Abstract
Objective
Surveys suggest prolonged administration of prophylactic antibiotics is common after mastectomy with reconstruction. We determined utilization, predictors, and outcomes of post-discharge prophylactic antibiotics after mastectomy ± immediate breast reconstruction.
Design
Retrospective cohort.
Patients
Commercially insured women aged 18–64 years coded for mastectomy from 1/2004–12/2011. Women with a preexisting wound complication or septicemia were excluded.
Methods
Predictors of prophylactic antibiotics within 5 days after discharge were identified in women with one year prior insurance enrollment; relative risks (RR) were calculated using generalized estimating equations.
Results
12,501 mastectomy procedures were identified, with immediate reconstruction in 7,912 (63.3%). Post-discharge prophylactic antibiotics were used in 4,439 (56.1%) procedures with and 1,053 (22.9%) without immediate reconstruction (p < .001). The most common antibiotics were cephalosporins (75.1%) and fluoroquinolones (11.1%). Independent predictors of post-discharge antibiotics were implant reconstruction (RR 2.41, 95% confidence interval [CI] 2.23, 2.60), autologous reconstruction (RR 2.17, 95% CI 1.93, 2.45), autologous reconstruction plus implant (RR 2.11, 95% CI 1.92, 2.31), hypertension (RR 1.05, 95% CI 1.00, 1.10), tobacco use (RR 1.07, 95% CI 1.01, 1.14), surgery at an academic hospital (RR 1.14, 95% CI 1.07, 1.21), and receipt of home health care (RR 1.11, 95% CI 1.04, 1.18). Post-discharge prophylactic antibiotics were not associated with SSI after mastectomy ± immediate reconstruction (both p > 0.05).
Conclusions
Prophylactic post-discharge antibiotics are common after mastectomy, with immediate reconstruction the strongest predictor. Stewardship efforts in this population to limit continuation of prophylactic antibiotics after discharge are needed to limit antimicrobial resistance.
Introduction
Surgical site infections (SSIs) are the most common healthcare-associated infections in the United States, with an estimated 157,500 SSIs occurring annually.1 The Centers for Disease Control and Prevention (CDC) guidelines for the prevention of SSI recommend the use of pre-operative antibiotic prophylaxis for procedures in which there are data supporting the benefit of prophylactic antibiotics, those that involve implantation of a medical device, or in surgeries where an SSI is potentially catastrophic.2 The most recent CDC guidelines recommend against administration of prophylactic antibiotics in clean surgeries after the surgical incision is closed, even in the presence of surgical drains,3 due to lack of data showing benefit for this practice. Other national and international organizations recommend stopping prophylactic antibiotics within 24 hours of non-cardiothoracic surgery.2,4,5 In practice, compliance with limiting the duration of prophylactic antibiotic after surgery was found to vary by 58–100% in a study of National Surgical Quality Improvement Program hospitals.6,7 A major concern with the use of post-discharge antibiotic prophylaxis is exposing patients to unnecessary antibiotics. The use of unnecessary antibiotics may result in additional costs and adverse events, in addition to selection of antibiotic-resistant bacteria8 and Clostridium difficile infection.9
Post-discharge antibiotic prophylaxis use is common in mastectomy10-13 and breast augmentation,14,15 in which surgeons often continue antibiotics until removal of all surgical drains. The American Society of Plastic Surgeons practice guidelines for expander/implant breast reconstruction recommend that antibiotics should be discontinued < 24 hours after surgery unless drains are present, in which case prophylaxis duration is left to surgeon preference.16 One survey of 460 plastic surgeons found that 72% prescribed outpatient antibiotics after mastectomy with breast reconstruction.17 Another survey of 253 plastic surgeons who performed primarily cosmetic breast augmentation reported that 84% prescribed postoperative antibiotics, with 73% continuing them for >3 days.18
We utilized a large commercial health insurer database to analyze use and outcomes of post-discharge prophylactic antibiotics in a cohort of women undergoing mastectomy with and without immediate reconstruction. We aimed to determine the prevalence of post-discharge prophylactic antibiotics in uncomplicated procedures, identify patient and operative factors associated with this utilization, and in a secondary analysis, determine whether post-discharge antibiotic use was associated with decreases in SSI and noninfectious wound complications (NIWCs). Use of large generalizable health insurer claims data allows for better understanding of practice regarding continuation of prophylactic antibiotics post-hospital discharge among a wide variety of surgeons and potential stewardship opportunities to limit this practice in breast surgery. The large database also provides the statistical power lacking from existing studies to determine the association of post-discharge antibiotics and the incident SSIs and NIWCs.
Methods
Primary Data Source
We conducted a retrospective cohort study using all fully-adjudicated paid claims submitted for reimbursement from providers, facilities, and outpatient pharmacies linked to health plan enrollment information from 12 Anthem-affiliated plans in the HealthCore Integrated Research Database (Appendix).19 Fully-insured women with enrollment in a fee-for-service plan with medical coverage of hospital and physician services and prescription drug coverage were eligible for inclusion in the cohort. Women with end-stage renal disease, prior organ transplant, or HIV infection were excluded due to the rare nature of the conditions and for privacy concerns.
