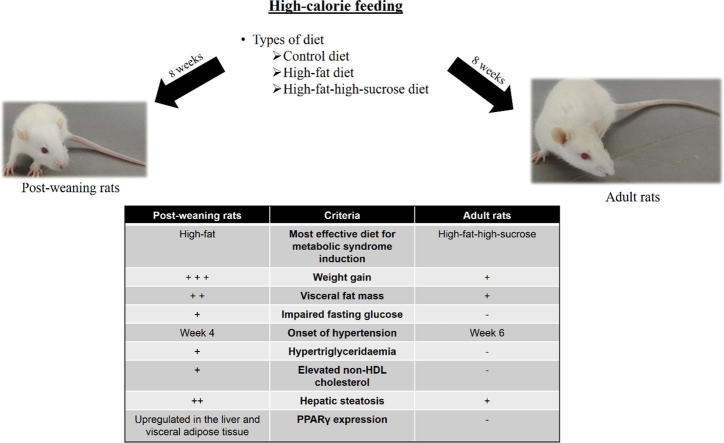
Graphical abstract
The effective high-calorie diet for metabolic syndrome induction is different between Sprague Dawley rats of different developmental stages. The post-weaning rats on high-fat diet for 8 weeks developed all phenotypes of metabolic syndrome while the adult rats on high-fat-high-sucrose diet merely became obese and hypertensive. The post-weaning rats on high-fat diet is a better and less time-consuming model for metabolic syndrome research.
Keywords: Dyslipidaemia, Hepatic steatosis, High-fat diet, Hypertension, Obesity, Peroxisome proliferator-activated receptor
Abstract
The present study aimed to examine the effects of the types of high-calorie diets (high-fat and high-fat-high-sucrose diets) and two different developmental stages (post-weaning and young adult) on the induction of metabolic syndrome. Male, post-weaning and adult (3- and 8-week old, respectively) Sprague Dawley rats were given control, high-fat (60% kcal), and high-fat-high-sucrose (60% kcal fat + 30% sucrose water) diets for eight weeks (n = 6 to 7 per group). Physical, biochemical, and transcriptional changes as well as liver histology were noted. Post-weaning rats had higher weight gain, abdominal fat mass, fasting glucose, high density lipoprotein cholesterol, faster hypertension onset, but lower circulating advanced glycation end products compared to adult rats. This is accompanied by upregulation of peroxisome proliferator-activated receptor (PPAR) α and γ in the liver and receptor for advanced glycation end products (RAGE) in the visceral adipose tissue. Post-weaning rats on high-fat diet manifested all phenotypes of metabolic syndrome and increased hepatic steatosis, which are linked to increased hepatic and adipocyte PPARγ expression. Adult rats on high-fat-high-sucrose diet merely became obese and hypertensive within the same treatment duration. Thus, it is more effective and less time-consuming to induce metabolic syndrome in male post-weaning rats with high-fat diet compared to young adult rats. As male rats were selectively included into the study, the results may not be generalisable to all post-weaning rats and further investigation on female rats is required.
Introduction
Metabolic syndrome (MetS) is a multiplex risk factors for cardiovascular disease and type 2 diabetes mellitus. The defining clinical criteria of MetS include central obesity, dyslipidaemia, hypertension, and glucose intolerance [1]. People with MetS are twice as likely to develop cardiovascular disease and up to five times as likely to become diabetic than those without the condition [2]. In addition, other comorbidities of MetS are also increasingly recognized, such as polycystic ovary syndrome [3], cancer [4], and cognitive degenerative disease [5]. MetS is deemed to be a worldwide health threat not only because of its devastating complications, but also due to the widespread global prevalence. In most developed and developing countries, approximately 20% of the adult population have MetS [6]. The statistic is undoubtedly an underestimation due to the exclusion of the rapidly-escalating paediatric and adolescent cases. Thus, studying the disease is crucial for us to propose potential solutions to this emerging epidemic.
One major challenge in MetS research is to create a clinically relevant disease model. In this context, metabolic dysfunction is commonly induced in rodents, particularly rats and mice, via different approaches, which include the use of genetic models like ob/ob and db/db mice as well as diet-induced models. The animal models of MetS are well-summarized by Panchal and Brown [7] and Aydin et al. [8]. Diet-induced models are usually preferred considering that over-nutrition is one of the key contributors to MetS. Nonetheless, the lack of standardised methodology for MetS induction results in a large variety of high-calorie diets being used, each with different preparation methods, formulations, and nutritional values. Some of the most popular diets are those high in saturated fat, fructose, sucrose or a combination of these macronutrients. This is because increased dietary lipid and fructose markedly upregulates de novo lipogenesis and promotes ectopic lipid deposition, which in turn, leads to peripheral insulin resistance, inflammatory response, chronic oxidative stress insult, and progressive organ damage [9], [10]. Nonetheless, due to the diverse dietary compositions and feeding approaches, making comparisons between different diets and studies is often difficult. The issue of diet choice is further complicated by other concerns: purified ingredient-based diet versus chow-based diet [11], mismatched control diet [12], and feeding duration.
Additionally, other factors such as the animal species, strain, gender, and age should also be taken into consideration. Most studies employ male, adult rodents with a varying starting age. The feeding duration ranges from two to 18 weeks [8]. Given that the starting age could potentially influence the progression of MetS in both rodents [13], [14] and humans [15], translating the experimental findings obtained from animal studies to mismatched age groups ought to be carried out cautiously. Furthermore, as mentioned earlier, the prevalence of metabolic syndrome among children and adolescents has drastically increased over recent years. Animal studies employing younger rats may be of interest since the experimental findings can potentially be applicable on paediatric population. Essentially, despite the extensive use of diet-induced models in MetS research, there are still a lot of unresolved issues and room for improvement to create better models.
Considering the vast diversity of determinants which may affect MetS progression, comparative animal studies are very useful for the optimisation of disease model creation. In the present study, we are particularly interested in the effects of age and dietary composition on the initiation of MetS. The other variables, such as the species, strain, and gender were kept constant by the use of male Sprague Dawley rats, which are one of the most commonly used animals in MetS research. The efficiency of two widely employed high-calorie diets (high-fat and high-fat-high-sucrose) in the induction of MetS in rats was examined. The differences in MetS progression that could be affected by the two developmental stages (post-weaning versus young adult) were also investigated. The findings will be discussed with reference to physical, biochemical, and transcriptional variables. The output of this study may help to put forward some key parameters for the establishment of a better MetS model.
Material and methods
Animal ethics and housing conditions
The use and handling of animals in the research have been approved by Monash University Monash Animal Research Platform Animal Ethics Committees (AEC approval No.: MARP/2015/060) in compliance to the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes outlined by National Health and Medical Research Council. Thirty-nine male Sprague Dawley rats (Rattus norvergicus) including 18 post-weaning (3-week old) and 21 young adult (8-week old) were obtained from Monash University Malaysia Animal Facility. The rats were kept individually at 23 ± 1 °C with 12-h light/dark cycle. They were given ad libitum access to homemade purified ingredient-based diet and drinking water throughout the entire experiment.
