Abstract
Highly selective probes hybridize only to fully complementary DNA or RNA sequences and, therefore, often fail to recognize mutated viral genomes. Here we designed a probe that possesses two seemingly incompatible properties: it tolerates some point mutations in genome, while it remains selective towards others. An OR deoxyribozyme logic gate was designed to fluorescently report the sequences of enterovirus 71 (EV71) covering ~90% of all known EV71 strains. Importantly, sequences of closely related coxsackieviruses that differed by single nucleotides were reliably differentiated in 7 out of 8 cases.
Hybridization probes are short oligonucleotide sequences designed to be complementary to targeted RNA or DNA analytes. For differentiation of closely related target sequences, e.g. those that differed by single nucleotide substitutions (SNS), a hybridization probe should form a stable hybrid only with a fully matched analyte, while it should remain unbound even if a single base mismatch is present. This approach has become an invaluable tool in the analysis of SNS in the human genome1 and distinguishing drug resistant from drug susceptible bacterial strains,2 but has often failed in the analysis of viral sequences.3 Indeed, viral genomes are particularly prone to mutations, due to the absence of the proofreading activities of viral nucleic acid polymerases, a strategy that enables escape from the immune system and the rapid development of drug resistance.4 This makes the frequent need to differentiate between two closely related viral species even more complicated.5 Therefore, an ideal hybridization probe should be resistant to some point mutations, while remaining highly selective to others. Here we resolved the conflict of these contradictory requirements by introducing a mutation-resistant hybridization sensor, named a deoxyribozyme (DZ) OR gate. Unlike expensive equipment-based high resolution melting assays,6 the DZ OR gate is compatible with low-cost point-of-care assays and has the potential to be more accurate than current technologies.
As a model target, we selected enterovirus 71 (EV71). The genome of the enterovirus consists of approximately 7500 nucleotides that include an open reading frame (ORF) and untranslated regions (UTRs) flanked by 5′ and 3′ terminal untranslated regions. The ORF was divided into three domains, P1, P2, and P3, which encode VP1–4, 2A–2C and 3A–3D proteins, respectively.8 EV71 and coxsackievirus group A (CV-A) are known as widespread human enterovirus species, which cause Hand-Foot-and-Mouth disease (HFMD).9 However, EV71 frequently causes more severe neurological complications compared with CV-A and other enterovirus serotypes. Therefore, there is a clinical need to distinguish EV71 from non-EV71 serotypes. The EV71-specific fragments were selected on the basis of the enterovirus sequences deposited in GenBank. Because of the highly mutated sequences of the enterovirus, it was found that 15-base was the longest fragment conserved in about 70% of the EV71 strains. We, therefore, performed detection of the three selected fragments in an OR logic gate mode in which a high output signal (digital 1) was produced if at least one of the 3 fragments was present. This approach enables detection of > 90% of all known EV71 strains (Table S1, ESI†).
Logic gates made of DNA were introduced as computational media capable of complex analysis of specific DNA or RNA inputs.10 It is believed that this approach can potentially help in the analysis of the complex patterns of biological markers in biomedical applications.11
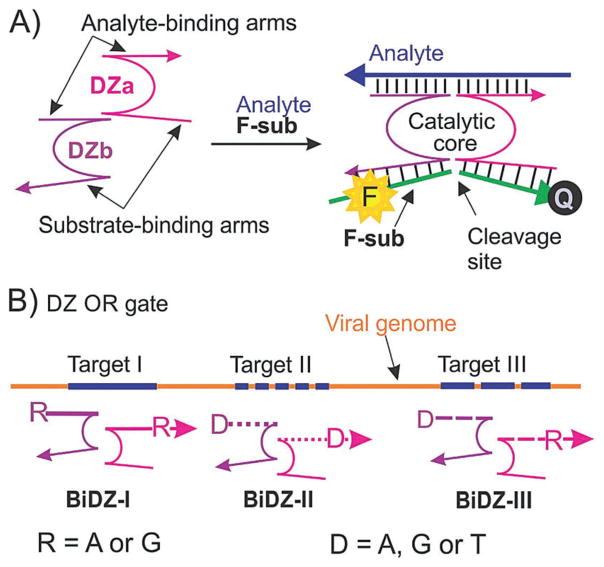
The DZ OR gate was designed based on a binary DZ sensor (BiDZ) developed earlier (Fig. 1A).7 BiDZ consists of two DNA strands DZa and DZb, which hybridize to a targeted analyte and form a catalytic core of RNA-cleaving DZ. The DZ cleaves a fluorophore and a quencher labelled fluorogenic substrate (F-sub, Table S3, ESI†), followed by an increase in fluorescence. We believe that the BiDZ probe is one of the most promising hybridization-based sensors developed to date due to its (i) high selectivity and (ii) improved sensitivity. Indeed, the two-component design enables high selectivity even at ambient temperatures.7 The selectivity is improved due to the catalytic signal amplification: one activated DZ core can cleave multiple F-sub molecules, thus increasing the output signal over time.