Mastectomy Population
We identified inpatient mastectomy operations among insured members aged 18–64 years from 1/1/2004–12/31/2011 using International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) and Current Procedural Terminology, 4th edition (CPT-4) procedure codes from facilities and providers, as described previously.19 Classification of the mastectomy and reconstruction has been described in detail previously.19,20 To limit identification of antibiotics to those prescribed for prophylaxis (rather than therapeutic indications), we excluded operations for which SSI,19 cellulitis,19 NIWCs,21 port infection (ICD-9-CM diagnosis 996.62), or septicemia (ICD-9-CM diagnosis 038.0–038.9, 790.7) were coded from 30 days before mastectomy through 5 days after discharge from the mastectomy hospitalization (see below).
Post-Discharge Prophylactic Antibiotics
We defined post-discharge prophylactic antibiotics as any of the following antibiotics from paid claims in the outpatient pharmacy file from 0–5 days after mastectomy discharge: cephalosporins, fluoroquinolones, lincosamides, macrolides, penicillins, rifampin, sulfamethoxazole-trimethoprim, and tetracyclines.
Predictors of Prophylactic Antibiotic Use
To identify predictors of post-discharge prophylactic antibiotic use in univariable and multivariable analyses, we limited the population to women with insurance coverage 365 days before through 30 days after surgery, the first mastectomy per patient, and procedures with non-missing information on patient region of residence (Appendix, Figure).
Potential predictors of prophylactic antibiotic use included demographics, comorbidities (primarily using the Elixhauser classification),22 medications, cancer, operative factors, and facility factors with clinical or biologic plausibility for association with antibiotic use and/or risk for SSI, as described previously.20,21 We included postoperative home health care within 10 days after discharge, unless the home health visits were first coded at the time of or after an SSI or NIWC, as these visits were likely due to wound care.
Complications After Mastectomy
SSIs and NIWCs first recorded from 6 days after mastectomy discharge through 90 days after mastectomy were identified using ICD-9-CM diagnosis codes from inpatient and outpatient facilities and provider claims, as reported previously.19,21 NIWCs included fat necrosis (567.82, 611.3), dehiscence (875.0, 875.1, 879.0, 879.1, 998.3, 998.32), hematoma (998.12), and tissue necrosis (998.83). We censored the observation period to capture complications before a subsequent surgical procedure, as described previously.19 We captured C. difficile infection (ICD-9-CM diagnosis code 008.45 or prescription for oral vancomycin) through 30 days after discharge from the mastectomy hospitalization. For persons with prophylactic antibiotic use, we only included C. difficile coded on or after the first post-discharge antibiotic date.
Statistical Analyses
We determined potential predictors of prophylactic antibiotic use using chi-square tests for binary variables and a generalized estimating equations (GEE) model for multicategory variables. Any predictors with a cell size < 5 were excluded from analysis. Among factors with p < .2 in univariate analysis, we assessed multicollinearity by examining the tolerance values in each model to ensure independence of explanatory variables. A GEE model was used to estimate adjusted relative risks since the prevalence of prophylactic antibiotics was high. Backward selection was used with a cutoff of p < .05. For our secondary aim, we compared the incidence of SSI and NIWCs after mastectomy according to prophylactic antibiotic use with a logistic regression model, controlling for type of breast reconstruction. All data management and analyses were performed using SAS v9.4 (SAS Institute Inc., Cary, NC). We performed post-hoc power calculations using PASS 14 Power Analysis and Sample Size Software (NCSS, LLC, Kaysville, UT). This study was approved by the Washington University Human Research Protection Office.
Results
Starting with our previously described population of 18,696 mastectomy procedures,19 12,501 inpatient mastectomy procedures among 12,198 patients with medical and prescription drug coverage met eligibility criteria for this study (Appendix). Immediate reconstruction was performed in 7,912 (63.3%) procedures. Prophylactic antibiotics were prescribed post-discharge after 5,492 (43.9%) procedures overall. Of 5,651 unique prescriptions within 5 days post-discharge, cephalosporins were the most commonly prescribed antibiotic class (75.1%), followed by fluoroquinolones (11.1%), lincosamides (4.8%), and penicillins (3.5%). Utilization of post-discharge prophylactic antibiotics was more common after mastectomy with immediate reconstruction than after mastectomy only (4,439 [56.1%] versus 1,053 [22.9%]; p < .001).
