Abstract
The PTPN22R620W single nucleotide polymorphism increases the risk of developing multiple autoimmune diseases including type 1 diabetes, rheumatoid arthritis and lupus. PTPN22 is highly expressed in antigen presenting cells (APCs) where the expression of the murine disease associated variant orthologue (Ptpn22R619W) is reported to dysregulate pattern recognition receptor signalling in dendritic cells (DCs) and promote T-cell proliferation. Because T-cell activation is dependent on DC antigen uptake, degradation and presentation, we analysed the efficiency of these functions in splenic and GM-CSF bone marrow derived DC from wild type (WT), Ptpn22-/- or Ptpn22R619W mutant mice. Results indicated no differential ability of DCs to uptake antigen via macropinocytosis or receptor-mediated endocytosis. Antigen degradation and presentation was also equal as was WT T-cell conjugate formation and subsequent T-cell proliferation. Despite the likely presence of multiple phosphatase-regulated pathways in the antigen uptake, processing and presentation pathways that we investigated, we observed that Ptpn22 and the R619W autoimmune associated variant were dispensable. These important findings indicate that under non-inflammatory conditions there is no requirement for Ptpn22 in DC dependent antigen uptake and T-cell activation. Our findings reveal that perturbations in antigen uptake and processing, a fundamental pathway determining adaptive immune responses, are unlikely to provide a mechanism for the risk associated with the Ptpn22 autoimmune associated polymorphism.
Introduction
The C1858T polymorphism within the protein tyrosine phosphatase PTPN22 (encoding PTPN22R620W) is a major risk factor for the development of multiple autoimmune diseases, including rheumatoid arthritis (RA), type I diabetes, lupus and juvenile idiopathic arthritis (JIA) [1]. PTPN22 (Ptpn22 in the mouse) negatively regulates Src and Syk family kinase (SFK) activity downstream of the T-cell antigen receptor (TCR) [2]. As a result, T-cells from Ptpn22-/- mice exhibit enhanced TCR signalling resulting in homeostatic expansion of CD4+ effector T-cells [3]. In addition to TCR signalling, PTPN22 regulates many pathways in different cell types including the B-cell receptor [4], the αLβ2 integrin LFA-1 [5] and Toll-Like Receptor (TLR) signalling pathways [6–9]. Furthermore, Ptpn22 also functions to alter SFK independent signalling events by modulating TRAF ubiquitination [8]. It has become widely accepted that the autoimmune associated PTPN22R620W variant displays reduced binding to the tyrosine kinase Csk, due to a missense mutation in the P1 domain [2,10], but precisely how the PTPN22-R620W variant affects the functions of immune cell subsets is more complex. Both gain- and loss-of-phosphatase function effects have been reported, depending on the cellular context and signalling pathway under investigation [4,5,9–11].
Antigen presenting cells (APCs) are critical for pathogen sensing and antigen uptake to effectively clear infection. Ptpn22 is highly expressed in myeloid cells and a functional role for Ptpn22 in the regulation of multiple pattern recognition receptors has been established [6,8,9]. Antigen uptake occurs via non-specific pinocytosis or via receptor-mediated phagocytosis, and is critical for APC mediated T-cell activation. Despite this, investigations assessing the capability of Ptpn22 to regulate antigen uptake and presentation are limited. A recent study reported that Ptpn22R619W (the murine orthologue of the human autoimmune associated variant R620W) expression in macrophages under non-inflammatory conditions increased wild type (WT) OT-II T-cell activation and proliferation. It was suggested that this was caused by an enhanced phagocytic capability of Ptpn22R619W macrophages [12]. A further study documented that Ptpn22R619W expressing BMDC matured with LPS also induce enhanced WT OT-II T-cell proliferation compared to WT controls, attributed to enhanced co-stimulation rather than phagocytosis [9]. Src and Syk family kinases regulate multiple processes that determine antigen uptake, processing and presentation, and ultimately, the capability of APCs to activate T-cell responses [13]. For example, phosphorylation of FcγR and dectin-1 associated ITAM motifs by SFKs regulates receptor-mediated phagocytosis [14–16], whilst SFKs also mediate tyrosine phosphorylation of cortactin leading to cytoskeletal rearrangement which facilitates engulfment of antigen [14]. Enhanced antigen phagocytosis and processing may, in addition, lead to enhanced T-cell activation, thus further contributing to the expansion of autoreactive T-cells in vivo [4,11]. We therefore sought to systematically assess the role of Ptpn22 in antigen phagocytosis and processing, and its impact on T-cell activation.
Methods
Ethics statement
All animal work has been conducted according to The Animals (Scientific Procedures) Act 1986 under Home Office License 70/8792. The King’s College London Animal Welfare and Ethical Review Body (AWERB) approved the research. Animals were sacrificed by a rising CO2 concentration and death was confirmed by cervical dislocation.
Mice
Wild type (WT) C57BL/6, Ptpn22-/-, Ptpn22R619W mice, OT-II, and OT-II x Ly5.2 (CD45.1) were housed under specific pathogen free (SPF) conditions and used in experiments according to UK Home Office approved protocols. Ptpn22-/- mice and Ptpn22R619W mutant mice were backcrossed for more than 10 generations to the C57BL/6 strain. Their generation, genotype and phenotype have been previously described in detail [4,17] Age and gender-matched mice were used in all experiments.
Bone marrow derived dendritic cell (BMDC) culture
BMDC were produced using a protocol adapted from Inaba et al [18]. Bone marrow was flushed from femurs and tibias of WT, Ptpn22-/- or Ptpn22R619W mice with RPMI-1640 supplemented with L-glutamine (Corning) containing 1% FBS and penicillin/streptomycin (100 μg/ml). Bone marrow cells were incubated for 30 minutes at 37°C in 5% CO2 on Petri dishes to remove adherent macrophages. Non-adherent progenitor cells were seeded at 1.5 x 106 cells/ml in 24-well tissue culture plates in RPMI-1640 with L-glutamine supplemented with 10% heat-inactivated FBS, β-mercaptoethanol (50 μM), penicillin/streptomycin (100 μg/ml) and 1% murine GM-CSF; GM-CSF was produced from the B78H1/GMCSF.1 cell line. BMDC were cultured for 6 days at 37°C and 5% CO2 and medium replaced on days 3 and 4. BMDCs were used in functional assays on days 6 and 7.
BMDC phenotype
BMDCs were harvested and stained using anti-mouse CD11c-PE/Cy7 (clone N418; Biolegend), anti-mouse CD11b-APC/Cy7 (clone M1/70; Biolegend), MHC class II I-Ab-FITC (clone AF6-120.1; Biolegend), CD206-PE (clone C068C2; Biolegend) and Zombie UV Fixable Viability (Biolegend) in PBS containing anti-mouse CD16/CD32 (clone 93; Biolegend). Cells were washed with FACS buffer (5% FBS, 1 mM EDTA, 0.01% NaN3 in PBS) and fixed using 1% paraformaldehyde (PFA, Electron Microscopy Services) in PBS.
Pinocytosis assay
BMDC were stimulated overnight in the presence or absence of LPS (100 ng/ml, Invivogen). BMDC (2 x 105 cells/FACS tube) were incubated with Lucifer yellow (LY, Invitrogen, 1 mg/ml) at 37°C for the indicated time points. Samples were returned to ice and washed twice with cold PBS to prevent further uptake. Cells were stained with anti-mouse CD11c-PE/Cy7 and Zombie UV Fixable Viability dye for 30 minutes on ice. Cells were washed with FACS buffer and fixed using 1% PFA in PBS.
Receptor-mediated endocytosis assay
BMDC (2 x 105) were added to FACS tubes and incubated with ovalbumin-AF488 (Invitrogen, 10 μg/ml) or Zombie UV Fixable Viability dye labelled heat killed Listeria monocytogenes (HKLM, Invivogen, 1 DC:30 HKLM) on ice for 45 minutes. Cells were washed with cold FACS buffer and incubated at 37°C for various time points. Samples were returned to ice and washed with cold PBS to prevent further uptake. Cells were stained with anti-mouse CD11c-PE/Cy7 and Fixable Viability Dye eFluor 506 (eBioscience) for 30 minutes on ice. Cells were washed with FACS buffer and fixed using 1% PFA in PBS.