Fig. 1.
Design of a deoxyribozyme (DZ) OR gate for detection of enterovirus 71 (EV71) strains. (A) Binary DZ (BiDZ) sensor for fluorescence detection of specific DNA or RNA analytes.7 DNA strands DZa and DZb hybridize to a targeted analyte and form a DZ catalytic core, which cleaves a fluorophore (F) and a quencher (Q) labelled F-sub followed by separation of F from Q associated with an increased fluorescence. (B) Three EV71-specific fragments (targets I, II and III) were chosen to be targeted by the DZ OR gate, which consisted of 3 series of BiDZ sensors: DZ-I, DZ-II and DZ-III. Each DZa and DZb strand in each sensor contained a degenerative position: R or D (see Table S3 for all sequences, ESI†), thus comprising a total of 19 individual BiDZ sensors.
The conservative 15-nt EV71 targeted fragments selected by us were insufficiently long to bind with the two adaptor strands (DZa and DZb) stably. Thus we made 3-nt extensions at both 5′ and 3′ terminals of the 15-nt targeted fragments to produce 21-nt targets without altering the coverage rate. To achieve this, each fragment had 2 variable nucleotides residing at the 1st and 19th sites (numbered by 5′ → 3′), in accordance with the varied nucleotides present in clinical isolates (Table S2, ESI†). The details of target selection are provided in ESI† (Table S1), with the resulting targeted fragments shown in Table S2.
In order to recognize a diverse set of targets and the three targeted loci in the EV71 genome, we designed a DZ OR gate, which, according to the Boolean logic, would respond by producing a high signal (digital 1) in the presence of any EV71 target. This signalling mode would inform about the presence of the EV71 no matter which viral strain is present, thus prompting appropriate medical care. The DZ OR gate consisted of three sets of BiDZ probes targeting the three selected EV71-specific loci. Addition of the degenerative nucleotides (D and R) in the DZa and DZb was necessary to enable recognition of all possible strains containing point mutations in the 21-nt fragments (Fig. 1B and Table S3, ESI†). For example, each DZa-I and DZb-I contained one site R, where R is either A or G. Therefore, a BiDZ sensor made of DZa-I and DZb-I is able to specifically recognize four EV71 sequences (EV-I-1, EV-I-2, EV-I-3 and EV-I-4, Table S3, ESI†), which correspond to all natural combinations of the EV71 strains. Taking into account all variable positions, the mixture of the three BiDZ sensors could detect the sequences of 19 EV71 strains.
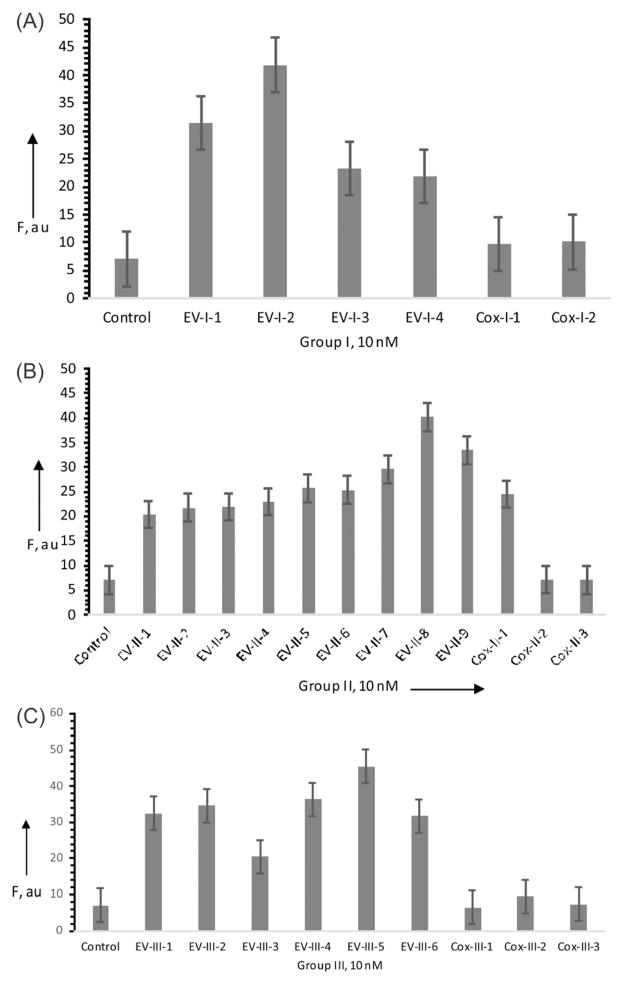
Indeed, the DZ OR gate produced fluorescence significantly above the background in the presence of each possible analyte sequence (Fig. 2). Importantly, the DZ OR gate produced only the background fluorescence in the presence of 7 out of 8 coxsackievirus sequences (Fig. 2), which contained one to three nucleotides differing from the EV71 sequences (see Table S3, ESI†). The DZ OR gate failed to differentiate the Cox-II-1 sequence, despite having an A–G mismatch, which is known to be one of the least destabilizing mismatches.12 