Abstract
When we search for visual targets in a cluttered background we systematically move our eyes around to bring different regions of the scene into foveal view. We explored how visual search behavior changes when the fovea is not functional, as is the case in scotopic vision. Scotopic contrast sensitivity is significantly lower overall, with a functional scotoma in the fovea. We found that in scotopic search, for a medium- and a low-spatial-frequency target, individuals made longer lasting fixations that were not broadly distributed across the entire search display but tended to peak in the upper center, especially for the medium-frequency target. The distributions of fixation locations are qualitatively similar to those of an ideal searcher that has human scotopic detectability across the visual field, and interestingly, these predicted distributions are different from those predicted by an ideal searcher with human photopic detectability. We conclude that although there are some qualitative differences between human and ideal search behavior, humans make principled adjustments in their search behavior as ambient light level decreases.
Keywords: visual search, scotopic vision, scotopic sensitivity, eye movements
1. Introduction
Looking for a specific object in the environment is an everyday task. Searching for a known face in a crowd, some pen on a cluttered desk, or – from a more evolutionary perspective – a fruit hidden in foliage are only few examples. Visual search is considered to be one of the most fundamental perceptual tasks (Geisler, Perry, & Najemnik, 2006; Wolfe, 2010). Humans have a large field of view, but visual acuity is not uniformly good across the retina. It shows a maximum within the central 2° of visual angle, and declines continuously into the periphery (e.g. Pointer & Hess, 1989). In most cases the visual scene under consideration is larger than the area that can be inspected during one single fixation. Thus, this area of highest resolution, the fovea, has to be guided to multiple locations in the scene during active visual search in order to find the desired object. Humans use saccades, high-speed eye movements, to bring the projected images of possible target locations onto the fovea. The underlying mechanisms (e.g. how fixation locations are selected or how the specific moment of saccade execution is determined by the visual system) and the efficiency of the search process are a matter of extensive research (e.g. Eckstein, Beutter, & Stone, 2001; Findlay, 1997; Najemnik & Geisler, 2005; 2008; 2009; Motter & Belky, 1998; Wolfe, 2007; Wolfe & Gencarz, 1996).
Guiding the fovea to different locations in the visual field in order to find a target object seems an optimal strategy given the drastic decrease in resolution towards the periphery. Moreover, moving the eyes and thus the fovea seems the only way to effectively use the given combination of a large field of view with only a small spot of high resolution. However, different natural conditions can have fundamental effects on how target visibility varies across the visual field. Due to the distribution of photoreceptors in the retina, humans experience a central scotoma when exposed to scotopic lighting conditions. Scotopic vision, often also referred to as night vision, is defined as low-light vision that lies below cone threshold and is thus mediated only by rods, for example under starlight. Since the fovea solely contains cones, this region with highest resolution under photopic conditions, is not operating under dim scotopic illumination. This can easily be experienced when trying to fixate a dim star on the night sky — its image vanishes as soon as the fovea lands on it.
Scotopic visual search must thus rely on peripheral information. By using gaze-contingent displays it has been shown that search performance deteriorates when vision in the periphery is limited (Geisler et al., 2006; Loschky & McConkie, 2002). This suggests that peripheral information can be used effectively in photopic visual search tasks, presumably both to detect the target and guide the eye movements. However, variations in visibility within gaze-contingent displays are artificial, whereas the central scotoma in scotopic vision is natural. It is arguable whether both types are treated similarly by the visual system or whether for instance rather explicit but unfamiliar changes in the visibility map affect search behavior in a different manner than natural, previously experienced but presumably more subtle changes. More importantly, scotopic vision is different from peripheral photopic vision in that the latter is mediated by cones. As stated above, rods have a higher luminous sensitivity than cones and they are differently distributed on the retina. Cones peak in the fovea, where there are no rods, whereas the rods are more evenly distributed with a shallow peak at about 20° in the periphery (Curcio, Sloan, Kalina & Hendrickson, 1990). Furthermore, both types of photoreceptors have different spectral, spatial and temporal characteristics. The spectral sensitivity of rods peaks at about 505 nm (Wyszecki & Stiles, 1982), the ones of cones peak at about 570 nm, 545 nm, and 445 nm, for red, green and blue cones respectively (2° fundamentals; Stockman & Sharpe, 2000). Due to the different pathways that the rod and cone signals enter, rod vision is slower than cone vision; it is delayed by about 67 ms (MacLeod, 1972), though the actual delay may depend on the adaptation state (Sharpe, Stockman, & MacLeod, 1989). Since rods show a larger neural convergence than cones the photoreceptors also differ in their spatial sensitivity. On average, rods peak at 0.9 cpd and cones at 2.8 cpd (D’Zmura & Lennie, 1986), but spatial sensitivity also depends on the eccentricity (Hess, Nordby, Pointer, 1987).
Taken together, having two types of photoreceptors with different luminous sensitivity enables the visual system to work effectively under a huge range of illuminations, but it also entails many other substantial differences between photopic and scotopic vision. A foveated visual search strategy optimized for cone vision will be suboptimal or even unsuccessful under scotopic illumination since the observer would make eye movements to bring probable target locations onto the fovea but the foveal image of this position would fade as soon as the eyes land there. Here, we address the question whether the human visual system modifies its search strategy to meet the properties of rod vision. Adaptation of the search strategy to different lighting conditions would require the visual system to have representations of separate scotopic and photopic (and potentially also mesopic) visibility maps that can be used in order to guide visual search. An adjusted scotopic search strategy would – depending on the task and the size of the target – not aim to bring images of probable target locations onto the fovea, but, presumably, onto a different part of the retina. This could either mean that eye movements are used to bring these images onto the peripheral part of the retina with the highest visibility for a given target (e.g., the development of a pseudo fovea) or that no eye movements are executed if the target is adequately visible in all parts of the periphery. The purpose of the present study was to directly address this question by letting observers do a visual search task similar to the one reported by Najemnik and Geisler (2005; 2008; 2009) under photopic as well as scotopic viewing conditions.
2. Methods and materials
To compare saccadic eye movements in visual search under scotopic and photopic viewing conditions, two experiments were conducted. The first one was a detection experiment to measure the contrast sensitivity of our observers for the targets at different locations in the visual field. The second one was a visual search paradigm, directly measuring different variables of search behavior. Each experiment included four conditions, two scotopic ones and two photopic ones, which varied in the spatial frequency and contrast of the target as well as the size of the visual field under investigation.
2.1. General methods
2.1.1. Observers
Observers were four female students of the University of Gießen and one of the authors (VCP). On average they were 21.8 years old (SD = 1.3). All observers gave their informed consent to participate and were naïve to the aims of the study (except for the author). In two conditions (low-sf and high-sf) only two observers participated (one of them the author and one naïve subject). Visual acuity of the observers was measured with Landolt rings prior to their participation in the experiment. All five participants had a visual acuity of 1.4 and had normal night vision according to subjective reports. For their participation the naïve subjects received either money (eight euro per hour) or course credit. The experiments were carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) and approved by the local ethics committee LEK FB06 at Giessen University (proposal number 2009-0008). Informed consent was obtained from all observers.
2.1.2. Apparatus and eye movement recordings
Eye positions were recorded with a head-mounted eye tracker (EyeLink II; SR Research, Osgoode, Ontario, Canada) at a sampling frequency of 500 Hz. Calibration was done prior to every block using a 9-fixation-point-procedure. Larger calibration targets were used in the scotopic conditions to enable subjects to accurately fixate its center even if it fades under this viewing condition, because they could use the endpoints as anchors, i.e. either a white cross (2° × 2°) or black and white concentric circles (largest diameter 2°). The eye tracker was calibrated so that the accuracy of calibration was not worse than 0.35° in the photopic condition and 0.70° in the scotopic condition. When required, the calibration procedure could be repeated during a block, but this was rarely necessary. In addition, drift correction was done prior to every trial.
Visual stimuli were presented on a calibrated CRT screen with a resolution of 1280 × 1024 pixels and a refresh rate of 100 Hz. A correction of the monitor’s gamma nonlinearity was applied based on prior photometric measures. Subjects’ head positions were stabilized with a chin rest. Stimuli were viewed binocularly from a distance of 47 cm. The experiment took place in a windowless light-tight room. The walls of the room were black and all potential light sources or highly reflective surfaces were either removed or covered. In addition to the light from the monitor the room was dimly lit by a small lamp in the photopic conditions. For the scotopic conditions four neutral density filters (LEE Filters, Burbank, CA) were mounted in front of the monitor, all other light sources were switched off. The remaining light emitted from a black patch displayed on the monitor had a luminance of 0.0001 cd/m2 and for a white patch 0.0156 cd/m2. The luminance for a mid grey patch was 0.008 cd/m2. In the photopic condition the luminance for the grey patch on the monitor was 30.64 cd/m2, the luminance for a white area was 60.09 cd/m2 and for a black patch 0.0002 cd/m2. To achieve asymptotic scotopic sensitivity subjects dark adapted for 20 minutes prior to each experimental task of scotopic viewing conditions.
