Abstract
Molecular phylogenetic analyses of ITS-LSU rDNA sequence data demonstrate that Melanconis species occurring on Juglandaceae are phylogenetically distinct from Melanconis s.str., and therefore the new genus Juglanconis is described. Morphologically, the genus Juglanconis differs from Melanconis by light to dark brown conidia with irregular verrucae on the inner surface of the conidial wall, while in Melanconis s.str. they are smooth. Juglanconis forms a separate clade not affiliated with a described family of Diaporthales, and the family Juglanconidaceae is introduced to accommodate it. Data of macro- and microscopic morphology and phylogenetic multilocus analyses of partial nuSSU-ITS-LSU rDNA, cal, his, ms204, rpb1, rpb2, tef1 and tub2 sequences revealed four distinct species of Juglanconis. Comparison of the markers revealed that tef1 introns are the best performing markers for species delimitation, followed by cal, ms204 and tub2. The ITS, which is the primary barcoding locus for fungi, is amongst the poorest performing markers analysed, due to the comparatively low number of informative characters. Melanconium juglandinum (= Melanconis carthusiana), M. oblongum (= Melanconis juglandis) and M. pterocaryae are formally combined into Juglanconis, and J. appendiculata is described as a new species. Melanconium juglandinum and Melanconis carthusiana are neotypified and M. oblongum and Diaporthe juglandis are lectotypified. A short description and illustrations of the holotype of Melanconium ershadii from Pterocarya fraxinifolia are given, but based on morphology it is not considered to belong to Juglanconis. A key to all treated species of Juglanconis is provided.
Keywords: Ascomycota, Diaporthales, molecular phylogeny, new species, pathogen, systematics
INTRODUCTION
Melanconis is a well-known genus of Diaporthales, being the generic type of the family Melanconidaceae. However, its circumscription has substantially changed over the years. In his monograph of Melanconis, Wehmeyer (1941) used a wide generic concept. He included the genera Macrodiaporthe, Melanconiella, Pseudovalsella and even some species of Prosthecium and Pseudovalsa, making the genus very heterogeneous. This concept was largely accepted by Müller & Von Arx (1962). Subsequent researchers (e.g. Barr 1978) did not follow this wide concept, restricting the genus Melanconis mostly to Wehmeyer’s (1941) subg. Eumelanconis. In this restricted sense, the genus Melanconis was defined by a distinct ectostromatic disc, more or less well-developed entostroma, two-celled hyaline to brown ascospores with or without appendages, in combination with melanconium- or discosporium-like asexual morphs (Barr 1978).
In the phylogenetic analyses of Castlebury et al. (2002), several species traditionally classified within the genus Melanconis were shown to be phylogenetically scattered throughout the Diaporthales, demonstrating the need of a critical taxonomic revision of the genus. It became evident that the genus Melanconis, based on the type species M. stilbostoma, has to be restricted to only few species (Castlebury et al. 2002, Rossman et al. 2007). All five Melanconis species currently accepted in the genus occur on Alnus and Betula (Betulaceae; Fan et al. 2016).
Following the results of phylogenetic analyses, several genera were recently segregated from Melanconis. Based on detailed molecular phylogenetic and morphological investigations, Voglmayr et al. (2012) re-established the genus Melanconiella, widened its circumscription and transferred several species of Melanconis to Melanconiella. These investigations also revealed an unexpectedly high species biodiversity. As a result, several previously synonymised taxa were recognised as distinct species, and several species were described as new. Another species placed in Melanconis by Wehmeyer (1941), M. appendiculata, has recently been shown to belong to the Diaporthaceae (Voglmayr & Jaklitsch 2014), and the genus Phaeodiaporthe described by Petrak (1919) was re-established. In the phylogenetic analyses of Castlebury et al. (2002), Melanconis desmazieri was also shown to be unrelated to Melanconis but formed an isolated lineage together with Hercospora tiliae. When describing Melanconis desmazieri, Petrak (1938) made the connection with its asexual morph, Melanconium desmazieri, for which Grove (1937) established the monotypic genus Lamproconium. Following the recent changes of the ICN, Lamproconium desmazieri is therefore the name to be used for M. desmazieri. Acknowledging their isolated phylogenetic position, Norphanphoun et al. (2016) placed Lamproconium and Hercospora in a new family Lamproconiaceae.
These results demonstrate the need of detailed investigations on the remaining Melanconis species for which no sequence data are yet available. In this respect, species on Juglandaceae are of particular interest. This group contains economically important pathogens of Juglans spp., causing black pustular dieback disease of walnut (Graves 1923, Belisario 1999). Two species are commonly known on Juglans spp., Melanconis carthusiana, distributed from Europe to Central Asia, and M. juglandis, occurring in North America and Eastern Asia, with their asexual morphs described as Melanconium juglandinum and M. oblongum, respectively (Wehmeyer 1941).
Due to their economic importance, several studies dealing with Melanconis on Juglandaceae are available. Graves (1923) provided a detailed account on pathogenicity and taxonomy of Melanconium oblongum. He proved the connection of the sexual and asexual morphs by pure culture studies, provided detailed descriptions and combined the sexual morph, Diaporthe juglandis, in Melanconis, in analogy to the European Melanconis carthusiana. He also considered the North American Melanconium oblongum to be morphologically distinct from the European Melanconium juglandinum. In addition, he confirmed its pathogenicity on Juglans cinerea as a serious disease by inoculation experiments.
In his monograph on Melanconis, Wehmeyer (1941) recognised M. carthusiana and M. juglandis within his subg. Eumelanconis sect. Chrysostromae. He treated them as distinct species with some misgivings, as he considered the differences to be minor and probably insufficient for separation at the species level. Within M. juglandis, he described var. caryae from Carya glabra (Wehmeyer 1940), which differed in its host and the absence of a melanconium-like asexual morph, and var. tiliae from Tilia americana (Wehmeyer 1941), which he considered to be synonymous with the European Melanconis desmazieri, although he recognised the asexual morphs of American and European collections to be different.
Based on detailed morphological and pure culture studies, Kobayashi (1970) recorded, described and illustrated M. juglandis from Japan, stating that the Japanese collections agreed well with North American material. In addition, he reported the sexual morph of Melanconium pterocaryae and described it as Melanconis pterocaryae.
The lack of molecular phylogenetic investigations and of a taxonomic revision of Melanconis on Juglandaceae prompted us to initiate detailed molecular phylogenetic and morphological investigations, the results of which are presented here.
MATERIALS AND METHODS
Sample sources
The altogether 18 isolates of Melanconis from Juglandaceae included in this study either originated from ascospores or conidia of fresh specimens or from culture collections. Details of the strains including NCBI GenBank accession numbers of gene sequences used to compute the phylogenetic trees are listed in Table 1. Strain acronyms other than those of official culture collections are used here primarily as strain identifiers throughout the work. Representative isolates have been deposited at the CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands (CBS). Details of the specimens used for morphological investigations are listed in the Taxonomy section under the respective descriptions. Herbarium acronyms are according to Thiers (2016). Freshly collected specimens have been deposited in the Fungarium of the Department of Botany and Biodiversity Research, University of Vienna (WU).