Further optimizations are needed to achieve differentiation of Cox-II-1 by the DZ OR gate sensor presented here. These may be achieved by shortening of the analyte binding arms or introducing conformational constraints as was explored by us earlier for other multicomponent probes.13 The position of the mutation is also important. As was demonstrated earlier, binary probes achieve best differentiations of point mutations if the mismatch position is located in the middle of one of the two probe–analyte complexes.14
Fig. 2.
Fluorescence response of the DZ OR gate in the presence of EV71 and coxsackievirus sequences. F-Sub, DZa-I, DZb-I, DZa-II, DZb-II, DZa-III and DZb-III were incubated in the absence (control) or presence of 10 nM EV-I or Cox-I series (A); 10 nM EV-II or Cox-II series (B); and 10 nM EV-III or Cox-III series (C). Fluorescence at 517 nm (λex = 485 nm) was registered after 3 h of incubation at 28°C. The data of 3 independent experiments with standard deviations are presented.
Furthermore, we measured the limits of detection of the DZ OR gate in recognition of two representative analytes: EV-II-1, which produced the lowest signal, and EV-III-5, which produced the highest signal (Fig. 2). The LOD were found to be 374 pM after 1 h and 134 pM after 3 h for EV-II-1 and 51.8 pM after 1 h and 16.1 pM after 3 h for EV-III-5 (Fig. S2, ESI†). These LOD are at least 1 order of magnitude lower than those of molecular beacon probes, which are conventionally used for nucleic acid analysis.15
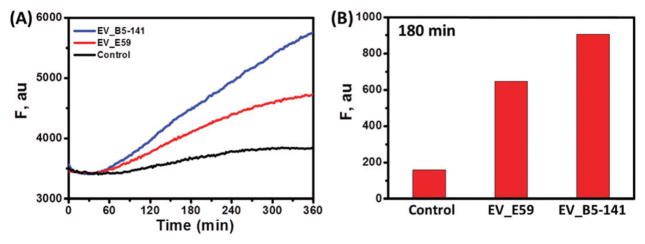
In order to prove the practical significance of the developed strategy, we interrogated the genuine enterovirus sequences obtained by reverse transcription/amplification of viral RNA (Fig. 3). The fluorescence increases of the DZ OR gate (after 3 h) produced in the presence of E59 and B5/141 isolates were obviously greater than that of the control. The long GC-rich viral sequence presumably folded at 28 °C, which led to a low hybridization rate of the target with adaptor strands and a slowly increasing fluorescence.
Fig. 3.
(A) Time-course fluorescence of the DZ OR gate in the absence (black) and presence of EV_E59 (red) and EV_B5-141 (blue) sequences. For all curves, the initial fluorescence (t = 0) is represented at same value for a clear comparison. (B) Histogram representing the magnitudes of the fluorescence at 180 min yielded by the control and the two EV71 isolates.
We proposed and experimentally validated a strategy for the highly selective analysis of mutation-prone sequences. The approach enabled detection of 90% of the sequences of all known EV71 strains and distinguished them from sequences containing known point mutations. One limitation of the approach is the requirement to know the position (and preferably the type) of the mutation for accurate design of an OR gate. However, considering the availability, speed and cost efficiency of modern sequencing techniques this limitation should not create a significant obstacle in transferring this technology to the clinical analysis of mutation-prone pathogens.