Antigen uptake by splenic DCs
Spleens from WT, Ptpn22-/- or Ptpn22R619W mice were digested in 1 ml of RPMI 1640, containing DNase and Liberase (both at 0.1mg/ml, Roche) on 24-well plates for 30 minutes at 37°C in 5% CO2; 10 mM EDTA was added for the final 5 minutes. Splenocyte cell suspensions were resuspended in 2 ml of red blood cell lysis buffer (Biolegend), washed in PBS and pelleted (1600 rpm, 5 minutes). 5 x 106 splenocytes were added per FACS tube. Splenocytes were cooled and incubated with ovalbumin-AF647 (Invitrogen, 50 μg/ml) for 45 minutes on ice. Cells were washed and transferred to 37°C for 45 minutes to allow for uptake. Cells were stained to identify splenic DCs and were identified as singlet, live, lineage- (excluding CD3, CD19, Ter119, NK1.1, GR1, B220+ splenocytes), CD11c-PE/Cy7+, MHC class II I-Ab-FITC+.
Antigen processing assay
3 μm polystyrene beads (Polysciences) were coated overnight at 4°C with ovalbumin-AF594 (Invitrogen, 0.5 mg/ml). 1 x 105 BMDC and 2 x 106 beads were added to duplicate wells of a 96-well round bottom plate (Nunc, 1 DC:20 beads) and pelleted (2000 rpm, 5 minutes). BMDC were incubated at 37°C, 5% CO2 for 0–5 hour time points. To exclude non-internalised beads, cells were stained with rabbit anti-ovalbumin (Sigma, 50 μg/ml) for 20 minutes on ice, then washed and stained with F(ab’)2 goat anti-rabbit IgG-AF647 (Invitrogen, 4 μg/ml) for 20 minutes on ice. Cells were washed with FACS buffer and lysed for 10 minutes on ice (0.5% NP-40, 50 mM Tris, 150 mM NaCl). Beads were washed twice with FACS buffer and transferred to FACS tubes.
CD4+ T-cell isolation
MACS negative selection kits (Miltenyi Biotech) were used to isolate CD4+ T-cells from the lymph nodes (LN) and spleens of 8–16 week old WT OT-II or WT OT-II CD45.1 mice. T-cells (2 x 107 cells/ml) were labelled with 2 μM CellTrace Violet (CTV, Invitrogen) for 20 minutes at 37°C.
DC:T-cell conjugate assay
BMDC were incubated overnight with LPS (100 ng/ml, Invivogen) and OVA323-339 peptide (10 μM, Invivogen). Cells were harvested, washed and resuspended at 1 x 107/ml in PBS prior to staining with 1 μM CellTrace Far Red (CTFR, Invitrogen) for 20 minutes at 37°C, followed by quenching in culture media for 20 minutes at 37°C. 1 x 105 CTFR labelled BMDC and 2 x 105 CTV labelled WT OT-II T-cells (in a total volume of 50μl) were added to each 1.5 ml tube, centrifuged for 2 minutes at 500 rpm and incubated at 37°C for the indicated times. Cells were fixed with 3% PFA in PBS for 15 minutes at room temperature, transferred to FACS tubes and acquired using a medium flow rate on a FACS Canto (BD). Conjugates were identified as CTV+ CTFR+ cells.
Antigen presentation assay
2 x 105 BMDC were incubated on 96-well U bottom plates with 0.3 mg/ml GFP-Eα (a kind gift from Erwan Boëdec, Paris 7 University) for 18 hours at 37°C. BMDC were harvested, washed and stained with anti-CD11c-PE/Cy7, anti-Eα52-68-biotin (eBioscience) and Fixable Viability Dye eFluor 506 for 30 minutes on ice. Cells were washed with FACS buffer and stained with PBS containing streptavidin-APC (Biolegend) at room temperature for 10 minutes. Cells were washed and fixed in 1% PFA in PBS prior to flow cytometry. BMDC were identified as live, CD11c+ singlets.
In vitro co-cultures
WT and Ptpn22-/- BMDC were pulsed overnight with ovalbumin (Invivogen) or OVA323-339 (Invivogen) at 0.01–1 μM. BMDC were harvested, washed and resuspended in RPMI-1640 with L-glutamine supplemented with 10% heat-inactivated FBS, β-mercaptoethanol (50 μM), penicillin/streptomycin (100 μg/ml). CD4+ T-cells were isolated and CTV labelled as described above. BMDC were co-cultured with CTV labelled CD4+ T-cells at 1:2 BMDC:T-cell ratio (1 x 105 BMDC:2 x 105 T-cells) in 96-well U bottom plates for 1–6 days.
T-cell adoptive transfer
1 x 106 CTV labelled CD4+ TCR Vα2+ Vβ5+ WT OT-II T-cells were injected intravenously into WT or Ptpn22-/- mice. The following day mice were immunised with ovalbumin (10 μg/mouse) or PBS via intraperitoneal injection. After 72 hours spleens were harvested and single cell suspensions were stained using antibodies against: TCR Vα2-PE (clone MR9-4; Biolegend), TCR Vβ5-APC (clone B20.1; Biolegend), CD4-APC/Cy7 (clone GK1.5; Biolegend), CD45.1-PE/Cy7 (clone A20; Biolegend), CD45.2-BV785 (clone 104; Biolegend). CTV dilution was determined within the live, singlet, CD4+, Vα5+Vβ2+, CD45.1+, CD45.2- population.
Cytokine immunoassays and cell phenotyping
Cell-free co-culture supernatants were harvested at the indicated times. IL-2 and IFNγ were determined by immunoassay. Antibody pairs were purchased from Biolegend. Cytokine levels were determined using streptavidin-europium and enhancement solution (both from Perkin Elmer) and detected on a Victor 1420 multilabel counter (Perkin Elmer). Day 1 or 6 BMDC and OT-II T-cell co-cultures were harvested and stained with anti-CD3ε-FITC (clone 145.2C11; Biolegend), anti-CD4-PerCP (clone RM4-5; Biolegend), anti-CD69 (clone H1.2F3; Biolegend), anti-CD25 (PC61; Biolegend) and Fixable Viability Dye eFluor 506. Cells were fixed in 1% PFA in PBS. Proliferating and activated T-cell populations were determined gating on live, singlet, CD3+, CD4+ cells and by CTV dilution.
Flow cytometry
Gates were determined by fluorescence minus one (FMO) controls. Cells were acquired using Becton Dickinson Fortessa or FACSCanto II flow cytometers and data analysed using FlowJo Version 8.7.
BMDC genotyping
Day 7 BMDCs were lysed in 50mM Tris (pH 8), 25mM EDTA, 100mM NaCl, 1% SDS and 0.4mg/ml Proteinase K (Roche) 1 hour at 56°C. DNA was extracted and a PCR was carried out using the following conditions: 94°C for 1min, followed by 29 cycles of 94°C for 15 seconds, 55°C for 15 seconds, 72°C for 12 seconds, all followed by a 10 minute extension step at 72°C. The following primers were used: 5'-AGCCAAGTTTCTTTGTTGAGAA-3' and 5'-CAGACACAACAAAGCCCAGA-3' (Sigma). PCR products were run on a 1% agarose gel and WT and Ptpn22-/- bands were identified (498bp and 221bp respectively).
Real-time PCR
Total RNA was extracted from BMDCs using TRIzol reagent, and cDNA was reverse transcribed using first strand cDNA synthesis using random hexamers. Gene expression was measured by TaqMan quantitative real-time PCR using FAM labelled Ptpn22 (Mm00501246_m1; Applied Biosystems) and VIC labelled 18S probe. Acquisition was conducted using the ABI 7900HT fast real-time PCR system (Applied Biosystems). Relative abundance of gene expression was calculated using 18S as an endogenous control, and the formula: relative abundance = 2(-dCt).
Statistical analysis
GraphPad Prism software was used for statistical analysis by unpaired T-test or two-way ANOVA with Sidak’s post-test (paired or unpaired; two-tails).