2.1.3. Stimuli
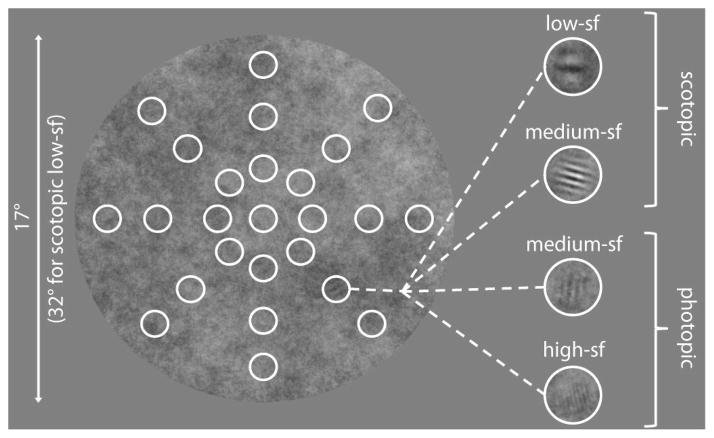
Target stimuli were circular achromatic Gabor patches (SD = 0.5°) with a spatial frequency of 2.5 cpd in the medium-sf conditions, 1 cpd in the low-sf condition, and 6 cpd in the high-sf condition. The medium-sf conditions were chosen because they are physically identical; low- and high-sf conditions were chosen to increase the differences in visibility across the visual field in the photopic and scotopic viewing. Orientation of the Gabor patches was randomized throughout the trials. Contrast of the Gabor patches varied in the different experiments and conditions (see below). The background was a circular region with a radius of 8.5° of visual angle (17° in the low-sf condition), filled with 1/f noise with a peak contrast of 50%, resulting in an average rms contrast of 15%. The display outside of the background was set to the mean luminance of the noise.
2.2. Detection experiment
2.2.1. Stimuli
A detection experiment was conducted to measure contrast sensitivity at different target locations in the noise background under scotopic and photopic viewing conditions. The detection experiment consisted of a two-alternative forced choice task (2AFC). Different stimuli were used in the four conditions. In the scotopic and photopic medium-sf condition the Gabor patches were presented at one of five positions (a subset of those used in the search experiment), i.e. centrally at 0° of visual angle and at 4.6° along all cardinal axes (-90°, 0°, 90°, 180°). In the other conditions targets were presented at 25 positions in eight directions along the cardinal and diagonal axes (-135°, -90°, -45°, 0°, 45°, 90°, 135°, 180°), i.e. all positions that were used in the search experiment, see figure 1. The high-sf target was presented at eccentricities of 0°, 2.3°, 4.6°, and 7°; the low-sf target was presented at 0°, 5°, 10°, and 15° of eccentricity. For one of the observers (VCP) measurements were also obtained at all 25 target locations in the medium-sf conditions.
Figure 1.
Search display. In all four conditions Gabor patches were embedded equally often at 25 possible target locations in the 1/f noise background, indicated by the white circles (they were not shown to the participants). The noise background had a diameter of 17 deg in the photopic high-sf and medium-sf as well as in the scotopic medium-sf condition. A larger diameter of 32 deg was used in the scotopic low-sf condition.
2.2.2. Task and procedure
To start a trial and initiate drift correction, subjects had to fixate a central fixation cross and press a button. The fixation cross remained at the center of the display and the background noise appeared for 0.75 s in the scotopic condition and 0.25 s in the photopic condition. Next, a blank screen appeared for 0.50 s, followed by the second stimulus interval containing the background noise that lasted for the same duration as the first interval. The target was embedded in either the first or the second background that was presented, whereby the background noise was distinct in both intervals. Subjects judged which stimulus interval contained the target and made their response with one of two keys of the keyboard. Subjects were instructed to fixate the central fixation cross throughout the whole trial, except for the conditions in which the target was presented at the center of the screen. In this case the cross disappeared when the noise background appeared. Nevertheless subjects were supposed to fixate the center of the screen. To prevent afterimages of the fixation cross, it was presented alternately in black and white in different trials. If subjects did not fixate within 1.5° of visual angle around the fixation cross, a warning signal sounded and those trials were excluded from further analysis.
One experimental block consisted of 50 trials, in which the same target was always presented at the same location. Prior to each block the eye tracker was calibrated and one trial was presented as an example, in which a white circle on the background indicated the targets’ location in the upcoming block. In the low- and high-sf condition this circle also appeared on the grey background in between consecutive trials. For each of the four conditions, the observers completed one block for every target position. If the resulting data was not sufficient to fit a psychometric function, a second block was conducted. Different conditions were tested in different sessions and the order of these sessions was varied across subjects.
2.2.3. Data analysis
To determine contrast thresholds of the subjects, an adaptive 2:1 staircase procedure (Levitt, 1971) was used for both medium-sf conditions to adjust the contrast of the Gabor patches online. The QUEST procedure (Watson & Pelli, 1983) was used to adjust the contrast of the Gabors in the low-sf and in the high-sf condition. The collected data was used to fit psychometric functions of detectability for every observer and all investigated target locations in all four conditions. This was done offline using the psignifit toolbox (Wichman & Hill, 2001) on Matlab R2007b (The MathWorks). Sensitivity data of the medium-sf conditions was compared with a 2 (viewing conditions) × 2 (eccentricities) repeated measures ANOVA using the statistical package R (R Development Core Team 2011).
2.3. Search experiment
2.3.1. Stimuli
The contrast of the Gabors varied between the conditions to account for the differences in visibility (see table 1): the scotopic low-sf target had a contrast of 30%, the scotopic medium-sf target had a contrast of 40%, the photopic medium-sf target had a contrast of 7.5%, and the photopic high-sf target had a contrast of 5%. We chose these contrasts to achieve reasonable search durations of about 3 seconds in all conditions. In each trial the target randomly appeared at one of 25 possible locations embedded in the background (see figure 1). It was presented at one of eight different directions at the cardinal and diagonal axes: -135°, -90°, -45°, 0°, 45°, 90°, 135°, and 180° and at one of four different eccentricities: 0°, 2.3°, 4.6°, and 7° of visual angle in the medium- and high-sf conditions and at eccentricities of 0°, 5°, 10° and 15° in the low-sf condition, see table 1.
Table 1.
Summary of the methodological details in the four experimental conditions of the search experiment.
| Scotopic | Photopic | |||
|---|---|---|---|---|
| white: 0.0156cd/m2, black: 0.0001cd/m2, grey: 0.008cd/m2 | white: 60.09cd/m2, black: 0.0002cd/m2, grey: 30.64cd/m2 | |||
|
| ||||
| LOW-SF | MEDIUM-SF | MEDIUM-SF | HIGH-SF | |
| Spatial frequency | 1.0 cpd | 2.5 cpd | 2.5 cpd | 6.0 cpd |
| Contrast | 30% | 40% | 7.5% | 5% |
| Noise radius | 17° | 8.5° | 8.5° | 8.5° |
| Eccentricities | 0°, 5°, 10°, 15° | 0°, 2.3°, 4.6°, 7° | 0°, 2.3°, 4.6°, 7° | 0°, 2.3°, 4.6°, 7° |
| Observers | N=2 | N=5 | N=5 | N=2 |
2.3.2. Task and procedure
The order of conditions was randomized between observers. The eye tracker was calibrated prior to each block and before the first block subjects were asked to do some practice trials. To ensure observers fixated the center of the screen at the beginning of each trial and for drift correction, a central fixation dot was presented on a uniform grey background previous to each trial. Subjects were instructed to fixate it and initiate the trial with a button press. Background noise with the embedded target appeared and the observers had to search the target and press a response button as soon as they located it. This determined the search time. Next, crosshairs appeared on the screen indicating the observer’s center of fixation. Subjects were now asked to fixate the target and press the button again to indicate the judged location of the target. If the eye position at that time was closer to the true target position than to any other of the 25 potential target locations, the response was considered correct, i.e. a hit. Otherwise the response was assigned to the closest target location and counted as a false alarm for this location. Hits and false alarms were used to calculate d′. At the end of each trial crosshairs disappeared and a white circle was presented around the correct target location, serving as feedback for the observers. The target was presented four times at each of the 25 possible locations in one experimental block, so that one block consisted of 100 trials. Each participant completed four blocks of each condition (i.e., 400 trials), except for the author who completed 6 blocks in the high- and medium-sf conditions.
2.3.3. Data analysis
Saccades were detected with the EyeLink saccade detector, which uses a combination of a velocity and acceleration criterion. Data were then analyzed using customized software in Matlab R2007b (The MathWorks). We determined the search time for correct trials, i.e. between the onset of the trial and the first button press, which indicated that the subject found the target, the percentage of correct responses and the ratio between these two as measures of the search performance. Fixations were analyzed with regard to their number, duration and location on the search display. Additionally, we analyzed the direction and amplitude of saccades. Saccades and fixations were only considered for analysis if they were recorded during the search time and only analyzed for hits. Where applicable, we computed individual means for each observer, viewing condition and eccentricity (averaged across angles) to compare the scotopic and photopic medium-sf condition with repeated measures 2 (viewing condition) ×4 (eccentricity) – ANOVAs using the statistical package R (R Development Core Team 2011). This analysis could not be conducted for the low-sf and high-sf condition as only two observers completed these conditions.
