Abstract
Direct international comparisons which aim to understand how factors associated with human papillomavirus (HPV) vaccine initiation and attitudes towards the HPV vaccination in parents differ are scarce. Parents (n = 179) of daughters aged 9–17 years in the US, UK and Australia completed an online survey in 2011 with questions measuring daughters' HPV vaccination status, HPV knowledge, HPV vaccination knowledge, and statements assessing attitude towards the HPV vaccine. The strongest factor associated with vaccination status across all countries was parental HPV knowledge (p < 0.001). Parents with both very low and very high knowledge scores were less likely to have vaccinated their daughters. Parents with higher HPV vaccination knowledge scores intended to vaccinate their daughters (if not already vaccinated) for protective reasons (p < 0.001), while those whose daughters were already vaccinated understood that vaccination protection was not 100% and that their daughters may still be at risk of getting HPV (p < 0.05). Compared to the UK and Australia, a higher proportion of parents with unvaccinated daughters from the US were worried about the side-effects of the HPV vaccination (US: 60.5%, UK: 36.4%, AUS: 15.4%; p < 0.05), believed that getting the vaccination might be a hassle (US: 21.1%, UK: 0%, AUS: 7.7%; p < 0.05), and that the vaccine was too new (US: 44.7%, UK: 22.7%, AUS: 7.7%; p < 0.05). This study adds to the understanding of how parents may influence vaccination uptake by demonstrating the effect of knowledge and the parental attitudes towards HPV vaccination across three countries.
Abbreviations: HPV, Human papillomavirus; US, United States; UK, United Kingdom; AUS, Australia
Keywords: Human papillomavirus, Vaccination, Attitudes, Knowledge, Parents, International
Highlights
-
•
The strongest predictor of HPV vaccination status is parents' HPV knowledge.
-
•
Very low and very high parental HPV knowledge scores were associated with decreased vaccination uptake.
-
•
Parents from the US are more worried about HPV vaccination side-effects than parents from the UK or Australia.
1. Introduction
High-risk types of human papillomavirus (HPV), the most common sexually transmitted infection (STI) internationally, can lead to development of cervical cancer as well as genital warts, anal, penile and oropharyngeal cancers (Forman et al., 2012). In 2006, the United States Food and Drug Administration (FDA) approved the quadrivalent vaccine for the prevention of HPV types 6, 11, 16 and 18 (U.S. Food and Drug Administration, 2006) associated with over of 70% of all cervical cancer cases and 90% of genital warts cases (Munoz et al., 2004). In 2008, the FDA further approved the bivalent HPV vaccination which prevents HPV types 16 and 18 protecting against cervical cancer but not strains of HPV that cause genital warts (U.S. Food and Drug Administration, 2006, U.S. Food and Drug Administration, 2009a). Recently, a nonavalent vaccine has been licensed for use in the European Union and the United States (US), providing protection against five additional high-risk types of HPV (Forster & Waller, 2016). Widespread use of this vaccine has not yet begun in the United Kingdom (UK), however, it is currently the only vaccine being administered in the US. Now, almost all high-income countries have one of the HPV vaccines available to girls and young women, although the choice of vaccine, the cohorts it is offered to, the way it is administered and the cost, varies. In the US, the HPV vaccine is predominantly available through physicians' clinics and medical centres, while in the UK and Australia, publically funded school-based programs offer the vaccine free to girls age 12–13. In 2007, Australia was the first country internationally to launch publically funded school-based programs, with the UK launching their program a year later in 2008. Australia also had catch-up programs targeting young women in schools and the community, and the UK had a similar catch-up program from 2008 to 2010.
Given that the primary target population for HPV vaccination programs is girls aged 9–13 years, typically before the initiation of sexual activity (World Health Organisation, 2013), parental knowledge and attitudes play an important role in the success of vaccination as consent is usually required for their adolescent children to be vaccinated. Research aimed at understanding HPV vaccine uptake has demonstrated that uptake of the HPV vaccine is generally high, however parents and girls often have insufficient knowledge and understanding about and have varying attitudes towards vaccination (Hendry et al., 2013). Alongside this, several studies conducted across different settings have aimed to examine factors influencing HPV vaccine uptake. Findings from these studies are wide-ranging, however, parental intentions have been shown to consistently be a strong predictor of their children's HPV vaccine uptake (Alberts et al., 2017, Mupandawana and Cross, 2016).
The understanding of parental psychosocial determinants that influence HPV vaccine initiation is a growing area of research (Allen et al., 2010, Krawczyk et al., 2015a, Krawczyk et al., 2015b, Trim et al., 2012), although no studies have directly compared countries that use different methods of administering the vaccine, such as school-based or non-school-based programs. Using online survey data collected across three countries, this study examined parents of 9–17 year-old girls in the US, UK and Australia to identify country-specific factors which are associated with their daughters' vaccination status (HPV vaccine initiation). It also compared attitude differences between countries and by HPV vaccination knowledge by using identical knowledge and attitude variables in order to help better identify how parents' attitudes and knowledge play a role in vaccination participation internationally.
2. Material and methods
2.1. Participants
Participants (n = 12,259) from the US, UK and Australia were directed to a web-based survey by Survey Sampling International (SSI). SSI has existing online panels of participants who sign up to take part in market research for points which go towards a small reward (e.g. coupons and discounts). Quotas were set to ensure adequate representation of different age-groups and genders. The initial target sample for the survey was 2400 participants aged 18–70 years with approximately 800 participants from each country, including an equal number of men and women. Participants meeting these criteria were recruited to the survey. Participants were asked additional demographic questions including whether they had a daughter aged 9–17 years of age (the HPV vaccination age-range). From this information, survey data was analysed from participants who indicated they had at least one daughter in the HPV vaccination age-range. Ethical approval was obtained from human research ethics committees at Indiana University, USA, University College London, UK and the University of Sydney, Australia.