Table 1.
Strains and NCBI GenBank accessions used in the phylogenetic analyses of the combined multigene matrix of Juglanconis. All sequences were generated during the present study.
| Taxon | Strain | Culture collection | Herbarium | Origin | Host | GenBank accession no. |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ITS-LSU | cal | his | ms204 | rpb1 | rpb2 | tef1 | tub2 | ||||||
| Juglanconis appendiculata | D140 | WU 35956 | Greece | Juglans regia | KY427138 | – | – | KY427157 | – | KY427188 | KY427207 | KY427226 | |
| D96 | WU 35954 | Austria | Juglans nigra | KY427139 | – | – | – | – | KY427189 | KY427208 | – | ||
| D96A | WU 35954 | Austria | Juglans nigra | KY427140 | – | – | KY427158 | – | KY427190 | KY427209 | – | ||
| MC | WU 32010 | Greece | Juglans regia | KY427141 | KY427242 | – | KY427159 | KY427174 | KY427191 | KY427210 | KY427227 | ||
| MC2 | WU 35957 | Spain | Juglans regia | KY427142 | KY427243 | – | KY427160 | KY427175 | KY427192 | KY427211 | KY427228 | ||
| MC4 | WU 35958 | Spain | Juglans regia | KY427143 | KY427244 | – | KY427161 | KY427176 | KY427193 | KY427212 | KY427229 | ||
| ME17, W.J.1665, A.R.3581 | CBS 123194 | WU 35951, BPI 840932 | Austria | Juglans regia | KY427144 | KY427245 | – | KY427162 | KY427177 | KY427194 | KY427213 | KY427230 | |
| Juglanconis juglandina | D142 | WU 35960 | Austria | Juglans regia | KY427145 | – | – | – | – | KY427195 | KY427214 | – | |
| MC1 | WU 35967 | Austria | Juglans regia | KY427146 | KY427246 | KY427128 | KY427163 | KY427178 | KY427196 | KY427215 | KY427231 | ||
| MC3 | WU 35968 | Spain | Juglans regia | KY427147 | KY427247 | KY427129 | KY427164 | KY427179 | KY427197 | KY427216 | KY427232 | ||
| ME16, W.J.1450, A.R.3420 | CBS 121083 | BPI 843622 | Austria | Juglans regia | KY427148 | KY427248 | KY427130 | KY427165 | KY427180 | KY427198 | KY427217 | KY427233 | |
| ME22, W.J.1500, A.R.3860 | CBS 133343 | WU 35959 | Austria | Juglans regia | KY427149 | KY427249 | KY427131 | KY427166 | KY427181 | KY427199 | KY427218 | KY427234 | |
| ME23 | WU 35965 | Austria | Juglans nigra | KY427150 | KY427250 | KY427132 | KY427167 | KY427182 | KY427200 | KY427219 | KY427235 | ||
| Juglanconis oblonga | ME14, A.R.4413 | CBS 133344 | – | USA | Juglans cinerea | KY427151 | KY427251 | KY427133 | KY427168 | KY427183 | KY427201 | KY427220 | KY427236 |
| ME15, A.R.4529 | CBS 133330 | – | USA | Juglans cinerea | KY427152 | KY427252 | KY427134 | KY427169 | KY427184 | KY427202 | KY427221 | KY427237 | |
| ME18, M4-1 | MAFF 410216 | TFM FPH 2623 | Japan | Juglans ailanthifolia | KY427153 | KY427253 | KY427135 | KY427170 | KY427185 | KY427203 | KY427222 | KY427238 | |
| ME19, M4-10 | MAFF 410217 | TFM FPH 3599, TFM FPH 3601 | Japan | Juglans ailanthifolia | KY427154 | KY427254 | KY427136 | KY427171 | KY427186 | KY427204 | KY427223 | KY427239 | |
| Juglanconis pterocaryae | ME20, LFP-M4-8 | MAFF 410079 | TFM FPH 3373 | Japan | Pterocarya rhoifolia | KY427155 | KY427255 | KY427137 | KY427172 | KY427187 | KY427205 | KY427224 | KY427240 |
| Melanconis stilbostoma | D143 | WU 35970 | Poland | Betula pendula | KY427156 | – | – | KY427173 | – | KY427206 | KY427225 | KY427241 | |
Morphology
Microscopic observations were made in tap water except where noted. Methods of microscopy included stereomicroscopy using a Nikon SMZ 1500 equipped with a Nikon DS-U2 digital camera, and Nomarski differential interference contrast (DIC) using a Zeiss Axio Imager.A1 compound microscope equipped with a Zeiss Axiocam 506 colour digital camera. Images and data were gathered using the NIS-Elements D v. 3.22.15 or Zeiss ZEN Blue Edition softwares. For certain images of ascomata the stacking software Zerene Stacker v. 1.04 (Zerene Systems LLC, Richland, WA, USA) was used. Measurements are reported as maxima and minima in parentheses and the range representing the mean plus and minus the standard deviation of a number of measurements given in parentheses. Due to poor or absent sporulation in pure culture, conidial and conidiophore morphology was only studied from natural substrates.
Culture preparation, DNA extraction, PCR and sequencing
Single ascospore or conidium isolates were prepared and grown on 2 % malt extract agar (MEA), or on 2 % corn meal agar plus 2 % w/v dextrose (CMD).
Growth of liquid culture and extraction of genomic DNA was performed as reported previously (Voglmayr & Jaklitsch 2011, Jaklitsch et al. 2012) using the DNeasy Plant Mini Kit (QIAgen GmbH, Hilden, Germany) or the modified CTAB method of Riethmüller et al. (2002).