Overall, 793 procedures were coded for SSI (6.3%) and 802 (6.4%) were coded for an NIWC within 90 days after operation. A total of 13 (0.1%) procedures had evidence for C. difficile infection within 30 days after hospital discharge. In mastectomy-only operations, the SSI rates were similar regardless of post-discharge prophylactic antibiotic use, but the rate of NIWCs was higher in women given post-discharge prophylactic antibiotics compared to women who did not receive post-discharge antibiotics (Figure 1). Post-discharge prophylactic antibiotic use was not associated with SSI or NIWCs following mastectomy with immediate reconstruction (Figure 1). After adjusting for type of procedure (i.e., mastectomy only, implant reconstruction, autologous flap reconstruction), receipt of post-discharge prophylactic antibiotic was not associated with SSI (odds ratio [OR] 0.87; 95% confidence interval [CI] 0.75, 1.01) nor NIWCs (OR 1.14; 95% CI 0.99, 1.33) compared to no prescription for post-discharge antibiotics. Based on the number of reconstruction procedures and assuming a 25% reduction in SSI associated with post-discharge antibiotic use, we had 81% power to detect a difference in the complication rates in the reconstruction population, but much lower power (18%) in the mastectomy only population, due to the lower percentage receiving post-discharge antibiotics and the lower baseline complication rates.
Figure 1. Incidence of Surgical Site Infection and Noninfectious Wound Complications by Post-Discharge Prophylactic Antibiotic Use and Presence of Immediate Breast Reconstruction (N = 12,501).
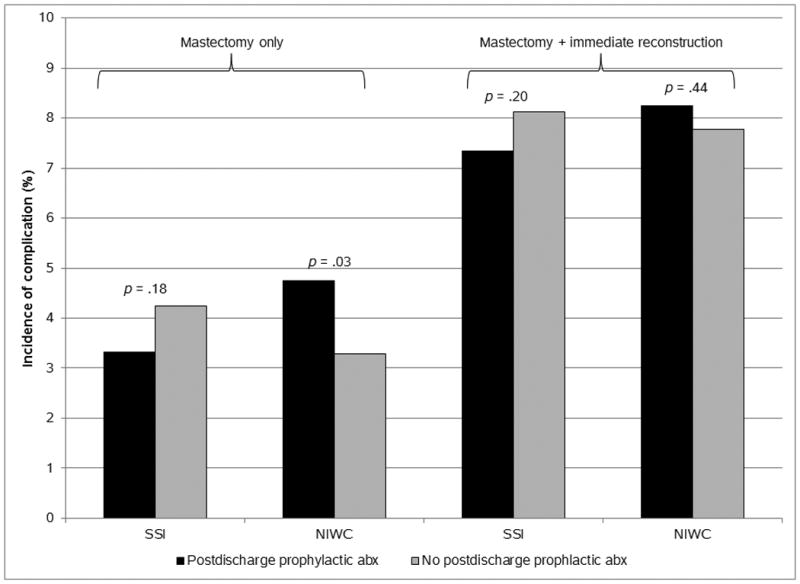
NOTE. Abx, antibiotic; NIWC, noninfectious wound complication; SSI, surgical site infection.
The population for analysis of risk factors associated with post-discharge antibiotic utilization was reduced to 9,188, after excluding those with less than one year of prior health insurance coverage, second mastectomy procedures, and 23 procedures with missing U.S. region (Appendix figure). In univariable analysis, women who received post-discharge prophylactic antibiotics were younger, in the higher income quartiles, and more likely to have preexisting depression, tobacco use disorder, skin disease, breast carcinoma in situ or undergoing prophylactic mastectomy, compared to women who did not receive post-discharge antibiotics (Table 1). Women who received post-discharge antibiotics were also more likely to undergo sentinel node dissection, implant, autologous flap, and autologous flap plus implant reconstruction, and less likely to have a modified radical mastectomy than women who did not receive antibiotics post-discharge. Several facility factors were associated with increased likelihood to receive post-discharge prophylactic antibiotics, including larger bed size, medical school affiliation, residency program, and facility location in the U.S. Northeast and Midwest regions (Table 1).
Table 1. Univariate Predictors of Post-Discharge Prophylactic Antibiotics after 9,188 Mastectomy Proceduresa.