2.2. Measures
Participants were given information which included the purpose of the study, confidentiality and anonymity information and contact details for researchers in their country. Initially participants were asked their age, gender and country of residence to determine if they were eligible to take part in the survey, based on the quota of n = 400 men and n = 400 women from each country. Before participants began the questions on HPV knowledge they were asked ‘how many daughters do you have between the ages of 9 and 17’. If participants had a daughter or daughters in this age-range they were asked for their daughters' specific age(s) and how many of those had received at least one dose of the HPV vaccine. If at least one of the participant's daughters had been vaccinated, the parent was classified as “vaccinated” for the outcome variable. All participants were then asked questions on their knowledge of HPV (16 questions) and HPV vaccination (7 questions), which were later validated (Waller et al., 2013). Response options for the two sets of knowledge questions were True/False/Don't know, and data on knowledge of HPV, HPV vaccination and HPV testing were analysed and results published (Dodd et al., 2014, Marlow et al., 2013).
If participants answered that they had a daughter in the vaccination age-range they were further directed to a set of purposely designed attitude statements on HPV and HPV vaccination (Supplementary Table 1) which were derived from a UK survey (Marlow et al., 2007). Statements were directed specifically to either participants with at least one unvaccinated daughter eligible for HPV vaccination (14 attitude statements) or participants with at least one daughter who had received one or more doses of vaccine (8 attitude statements), with both sets of parents receiving the same eight attitude statements (with the wording only differing slightly based on context). Parents with at least one unvaccinated daughter were given an additional six attitude statements. Response options for the attitude statements were given as a 5-point Likert scale (Strongly disagree, Disagree, Neither disagree nor agree, Agree, Strongly agree). Data analysis on attitudes to HPV and to HPV vaccination was performed on the responses of participants who indicated that they had a daughter who was of vaccination age.
Participants were removed from the final analysis if they did not know whether their daughter had received the HPV vaccination, if they had never heard of the HPV vaccination or if they did not answer any of the parental attitude statements on HPV or the HPV vaccination (n = 146).
2.3. Statistical analysis
Responses to attitude items were collapsed into three categories: Disagree (Strongly disagree and Disagree), Neither disagree nor agree, and Agree (Agree and Strongly agree). Parental HPV vaccination knowledge scores (ranging from 0 to 7) were combined into three categories: low (0–2 correct responses), medium (3–4), and high (5 +). Chi-square tests were conducted initially to investigate the association between knowledge, attitudes and daughters' vaccination status.
The association between the proportion with daughters “not vaccinated” and the demographic, knowledge (as a continuous measure) and attitude variables were investigated using logistic regression with odds ratios reported from these models. Initially all variables were fitted univariately with the outcome and then a multivariable model was constructed. We decided a priori on the most important variables to be included in the model and included all the attitude variables regardless of significance because of the small sample size. For the model building, all demographic factors were fitted and then backwards elimination applied. The knowledge measures were then included and backwards elimination used. Finally, all attitude variables were added to produce the final model. The attitude regarding genital warts was only measured in two countries so it was fitted separately where the data was available and adjusted for all factors in the final model. This is because at the time of data collection the US and Australia offered Gardasil which protects against genital warts and the UK offered Cervarix which did not protect against genital warts. Fractional polynomials were used to assess the linearity of the association between the continuous knowledge scores and vaccination status. Likelihood ratio p-values were used to assess statistical significance with p < 0.05 considered significant.
Data were analysed using SPSS V.21 (IBM, Armonk, NY). The logistic regression models were conducted in Stata 14 (StataCorp. 2015. Stata Statistical Software: Release 14. College Station).
3. Results
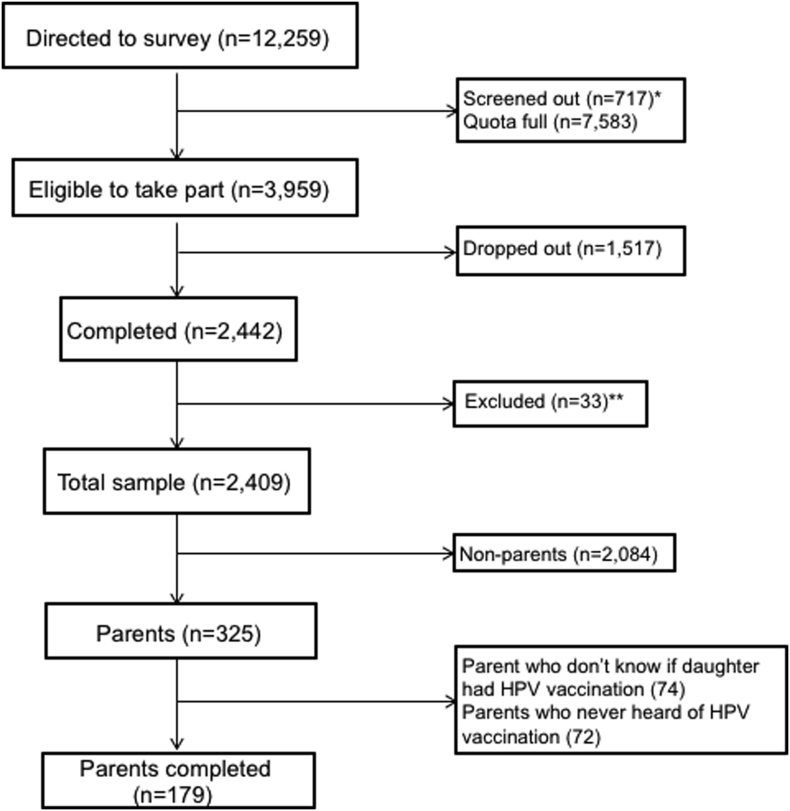
The survey had a 62% response rate, as a total of 12,259 men and women were directed to the HPV survey, 3959 were deemed eligible and invited to take part, and 2442 participants completed the survey. From that sample, 325 participants identified themselves as parents of daughters aged 9–17 years. Parents who did not know their daughters' vaccination status, had never heard of HPV or did not answer any questions or attitude statements were excluded, leaving 179 parents in the analysed sample (Fig. 1). Participant characteristics are shown in Table 1. Overall, 59.2% of parents reported that their daughter had received the HPV vaccine.