Supplementary Material
Acknowledgments
Funding from NIAID (R15AI10388001A1), NSF CCF 1423219, MOST 105-2113-M-029-008-MY2, MOST 104-2320-B-400-021-MY2, and PH-106-PP-05 is greatly appreciated. D. M. K. was partially supported by the ITMO University Fellowship and Professorship Program.
Footnotes
Electronic supplementary information (ESI) available: Detailed experimental procedures; sequence selection; RNA extraction; RT-PCR; PCR; data used for the calculation of LOD; and fluorescence responses for viral genome sequences. See DOI: 10.1039/c7cc05576e
Conflicts of interest
The authors declare no conflicts of interest.
Notes and references
- 1.(a) Knez K, Spasic D, Janssen KP, Lammertyn J. Analyst. 2014;139:353. doi: 10.1039/c3an01436c. [DOI] [PubMed] [Google Scholar]; (b) Wang L, Luhm R, Lei M. Adv Exp Med Biol. 2007;593:105. doi: 10.1007/978-0-387-39978-2_11. [DOI] [PubMed] [Google Scholar]
- 2.(a) Frickmann H, Masanta WO, Zautner AE. Biomed Res Int. 2014;2014:375681. doi: 10.1155/2014/375681. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Nakipoglu M, Yilmaz F, Icgen B. Environ Monit Assess. 2016;188:569. doi: 10.1007/s10661-016-5578-7. [DOI] [PubMed] [Google Scholar]; (c) Bengtson HN, Homolka S, Niemann S, Reis AJ, da Silva PE, Gerasimova YV, Kolpashchikov DM, Rohde KH. Biosens Bioelectron. 2017;94:176. doi: 10.1016/j.bios.2017.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Mani V, Wang S, Inci F, De Libero G, Singhal A, Demirci U. Adv Drug Delivery Rev. 2014;78:105. doi: 10.1016/j.addr.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Wu W, Tang YW. Clin Lab Med. 2009;29:673. doi: 10.1016/j.cll.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kamau E, Agoti CN, Lewa CS, Oketch J, Owor BE, Otieno GP, Bett A, Cane PA, Nokes DJ. J Clin Virol. 2017;88:21. doi: 10.1016/j.jcv.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Chen Q, Belmonte I, Buti M, Nieto L, Garcia-Cehic D, Gregori J, Perales C, Ordeig L, Llorens M, Soria ME, Esteban R, Esteban JI, Rodriguez-Frias F, Quer J. World J Gastroenterol. 2016;22:9604. doi: 10.3748/wjg.v22.i43.9604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Sanjuán R, Domingo-Calap P. Cell Mol Life Sci. 2016;73:4433. doi: 10.1007/s00018-016-2299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Faillace CA, Lorusso NS, Duffy S. Ecol Lett. 2017;20:524. doi: 10.1111/ele.12742. [DOI] [PubMed] [Google Scholar]; (c) Seifert D, Beerenwinkel N. Curr Top Micro-biol Immunol. 2016;392:181. doi: 10.1007/82_2015_462. [DOI] [PubMed] [Google Scholar]; (d) Koch N, Yahi N, Colson P, Fantini J, Tamalet C. J Virol Methods. 1999;80:25. doi: 10.1016/s0166-0934(99)00030-0. [DOI] [PubMed] [Google Scholar]; (e) Sacks D, Ledwaba J, Morris L, Hunt GM. J Clin Microbiol. 2017;55:122. doi: 10.1128/JCM.01291-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Fulton RW, d’Offay JM, Dubovi EJ, Eberle R. Virus Res. 2016;223:115. doi: 10.1016/j.virusres.2016.06.017. [DOI] [PubMed] [Google Scholar]; (b) Li L, He Y, Yang H, Zhu J, Xu X, Dong J, Zhu Y, Jin Q. J Clin Microbiol. 2005;43:3835–3839. doi: 10.1128/JCM.43.8.3835-3839.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Hsiao CC, Chang J, Wu JY, Liu WH, Han SY, Chen PJ, Yeh SH. Antivir Ther. 2012;17:291–303. doi: 10.3851/IMP2022. [DOI] [PubMed] [Google Scholar]; (b) Zhao XT, Zhou DQ, Wu S, Chen YW, Shao Y, Zhang J, Xia CS, Wang KP, Yang H, Wan J, Yu B, Zhang Z, Zhang W. Arch Virol. 