Diet preparation, composition, and treatment
Both post-weaning and adult rats were randomly assigned into three groups (n = 6 for post-weaning rats and n = 7 for adult rats per group), which were provided with control diet (CD), high-fat diet (HFD), and high-fat-high-sucrose diet (HFSD), respectively, for 8 weeks. The compositions of the diets, which were formulated based on AIN-93G diet [16] are shown in Table 1. All the ingredients, except for milk fat (Promac Enterprises Sdn. Bhd., Kuala Lumpur, Malaysia) and sucrose (MSM Malaysia Holdings Bhd., Kuala Lumpur, Malaysia), were purchased from MP Biomedical, Santa Ana, USA. The diets were prepared by mixing the ingredients thoroughly, followed by oven-baking for 10 min at 160 °C. For HFSD group, 30% (w/v) sucrose water was supplemented in addition to the HFD. The food and water were replenished every day. Body weight, food and water intake were measured daily.
Table 1.
Macronutrient composition and ingredients of control, high-fat and high-fat-high-sucrose diets.
| Macronutrient | Control diet | High-fat diet | High-fat-high-sucrose diet |
|---|---|---|---|
| Protein (kcal%) | 20 | 20 | 20 |
| Carbohydrate (kcal%) | 70 | 20 | 20 |
| Lipid (kcal%) | 10 | 60 | 60 |
| Saturated (%) | 36.6 | 57.9 | 57.9 |
| Monounsaturated (%) | 29.0 | 28.8 | 28.8 |
| Polyunsaturated (%) | 32.0 | 8.4 | 8.4 |
| Trans (%) | 1.8 | 3.6 | 3.6 |
| Energy content (kcal/g) | 3.9 | 5.3 | 5.3 + 1.2 kcal/mL from sucrose water |
| Ingredient | Mass (g) | ||
| Casein | 200 | 200 | 200 |
| l-cystine | 3 | 3 | 3 |
| Corn starch | 525.5 | 18 | 18 |
| Maltodextrin | 125 | 125 | 125 |
| Sugar | 50 | 50 | 50 |
| Cellulose | 50 | 50 | 50 |
| Milk fat | 20 | 245 | 245 |
| Corn oil | 25 | 25 | 25 |
| AIN-93G Mineral mix | 35 | 35 | 35 |
| AIN-93-VX Vitamin mix | 10 | 10 | 10 |
| Choline bitartrate | 2 | 2 | 2 |
| t-butylhydroquinone | 0.014 | 0.014 | 0.014 |
| Additional supplement | – | – | 30% (w/v) sucrose water |
Food was withdrawn while sucrose water of the HFSD group was replaced with tap water 12 h prior to humane sacrifice. The rats were euthanized by exsanguination via cardiac puncture under the influence of ketamine (75 mg/kg) and xylazine hydrochloride (10 mg/kg) administered intraperitoneally. Blood samples were collected in tubes with 0.5 M ethylenediaminetetraacetic acid (EDTA). Plasma was obtained by centrifugation of the blood samples at 4 °C, 2000×g for 20 min. The supernatant (plasma) was snap frozen in liquid nitrogen and stored at −80 °C until further use. About 1 cm × 1 cm of the liver tissues were excised and stored in 10% neutral buffered formalin for fixation and histology. Retroperitoneal white adipose tissue (rWAT) and liver were harvested promptly, snap frozen in liquid nitrogen and stored at −80 °C.
Blood pressure measurement
Systolic and diastolic blood pressure was measured with Mouse and Rat Tail Cuff Blood Pressure System (IITC Life Sciences, Los Angeles, USA). The rats were placed into a plastic restrainer one at a time to restrict their movement throughout the measurement. A tail-cuff with a pulse transducer was applied onto the tail of the restrained rats. The rat was then placed into a well-ventilated chamber equilibrated at 32 °C for 15–20 min to facilitate the dilatation of caudal arteries. Next, the triplicate readings of the systolic and diastolic blood pressure were recorded. The procedure was performed before the experiment (Week 0) and every two weeks (Week 2, 4, 6, and 8).
Biochemical assays
The glycaemic parameters and lipid profile at the end of the eight-week treatment were measured. Fasting plasma glucose was determined using Trinder’s glucose oxidase test while fasting plasma insulin was determined using Mercodia Ultrasensitive Rat Insulin ELISA (Mercodia, Uppsala, Sweden). Based on the fasting plasma glucose and insulin levels, homeostasis model assessment of the insulin resistance (HOMA-IR), β-cell function (HOMA %β), and insulin sensitivity (HOMA %S) were evaluated using HOMA calculator [17]. Glycated haemoglobin A1c (HbA1c) and advanced glycation end product (AGE) levels were determined with Rat Haemoglobin A1c (HbA1c) kit (Crystal Chem, Downers Grove, USA) and OxiSelect™ Advanced Glycation End Product (AGE) Competitive ELISA kit (Cell Biolabs, San Diego, USA), respectively. Circulating triglyceride, total cholesterol (TC), and free fatty acid (FFA) levels were measured using Randox TR1607 Triglycerides, CH200 Cholesterol and FA115 Non-esterified Fatty Acids kits (Randox, Dublin, UK). Chylomicron, low density lipoprotein (LDL) and very low density lipoprotein (VLDL) were precipitated from the plasma specimens using Randox CH203 HDL-cholesterol Precipitant kit (Randox, Dublin, UK) and the remaining fraction was subjected to CH200 Cholesterol kit for the determination of high density lipoprotein (HDL)-cholesterol. Non HDL-cholesterol was calculated by subtracting HDL-cholesterol from TC. All assays with commercial kits were performed in duplicate according to the manufacturers’ instructions.
RNA extraction and cDNA synthesis
Total RNA extraction of the liver was conducted using Qiagen RNeasy Mini Kit (Qiagen, Hilden, Germany), whereas that of rWAT was isolated with Tri-RNA reagent (Favorgen, Ping-Tung, Taiwan) and Qiagen RNeasy Mini Kit. The concentration and purity of the RNA were determined by measuring the absorbance at 260 nm and 280 nm with Infinite® 200 PRO (TECAN, Zürich, Switzerland). RNA integrity was examined with agarose gel electrophoresis to check 18S and 28S ribosomal RNA. RNase-free DNase I (ThermoFisher Scientific, Waltham, USA) treatment was performed prior to cDNA synthesis, which was carried out with Qiagen Omniscript Reverse Transcription Kit (Qiagen, Hilden, Germany).
Quantitative PCR (qPCR)
Rotor-Gene Q (Qiagen, Hilden, Germany) was used to carry out qPCR of peroxisome proliferator-activated receptors (PPAR) α and γ, lipoprotein lipase (LPL), and receptor for advanced glycation end product (RAGE) of the liver and adipose tissue. Hypoxanthine phosphoribosyltransferase 1 (HPRT1) and β-actin, which have been demonstrated to express stably in the target tissues, were selected as the reference genes for normalisation of the target genes [18]. JumpStart™ Taq ReadyMix (Sigma-Aldrich, St. Louis, USA) was used for the qPCR reactions. All primers and hydrolysis probes were synthesised by First BASE Laboratories, Malaysia. The nucleotide sequences of the primers and hydrolysis probes are outlined in Table 2. Normalised Ct or ΔCt values of the genes of interest were calculated using the following formula:
Table 2.