Fig. 1.
Participant recruitment.
⁎Participants outside the age range 18–70 years.
⁎⁎Data involved inconsistencies therefore survey may not have been completed properly.
Table 1.
Participant characteristics (n = 179).
| Characteristic | USA (n = 67) | UK (n = 59) | Australia (n = 53) |
|---|---|---|---|
| Age [mean (SD)] | 37.8 (9.97) | 39.4 (8.72) | 42.5 (8.92) |
| Gender [n (%)] | |||
| Men | 21 (31.3) | 21 (35.6) | 24 (45.3) |
| Women | 46 (68.7) | 38 (64.4) | 29 (54.7) |
| Daughter's vaccination status [n (%)] | |||
| Vaccinated | 29 (43.3) | 37 (62.7) | 40 (75.5) |
| Not vaccinated | 38 (56.7) | 22 (37.3) | 13 (24.5) |
| Ethnicitya [n (%)] | |||
| Majority | 50 (74.6) | 50 (84.7) | 42 (79.2) |
| Minority | 17 (25.4) | 9 (15.3) | 11 (20.8) |
| Educationb [n (%)] | |||
| High | 49 (73.1) | 29 (49.2) | 19 (35.8) |
| Low | 18 (26.9) | 30 (50.8) | 34 (64.2) |
| Relationship status [n (%)] | |||
| Married/partner | 49 (73.1) | 46 (78.0) | 43 (81.1) |
| Dating/single | 18 (26.9) | 13 (22.0) | 10 (18.9) |
| Religious engagement [n (%)] | |||
| Often | 34 (50.7) | 13 (22.0) | 12 (22.6) |
| Sometimes | 20 (29.9) | 8 (13.6) | 7 (13.2) |
| Rarely or never | 13 (19.4) | 38 (64.4) | 34 (64.2) |
| Average HPV knowledge score [mean (SD)]c | 9.75 (4.02) | 9.25 (3.43) | 9.47 (2.99) |
| Average HPV vaccination knowledge score [mean (SD)]d | 4.18 (1.72) | 4.10 (1.64) | 4.34 (1.07) |
Majority in USA = white non-Hispanic, UK = white British, AUS = white Australian.
Education was coded as follows: High: college graduate/graduate school, some college/associate degree (USA), degree/post-graduate degree, vocational/A-levels/other qualification < degree (UK), any university education, vocational qualification (AUS); Low: high school, CED or below (USA), no formal education/GCSEs (UK), no formal education/high school (AUS).
HPV knowledge scores ranged from 0 to 16 and was measured on the number of correct answers out of 16 questions.
HPV vaccination knowledge score ranged from 0 to 7 and was measured on the number of correct answers out of 7.
3.1. Factors associated with non-vaccinated status across the entire sample
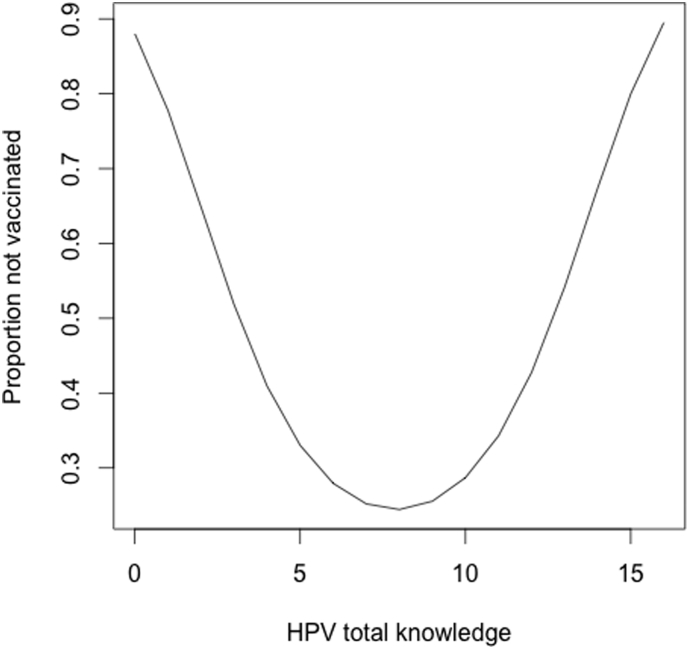
The strongest factor associated with daughters' vaccination status across the entire sample was parents' HPV knowledge (p < 0.001; Table 2). As shown in Fig. 2, parents' HPV knowledge scores displayed a non-linear relationship; parents with low knowledge scores and parents with high knowledge scores were less likely to have vaccinated their daughters. HPV vaccination specific knowledge was also a significant univariate factors associated with of vaccination status (p < 0.05) and displayed a non-linear relationship; parents with lower levels of vaccination specific knowledge and very high levels of vaccination specific knowledge were also less likely to have vaccinated their daughters. Parents' demographic characteristics including their country of origin (OR = 2.204, 95% CI 1.07–4.50; p < 0.05) and gender (OR = 0.492 95% CI [0.259, 0.935], p < 0.05) were also factors associated with non-vaccination in our sample, with parents in the US and men (across all countries) being less likely to vaccinate their daughters.
