Abstract
BACKGROUND
Peripartum cardiomyopathy (PPCM) remains a major cause of maternal morbidity and mortality.
OBJECTIVES
This study sought to prospectively evaluate recovery of the left ventricular ejection fraction (LVEF) and clinical outcomes in the multicenter IPAC (Investigations of Pregnancy Associated Cardiomyopathy) study.
METHODS
We enrolled and followed 100 women with PPCM through 1 year post-partum. The LVEF was assessed by echocardiography at baseline and at 2, 6, and 12 months post-partum. Survival free from major cardiovascular events (death, transplantation, or left ventricular [LV] assist device) was determined. Predictors of outcome, particularly race, parameters of LV dysfunction (LVEF), and remodeling (left ventricular end-diastolic diameter [LVEDD]) at presentation, were assessed by univariate and multivariate analyses.
RESULTS
The cohort was 30% black, 65% white, 5% other; the mean patient age was 30 ± 6 years; and 88% were receiving beta-blockers and 81% angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. The LVEF at study entry was 0.35 ± 0.10, 0.51 ± 0.11 at 6 months, and 0.53 ± 0.10 at 12 months. By 1 year, 13% had experienced major events or had persistent severe cardiomyopathy with an LVEF <0.35, and 72% achieved an LVEF ≥0.50. An initial LVEF <0.30 (p = 0.001), an LVEDD ≥6.0 cm (p < 0.001), black race (p = 0.001), and presentation after 6 weeks postpartum (p = 0.02) were associated with a lower LVEF at 12 months. No subjects with both a baseline LVEF <0.30 and an LVEDD ≥6.0 cm recovered by 1 year post-partum, whereas 91% with both a baseline LVEF ≥0.30 and an LVEDD <6.0 cm recovered (p < 0.00001).
CONCLUSIONS
In a prospective cohort with PPCM, most women recovered; however, 13% had major events or persistent severe cardiomyopathy. Black women had more LV dysfunction at presentation and at 6 and 12 months post-partum. Severe LV dysfunction and greater remodeling at study entry were associated with less recovery. (Investigations of Pregnancy Associated Cardiomyopathy [IPAC]; NCT01085955)
Keywords: heart failure, myocardial recovery, race, remodeling
Peripartum cardiomyopathy (PPCM) is an uncommon complication of pregnancy that remains a major cause of maternal morbidity and mortality (1). Although older studies estimate its prevalence in the United States at 1 in 4,000 live births, with increased recognition, more recent studies place this estimate closer to 1 in 2,000 (2). PPCM is endemic in Haiti (3) and parts of Africa (4), and race remains a major risk factor for its development (5,6). The clinical presentation is similar to that of other forms of nonischemic cardiomyopathy, with the onset in the later part of pregnancy or the first few months post-partum (7). Although the etiology remains uncertain, an autoimmune inflammatory pathogenesis triggered by fetal or placental antigens has been suspected (8,9). More recently, both genetic (10,11) and vascular (12) etiologies have been postulated to play a significant role.
Outcomes of PPCM are markedly heterogeneous. Previous investigations have demonstrated that many women with PPCM recover left ventricular (LV) function completely; however, a substantial percentage is left with persistent dilated cardiomyopathy and chronic progressive heart failure (13). Given the low prevalence of the disorder, most single-center reports are limited in study number and being retrospective, and there is minimal prospective data on clinical outcomes of contemporary evidence-based therapy (14,15). The utility of demographics or clinical phenotype for predicting myocardial recovery has not been prospectively evaluated.
The Peripartum Cardiomyopathy Network was formed as a 30-center collaborative group to facilitate research on this disorder. The IPAC (Investigations of Pregnancy Associated Cardiomyopathy) study was initiated in 2009 as a National Heart, Lung, and Blood Institute–funded multicenter, prospective investigation of the demographic characteristics, inflammatory pathogenesis, treatment, and clinical predictors of outcomes for PPCM patients in North America. We now report the clinical characteristics of the IPAC cohort, the subsequent outcomes during the first year post-partum, and the clinical and demographic predictors of myocardial recovery.
METHODS
COHORT
Between December 2009 and September 2012, 100 women with newly diagnosed PPCM were enrolled within the first 13 weeks post-partum at 30 centers (Online Appendix). All women were at least 18 years of age and had no history of cardiac disease, an estimated clinical LV ejection fraction (LVEF) ≤0.45 at the time of enrollment, and an evaluation consistent with idiopathic nonischemic cardiomyopathy. Women with significant valvular disease, coronary disease (>50% stenosis of a major epicardial vessel or a positive noninvasive study), evidence of ongoing bacterial septicemia (positive blood cultures), ongoing drug or alcohol abuse, history of chemotherapy or chest radiation within 5 years of enrollment, or a history of cardiomyopathy were excluded.
PROTOCOL
The study protocol was approved by the institutional review boards at all participating centers, and informed consent was obtained from all subjects. At the time of enrollment, demographic information (including self-designated race), previous clinical evaluation, and current medical therapy were recorded. Women were followed until 1 year postpartum. All hospitalizations and major cardiac events, including death, cardiac transplantation, and implantation of a left ventricular assist device (LVAD), were recorded.
LV FUNCTION
All subjects had an echocardiogram to assess LVEF at the time of enrollment, which was repeated at 6 and 12 months post-partum. Women enrolled early (within 6 weeks post-partum, n = 66) had a repeat assessment of LV function at 2 months. Echocardiograms were reviewed by a core laboratory at the University of Pittsburgh for assessment of ventricular volumes and calculation of ejection fraction. LV volumes and LVEFs were assessed by biplane Simpson’s rule using manual tracing of digital images. Left ventricular end-diastolic diameter (LVEDD) was assessed in the parasternal long-axis view. Due to format, a subset of echocardiograms were not available for assessment by the core laboratory (22 of 310, 7%); for these studies, the LVEF calculated locally was used.