2.4. Ideal searcher
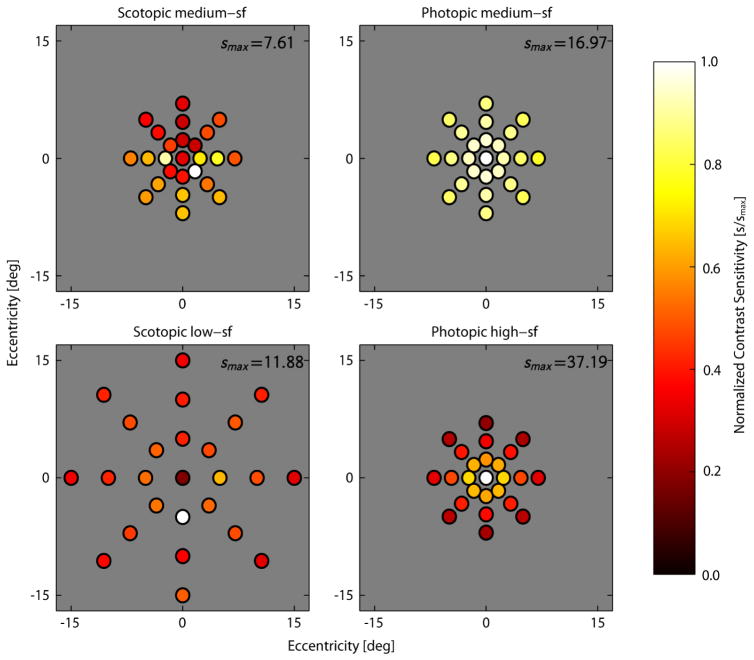
Simulation of the ideal searcher requires knowing the detectability of the target as a function of retinal location (i.e., the d′ map), where the detectability is measured with minimal position uncertainty (the location of the target is known/cued). We estimated the visibility map from the contrast sensitivity values for subject VCP shown in Fig. 3. We do not have full sets of contrast sensitivity data for the other subjects, but the data we do have are similar to those of subject VCP. To estimate the visibility map for each search condition, we interpolated or extrapolated the contrast sensitivity values in Figure 3. First consider the scotopic contrast sensitivities. Inside the outermost target locations we used linear interpolation. Outside these locations we extrapolated. To do this we found the average contrast threshold (i.e., 1/sensitivity) value in the outer two rings of target locations. These two averages define a slope (rate of change of contrast threshold with eccentricity). For each spoke we extrapolated from the contrast threshold at the outer location using the slope based on the outer two rings. The contrast threshold and sensitivity at all locations beyond the outermost target locations was then obtained by linear interpolation. Next consider the photopic contrast sensitivities. Here we fit an exponential (log-linear) function (as in Peli, Yang & Goldstein, 1991; Geisler, et al., 2006; and Michel & Geisler, 2011) to the thresholds along each spoke and then interpolated the resulting functions. Obviously, there are a number of approximations here, but the visibility maps should be sufficient to determine approximate ideal searcher performance.
Figure 3.
Estimated contrast sensitivity at each potential target location in each search condition for subject VCP. To better illustrate the pattern of target visibility, the contrast sensitivity values (s) within each panel have been normalized by the maximum sensitivity (smax) for the corresponding search condition.
Ideal search performance was computed as in the “dynamic noise” case in Najemnik and Geisler (2005; see their paper for details). Unlike in their experiment, the orientation of the Gabor was random, i.e. unknown, in our search experiment. However, because the same orientation uncertainty was present in the sensitivity measurements that we used to model the sensory noise of the ideal searcher, no additional adjustment of the model was necessary to account for this difference. Here, we simulated an ideal searcher under two different assumptions. In one case (the true ideal), the searcher knows precisely the 25 potential target locations and is able to disregard information from anywhere else in the display. We refer to this first case as the “true distribution of priors” case. Keeping in mind that the background is a uniform field of 1/f noise with no indication of potential target locations, we also considered the case where the searcher erroneously assumes that the target could be anywhere in the search area (a dense tiling of potential target locations). We refer to this second case as the “spatially dense distribution of priors” case. We simulated the “spatially dense distribution of priors” by having the simulated observer assume that the target location was selected randomly from a uniform triangular grid with nonoverlapping potential target locations separated by 1° of visual angle (for a total of 167 potential locations in the 7° display conditions and 831 potential locations in the 15° display condition).
We find that in the search experiments, humans made somewhat more fixations than the ideal searchers, when the ideal searcher’s error rate was forced to match that of the humans. To better compare the density of fixations and saccade vectors we scaled the contrast sensitivity of the ideal searcher, by a fixed factor at all retinal eccentricities, so that the median numbers of fixations for humans and ideal were the same. The scaled contrast sensitivies were propagated to d′ values using the measured average psychometric function shape (steepness) parameter. Table 2 shows the scale factors.
Table 2.
Scaling adjustments made to measured contrast sensitivities required to degrade ideal search performance to human levels
| Scotopic | Photopic | |||
|---|---|---|---|---|
|
| ||||
| LOW-SF | MEDIUM-SF | MEDIUM-SF | HIGH-SF | |
| “True distribution of priors” | 55% | 55% | 80% | 70% |
| “Spatially dense distribution of priors” | 65% | 65% | 90% | 80% |
Results
3.1. Detection experiment
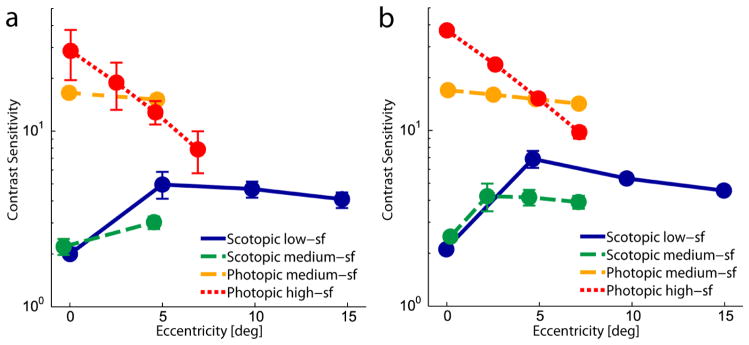
As expected, we observed large differences in contrast sensitivity between scotopic and photopic conditions, see Figure 2. In the scotopic conditions the sensitivity was minimal at the center of the visual field (low-sf: M = 2.01 ± 0.08 (mean ± one SEM); medium-sf: M = 2.20 ± 0.23) and increased to about five degrees in the periphery (low-sf: M = 4.96 ± 0.87; medium-sf: M = 3.03 ± 0.24). For the low-sf target the contrast sensitivity remained relatively stable from five to fifteen degrees in the periphery (between M = 4.64 ± 0.50 and M = 4.05 ± 0.41). On the contrary, in the photopic conditions the sensitivity was maximal within the fovea (high-sf: M = 28.43 ± 9.07; medium-sf: M = 16.50 ± 1.06.). In the high-sf condition the sensitivity decreased drastically with eccentricity (M = 7.81 ± 2.11 at 7°). This was less so for the medium-sf target (M = 15.14 ± 1.02 at 4.7°). The differences between viewing conditions were also statistically significant when comparing the medium-sf conditions (F(1,4) = 160.42, p < .001). As expected, this comparison also revealed a significant interaction between viewing condition and eccentricity (F(1,4) = 20.75, p < .05). All of these results are in line with the differential distribution of rods and cones across the visual field.
Figure 2.
(a) Mean contrast sensitivity for the different search targets at multiple eccentricities in the visual field averaged across subjects and across target directions. (b) Measurements for the one subject (VCP) where thresholds were measured at every potential target location. Error bars show one SEM. For better discriminability data points are shifted slightly apart on the horizontal axis.
Visibility measurements are required to determine the optimal way to move the eyes over the search display. Making many saccades to guide the fovea to multiple locations in order to find the target is only optimal in case that detectability varies across the visual field. If detectability does not vary, then it is optimal not to move the eyes, because little or no information is gained during the saccades, but perception is partially suppressed instead (Burr, Morrone, & Ross, 1994). Thus, if saccades are executed, time is effectively lost to the eye movements rather than used for the search. In the photopic conditions detectability varies most strongly for the high-sf Gabor, being highest in the fovea and rapidly falling off towards the periphery. For the scotopic conditions, detectability varies in the opposite way, with a central scotoma surrounded by a large region of higher sensitivity.
Recall that for subject VCP detectability data was obtained at all the potential target locations for all conditions in the search experiment. The data from subject VCP (Fig. 2b) are generally in agreement with those of the other subjects, and thus we used the data from subject VCP to estimate the contrast sensitivity at each potential target location, for the four search conditions. Specifically, we first fit the psychometric data at each target location in each of the four conditions with a Weibull function: ψ(c) = 0.5 + 0.5exp[−(c/ct)β]. We allowed the threshold parameter ct to be different for each location, but constrained the steepness parameter β to be the same (estimates of separate steepness parameters are too noisy, given the number of trials). We then set the contrast of the target to the value used in the search experiment (see Methods) and calculated the contrast sensitivity at each location from the psychometric function at that location. The resulting values are shown in figure 3. In the scotopic conditions contrast sensitivity is lower in the fovea and in the upper visual field. In the medium-sf photopic conditions contrast sensitivity values are approximately radially symmetric.
In the high spatial frequency photopic conditions the d′ values are lowest in the upper visual field, highest in the nasal and temporal visual fields and intermediate in the lower visual field (similar to Najemik & Geisler 2005, who also used a high spatial frequency target). We used the contrast sensitivity values in Figure 3, to generate the continuous d′ maps used to determine the performance of the ideal searcher (see Discussion).