The following loci were amplified and sequenced: the complete internal transcribed spacer region (ITS1-5.8S-ITS2) and a c. 900 bp fragment of the large subunit nuclear ribosomal DNA (nuLSU rDNA), amplified and sequenced as a single fragment with primers V9G (De Hoog & Gerrits van den Ende 1998) and LR5 (Vilgalys & Hester 1990); a 450–454 bp fragment of the calmodulin (cal) gene with primers CAL-228F and CAL-737R (Carbone & Kohn 1999); a 441–445 bp fragment of the histone H3 (his) gene with primers CYLH3F (Crous et al. 2004) and H3-1b (Glass & Donaldson 1995); a c. 1 kb fragment of the guanine nucleotide-binding protein subunit beta (ms204) gene with primers MS-E1F1 and MS-E5R1 (Walker et al. 2012); a 711 bp fragment of the RNA polymerase II subunit 1 (rpb1) gene with primers RPB1-Af and RPB1-Cr (Stiller & Hall 1997); a c. 1.2 kb fragment of the RNA polymerase II subunit 2 (rpb2) gene with primers fRPB2-5f and fRPB2-7cr (Liu et al. 1999) or dRPB2-5f and dRPB2-7cr (Voglmayr et al. 2016); a c. 1.3 kb fragment of the translation elongation factor 1-alpha (tef1) gene containing introns 4 and 5 and part of the exon with primers EF1-728F (Carbone & Kohn 1999) and TEF1LLErev (Jaklitsch et al. 2005); and a 441–445 bp fragment of the β-tubulin (tub2) gene with primers T1 (O’Donnell & Cigelnik 1997) and the newly designed BtHV2r (5’ CATCATRCGRTCNGGGAACTC 3’). PCR products were purified using an enzymatic PCR cleanup (Werle et al. 1994) as described in Voglmayr & Jaklitsch (2008). DNA was cycle-sequenced using the ABI PRISM Big Dye Terminator Cycle Sequencing Ready Reaction Kit v. 3.1 (Applied Biosystems, Warrington, UK) and the PCR primers; in addition, primers ITS4 (White et al. 1990) and LR3 (Vilgalys & Hester 1990) were used as internal sequencing primers for the ITS-LSU rDNA region. Sequencing was performed on an automated DNA sequencer (ABI 3730xl Genetic Analyzer, Applied Biosystems).
Data analysis
To reveal the phylogenetic position of Melanconis species occurring on Juglandaceae within the Diaporthales, a phylogenetic analysis was performed with nuLSU rDNA sequences. Sequences of representative species were selected from Castlebury et al. (2002) and supplemented with sequences from GenBank. Gaeumannomyces graminis and Kohlmeyeriopsis medullaris (Magnaporthaceae) were included as outgroups. GenBank accession numbers of the sequences selected are given in the phylogenetic tree (Fig. 1). In addition, an ITS-LSU matrix was produced with a subset of taxa according to the results of the LSU analyses, including selected members of Cryphonectriaceae, Gnomoniaceae, Harknessiaceae, Melanconidaceae and Schizoparmaceae; for GenBank accession numbers see Table 1. For detailed investigations of species relationships and delimitation within Melanconis species from Juglandaceae, a combined matrix of eight loci (partial SSU-ITS-LSU rDNA, cal, his, ms204, rpb1, rpb2, tef1 and tub2) was produced for phylogenetic analyses, with Melanconis stilbostoma as the outgroup. The GenBank accession numbers of sequences used in these analyses are given in Table 2.
Fig. 1.
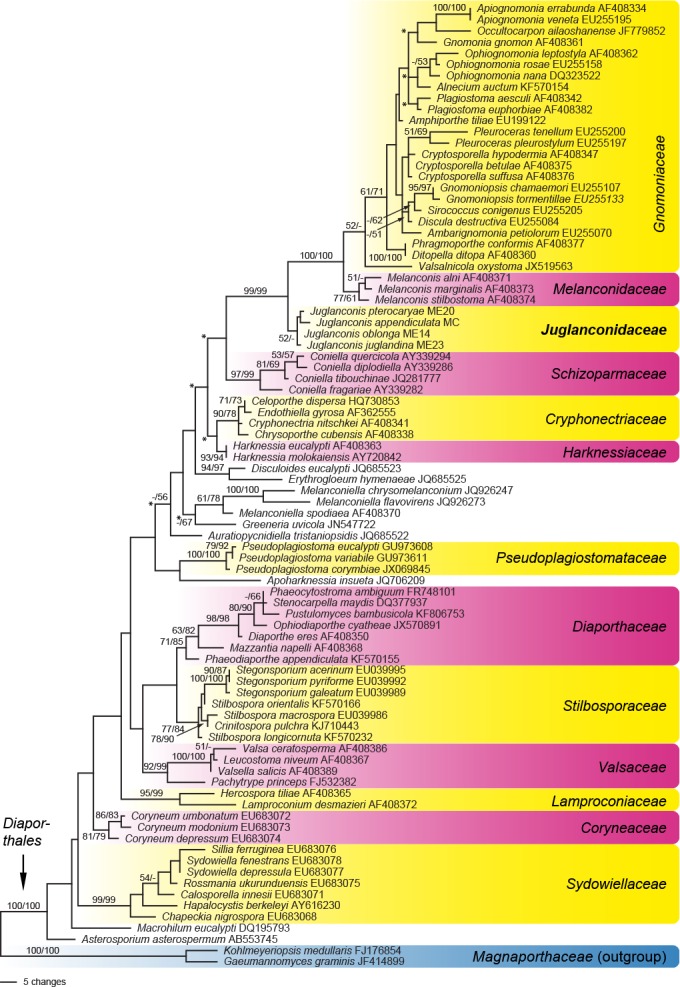
Phylogram of one of 240 MP trees of 894 steps (CI = 0.41, RI = 0.81, RC = 0.33) revealed by PAUP from an analysis of the LSU matrix of selected Diaporthales, showing the phylogenetic position of Juglanconis. MP and ML bootstrap support above 50 % are given at the first and second position, respectively, above or below the branches. GenBank accession numbers are given following the taxon names; nodes marked by an asterisk (*) collapsed in the strict consensus of all MP trees.
Table 2.
Strains and NCBI GenBank accession numbers of the ITS and LSU sequences used in the phylogenetic analyses of the ITS-LSU matrix. Isolates/sequences in bold were isolated/sequenced in the present study.