Nucleotide sequences of the primers and hydrolysis probes.
| Target gene | Nucleotide sequence (5′ → 3′) |
||
|---|---|---|---|
| Forward primer | Reverse primer | Hydrolysis probe | |
| β-actina | GTA TGG GTC AGA AGG ACT CC | GTT CAA TGG GGT ACT TCA GG | [TET] CCT CTC TTG CTC TGG GC [BHQ1] |
| HPRT1a | CTG GAA AGA ACG TCT TGA TTG | GTA TCC AAC ACT TCG AGA GG | [6FAM] AGC CCC AAA /[ZEN]/ATG GTT AAG GTT GCA AG [Iowa Black® FQ] |
| RAGE | CCC TGA CCT GTG CCA TCT CT | GGG TGT GCC ATC TTT TAT CCA | [6FAM] CCC AGC CTC CCC CTC AAA TCC A [BHQ1] |
| PPARα | TGT GGA GAT CGG CCT GGC CTT | CCG GAT GGT TGC TCT GCA GGT | [6FAM] TGC AGG AGG GGA TTG TGC ACG TGC TCA [BHQ1] |
| PPARγ | CCC TGG CAA AGC ATT TGT AT | GGT GAT TTG TCT GTT GTC TTT C | [6FAM] TCC TTC CCG CTG ACC A [BHQ1] |
| LPL | CAG CAA GGC ATA CAG GTG | CGA GTC TTC AGG TAC ATC TTA C | [6FAM] TTC TCT TGG CTC TGA CC [BHQ1] |
HPRT1, hypoxanthine phosphoribosyltransferase 1; LPL, lipoprotein lipase; PPAR, peroxisome proliferator-activated receptor; RAGE, receptor for advanced glycation end product.
Denotes reference genes.
Tissue processing and histology
The well-fixed liver specimens were subjected to conventional tissue processing and embedded in paraffin wax. Thin sections (5 μm) were produced and stained with haematoxylin and eosin (H&E) to visualise the morphology. Three microscopic images at 200× magnification for each rat were captured with Nikon Eclipse TS100 (Nikon, Tokyo, Japan) and analysed with ImageJ to calculate the area of steatosis in the liver [19].
Statistical analysis
Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) 22.0. Dependent variables with repeated measures like cumulative weight gain and blood pressure were analysed using mixed model ANOVA using “time” as the within-subjects factor while “age” and “types of diets” as the between-subjects factors. Intergroup comparisons of other variables, including calorie intake, rWAT mass, glycaemic indices, lipid profile, and ΔCt values were analysed with two-way ANOVA with “age” and “types of diets” as the between-subjects factors. Pairwise comparisons were performed with Bonferroni correction. The level of statistical significance was pre-determined at P ≤ 0.05.
Results
Differential obesity-inducing effects of HFD and HFSD on the post-weaning and adult rats
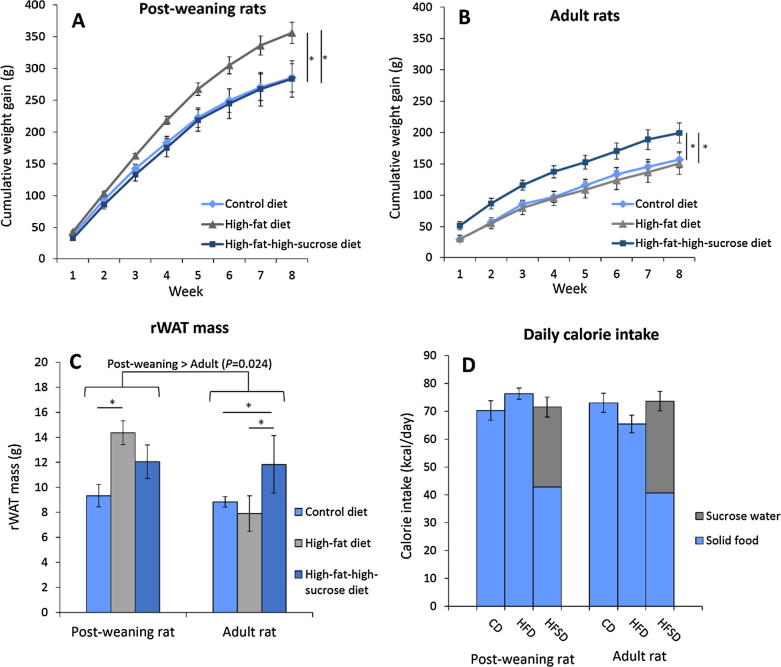
The initial weight of the rats within the same age groups, namely the post-weaning rats (64.5 ± 2.0 g, 63.0 ± 1.7 g, and 64.0 ± 1.9 g for CD, HFD, and HFSD) and the adult rats (222.2 ± 3.9 g, 219.4 ± 5.6 g, and 217.7 ± 3.2 g for CD, HFD, and HFSD), were not significantly different from each other. However, the post-weaning and adult rats gained weight at varying rates when exposed to different types of high-calorie diets. Based on Fig.1A and B, the post-weaning and adult rats fed on HFD and HFSD, respectively, were more prone to accelerated weight gain. For the post-weaning rats on HFD, weight gain increased by about 25% over a course of eight weeks compared to those on CD and HFSD. Similar trend was also observed in the adult rats on HFSD compared to those on CD and HFD. Furthermore, the post-weaning rats also gained weight much faster than the adult rats (P < 0.001) which could be partly attributed to growth.
Fig. 1.
Cumulative weight gain of the post-weaning (A) and adult rats (B) as well as the retroperitoneal white adipose tissue mass (C) and daily calorie intake (D) of the rats on different diets for eight weeks. Error bars indicate SEM. The sample size was n = 6–7 per group. * indicates P < 0.05 between groups. CD, control diet; HFD, high-fat diet; HFSD, high-fat-high-sucrose diet; rWAT, retroperitoneal white adipose tissue.
Increased weight gain is associated with increased rWAT mass as illustrated in Fig.1C. To elucidate, the rWAT fat depot was increased by more than 50% in the post-weaning rats on HFD compared to the control group. Similar trend was observed in the adult rats on HFSD. Thus, it is justified to say that these rats developed central obesity after feeding on the corresponding high-calorie diets for eight weeks. The rWAT mass (±SEM) of the post-weaning rats (11.91 ± 0.76 g) was significantly higher than that of the adult rats (9.49 ± 0.68 g) (P = 0.024), suggesting that younger rats may be more susceptible to obesity induction. Nevertheless, calorie intake per day was similar across all groups (Fig.1D), indicating that the increased weight gain and rWAT mass were independent of calorie consumption. It is also worth mentioning that for the HFSD group, both the post-weaning and adult rats obtained more than two fifths of their daily calorie intake from the sucrose water. Carbohydrate preference over lipid was noted in adult rats as suggested by significant increase in the consumption of sucrose water (P < 0.001) (Table 3).
Table 3.
