann EC, Braskie MN, Landau SM, O’Neil JP, Jagust WJ. Dopamine and frontostriatal networks in cognitive aging. Neurobiol Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Allard S, Scardochio T, Cuello AC, Ribeiro-da-Silva A. Correlation of cognitive performance and morphological changes in neocortical pyramidal neurons in aging. Neurobiol Aging. 2012;33:1466–1480. doi: 10.1016/j.neurobiolaging.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Segovia G, Porras A, Del Arco A, Mora F. Glutamatergic neurotransmission in aging: a critical perspective. Mechanisms of Ageing and Development. 2001;122:1–29. doi: 10.1016/s0047-6374(00)00225-6. [DOI] [PubMed] [Google Scholar]
- 127.Mora F, Segovia G, Del Arco A. Glutamate-dopamine-GABA interactions in the aging basal ganglia. Brain Res Rev. 2008;58:340–353. doi: 10.1016/j.brainresrev.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 128.Dreher JC, Meyer-Lindenberg A, Kohn P, Berman KF. Age-related changes in midbrain dopaminergic regulation of the human reward system. PNAS. 2008;105:15106–15111. doi: 10.1073/pnas.0802127105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mata R, et al. Ecological rationality: a framework for understanding and aiding the aging decision maker. Front Neurosci. 2012;6:19. doi: 10.3389/fnins.2012.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Horn JL, Cattell RB. Age differences in fluid and crystallized intelligence. Acta psychologica. 1967;26:107–129. doi: 10.1016/0001-6918(67)90011-x. [DOI] [PubMed] [Google Scholar]
- 131.Agarwal S, Driscoll JC, Gabaix X, Laibson DI. The Age of Reason: Financial Decisions over the Life-Cycle with Implications for Regulation. Brookings Papers on Economic Activity. 2009;40:51–117. Adapts classic findings on age differences in fluid and crystallized abilities to explain adult age differences in optimal financial decisions in the real world. [Google Scholar]
- 132.Li Y, et al. Sound credit scores and financial decisions despite cognitive aging. Proc Natl Acad Sci USA. 2014 doi: 10.1073/pnas.1413570112. 201413570. [DOI] [PMC free article] [PubMed]
- 133.Löckenhoff CE, Carstensen LL. Aging, emotion, and health-related decision strategies: motivational manipulations can reduce age differences. Psychol Aging. 2007;22:134–146. doi: 10.1037/0882-7974.22.1.134. [DOI] [PubMed] [Google Scholar]
- 134.Westbrook A, Martins BS, Yarkoni T, Braver TS. Strategic insight and age-related goal-neglect influence risky decision-making. Front Neurosci. 2012;6:68. doi: 10.3389/fnins.2012.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Reyna VF, Lloyd FJ. Physician decision making and cardiac risk: effects of knowledge, risk perception, risk tolerance, and fuzzy processing. J Exp Psychol Appl. 2006;12:179–195. doi: 10.1037/1076-898X.12.3.179. [DOI] [PubMed] [Google Scholar]
- 136.Reyna VF, Farley F. Risk and Rationality in Adolescent Decision Making: Implications for Theory, Practice, and Public Policy. Psychological Science in the Public Interest. 2006;7:1–44. doi: 10.1111/j.1529-1006.2006.00026.x. [DOI] [PubMed] [Google Scholar]
- 137.Worthy DA, Maddox WT. Age-based differences in strategy use in choice tasks. Front Neurosci. 2012;5:145. doi: 10.3389/fnins.2011.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mata R, Nunes L. When less is enough: Cognitive aging, information search, and decision quality in consumer choice. Psychol Aging. 2010;25:289–298. doi: 10.1037/a0017927. [DOI] [PubMed] [Google Scholar]
- 139.Lindenberger U. Human cognitive aging: corriger la fortune? Science. 2014;346:572–578. doi: 10.1126/science.1254403. [DOI] [PubMed] [Google Scholar]
- 140.Gutchess A. Plasticity of the aging brain: new directions in cognitive neuroscience. Science. 2014;346:579–582. doi: 10.1126/science.1254604. [DOI] [PubMed] [Google Scholar]
- 141.Knutson B, Samanez-Larkin GR, Kuhnen CM. Gain and loss learning differentially contribute to life financial outcomes. PLoS ONE. 2011;6:e24390. doi: 10.1371/journal.pone.0024390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Denburg NL, et al. The orbitofrontal cortex, real-world decision-making, and normal aging. Ann N Y Acad Sci. 2007 doi: 10.1196/annals.1401.031. [DOI] [PMC free article] [PubMed]
- 143.Fighting Fraud 101. SaveAndInvest.org; 2011. pp. 1–14. [Google Scholar]
- 144.Korniotis GM, Kumar A. Do Older Investors Make Better Investment Decisions? The Review of Economics and Statistics. 2011;93:244–265. [Google Scholar]