STATISTICAL ANALYSIS
The Student t and Fisher exact tests were used to compare continuous and categorical variables by self-identified race (“black” vs. “white and other”). The Kaplan-Meier method was next used to estimate survival free from events (cardiac transplantation and need for mechanical circulatory support). Using the exact log-rank test, event-free survival was compared between racial subsets. For analysis of event rate by baseline LVEF, an initial LVEF <0.30 delineated approximately one-third of the cohort with the most severe LV dysfunction on presentation (initial LVEF <0.30, n = 30), and this subset was compared with those with moderate LV dysfunction (LVEF ≥0.30, n = 70). Finally, we compared by race and by initial LVEF the percentage of subjects who achieved the following status at the end of study: recovery (last LVEF ≥0.50), partial recovery (last LVEF 0.35 to 0.49), no recovery (last LVEF <0.35), and those with major events (death, transplantation, or LVAD).
A regression model with LVEF at 12 months as a continuous outcome and baseline LVEF as a predictor was used to examine the relationship between these 2 variables. We next used analysis of covariance to examine how race and baseline LVEF affect the final LVEF at 12 months. When this analysis is significant (p < 0.05), the reported beta coefficient (B) represents the slope or the number of units the outcome variable changes with a 1-unit change in the predictor variable. We then examined the univariate effects of different clinical and demographic factors on LVEF at 12 months using analysis of variance or regression models for categorical and continuous variables, respectively. After their identification at 12 months, the significant univariate predictors of LVEF were included in a multivariate analysis model. In a multivariate model, B is interpreted as the effect when holding all other variables constant. These analyses were performed using SPSS version 21 (IBM, Armonk, New York). Next we examined the change in LVEF (ΔLEVF, computed as LVEF at 12 months baseline LVEF) as a continuous outcome and tested the effects of baseline LVEF and race on LVEF change using linear regression models.
The presence of repeated measures of LVEF at 6 and 12 months post-partum allowed us to use both as outcome measures and evaluate LVEF recovery over time using a random-effects model that takes into account the correlation among data collected from the same subject. We first used a univariate analysis to assess the association of each variable with LVEF over time (both 6- and 12-month LVEF). After identifying significant univariate predictors of outcome, we used these factors to build a multivariate mixed-effects model. The Akaike information criteria were used for model comparison. Statistical significance was evaluated by likelihood ratio tests and the type I error controlled at 5%. All statistical analyses were conducted using R version 3.0.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
DEMOGRAPHIC AND CLINICAL CHARACTERISTICS
The cohort of 100 women included 30% with a self-designated race as black, 65% as white, and 5% as other, with a mean age of 30 ± 6 years (range, 18 to 43 years), gravida of 2.8 ± 1.9 (range, 1 to 10), and para of 2.2 ± 1.3 (range, 1 to 6). Two women were enrolled on the day of delivery and the rest post-partum, with the mean time post-partum at study entry of 31 ± 24 days (range, 0 to 95) (Table 1). The percentage of subjects in each New York Heart Association functional class (I to IV) at study entry was 12%/46%/25%/17%, respectively. Baseline vital signs were notable for a mean systolic blood pressure (BP) of 112 ± 17 mm Hg, diastolic BP of 70 ± 13 mm Hg, and heart rate of 86 ± 16 beats/min. At the time of study entry, 88% were receiving beta-blockers, 81% received angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, 15% were receiving inotropic therapy, and 2 were on an intra-aortic balloon pump. For the entire cohort, 10% had a family history of dilated cardiomyopathy, 11% were diabetic, 34% were smokers, 45% had a history of chronic or gestational hypertension, and 5% had a history of autoimmune illness. Only 1 woman was treated with bromocriptine, and 15% were breastfeeding at the time of enrollment. Overall, 66 women were enrolled early post-partum (median time from delivery to study entry, 14 days; 25% and 75% quartiles: 8 and 24 days, respectively), and 34 women were enrolled more than 6 weeks post-partum (median, 59 days; quartile range, 51 to 70 days).
TABLE 1.
Clinical Characteristics and Myocardial Recovery by Race
| All (N = 100) | Black (n = 30) | White or Other* (n = 70) | p Value | |
|---|---|---|---|---|
| Age, yrs | 30 ± 6 | 30 ± 6 | 30 ± 6 | 0.85 |
| NYHA functional class (I/II/III/IV) | 12/46/25/17 | 20/40/33/7 | 9/49/21/21 | 0.15 |
| % Familial | 10 | 9 | 13 | 0.48 |
| % With diabetes | 11 | 17 | 9 | 0.30 |
| % With hypertension | 45 | 70 | 34 | 0.002 |
| Days PP at entry, | 31 ± 24 | 42 ± 25 | 26 ± 22 | 0.002 |
| HR, beats/min | 86 ± 16 | 88 ± 13 | 85 ± 18 | 0.36 |
| SBP, mm Hg | 112 ± 17 | 116 ± 19 | 110 ± 16 | 0.09 |
| DBP, mm Hg | 71 ± 13 | 76 ± 12 | 68 ± 13 | 0.009 |
| On ACEi/ARB, % | 81 | 83 | 80 | 0.78 |
| On BB, % | 88 | 97 | 84 | 0.10 |
| LVEF at study entry | 0.35 ± 0.10 | 0.31 ± 0.09 | 0.36 ± 0.09 | 0.009 |
| LVEDD at study entry | 5.5 ± 0.07 | 5.8 ± 0.07 | 5.5 ± 0.07 | 0.04 |
| LVEF at 6 months | 0.51 ± 0.11 | 0.46 ± 0.14 | 0.53 ± 0.08 | 0.006 |
| LVEF at 12 months | 0.53 ± 0.10 | 0.47 ± 0.14 | 0.56 ± 0.07 | 0.001 |
| ΔLVEF at 12 months | 0.18 ± 0.11 | 0.17 ± 0.12 | 0.18 ± 0.10 | 0.68 |
| Recovered, % | 72 | 59 | 77 | 0.13 |
Values are mean ± SD unless otherwise indicated.