Effects of HFD and HFSD on the consumption of food and water and glycaemic parameters of post-weaning and adult rats after eight-week long treatment.
| Parameter | Post-weaning rat |
Adult rat |
Post-weaning rat vs. Adult rat | ||||
|---|---|---|---|---|---|---|---|
| CD | HFD | HFSD | CD | HFD | HFSD | ||
| Food intake (g/day) | 18.11 ± 0.90 | 14.36 ± 0.37** | 8.06 ± 0.77***,††† | 18.82 ± 0.89 | 12.31 ± 0.59*** | 7.65 ± 0.52***,††† | NS (P = 0.330) |
| Water intake (mL/day) | 21.59 ± 0.44 | 21.38 ± 0.73 | 23.71 ± 1.19 | 20.19 ± 0.90 | 19.70 ± 0.78 | 28.96 ± 1.17***,††† | NS (P = 0.552) |
| FPG (mmol/L) | 5.52 ± 0.12 | 6.53 ± 0.15** | 5.84 ± 0.21† | 5.02 ± 0.18 | 5.12 ± 0.17 | 5.54 ± 0.20 | Post-weaning > Adult (P < 0.001) |
| HbA1c (%) | 3.81 ± 0.42 | 5.95 ± 0.51** | 5.72 ± 0.63* | 5.01 ± 0.31 | 5.73 ± 0.50 | 5.75 ± 0.32 | NS (P = .378) |
| AGE (µg/mL) | 61.23 ± 15.45 | 79.85 ± 5.62 | 43.48 ± 7.41† | 104.56 ± 23.66 | 114.46 ± 17.81 | 61.85 ± 7.80† | Post-weaning < Adult (P = 0.011) |
| FPI (mU/L) | 10.96 ± 1.95 | 5.69 ± 0.70 | 7.94 ± 1.06 | 6.51 ± 1.37 | 10.47 ± 3.46 | 8.91 ± 3.28 | NS (P = 0.543) |
| HOMA-IR | 1.26 ± 0.21 | 0.72 ± 0.10 | 0.82 ± 0.08 | 0.63 ± 0.16 | 0.87 ± 0.23 | 0.67 ± 0.19 | NS (P = 0.158) |
| HOMA%β (%) | 108.0 ± 12.9 | 40.8 ± 2.9** | 63.8 ± 3.9 | 81.4 ± 17.5 | 80.0 ± 21.7 | 47.9 ± 8.0 | NS (P = 0.917) |
| HOMA%S (%) | 92.9 ± 19.9 | 132.4 ± 13.4 | 116.5 ± 13.2 | 129.0 ± 20.0 | 89.8 ± 14.2 | 119.3 ± 32.8 | NS (P = 0.943) |
Values are expressed as mean ± SEM. The sample size was n = 6–7 per group.
AGE, advanced glycation end products; CD, control diet; FPG, fasting plasma glucose; FPI, fasting plasma insulin; HbA1c, glycated haemoglobin A1c; HFD, high-fat diet; HFSD, high-fat-high-sucrose diet; HOMA %β, homeostasis model assessment of β-cell function; HOMA%S, homeostasis model assessment of insulin sensitivity; HOMA-IR, homeostasis model assessment of insulin resistance; NS, non-significant.
P < 0.05 compared to CD.
P < 0.01 compared to CD.
P < 0.001 compared to CD.
P < 0.05 compared to HFD.
P < 0.001 compared to HFD.
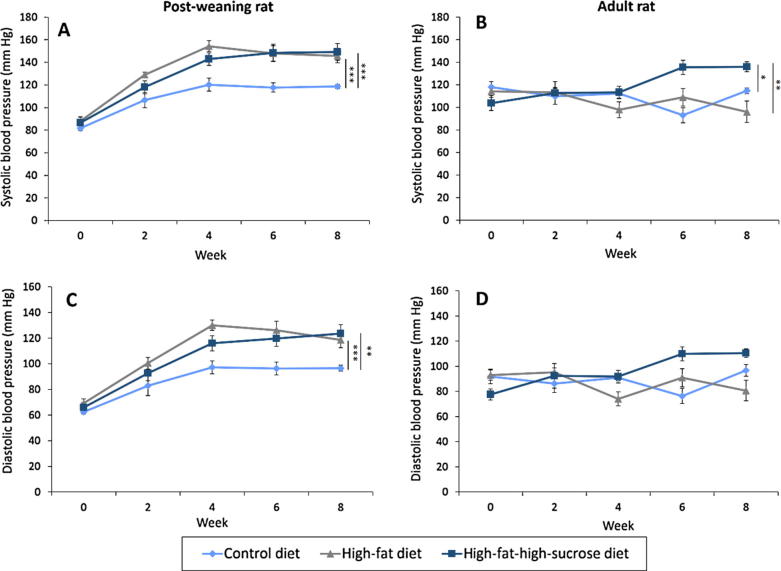
High calorie diets induced systolic and diastolic hypertension
Like the initial body weight, the starting systolic and diastolic blood pressure levels of the rats within the same age groups were comparable. Both the HFD and HFSD triggered systolic and diastolic hypertension (Fig.2A, C) in the post-weaning rats. At the end of the experiment, the systolic blood pressure levels were 146 ± 6 mmHg and 149 ± 7 mmHg in the HFD- and HFSD-treated post-weaning rats compared to 119 ± 2 mmHg in those on CD. Conversely, the diastolic blood pressure levels increased from 97 ± 2 mmHg in the CD group to 119 ± 6 mmHg and 124 ± 7 mmHg in the HFD and HFSD groups, respectively. The escalated blood pressure took place from week 4 onwards.
Fig. 2.
Systolic and diastolic blood pressure of the post-weaning (A and C) and adult (B and D) rats on different diets over eight weeks. Error bars indicate SEM. The sample size was n = 6–7 per group. * indicates P < 0.05, ** indicates P < 0.01 and *** indicates P < 0.001 between groups.
In contrast, the hypertensive effect of the high calorie diets was less prominent among the adult rats. Such an effect was observed only in the systolic blood pressure of those on HFSD whereby the blood pressure was elevated from 115 ± 3 mmHg in the CD group to 136 ± 4 mmHg in the HFSD group at the end of the experiment (Fig.2B). The onset of the systolic hypertension occurred in week 6 which was slower compared to the post-weaning rats. In addition, no difference in the diastolic blood pressure of adult rats was detected (Fig.2D). The results show that younger rats are more vulnerable to high-calorie diet-induced hypertension.
High-fat diet caused hyperglycaemia, dyslipidaemia and hepatic steatosis in the post-weaning rats
Based on Table 3, the food consumed per day was significantly lower in HFD- and HFSD-treated groups (P < 0.001). This was to compensate for the increased calorie content of the diets. The post-weaning rats given HFD became hyperglycaemic at the end of the experiment as demonstrated by the elevated fasting plasma glucose level compared to CD- and HFSD-treated rats. This is further supported by a significant increase in HbA1c % which is suggestive of chronic hyperglycaemia. The contributing factor could be an impairment in β-cell function as indicated by a 62% reduction in HOMA %β compared to the CD-treated rats. However, prolonged hyperglycaemia did not lead to increased circulating AGE level in the post-weaning rats on HFD. More surprisingly, compared to CD, HFSD significantly reduced plasma AGEs by 29% in the post-weaning rats.
On the other hand, the carbohydrate metabolism of the adult rats was mildly affected by the high calorie feeding because no difference was found between groups in terms of the glycaemic indices. Similar AGE-lowering effect of HFSD was also observed in the adult rats. Between different age groups, the post-weaning rats had significantly higher fasting plasma glucose level of 5.96 ± 0.11 mmol/L in comparison to 5.23 ± 0.10 mmol/L in the adult rats, suggesting that older rats are more resistant to metabolic derangement. However, the older rats had higher circulating AGEs (93.62 ± 8.16 μg/mL) compared to the young rats (61.52 ± 8.58 μg/mL) (P = 0.011), denoting a positive correlation between age and AGE accumulation.