Results
Ptpn22 does not impact antigen uptake via macropinocytosis
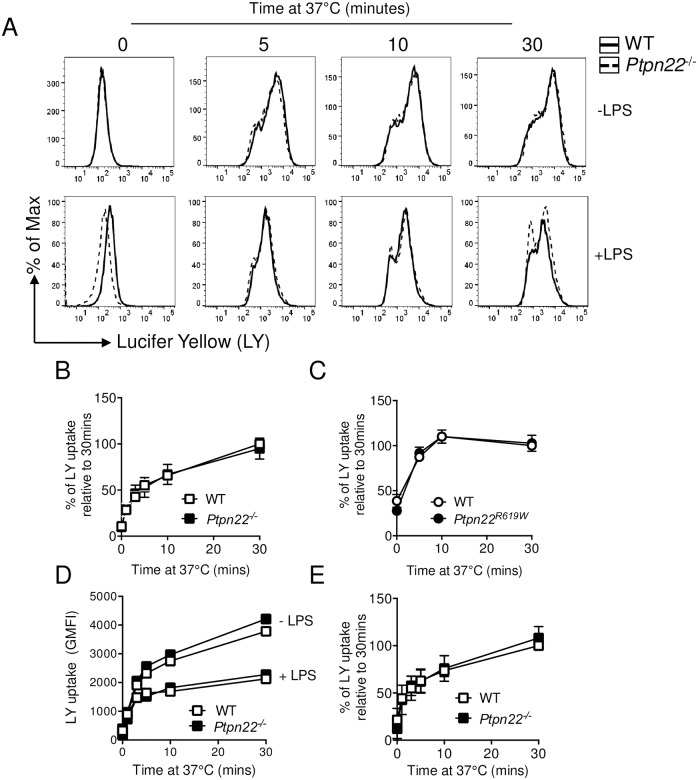
Macropinocytosis occurs constitutively in APCs allowing for non-specific uptake of soluble molecules, nutrients and antigens [14,19]. Pinocytosis requires actin-dependent membrane ruffling, ultimately leading to MHC class I and MHC class II dependent antigen presentation to T-cells [19–21]. First, we assessed if Ptpn22 variants could regulate antigenic uptake via pinocytosis. To address this, we generated BMDC in the presence of GM-CSF from WT, Ptpn22-/- and Ptpn22R619W mice; Ptpn22 expression was verified by genotyping and PCR (Panels A and B in S1 Fig). No genotype specific differences were observed with respect to the proportion or number of CD11c+ DC, (or macrophage like CD11c+, CD11bHi, I-AbInt cells) generated within these cultures (Panels A and B in S2 Fig) [14]. Macropinocytosis was assessed by the capability of WT and Ptpn22-/- BMDC to take up Lucifer yellow (LY). WT and Ptpn22-/- BMDC were incubated in the presence of LY at 37°C for time points up to 30 minutes. As expected, the LY geometric mean fluorescent intensity (GMFI) signal increased over time (Fig 1A), and internalisation of LY by WT, Ptpn22R619W, and Ptpn22-/- BMDC was comparable (Fig 1A–1C). Following TLR mediated maturation, macropinocytosis is decreased [14,21,22]. Accordingly, we assessed if the absence of Ptpn22 altered the capability of BMDC to downregulate LY uptake following LPS mediated maturation. We observed that, as previously reported [21], BMDC pinocytosis was significantly reduced following LPS treatment (Fig 1D), however WT and Ptpn22-/- BMDC had an equal reduction in LY uptake (Fig 1D and 1E). We therefore conclude that altered Ptpn22 function does not impact macropinocytosis.
Fig 1. Macropinocytosis does not require Ptpn22.
(A-E) Wild type (WT), Ptpn22-/- and Ptpn22R619W derived bone marrow derived dendritic cells (BMDC) were harvested and stimulated at 37°C for 0–30 minutes with Lucifer Yellow (LY). LY expression was determined by flow cytometry by gating on live, singlet, CD11c+ cells. (A) Representative flow cytometry plots showing (LY) uptake by WT (black solid line) and Ptpn22-/- (black dashed line), immature (top row) and LPS matured BMDC (bottom row). (B) LY uptake by WT (white square) and Ptpn22-/- (black square) immature BMDC. Data shown are Geometric Mean Fluorescent Intensity (GMFI) relative to maximal WT uptake at 30 minutes; N = 4 independent experiments; line represents mean ± s.d. (C) LY uptake by WT (white circle) and Ptpn22R619W (black circle) immature BMDC. Data shown are GMFI relative to maximal WT uptake at 30 minutes; N = 3 independent experiments; line represents mean ± s.d. (D) LY uptake by immature (-LPS) or LPS matured (+LPS) WT (white square) and Ptpn22-/- (black square) BMDC, showing representative LY GMFI of 3 independent experiments. (E) LY uptake relative to maximal WT uptake at 30 minutes by LPS matured WT (white square) and Ptpn22-/- (black square) BMDC. Data shown are GMFI mean ± s.d; N = 3 independent experiments.
Ptpn22 does not influence mannose receptor-mediated antigen uptake
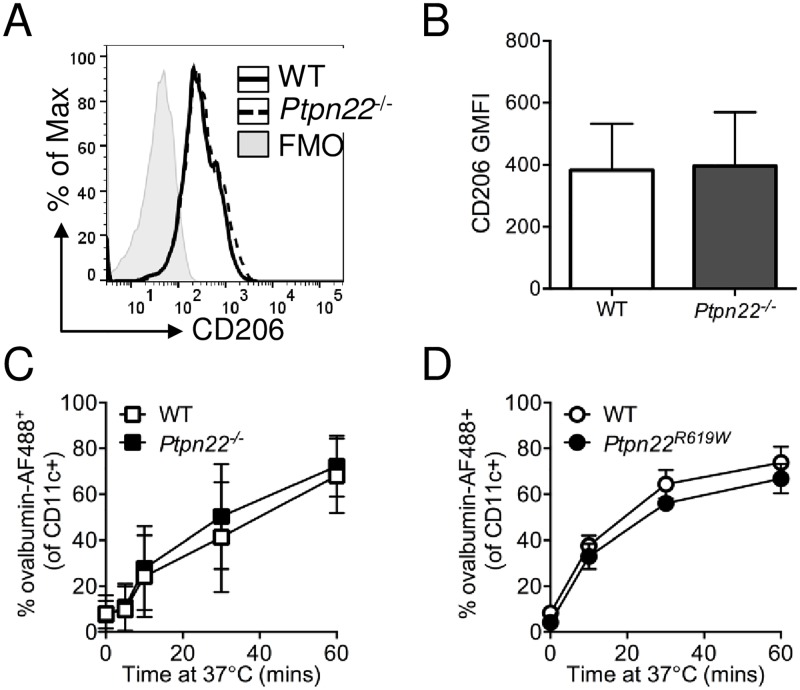
Phagocytic cell surface receptors expressed on APCs mediate uptake of specific types of antigen through recognition of specific pattern associated molecular patterns (PAMPs) [23]. Receptor-mediated endocytosis is dependent on a number of processes including receptor clustering, modulation of specific signalling pathways by ubiquitination and kinase-induced phosphorylation, in addition to actin and cytoskeletal rearrangement [23,24]. We assessed the involvement of Ptpn22 in mannose receptor (CD206) dependent phagocytosis of ovalbumin [24], since ovalbumin provides an ideal model with which to assess the impact of altered phagocytosis on antigen specific OT-II T-cell activation. We confirmed that there was no difference in cell surface expression of CD206 on immature WT versus Ptpn22-/- BMDC (Fig 2A and 2B) and WT versus Ptpn22R619W BMDC (Panel A in S3 Fig). We next assessed if binding and uptake of ovalbumin conjugated to AF488 (ovalbumin-AF488) was regulated by Ptpn22. Wild type and Ptpn22-/- BMDC were incubated with ovalbumin-AF488 at 37°C for up to 60 minutes and the presence of AF488 within CD11c+ cells determined. We observed over time that the proportion of AF488+ BMDC increased, but no differences were observed between the WT and Ptpn22 deficient BMDC (Fig 2C), or between WT and Ptpn22R619W BMDC (Fig 2D) at any time point. A lack of specific signalling motifs within the CD206 cytoplasmic domain suggests that CD206 dependent antigen uptake may not be directly dependent on SFKs [25], despite the requirement of SFKs in initiating initial cortical actin protrusions involved in early phagocytic events [26]. Therefore we assessed if Ptpn22 regulates the uptake of antigens via other pattern recognition receptors, such as TLRs. Listeria monocytogenes is phagocytosed via TLR2, and Candida albicans via dectin-1, both in a SFK dependent manner [26]. We observed no significant difference in the capability of WT or Ptpn22-/- BMDCs to uptake heat killed L. monocytogenes (Panel B in S3 Fig) or heat killed C. albicans (HKCA) (Panel C in S3 Fig). Together these data indicate that despite the presence of phosphatase dependent pathways, Ptpn22 does not impact antigen uptake via multiple phagocytic receptor pathways.