| Variable | Category | Post-discharge prophylactic antibiotic use | P | |
|---|---|---|---|---|
| Yes No. (%) |
No No. (%) |
|||
| Total | 4,071 | 5,117 | ||
| Patient factors | ||||
| Age | 18–35 years | 208 (5.1) | 204 (4.0) | .59 |
| 36–40 years | 356 (8.7) | 357 (7.0) | .68 | |
| 41–45 years | 673 (16.5) | 701 (13.7) | Ref | |
| 46–50 years | 913 (22.4) | 1,040 (20.3) | .20 | |
| 51–55 years | 797 (19.6) | 982 (19.2) | .02 | |
| 56–60 years | 664 (16.3) | 1,035 (20.2) | <.001 | |
| 61–64 years | 460 (11.3) | 798 (15.6) | <.001 | |
| Income quartile (based on median for zip code) | 0–25th percentile | 797 (19.6) | 1,254 (24.5) | <.001 |
| 26–50th percentile | 930 (22.8) | 1,224 (23.9) | <.001 | |
| 51–75th percentile | 1,036 (25.4) | 1,206 (23.6) | .06 | |
| 76–100th percentile | 1,176 (28.9) | 1,227 (24.0) | Ref | |
| Missing | 132 (3.2) | 206 (4.0) | .002 | |
| Comorbidities/medications | ||||
| Anticoagulopathy drugs | 60 (1.5) | 107 (2.1) | .03 | |
| Depression | 302 (7.4) | 333 (6.5) | .09 | |
| Diabetes | 229 (5.6) | 373 (7.3) | .001 | |
| Hypertension | 1,216 (29.9) | 1,623 (31.7) | .06 | |
| Liver disease | 13 (0.3) | 28 (0.5) | .10 | |
| Malnutrition/weight loss | 35 (0.9) | 60 (1.2) | .14 | |
| Cancer (other than breast) | 331 (8.1) | 490 (9.6) | .02 | |
| Obesity | 267 (6.6) | 373 (7.3) | .17 | |
| Skin disease | 102 (2.5) | 103 (2.0) | .11 | |
| Tobacco use disorder | 527 (12.9) | 608 (11.9) | .12 | |
| Smoking-related diseases | 107 (2.6) | 178 (3.5) | .02 | |
| Cancer-related | ||||
| Stage of breast cancer | Benign or prophylactic | 160 (3.9) | 146 (2.9) | .003 |
| Carcinoma in situ | 585 (14.4) | 589 (11.5) | <.001 | |
| Local | 2,465 (60.6) | 3,100 (60.6) | Ref | |
| Regional | 772 (19.0) | 1,144 (22.4) | .003 | |
| Metastatic | 89 (2.2) | 138 (2.7) | .15 | |
| Operative factors | ||||
| Type of procedure | Unilateral mastectomy only | 581 (14.3) | 1,844 (36.0) | Ref |
| Bilateral mastectomy only | 185 (4.5) | 640 (12.5) | .37 | |
| Mastectomy plus implant | 2,535 (62.3) | 1,878 (36.7) | <.001 | |
| Mastectomy plus flap | 570 (14.0) | 562 (11.0) | <.001 | |
| Mastectomy plus flap and implant | 200 (4.9) | 193 (3.8) | <.001 | |
| Modified radical mastectomy | 1,522 (37.4) | 2,271 (44.4) | <.001 | |
| Sentinel node dissection | 1,126 (27.7) | 1,233 (24.1) | <.001 | |
| Facility | ||||
| Number of beds | 1–299 | 839 (20.6) | 1,354 (26.5) | Ref |
| 300–499 | 881 (21.6) | 1,137 (22.2) | <.001 | |
| 500+ | 826 (20.3) | 838 (16.4) | <.001 | |
| Missingb | 1,525 (37.5) | 1,788 (34.9) | <.001 | |
| Location of facility | Urban | 2,460 (60.4) | 3,126 (61.1) | <.001 |
| Rural | 86 (2.1) | 203 (4.0) | Ref | |
| Missingb | 1,525 (37.5) | 1,788 (34.9) | <.001 | |
| Medical school affiliation | Yes | 1,638 (40.2) | 1,777 (34.7) | <.001 |
| No | 908 (22.3) | 1,552 (30.3) | Ref | |
| Missingb | 1,525 (37.5) | 1,788 (34.9) | <.001 | |
| Residency program | Yes | 1,424 (35.0) | 1,530 (29.9) | <.001 |
| No | 1,122 (27.6) | 1,799 (35.2) | Ref | |
| Missingb | 1,525 (37.5) | 1,788 (34.9) | <.001 | |
| Region of facilityc | Northeast | 984 (24.2) | 830 (16.2) | <.001 |
| South | 1,174 (28.8) | 1,749 (34.2) | .58 | |
| Midwest | 702 (17.2) | 786 (15.4) | <.001 | |
| West | 1,211 (29.7) | 1,752 (34.2) | Ref | |
| Postoperative factors | ||||
| Home health cared | 577 (14.2) | 601 (11.7) | .001 | |
NOTE. Ref, reference group.
The following factors had p ≥ 0.2 and were excluded from the table: alcohol abuse, blood loss anemia, coagulopathy, collagen vascular/ rheumatologic disease, congestive heart failure, deficiency anemia, disease-modifying anti-rheumatic drugs, oral steroids, peripheral vascular disease, pneumonia or urinary tract infection in UTI within 30 days prior to surgery, psychoses, renal failure, prior Staphylococcus aureus infection, inflammatory breast disease, history of breast cancer, and history of radiotherapy.
Missing facility information due to no facility claim for procedure, no match to a facility in the American Hospital Association Annual Survey of Hospitals, or a match to multiple facilities.
For those with missing facility information, we used patient home zip code when possible to determine the U.S. region of the facility.
Restricted to home health care before a surgical site infection or noninfectious wound complication
The independent predictors of prophylactic antibiotic use post-discharge with the largest effect sizes were operative factors, including immediate breast implant (relative risk [RR] 2.41), and autologous flap reconstruction with (RR 2.17) or without an implant (RR 2.11). Patient factors (hypertension, tobacco use disorder, and postoperative home health), and facility characteristics (surgery at a facility in the Northeast or Midwest and surgery at an academic hospital) were associated with slightly increased risk of post-discharge prophylactic antibiotic use after controlling for the type of procedure (Table 2).