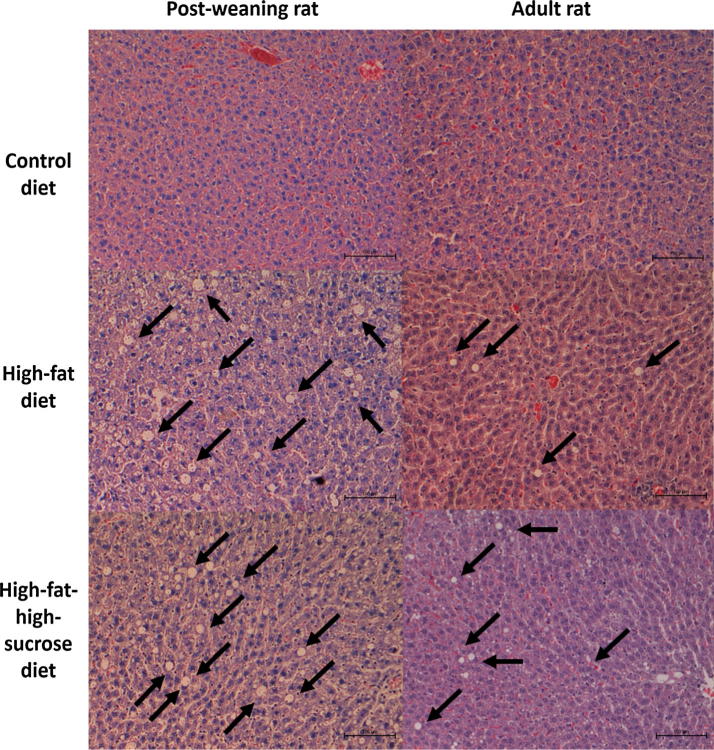
The lipid profile is outlined in Table 4. Basically, post-weaning rats on HFD also developed hypertriglyceridaemia and elevated non-HDL cholesterol level at the end of experiment. Such adverse effects were not observed in adult rats on the high-calorie diets, implying that HFD is more effective in disrupting glucose and lipid homeostasis in the young rats. The dyslipidaemic condition of the post-weaning rats on HFD and HFSD is further augmented by the increased hepatic lipid deposition shown in Fig. 3. Even though the extent of fatty liver in adult rats on HFSD also increased by almost 100%, the difference did not reach statistical significance (P = 0.085) when compared to CD.
Table 4.
Effects of HFD and HFSD on the lipid profile and hepatic lipid deposition of post-weaning and adult rats after eight-week long treatment.
| Parameter | Post-weaning rat |
Adult rat |
Post-weaning rat vs. Adult rat | ||||
|---|---|---|---|---|---|---|---|
| CD | HFD | HFSD | CD | HFD | HFSD | ||
| Triglycerides (mmol/L) | 1.08 ± 0.07 | 1.88 ± 0.29* | 1.15 ± 0.11 | 1.30 ± 0.19 | 0.95 ± 0.16 | 1.59 ± 0.17 | NS (P = 0.559) |
| Total cholesterol (mmol/L) | 1.80 ± 0.22 | 2.16 ± 0.20 | 1.59 ± 0.10 | 2.11 ± 0.21 | 1.79 ± 0.22 | 1.32 ± 0.07* | NS (P = 0.493) |
| HDL-cholesterol (mmol/L) | 1.54 ± 0.18 | 1.17 ± 0.08 | 0.94 ± 0.09* | 1.23 ± 0.21 | 1.12 ± 0.14 | 0.51 ± 0.07**,†† | Post-weaning > Adult (P = 0.025) |
| Non-HDL-cholesterol (mmol/L) | 0.55 ± 0.10 | 1.12 ± 0.32* | 0.71 ± 0.07 | 0.76 ± 0.05 | 0.58 ± 0.07 | 0.94 ± 0.09 | NS (P = 0.807) |
| NEFA (mmol/L) | 0.57 ± 0.05 | 0.38 ± 0.04 | 0.46 ± 0.07 | 0.66 ± 0.12 | 0.45 ± 0.07 | 0.42 ± 0.03* | NS (P = 0.505) |
| Hepatic lipid deposition (%) | 1.95 ± 0.33 | 4.26 ± 0.33*** | 4.14 ± 0.64** | 1.29 ± 0.17 | 1.80 ± 0.17 | 2.53 ± 0.33 | Post-weaning > Adult (P = 0.000) |
Values are expressed as mean ± SEM. The sample size was n = 6–7 per group.
CD, control diet; HFD, high-fat diet; HFSD, high-fat-high-sucrose diet; NEFA, non-esterified fatty acids; NS, non-significant.
P < 0.05 compared to CD.
P < 0.01 compared to CD.
P < 0.001 compared to CD.
P < 0.01 compared to HFD.
Fig. 3.
Representative H&E-stained liver sections (x200 magnification) of the post-weaning and adult rats given different diets for eight weeks. The black arrows indicate the lipid deposition sites in the liver tissues. The sample size was n = 6–7 per group.
Unexpectedly, inter-diet group comparison shows that HFSD significantly reduced circulating TC compared to the CD- (P = 0.043) and HFD-treated rats (P = 0.032). Similarly, when the rats were fed with HFSD, HDL-cholesterol was also reduced in comparison to the CD- (P < 0.001) and HFD-treated rats (P = 0.016). Moreover, both high-calorie diets significantly lowered FFA level (±SEM) to 0.41 ± 0.05 mmol/L and 0.44 ± 0.05 mmol/L in HFD- (P = 0.026) and HFSD-treated rats (P = 0.047), respectively, compared to 0.61 ± 0.05 mmol/L in the control group.