Table 2.
Factors associated with non-vaccination of daughters (9–17 years) with HPV vaccine (n = 179).
| Factors | Agreement | No. of parents | Univariate model |
Multivariate model |
||
|---|---|---|---|---|---|---|
| Adjusted OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value | |||
| Demographics | ||||||
| Age | – | 179 | 1.000 (0.969, 1.032) | 0.9892 | ||
| Gender | 0.0278 | |||||
| Male | – | 66 | 0.492 (0.259, 0.935)⁎ | |||
| Female (ref) | 113 | 1 | ||||
| Country | 0.0012 | 0.1039 | ||||
| USA | 67 | 2.204 (1.077, 4.507)⁎ | 1.337 (0.567, 3.154) | |||
| Australia | – | 53 | 0.547 (0.241, 1.239) | 0.496 (0.191, 1.288) | ||
| UK (ref) | 59 | 1 | 1 | |||
| Qualifications | 0.0958 | |||||
| High school grad or below | – | 82 | 0.599 (0.327, 1.099) | |||
| Uni/college or above (ref) | 97 | 1 | ||||
| Ethnicity | 0.7329 | |||||
| Other | – | 37 | 1.136 (0.547, 2.362) | |||
| White (ref) | 142 | 1 | ||||
| Relationship | 0.5317 | |||||
| Single/dating | – | 41 | 0.796 (0.387, 1.634) | |||
| Partner/married (ref) | 138 | 1 | ||||
| Religious services attendance | 0.5291 | |||||
| Sometimes | – | 35 | 1.467 (0.660, 3.259) | |||
| Often | 59 | 1.372 (0.697, 2.703) | ||||
| Rarely or never (ref) | 85 | 1 | ||||
| Knowledge | ||||||
| HPV knowledge | – | 179 | < 0.001 | < 0.001 | ||
| Linear term | 0.454 (0.296, 0.695)⁎⁎ | 0.431 (0.259, 0.716)⁎⁎ | ||||
| Square term | 1.051 (1.026, 1.077)⁎⁎ | 1.055 (1.026, 1.086)⁎⁎ | ||||
| HPV vaccination knowledge | – | 179 | 0.0042 | |||
| Linear term | 2.813 (0.585, 13.515) | |||||
| Square term | 0.561 (0.331, 0.950)⁎ | |||||
| Cube term | 1.067 (1.015, 1.122)⁎ | |||||
| Attitudesa | ||||||
| Having the HPV vaccination might make girls more like to have sex | Disagree (ref) | 111 | 1 | 0.4775 | 1 | 0.3674 |
| Neither agree nor disagree | 42 | 1.167 (0.571, 2.387) | 1.144 (0.366, 3.576) | |||
| Agree | 26 | 0.628 (0.252, 1.567) | 0.417 (0.096, 1.812) | |||
| Girls who have the HPV vaccination might be more likely to have unprotected sex | Disagree (ref) | 84 | 1 | 0.8785 | 1 | 0.7052 |
| Neither agree nor disagree | 63 | 1.103 (0.569, 2.139) | 0.907 (0.332, 2.481) | |||
| Agree | 32 | 0.882 (0.382, 2.039) | 1.578 (0.375, 6.636) | |||
| Cervical cancer is not something I worry about for my daughter | Disagree (ref) | 88 | 1 | 0.626 | 1 | 0.9925 |
| Neither agree nor disagree | 55 | 0.776 (0.390, 1.543) | 0.964 (0.415, 2.240) | |||
| Agree | 36 | 0.710 (0.319, 1.580) | 0.938 (0.310, 2.839) | |||
| My daughter may one day be at risk of getting HPV | Disagree (ref) | 18 | 1 | 0.0951 | 1 | 0.0992 |
| Neither agree nor disagree | 47 | 0.478 (0.155, 1.477) | 0.912 (0.168, 4.965) | |||
| Agree | 114 | 1.048 (0.386, 2.850) | 2.454 (0.582, 10.357) | |||
| I am very worried about the side effects of the HPV vaccination for my daughter | Disagree (ref) | 52 | 1 | 0.1221 | 1 | 0.1406 |
| Neither agree nor disagree | 60 | 1.500 (0.685, 3.283) | 2.503 (0.915, 6.848) | |||
| Agree | 67 | 2.184 (1.022, 4.665) | 2.245 (0.833, 6.055) | |||
| It is like that my daughter will get HPV one day | Disagree (ref) | 61 | 1 | 0.9657 | 1 | 0.504 |
| Neither agree nor disagree | 92 | 1.013 (0.525, 1.956) | 1.227 (0.509, 2.959) | |||
| Agree | 26 | 0.900 (0.351, 2.305) | 0.627 (0.180, 2.181) | |||
| It is possible that my daughter may get HPV in the future | Disagree (ref) | 16 | 1 | 0.0502 | 1 | 0.104 |
| Neither agree nor disagree | 68 | 0.248 (0.077, 0.797) | 0.175 (0.030, 1.013) | |||
| Agree | 95 | 0.303 (0.097, 0.942) | 0.191 (0.036, 1.000) | |||
| Genital warts are not something I worry about for my daughterb | Disagree (ref) | 55 | 1 | 0.0913 | 1 | 0.105c |
| Neither agree nor disagree | 41 | 0.683 (0.302, 1.544) | 0.674 (0.187, 2.421) | |||
| Agree | 24 | 0.321 (0.111, 0.932) | 0.185 (0.036, 0.941)⁎ | |||
8 attitude statements that were given to both groups of parents.