Table 2. Multivariable Predictors of Post-Discharge Prophylactic Antibiotics after Mastectomy Performed During an Inpatient Hospitalization.
| Variable | Category | Relative risk (95% confidence interval) |
|---|---|---|
| Patient factors | ||
| Hypertension | 1.05 (1.00, 1.10) | |
| Tobacco use disorder | 1.07 (1.01, 1.14) | |
| Operative factors | ||
| Type of procedure | Unilateral mastectomy only | 1.00 |
| Bilateral mastectomy only | 0.94 (0.81, 1.09) | |
| Mastectomy plus implant | 2.41 (2.23, 2.60) | |
| Mastectomy plus flap | 2.11 (1.92, 2.31) | |
| Mastectomy plus flap plus implant | 2.17 (1.93, 2.45) | |
| Location of facilitya | West | 1.00 |
| Northeast | 1.25 (1.18, 1.33) | |
| South | 1.04 (0.97, 1.10) | |
| Midwest | 1.19 (1.11, 1.28) | |
| Medical school affiliation | Yes | 1.14 (1.07, 1.21) |
| No | 1.00 | |
| Missing | 1.05 (0.99, 1.12) | |
| Postoperative factors | ||
| Home health care | 1.11 (1.04, 1.18) | |
Discussion
We determined variation and predictors of post-discharge prophylactic antibiotic use in a large population of women undergoing mastectomy using geographically diverse claims data. Despite recommendations in many surgical guidelines to discontinue administration of prophylactic antibiotics either immediately after surgery or within 24 hours post-procedure, antibiotics are frequently overused postoperatively, particularly after breast operations. We found that utilization of post-discharge prophylactic antibiotics ranged from 23% for mastectomy without immediate reconstruction to 56% for mastectomy with immediate reconstruction, aligning with survey data suggesting that plastic surgeons are more likely to continue patients on prophylactic antibiotics post-discharge than general breast surgeons.17,23 Given their common utilization of antibiotics, surgeons are positioned to be key partners in the success of hospital antibiotic stewardship programs.24
Factors associated with prolonged perioperative prophylaxis are critical to development of interventions to assure appropriate antibiotic use. We found that by far the most important predictors of post-discharge prophylactic antibiotics after mastectomy were autologous flap and implant reconstruction, associated with 2.1–2.4 fold increased risk of prolonged antibiotic use. Immediate reconstruction is preferred over delayed reconstruction by many plastic surgeons due to better cosmesis and perceived psychosocial benefits for the patient.25 Surgery performed in a teaching facility and in facilities located in the U.S. Northeast and Midwest regions were also associated with small increased risk of post-discharge prophylactic antibiotic use. This regional variation in use of prolonged antibiotics is important to investigate further, since it may have broad implications for regional differences in antibiotic stewardship programs as well as rates of antibiotic resistance in the community. In contrast patient characteristics were associated with only slightly increased risk of prolonged antibiotic utilization, suggesting that stewardship interventions to reduce post-discharge antibiotic utilization should target primarily plastic surgeons performing immediate reconstruction.
Three prior observational breast surgery studies reported significantly lower SSI rates in patients given post-discharge prophylactic antibiotics compared to either the recommended practice of a single dose of antibiotic before incision26 or prophylaxis limited to 24 hours after surgery.27,28 The study by Edwards included procedures performed by only two surgeons, one of whom always prescribed postoperative antibiotics until drains were removed while the other surgeon never used postoperative antibiotics. Therefore, confounding bias may be present in this study due to unmeasured differences in operative technique or other practices between the two surgeons.27 In the studies by Clayton26 and Avashia28 et al., retrospective comparison of SSI rates during time periods with different prophylactic antibiotic practices were performed. It is possible other infection control and operative practices varied between time periods, resulting in residual confounding. In the study by Avashia et al. a change in prophylactic antibiotic practice was prompted by a 31.6% infection rate in 19 women during the period when only perioperative antibiotics were used, raising the possibility that the decreased SSI rate after reverting back to prolonged antibiotic administrative was due to regression to the mean.28 The five observational studies10,29-32 and one randomized trial33 in the literature that did not find significantly different SSI rates associated with post-discharge antibiotic use compared to 24 hours or less of antibiotic administration were small and thus underpowered to detect a significant difference if it existed.34 The lack of high-quality data on the risk and benefits of post-discharge antibiotic prophylaxis likely perpetuates the continued use of inappropriate prolonged antibiotic prophylaxis beyond the recommended 24 hours of duration.