| Taxon | Isolate No.1,2 | Herbarium no.1 | Country | Host | GenBank accession numbers |
|
|---|---|---|---|---|---|---|
| ITS | LSU | |||||
| Alnecium auctum | CBS 124263ET | WU 30206 | Austria | Alnus glutinosa | KF570154 | KF570154 |
| Ambarignomonia petiolorum | CBS 121227ET | BPI 844274 | USA | Liquidambarstyraciflua | EU254748 | EU255070 |
| Amphiporthe tiliae | CBS 119289 | BPI 843515 | Austria | Tilia platyphylla | EU199178 | EU199122 |
| Apiognomonia hystrix | CBS 911.79 | CBS-H 11343 | Switzerland | Acer pseudoplatanus | DQ313549 | EU255180 |
| Apiognomonia veneta | CBS 897.79 | NA | Switzerland | Platanus orientalis | DQ313532 | EU255195 |
| Celoporthe dispersa | CBS 118782T | PREM 58896 | South Africa | Syzigium cordatum | NR_119569 | HQ730853 |
| Coniella diplodiella | CBS 111858ET | NA | France | Vitis vinifera | AY339323 | KX833335 |
| Coniella fragariae | CBS 172.49NT | NA | Belgium | Fragaria sp. | AY339317 | AY339282 |
| Coniella quercicola | CBS 904.69NT | NA | Netherlands | Quercus robur | KX833595 | KX833414 |
| Coniella tibouchinae | CBS 131594T | NA | Brazil | Tibouchina granulosa | JQ281774 | KX833418 |
| Cryphonectria parasitica | ATCC 38755 | NA | USA | Castanea dentata | AY141856 | EU199123 |
| Cryptosporella betulae | CBS 109763 | BPI 748448 | Austria | Betula alba | EU199180 | AF408375 |
| Cryptosporella hypodermia | CBS 171.69 | NA | Netherlands | Ulmus campestris | EU199225 | DQ862028 |
| Cryptosporella suffusa | CBS 121077 | BPI 871231 | Austria | Alnus incana | EU199184 | EU199124 |
| Discula destructiva | CBS 109771 | BPI 1107757 | USA | Cornus nuttallii | EU199186 | AF408359 |
| Ditopella ditopa | CBS 109748 | BPI 748439 | Austria | Alnus glutinosa | DQ323526 | AF408360 |
| Gnomonia gnomon | CBS 199.53 | NA | Italy | Corylus avellana | AY818956 | AF408361 |
| Gnomonia virginianae | CBS 121913T | BPI 844264 | USA | Ostrya virginiana | EU254801 | EU255105 |
| Gnomoniopsis chamaemori | CBS 803.79 | NA | Finland | Rubus chamaemorus | EU254808 | EU255107 |
| Gnomoniopsis tormentillae | CBS 904.79 | NA | Switzerland | Potentilla erecta | EU254856 | EU255133 |
| Harknessia eucalypti | CBS 342.97 | NA | Australia | Eucalyptus regnans | AY720745 | AF408363 |
| Harknessia leucospermi | CBS 775.97T | NA | South Africa | Leucospermum sp. | AY720727 | AY720824 |
| Harknessia molokaiensis | CBS 114877ET | NA | USA | Eucalyptus robusta | AY720749 | AY720842 |
| Harknessia syzygii | CBS 111124ET | NA | South Africa | Syzygium cordatum | AY720738 | AY720834 |
| Juglanconis appendiculata | D96T | WU 35954 | Austria | Juglans nigra | KY427139 | KY427139 |
| Juglanconis juglandina | ME23NT | WU 35965 | Austria | Juglans nigra | KY427150 | KY427150 |
| Juglanconis oblonga | ME14 | NA | USA | Juglans cinerea | KY427151 | KY427151 |
| Juglanconis pterocaryae | MAFF 410079T = ME20 | TFM FPH 3373 | Japan | Pterocarya rhoifolia | KY427155 | KY427155 |
| Melanconis alni | CBS 109773 | BPI 748444 | Austria | Alnus viridis | DQ323523 | AF408371 |
| Melanconis betulae | CFCC 50471 | BJFC-S1319 | China | Betula albosinensis | KT732952 | KT732971 |
| Melanconis itoana | CFCC 50474 | BJFC-S1322 | China | Betula albosinensis | KT732955 | KT732974 |
| Melanconis marginalis | CBS 109744 | BPI 748446 | Canada | Alnus rubra | EU199197 | AF408373 |
| Melanconis stilbostoma | CBS 121894 | NA | Austria | Betula alba | JQ926229 | JQ926229 |
| Occultocarpon ailaoshanense | CBS 129146T | BPI 879253 | China | Alnus nepalensis | JF779849 | JF779853 |
| Ophiognomonia leptostyla | CBS 844.79 | NA | Switzerland | Juglans regia | EU254910 | EU255149 |
| Ophiognomonia melanostyla | CBS 129144 | BPI 879257 | Germany | Tilia cordata | JF779850 | JF779854 |
| Ophiognomonia nana | CBS 883.79 | NA | Finland | Betula nana | DQ323534 | DQ323522 |
| Ophiognomonia rosae | CBS 121267 | BPI 877636 | USA | Rosa sp. | EU254936 | EU255158 |
| Phragmoporthe conformis | CBS 109783 | BPI 748450 | Canada | Alnus rubra | DQ323527 | AF408377 |
| Plagiostoma aesculi | CBS 109765 | BPI 748430 | Austria | Aesculus hippocastanum | EU199179 | AF408342 |
| Plagiostoma apiculatum | CBS 109775ET | BPI 747938 | Austria | Salix sp. | DQ323529 | AF408345 |
| Plagiostoma euphorbiae | CBS 340.78 | NA | Netherlands | Euphorbia palustris | EU199198 | AF408382 |
| Plagiostoma salicellum | CBS 121466E | BPI 843527 | Austria | Salix alba | EU254996 | EU255166 |
| Pleuroceras pleurostylum | CBS 906.79 | NA | Switzerland | Salix helvetica | EU255061 | EU255197 |
| Pleuroceras tenellum | CBS 121082 | BPI 871059 | USA | Acer rubrum | EU199199 | EU255202 |
| Sirococcus conigenus | CBS 101225 | BPI 871248 | Austria | Picea abies | EU199201 | EU199134 |
| Sirococcus piceicola | CBS 119621 | BPI 871166 | Switzerland | Picea abies | EU199202 | EU199135 |
1 ATCC: American Type Culture Collection, Manassas, VA, USA; BJFC: Museum of the Beijing Forestry University, Beijing, China; BPI: U.S. National Fungus Collections USDA-ARS MD USA; CBS: CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands; CFCC: China Forestry Culture Collection Center, Beijing, China; MAFF: MAFF Genebank, National Institute of Agrobiological Sciences, Ibaraki, Japan; NA: not applicable; PREM: South African National Collection of Fungi, Pretoria, South Africa; WU: Herbarium of the University of Vienna, Austria.
2 T: ex-type strain; ET: ex-epitype strain; NT: ex-neotype strain.
Sequence alignments for phylogenetic analyses were produced with the server version of MAFFT (www.ebi.ac.uk/Tools/mafft or http://mafft.cbrc.jp/alignment/server/), checked and refined using BioEdit v. 7.0.9.0 (Hall 1999). After exclusion of a 355 bp insertion in the LSU of Ditopella ditopa, the LSU matrix contained 1 337 characters and the ITS-LSU matrix 1 591 characters. The combined data matrix contained 7 767 characters; viz. 1 600 nucleotides of SSU-ITS-LSU, 455 nucleotides of cal, 449 nucleotides of his, 1 037 nucleotides of ms204, 711 nucleotides of rpb1, 1 150 nucleotides of rpb2, 1 395 nucleotides of tef1 and 970 nucleotides of tub2. Prior to phylogenetic analyses, the approach of Wiens (1998) was applied to test for significant levels of localised incongruence among the markers used for the combined analysis, using the level of bootstrap support (Sung et al. 2007) as described in Jaklitsch & Voglmayr (2014). For this, the 70 % maximum parsimony (MP) bootstrap consensus trees calculated for each individual partition, using the same parameters given below, were compared. No topological conflicts were observed between these bootstrap trees of the various genes, indicating the absence of significant incongruence and combinability of the eight loci (Wiens 1998).