Fig 2. Ptpn22 variants do not alter BMDC receptor mediated phagocytosis.
(A-B) Wild type (WT) and Ptpn22-/- derived bone marrow derived dendritic cells (BMDC) were harvested and cell surface CD206 was determined by flow cytometry. (A) Representative CD206 expression profiles on WT (black solid line) and Ptpn22-/- (black dashed line) BMDC. (B) CD206 Geometric Mean Fluorescent Intensity (GMFI). N = 11 independent experiments; bars represent mean ± s.e.m. (C) WT and Ptpn22-/- BMDC were incubated with ovalbumin-AF488 for 0–60 minutes at 37°C prior to CD11c staining. N = 4 independent experiments; line represents the mean ± s.d. (D) WT and Ptpn22R619W BMDC were incubated with ovalbumin-AF488 for 0–60 minutes at 37°C prior to CD11c staining. N = 3 independent experiments; line represents the mean ± s.d.
Splenic dendritic cell uptake of ovalbumin occurs independently of Ptpn22
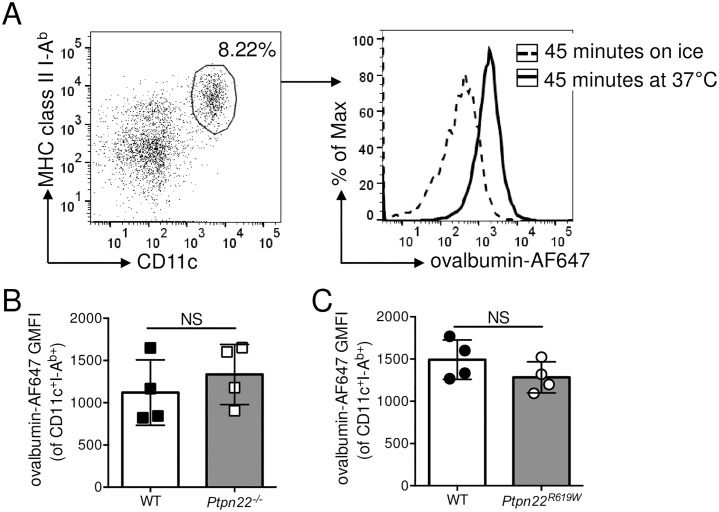
In vitro generated BMDC are a widely used tool to assess APC function, but differ from in vivo DC subsets. To address if in vivo ovalbumin antigen uptake by DCs was regulated by Ptpn22, we isolated the spleens of WT, Ptpn22-/- and Ptpn22R619W mice, and incubated splenocytes with ovalbumin-AF647 for 45 minutes either on ice or at 37°C. Following 45 minutes at 37°C there was an increase in the ovalbumin-AF647 GMFI of CD11c+ MHC class II+ splenic DCs (Fig 3A). However, when we assessed binding and uptake of ovalbumin-AF647 by WT, Ptpn22-/-, or Ptpn22R619W CD11c+ MHC class II+ splenic DCs we observed no differences (Fig 3B and 3C), indicating that, as observed with in vitro generated BMDC, the capability of splenic DC to take up ovalbumin is not dependent on Ptpn22.
Fig 3. Regulation of splenic DC receptor mediated phagocytosis of ovalbumin occurs independently of Ptpn22.
(A-B) Wild type (WT) and Ptpn22-/- splenocytes were incubated for 45 minutes with ovalbumin-AF647 on ice or at 37°C and stained for lineage-, CD11c+, I-Ab+ DC. (A) Representative flow cytometry staining of CD11c+ I-Ab+ splenic DCs (left hand panel, gated on live, singlet, lineage-) and ovalbumin-AF647 fluorescence in splenic DCs (right hand panel) incubated with ovalbumin-AF647 for 45 minutes on ice (black dashed line) or at 37°C (black solid line). (B) ovalbumin-AF647 Geometric Mean Fluorescent Intensity (GMFI) of WT and Ptpn22-/- CD11c+ I-Ab+ splenic DC incubated at 37°C for 45 minutes. Squares represent individual mice and bars represent the mean ± s.d. (C) Wild type (WT) and Ptpn22R619W splenocytes were incubated for 45 minutes with ovalbumin-AF647 at 37°C. Circles represent individual mice and bars represent the mean ± s.d. NS = not significant by unpaired T-test.
Ptpn22 is dispensable for antigen processing and presentation
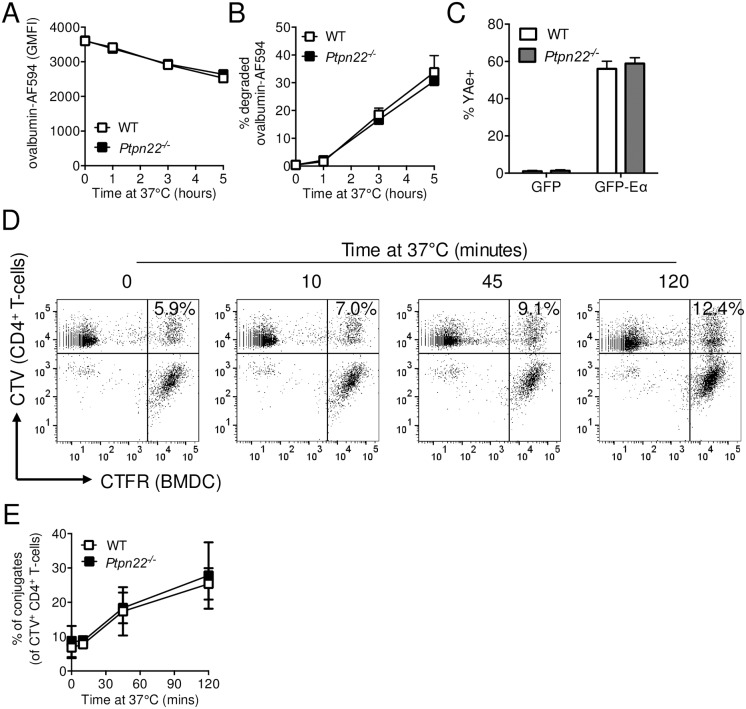
Following antigen uptake, protein antigens are processed into peptides by lysosomal degradation [27]. This process involves a complex series of events resulting in fusion of the phagocytic endosome with the acid rich lysosome. Perturbations of the lysosomal degradation pathway are associated with a variety of inflammatory and degenerative diseases [28]. To assess antigen processing, BMDCs were incubated with ovalbumin-AF594 coated beads for 0–5 hours at 37°C. Internalised beads were identified by the absence of staining using rabbit anti-ovalbumin. To determine antigen degradation, loss of ovalbumin fluorescence was assessed by flow cytometry. Over time, the intensity of ovalbumin-AF594 fluorescence reduced (Fig 4A), indicative of antigen degradation [29]. However, the amount of degradation (Fig 4A) and the proportion of internalised beads with degraded ova (Fig 4B) were similar for WT and Ptpn22-/- BMDC, indicating that Ptpn22 is dispensable for antigen processing.
Fig 4. Ptpn22 is dispensable for antigen degradation and presentation.
(A-B) Wild type (WT) and Ptpn22-/- BMDC were incubated with ovalbumin-AF594 coated beads for 0–5 hours at 37°C. Non-internalised beads were excluded by staining with rabbit anti-ovalbumin followed by F(ab’)2 anti-rabbit-AF647. Cells were lysed and the fluorescent intensity of AF594+ AF647- beads determined by flow cytometry. (A) Ovalbumin-AF594 Geometric Mean Fluorescent Intensity (GMFI). N = 2 independent experiments ± s.d. (B) Proportion of internalised beads from WT and Ptpn22-/- BMDC that have reduced ovalbumin-AF594 fluorescence. N = 2 independent experiments ± s.d. (C) WT and Ptpn22-/- BMDC were incubated with GFP or GFP-Eα for 18 hours followed by staining for Eα52–68 in I-Ab (YAe). The percentage of YAe+ live, singlet, CD11c+ BMDC was determined by flow cytometry. N = 3 independent experiments; bars represent mean ± s.d. (D-E) CellTrace Violet (CTV) labelled WT CD4+ OT.II T-cells were incubated with CellTrace Far Red (CFTR) stained OVA323-339 peptide pulsed LPS matured WT or Ptpn22-/- BMDC for 0–120 minutes at 37°C, and the proportion of CTV+ CTFR+ conjugates within the CTV+ T-cell population determined by flow cytometry. (D) Representative flow cytometry plots showing conjugates (top, right hand quadrant, CTV+ CTFR+). (E) Proportion of DC:T-cell conjugates within CTV+ gate. N = 4 independent experiments; line represents mean ± s.d. Data (C) determined non-significant by unpaired T-test. Differences between genotypes in (E) were deemed non-significant by two-way ANOVA with Sidak’s Multiple comparison test.