The white or other cohort by self-designated race is predominantly white (65 white and 5 other).
ACEi = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; BB = beta-blocker; DBP = diastolic blood pressure; HR = heart rate; LVEDD = left ventricular end-diastolic diameter; LVEF = left ventricular ejection fraction; NYHA = New York Heart Association; PP = post-partum; SBP = systolic blood pressure.
Comparing clinical characteristics by race, black women were more likely to be enrolled later (42 ± 25 days post-partum to study entry for black women vs. 26 ± 22 days for white or other; p = 0.002) and had a significantly lower LVEF at study entry (0.31 ± 0.09 mean LVEF at study entry for black women vs. 0.36 ± 0.09 for white or other; p = 0.009). Black women were also more likely to have a history of hypertension (70% vs. 34%; p = 0.002) and a higher mean diastolic BP at study entry (76 ± 12 mm Hg vs. 68 ± 13 mm Hg; p = 0.009). Medical therapy (percentage receiving angiotensin-converting enzyme inhibitors and beta-blockers) was similar between groups.
EVENT-FREE SURVIVAL
Survival and event data were available for 97 women at the 6-month and 91 women at the 12-month time points. During the first year post-partum, 6 women experienced 9 major events: 4 deaths, 4 LVAD implantations, and 1 heart transplantation. Of the 3 women who required LVAD support early (within the first 3 months postpartum), 2 died while on an LVAD, and 1 underwent a transplantation. One additional subject required mechanical support more than 11 months after delivery and remained on the LVAD at the time of 12-month follow up. Besides the 6 subjects with major events, only 3 additional women experienced cardiac hospitalizations. The event-free survival rate (survival without LVAD implantation or cardiac transplantation) at 1 year was 93% (event rate, 7%), whereas transplantation-free survival (not including LVAD implantation as an endpoint) was 95%. Event-free survival was similar between black and white or other women (p = 0.44), but significantly worse for women with a baseline LVEF <0.30, compared with those with LVEF ≥0.30 (1-year event-free survival rate, 82% vs. 99%; p = 0.004 (Figure 1).
FIGURE 1. Event Rate by Baseline LVEF.
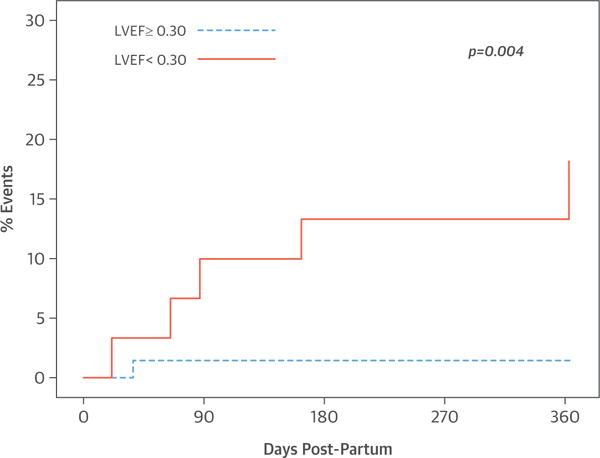
The percentage of women who died or underwent LVAD implantation during the first year post-partum. Dashed blue line = women with an initial LVEF ≥0.30; solid red line = women with an initial LVEF <0.30. The event rate was significantly higher in women with an LVEF <0.30, p = 0.004. LVAD = left ventricular assist device; LVEF = left ventricular ejection fraction.
IMPROVEMENT OF LVEF DURING THE FIRST YEAR POST-PARTUM
For the entire cohort, the mean LVEFs at study entry, 6 months, and 12 months post-partum were 0.35 ± 0.10, 0.51 ± 0.11, and 0.53 ± 0.10, respectively (Figure 2A). For women enrolled within the first few weeks post-partum, much of the recovery was noted early, by the 2-month assessment. For women enrolled early post-partum (n = 66), LVEFs at baseline, 2 months, 6 months, and 1 year were 0.35 ± 0.09, 0.48 ± 0.10, 0.53 ± 0.10, and 0.55 ± 0.08, respectively. For women enrolled later (after 6 weeks post-partum, n = 34), mean LVEFs at study entry were similar (0.34 ± 0.10; p = 0.55), but the 6- and 12-month LVEFs were significantly lower (mean LVEF for subjects enrolled late at 6 months = 0.47 ± 0.12; p = 0.02; 12 months = 0.49 ± 0.14; p = 0.03).
FIGURE 2. LVEF Over Time Post-Partum.
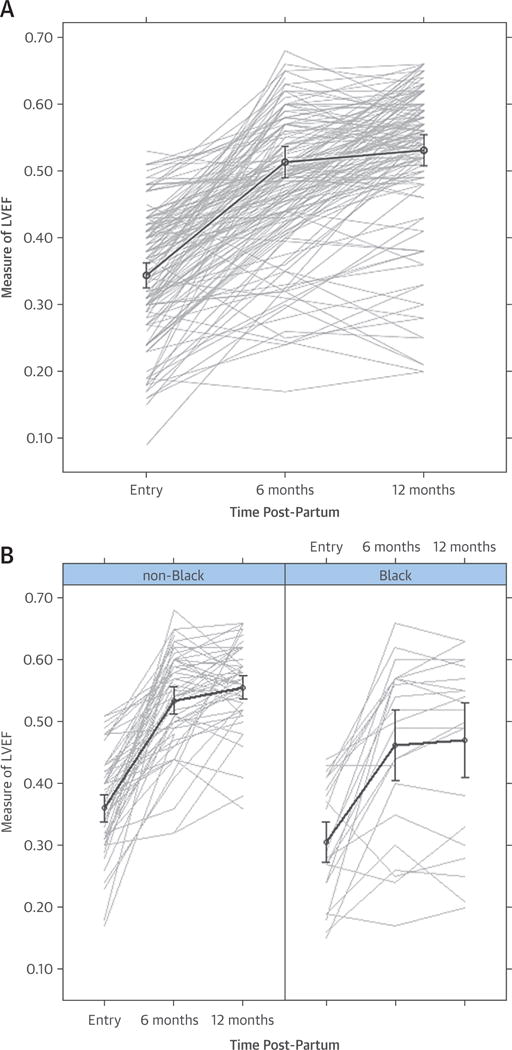
(A) Overall cohort. LVEF at study entry, 6, and 12 months post-partum for the entire cohort. (B) By race. The LVEF was significantly poorer in black women with PPCM at study entry (p = 0.009), 6 months (p = 0.006), and 12 months post-partum (p = 0.001). LVEF = left ventricular ejection fraction; PPCM = post-partum cardiomyopathy.