3.2. Search experiment
3.2.1. Search performance
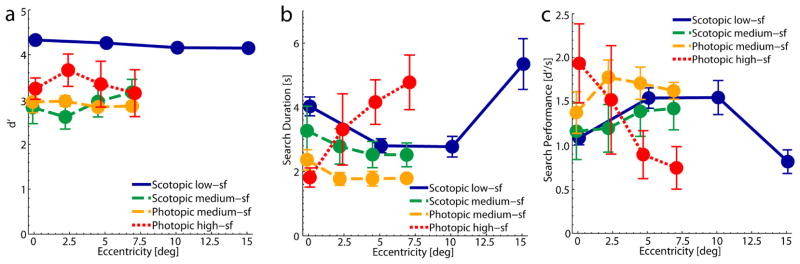
Visual search performance can be quantified by three different measures, sensitivity (defined as d′), speed, and the ratio between both, here defined as d′ per second. On average, the highest sensitivity was observed in the scotopic low-sf condition; d′ for the central target was 4.33. This sensitivity slowly decreased in the periphery to 4.15 ± 0.07 at 15°, see figure 4a. At the same time, search duration was rather long in this condition (longer than in the medium-sf conditions), see figure 4b. However, this might also be influenced by the larger size of the search display used in the scotopic low-sf condition. In this search condition the duration also depended on the eccentricity of the target, it was longer for the central (M = 4.03s ± 0.29s) and most peripheral target (M = 5.35s ± 0.79s) than for the targets hidden at 5° (M = 2.80s ± 0.22s) and 10° (M = 2.77s ± 0.32s). Accordingly, search performance defined as d′ per second, i.e. when taking speed and sensitivity into account, was not higher than in the other conditions. Search performance was lower for the central target than for ones at 5° and 10°, which resembles the pattern of sensitivity for this low-sf target. It was also lower for the most peripheral target compared to ones at 5° and 10°, despite the fact that there is little difference in contrast sensitivity for these eccentricities. Since target eccentricity was unknown in the search task, whereas it was cued in the detection task, the search for the more eccentric targets might be more difficult because a larger area had to be processed.
Figure 4.
Performance in the four search conditions. a) d′ as a function of target eccentricity. b) Mean search duration for correct trials as a function of the target’s eccentricity. c) Search performance in d′ per second as a function of the target’s eccentricity. All data were averaged across target directions and across participants. Error bars show one SEM. For better discriminability data points are shifted slightly apart on the horizontal axes.
An even larger fall-off in search performance with eccentricity was observed in the high-sf condition. The performance of 1.09 ± 0.08 d′/s for high-sf targets embedded in the center of the background noise decreased to 0.82 ± 0.13 d′/s at 7° in the periphery. This decrease in performance with eccentricity results from the increase of search duration along with the decrease in d′. Again, the pattern of search performance corresponds to the decrease in contrast sensitivity for the photopic high-sf target as measured in the detection experiment. In the scotopic and photopic medium-sf conditions there was no clear systematic difference in search performance between central and peripheral targets (F(3,12) = 1.70, p > .05). Performance was overall similar (F(1,4) = 2.82, p > .05) in these two viewing conditions which had physically the most similar targets. Also sensitivity (scotopic: M = 2.89 ± 0.17; photopic: M = 2.90 ± 0.08; F(1,4) = 0.02, p > .05) and speed (scotopic: M = 2.77s ± 0.29s; photopic: M = 1.93s ± 0.13s; F(1,4) = 3.56, p = .13) on their own did not differ much between scotopic and photopic visual search for the medium-sf target, although the higher search duration in the scotopic condition might be considered a trend. We also observed a significant interaction effect between eccentricities and viewing conditions on d′ (F(3,12) = 5.14, p < .05). Taken together, search performance was similar in the different viewing conditions, so the level of difficulty seems comparable across conditions. However, performance seemed to be modulated across eccentricities by the differences in target visibility that we measured in our detection experiment.
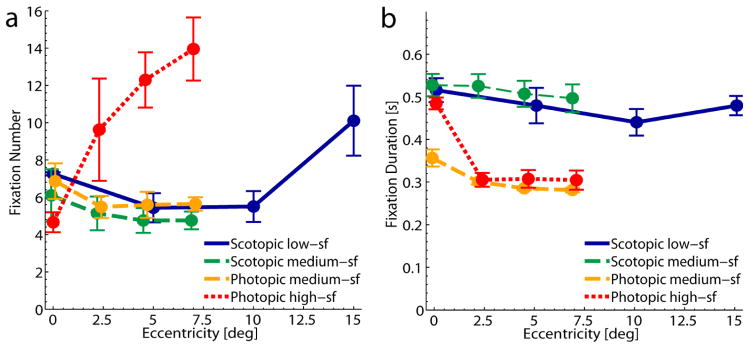
3.2.2. Fixations
The duration of a visual search trial is largely determined by the number of fixations (or, analogously, by the number of executed saccades) and this is indeed what we observed. The graphs of the number of fixations in figure 5a look highly similar to the graphs for the search durations in figure 4b. The further the high-sf target was hidden in the periphery the more fixations were needed to find it. In the low-sf condition the number of fixations increased for targets that were presented at 15° in the periphery, as did the time to find the target. For eccentricities between 0° and 10° the number of fixations was similar for the low-sf and both medium-sf conditions. We did not observe a main effect of either viewing condition (F(1,4) = 0.68, p > .05) or eccentricity (F(3,12) = 3.30, p = .06) when comparing search for the medium-sf targets. However, the duration of a visual search trial is not only determined by the number of fixations but also by their duration. We found that the duration of fixations was longer in scotopic compared to photopic visual search, see figure 5b. This difference was significant for the medium-sf targets (scotopic: M = 0.51s ± 0.02s vs. photopic: M = 0.31s ± 0.01s; F(1,4) = 52.38, p < .001). It is consistent with the longer integration time for rod vision. We also observed a main effect of eccentricity on fixation duration (F (1.32, 5.29) = 8.60, p = .002, Greenhouse-Geisser corrected). Interestingly, also in the photopic conditions fixation durations were longer when the target was hidden at the central position. In the photopic condition this effect vanishes when the first fixation is excluded from analysis.
Figure 5.
Number and duration of fixations in the different conditions of the visual search task. a) Mean number of fixations during the visual search task as a function of target eccentricity. b) Mean duration of fixations in one search trial as a function of target eccentricity. All data were averaged across target directions and across participants. Error bars show one SEM. For better discriminability data points are shifted slightly apart on the horizontal axes.
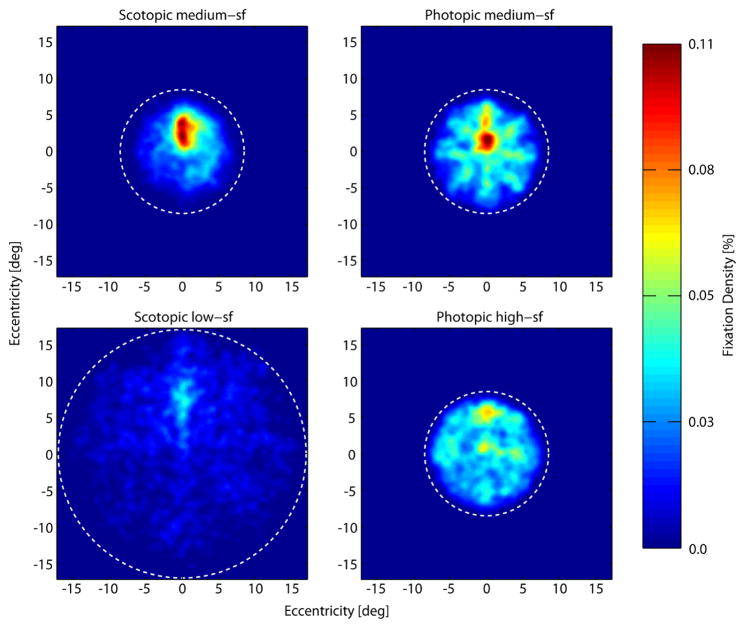
Figure 6 shows the density of fixations across the visual field in the different conditions aggregated across observers. Two observations are most striking: First, the majority of fixations is located in the upper center of the visual field. This is most prominent in the scotopic conditions especially during search for the medium-sf target, but can also be seen in the other search conditions. Second, fixations are more broadly distributed over the entire search display during photopic search and low-sf scotopic search. Besides a peak of fixations slightly above the center, fixations spread out toward the borders of the circular background, especially for the high-sf condition. This is not the case in the medium-sf scotopic search condition. Here, fixations were overall less distributed across the search background and fewer fixations were made towards the edge of the search display.
Figure 6.
Density of fixations across the search display (dotted white circles show outlines of the noise background) aggregated across all observers and trials in the four different conditions (scotopic medium-sf, photopic medium-sf, scotopic low-sf and photopic high-sf from top left to bottom right). Number of fixations at a given location increases from dark blue to red. For better comparison between conditions we normalized the data in each condition by the total number of fixations in that condition. Note that the first and last fixations of each trial were excluded here because the first one always appeared at the center of the search display and the last ones are biased towards the target locations. As a result, the plots shown here are based on 20179 fixations in total. Density plots were smoothed with a Gaussian low-pass filter (σ = 2 deg).