2012;157:475. doi: 10.1007/s00705-011-1173-y. [DOI] [PubMed] [Google Scholar]; (c) Arvia R, Corcioli F, Azzi A. J Virol Methods. 2013;189:265–270. doi: 10.1016/j.jviromet.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 7.(a) Kolpashchikov DM. ChemBioChem. 2007;8:2039. doi: 10.1002/cbic.200700384. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Mokany E, Bone SM, Young PE, Doan TB, Todd AV. J Am Chem Soc. 2010;132:1051. doi: 10.1021/ja9076777. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Gerasimova YV, Cornett E, Kolpashchikov DM. ChemBioChem. 2010;11:811. doi: 10.1002/cbic.201000006. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Gerasimova YV, Kolpashchikov DM. Chem Biol. 2010;17:104. doi: 10.1016/j.chembiol.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Gerasimova YV, Yakovchuk P, Dedkova LM, Hecht SM, Kolpashchikov DM. RNA. 2015;21:1834. doi: 10.1261/rna.052613.115. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Zhang L, Zhu J, Li T, Wang E. Anal Chem. 2011;83:8871–8876. doi: 10.1021/ac2006763. [DOI] [PubMed] [Google Scholar]; (g) Cox AJ, Bengtson HN, Gerasimova YV, Rohde KH, Kolpashchikov DM. ChemBioChem. 2016;21:2038–2041. doi: 10.1002/cbic.201600438. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Cox AJ, Bengtson HN, Rohde KH, Kolpashchikov DM. Chem Commun. 2016;52:14318–14321. doi: 10.1039/c6cc06889h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oberste MS, Peñaranda S, Maher K, Pallansch MA. J Gen Virol. 2004;85:1597. doi: 10.1099/vir.0.79789-0. [DOI] [PubMed] [Google Scholar]
- 9.(a) Chan KP, Goh KT, Chong CY, Teo ES, Lau G, Ling AE. Emerging Infect Dis. 2003;9:78. doi: 10.3201/eid1301.020112. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wu Y, Yeo A, Phoon MC, Tan EL, Poh CL, Quak SH, Chow VT. Int J Infect Dis. 2010;14:e1076. doi: 10.1016/j.ijid.2010.07.006. [DOI] [PubMed] [Google Scholar]; (c) Liu MY, Liu W, Luo J, Liu Y, Zhu Y, Berman H, Wu J. PLoS One. 2011;6:e25287. doi: 10.1371/journal.pone.0025287. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Xu M, Su L, Cao L, Zhong H, Dong N, Dong Z, Xu J. PLoS One. 2015;10:e0138514. doi: 10.1371/journal.pone.0138514. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Huang CC, Liu CC, Chang YC, Chen CY, Wang ST, Yeh TF. N Engl J Med. 1999;341:936. doi: 10.1056/NEJM199909233411302. [DOI] [PubMed] [Google Scholar]
- 10.(a) Stojanovic MN, Mitchell TE, Stefanovic D. J Am Chem Soc. 2002;124:3555. doi: 10.1021/ja016756v. [DOI] [PubMed] [Google Scholar]; (b) Okamoto A, Tanaka K, Saito I. J Am Chem Soc. 2004;126:9458. doi: 10.1021/ja047628k. [DOI] [PubMed] [Google Scholar]; (c) Frezza BM, Cockroft SL, Ghadiri MR. J Am Chem Soc. 2007;129:14875. doi: 10.1021/ja0710149. [DOI] [PubMed] [Google Scholar]; (d) Lake A, Shang S, Kolpashchikov DM. Angew Chem, Int Ed Engl. 2010;122:4561. doi: 10.1002/anie.200907135. [DOI] [PubMed] [Google Scholar]; (e) Gerasimova YV, Kolpashchikov DM. Chem – Asian J. 2012;7:534. doi: 10.1002/asia.201100664. [DOI] [PubMed] [Google Scholar]; (F) Gerasimova YV, Kolpashchikov DM. Angew Chem, Int Ed Engl. 2016;55:10244. doi: 10.1002/anie.201603265. [DOI] [PubMed] [Google Scholar]; (g) Gerasimova YV, Kolpashchikov DM. Chem Commun. 2015;51:870. doi: 10.1039/c4cc08241a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Guz N, Fedotova TA, Fratto BE, Schlesinger O, Alfonta L, Kolpashchikov DM, Katz E. ChemPhysChem. 2016;17:2247. doi: 10.1002/cphc.201600129. [DOI] [PubMed] [Google Scholar]