Participants from the UK were not given this question, therefore 59 participants missing.
Estimated in the USA and AUS only in a separate model adjusting for all other variables from the multivariable model.
p < 0.05.
p < 0.01.
Fig. 2.
Estimated proportion of daughters not vaccinated as a quadratic function of parents' HPV knowledge.
3.2. Parental attitude differences by country
As shown in Table 3 (and in Supplementary Table 2), among parents with unvaccinated daughters, worry about the side-effects of the vaccine was significantly more prevalent in the US than the UK or Australia (US 60.5% agree, UK 36.4%, AUS 15.4%, p < 0.05). Non-vaccinating parents from the US were also more likely to agree that getting all 3 doses of the HPV vaccine would be a big hassle (US 21.1% agree, UK 0%, AUS 7.7%, p < 0.05) and that the HPV vaccine is so new that they would want to wait before deciding to get it for their daughter (US 44.7% agree, UK 22.7%, AUS 7.7%, p < 0.05).
Table 3.
Significant parental attitude differences by country.
| Unvaccinated daughters (n = 73) |
Vaccinated daughters (n = 106) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Attitudes | Country | Agreement (%)a |
p-Valueb | Attitudes | Country | Agreement (%)a |
p-Valueb | ||||
| Disagree | Neither agree nor disagree | Agree | Disagree | Neither agree nor disagree | Agree | ||||||
| I would be very worried about the side effects of the HPV vaccination for my daughter | USA | 13.2 | 26.3 | 60.5 | 0.029 | Now that my daughter has been vaccinated, genital warts are not something I worry about for her | USA | 37.9 | 20.7 | 41.4 | 0.025 |
| UK | 22.7 | 40.9 | 36.4 | UKc | – | – | – | ||||
| AUS | 46.2 | 38.5 | 15.4 | AUS | 40.0 | 45.0 | 15.0 | ||||
| Getting my daughter all 3 doses of the HPV vaccine would be a big hassle | USA | 57.9 | 21.1 | 21.1 | 0.042 | ||||||
| UK | 54.5 | 45.5 | 0.0 | ||||||||
| AUS | 76.9 | 15.4 | 7.7 | ||||||||
| The HPV vaccine is so new that I want to wait before deciding to get it for my daughter | USA | 26.3 | 28.9 | 44.7 | 0.014 | ||||||
| UK | 40.9 | 36.4 | 22.7 | ||||||||
| AUS | 76.9 | 15.4 | 7.7 | ||||||||
Row percentages for comparison across countries.
Pearson chi-square test used.
Participants from the UK were not given this question.
Parents with vaccinated daughters from the US were more likely than their Australian counterparts to agree that genital warts are not something they worry about for their daughters now that they are vaccinated (US 41.4%, AUS 15.0%, p < 0.05). UK parents were not given this question.
3.3. Association between HPV vaccination knowledge and parental attitudes
The significant associations between parental attitudes and HPV vaccination knowledge are presented in Table 4 (and in Supplementary Table 3). Compared to parents of unvaccinated daughters with low knowledge scores, those with higher knowledge scores were more likely to believe that they want to be on the safe side and vaccinate their daughters (low knowledge = 27.3%, medium = 44.1%, high = 74.1%; p ≤ 0.001). Compared to parents of vaccinated daughters with low knowledge scores, those with higher knowledge scores believed that their daughters may still one day be at risk of getting HPV (low knowledge = 22.2% vs medium = 61.7% vs high = 62.0%; p < 0.05) and that it is still possible for their daughters to get HPV in the future (low knowledge = 11.1% vs medium = 53.2% vs high = 62.0%; p < 0.05).
Table 4.
Significant parental attitude differences by HPV vaccination specific knowledge scores.
| Unvaccinated daughters (n = 73) |
Vaccinated daughters (n = 106) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Attitudes | HPV knowledge score | Agreement (%)a |
p-Valueb |
Attitudes | HPV knowledge score | Agreement (%)a |
p-Valueb | ||||
| Disagree | Neither agree nor disagree | Agree | Disagree | Neither agree nor disagree | Agree | ||||||
| I would want to be on the safe side and vaccinate my daughter | 0–2 | 0.0 | 72.7 | 27.3 | < 0.001 | Although my daughter has had the HPV vaccination, she may one day be at risk of getting HPV | 0–2 | 11.1 | 66.7 | 22.2 | 0.044 |
| 3–4 | 32.4 | 23.5 | 44.1 | 3–4 | 14.9 | 23.4 | 61.7 | ||||
| 5 + | 11.1 | 14.8 | 74.1 | 5 + | 4.0 | 34.0 | 62.0 | ||||
| Although my daughter has had the HPV vaccination, it is still possible that she may get HPV in the future | 0–2 | 11.1 | 77.8 | 11.1 | 0.023 | ||||||
| 3–4 | 8.5 | 38.3 | 53.2 | ||||||||
| 5 + | 0.0 | 38.0 | 62.0 | ||||||||
Row percentages reported for comparison across knowledge levels.
Pearson chi-square test used.