Follow-up LVEFs differed by race at 6 months (black = 0.46 ± 0.14, white or other = 0.53 ± 0.08; p = 0.006) and 12 months (black = 0.47 ± 0.14, white or other = 0.56 ± 0.07; p = 0.001) (Figure 2B). Women with more severe LV dysfunction at study entry (LVEF <0.30) had significantly lower LVEFs at 6 and 12 months. For women with an LVEF <0.30 at study entry, mean LVEFs at baseline and 6 and 12 months were 0.23 ± 0.05, 0.45 ± 0.14, and 0.46 ± 0.14, respectively; whereas for women with an LVEF at study entry ≥0.30, the mean LVEFs at study entry and 6 and 12 months were 0.39 ± 0.06 (p < 0.001), 0.54 ± 0.08 (p < 0.001), and 0.56 ± 0.07 (p < 0.001), respectively (Figure 3A). Women with an LVEDD ≥6.0 cm (n = 25) at study entry, compared with those with an LVEDD <6.0 cm (n = 74), had a lower LVEF at baseline (mean LVEF at study entry = 0.31 ± 0.10 vs. 0.36 ± 0.09) (p = 0.04), and at 6 and 12 months (mean LVEF at 6 months = 0.40 ± 0.13 vs. 0.55 ± 0.07; p < 0.001; 12 months = 0.42 ± 0.13 vs. 0.56 ± 0.07; p < 0.001) (Figure 3B).
FIGURE 3. Initial LVEF and LVEDD and LVEF Over Time.
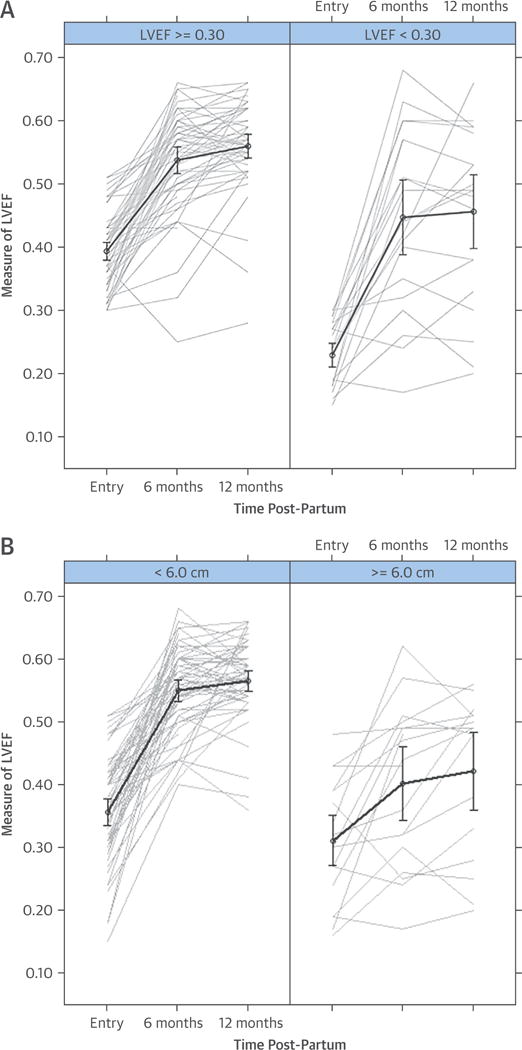
(A) Initial LVEF <0.30 or ≥0.30. In women with severe LV dysfunction at study entry, poorer LV function persists at 6 months (p < 0.001) and 12 months (p < 0.001) postpartum. (B) Initial LVEDD <6.0 or ≥6.0 cm. Women with greater LV remodeling at entry (LVEDD ≥6.0 cm) demonstrate a lower LVEF at study entry (p = 0.04) and less recovery at 6 months (p < 0.001) and 12 months (p < 0.001) post-partum. LV = left ventricular; LVEDD = left ventricular end-diastolic diameter.
STATUS AT THE END OF THE STUDY BY RACE AND INITIAL LVEF
Of the 92 women with either an event or complete recovery data, poor outcomes were evident in only 12 (13%), including 6 with major events and 6 with persistent severe cardiomyopathy. Partial recovery was evident in 14 women (15%) and complete recovery in 66 women (72%). Among women with a baseline LVEF <0.30, 37% achieved a final LVEF ≥0.50, whereas an equal number (37%) either had an event or a final LVEF <0.35. In contrast, for women with an initial LVEF ≥0.30, 86% achieved a final LVEF >0.50, whereas only 3% had an event or final LVEF <0.35 (p < 0.001) (Figure 4). Final status was significantly worse in black women (n = 27), as only 59% achieved a final LVEF ≥0.50 versus 77% of whites or others (n = 65), whereas 26% of blacks had either an event or a final LVEF <0.35 versus only 8% of whites or others (p = 0.03). Combining baseline LVEF and LVEDD allowed a more accurate prediction of recovery. For women with both an LVEF ≥0.30 and an LVEDD <6.0 cm on presentation (n = 55), 91% recovered to a final LVEF ≥0.50 compared with just 62% of women with only 1 of these characteristics (n = 26), and 0% (n = 10) of women with both an LVEF <0.30 and an LVEDD ≥6.0 cm (p < 0.00001).