We next assessed whether Ptpn22 was required for loading of peptides onto MHC class II. WT and Ptpn22-/- BMDC were incubated for 18 hours at 37°C with a soluble fusion protein containing the Eα peptide epitope, GFP-Eα. Cell surface I-Ab restricted presentation of Eα peptide was determined by staining with anti-YAe-biotin (specific for the Eα52–68:IAb complex), followed by streptavidin-APC [30]. Although Eα52–68:IAb was detected on more than 50% of BMDC, the absence of Ptpn22 expression had no effect on the ability of BMDC to present Eα peptides (Fig 4C). In addition, cell surface expression of I-Ab was similar between WT and Ptpn22-/- BMDC [31]. Having established that antigen degradation and loading onto MHC class II were similar between WT and Ptpn22-/- BMDC, we next assessed if Ptpn22 altered the formation of DC:T-cell conjugates. BMDC were activated overnight with LPS to upregulate cell surface MHC class II expression, pulsed with OVA323-339 peptide, and labelled with CellTrace Far Red (CTFR). CTFR labelled BMDC were then co-cultured with CellTrace Violet (CTV) labelled WT OT-II T-cells for up to 120 mins at 37°C. The proportion of CTV+ CTFR+ cells increased over time, in keeping with the formation of DC:T-cell conjugates (Fig 4D). However, we did not observe any difference in the capability of WT and Ptpn22-/- BMDC to form conjugates (Fig 4E), indicating that Ptpn22 is dispensable for DC:T-cell conjugate formation when Ptpn22 deficiency is confined to the DC. Together these data indicate that Ptpn22 does not regulate antigen phagocytosis, processing and presentation.
Ptpn22 is redundant for dendritic cell activation of antigen specific T-cells
It has previously been reported that Ptpn22R619W BMDC are able to enhance antigen specific T-cell activation [9,12]. Given that we found no evidence that Ptpn22 regulated antigen phagocytosis, processing and presentation, we next sought to assess if APC induced T-cell activation is indeed dependent on Ptpn22. We compared the capability of WT and Ptpn22-/- BMDC pulsed overnight with ovalbumin or OVA323-339 peptide at concentrations between 0.01–1 μM in the absence of TLR stimulation. BMDC were harvested, washed and co-cultured with WT CTV labelled CD4+ OT-II for up to 6 days. We observed that increasing concentrations of ovalbumin or OVA323-339 increased expression of T-cell early activation markers CD69 and CD25 (Fig 5A and panel A in S4 Fig), with OVA323-339 being more potent. WT and Ptpn22-/- BMDC were equally capable of inducing the upregulation of both CD69 and CD25 regardless of peptide or protein concentration. Moreover, comparison of Ptpn22R619W BMDC to WT controls did not reveal any differences in the capability to induce early T-cell activation (Fig 5B and panel B in S4 Fig). IL-2 is rapidly secreted by T-cells following T-cell activation. Although IL-2 secretion increased, depending on the dose of ovalbumin peptide or protein, no differences between BMDC genotypes were observed (Fig 5C and panel C in S4 Fig). In addition we observed no Ptpn22 dependent difference in IFNγ secretion over the 6 days of co-culture (Fig 5D). Surprisingly, and contrary to previous reports [9], no differences were observed in the capability of Ptpn22 variant DC to induce WT T-cell proliferation at multiple peptide or protein concentrations when compared to WT BMDC (Fig 5E and panels D and E in S4 Fig) or when BMDC were matured in the presence of LPS (Panel F in S4 Fig). This is in contrast to the enhanced T-cell proliferation observed when Ptpn22 variants are expressed in the T-cell compartment [17,32].
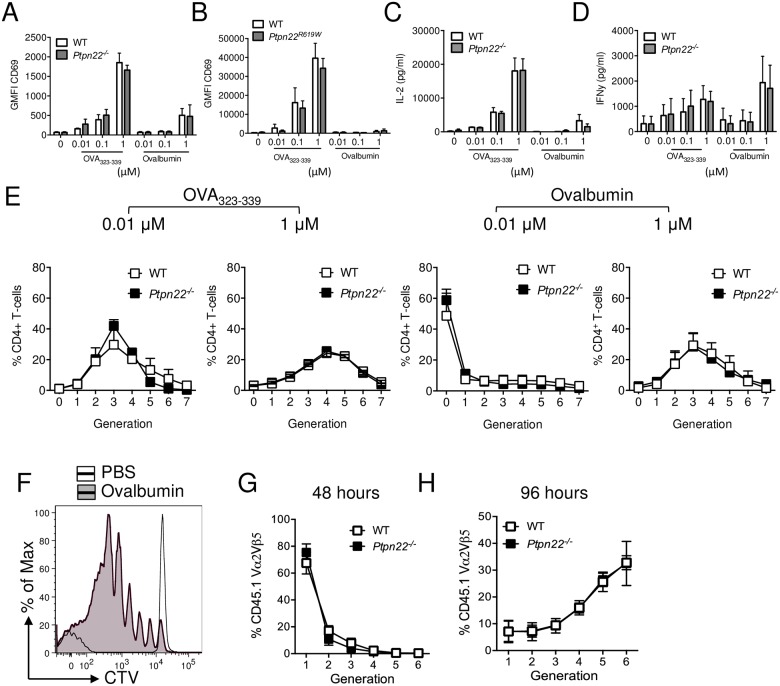
Fig 5. Ptpn22 variants do not modulate BMDC dependent OT.II T-cell activation.
(A-E) Wild type (WT), Ptpn22-/- and Ptpn22R619W BMDC were stimulated overnight in the presence or absence of OVA323-339 (0.01–1 μM) or ovalbumin (0.01–1 μM). BMDC were harvested and co-cultured with CellTrace Violet (CTV) labelled CD4+ OT.II T-cells at a 1:2 BMDC:T-cell ratio. (A-B) 24 hour Geometric Mean Fluorescent Intensity (GMFI) surface expression of CD69 determined on live, singlet, CD4+ T-cells. (A) N = 3 independent experiments and (B) N = 4 independent experiments; bars represent mean ± s.d. (C-D) Co-culture supernatants were assessed for (C) IL-2 after 24 hours and (D) IFNγ after 144 hours. N = 3 independent experiments; bars represent mean ± s.d. (E) At day 6 the proportion of CD4+ T-cells within each CTV generation was determined by flow cytometry. N = 3 independent experiments, lines represent mean ± s.d. (F-H) CTV labelled CD45.1+ CD4+ TCR Vα2+Vβ5+ OT.II T-cells were adoptively transferred i.v. into CD45.2+ WT or Ptpn22-/- recipient mice followed by i.p. immunisation of PBS or ovalbumin (100 μg/mouse). Spleens were assessed after 48h (G) or 96h (F, H) for CTV dilution within the CD45.1+ CD4+ TCR Vα2+Vβ5+ population by flow cytometry. (F) Representative flow cytometry plot showing CTV dilution following i.p. of PBS (black dashed line) or 10 μg ovalbumin (black line, grey fill). (G-H) Lines represent mean ± s.d., N = 3 (G) and N = 5 (H). Differences between genotypes (A-E, G, H) were deemed non-significant by two-way ANOVA with Sidak’s Multiple comparison test.
Finally, we sought to address if Ptpn22 was dispensable for APC mediated activation of T-cells in vivo. To address this, CTV labelled WT CD4+ CD45.1+ OT-II T-cells were adoptively transferred into CD45.2+ WT or Ptpn22-/- mice and immunised with ovalbumin. No difference in ex vivo CD11c+ MHC class II IAb+ CD40 or CD86 expression was observed (Panels A and B in S5 Fig). 48 or 96 hours after immunisation, splenic CD45.1+ TCR Vα2+Vβ5+ CD4+ T-cells were assessed for CTV dilution by flow cytometry (Fig 5F). Consistent with our in vitro experiments, we observed no differences in the capability of Ptpn22-/- APC to enhance the proliferation of adoptively transferred WT OT-II T-cells at either time point (Fig 5G and 5H). Furthermore no difference in OT-II T-cell proliferation was observed when T-cells were transferred into WT vs Ptpn22R619W mice (Panel C in S5 Fig). Together these data demonstrate that Ptpn22 and the autoimmune associated Ptpn22R619W variant does not impact the uptake, processing, and presentation of antigen by DCs, and that under non-inflammatory conditions the resultant T-cell priming is unaltered.