Maximum parsimony (MP) analyses were performed with PAUP v. 4.0a150 (Swofford 2002). All molecular characters were unordered and given equal weight; analyses were performed with gaps treated as missing data; the COLLAPSE command was set to MINBRLEN. For the LSU and the ITS-LSU matrices, first a parsimony ratchet approach was used. For this, nexus files were prepared using PRAP v. 2.0b3 (Müller 2004), implementing 1 000 ratchet replicates with 25 % of randomly chosen positions upweighted to 2, which were then run with PAUP. In a second step, the best trees obtained by the parsimony ratchet analyses were loaded in PAUP and subjected to heuristic search using TBR branch swapping (MULTREES option in effect, steepest descent option not in effect). MP analysis of the combined multilocus matrix was done using 1 000 replicates of heuristic search with random addition of sequences and subsequent TBR branch swapping (MULTREES option in effect, steepest descent option not in effect). Bootstrap analyses with 1 000 replicates were performed in the same way, but using 5 rounds of random sequence addition and subsequent branch swapping during each bootstrap replicate; in addition, each replicate was limited to 1 million rearrangements in the LSU and ITS-LSU matrices.
Maximum likelihood (ML) analyses were performed with RAxML (Stamatakis 2006) as implemented in raxmlGUI v. 1.3 (Silvestro & Michalak 2012), using the ML + rapid bootstrap setting and the GTRGAMMAI substitution model with 1 000 bootstrap replicates. The matrix was partitioned for the different gene regions included in the combined multilocus analyses.
The sequence markers used for the multilocus analyses were also individually compared for their phylogenetic resolution within Juglanconis. Because several markers were not available for the outgroup taxon (Melanconis stilbostoma), only the accessions of Juglanconis were compared. The data matrices of the individual genes were subjected to MP bootstrap analyses with the same settings as in the analyses of the multilocus matrix and the resulting bootstrap support values of species and internal nodes were compared.
RESULTS
Molecular phylogeny
Of the 1 337 characters of the LSU matrix, 219 were parsimony informative. MP analyses revealed 240 MP trees of score 894, one of which is shown in Fig. 1; tree topologies of all MP trees were identical except for some nodes within Gnomoniaceae and some deeper nodes in the tree (see nodes marked by an asterisk in Fig. 1). In both MP and ML analyses, the Juglanconis-Gnomoniaceae-Melanconidaceae clade was highly supported. The subclade containing Melanconis (Melanconidaceae s.str.) and Gnomoniaceae received maximum support, but Melanconidaceae and Gnomoniaceae received only low or insignificant support. The genus Juglanconis was revealed as sister clade to the Gnomoniaceae-Melanconidaceae s.str. clade and received low or insignificant support as well.
Of the 1 591 characters included in the ITS-LSU analyses, 291 were parsimony informative. MP analyses revealed two MP trees 1 156 steps long, one of which is shown in Fig. 2. Tree topologies of the two MP trees differed in an interchanged position of Ophiognomonia rosae and O. nana. The ML tree revealed by RAxML showed the same relationships between the families as the MP trees, but differed in some unsupported nodes within the Gnomoniaceae (not shown). The clade containing Juglanconidaceae, Melanconidaceae and Gnomoniaceae received maximum support in both analyses, and Juglanconidaceae were revealed as sister group to the highly supported clade containing Melanconidaceae and Gnomoniaceae. Juglanconidaceae received maximum and medium (70 %) support in MP and ML analyses, respectively.
Fig. 2.
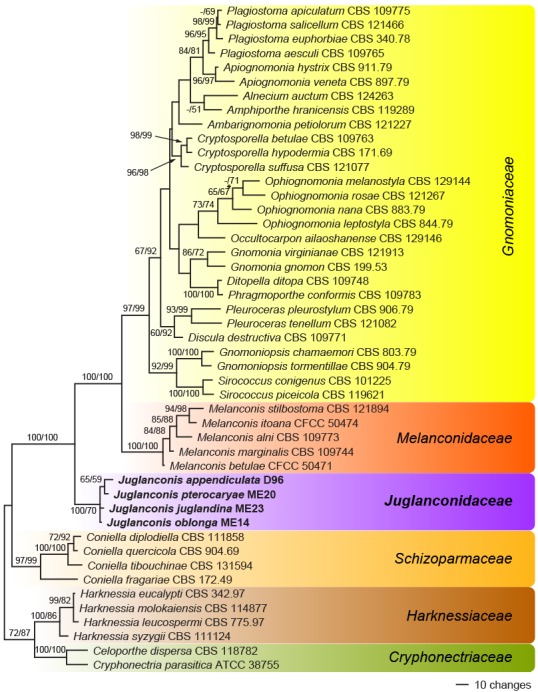
Phylogram showing one of two MP trees of 1 156 steps (CI = 0.47, RI = 0.78, RC = 0.36) revealed by PAUP from an analysis of the ITS-LSU matrix of selected Diaporthales, showing the phylogenetic position of Juglanconis. MP and ML bootstrap support above 50 % are given at the first and second position, respectively, above or below the branches. Strain/culture numbers are given following the taxon names. The node marked by an asterisk (*) collapsed in the strict consensus of the two MP trees.
Of the 7 767 characters included in the combined multilocus analyses, 315 were parsimony informative (16 from ITS-LSU, 32 from cal, 10 from his, 52 from ms204, 26 from rpb1, 45 from rpb2, 71 from tef1 and 63 from tub2). The his gene consistently failed to amplify in Juglanconis appendiculata and is therefore missing for this species. The MP analysis revealed five MP trees 907 steps long, one of which is shown in Fig. 3. Tree topologies of all MP trees were identical except for minor differences within J. appendiculata. The ML tree revealed by RAxML was identical to the MP tree shown. All four species of Juglanconis received maximum support in both analyses, as well as the relationships between the species. Juglanconis juglandina and J. oblonga are revealed as closely related but distinct species, and conspecificity of Japanese and North American accessions of J. oblonga is confirmed.
Fig. 3.