FIGURE 4. Final Status Based on the Initial LVEF.
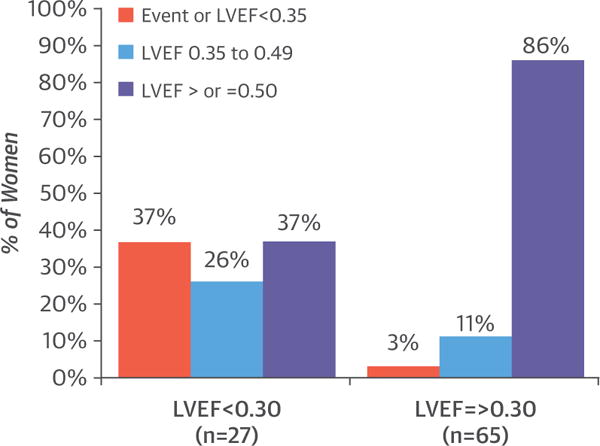
Comparison of status at the end of the study based on the initial LVEF. Red column, percentage of women with no recovery (event or final LVEF <0.35); blue column, percentage of women with partial recovery (final LVEF 0.35 to 0.49); purple column, percentage of women with complete recovery (LVEF ≥0.50). Recovery was evident in 86% of women with a baseline LVEF ≥0.30, compared with 37% of those with an LVEF <0.30, p < 0.001. LVEF = left ventricular ejection fraction.
EFFECT OF RACE AND BASELINE LVEF ON LVEF AT 12 MONTHS
Panel A of the Central Illustration shows the analysis of covariance model examining the joint effects of baseline LVEF and race on 12-month LVEF values. Race (B = 31.68; SE = 8.56; p < 0.0001) and baseline LVEF (B = 0.882; SE = 0.203; p < 0.0001) were both significant predictors of LVEF at 12 months. In addition, we observed a significant interaction between race and LVEF at baseline (B = 0.789; SE = 0.25; p = 0.002). In subgroup analyses, we observed a significant association between baseline LVEF and LVEF at 12 months only in blacks (B = 0.88; SE = 0.28; p = 0.005) but not in whites and others (B = 0.093; SE = 0.12; p = 0.44).
CENTRAL ILLUSTRATION. Myocardial Recovery and Outcomes in Peripartum Cardiomyopathy: Baseline LVEF, Race, and 12-Month Outcome—Absolute LVEF and Change in LVEF From Baseline.
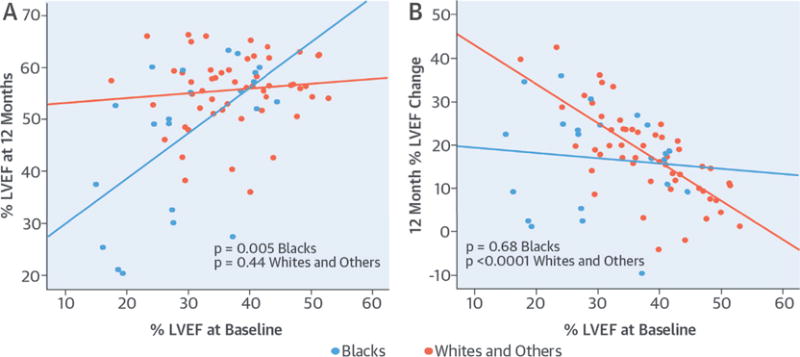
(A) Absolute LVEF at 12 months. The y-axis shows the LVEF at 12 months; the baseline LVEF is shown on the x-axis. A significant association was seen in blacks (p < 0.005) but not in whites and others (p = 0.44). (B) Change in LVEF from baseline to 12 months. The change in LVEF is shown on the y-axis and baseline LVEF is shown on the x-axis. A significant association was seen in whites and others (p < 0.0001) but not in blacks (p = 0.68). LVEF = left ventricular ejection fraction.
EFFECT OF RACE AND BASELINE LVEF ON 12-MONTH CHANGE IN LVEF
The change in LVEF at 12 months (ΔLVEF) was significantly associated with baseline LVEF only (B = −0.52; SE = 0.12; p < 0.001), as a lower baseline LVEF was associated with more improvement (greater ΔLVEF). Subset analysis by race demonstrated that baseline LVEF was significantly associated with ΔLVEF at 12 months in whites and others (B = −0.91; SE = 0.12; p < 0.0001) but showed no association with ΔLVEF in blacks (B = −0.118; SE = 0.28; p = 0.68 (Central Illustration, panel B). As white subjects had a greater tendency to recover, a lower LVEF was associated with a greater ΔLVEF at 12 months. In contrast, the ΔLVEF for black women appeared independent of their LVEF at study entry. The ΔLVEF at 12 months for the overall cohort appeared similar by race (ΔLVEF for blacks 0.17 ± 0.12 versus whites 0.18 ± 0.10; p = 0.68) (Table 1). However, the ΔLVEF was significantly higher for whites with a lower initial LVEF (ΔLVEF whites with initial LVEF <0.35 = 0.25 0.09) compared with whites with a higher initial LVEF (initial LVEF ≥0.35, ΔLVEF = 0.13 ± 0.08; p < 0.0001) to a much greater degree than was evident in black women (ΔLVEF with initial LVEF <0.35 vs. ≥0.35 in blacks = 0.18 ± 0.13 vs. 0.15 ± 0.11; p = 0.47).