Discussion
PTPN22R620W is associated with an enhanced risk of developing multiple autoimmune diseases and, as such, has prompted investigations into which cells and pathways may be perturbed by carrying the polymorphism, thereby promoting autoimmunity. Adaptive immune responses are critical to the pathogenesis of autoimmune diseases associated with the PTPN22R620W polymorphism. Furthermore, SFK dependent pathways regulate antigen processing and presentation in dendritic cells. Here we took a systematic approach to assess if Ptpn22 variants are capable of regulating APC antigen uptake, processing, presentation and APC-driven T-cell activation under non-inflammatory conditions. Unexpectedly, we observed that Ptpn22 and the autoimmune associated variant are dispensable for each of these processes both in vitro and in vivo.
Despite antigen uptake requiring a balance of SFK dependent phosphorylation and phosphatase-regulated dephosphorylation, our data indicate that Ptpn22 is dispensable in this process. In contrast to a previous report observing enhanced uptake of E.coli by Ptpn22R619W macrophages [14], we observed no differences in receptor-mediated antigen uptake by dendritic cells across a range of receptor pathways. The cause of this discrepancy may reflect differences in cell-mediated uptake in macrophages versus dendritic cells, or may reflect differences in mouse health status/microbiota, or may indicate that Ptpn22 specifically regulates E.coli dependent uptake rather than broadly regulating receptor-mediated endocytosis pathways. Overall, our combined data strongly support the conclusion that alternative phosphatases are likely to operate as primary, or redundant regulators of antigen processing pathways. Indeed, phosphatases including SHIP-1, SHP-1, and CD45 have all been observed to regulate processes required for antigen uptake and processing [15,33,34].
Again contrary to previous reports [9,12], we observed no difference in the capability of Ptpn22 deficient or Ptpn22R619W BMDC or splenic DC versus the equivalent WT-derived populations to induce WT OT-II T-cell proliferation. The reason for this observed discrepancy in T-cell activation compared with previous studies is unclear. It may be due to subtle differences in the thresholds for signalling dependent on the dosing concentrations of BMDC stimuli, the age of animals studied or the possibility that previous work utilised cells from animals with active inflammatory changes. Most strikingly, and consistent with the interpretation that Ptpn22 has little impact on APC function in a physiological setting, our in vivo data demonstrated equivalent activation of WT OT-II CD4+ T-cells following adoptive transfer into Ptpn22 deficient or WT control mice (Fig 5G and 5H). As we, and others, have previously reported, Ptpn22R619W mice exhibit increased responses to ova immunisation and marked changes in T-cell activation [4,17,35]. Thus, although our data revealed no difference in conjugate formation between WT and Ptpn22 variant DC with WT T-cells, Ptpn22 is likely to modulate MHC class II restricted DC:T-cell conjugate formation in a T-cell dependent manner (rather than through effects on the DC). Consistent with this idea, Ptpn22 deficient CD8+ OT-I T-cells have an enhanced capability to form conjugates with WT APCs in response to low affinity peptide antigen, leading to enhanced T-cell activation in an LFA-1 dependent manner [5,32].
In conclusion we show that Ptpn22 deficiency or the expression of the autoimmune associated variant of Ptpn22 does not regulate the phagocytic capability of in vitro generated BMDC or splenic DCs. Furthermore, as systematically demonstrated here, using a broad range of models, Ptpn22 is redundant for the processing and presentation of protein antigen and that Ptpn22 variant DC were not altered in their capability to induce T-cell activation under non-inflammatory conditions either in vitro or in vivo. Antigen uptake, processing and presentation are fundamental to the induction of adaptive T-cell responses. Our data reveal that perturbations caused by the autoimmune associated risk variant of Ptpn22 are unlikely to promote autoimmunity via these pathways in DC.
Supporting information
(A) Day 6 WT, Ptpn22-/- and Ptpn22R619W BMDC were lysed and DNA extracted. Ptpn22 PCR product was run on a 1% agarose gel and WT and Ptpn22-/- bands were identified (498bp and 221bp respectively). (B) RNA was extracted and cDNA synthesised from day 6 WT, Ptpn22-/- and Ptpn22R619W BMDC. Expression of Ptpn22 was determined by real-time PCR and normalised to expression of 18S. Bars represent the mean of 3 individual mice + s.d.
(PDF)
Day 6 WT and Ptpn22-/- BMDC were harvested and cell surface stained for CD11c, CD11b, and MHC class II I-Ab and the proportion of MHC class II+ CD11c+, MHC class IIHi CD11bInt DC, and MHC class IIInt CD11bHI macrophages determined by flow cytometry. (A) Representative gating of live, singlets, followed by CD11c+ MHC class II+ gate (left plot), followed by DC gate (CD11bInt MHC class IIHI) and macrophage gate (CD11bHI MHC class IIInt). (B) Data show percentages of each population within WT and Ptpn22-/- BMDC cultures. Data are of 8 independent experiments. Bars represent mean + s.d. Differences between genotypes were deemed non-significant by two-way ANOVA with Sidak’s Multiple comparison test.
(PDF)
(A) Day 6 WT and Ptpn22R619W BMDC were harvested and cell surface stained for CD206. Live singlet CD11c+ cells were gated and CD206 Geometric Mean Fluorescent Intensity (GMFI) determined by flow cytometry. N = 3 independent experiments; bars represent mean + s.d. (B) WT and Ptpn22-/- BMDC were incubated with labelled heat killed L. monocytogenes (HKLM) at 37°C for 0–60 minutes. The percentage of CD11c+ HKLM+ BMDC was determined by flow cytometry. N = 5 independent experiments; bars represent mean + s.d. (C) Day 6 BMDC were generated from WT or Ptpn22-/- mice. BMDC were incubated with labelled heat killed C. albicans (HKCA) at 4°C or 37°C for 1 hour. The percentage of CD11c+ HKCA+ BMDC was determined by flow cytometry. N = 4; bars represent mean + s.d. Differences between genotypes were deemed non-significant by unpaired T-test (A, C) and two-way ANOVA with Sidak’s Multiple comparison test (B).
(PDF)
WT, Ptpn22-/- and Ptpn22R619W BMDC were stimulated overnight in the presence or absence of OVA323-339 (0.01–1 μM) or ovalbumin (0.01–1 μM). BMDC were harvested and co-cultured with CellTrace Violet (CTV) labelled CD4+ OT-II T-cells at a 1:2 BMDC:T-cell ratio. (A-B) 24 hour Geometric Mean Fluorescent Intensity (GMFI) surface expression of CD25 determined on live, singlet, CD4+ T-cells. (A) N = 3 independent experiments; (B) N = 4 independent experiments; bars represent mean ± s.d. (C) Co-culture supernatants were assessed for IL-2 after 24 hours. N = 4 independent experiments; bars represent mean + s.d. (D-E) WT and Ptpn22R619W BMDC pulsed overnight with (D) OVA323-339 (1 μM) or (E) ovalbumin (1 μM) were co-cultured with CTV labelled CD4+ OT-II T cells. At day 6 the proportion of CD4+ T-cells within each CTV generation was determined by flow cytometry. N = 4 independent experiments; lines represent mean ± s.d. Differences between genotypes were deemed non-significant by two-way ANOVA with Sidak’s Multiple comparison test. (F) WT and Ptpn22-/- BMDC were stimulated overnight in the presence or absence LPS in the presence of ovalbumin (1μM). BMDC were harvested and co-cultured with CTV labelled CD4+ OT-II T-cells at a 1:2 BMDC:T-cell ratio. At day 6 the proportion of CD4+ T-cells within each CTV generation was determined by flow cytometry N = 7 independent experiments; bars represent mean + s.d.
(PDF)
(A-B) Splenocytes from WT or Ptpn22-/- mice were surface stained and mean fluorescent intensity of CD40 and CD86 on live, singlet, Lin-, CD11c+, MHC class II IAb+ cells was determined by flow cytometry. Bars represent mean ± s.d, each point represents an individual mouse. (C) CTV labelled CD45.1+ CD4+ TCR Vα2+Vβ5+ OT.II T-cells were adoptively transferred i.v. into CD45.2+ WT or Ptpn22R619W recipient mice followed by i.p. immunisation of PBS or ovalbumin (100 μg/mouse). Spleens were assessed after 96h for CTV dilution within the CD45.1+ CD4+ TCR Vα2+Vβ5+ population by flow cytometry. Bars represent mean + s.d., N = 2/3 per group.