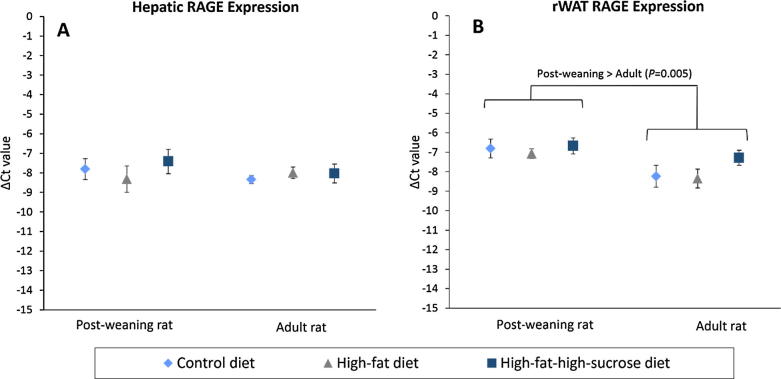
Overexpression of rWAT RAGE and hepatic PPARs in the post-weaning rats
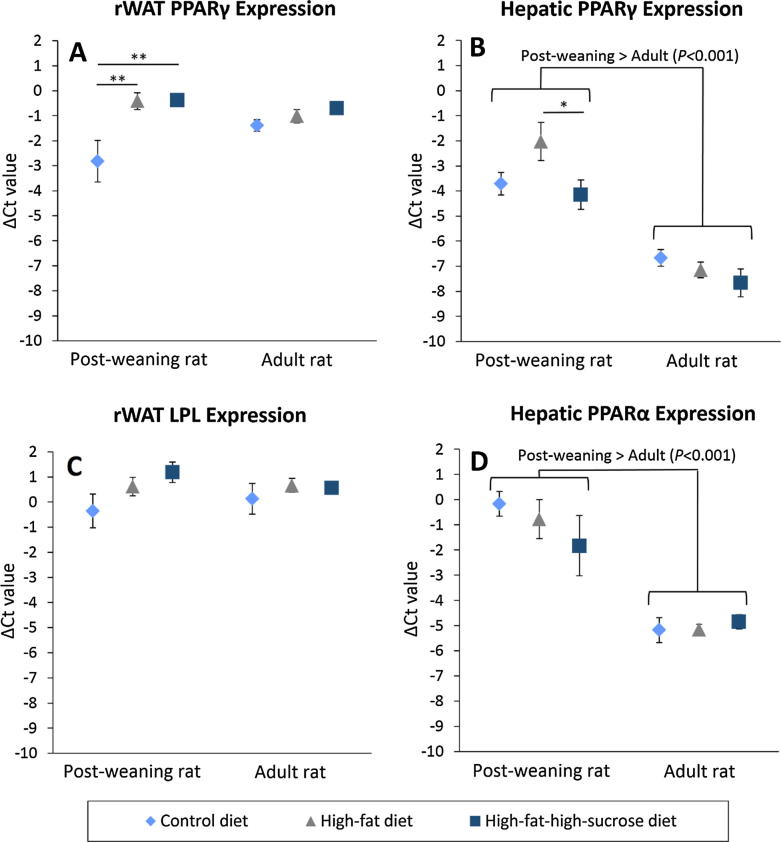
No difference in RAGE expression in the liver and rWAT was found (Fig.4A, B). Nonetheless, rWAT RAGE expression of the post-weaning rats was upregulated twofold compared to the adult rats (P = .005). On the other hand, the post-weaning rats given HFD and HFSD significantly overexpressed PPARγ by more than fivefold in the rWAT compared to those on CD (Fig.5A), but LPL expression remained unchanged (Fig.5C). In the liver, PPARγ expression of the HFD-treated post-weaning rats also upregulated by 220% and 333% compared to the CD- and HFSD-treated rats. Additionally, hepatic lipid metabolism of the post-weaning rats was much more active than that of the adult rats because the PPARα and γ expression was increased by more than 14- and 17-fold, respectively in the young rats (Fig.5B, D).
Fig. 4.
Normalized Ct values (ΔCt) of RAGE expression in the liver (A) and rWAT (B) of the post-weaning and adult rats on different diets at the end of eight-week treatment. HPRT1 and β-actin were used as reference genes. Error bars indicate SEM. The sample size was 6–7 per group. RAGE, receptor for advanced glycation end products; rWAT, retroperitoneal white adipose tissue.
Fig. 5.
Normalized Ct values (ΔCt) of PPARγ in the rWAT (A) and liver (B), LPL in the rWAT (C) and PPARα in the liver (D) of post-weaning and adult rats on different diets at the end of eight-week treatment. HPRT1 and β-actin were used as reference genes. Error bars indicate SEM. The sample size was 6–7 per group. * indicates P < 0.05, ** indicates P < 0.01 between groups. LPL, lipoprotein lipase; PPARα, peroxisome proliferator-activated receptor α; PPARγ, peroxisome proliferator-activated receptor γ; rWAT, retroperitoneal white adipose tissue.
Discussion
Diets enriched with lipids and/or carbohydrates have been widely used to induce MetS in rodents with varying degrees of success at inducing the key symptoms, namely central obesity, hypertension, hyperglycaemia and dyslipidaemia [7], [8]. In this study, we demonstrate that the interplay between the developmental stage of the rats and the types of diet plays a crucial role in disease induction. Three-week old post-weaning rats given HFD for eight weeks developed all the phenotypes of MetS whereas adult rats on HFSD merely became obese and hypertensive, making the former a more time-saving and cost-effective MetS model.
Although diet-induced obese model using post-weaning rats has been developed [20], comparative study between post-weaning rats and the commonly used young adult rats is rather limited. This study shows that post-weaning rats gained weight more rapidly to become centrally obese. This is accompanied by elevated systolic and diastolic blood pressure. In fact, increased susceptibility to hypertension upon HFD-feeding in younger rats has also been described previously [21]. Although the adult rats given HFSD diet also developed these features, the effects were relatively less severe (slower weight gain, lower rWAT mass and delayed systolic hypertension onset). Furthermore, the post-weaning rats also developed impaired fasting glucose which could be caused by impaired β-cell function as indicated by low HOMA β%. In contrast, Pagliassotti and coworkers reported that older rats have reduced glucose disposal rate in the skeletal muscle and adipose tissues, thus, they are more susceptible to glucose intolerance due to insulin resistance [22]. This suggests that the development stage may affect the pathophysiology of glucose dysregulation. Even though insulin resistance is a major pathophysiology of glucose metabolic dysregulation in MetS, reduced β-cell function has also been identified as an important contributing factor, particularly in obese children and adolescents [23], [24]. As such, our model could potentially be used to mimic childhood and adolescent MetS.
The overexpression of hepatic PPARα and γ, which is consistently found in obese murine models [25], was detected in the young rats. To elucidate, PPARα is primarily responsible for transcriptional regulation of the genes for fatty acid uptake and oxidation in the liver [26] whereas hepatic PPARγ is usually expressed at low levels but is markedly upregulated when there is an increased lipid flux into the liver [27]. This leads to an increased lipid accumulation in the liver which is also known as hepatic steatosis as observed in the post-weaning rats on HFD and HFSD in the present study. Increased liver steatosis could serve to modulate the triglyceride level and prevent ectopic fat deposition at other tissues [28] which may explain why the post-weaning rats on HFSD were able to maintain a normal lipid profile despite the severe hepatic steatosis. Normal hepatic PPARγ expression among the adult rats also suggests a state of lipid homeostasis, but the same cannot be said for the HFD-treated post-weaning rats.
In this study, post-weaning rats were selected to compare to adult rats because the young rats could be used to mimic childhood condition. Furthermore, the eight-week treatment covered Day 21 to Day 77 of their postnatal life which corresponds to the childhood, adolescence and early adulthood in human beings [29] and so, the onset of metabolic dysfunction in the post-weaning rats could potentially be used to model paediatric MetS. Additionally, considering their rapid growth and high basal metabolic rate, it is speculated that younger rats may be more sensitive to nutritional cues of the high-calorie diets. Indeed, we observed an immerse difference in term of the effectiveness of MetS induction between different developmental stages which strongly suggests an inherent metabolic regulatory difference. This may be linked to programmed MetS. Principally, the concept of programmed MetS suggests that nutritional insults (eg. starvation and over-nutrition) during gestation [30] or immediate postnatal [31] may induce epigenetic modifications of key metabolic regulatory genes that substantially enhance the risk of metabolic diseases. Certain studies have demonstrated that the window for metabolic programming could extend into early childhood [32], [33]. In this context, PPARs is known to be a key player in metabolic programming because a vast array of PPAR target genes, including Hdac and Sirt7 which are both epigenetic regulators, are well-implicated in programmed metabolic syndrome [34]. This is in line with the expression assays of the present study which show the significant upregulation of PPARs in the liver of post-weaning rats but not the adult rats. Hence, programmed MetS could lend support to explain the susceptibility of post-weaning rats on HFD to the disorder, but further investigation on the epigenetic modification is warranted to explore the possible mechanism.