UNIVARIATE AND MULTIVARIATE ANALYSES OF BASELINE PREDICTORS OF LVEF AT 12 MONTHS
On univariate analysis of the predictors of LVEF at 12 months, initial LVEF at study entry, LVEDD, race, body mass index (BMI), and time post-partum to presentation were univariate predictors of subsequent LVEF at 12 months (Table 2). There was no difference in LVEF at 12 months for women who breastfed (n = 15) compared with those who did not. There was no apparent difference in 12-month LVEF for women with a family history of dilated cardiomyopathy in a primary relative (n = 10) versus women with no such history. In addition, there was no apparent difference in recovery on the basis of multiparity, maternal age, BP, or New York Heart Association functional class at presentation. Multivariate analysis demonstrated a significant interaction between black race and baseline LVEF for their impact on 12-month LVEF (B = 0.72; SE = 0.23; p = 0.002). This interaction mirrors the results seen in the linear regression shown in panel A of the Central Illustration, and supports the observation that baseline LVEF has a much greater impact on 12-month LVEF in black women than it does in white women. A multivariate model with all significant univariate predictors, which accounted for the interaction of race and baseline LVEF, demonstrated that race and LVEDD remained significant predictors of LVEF at 12 months, whereas baseline LVEF, BMI, and time to presentation were no longer significant (Table 2).
TABLE 2.
Univariate and Multivariate Analysis Predictors of 12-Month LVEF
| Fixed Effect | Beta | SE | p Value |
|---|---|---|---|
| Univariate
| |||
| Black | −8.65 | 2.44 | 0.001 |
| Hypertension | 0.102 | 2.39 | 0.966 |
| Family history of DCM | −3.21 | 4.12 | 0.44 |
| Diabetes | −5.01 | 3.26 | 0.16 |
| Breastfeeding | 4.94 | 2.95 | 0.10 |
| NYHA functional class | |||
| II | 1.61 | 3.68 | 0.66 |
| III | −7.21 | 4.08 | 0.08 |
| IV | −3.73 | 4.29 | 0.39 |
| ACEi/ARB | 3.91 | 3.15 | 0.22 |
| Beta-blocker | −1.602 | 4.17 | 0.70 |
| Days post-partum at entry | −0.11 | 0.048 | 0.02 |
| Baseline LVEF | 0.481 | 0.12 | <0.0001 |
| LVEDD | −0.82 | 0.15 | <0.0001 |
| Age | 0.127 | 0.189 | 0.50 |
| Parity | −0.34 | 0.94 | 0.72 |
| SBP, mm Hg | 0.016 | 0.07 | 0.83 |
| DBP, mm Hg | −0.044 | 0.102 | 0.66 |
| Heart rate, beats/min | −0.08 | 0.07 | 0.26 |
| BMI | −0.379 | 0.177 | 0.04 |
|
Multivariate | |||
| Baseline LVEF | 0.04 | 0.14 | 0.79 |
| Baseline LVEDD | −0.66 | 0.17 | <0.0001 |
| Black | −26.03 | 7.99 | 0.002 |
| BMI | −0.04 | 0.15 | 0.77 |
| Time post-partum | −0.06 | 0.04 | 0.18 |
Significant univariate predictors of LVEF at 12 months included race, baseline LVEF, BMI, and days post-partum to study entry. The beta coefficient represents the slope or the number of units the outcome variable changes with 1-unit change in the predictor variable. These factors were then included in a multivariate model that also included an interaction term for race and baseline LVEF (significant interaction of race baseline LVEF for their impact on 12-month LVEF: beta = 0.72; SE = 0.23; p = 0.002). Only race and LVEDD remain significant in the multivariate analysis.
BMI = body mass index; DCM = dilated cardiomyopathy; other abbreviations as in Table 1.
ANALYSIS OF BASELINE PREDICTORS OF LVEF OVER TIME (COMBINED ENDPOINTS OF 6- AND 12-MONTH LVEFs)
Evaluating both 6- and 12-month LVEFs as combined endpoints (random-effects model) mirrors the findings with the 12-month endpoint alone. Univariate analysis determined that baseline LVEF (B = 0.44; SE = 0.11; p < 0.001), LVEDD (B = −0.83; SE = 0.13; p < 0.001), black race (B = −7.46; SE = 2.25; p = 0.001), BMI (B = −0.42; SE = 0.15; p = 0.008), and days post-partum to presentation (B = 0.11; SE = 0.04; p = 0.02) were again significant predictors. Multivariate analysis accounting for the interaction of race and baseline LVEF to LVEF over time (B = 0.54; SE = 0.21; p = 0.01) demonstrated once more that the most significant predictor was LVEDD (B = −0.68; SE = 0.21; p < 0.0001) followed by race (B = −18.82; SE = 7.19; p = 0.01), whereas baseline LVEF (B = 0.01; SE = 0.13; p = 0.92), BMI (B = −0.15; SE = 0.13; p = 0.27), and time to presentation (B = −0.07; SE = 0.0.4; p = 0.18) were not significant.
DISCUSSION
In this prospective cohort of women with PPCM treated with standard heart failure therapy, the overwhelming majority recovered. In 66 women, representing 72% of those with a complete assessment, LVEF improved to ≥0.50 by 12 months. Indeed, initial echocardiographic assessment delineated a large subset of women (>50% of the total cohort) presenting without significant remodeling (LVEDD <6.0 cm) or severe LV dysfunction (LVEF ≥0.30), in whom the probability of recovery exceeded 90%. Although this degree of recovery is encouraging, poor outcomes were still evident in 12 women (13%), including 6 who had a persistent severe cardiomyopathy and another 6 who either died or required LVAD implantation by 1 year. Of the clinical and demographic variables evaluated, LV remodeling (LVEDD) at entry and black race, demonstrated the strongest association with lower LVEF at 12 months post-partum.
There was a clear interaction of race and LV recovery over time. Although the mean change in LVEF at 12 months was similar in black and white subjects, in blacks, the initial LVEF was predictive of the 12-month LVEF, whereas the change of LVEF was independent of the initial LVEF. In contrast, in whites, a lower initial LVEF was associated with greater improvement at 12 months, and therefore the LVEF at 12 months appears independent of LVEF at baseline. As a result, black women presented with a lower initial LVEF that persisted at 6 and 12 months post-partum. Black race has been a persistent risk factor for the development of PPCM in the United States (16), and the findings from this analysis support previous retrospective studies regarding the impact of race on myocardial recovery in PPCM (13). Previous studies have suggested that poorer LV recovery in black subjects with recent-onset non-ischemic cardiomyopathy may not be limited to PPCM. In the IMAC2, a prospective study of LV recovery in recent-onset nonischemic cardiomyopathy, poorer recovery was noted in black men, black women with cardiomyopathy not associated with pregnancy, and black women with PPCM compared with white subjects (17,18).