In contrast to the three studies above that reported lower SSI rates associated with post-discharge prophylactic antibiotic use, we found that the rates of SSI and NIWCs did not differ according to use of post-discharge prophylactic antibiotics, after controlling for procedure type. Importantly, we had sufficient power (>80%) in the reconstruction population to detect a difference between post-discharge prophylactic antibiotic use and the incidence of complications.10,26-33 Cephalosporins were the antibiotic class most commonly prescribed for post-discharge prophylaxis in our cohort, followed by fluoroquinolones. Both of these antibiotic classes are associated with selection for colonization by and infection with antibiotic-resistant bacteria.35 Fluoroquinolones are also increasingly reported to be associated with serious adverse events.36-38
The coding algorithms we used based on ICD-9-CM and CPT-4 codes have relatively good sensitivity to identify SSI after breast procedures39 and C. difficile infection.40 Despite this, our estimates of SSI, NIWCs, and C. difficile infection rates are almost certainly underestimates of the true incidence, since the claims algorithm is unlikely to detect most minor infections, especially during the global 90-day major surgery reimbursement period. It is possible that not all post-discharge antibiotic use in the absence of diagnosis codes for infection or NIWCs represented continuation of antibiotic prophylaxis, although this is unlikely since our window to identify post-discharge prophylaxis was very short (within 5 days after discharge). Outpatient antibiotics were identified by paid pharmacy claims and thus we could not detect antibiotics given as samples or in the absence of a prescription drug claim, and cannot confirm that antibiotics were taken as directed. Although facility characteristics were missing for approximately one-third of procedures, we think differential misclassification is unlikely, such that certain types of facilities would be disproportionally more or less likely to have a paid claim in the database or match to the AHA database. We were however able to impute the facility region by using patient zip code, which was important since regional utilization of post-discharge antibiotics varied.
Strengths of our study include the large population of women undergoing mastectomy, resulting in sufficient power to detect differences in complication rates in the reconstruction population. Our population was enriched in women undergoing breast reconstruction because of the use of commercial claims data, since younger women are more likely to undergo immediate reconstruction.41 Additional strengths include the ability to detect regional differences in antibiotic use because of the broad geographic coverage in the insurer claims data, and analysis of variation in use of prophylactic antibiotics by operative, patient, and facility characteristics.
In summary, we found that post-discharge prophylactic antibiotic use in the absence of infectious or noninfectious wound complications is common after mastectomy, and driven primarily by immediate reconstruction rather than patient-level characteristics. We found no benefit to continuation of prophylactic antibiotics post-discharge, with similar SSI and NIWC complication rates in women who received post-discharge antibiotics versus women who did not receive continued prophylactic antibiotics. Stewardship efforts to limit the duration of prophylactic antibiotics after surgery and discourage continuation of antibiotics after hospital discharge are essential to avoid further increases in antimicrobial resistance.
Supplementary Material
Acknowledgments
We thank Cherie Hill for database and computer management support.
Funding: Funding for this project was provided by the National Institutes of Health (NIH) (5R01CA149614 to MAO). Additional support was provided by the Centers for Disease Control and Prevention (CDC) Epicenters Program (U54CK000162 to VJF), grant UL1 TR000448 from the National Center for Advancing Translational Sciences (NCATS) of the NIH, and grant number R24 HS19455 from the Agency for Healthcare Research and Quality (AHRQ). The findings and conclusions in this document are those of the authors, who are responsible for its content, and do not necessarily represent the official view of NIH, CDC, or AHRQ.
Footnotes
This work was presented at the International Conference on Pharmacoepidemiology & Therapeutic Risk Management in Dublin, Ireland in August 2016.
Conflicts of interest: MAO reports consultant work with Merck, Pfizer, and Sanofi Pasteur and grant funding through Pfizer, and Sanofi Pasteur for work outside the submitted manuscript. AEW is an employee of HealthCore, a wholly-owned subsidiary of Anthem, Inc., a health insurance company; she has received Anthem stock options and participated in an Anthem employee stock purchase plan. VJF reports her spouse is the Senior Vice President and Chief Medical Officer for Express Scripts; she has received grants from the Foundation for Barnes-Jewish Hospital, CDC, and the Doris Duke Foundation. DKW reports consultant work with Centene Corp., Worrell Inc., Novaerus, and Carefusion and is a sub-investigator for a Cepheid Inc.-sponsored study for work outside the submitted manuscript. No other authors report conflicts of interest relevant to this article.
References
- 1.Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370:1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol. 1999;20:250–278. doi: 10.1086/501620. [DOI] [PubMed] [Google Scholar]
- 3.Berrios-Torres SI, Umscheid CA, Bratzler DW, et al. Centers for Disease Control and Prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg. 2017 doi: 10.1001/jamasurg.2017.0904. [DOI] [PubMed] [Google Scholar]
- 4.Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm. 2013;70:195–283. doi: 10.2146/ajhp120568. [DOI] [PubMed] [Google Scholar]
- 5.European Centre for Disease Prevention and Control. Systematic review and evidence-based guidance on perioperative antibiotic prophylaxis. [Accessed 6/16/2016]; http://ecdc.europa.eu/en/publications/Publications/Perioperative%20antibiotic%20prophylaxis%20-%20June%202013.pdf. Published 2013.