4. Discussion
Our study contributes to the literature by providing an international comparison of correlates of HPV vaccination uptake using identical knowledge and attitude variables across three countries. It found that shortly after introduction of the HPV vaccine, HPV knowledge level was associated with vaccination uptake, and parental attitudes differed by both country and level of HPV vaccination knowledge.
Across all three countries (US, UK and Australia), parents were more likely to have chosen not to vaccinate their daughters if they scored very low or very high on HPV knowledge and HPV vaccination specific knowledge questions. Parents of unvaccinated daughters with higher vaccination knowledge scores in our sample did, however, express attitudes favouring vaccinating their daughters in the future. Evidence varies across a number of countries and settings as to whether knowledge impacts vaccination uptake. Some studies report a correlation between higher levels of parental HPV knowledge and vaccine acceptability (de Visser and McDonnell, 2008, Woodhall et al., 2007), and others reporting no correlation (Allen et al., 2010, Dempsey et al., 2006, Fishman et al., 2014, Hausdorf et al., 2007, Lenselink et al., 2008). We also found that country of origin and gender were associated with HPV vaccine initiation with parents residing in the US and males in all three countries being less likely to have daughters who were vaccinated (where vaccination is not part of a school-based program).
In regards to parental attitudes, we found that parents of unvaccinated daughters in the US were more worried about the side effects of the HPV vaccine than parents of unvaccinated girls in the UK and Australia. This supports the finding from this study that more parents with daughters in the vaccination age-range from the US in our sample compared to the UK and Australia were not vaccinated. Previous studies have shown that parents in the US express serious concerns about vaccines for their children. A study conducted in the US with 1552 parents found that more than half (54%) still worry about vaccine side effects (Freed et al., 2010), and overall, 11.5% of parents had refused at least one vaccine their doctor had recommended, with the HPV vaccine being the most commonly refused (56%). Furthermore, a recent systematic review on barriers to the HPV vaccination in the US found that parental attitudes to the vaccine included the need for parents in the US to receive more information before vaccinating their child, and concerns about adverse effects of the vaccine (Holman et al., 2014). Parents from the US with unvaccinated daughters more often believed that getting all three doses of the HPV vaccine would be a significant obstacle, not surprisingly as the HPV vaccine distribution in the US is predominantly available through physicians' clinics and medical centres, whereas in the UK and Australia free school-based and catch-up programs are offered (Wong et al., 2011). Parents of unvaccinated daughters from the US also believed that the vaccine was so new that they would want to wait before deciding to get it for their daughter. This finding was unexpected given that advertising about HPV contributed to an increased awareness of HPV in the US at the time of data collection (Cates et al., 2010, Hughes et al., 2009). It is important to note however, that this survey was conducted in 2011, and therefore attitudes of US parents now may differ.
Parental vaccination knowledge was also shown to be associated with attitudes towards the HPV vaccine. Our study found that parents of unvaccinated daughters with higher vaccination knowledge scores expressed attitudes that they intended to vaccinate their daughters, and be on the safe side. This finding might help explain why many of the parents with higher HPV knowledge and HPV vaccine knowledge scores in our sample had daughters who had not yet been vaccinated. For parents of vaccinated daughters with higher knowledge scores, they understood that although they have vaccinated their daughters they may still be at risk of getting HPV in the future. This demonstrates that vaccinated parents in our study with higher HPV vaccination knowledge had better understanding of the risks of HPV.
By recruiting parents online through a wider survey, this study was able to directly compare parental attitudes across three countries, although recruitment using online panel samples and the use of quotas to ensure adequate representation of different age-groups and genders means that the sample may not be representative of parents in the US, UK and Australia. The use of quotas did, however, ensure that respondents with lower levels of education were well represented as shown with our sample. Furthermore, using a survey design allowed us to be able to separate parents who had vaccinated and unvaccinated daughters in our analysis to determine factors associated with vaccination status and compare attitudinal differences within the groups, as survey questions were tailored to parents based on their daughters' vaccination status.
Our study was limited by the relatively small proportion of respondents who identified as parents of daughters aged 9–17 years. Having a larger group of parents included in the study may have provided more significant results, as many of our attitudinal results were approaching significance. However, importantly, we included both women and men in our sample. Previous studies on HPV parental attitudes have mainly focused on mothers, although with the HPV vaccination now provided to school-aged boys (U.S. Food and Drug Administration, 2009b), fathers' knowledge and attitudes may be increasingly important.
Furthermore, our study focused on intrapersonal factors that may be associated with HPV vaccine initiation. Healthcare provider (HCP) recommendations, along with various interpersonal and environmental influences have also been shown to be associated with initiation and are important to understand. As demonstrated across several studies (Ferrer et al., 2014, Gamble et al., 2010, Mohammed et al., 2017, Staras et al., 2014, Stephens et al., 2016), HCPs' influence on vaccine uptake appears to often outweigh parental knowledge and attitudes.
Since acceptance of HPV vaccination varies internationally, and many adolescents are still not getting the HPV vaccine in various countries (Wigle et al., 2016), it is important to understand why some parents choose to vaccinate their children and some parents do not in order to continue to increase vaccination uptake. Importantly, our study focuses on parental factors associated with the HPV vaccination across three countries following vaccination introduction and adds to the growing body of existing research which explores how parents' knowledge and attitudes may influence vaccine uptake. This is the first study to directly assess parental knowledge and attitudes across different countries and between school based and non-school based programs. This understanding is important in order to target interventions aimed at improving knowledge and changing attitudes which, in turn, may help increase vaccination participation internationally and in the individual countries included in this study. In particular, US-based research has shown HCPs to be influential over parental decisions about the HPV vaccine, and this study has identified areas which could be useful to focus discussions with parents about their child receiving the HPV vaccine. Furthermore, the UK and Australia have universal health care systems with vaccination policy determined centrally, whereas in the US vaccination policy is determined on a far less coherent state-to-state basis and may therefore allow parental attitudes to be particularly influential in vaccination decisions.