A recent retrospective analysis of a predominantly African-American cohort reported worse outcomes than have historically been reported, with high mortality and complete recovery in only 23% of women (19). In contrast, in the current study, nearly 60% of black women did achieve complete recovery (final LVEF ≥0.50). Furthermore, event-free survival did not differ by race, demonstrating the importance of prospective data in defining prognosis. Given the 8 patients lost to follow-up, it is possible that the overall event rate was higher than the 7% reported at 1 year. However, losses to follow-up did not differ by race and are unlikely to have influenced this analysis.
STUDY LIMITATIONS
One limitation of the current study is the variation in the time post-partum to study entry, from the day of delivery to nearly 3 months post-partum. In general, women who presented later had a lower LVEF at 12 months postpartum, but whether this represented a difference in initial disease severity cannot be determined. Black women, on average, presented later post-partum, as more than one-half were enrolled more than 6 weeks post-partum compared with only 22% of white women. In addition, black women had a much higher prevalence of hypertension (70% vs. 34%). Although medical therapy at entry was similar in racial subsets, whether the delay in presentation or the increased prevalence of hypertension among black women contributed to lower LVEF at entry remains to be determined.
Recently, the use of bromocriptine to inhibit proactin release has been advocated as a targeted therapy for PPCM (20,21). A 2013 report of a large series from Germany (22) found that the greatest improvement occurred in PPCM patients receiving a combination of conventional therapy (beta-blockers and angiotensin-converting enzyme inhibitors) plus bromocriptine. However, these investigators did not find any difference in the percentage of PPCM women who reached full recovery after receiving bromocriptine compared with those who did not. Indeed, the recovery rate in this German study is comparable to that of the current investigation on conventional therapy alone. A randomized trial to address the role of bromocriptine therapy in PPCM is currently in progress in Europe.
Breastfeeding prolongs the increase in prolactin in the post-partum period, and some have advocated avoidance of breastfeeding as a recommendation for PPCM mothers. Given the importance of breastfeeding to infant survival (23) in developing nations, this prohibition comes at a substantial cost in geographic areas of Africa, Asia, and the Caribbean, where PPCM is more prevalent. The immunological, developmental, and nutritional advantages of breastfeeding over the alternatives are increasingly recognized, and recommendations limiting this practice would also have hidden costs in more developed countries. In the current study, breastfeeding was not associated with any diminishment of recovery, and a previous retrospective Internet-based study suggested better outcomes in women who breastfed compared with those who did not (24). The absence of a hazard for breastfeeding in IPAC suggests a relatively weaker influence of prolactin or prolactin metabolites in the pathogenesis of PPCM than has been currently postulated and supports the need for additional study before solidifying recommendations of breastfeeding for PPCM mothers.
Although genetic predispositions have been increasingly recognized as a risk factor for the development of PPCM (25), the role of genetics for predicting subsequent recovery remains unknown. A family history of dilated cardiomyopathy in IPAC did not predict a poorer subsequent recovery. The small percentage of women (10%) reporting a family history likely underestimates the importance of genetics in the development of PPCM. Future analysis through molecular diagnostics will help to clarify the role of genetic predisposition, both for the development of PPCM and in predicting subsequent recovery.
CONCLUSIONS
The results of the prospective IPAC study demonstrated significant recovery in the majority of women with PPCM and should be very encouraging to women presenting with this disorder and to their physicians. Unfortunately, adverse outcomes remain unacceptably high, with 6% dead, having undergone transplantation, or on an LVAD at 1 year, and more than 20% left with some degree of chronic cardiomyopathy. Black women, already at greater risk of the development of this disorder, demonstrated a lower mean LVEF, both at presentation and 12 months post-partum. There remains a great need for more targeted therapies to improve outcomes in those women whose probability of recovery on conventional therapy is diminished. A poor LVEF at presentation (<0.30) and a greater degree of LV dilation (LVEDD ≥6.0 cm) appear to predict poorer subsequent recovery with conventional therapy. Trials that target PPCM women with these 2 characteristics may permit a better assessment of novel therapeutic interventions.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE
The majority of women presenting with PPCM recover myocardial function completely with conventional heart failure therapy during the first year post-partum. Women with more severe LV dysfunction or greater LV remodeling at presentation have less chance of full recovery.
TRANSLATIONAL OUTLOOK
Given the high rate of recovery of LV function with conventional therapy alone, additional studies are needed to identify predictors of poorer outcome as a target for future trials of novel therapeutic interventions.
Acknowledgments
This investigation was supported by the National Heart, Lung, and Blood Institute through contract HL102429.
ABBREVIATIONS AND ACRONYMS
- BMI
body mass index
- BP
blood pressure
- LV
left ventricular
- LVAD
left ventricular assist device
- LVEDD
left ventricular end-diastolic diameter
- LVEF
left ventricular ejection fraction
- ΔLVEF
change in left ventricular ejection fraction
- PPCM
peripartum cardiomyopathy
APPENDIX
For a complete list of the IPAC investigators, please see the online version of this article.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclosure.
Listen to this manuscript’s audio summary by JACC Editor-in-Chief Dr. Valentin Fuster.