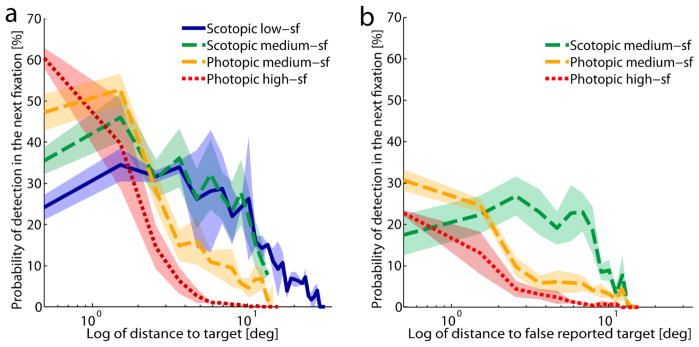
Taken together, the number of fixations was similar in photopic and scotopic visual search. During scotopic visual search, however, fixations lasted longer and were distributed less broadly across the display, especially in the medium-sf condition. The number and distribution of fixations in the scotopic conditions seems remarkable, since these targets can be detected in the periphery as shown in the detection experiment. As a measure of how close to the target the observers actually fixated when they detected it we calculated the probability of detecting the target during the upcoming fixation as a function of the current distance to the target, see Figure 7a. This was done by calculating the frequency of distances between target and gaze position for all fixations in successful trials and then computing the ratio between the frequency of distances in the second to last fixation and all previous fixations. As measures of how often the targets were detected dependent on the distance to the target the curves in figure 7a might be regarded as empirical or oculomotor sensitivity functions complementary to the sensitivity functions derived in our detection experiment. In both photopic search conditions there was a clear decrease in the probability of target detection with increasing distance to the target, i.e. the closer the gaze position was to the target, the larger the probability of detection. In the scotopic conditions on the other hand, detection probability was lower when the distance to the target was very small but did decrease only modestly for eccentricities between one and ten degrees in the periphery. Thus, in these conditions observers often moved their eyes closer to the target than necessary. Interestingly, we observed a similar pattern for incorrect trials regarding the distance to the erroneously reported target location, see figure 7b. In the photopic conditions the probability of reporting the (wrong) target was larger the closer the gaze position was to that wrong position. However, comparable to correct trials, the probability of detection in the incorrect scotopic trials was lower for smaller distances to the false reported target position and higher for a greater range of larger distances.
Figure 7.
a) Probability of detecting the target in the upcoming fixation (n+1) in percent as a function of the distance to the target at the location of the current fixation (n) for successful trials (hits). The probability was calculated from the ratio between the frequency of distances from the second last fixation location to the target and the frequency of distances of all other fixations. Distances were binned in steps of 1°, bins with fewer than 10 trials were excluded. Error bands show ± 1 SEM. b) Probability of (incorrect) target detection in the upcoming fixation (n+1) in percent as a function of the distance to the false reported target location of the current fixation (n) in incorrect trials (misses). The probability was calculated as in a), but for the distance to the erroneously reported target location, not the true target location. The scotopic low-sf condition is not presented in this graph because there were too few error trials. Error bands show ± 1 SEM.
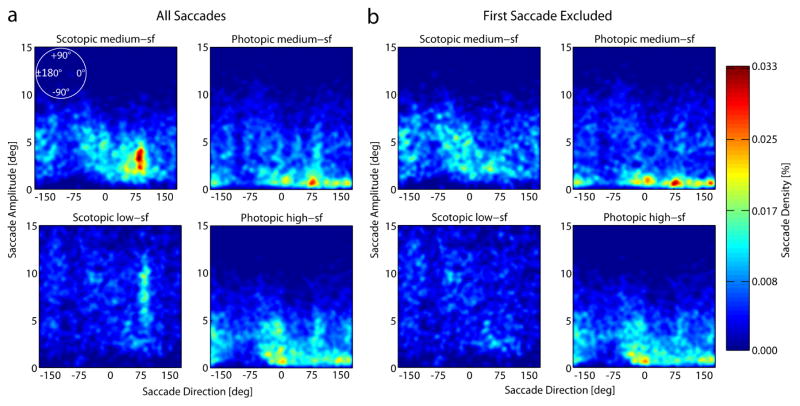
3.2.3. Saccades
Figure 8 shows the density of saccade amplitudes in a given direction for all saccades aggregated across observers (a) and for all but the first saccade made in each search trial (b). In both photopic conditions saccades were distributed over the whole range of directions, with peaks in the upward and horizontal directions, and the majority of them had amplitudes between one and five degrees of visual angle, see figure 8a. However, it seems as if there were fewer saccades going downwards than in all other directions. In the scotopic search conditions there is a clear peak of saccades with a direction of 90°, i.e. upwards. Figure 8b reveals that this effect is mainly due to the first saccade in each trial. If the first saccade is excluded from analysis (see figure 8B) this peak at around 90° vanishes. Thus, in the majority of scotopic search trials the first saccade was made towards the center of the upper half of the search display, i.e., with an amplitude of about 4° of visual angle in the medium-sf condition and an amplitude of about 8° of visual angle in the low-sf condition with the larger diameter of the search display. This might also explain the corresponding peak of fixations in the upper center of the visual field for the scotopic conditions (see figure 6). However, please note that figure 6 shows the density of all fixations and this pattern could have occurred through an infinite number of combinations of saccade directions and amplitudes.
Figure 8.
Density of saccade amplitudes as a function of saccade directions across the search display (zero indicates saccades directed horizontally to the right, positive numbers: counter clockwise; negative numbers: clockwise). The number of saccades of a given amplitude and direction increases from dark blue to red. a) Density of saccade amplitudes as a function of saccade directions aggregated across all observers and trials in each of the four different conditions (scotopic medium-sf, photopic medium-sf, scotopic low-sf and photopic high-sf from top left to bottom right). Plots are based on a total of 23.969 saccades. b) Shows the same data but the first saccade that was made in each trial was excluded. For better comparison between conditions we normalized the data in each condition by the total number of saccades in that condition (for B by the total number of saccades included in the analysis). Density plots were smoothed with a Gaussian low-pass filter (σ = 2 deg).
It is also noticeable in figure 8 that the large number of small saccades under photopic conditions is essentially absent under scotopic conditions. These small saccades, probably a mixture of microsaccades and corrective saccades, serve to optimize the foveal position of the target on the retina. Under scotopic conditions it would not make sense to execute them and the visual system indeed behaves in this manner. This interpretation is supported by the fact that the probability to detect the target in scotopic conditions is at small distances lower and at large distances higher than in the photopic conditions (figure 7a).
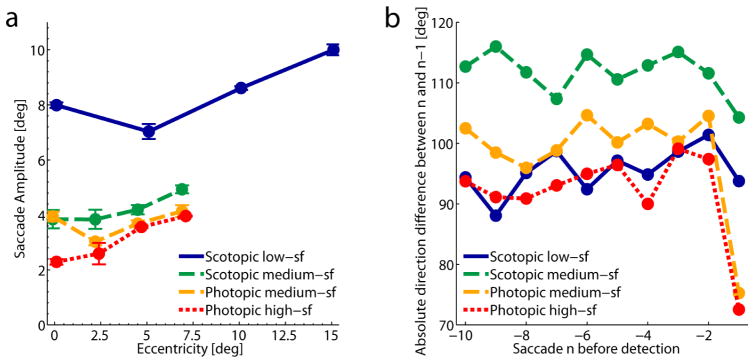
Overall, observers made larger saccades in the low-sf condition, however, the search display also had a diameter that was twice as large as in the other conditions. In all conditions saccade amplitudes increased as a function of target eccentricity, i.e. with the area that needed to be searched in order to find the target, see figure 9a. For the medium-sf conditions this also showed up as a main effect of eccentricity (F(1.11,4.43) = 10.44, p < .05, Greenhouse-Geisser corrected). Additionally, this ANOVA revealed an interaction effect between eccentricity and viewing condition (F(1.77,7.08) = 6.02, p < .05, Greenhouse-Geisser corrected), presumably because of the slightly modulated eccentricity effect in the photopic condition. However, irrespective of the increase of amplitudes with target eccentricity, saccade amplitudes were larger in the low-sf condition with the larger search display.
Figure 9.
a) Mean saccade amplitudes as a function of target eccentricity. All data were averaged across target directions and across participants. Error bars show one SEM. For better discriminability data points are shifted slightly apart on the horizontal axes. b) Mean absolute directional difference between two successive saccades for the last ten saccades before target detection in all four search conditions. Random changes in the direction between two saccades lead to an average direction difference of about 90°, lower values arise through a systematic undershoot of saccades, higher values represent successive saccades that systematically change direction about more than 90°.
Figure 9b shows the direction difference between two successive saccades for the last ten saccades before target detection in all four search conditions. Random changes in the direction of the successive saccades would result in an average difference of about 90°. This is what we observe for differences between saccades until the second last saccade in the low-sf, high-sf and photopic medium-sf condition. However, in both photopic conditions we observed smaller differences between the two last saccades. This might reflect a systematic undershoot of the second to last saccade towards the target with a subsequent corrective saccade in the same or similar direction. Such a corrective saccade, which is typically executed in order to bring the fovea directly onto the desired object, was not performed in the scotopic conditions. In the scotopic medium-sf condition direction differences between subsequent saccades were larger than can be expected from random changes in direction. Instead, the data point to a systematic tendency for successive saccades to go into largely diverging or even reversing directions, i.e. differing more than 90°.