5. Conclusion
Our study demonstrates that both low and high HPV knowledge may be associated with lower rates of vaccination, with parents' country and gender also being influential factors. It also demonstrates that parental attitudes towards the HPV vaccine differ by country and knowledge. These findings can contribute to the way the vaccine is promoted and/or administered in the future across different countries.
The following are the supplementary data related to this article.
Survey attitude statements.
Parental attitude statement differences by country.
Parental attitude statement differences by HPV vaccination specific knowledge scores.
Conflict of interest statement
Authors declare no conflicts of interest.
Funding statement
Funding for data collection came from departmental funds from the Section of Adolescent Medicine, Department of Pediatrics, Indiana University and the Cancer Research UK Health Behaviour Research Centre, Department of Behavioural Science and Health, UCL.
Contributors
JW, LM, RO, GDZ, KM contributed to the planning and design of the study. LM assembled the data. BN and RMT performed the statistical analysis. BN, RHD, RMT and KM interpreted the data. BN and KM drafted the article. All authors assisted with revisions of the article, read and approved the final article.
Acknowledgments
Acknowledgments
We would like to thank all study participants.
References
- Alberts C.J., van der Loeff M.F., Hazeveld Y. A longitudinal study on determinants of HPV vaccination uptake in parents/guardians from different ethnic backgrounds in Amsterdam, the Netherlands. BMC Public Health. 2017;17(1):220. doi: 10.1186/s12889-017-4091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J.D., Othus M.K., Shelton R.C. Parental decision making about the HPV vaccine. Cancer Epidemiol. Biomark. Prev. 2010;19(9):2187–2198. doi: 10.1158/1055-9965.EPI-10-0217. [DOI] [PubMed] [Google Scholar]
- Cates J.R., Shafer A., Carpentier F.D. How parents hear about human papillomavirus vaccine: implications for uptake. J. Adolesc. Health. 2010;47(3):305–308. doi: 10.1016/j.jadohealth.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Visser R., McDonnell E. Correlates of parents' reports of acceptability of human papilloma virus vaccination for their school-aged children. Sex. Health. 2008;5(4):331–338. doi: 10.1071/sh08042. [DOI] [PubMed] [Google Scholar]
- Dempsey A.F., Zimet G.D., Davis R.L., Koutsky L. Factors that are associated with parental acceptance of human papillomavirus vaccines: a randomized intervention study of written information about HPV. Pediatrics. 2006;117(5):1486–1493. doi: 10.1542/peds.2005-1381. [DOI] [PubMed] [Google Scholar]
- Dodd R.H., McCaffery K.J., Marlow L.A., Ostini R., Zimet G.D., Waller J. Knowledge of human papillomavirus (HPV) testing in the USA, the UK and Australia: an international survey. Sex. Transm. Infect. 2014;90(3):201–207. doi: 10.1136/sextrans-2013-051402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer H.B., Trotter C., Hickman M., Audrey S. Barriers and facilitators to HPV vaccination of young women in high-income countries: a qualitative systematic review and evidence synthesis. BMC Public Health. 2014;14 doi: 10.1186/1471-2458-14-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman J., Taylor L., Kooker P., Frank I. Parent and adolescent knowledge of HPV and subsequent vaccination. Pediatrics. 2014;134(4):e1049–1056. doi: 10.1542/peds.2013-3454. [DOI] [PubMed] [Google Scholar]
- Forman D., de Martel C., Lacey C.J. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30(Suppl. 5):F12–23. doi: 10.1016/j.vaccine.2012.07.055. [DOI] [PubMed] [Google Scholar]
- Forster A.S., Waller J. Taking stock and looking ahead: behavioural science lessons for implementing the nonavalent human papillomavirus vaccine. Eur. J. Cancer. 2016;62:96–102. doi: 10.1016/j.ejca.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed G.L., Clark S.J., Butchart A.T., Singer D.C., Davis M.M. Parental vaccine safety concerns in 2009. Pediatrics. 2010;125(4):654–659. doi: 10.1542/peds.2009-1962. [DOI] [PubMed] [Google Scholar]
- Gamble H.L., Klosky J.L., Parra G.R., Randolph M.E. Factors influencing familial decision-making regarding human papillomavirus vaccination. J. Pediatr. Psychol. 2010;35(7):704–715. doi: 10.1093/jpepsy/jsp108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausdorf K., Newman B., Whiteman D., Aitken J., Frazer I. HPV vaccination: what do Queensland parents think? Aust. N. Z. J. Public Health. 2007;31(3):288–289. doi: 10.1111/j.1467-842x.2007.00066.x. [DOI] [PubMed] [Google Scholar]
- Hendry M., Lewis R., Clements A., Damery S., Wilkinson C. “HPV? Never heard of it!”: a systematic review of girls' and parents' information needs, views and preferences about human papillomavirus vaccination. Vaccine. 2013;31(45):5152–5167. doi: 10.1016/j.vaccine.2013.08.091. [DOI] [PubMed] [Google Scholar]