(PDF)
Acknowledgments
The authors thank Dr Esperanza Perucha and Dr Tamlyn Peel for helpful discussions and Wing Wu for technical help.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by Arthritis Research UK (http://www.arthritisresearchuk.org) grants 20218 (awarded to H.A.P and A.P.C), 20525 (awarded to G.H.C, P.G, R.Z and A.P.C), Wellcome Trust (https://wellcome.ac.uk) Investigator Award 096669AIA (awarded to R.Z) and National Institutes of Health (https://www.nih.gov): DP3-DK097672 and DP3-DK111802 (to D.J.R). Additional support was provided by the Children's Guild Association Endowed Chair in Pediatric Immunology and the Benaroya Family Gift Fund (to D.J.R.). This work was also supported by infrastructure funded by the National Institute for Health Research (NIHR) BioResource Clinical Research facility and Biomedical Research Centre based at Guy’s and St. Thomas’ NHS Foundation Trust and King’s College London (reference: guysbrc-2012-17). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Burn GL, Svensson L, Sanchez-Blanco C, Saini M, Cope AP. Why is PTPN22 a good candidate susceptibility gene for autoimmune disease? FEBS Lett. 2011;585: 3689–98. doi: 10.1016/j.febslet.2011.04.032 [DOI] [PubMed] [Google Scholar]
- 2.Cloutier JF, Veillette A. Cooperative inhibition of T-cell antigen receptor signaling by a complex between a kinase and a phosphatase. J Exp Med. The Rockefeller University Press; 1999;189: 111–21. Available: http://www.ncbi.nlm.nih.gov/pubmed/9874568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hasegawa K, Martin F, Huang G, Tumas D, Diehl L, Chan AC. PEST domain-enriched tyrosine phosphatase (PEP) regulation of effector/memory T cells. Science. 2004;303: 685–9. doi: 10.1126/science.1092138 [DOI] [PubMed] [Google Scholar]
- 4.Dai X, James RG, Habib T, Singh S, Jackson S, Khim S, et al. A disease-associated PTPN22 variant promotes systemic autoimmunity in murine models. J Clin Invest. American Society for Clinical Investigation; 2013;123: 2024–2036. doi: 10.1172/JCI66963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burn GL, Cornish GH, Potrzebowska K, Samuelsson M, Griffié J, Minoughan S, et al. Superresolution imaging of the cytoplasmic phosphatase PTPN22 links integrin-mediated T cell adhesion with autoimmunity. Sci Signal. 2016;9: ra99 doi: 10.1126/scisignal.aaf2195 [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Ewart D, Crabtree JN, Yamamoto A, Baechler EC, Fazeli P, et al. PTPN22 Variant R620W Is Associated With Reduced Toll-like Receptor 7-Induced Type I Interferon in Systemic Lupus Erythematosus. Arthritis Rheumatol (Hoboken, NJ). 2015;67: 2403–14. doi: 10.1002/art.39211 [DOI] [PubMed] [Google Scholar]
- 7.Holmes DA, Suto E, Lee WP, Ou Q, Gong Q, Smith HRC, et al. Autoimmunity-associated protein tyrosine phosphatase PEP negatively regulates IFN-α receptor signaling. J Exp Med. 2015;212: 1081–93. doi: 10.1084/jem.20142130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Shaked I, Stanford SM, Zhou W, Curtsinger JM, Mikulski Z, et al. The autoimmunity-associated gene PTPN22 potentiates toll-like receptor-driven, type 1 interferon-dependent immunity. Immunity. 2013;39: 111–22. doi: 10.1016/j.immuni.2013.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang J, Zahir N, Jiang Q, Miliotis H, Heyraud S, Meng X, et al. The autoimmune disease-associated PTPN22 variant promotes calpain-mediated Lyp/Pep degradation associated with lymphocyte and dendritic cell hyperresponsiveness. Nat Genet. 2011;43: 902–907. doi: 10.1038/ng.904 [DOI] [PubMed] [Google Scholar]
- 10.Fiorillo E, Orrú V, Stanford SM, Liu Y, Salek M, Rapini N, et al. Autoimmune-associated PTPN22 R620W variation reduces phosphorylation of lymphoid phosphatase on an inhibitory tyrosine residue. J Biol Chem. American Society for Biochemistry and Molecular Biology; 2010;285: 26506–18. doi: 10.1074/jbc.M110.111104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rieck M, Arechiga A, Onengut-Gumuscu S, Greenbaum C, Concannon P, Buckner JH. Genetic variation in PTPN22 corresponds to altered function of T and B lymphocytes. J Immunol. 2007;179: 4704–10. Available: http://www.ncbi.nlm.nih.gov/pubmed/17878369 [DOI] [PubMed] [Google Scholar]
- 12.Li M, Beauchemin H, Popovic N, Peterson A, d’Hennezel E, Piccirillo CA, et al. The common, autoimmunity-predisposing 620Arg > Trp variant of PTPN22 modulates macrophage function and morphology. J Autoimmun. 2017;79: 74–83. doi: 10.1016/j.jaut.2017.01.009 [DOI] [PubMed] [Google Scholar]
- 13.Lowell CA. Src-family and Syk kinases in activating and inhibitory pathways in innate immune cells: signaling cross talk. Cold Spring Harb Perspect Biol. Cold Spring Harbor Laboratory Press; 2011;3 doi: 10.1101/cshperspect.a002352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu J, Yu D, Zeng X-C, Zhou K, Zhan X. Receptor-mediated endocytosis involves tyrosine phosphorylation of cortactin. J Biol Chem. American Society for Biochemistry and Molecular Biology; 2007;282: 16086–94. doi: 10.1074/jbc.M701997200 [DOI] [PubMed] [Google Scholar]
- 15.Goodridge HS, Reyes CN, Becker CA, Katsumoto TR, Ma J, Wolf AJ, et al. Activation of the innate immune receptor Dectin-1 upon formation of a “phagocytic synapse”. Nature. 2011;472: 471–5. doi: 10.1038/nature10071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki T, Kono H, Hirose N, Okada M, Yamamoto T, Yamamoto K, et al. Differential Involvement of Src Family Kinases in Fcγ Receptor-Mediated Phagocytosis. J Immunol. 2000;165 Available: http://www.jimmunol.org/content/165/1/473 [DOI] [PubMed] [Google Scholar]
- 17.Brownlie RJ, Miosge LA, Vassilakos D, Svensson LM, Cope A, Zamoyska R. Lack of the phosphatase PTPN22 increases adhesion of murine regulatory T cells to improve their immunosuppressive function. Sci Signal. 2012;5: ra87 doi: 10.1126/scisignal.2003365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176: 1693–702. Available: http://www.ncbi.nlm.nih.gov/pubmed/1460426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim JP, Gleeson PA. Macropinocytosis: an endocytic pathway for internalising large gulps. Immunol Cell Biol. Nature Publishing Group; 2011;89: 836–843. doi: 10.1038/icb.2011.20 [DOI] [PubMed] [Google Scholar]
- 20.Norbury CC, Chambers BJ, Prescott AR, Ljunggren H-G, Watts C. Constitutive macropinocytosis allows TAP-dependent major histocompatibility compex class I presentation of exogenous soluble antigen by bone marrow-derived dendritic cells. Eur J Immunol. 1997;27: 280–288. doi: 10.1002/eji.1830270141 [DOI] [PubMed] [Google Scholar]
- 21.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182: 389–400. Available: http://www.ncbi.nlm.nih.gov/pubmed/7629501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Platt CD, Ma JK, Chalouni C, Ebersold M, Bou-Reslan H, Carano RAD, et al. Mature dendritic cells use endocytic receptors to capture and present antigens. Proc Natl Acad Sci U S A. National Academy of Sciences; 2010;107: 4287–92. doi: 10.1073/pnas.0910609107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freeman SA, Grinstein S. Phagocytosis: receptors, signal integration, and the cytoskeleton. Immunol Rev. 2014;262: 193–215. doi: 10.1111/imr.12212 [DOI] [PubMed] [Google Scholar]
- 24.Doherty GJ, McMahon HT. Mechanisms of Endocytosis. Annu Rev Biochem. 2009;78: 857–902. doi: 10.1146/annurev.biochem.78.081307.110540 [DOI] [PubMed] [Google Scholar]