3.3. Ideal searcher
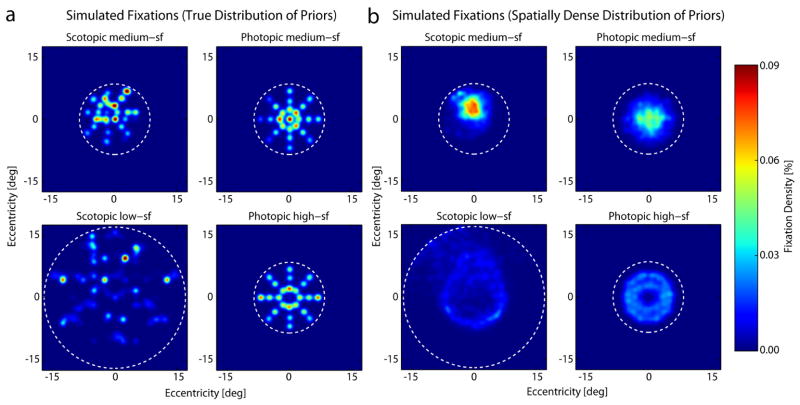
Figure 10a shows the distribution of the fixation locations predicted by the ideal searcher using the “true distribution of priors”, and figure 10b the distribution predicted by the searcher using the “spatially dense distribution of priors”. Comparison with figure 6 shows that there are some similarities and differences with the human searchers. For the scotopic conditions the ideal, like the humans, shows little hint of the annulus distribution. In other words, the ideal searcher predicts an annulus distribution for the conditions of Najemnik and Geisler (2005), but not for the current scotopic conditions. Only in the low-sf condition and only with a spatially dense distribution of priors does the ideal searcher predict a slightly ring-shaped fixation pattern for scotopic search, that we do not observe in humans. However, this ring is elongated towards the upper visual field similar to the peak in the human fixation distribution. In the scotopic medium-sf condition the spatially dense distribution of priors predicts a more condensed distribution of fixations in the upper visual field than the true prior and thus, resembles more the human searcher. In all cases the ideal searcher predicts the qualitative facts that fixations in scotopic search tend to be concentrated in the upper visual field, and tend to be more dispersed in low-sf condition than in the medium-sf condition.
Figure 10.
Density of fixations predicted by the ideal searcher given the contrast sensitivity maps in Figure 3. a) Predicted fixations for an ideal searcher aware of the true distribution of possible target locations. b) Predicted fixations for an otherwise ideal observer that assumes a dense uniform tiling of possible target locations. The dashed circles represent the search region. As in figure 6, the first and last fixations have been excluded.
For the photopic conditions the ideal searcher using the true distribution of priors predicts a distribution of fixations that closely mirrors the spoke-like arrangement of possible target locations, while the dense prior searcher predicts somewhat less structured distributions. For the medium-sf target it predicts a non-annulus distribution, similar to the human data, but much more centered than the fixations of our observers. For the high-sf target, on the other hand, it predicts an annulus distribution much like in the task of Najemnik and Geisler (2005), but interestingly less like the human observes in our task.
Both the human and the ideal searchers display a weaker bias for the upper visual field in the photopic conditions than in the scotopic conditions, but the predicted difference in bias for the ideal searchers is larger than that of the human searchers.
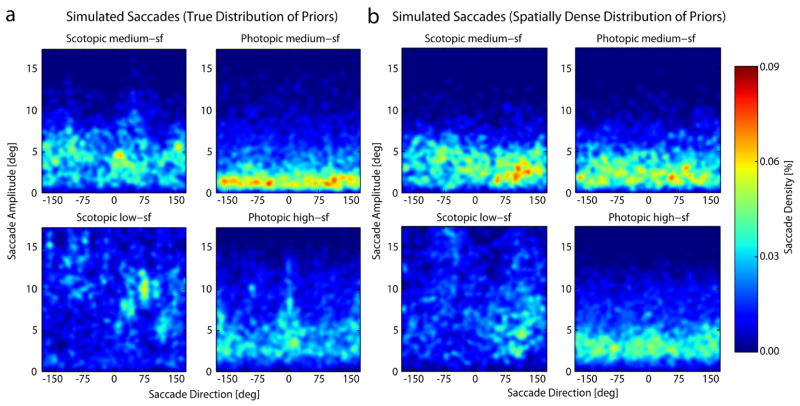
Figure 11 shows the distribution of saccade amplitudes as a function of saccade direction, for the ideal searchers. Again there are qualitative similarities and differences between the ideal and human distributions. For the scotopic conditions humans are like the ideal searcher in that (1) there is a wide range of saccade directions, (2) with a peak in vertical (near +90 deg), and (3) the saccades are longer and more distributed in the low-sf condition.
Figure 11.
Predicted density of saccade amplitudes as a function of saccade directions across the search display for a searcher using the true distribution of priors (a) and a spatially dense distribution of priors (b). Compare with Figure 8a.
For the photopic conditions there are fewer similarities between human and ideal. Like the ideal, the most common human saccade lengths are shorter in the medium-sf condition than in the high-sf condition, but the human peak densities are not concentrated in the same directions as they are for the ideal (although both are fairly widely distributed). Note also that for the conditions of Najemnik and Geisler (2005; 2008) the ideal searcher predicts that horizontal saccades are more common than any other direction, whereas the same ideal searcher makes quite different predictions for the current stimulus conditions. In other words, the ideal searcher predicts that both the distribution of fixation locations and distribution of saccade vectors depend on the specific target and the specific configuration of potential target locations. Here, for example, there were 25 possible target locations along eight directions, whereas in the task of Najemnik and Geisler (2005; 2008) the target could be hidden at one of 85 possible positions tiling the entire search background.
4. Discussion
We investigated how differences in visual sensitivity between photopic and scotopic conditions affect visual search behavior. Under photopic conditions, we replicated earlier results that poorly visible peripheral targets require more saccades than central targets (e.g. Findlay, 1997; Geisler et al., 2006; Loschky & McConkie, 2002; Najemnik & Geisler, 2005; 2008). Under scotopic conditions, we observed longer fixation periods that were less spread across the search field, but focused in the upper center of the display (mostly because in the majority of trials the first saccade was made towards this area). Here, the probability of target detection did not depend as much on the distance between gaze and target as it did during photopic search. Overall, it seems as if our observers did employ two different strategies in their search.
4.1. Visual search strategies
4.1.1. Photopic search
As expected for a foveated visual system, it seems as if in the photopic conditions observers overtly shifted their attention to multiple locations on the search display by making saccades and then scanned mainly the small foveal region of the image to find the target. Accordingly, the probability of detecting the target was larger the closer the gaze position was to the target. This was true even for the medium-sf target for which the detectability decreased only modestly in the periphery. With this foveal search strategy observers needed to make more fixations the further the high-sf target was hidden in the periphery and resolution was only poor there (high-sf condition). This effect has already been reported in previous literature for a similar search task with a 6 cpd target (Najemnik & Geisler, 2005). A fixation lasted about 300 ms before the next saccade was executed, resembling the 280 ms fixation length reported by Najemnik and Geisler (2005) and the general pattern of about three saccades per second. Photopic fixations were distributed broadly across the search display and showed a peak in the upper center. This was also the case in Najemnik and Geisler’s task but they additionally observed a second peak at the lower half of the display and an overall ‘donut’-shaped pattern of fixations which we did not find here. The distribution of fixations in the photopic medium-sf condition suggests that observers might have some sense of the spoke-like arrangement of the target locations, but it is not very pronounced, neither is it visible in any other condition. Thus, in our task humans did not seem to perfectly use location priors to guide their search as it has been suggested from other literature (e.g. Peterson & Kramer, 2001; Walthew & Gilchrist, 2006). Note that, the last fixation of any given search trial, which would of course show the effect, has been excluded in figure 6. In both tasks, saccades were rather small, mostly below 5°. Here, if a saccade undershot the target a correcting saccade was executed towards the same or similar direction, presumably to bring the fovea directly onto the target. In our experiment, we did not find as strong a preference for horizontal saccades as in theirs (Najemnik & Geisler, 2008), especially in the current medium-sf condition (figure 8). Interestingly, given the current visibility map and stimulus conditions, the ideal searchers also showed only a modest preference for horizontal saccades in the high-sf condition and essentially no preference in the low-sf condition (figure 11). The large proportion of horizontal saccades in Najemnik and Geisler’s data might be a consequence of the horizontally extended visibility map, leading to more locations with high posterior probabilities in the horizontal than in the vertical direction. In our data the shape of the visibility map was more circular, leading to a more homogeneous distribution of locations with high posterior probability.
4.1.2. Scotopic search
A different strategy seems to have emerged during scotopic visual search. It was characterized by a similar number of fixations as in photopic search, but these fixations lasted much longer and were not distributed so uniformly across the entire circular background (at least in the middle-sf condition). The longer fixation durations during scotopic search might be due to the delay within the rod pathways. However, this cannot be the only factor because temporal lag in the neuronal connections cannot produce a difference in fixation length of about 200 ms. Another likely factor is the longer temporal integration in the rod system. Because of the longer integration time there may be a benefit (increased target detectability) from extending the fixation duration. Another possibility is that observers fixated longer in the scotopic conditions to covertly shift their attention across a larger peripheral area surrounding the fovea in order to find the target. Several of our results can give a hint on the size of this ring-shaped window of attention. First, the detection experiment revealed that sensitivity for our scotopic targets varied only modestly along the periphery up to 15°. However, in that experiment the location of the target was known, this might have facilitated the detection. Second, the probability of target detection in the search experiment was similar for scotopic targets up to 10° in the periphery, although there is likely to be variation with direction in the visual field (figure 3). We might consider this distribution as an empirical sensitivity function that takes the uncertainty about the target position during the search process into account. If the window of attention during search would span a radius of up to 10°, this area is even larger than the search display in the medium-sf condition, which had a radius of 8.5°. It thus seems as if in this condition the area that could covertly be searched within a single fixation was limited by the search display itself and not by resolution (except for the center) or attentional matters. This is not the case in the low-sf condition in which the noise background had a radius of 17°. Accordingly, observers made larger saccades with an amplitude of about 8°-10°, i.e. about half of the area they could scan within one fixation, to shift their attentional window over the display. In the majority of trials in both scotopic conditions the first saccade was made towards the center of the upper half of the display. Presumably, this saccade was either successful and the target was found in the upper half of the display or more saccades were executed in various directions.