- Holman D.M., Benard V., Roland K.B., Watson M., Liddon N., Stokley S. Barriers to human papillomavirus vaccination among US adolescents: a systematic review of the literature. JAMA Pediatr. 2014;168(1):76–82. doi: 10.1001/jamapediatrics.2013.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J., Cates J.R., Liddon N., Smith J.S., Gottlieb S.L., Brewer N.T. Disparities in how parents are learning about the human papillomavirus vaccine. Cancer Epidemiol. Biomark. Prev. 2009;18(2):363–372. doi: 10.1158/1055-9965.EPI-08-0418. [DOI] [PubMed] [Google Scholar]
- Krawczyk A., Knauper B., Gilca V. Parents' decision-making about the human papillomavirus vaccine for their daughters: I. Quantitative results. Hum. Vaccin. Immunother. 2015;11(2):322–329. doi: 10.1080/21645515.2014.1004030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk A., Perez S., King L., Vivion M., Dube E., Rosberger Z. Parents' decision-making about the human papillomavirus vaccine for their daughters: II. Qualitative results. Hum. Vaccin. Immunother. 2015;11(2):330–336. doi: 10.4161/21645515.2014.980708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenselink C.H., Gerrits M.M., Melchers W.J., Massuger L.F., van Hamont D., Bekkers R.L. Parental acceptance of human papillomavirus vaccines. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008;137(1):103–107. doi: 10.1016/j.ejogrb.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Marlow L.A., Waller J., Wardle J. Parental attitudes to pre-pubertal HPV vaccination. Vaccine. 2007;25(11):1945–1952. doi: 10.1016/j.vaccine.2007.01.059. [DOI] [PubMed] [Google Scholar]
- Marlow L.A., Zimet G.D., McCaffery K.J., Ostini R., Waller J. Knowledge of human papillomavirus (HPV) and HPV vaccination: an international comparison. Vaccine. 2013;31(5):763–769. doi: 10.1016/j.vaccine.2012.11.083. [DOI] [PubMed] [Google Scholar]
- Mohammed K.A., Vivian E., Loux T.M., Arnold L.D. Factors associated with parents' intent to vaccinate adolescents for human papillomavirus: findings from the 2014 National Immunization Survey—Teen. Prev. Chronic Dis. 2017;14 doi: 10.5888/pcd14.160314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz N., Bosch F.X., Castellsague X. Against which human papillomavirus types shall we vaccinate and screen? The international perspective. Int. J. Cancer. 2004;111(2):278–285. doi: 10.1002/ijc.20244. [DOI] [PubMed] [Google Scholar]
- Mupandawana E.T., Cross R. Attitudes towards human papillomavirus vaccination among African parents in a city in the north of England: a qualitative study. Reprod. Health. 2016;13(1):97. doi: 10.1186/s12978-016-0209-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staras S.A., Vadaparampil S.T., Patel R.P., Shenkman E.A. Parent perceptions important for HPV vaccine initiation among low income adolescent girls. Vaccine. 2014;32(46):6163–6169. doi: 10.1016/j.vaccine.2014.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp . College Station, T. S. L.; 2015. Stata Statistical Software: Release 14. [Google Scholar]
- Stephens D.P., Tamir H., Thomas T.L. Factors motivating HPV vaccine uptake among vaccinated and nonvaccinated Hispanic young adult women. Hisp. Health Care Int. 2016;14(4):184–191. doi: 10.1177/1540415316679808. [DOI] [PubMed] [Google Scholar]
- Trim K., Nagji N., Elit L., Roy K. Parental knowledge, attitudes, and behaviours towards human papillomavirus vaccination for their children: a systematic review from 2001 to 2011. Obstet. Gynecol. Int. 2012;2012 doi: 10.1155/2012/921236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration . 2006. Approved Products: Gardasil. (Retrieved from Silver Spring, MD, USA) [Google Scholar]
- U.S. Food and Drug Administration . 2009. Approved Products: Cervarix. (Retrieved from Silvers Spring, MD, USA) [Google Scholar]
- U.S. Food and Drug Administration FDA Approves New Indication for Gardasil to Prevent Genital Warts in Men and Boys (Press Release) 2009. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm187003.htm [Press release] Retrieved from.
- Waller J., Ostini R., Marlow L.A., McCaffery K., Zimet G. Validation of a measure of knowledge about human papillomavirus (HPV) using item response theory and classical test theory. Prev. Med. 2013;56(1):35–40. doi: 10.1016/j.ypmed.2012.10.028. [DOI] [PubMed] [Google Scholar]
- Wigle J., Fontenot H.B., Zimet G.D. Global delivery of human papillomavirus vaccines. Pediatr. Clin. N. Am. 2016;63(1):81–95. doi: 10.1016/j.pcl.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Wong C.A., Saraiya M., Hariri S. Approaches to monitoring biological outcomes for HPV vaccination: challenges of early adopter countries. Vaccine. 2011;29(5):878–885. doi: 10.1016/j.vaccine.2010.10.018. [DOI] [PubMed] [Google Scholar]
- Woodhall S.C., Lehtinen M., Verho T., Huhtala H., Hokkanen M., Kosunen E. Anticipated acceptance of HPV vaccination at the baseline of implementation: a survey of parental and adolescent knowledge and attitudes in Finland. J. Adolesc. Health. 2007;40(5):466–469. doi: 10.1016/j.jadohealth.2007.01.005. [DOI] [PubMed] [Google Scholar]
- World Health Organisation . Comprehensive Cervical Cancer Control: A Guide to Essential Practice (C4 GEP) 2013. Chapter 4: HPV vaccination. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Survey attitude statements.
Parental attitude statement differences by country.
Parental attitude statement differences by HPV vaccination specific knowledge scores.