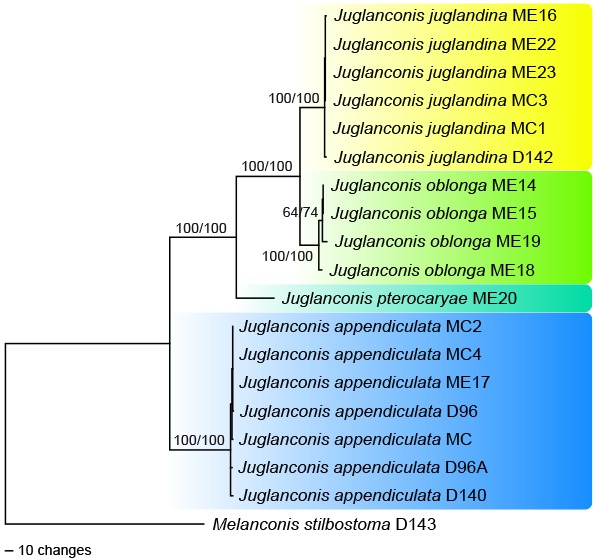
Phylogram showing one of five MP trees of 907 steps (CI = 0.97, RI = 0.98, RC = 0.95) revealed by PAUP from an analysis of the combined ITS-LSU-cal-his-ms204-rpb1-rpb2-tef1-tub2 matrix of Juglanconis, with Melanconis stilbostoma selected as outgroup. MP and ML bootstrap support above 50 % are given at the first and second position, respectively, above or below the branches. Strain numbers are given following the taxon names.
The number of alignment characters, the number and percentage of parsimony informative characters of the different markers and the percentage of MP bootstrap support for species and internal nodes revealed in the phylogenetic analyses are shown in Table 3. A comparison of the markers focusing on bootstrap support shows that the tef1 fragment containing introns 4 and 5 is the best resolving marker with 69 (5.1 %) parsimony informative characters (pic) and all nodes supported by 100 %, followed by cal with 32 (7 %) pic and support at all nodes above 98 %, except for J. oblonga with 94 %. Then followed ms204 and tub2 with 51 (5 %) and 62 (6.4 %) pic, respectively; all nodes were highly supported above 99 %, except for J. oblonga where support decreased to 87 % (ms204) and 86 % (tub2). In the rpb2, with 45 (3.9 %) pic, J. oblonga is supported by 95 %, whereas support of J. juglandina drops to 65 %. In the residual markers (rpb1, ITS and his) support of at least one node is absent.
Table 3.
Comparison of the phylogenetic markers used for the multigene analyses of Juglanconis. The markers were compared within Juglanconis. For the MP bootstrap support (% bts) of the respective clades, MP bootstrap analyses of the matrices of the respective markers were performed, applying the same rooting as in the multigene analyses (Fig. 3).
| ITS | LSU | cal | his | ms204 | rpb1 | rpb2 | tef1 | tub2 | |
|---|---|---|---|---|---|---|---|---|---|
| No. of alignment characters | 518 | 910 | 455 | 449 | 1030 | 711 | 1144 | 1361 | 970 |
| No. of variable characters | 15 | 5 | 37 | 31 | 65 | 27 | 55 | 77 | 72 |
| No. of parsimony-informative characters (pic) | 13 | 2 | 32 | 10 | 51 | 26 | 45 | 69 | 62 |
| % parsimony informative characters | 2.5 | 0.2 | 7.0 | 2.2 | 5.0 | 3.7 | 3.9 | 5.1 | 6.4 |
| % bts oblonga | – | – | 94 | 71 | 87 | 95 | 95 | 100 | 86 |
| % bts juglandina | 69 | 63 | 99 | 100 | 99 | – | 65 | 100 | 99 |
| % bts oblonga+juglandina | 84 | 61 | 98 | – | 100 | 100 | 100 | 100 | 100 |
| % bts pterocaryae+oblonga+juglandina | 100 | – | 100 | – | 100 | 100 | 100 | 100 | 100 |
Taxonomy
Juglanconidaceae Voglmayr & Jaklitsch, fam. nov. — MycoBank MB819587
Etymology. Referring to the name of the type genus.
Type genus. Juglanconis Voglmayr & Jaklitsch.
Family of Diaporthales. Pseudostromata consisting of an inconspicuous ectostromatic disc causing a more or less pustulate bark surface. Central column beneath the disc more or less conical. Stromatic zones lacking. Perithecia surrounding the ectostromatic disc, with long lateral ostioles that emerge at the margin or within the ectostromatic disc. Paraphyses deliquescent at maturity. Asci octosporous, with an apical ring, becoming detached from their base. Ascospores hyaline, bicellular, with or without gelatinous appendages. Asexual morph melanconium-like. Conidiomata acervular, with ectostromatic disc and central column. Conidiophores aseptate or few-celled, smooth, hyaline to brownish. Conidiogenous cells annellidic. Conidia brown, with gelatinous sheath. Conidial wall smooth on the outer surface, with inconspicuous to distinct irregular verrucae on the inner surface.
Note — We describe this family, because the genus Juglanconis is consistently placed outside described families of Diaporthales in phylogenetic analyses.
Juglanconis Voglmayr & Jaklitsch, gen. nov. — MycoBank MB819582
Etymology. Referring to its occurrence on Juglandaceae.
Type species. Juglanconis juglandina (Kunze) Voglmayr & Jaklitsch.
Genus of Diaporthales. Pseudostromata consisting of an inconspicuous, erumpent, light to dark coloured ectostromatic disc causing a more or less pustulate bark surface. Ectostromatic disc convex, flat or concave, greyish to brownish, surrounded by bark flaps. Central column beneath the disc more or less conical. Stromatic zones lacking. Perithecia inconspicuous at the bark level, surrounding the ectostromatic disc, oblique or horizontal, usually more or less irregularly scattered, sometimes arranged in a circle around the central column, with long lateral ostioles that converge at the margin of the column and emerge at the margin or within the ectostromatic disc. Ostioles flat in the disc or slightly projecting, rarely distinctly projecting and cylindrical, often obscuring the disc, sometimes covered by a distinct white crust. Paraphyses deliquescent at maturity. Asci oblong or fusoid, octosporous, with a more or less distinct apical ring becoming inconspicuous in old herbarium specimens; asci becoming detached from their base. Ascospores hyaline, ellipsoid, symmetrical to asymmetrical, straight to curved, bicellular, with a central or slightly eccentric septum, constricted at the septum, smooth, with or without blunt or pointed appendages.
Asexual morph: melanconium-like. Conidiomata acervular, covered by the bark, erumpent at maturity; possessing the same type of ectostromatic disc and central column as the sexual morph, usually preceding it. Conidiophores branched only at the base, mostly aseptate, sometimes few-celled, smooth, hyaline when young, brownish with age. Conidiogenous cells distinctly annellidic, successively producing several generations of conidia. Conidia brown, variable in shape, subglobose, ellipsoid, elongate pyriform, pip-shaped to fusoid, with distinct gelatinous sheath when fresh. Conidial wall smooth on the outer surface, with inconspicuous to distinct, sometimes confluent irregular verrucae on the inner surface.
Juglanconis appendiculata Voglmayr & Jaklitsch, sp. nov. — MycoBank MB819583; Fig. 4
Fig. 4.