Apart from that, an age-dependent increase in the circulating AGE level was observed in spite of the elevated glucose level among the post-weaning rats, indicating a predominant role of ageing to AGE aggregation [35]. However, RAGE expression in the rWAT was discordant with AGE level. Such an upregulation of RAGE in the rWAT of post-weaning rats could be driven by other non-glycated peptide ligands. Studies have demonstrated the integral role of adipocyte RAGE in regulation of adiposity and atherosclerotic risk [36], [37]. Therefore, enhanced adipocyte RAGE expression of the post-weaning rats could add another piece to the puzzle of their predisposition to MetS.
The daily calorie intake of the rats was relatively constant regardless of the age groups and the types of diet provided. This finding is consistent with previous study [38] and suggests that the observed metabolic perturbations are dependent on the components of the high-calorie diets, namely sucrose and lipids. The rats also showed a striking preference for carbohydrates over the lipids, as evidenced by the heavy reliance on the sucrose water in the HFSD group. As a result, the consumption of other macro- and micronutrients, most notably proteins, was substantially diluted (20% kcal in CD and HFD vs. 12% kcal in HFSD). The reduced protein intake may account for the lowering effect of cholesterol, FFA and AGE in the HFSD group simply because these parameters are dependent on the amount of transport proteins in the circulation.
Surprisingly, FFA level was lowered in both post-weaning and adult rats on high-calorie diets. This is consistent with a recent study which showed that FFA was significantly lowered in obese insulin resistant rodents [39]. This finding challenges the notion about the devastating insulin-desensitizing effect of FFA in obesity and MetS. In fact, Karpe et al. reviewed a number of clinical data and concluded that the causal relationship of increased systemic FFA and insulin resistance may not always be true [40]. Considering the emerging counter evidence, it may be wise to re-examined the role of circulating FFA in the pathogenesis of various metabolic disorders.
In the present study, even though the HFSD-treated rats consumed similar amount of calories, the protein intake was much less because of their dependence on sucrose-enriched water. This might lead to unintended metabolic changes due to protein malnutrition. Future studies should make sure the protein intake is comparable to the control group. Although the post-weaning rats seemed to be more vulnerable to MetS upon high-fat feeding, the impact of developmental stage cannot the fully elaborated without the rats of different ages namely, young, adult, middle- and old-aged. Further studies should also focus on the oxidative stress level, proinflammatory response, cytokine profile, sex and stress hormones so as to explore the possible mechanisms of the observed vulnerability.
Conclusions
To conclude, compared to the young adult rats which are commonly used in MetS study, the post-weaning rats were more vulnerable to metabolic dysfunctions. Notably, the post-weaning rats on HFD for eight weeks exhibited all key manifestations of MetS, including central obesity, systolic and diastolic hypertension, impaired fasting glucose, hypertriglyceridaemia, and elevated non-HDL cholesterol level. The expression of RAGE and PPARs were upregulated in the post-weaning rats compared to the adult rats, more so for those on HFD, leading to the postulation that nutritional insults during early childhood may have detrimental long-lasting effects on metabolism. Male, post-weaning rats on HFD will be a useful MetS model. However, the selective use of male post-weaning rats in this study limits the generalisation of the results to female rats. Thus, further studies should attempt to clarify the susceptibility of female post-weaning rats to MetS besides examining the pathophysiology of the model to explore the potential linkages with childhood obesity and MetS.
Acknowledgements
The work was supported by the Ministry of Science, Technology and Innovation, Malaysia (grant no.: 02-02-10-SF0249); and the School of Science, Monash University Malaysia. We would like to acknowledge Mr. Andrew Leong Kum Loong and Mr. Zulkhaili Zainal Abidin for their assistance in animal handling. We would also like to thank Mr. Derick Sim Kai Cheng and Ms. Lee Zhi Wei for their contribution to the project.
Acknowledgments
Conflict of interest
The authors have declared no conflict of interest.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Alberti K.G.M.M., Eckel R.H., Grundy S.M., Zimmet P.Z., Cleeman J.I., Donato K.A. Harmonizing the metabolic syndrome. A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 2.Ford E.S. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. 2005;28:1769–1778. doi: 10.2337/diacare.28.7.1769. [DOI] [PubMed] [Google Scholar]
- 3.Baranova A., Tran T.P., Birerdinc A., Younossi Z.M. Systematic review: association of polycystic ovary syndrome with metabolic syndrome and non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2011;33:801–814. doi: 10.1111/j.1365-2036.2011.04579.x. [DOI] [PubMed] [Google Scholar]
- 4.Esposito K., Chiodini P., Colao A., Lenzi A., Giugliano D. Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care. 2012;35:2402–2411. doi: 10.2337/dc12-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frisardi V., Solfrizzi V., Seripa D., Capurso C., Santamato A., Sancarlo D. Metabolic-cognitive syndrome: a cross-talk between metabolic syndrome and Alzheimer's disease. Ageing Res Rev. 2010;9:399–417. doi: 10.1016/j.arr.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 6.O'Neill S., O'Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes Rev. 2015;16:1–12. doi: 10.1111/obr.12229. [DOI] [PubMed] [Google Scholar]
- 7.Panchal S.K., Brown L. Rodent models for metabolic syndrome research. J Biomed Biotechnol. 2011;2011:351982. doi: 10.1155/2011/351982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aydin S., Aksoy A., Aydin S., Kalayci M., Yilmaz M., Kuloglu T. Today's and yesterday's of pathophysiology: Biochemistry of metabolic syndrome and animal models. Nutrition. 2014;30:1–9. doi: 10.1016/j.nut.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Zlobine I., Gopal K., Ussher J.R. Lipotoxicity in obesity and diabetes-related cardiac dysfunction. Biochim Biophys Acta. 2016;1861:1555–1568. doi: 10.1016/j.bbalip.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 10.Lim J.S., Mietus-Snyder M., Valente A., Schwarz J.-M., Lustig R.H. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat Rev Gastroenterol Hepatol. 2010;7:251–264. doi: 10.1038/nrgastro.2010.41. [DOI] [PubMed] [Google Scholar]