A preference for the first saccade to go upwards has often been reported for visual search tasks (Chedru, Leblanc, & Lhermitte, 1973; Durgin, Doyle, & Egan, 2008; Gould & Schaffer, 1965; Previc, 1996; Zelinsky, 1996; Schütz, 2014), although in some cases an additional lateral bias towards one or the other side has been reported. However, if the bias is caused for example by a generalization of learned oculomotor reading behavior (Gould & Schaffer, 1965), more global cognitive strategies (Durgin, et al., 2008) or has some other origin is still inconclusive. Interestingly, in our study this upwards bias of the first saccade was primarily present in the scotopic search conditions, whereas the gaze shifted in various directions at the beginning of photopic search (although fewest saccades were made downwards). To this point it is unclear what drives the bias in our task under scotopic but not photopic conditions, although the fact that this differential bias is predicted by the ideal searchers suggests that it may represent a strategy to maximize the information gained on the first saccade.
However, another question that arises from our results is why observers make so many saccades. Obviously, an eye movement needs to be executed if the target is hidden at the center of the display, because otherwise it cannot be detected. However, all targets in the medium-sf condition and most targets in the low-sf condition (except for those at 15°) should have been visible to some degree when fixating the center of the screen, although visibility would be less than indicted in Figs. 2 & 3 because of the effect of position uncertainty (Pelli 1985). Nevertheless, our observers made on average about five saccades during a scotopic search trial (about 4 in the medium-sf conditions and six in the low-sf condition). The ideal searcher automatically takes into account the position uncertainty, but makes fewer fixations than the human searchers, especially in the scotopic conditions (see Table 2). In all, about 10% of the search time was ‘lost’ to saccades. Possibly, the extra saccades were executed because the visual system cannot fully adapt to the requirements of scotopic search or has a poorer representation of the scotopic visibility distribution. However, it might be that these saccades were executed to serve a different purpose that we have not directly investigated here. Since we did not observe any microsaccades under scotopic vision, it might be the function of these larger saccades then to prevent fading of the image during the long lasting fixations.
4.2. Optimal search behavior
Much research has focused on the question of what guides eye movements during visual search (see Eckstein, 2011, for a review). One recent approach compares human search behavior to statistically optimal eye movements (Najemnik & Geisler, 2005; 2009). The goal of an optimal eye movement is to pick the fixation location that either maximizes the expected accuracy of identifying the location of the target after the fixation is made (Najemnik & Geisler, 2005), or similarly minimizes the expected entropy of posterior probability distribution (uncertainty) across the potential target locations (Najemnik & Geisler, 2009).
For a task similar to ours, Najemnik & Geisler (2005) compared the Bayesian ideal searcher to practiced human searchers. The ideal searcher takes the current posterior probability distribution over potential target locations and the visibility (d′) map of the observer as input, and calculates the optimal next fixation location. Najemnik and Geisler (2005) observed similarities between human and Bayesian ideal search patterns and a nearly optimal search performance for the human observers. Under their conditions, (1) human (photopic) visual search behavior was close to optimal, (2) the distribution of fixation locations (with first fixation excluded) formed an annulus with most fixations in the upper and (to a lesser extent) lower visual field, and (3) there were more horizontal than vertical saccades and longer vertical saccades than horizontal saccades.
The results of the present study are qualitatively different (see Figures 6 and 8). However, the present experimental paradigm also differs in some ways from Najemnik & Geisler (2005). Most obviously, the current experiment included scotopic conditions where the visibility of the target in the fovea is greatly reduced. Another potentially important difference is that in Najemnik & Geisler (2005) there were 85 potential target locations that uniformly tiled the search region, whereas in the current study there were 25 potential target locations along 8 directions (spokes) radiating from the center of the search region (see Fig. 1). Therefore, we simulated ideal search behavior under the current conditions.
The overall conclusion from Figures 10 and 11 is that many, but not all, of the qualitative properties of human fixation and saccade statistics measured in the current study are consistent with those of a rational (ideal) searcher, and that many of the striking differences between the current results and those of Najemnik and Geisler (2005; 2009) are expected given the differences in the visibility maps and configurations of potential target locations. These differences were not intuitive beforehand, underscoring the value of working out the predictions of normative models.
We simulated two searchers, one with the true distribution of priors of potential target locations and one with a spatially dense distribution of priors, under the assumption that observers may not know the exact potential locations in the uniform 1/f noise backgrounds. Comparison of the fixation and saccade distributions of the human and ideal searchers suggests that humans fall somewhere between true-prior and dense-prior models.
Besides the ideal searcher, there are of course also other formal models describing search behavior. The entropy limit minimization model (ELM) is a computationally simpler approximation of the ideal searcher that chooses fixation locations to minimize uncertainty (Renninger et al., 2007; Najemnik & Geisler, 2009). We did not test the ELM model in this study, because its predictions would be very close to the ideal searcher so that it could not explain the larger differences to the search behavior of human observers. A maximum-aposteriori (MAP) searcher, which moves the fovea to locations with the highest posterior probability, however leads to qualitatively different predictions than the ideal searcher under photopic conditions (Najemnik & Geisler, 2008). Under scotopic conditions, one would have to assume another preferred retinal location (PRL) than the fovea that is moved to the peaks of the posterior probability. The location of this PRL is not clearly defined, because we did not measure the visibility map in high resolution and because the PRL does not necessarily have to coincide with the peak in the visibility map. We did not test the MAP searcher because of this lack of constraints.
Of course, the fact that humans display some of the properties of the ideal searcher does not imply that the brain is actually performing the ideal observer’s computations. More likely is that some of the heuristics used by the brain capture some of the key computational principles of the ideal observer. Further, the heuristics used by the brain are not likely to be totally flexible (Morvan & Maloney, 2012; Verghese 2012), but are most likely to be optimized for search conditions similar to those that occur in the natural environment or in special conditions where the human searcher has extensive experience (e.g., a favorite video game). Human observers have, for instance, vast experience in looking at faces and it has been shown that they choose an optimal location below the eyes for their first fixation (Peterson & Eckstein, 2012). This strategy maximizes individual face identification (Peterson & Eckstein, 2013). Learning of an artificial optimal eye movement strategy for face identification was possible but difficult (Peterson & Eckstein, 2014).
Recent studies have identified conditions under which humans are clearly not optimal. For example, humans are relatively inefficient when searching for multiple targets in displays where the potential target locations fall at discrete cued locations on a circle (Verghese, 2012), but can become more efficient with appropriate feedback (Verghese & Ghahghaei, 2013). These kinds of conditions are likely to be less common in the normal environment than conditions like those the present experiment, where the context specifies an extended region of search (e.g., a tree or the ground around one’s feet) where a single target (e.g., a squirrel or a dropped coin) may be anywhere within the search region. Human observers are also reported to deviate from an ideal searcher in a task in which targets in some regions of the visual field are rewarded (Ackermann & Landy, 2013). In this study, humans adjusted their search strategy depending on the rewarded location and showed some qualitative similarity with eye movement patterns of an ideal observer, but their choice of fixation positions was overall suboptimal. Saccades executed at short latencies in particular are dominated by salience and often miss the reward (Schütz, Trommershäuser & Gegenfurtner, 2012).
Similarly, subjects are inefficient when the task is to select between three potential fixation locations, where the target is presented after the subject selects the fixation location (Morvan & Maloney, 2012). Under these conditions subjects often do not to take into account the falloff in detectability with retinal eccentricity. However, under more natural conditions such as searching over a defined region of space, they clearly do take the falloff into account. Perhaps the simplest evidence is that subjects rarely fixate near the edge of a continuous search region (whatever its shape) even when the target is just as likely to be near the edge. Fixating close to the edge would be inefficient because it would waste the part of the fovea and/or parafovea that falls outside the search region. This behavior is modified based on the detectability map of the search target (see photopic fixation densities in Fig. 6), and is very intuitive. For example, if one is told there may be a grain of sand on a table top the search will certainly extend to near edge of the table (because of implicit knowledge of the rapid falloff of detectability with eccentricity), but if one is told that there may be a marble on the same table the fixations will be fewer and rarely venture near the edge of the table. This is likely to be fairly automatic behavior that has evolved or has been learned over a long period time, but that captures a key component of the ideal strategy of maximizing information gain. An important direction for future research will be to better identify which optimal components are incorporated into the mechanisms of visual search and how flexible those components are across search conditions.
5. Conclusion
Vision seems near optimal in many regards. The distribution and neural connection of the photoreceptors in the retina allow us to combine a large field of view with a high resolution window. Eye movements make this combination functional by enabling us to continuously replace this area of highest resolution across the visual world. The existence of two types of photoreceptors with distinct properties lets us see under diverse lighting conditions. Previous results suggest that under photopic conditions human searchers share many properties with an ideal searcher, at least for conditions where a single target is randomly located in naturalistic backgrounds. Our current results suggest that human searchers also share many properties with an ideal searcher under scotopic conditions, even though the ideal searcher behaves differently under scotopic conditions than under photopic conditions. The results suggest that humans make at least some appropriate adjustments in search behavior as ambient light level decreases.
Highlights.
Scotopic contrast sensitivity is lower, with a functional scotoma in the fovea.
Scotopic visual search shows longer lasting and less distributed fixations.
An ideal searcher behaves differently under photopic and scotopic conditions.
Human fixation patterns are qualitatively similar to ones of an ideal searcher.
The brain adjusts visual search behavior to meet some properties of rod vision.