Juglanconis appendiculata. a–c. Ectostromatic discs and ostioles in surface view; d. ectostromatic disc in side view showing protruding ostioles; e. pseudostroma in vertical section; f, g. transverse sections below ectostromatic disc; h. pseudostroma in transverse section, showing perithecia and indistinct whitish to light brown entostroma; i–l. mature asci with apical ascal ring (i, j vital, k, l dead); m, n. ascus apex with apical ring (m vital, n dead); o–u. vital ascospores with cylindrical gelatinous appendages; v–z. dead ascospores (v–x showing gelatinous appendages); a1. conidioma in surface view; b1. transverse section of conidioma, showing central column; c1–e1. conidiophores (annellides) with conidia (c1, d1); f1–o1. conidia (showing gelatinous sheath in f1–k1; f1–j1 vital, k1–o1 dead); p1. detail of verruculose inner conidial wall. All in water, except k, l, n, y, z, c1–e1, l1–p1 in 3 % KOH ( a, c–e, h, u, a1, b1: WU 35955; b, g, k, l, n, w–z, c1–e1, j1–o1: WU 32010; f, i, j, o–s, f1–i1: WU 35954 (holotype); m, t: WU 35956; v: WU 35958, p1: WU 29730). — Scale bars: a = 1 mm; b, c, e, h = 0.5 mm; d, f, g, a1, b1 = 300 μm; i–l, c1–e1 = 20 μm; m–z, f1–o1 = 10 μm; p1 = 2 μm.
Etymology. Referring to its gelatinous ascospore appendages.
Holotype. AUSTRIA, Niederösterreich, Mühlleiten, Herrnau, on corticated branches of Juglans nigra, 28 Feb. 2015, H. Voglmayr (WU 35954; ex-epitype culture D96 (ex sexual morph) and culture D96A (ex asexual morph)).
Pseudostromata 1.5–3 mm diam, typically distinct, circular, projecting up to 0.3 mm beyond the host surface, without perithecial bumps. Ectostromatic disc distinct, circular or oblong, dark grey, brown or black, 0.3–2 mm diam, sometimes concealed by densely arranged ostioles, pulvinate. Central column yellowish, greenish to brownish grey. Entostroma indistinct. Ostioles 1–15 per disc, (80–)92–127(–154) μm diam (n = 34), plane or slightly papillate, black, sometimes covered by distinct white crust. Perithecia (380–)420–520(–560) μm diam (n = 20), arranged in various configurations. Asci (121–)131–147(–168) × (19.5–)20.5–24.2(–27.8) μm (n = 58), clavate to fusoid, containing 8 uni- to biseriate ascospores, with distinct funnel-shaped apical ring when fresh, 4.5–5.1 μm diam, 1.8–3.2 μm high, becoming faint in older herbarium specimens. Ascospores (23–)26–32(–38.5) × (7.7–)9.5–11.0(–13) μm, l/w = (2–)2.5–3.2(–4) (n = 141), hyaline, ellipsoid or broadly fusoid, symmetric to slightly asymmetric, distinctly constricted at the septum, with distinct appendages (1.6–)2.1–3.4(–4.2) μm long, (2.0–)2.2–2.6(–3.1) μm wide (n = 44); cells monomorphic to dimorphic with larger upper cell, with rounded to subacute ends, multiguttulate, often containing one large and numerous small guttules per cell; wall c. 0.5–0.7 μm thick, not swelling.
Asexual morph. Conidiomata acervular, 0.4–1 mm diam, dark brown to blackish, inconspicuous, scattered, with central or eccentric stromatic column; at maturity covered by brown to blackish discharged conidial masses. Conidiophores (25–)30–43(–52) × (3.0–)4.2–6.0(–7.5) μm (n = 48), narrowly cylindrical to lageniform, simple or branched at the base, smooth, subhyaline to pale brown. Conidiogenous cells annellidic with distinct annellations, integrated. Conidia (17.3–)21.3–26.2(–34.0) × (7–)8.6–10.2(–13) μm, l/w = (1.6–)2.2–2.9(–3.7) (n = 357), unicellular, hyaline when immature, brown when mature, variable in shape, pip-shaped, narrowly ellipsoid, elongate to suballantoid, truncate with distinct abscission scar at the base, densely multiguttulate, thin-walled; wall c. 0.5–0.7 μm, with in-distinct ornamentation on the inside of the wall consisting of small irregular verrucae 0.2–0.5 μm diam, with 0.6–0.8 μm wide gelatinous sheath.
Habitat & Host range — Dead corticated twigs and branches of Juglans spp. attached to the tree.
Distribution — Europe; apparently common in Southern Europe.
Additional specimens examined (all on corticated branches of Juglans regia except where noted). AUSTRIA, Kärnten, St. Margareten im Rosental, Trieblach, 7 July 2013, W. Jaklitsch (WU 35950); St. Margareten im Rosental, Wograda, 27 Oct. 2000, W. Jaklitsch W.J. 1665 (WU 35951, BPI 840932, culture CBS 123194); ibid., 7 July 2013, W. Jaklitsch (WU 35952); St. Margareten im Rosental, 20 June 2015, H. Voglmayr (WU 35953); Niederösterreich, Orth an der Donau, near Uferhaus, on corticated branches of Juglans nigra, soc. J. juglandina, 5 Mar. 2003, H. Voglmayr (WU 29730); ibid., soc. J. juglandina, 15 May 2016, H. Voglmayr (WU 35955). – FRANCE, Ariège, Rimont, Las Muros, 20 Sept. 2008, J. Fournier (J.F. 08180). – GREECE, Crete, Pananiana, 4 June 2015, H. Voglmayr & W. Jaklitsch (WU 35956, culture D140); Crete, Vrysses, 26 Nov. 2011, W. Jaklitsch (WU 32010, culture MC). – SPAIN, Asturias, Planadera, near the crossing to Valbona and Borreras, 5 June 2013, W. Jaklitsch & H. Voglmayr (WU 35958, culture MC4); Asturias, Pineda, 27 Apr. 2013, Enrique Rubio (WU 35957, culture MC2).
Notes — Juglanconis appendiculata is easily distinguished from the other Juglanconis species by its conspicuous cylindrical ascospore appendages; the other species with appendaged ascospores, J. pterocaryae, has tapering appendages with rounded to subacute tips and also differs by the hosts, Pterocarya spp. Additional differences from the sympatric J. juglandina include distinct pseudostromata and light brown, distinctly narrower conidia (typically 8.6–10.2 μm wide, l/w = 2.2–2.9, vs 12.0–14.5 μm, l/w = 1.4–1.8). In contrast to J. juglandina its sexual morph is produced abundantly, whereas its asexual morph is inconspicuous. Remarkably, it has remained undetected until now, although it appears to be a common species in Southern Europe where it replaces J. juglandina; in eastern and southern Austria it is commonly co-occurring with J. juglandina on the same branches. Observational evidence suggests that it is currently expanding its range northwards which could be due to global warming.