- 11.Warden C.H., Fisler J.S. Comparisons of diets used in animal models of high-fat feeding. Cell Metab. 2008;7:277. doi: 10.1016/j.cmet.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benoit B., Plaisancié P., Awada M., Géloën A., Estienne M., Capel F. High-fat diet action on adiposity, inflammation, and insulin sensitivity depends on the control low-fat diet. Nutr Res. 2013;33:952–960. doi: 10.1016/j.nutres.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 13.Busserolles J., Mazur A., Gueux E., Rock E., Rayssiguier Y. Metabolic syndrome in the rat: females are protected against the pro-oxidant effect of a high sucrose diet. Exp Biol Med. 2002;227:837–842. doi: 10.1177/153537020222700918. [DOI] [PubMed] [Google Scholar]
- 14.Ghezzi A.C., Cambri L.T., Botezelli J.D., Ribeiro C., Dalia R.A., de Mello M.A.R. Metabolic syndrome markers in wistar rats of different ages. Diabetol Metab Syndr. 2012;4:16. doi: 10.1186/1758-5996-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuk J.L., Ardern C.I. Age and sex differences in the clustering of metabolic syndrome factors. Diabetes Care. 2010;33:2457–2461. doi: 10.2337/dc10-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reeves P.G. Components of the AIN-93 diets as improvements in the AIN-76A diet. J Nutr. 1997;127:838S–841S. doi: 10.1093/jn/127.5.838S. [DOI] [PubMed] [Google Scholar]
- 17.Levy J.C., Matthews D.R., Hermans M.P. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21:2191–2192. doi: 10.2337/diacare.21.12.2191. [DOI] [PubMed] [Google Scholar]
- 18.Svingen T., Letting H., Hadrup N., Hass U., Vinggaard A.M. Selection of reference genes for quantitative RT-PCR (RT-qPCR) analysis of rat tissues under physiological and toxicological conditions. PeerJ. 2015;3:e855. doi: 10.7717/peerj.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lalanza J.F., Caimari A., del Bas J.M., Torregrosa D., Cigarroa I., Pallàs M. Effects of a post-weaning cafeteria diet in young rats: metabolic syndrome, reduced activity and low anxiety-like behaviour. PLoS ONE. 2014;9:e85049. doi: 10.1371/journal.pone.0085049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erdos B., Kirichenko N., Whidden M., Basgut B., Woods M., Cudykier I. Effect of age on high-fat diet-induced hypertension. Am J Physiol Heart Circ Physiol. 2011;301 doi: 10.1152/ajpheart.01289.2010. H164–H72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pagliassotti M.J., Gayles E.C., Podolin D.A., Wei Y., Morin C.L. Developmental stage modifies diet-induced peripheral insulin resistance in rats. Am J Physiol Regul Integr Comp Physiol. 2000;278:R66–R73. doi: 10.1152/ajpregu.2000.278.1.R66. [DOI] [PubMed] [Google Scholar]
- 23.Weiss R., Caprio S., Trombetta M., Taksali S.E., Tamborlane W.V., Bonadonna R. Β-cell function across the spectrum of glucose tolerance in obese youth. Diabetes. 2005;54:1735–1743. doi: 10.2337/diabetes.54.6.1735. [DOI] [PubMed] [Google Scholar]
- 24.Weigensberg M.J., Ball G.D.C., Shaibi G.Q., Cruz M.L., Goran M.I. Decreased β-cell function in overweight Latino children with impaired fasting glucose. Diabetes Care. 2005;28:2519–2524. doi: 10.2337/diacare.28.10.2519. [DOI] [PubMed] [Google Scholar]
- 25.Memon R.A., Tecott L.H., Nonogaki K., Beigneux A., Moser A.H., Grunfeld C. Up-regulation of peroxisome proliferator-activated receptors (PPAR-α) and PPAR-γ messenger ribonucleic acid expression in the liver in murine obesity: Troglitazone induces expression of PPAR-γ-responsive adipose tissue-specific genes in the liver of obese diabetic mice. Endocrinology. 2000;141:4021–4031. doi: 10.1210/endo.141.11.7771. [DOI] [PubMed] [Google Scholar]
- 26.Leone T.C., Weinheimer C.J., Kelly D.P. A critical role for the peroxisome proliferator-activated receptor α (PPARα) in the cellular fasting response: The PPARα-null mouse as a model of fatty acid oxidation disorders. Proc Natl Acad Sci U S A. 1999;96:7473–7478. doi: 10.1073/pnas.96.13.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inoue M., Ohtake T., Motomura W., Takahashi N., Hosoki Y., Miyoshi S. Increased expression of PPARγ in high fat diet-induced liver steatosis in mice. Biochem Biophys Res Commun. 2005;336:215–222. doi: 10.1016/j.bbrc.2005.08.070. [DOI] [PubMed] [Google Scholar]
- 28.Gavrilova O., Haluzik M., Matsusue K., Cutson J.J., Johnson L., Dietz K.R. Liver peroxisome proliferator-activated receptor γ contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J Biol Chem. 2003;278:34268–34276. doi: 10.1074/jbc.M300043200. [DOI] [PubMed] [Google Scholar]
- 29.Sengupta P. The laboratory rat: relating its age with human's. Int J Prev Med. 2013;4:624–630. [PMC free article] [PubMed] [Google Scholar]
- 30.Symonds M.E., Sebert S.P., Hyatt M.A., Budge H. Nutritional programming of the metabolic syndrome. Nat Rev Endocrinol. 2009;5:604–610. doi: 10.1038/nrendo.2009.195. [DOI] [PubMed] [Google Scholar]
- 31.Patel M.S., Srinivasan M. Metabolic programming due to alterations in nutrition in the immediate postnatal period. J Nutr. 2010;140:658–661. doi: 10.3945/jn.109.110155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vickers M.H., Breier B.H., Cutfield W.S., Hofman P.L., Gluckman P.D. Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am J Physiol Endocrinol Metab. 2000;279 doi: 10.1152/ajpendo.2000.279.1.E83. E83–E7. [DOI] [PubMed] [Google Scholar]
- 33.Tain Y.-L., Sheen J.-M., Yu H.-R., Chen C.-C., Tiao M.-M., Hsu C.-N. Maternal melatonin therapy rescues prenatal dexamethasone and postnatal high-fat diet induced programmed hypertension in male rat offspring. Front Physiol. 2015;6 doi: 10.3389/fphys.2015.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tain Y.-L., Hsu C.-N., Chan J. PPARs link early life nutritional insults to later programmed hypertension and metabolic syndrome. Int J Mol Sci. 2016;17:20. doi: 10.3390/ijms17010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schleicher E.D., Wagner E., Nerlich A.G. Increased accumulation of the glycoxidation product N(epsilon)-(carboxymethyl)lysine in human tissues in diabetes and aging. J Clin Invest. 1997;99:457–468. doi: 10.1172/JCI119180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ueno H., Koyama H., Shoji T., Monden M., Fukumoto S., Tanaka S. Receptor for advanced glycation end-products (RAGE) regulation of adiposity and adiponectin is associated with atherogenesis in apoE-deficient mouse. Atherosclerosis. 2010;211:431–436. doi: 10.1016/j.atherosclerosis.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 37.Monden M., Koyama H., Otsuka Y., Morioka T., Mori K., Shoji T. Receptor for advanced glycation end products regulates adipocyte hypertrophy and insulin sensitivity in mice. Diabetes. 2013;62:478–489. doi: 10.2337/db11-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Warwick Z.S., Schiffman S.S. Role of dietary fat in calorie intake and weight gain. Neurosci Biobehav Rev. 1992;16:585–596. doi: 10.1016/s0149-7634(05)80198-8. [DOI] [PubMed] [Google Scholar]
- 39.Jiang J., Wu Y., Wang X., Lu L., Wang L., Zhang B. Blood free fatty acids were not increased in high-fat diet induced obese insulin-resistant animals. Obes Res Clin Pract. 2016;10:207–210. doi: 10.1016/j.orcp.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 40.Karpe F., Dickmann J.R., Frayn K.N. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes. 2011;60:2441–2449. doi: 10.2337/db11-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]