- 6.Ingraham AM, Cohen ME, Bilimoria KY, et al. Association of surgical care improvement project infection-related process measure compliance with risk-adjusted outcomes: implications for quality measurement. J Am Coll Surg. 2010;211:705–714. doi: 10.1016/j.jamcollsurg.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Wang Z, Chen F, Ward M, Bhattacharyya T. Compliance with Surgical Care Improvement Project measures and hospital-associated infections following hip arthroplasty. J Bone Joint Surg Am. 2012;94:1359–1366. doi: 10.2106/JBJS.K.00911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harbarth S, Samore MH, Lichtenberg D, Carmeli Y. Prolonged antibiotic prophylaxis after cardiovascular surgery and its effect on surgical site infections and antimicrobial resistance. Circulation. 2000;101:2916–2921. doi: 10.1161/01.cir.101.25.2916. [DOI] [PubMed] [Google Scholar]
- 9.Poeran J, Mazumdar M, Rasul R, et al. Antibiotic prophylaxis and risk of Clostridium difficile infection after coronary artery bypass graft surgery. J Thorac Cardiovasc Surg. 2016;151:589–597. doi: 10.1016/j.jtcvs.2015.09.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Throckmorton AD, Boughey JC, Boostrom SY, et al. Postoperative prophylactic antibiotics and surgical site infection rates in breast surgery patients. Ann Surg Oncol. 2009;16:2464–2469. doi: 10.1245/s10434-009-0542-1. [DOI] [PubMed] [Google Scholar]
- 11.Hedrick TL, Smith PW, Gazoni LM, Sawyer RG. The appropriate use of antibiotics in surgery: a review of surgical infections. Curr Probl Surg. 2007;44:635–675. doi: 10.1067/j.cpsurg.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Halvorson EG, Disa JJ, Mehrara BJ, Burkey BA, Pusic AL, Cordeiro PG. Outcome following removal of infected tissue expanders in breast reconstruction: a 10-year experience. Ann Plast Surg. 2007;59:131–136. doi: 10.1097/01.sap.0000252716.73356.68. [DOI] [PubMed] [Google Scholar]
- 13.Macadam SA, Clugston PA, Germann ET. Retrospective case review of capsular contracture after two-stage breast reconstruction: is colonization of the tissue expander pocket associated with subsequent implant capsular contracture? Ann Plast Surg. 2004;53:420–424. doi: 10.1097/01.sap.0000130705.19174.d4. [DOI] [PubMed] [Google Scholar]
- 14.Khan UD. Breast augmentation, antibiotic prophylaxis, and infection: comparative analysis of 1,628 primary augmentation mammoplasties assessing the role and efficacy of antibiotics prophylaxis duration. Aesthetic Plast Surg. 2010;34:42–47. doi: 10.1007/s00266-009-9427-8. [DOI] [PubMed] [Google Scholar]
- 15.Perrotti JA, Castor SA, Perez PC, Zins JE. Antibiotic use in aesthetic surgery: a national survey and literature review. Plast Reconstr Surg. 2002;109:1685–1693. doi: 10.1097/00006534-200204150-00033. [DOI] [PubMed] [Google Scholar]
- 16.Alderman A, Gutowski K, Ahuja A, Gray D. ASPS clinical practice guideline summary on breast reconstruction with expanders and implants. Plast Reconstr Surg. 2014;134:648e–655e. doi: 10.1097/PRS.0000000000000541. [DOI] [PubMed] [Google Scholar]
- 17.Phillips BT, Wang ED, Mirrer J, et al. Current practice among plastic surgeons of antibiotic prophylaxis and closed-suction drains in breast reconstruction: experience, evidence, and implications for postoperative care. Ann Plast Surg. 2011;66:460–465. doi: 10.1097/SAP.0b013e31820c0593. [DOI] [PubMed] [Google Scholar]
- 18.Chopra K, Gowda AU, McNichols CH, Brown EN, Slezak S, Rasko Y. Antimicrobial prophylaxis practice patterns in breast augmentation: a national survey of current practice. Ann Plast Surg. 2016 doi: 10.1097/SAP.0000000000000942. [DOI] [PubMed] [Google Scholar]
- 19.Olsen MA, Nickel KB, Fox IK, et al. Incidence of surgical site infection following mastectomy with and without immediate reconstruction using private insurer claims data. Infect Control Hosp Epidemiol. 2015;36:907–914. doi: 10.1017/ice.2015.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olsen MA, Nickel KB, Margenthaler JA, et al. Development of a risk prediction model to individualize risk factors for surgical site infection after mastectomy. Ann Surg Oncol. 2016;23:2471–2479. doi: 10.1245/s10434-015-5083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nickel KB, Fox IK, Margenthaler JA, Wallace AE, Fraser VJ, Olsen MA. Effect of noninfectious wound complications after mastectomy on subsequent surgical procedures and early implant loss. J Am Coll Surg. 2016;222:844–852. doi: 10.1016/j.jamcollsurg.2016.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elixhauser A, Steiner C, Harris R, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Brahmbhatt RD, Huebner M, Scow JS, et al. National practice patterns in preoperative and postoperative antibiotic prophylaxis in breast procedures requiring drains: survey of the american society of breast surgeons. Ann Surg Oncol. 2012;19:3205–3211. doi: 10.1245/s10434-012-2477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sartelli M, Duane TM, Catena F, et al. Antimicrobial Stewardship: A Call to Action for Surgeons. Surg Infect (Larchmt) 2016;17:625–631. doi: 10.1089/sur.2016.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serletti JM, Fosnot J, Nelson JA, Disa JJ, Bucky LP. Breast reconstruction after breast cancer. Plast Reconstr Surg. 2011;127:124e–135e. doi: 10.1097/PRS.0b013e318213a2e6. [DOI] [PubMed] [Google Scholar]