References
- 1.Elkayam U. Clinical characteristics of peripartum cardiomyopathy in the United States: diagnosis, prognosis, and management. J Am Coll Cardiol. 2011;58:659–70. doi: 10.1016/j.jacc.2011.03.047. [DOI] [PubMed] [Google Scholar]
- 2.Mielniczuk LM, Williams K, Davis DR, et al. Frequency of peripartum cardiomyopathy. Am J Cardiol. 2006;97:1765–8. doi: 10.1016/j.amjcard.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 3.Fett JD, Christie LG, Carraway RD, et al. Five-year prospective study of the incidence and prognosis of peripartum cardiomyopathy at a single institution. Mayo Clin Proc. 2005;80:1602–6. doi: 10.4065/80.12.1602. [DOI] [PubMed] [Google Scholar]
- 4.Sliwa K, Förster O, Libhaber E, et al. Peripartum cardiomyopathy: inflammatory markers as predictors of outcome in 100 prospectively studied patients. Eur Heart J. 2006;27:441–6. doi: 10.1093/eurheartj/ehi481. [DOI] [PubMed] [Google Scholar]
- 5.Brar SS, Khan SS, Sandhu GK, et al. Incidence, mortality, and racial differences in peripartum cardiomyopathy. Am J Cardiol. 2007;100:302–4. doi: 10.1016/j.amjcard.2007.02.092. [DOI] [PubMed] [Google Scholar]
- 6.Harper MA, Meyer RE, Berg CJ. Peripartum cardiomyopathy: population-based birth prevalence and 7-year mortality. Obstet Gynecol. 2012;120:1013–9. doi: 10.1097/aog.0b013e31826e46a1. [DOI] [PubMed] [Google Scholar]
- 7.Pearson GD, Veille JC, Rahimtoola S, et al. Peripartum cardiomyopathy: National Heart, Lung, and Blood Institute and Office of Rare Diseases (National Institutes of Health) workshop recommendations and review. JAMA. 2000;283:1183–8. doi: 10.1001/jama.283.9.1183. [DOI] [PubMed] [Google Scholar]
- 8.Gleicher N, Elkayam U. Peripartum cardiomyopathy, an autoimmune manifestation of allograft rejection? Autoimmun Rev. 2009;8:384–7. doi: 10.1016/j.autrev.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Pulerwitz TC, Cappola TP, Felker GM, et al. Mortality in primary and secondary myocarditis. Am Heart J. 2004;147:746–50. doi: 10.1016/j.ahj.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 10.Morales A, Painter T, Li R, et al. Rare variant mutations in pregnancy-associated or peripartum cardiomyopathy. Circulation. 2010;121:2176–82. doi: 10.1161/CIRCULATIONAHA.109.931220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Spaendonck-Zwarts KY, van Tintelen JP, van Veldhuisen DJ, et al. Peripartum cardiomyopathy as a part of familial dilated cardiomyopathy. Circulation. 2010;121:2169–75. doi: 10.1161/CIRCULATIONAHA.109.929646. [DOI] [PubMed] [Google Scholar]
- 12.Patten IS, Rana S, Shahul S, et al. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature. 2012;485:333–8. doi: 10.1038/nature11040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goland S, Modi K, Bitar F, et al. Clinical profile and predictors of complications in peripartum cardiomyopathy. J Cardiac Fail. 2009;15:645–50. doi: 10.1016/j.cardfail.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 15.Authors/Task Force Members. McMurray JJ, Adamopoulos S, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012. Eur Heart J. 2012;33:1787–847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 16.Kao DP, Hsich E, Lindenfeld J. Characteristics, adverse events, and racial differences among delivering mothers with peripartum cardiomyopathy. J Am Coll Cardiol HF. 2013;1:409–16. doi: 10.1016/j.jchf.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McNamara DM, Starling RC, Cooper LT, et al. for the IMAC Investigators Clinical and de mographic predictors of outcomes in recent onset dilated cardiomyopathy: results of the IMAC (Intervention in Myocarditis and Acute Cardiomyopathy)-2 Study. J Am Coll Cardiol. 2011;58:1112–8. doi: 10.1016/j.jacc.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooper LT, Mather PJ, Alexis JD, et al. Myocardial recovery in peripartum cardiomyopathy; prospective comparison with recent onset cardiomyopathy in men and nonperipartum women. J Card Fail. 2012;18:28–33. doi: 10.1016/j.cardfail.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pillarisetti J, Kondur A, Alani A, et al. Peripartum cardiomyopathy: predictors of recovery and current state of implantable cardioverter-defibrillator use. J Am Coll Cardiol. 2014;63:2831–9. doi: 10.1016/j.jacc.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 20.Hilfiker-Kleiner D, Kaminski K, Podewski E, et al. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell. 2007;128:589–600. doi: 10.1016/j.cell.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 21.Sliwa K, Blauwet L, Tibazarwa K, et al. Evaluation of bromocriptine in the treatment of acute severe peripartum cardiomyopathy: a proof-of-concept pilot study. Circulation. 2010;121:1465–73. doi: 10.1161/CIRCULATIONAHA.109.901496. [DOI] [PubMed] [Google Scholar]
- 22.Haghikia A, Podewski E, Libhaber E, et al. Phenotyping and outcome on contemporary management in a German cohort of patients with peripartum cardiomyopathy. Basic Res Cardiol. 2013;108:366. doi: 10.1007/s00395-013-0366-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathur NB, Dhingra D. Breastfeeding. Indian J Pediatr. 2014;81:143–9. doi: 10.1007/s12098-013-1153-1. [DOI] [PubMed] [Google Scholar]
- 24.Safirstein JG, Ro AS, Grandhi S, et al. Predictors of left ventricular recovery in a cohort of peripartum cardiomyopathy patients recruited via the internet. Int J Cardiol. 2012;154:27–31. doi: 10.1016/j.ijcard.2010.08.065. [DOI] [PubMed] [Google Scholar]
- 25.van Spaendonck-Zwarts KY, Posafalvi A, van den Berg MP, et al. Titin gene mutations are common in families with both peripartum cardiomyopathy and dilated cardiomyopathy. Eur Heart J. 2014;35:2165–73. doi: 10.1093/eurheartj/ehu050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