- 11.(a) Cornett EM, Campbell EA, Gulenay G, Peterson E, Bhaskar N, Kolpashchikov DM. Angew Chem, Int Ed Engl. 2012;51:9075. doi: 10.1002/anie.201203708. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hemphill J, Deiters A. J Am Chem Soc. 2013;135:1012. doi: 10.1021/ja404350s. [DOI] [PubMed] [Google Scholar]; (c) Lai YH, Liao YC, Mu JJ, Kuo TM, Hsu YH, Chuang MC. Chem Commun. 2014;50:12018. doi: 10.1039/c4cc01108b. [DOI] [PubMed] [Google Scholar]; (d) Poje JE, Kastratovic T, Macdonald AR, Guillermo AC, Troetti SE, Jabado OJ, Fanning ML, Stefanovic D, Macdonald J. Angew Chem, Int Ed Engl. 2014;53:9222. doi: 10.1002/anie.201402698. [DOI] [PubMed] [Google Scholar]; (e) Lai YH, Lee CC, King CC, Chuang MC, Ho JAA. Chem Sci. 2014;5:4082. [Google Scholar]; (F) Chen Y, Song Y, Wu F, Liu W, Fu B, Feng B, Zhou X. Chem Commun. 2015;51:6980. doi: 10.1039/c5cc01389e. [DOI] [PubMed] [Google Scholar]; (g) Lee CC, Liao YC, Lai YH, Lee CC, Chuang MC. Anal Chem. 2015;87:5410. doi: 10.1021/acs.analchem.5b00810. [DOI] [PubMed] [Google Scholar]; (h) Chen L, Zeng X, Dandapat A, Chi Y, Kim D. Anal Chem. 2015;87:8851. doi: 10.1021/acs.analchem.5b01916. [DOI] [PubMed] [Google Scholar]; (i) Chen Y, Song Y, Wu F, Liu W, Fu B, Feng B, Zhou X. Chem Commun. 2015;51:6980. doi: 10.1039/c5cc01389e. [DOI] [PubMed] [Google Scholar]; (j) Groves B, Chen YJ, Zurla C, Pochekailov S, Kirschman JL, Santangelo PJ, Seelig G. Nat Nanotechnol. 2016;11:287. doi: 10.1038/nnano.2015.278. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Bi S, Ye J, Dong Y, Li H, Cao W. Chem Commun. 2016;52:402. doi: 10.1039/c5cc07046e. [DOI] [PubMed] [Google Scholar]; (l) Lee Y, Roslan R, Azizan S, Firdaus-Raih M, Ramlan EI. BMC Bioinf. 2016;17:438. doi: 10.1186/s12859-016-1297-x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (m) Vijayakumar P, Macdonald J. Chem Phys Chem. 2017;18:1735. doi: 10.1002/cphc.201700072. [DOI] [PubMed] [Google Scholar]
- 12.(a) Lokhov SG, Pyshnyi DV. FEBS Lett. 1997;420:134–138. doi: 10.1016/s0014-5793(97)01502-0. [DOI] [PubMed] [Google Scholar]; (b) Berashevich J, Chakraborty T. J Chem Phys. 2009;130:015101. doi: 10.1063/1.3050107. [DOI] [PubMed] [Google Scholar]
- 13.(a) Cornett EM, Kolpashchikov DM. Methods Mol Biol. 2013;1039:81. doi: 10.1007/978-1-62703-535-4_6. [DOI] [PubMed] [Google Scholar]; (b) Gerasimova YV, Hayson A, Ballantyne J, Kolpashchikov DM. ChemBioChem. 2010;11:1762. doi: 10.1002/cbic.201000287. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Nguyen C, Grimes J, Gerasimova YV, Kolpashchikov DM. Chemistry. 2011;17:13052. doi: 10.1002/chem.201101987. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Gerasimova YV, Kolpashchikov DM. Biosens Bioelectron. 2013;15:386. doi: 10.1016/j.bios.2012.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.(a) Kolpashchikov DM. J Am Chem Soc. 2005;127:12442. doi: 10.1021/ja0529788. [DOI] [PubMed] [Google Scholar]; (b) Kolpashchikov DM. J Am Chem Soc. 2006;128:10625. doi: 10.1021/ja0628093. [DOI] [PubMed] [Google Scholar]
- 15.(a) Tyagi S, Kramer FR. Nat Biotechnol. 1996;14:303. doi: 10.1038/nbt0396-303. [DOI] [PubMed] [Google Scholar]; (b) Kolpashchikov DM. Scientifica. 2012;2012:928783. doi: 10.6064/2012/928783. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.