- 25.Taylor PR, Gordon S, Martinez-Pomares L. The mannose receptor: linking homeostasis and immunity through sugar recognition. Trends Immunol. 2005;26: 104–110. doi: 10.1016/j.it.2004.12.001 [DOI] [PubMed] [Google Scholar]
- 26.Sousa S, Cabanes D, Bougnères L, Lecuit M, Sansonetti P, Tran-Van-Nhieu G, et al. Src, cortactin and Arp2/3 complex are required for E-cadherin-mediated internalization of Listeria into cells. Cell Microbiol. Blackwell Publishing Ltd; 2007;9: 2629–2643. doi: 10.1111/j.1462-5822.2007.00984.x [DOI] [PubMed] [Google Scholar]
- 27.Schwake M, Schröder B, Saftig P. Lysosomal Membrane Proteins and Their Central Role in Physiology. Traffic. John Wiley & Sons A/S; 2013;14: 739–748. doi: 10.1111/tra.12056 [DOI] [PubMed] [Google Scholar]
- 28.Settembre C, Fraldi A, Medina DL, Ballabio A. Signals from the lysosome: a control centre for cellular clearance and energy metabolism. Nat Rev Mol Cell Biol. Nature Publishing Group; 2013;14: 283–296. doi: 10.1038/nrm3565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Savina A, Vargas P, Guermonprez P, Lennon A-M, Amigorena S. Measuring pH, ROS Production, Maturation, and Degradation in Dendritic Cell Phagosomes Using Cytofluorometry-Based Assays. 2010. pp. 383–402. doi: 10.1007/978-1-60761-421-0_25 [DOI] [PubMed] [Google Scholar]
- 30.Viret C, Janeway CA. Functional and Phenotypic Evidence for Presentation of Eα52–68 Structurally Related Self-Peptide(s) in I-Eα-Deficient Mice. J Immunol. 2000;164 Available: http://www.jimmunol.org/content/164/9/4627.long [DOI] [PubMed] [Google Scholar]
- 31.Purvis HA, Clarke F, Jordan CK, Blanco CS, Cornish GH, Dai X, et al. Protein Tyrosine Phosphatase PTPN22 regulates IL-1β dependent Th17 responses by modulating dectin-1 signaling in mice. Eur J Immunol. 2017; doi: 10.1002/eji.201747092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salmond RJ, Brownlie RJ, Morrison VL, Zamoyska R. The tyrosine phosphatase PTPN22 discriminates weak self peptides from strong agonist TCR signals. Nat Immunol. Nature Publishing Group; 2014;15: 875–83. doi: 10.1038/ni.2958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gómez CP, Tiemi Shio M, Duplay P, Olivier M, Descoteaux A. The Protein Tyrosine Phosphatase SHP-1 Regulates Phagolysosome Biogenesis. J Immunol. 2012;189 Available: http://www.jimmunol.org/content/189/5/2203 [DOI] [PubMed] [Google Scholar]
- 34.Cox D, Dale BM, Kashiwada M, Helgason CD, Greenberg S. A regulatory role for Src homology 2 domain-containing inositol 5’-phosphatase (SHIP) in phagocytosis mediated by Fc gamma receptors and complement receptor 3 (alpha(M)beta(2); CD11b/CD18). J Exp Med. The Rockefeller University Press; 2001;193: 61–71. Available: http://www.ncbi.nlm.nih.gov/pubmed/11136821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maine CJ, Marquardt K, Cheung J, Sherman LA. PTPN22 controls the germinal center by influencing the numbers and activity of T follicular helper cells. J Immunol. NIH Public Access; 2014;192: 1415–24. doi: 10.4049/jimmunol.1302418 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Day 6 WT, Ptpn22-/- and Ptpn22R619W BMDC were lysed and DNA extracted. Ptpn22 PCR product was run on a 1% agarose gel and WT and Ptpn22-/- bands were identified (498bp and 221bp respectively). (B) RNA was extracted and cDNA synthesised from day 6 WT, Ptpn22-/- and Ptpn22R619W BMDC. Expression of Ptpn22 was determined by real-time PCR and normalised to expression of 18S. Bars represent the mean of 3 individual mice + s.d.
(PDF)
Day 6 WT and Ptpn22-/- BMDC were harvested and cell surface stained for CD11c, CD11b, and MHC class II I-Ab and the proportion of MHC class II+ CD11c+, MHC class IIHi CD11bInt DC, and MHC class IIInt CD11bHI macrophages determined by flow cytometry. (A) Representative gating of live, singlets, followed by CD11c+ MHC class II+ gate (left plot), followed by DC gate (CD11bInt MHC class IIHI) and macrophage gate (CD11bHI MHC class IIInt). (B) Data show percentages of each population within WT and Ptpn22-/- BMDC cultures. Data are of 8 independent experiments. Bars represent mean + s.d. Differences between genotypes were deemed non-significant by two-way ANOVA with Sidak’s Multiple comparison test.
(PDF)
(A) Day 6 WT and Ptpn22R619W BMDC were harvested and cell surface stained for CD206. Live singlet CD11c+ cells were gated and CD206 Geometric Mean Fluorescent Intensity (GMFI) determined by flow cytometry. N = 3 independent experiments; bars represent mean + s.d. (B) WT and Ptpn22-/- BMDC were incubated with labelled heat killed L. monocytogenes (HKLM) at 37°C for 0–60 minutes. The percentage of CD11c+ HKLM+ BMDC was determined by flow cytometry. N = 5 independent experiments; bars represent mean + s.d. (C) Day 6 BMDC were generated from WT or Ptpn22-/- mice. BMDC were incubated with labelled heat killed C. albicans (HKCA) at 4°C or 37°C for 1 hour. The percentage of CD11c+ HKCA+ BMDC was determined by flow cytometry. N = 4; bars represent mean + s.d. Differences between genotypes were deemed non-significant by unpaired T-test (A, C) and two-way ANOVA with Sidak’s Multiple comparison test (B).
(PDF)
WT, Ptpn22-/- and Ptpn22R619W BMDC were stimulated overnight in the presence or absence of OVA323-339 (0.01–1 μM) or ovalbumin (0.01–1 μM). BMDC were harvested and co-cultured with CellTrace Violet (CTV) labelled CD4+ OT-II T-cells at a 1:2 BMDC:T-cell ratio. (A-B) 24 hour Geometric Mean Fluorescent Intensity (GMFI) surface expression of CD25 determined on live, singlet, CD4+ T-cells. (A) N = 3 independent experiments; (B) N = 4 independent experiments; bars represent mean ± s.d. (C) Co-culture supernatants were assessed for IL-2 after 24 hours. N = 4 independent experiments; bars represent mean + s.d. (D-E) WT and Ptpn22R619W BMDC pulsed overnight with (D) OVA323-339 (1 μM) or (E) ovalbumin (1 μM) were co-cultured with CTV labelled CD4+ OT-II T cells. At day 6 the proportion of CD4+ T-cells within each CTV generation was determined by flow cytometry. N = 4 independent experiments; lines represent mean ± s.d. Differences between genotypes were deemed non-significant by two-way ANOVA with Sidak’s Multiple comparison test. (F) WT and Ptpn22-/- BMDC were stimulated overnight in the presence or absence LPS in the presence of ovalbumin (1μM). BMDC were harvested and co-cultured with CTV labelled CD4+ OT-II T-cells at a 1:2 BMDC:T-cell ratio. At day 6 the proportion of CD4+ T-cells within each CTV generation was determined by flow cytometry N = 7 independent experiments; bars represent mean + s.d.
(PDF)
(A-B) Splenocytes from WT or Ptpn22-/- mice were surface stained and mean fluorescent intensity of CD40 and CD86 on live, singlet, Lin-, CD11c+, MHC class II IAb+ cells was determined by flow cytometry. Bars represent mean ± s.d, each point represents an individual mouse. (C) CTV labelled CD45.1+ CD4+ TCR Vα2+Vβ5+ OT.II T-cells were adoptively transferred i.v. into CD45.2+ WT or Ptpn22R619W recipient mice followed by i.p. immunisation of PBS or ovalbumin (100 μg/mouse). Spleens were assessed after 96h for CTV dilution within the CD45.1+ CD4+ TCR Vα2+Vβ5+ population by flow cytometry. Bars represent mean + s.d., N = 2/3 per group.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.