- 26.Clayton JL, Bazakas A, Lee CN, Hultman CS, Halvorson EG. Once is not enough: withholding postoperative prophylactic antibiotics in prosthetic breast reconstruction is associated with an increased risk of infection. Plast Reconstr Surg. 2012;130:495–502. doi: 10.1097/PRS.0b013e31825dbefe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edwards BL, Stukenborg GJ, Brenin DR, Schroen AT. Use of prophylactic postoperative antibiotics during surgical drain presence following mastectomy. Ann Surg Oncol. 2014;21:3249–3255. doi: 10.1245/s10434-014-3960-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Avashia YJ, Mohan R, Berhane C, Oeltjen JC. Postoperative antibiotic prophylaxis for implant-based breast reconstruction with acellular dermal matrix. Plast Reconstr Surg. 2013;131:453–461. doi: 10.1097/PRS.0b013e31827c6d90. [DOI] [PubMed] [Google Scholar]
- 29.Liu DZ, Dubbins JA, Louie O, Said HK, Neligan PC, Mathes DW. Duration of antibiotics after microsurgical breast reconstruction does not change surgical infection rate. Plast Reconstr Surg. 2012;129:362–367. doi: 10.1097/PRS.0b013e31823ae8ce. [DOI] [PubMed] [Google Scholar]
- 30.McCullough MC, Chu CK, Duggal CS, Losken A, Carlson GW. Antibiotic prophylaxis and resistance in surgical site infection after immediate tissue expander reconstruction of the breast. Ann Plast Surg. 2016;77:501–505. doi: 10.1097/SAP.0000000000000275. [DOI] [PubMed] [Google Scholar]
- 31.Townley WA, Baluch N, Bagher S, et al. A single pre-operative antibiotic dose is as effective as continued antibiotic prophylaxis in implant-based breast reconstruction: A matched cohort study. J Plast Reconstr Aesthet Surg. 2015;68:673–678. doi: 10.1016/j.bjps.2014.12.041. [DOI] [PubMed] [Google Scholar]
- 32.Drury KE, Lanier ST, Khavanin N, et al. Impact of postoperative antibiotic prophylaxis duration on surgical site infections in autologous breast reconstruction. Ann Plast Surg. 2016;76:174–179. doi: 10.1097/SAP.0000000000000514. [DOI] [PubMed] [Google Scholar]
- 33.Phillips BT, Fourman MS, Bishawi M, et al. Are prophylactic postoperative antibiotics necessary for immediate breast reconstruction? results of a prospective randomized clinical trial. J Am Coll Surg. 2016;222:1116–1124. doi: 10.1016/j.jamcollsurg.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 34.Olsen MA, Nickel KB, Fox IK. Surveillance and prevention of surgical site infections in breast oncologic surgery with immediate reconstruction. Curr Treat Options Infect Dis. 2017 doi: 10.1007/s40506-017-0117-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paterson DL. “Collateral damage” from cephalosporin or quinolone antibiotic therapy. Clin Infect Dis. 2004;38(Suppl 4):S341–345. doi: 10.1086/382690. [DOI] [PubMed] [Google Scholar]
- 36.Wise BL, Peloquin C, Choi H, Lane NE, Zhang Y. Impact of age, sex, obesity, and steroid use on quinolone-associated tendon disorders. Am J Med. 2012;125:1228.e1223–1228.e1228. doi: 10.1016/j.amjmed.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lapi F, Wilchesky M, Kezouh A, Benisty JI, Ernst P, Suissa S. Fluoroquinolones and the risk of serious arrhythmia: a population-based study. Clin Infect Dis. 2012;55:1457–1465. doi: 10.1093/cid/cis664. [DOI] [PubMed] [Google Scholar]
- 38.Etminan M, Brophy JM, Samii A. Oral fluoroquinolone use and risk of peripheral neuropathy: a pharmacoepidemiologic study. Neurology. 2014;83:1261–1263. doi: 10.1212/WNL.0000000000000846. [DOI] [PubMed] [Google Scholar]
- 39.Olsen MA, Fraser VJ. Use of diagnosis codes and/or wound culture results for surveillance of surgical site infection after mastectomy and breast reconstruction. Infect Control Hosp Epidemiol. 2010;31:544–547. doi: 10.1086/652155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dubberke ER, Butler AM, Yokoe DS, et al. Multicenter study of surveillance for hospital-onset Clostridium difficile infection by the use of ICD-9-CM diagnosis codes. Infect Control Hosp Epidemiol. 2010;31:262–268. doi: 10.1086/650447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jagsi R, Jiang J, Momoh AO, et al. Trends and variation in use of breast reconstruction in patients with breast cancer undergoing mastectomy in the United States. J Clin Oncol. 2014;32:919–926. doi: 10.1200/JCO.2013.52.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