Acknowledgments
This work was largely supported by the Deutsche Forschungsgemeinschaft (DFG) grant GE 879/10 and the DFG International Research Training Group IRTG 1901 “The Brain in Action - BrainAct”, and in part by NIH grants EY02688 and EY11747 to WSG. We would like to thank Miguel Eckstein for his thorough and helpful review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackermann JF, Landy MS. Choice of saccade endpoint under risk. Journal of Vision. 2013;13(3):27, 1–20. doi: 10.1167/13.3.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr DC, Morrone MC, Ross J. Selective suppression of the magnocellular visual pathway during saccadic eye movements. Nature. 1994;371(6497):511–513. doi: 10.1038/371511a0. [DOI] [PubMed] [Google Scholar]
- Chedru F, Leblanc M, Lhermitte F. Visual searching in normal and brain-damaged subjects (Contribution to the study of unilateral inattention) Cortex. 1973;9:94–111. doi: 10.1016/s0010-9452(73)80019-x. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Sloan KR, Kalina RE, Hendrickson AE. Human photoreceptor topography. The Journal of Comparative Neurology. 1990;292:797–523. doi: 10.1002/cne.902920402. [DOI] [PubMed] [Google Scholar]
- D’Zmura M, Lennie P. Shared pathways for rod and cone vision. Vision Research. 1986;26(8):1273–1280. doi: 10.1016/0042-6989(86)90108-2. [DOI] [PubMed] [Google Scholar]
- Durgin FH, Doyle E, Egan L. Upper-left gaze bias reveals competing search strategies in a reverse Stroop task. Acta Psychologica. 2008;127:428–448. doi: 10.1016/j.actpsy.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Eckstein MP. Visual Search: A retrospective. Journal of Vision. 2011;11(5):1–36. doi: 10.1167/11.5.14. [DOI] [PubMed] [Google Scholar]
- Eckstein MP, Beutter BR, Stone LS. Quantifying the performance limits of human saccadic targeting during visual search. Perception. 2001;30:1389–1401. doi: 10.1068/p3128. [DOI] [PubMed] [Google Scholar]
- Findlay JM. Saccade target selection during visual search. Vision Research. 1997;5:617–631. doi: 10.1016/s0042-6989(96)00218-0. [DOI] [PubMed] [Google Scholar]
- Geisler WS, Perry JS, Najemnik J. Visual search: the role of peripheral information measured using gaze-contingent displays. Journal of Vision. 2006;6:858–873. doi: 10.1167/6.9.1. [DOI] [PubMed] [Google Scholar]
- Gould JD, Schaffer A. Eye-movement patterns in scanning numeric displays. Perceptual and Motor Skills. 1965;20:521–535. doi: 10.2466/pms.1965.20.2.521. [DOI] [PubMed] [Google Scholar]
- Hess RF, Nordby K, Pointer JS. Regional variation of contrast sensitivity across the retina of the achromat: sensitivity of human rod vision. Journal of Physiology. 1987;388:101–119. doi: 10.1113/jphysiol.1987.sp016604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. Journal of the Acoustical Society of America. 1971;49(2):467–477. [PubMed] [Google Scholar]
- Loschky LC, McConkie GW. Investigating spatial vision and dynamic attentional selection using a gaze-contingent multiresolutional display. Journal of Experimental Psychology: Applied. 2002;8(2):99–117. [PubMed] [Google Scholar]
- MacLeod DI. Rods cancel cones in flicker. Nature. 1972;235:173–174. doi: 10.1038/235173a0. [DOI] [PubMed] [Google Scholar]
- Michel MM, Geisler WS. Intrinsic position uncertainty explains detection and localization performance in peripheral vision. Journal of Vision. 2011;11(1):18, 1–18. doi: 10.1167/11.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morvan C, Maloney LT. Human visual search does not maximize the post-saccadic probability of identifying targets. PLoS Computational Biology. 2012;8(2):e1002342. doi: 10.1371/journal.pcbi.1002342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motter BC, Belky EJ. The guidance of eye movements during active visual search. Vision Research. 1998;38:1805–1815. doi: 10.1016/s0042-6989(97)00349-0. [DOI] [PubMed] [Google Scholar]
- Najemnik J, Geisler WS. Optimal eye movement strategies in visual search. Nature. 2005;434:387–391. doi: 10.1038/nature03390. [DOI] [PubMed] [Google Scholar]
- Najemnik J, Geisler WS. Eye movement statistics in humans are consistent with an optimal search strategy. Journal of Vision. 2008;8(3):1–14. doi: 10.1167/8.3.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najemnik J, Geisler WS. Simple summation rule for optimal fixation selection in visual search. Vision Research. 2009;49:1286–1294. doi: 10.1016/j.visres.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Peli E, Yang J, Goldstein RB. Image invariance with changes in size: the role of peripheral contrast thresholds. Journal of the Optical Society of America A. 1991;8:1762–1774. doi: 10.1364/josaa.8.001762. [DOI] [PubMed] [Google Scholar]
- Pelli DG. Uncertainty explains many aspects of visual contrast detection and discrimination. Journal of the Optical Society of America A. 1985;2(9):1508–1532. doi: 10.1364/josaa.2.001508. [DOI] [PubMed] [Google Scholar]
- Peterson MF, Eckstein MP. Looking just below the eyes is optimal across face recognition tasks. Proceedings of the National Academy of Sciences. 2012;109(48):E3314–E3323. doi: 10.1073/pnas.1214269109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson MF, Eckstein MP. Individual differences in eye movements during face identification reflect observer-specific optimal points of fixation. Psychological Science. 2013;24(7):1216–1225. doi: 10.1177/0956797612471684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson MF, Eckstein MP. Learning optimal eye movements to unusual faces. Vision Research. 2014;99:57–68. doi: 10.1016/j.visres.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson MS, Kramer AF. Attentional guidance of the eyes by contextual information and abrupt onsets. Perception & Psychophysics. 2001;63(7):1239–1249. doi: 10.3758/bf03194537. [DOI] [PubMed] [Google Scholar]
- Pointer JS, Hess RF. The contrast sensitivity gradient across the human visual field: with emphasis on the low spatial frequency range. Vision Research. 1989;29(9):1133–1151. doi: 10.1016/0042-6989(89)90061-8. [DOI] [PubMed] [Google Scholar]
- Previc FH. Attentional and oculomotor influences on visual field anisotropies in visual search performance. Visual Cognition. 1996;3(3):277–301. [Google Scholar]
- Renninger LW, Verghese P, Coughlan J. Where to look next? Eye movements reduce local uncertainty. Journal of Vision. 2007;7(3):6, 1–17. doi: 10.1167/7.3.6. [DOI] [PubMed] [Google Scholar]
- Schütz AC. Interindividual differences in preferred directions of perceptual and motor decisions. Journal of Vision. 2014;14(12):16, 1–17. doi: 10.1167/14.12.16. [DOI] [PubMed] [Google Scholar]
- Schütz AC, Trommershäuser &, Gegenfurtner KR. Dynamic integration of information about salience and value for saccadic eye movements. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(19):7547–7552. doi: 10.1073/pnas.1115638109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe LT, Stockman A, MacLeod DI. Rod flicker perception: scotopic duality, phase lags and destructive interference. Vision Research. 1989;29(11):1539–1559. doi: 10.1016/0042-6989(89)90137-5. [DOI] [PubMed] [Google Scholar]
- Stockman A, Sharpe LT. Spectral sensitivities of the middle- and long-wavelength sensitive cones derived from measurements in observers of known genotype. Vision Research. 2000;40:1711–1737. doi: 10.1016/s0042-6989(00)00021-3. [DOI] [PubMed] [Google Scholar]
- Verghese P. Active search for multiple targets is inefficient. Vision Research. 2012;74:61–71. doi: 10.1016/j.visres.2012.08.008. [DOI] [PubMed] [Google Scholar]
- Verghese P, Ghahghaei S. Immediate feedback improves saccade efficiency. Journal of Vision. 2013;13(9) [Google Scholar]
- Walthew C, Gilchrist ID. Target location probability effects in visual search: an effect of sequential dependencies. Journal of Experimental Psychology: Human Perception and Performance. 2006;32(5):1294. doi: 10.1037/0096-1523.32.5.1294. [DOI] [PubMed] [Google Scholar]
- Watson AE, Pelli DG. QUEST: A Bayesian adaptive psychometric method. Perception & Psychophysics. 1983;33(2):113–120. doi: 10.3758/bf03202828. [DOI] [PubMed] [Google Scholar]
- Wichman FA, Hill NJ. The psychometric fuction: 1. Fitting, sampling, and goodness of a fit. Perception and Psychophysics. 2001;68:1293–1313. doi: 10.3758/bf03194544. [DOI] [PubMed] [Google Scholar]
- Wolfe JM. Guided Search 4.0: Current progress with a model of visual search. In: Gray W, editor. Integrated models of cognitivesystems. New York: Oxford; 2007. pp. 99–119. [Google Scholar]
- Wolfe JM. Visual Search. Current Biology. 2010;20(8):R346–349. doi: 10.1016/j.cub.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe JM, Gencarz G. Guided Search 3.0: A model of visual search catches up with Jay Enoch 40 years later. In: Lakshminarayanan V, editor. Basic and clinical applications of vision science. Dordrecht, The Netherlands: Kluwer Academic; 1996. pp. 189–192. [Google Scholar]
- Wyszecki G, Stiles WS. Color Science: concepts and methods, quantitative data and formulae. 2. New York: Wiley; 1982. [Google Scholar]
- Zelinsky GJ. Using eye saccades to assess the selectivity of search movements. Vision Research. 1996;36(4):2177–2187. doi: 10.1016/0042-6989(95)00300-2. [DOI] [PubMed] [Google Scholar]