Juglanconis juglandina (Kunze) Voglmayr & Jaklitsch, comb. nov. — MycoBank MB819584; Fig. 5, 6
Fig. 5.
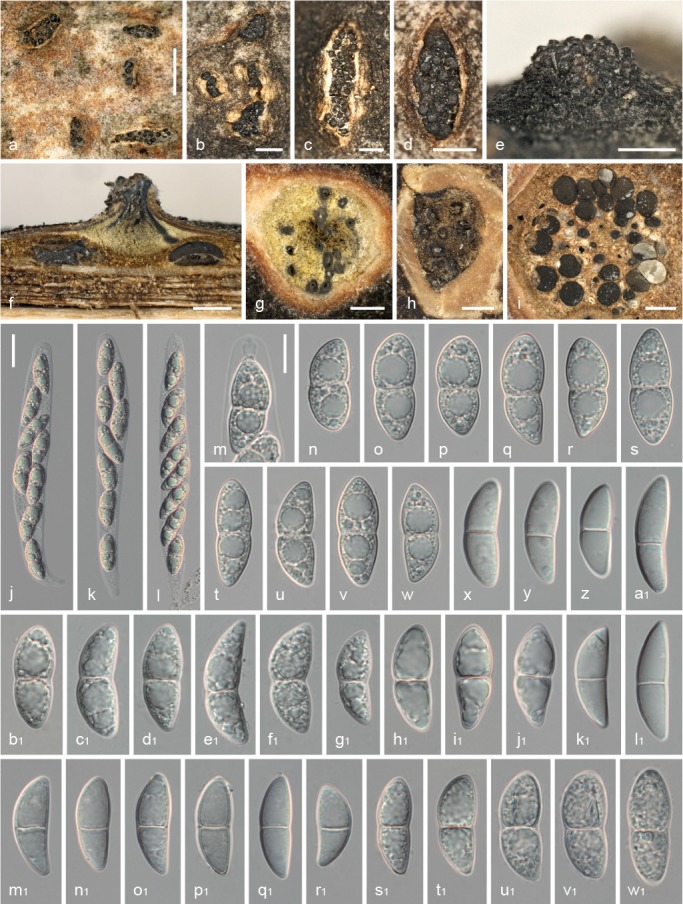
Juglanconis juglandina, sexual morph. a–d. Ectostromatic discs and ostioles in surface view; e. ectostromatic disc in side view showing protruding ostioles; f. pseudostroma in vertical section; g, h. transverse sections below ectostromatic disc; i. pseudostroma in transverse section, showing perithecia and indistinct whitish to light brown entostroma; j–l. mature vital asci with apical ascal ring; m. vital ascus apex with apical ring; n–w. vital ascospores; x–w1. dead ascospores. All in water, except b1–j1, s1–w1 in 3 % KOH (a, f, i, h1–j1: WU 35965 (neotype); b–e, g, x–g1: WU 35961; h: PC 0723585; j–w: WU 35966; k1, l1: WU 35959; m1–t1: WU 35964; u1–w1: BPI 614906). — Scale bars: a = 1 mm; b–i = 0.5 mm; j–l = 20 μm; m–w1 = 10 μm.
Fig. 6.

Juglanconis juglandina, asexual morph. a. Conidiomata in surface view; b, c. transverse (b) and vertical (c) sections of conidiomata, showing central column; d–h. conidiophores (annellides) with conidia; i–o1. conidia (showing gelatinous sheath in i–q, a1–g1; i–q vital, r–o1 dead; in j1–o1 showing verruculose inner conidial wall); p1–r1. detail of inner conidial wall, showing confluent verrucae. All in 3 % KOH, except d, e, i–q, j1 in water (a–c, f–h, w, x, m1: WU 35961; d, e, i–o, j1: WU 35966; p, q: WU 35960; r–v, k1: PC 0723585; y, z: BPI 614909; a1–e1: WU 35968; f1–i1, q1: WU 35967; l1, p1: PC 0723587; n1: BPI 614907; o1: BPI 614910; r1: BPI 614908). — Scale bars: a = 1 mm; b, c = 0.5 mm; d–o1 = 10 μm; p1–r1 = 2 μm.
Basionym. Melanconium juglandinum Kunze, in Schubert & Ficinus, Fl. Dresd., 2. Aufl.: 260. 1823.
Synonyms. Melanconidium juglandinum (Kunze) Kuntze, Revis. Gen. Pl. (Leipzig) 3, 2: 493. 1898.
Melanconium magnum (Grev.) Berk. subsp. juglandinum (Kunze) Sacc., Syll. Fung. (Abellini) 25: 580. 1931.
Melanconium juglandis Corda, Icon. Fungorum (Prague) 3: 21. 1839.
Melanconium juglandis Corda forma diffusa Corda, Icon. Fungorum (Prague) 3: 22. 1839.
Melanconium juglandinum Kunze forma diffusa (Corda) Sacc., Syll. Fung. (Abellini) 3: 753. 1884.
Melanconis carthusiana Tul. & C. Tul., Ann. Sci. Nat., Bot., sér. 4 5: 110. 1856.
Typification. AUSTRIA, Niederösterreich, Orth an der Donau, near Uferhaus, on corticated branches of Juglans nigra, 19 May 2013, W. Jaklitsch & H. Voglmayr (WU 35965, neotype of Melanconium juglandinum (MBT374383) and Melanconis carthusiana (MBT374385) here designated; culture ME23, culture lost).
Pseudostromata 0.8–2 mm diam, typically inconspicuous, sometimes distinct, circular, slightly projecting, without perithecial bumps. Ectostromatic disc indistinct, circular or oblong, dark grey, brown or black, 0.5–1.2(–2.3) mm diam, often concealed by densely arranged ostioles, often pulvinate. Central column yellowish, greenish to brownish grey. Entostroma indistinct. Ostioles 1–25 per disc, (94–)129–176(–208) μm diam (n = 30), plane or slightly papillate, black. Perithecia (375–)440–565 (–655) μm diam (n = 36), arranged in various configurations. Asci (126–)138–161(–184) × (14.2–)17.3–22.3(–25.0) μm (n = 75), clavate to fusoid, containing 8 uni- to irregularly biseriate ascospores, with indistinct apical ring when fresh 3.7–4.4 μm diam, 3.3–4.1 μm high, ring not visible in older herbarium specimens. Ascospores (20.5–)24.3–29.0(–36.5) × (6.7–) 8.5–11.0(–12.7) μm, l/w = (2.0–)2.3–3.2(–4.7) μm (n = 359), hyaline, inequilaterally ellipsoid or broadly fusoid, asymmetric, usually slightly to distinctly curved, distinctly constricted at the septum, without appendages; cells usually distinctly dimorphic, upper cell mostly larger, with rounded to subacute end, lower cell subacute to narrowly rounded, multiguttulate, containing mostly one large and numerous small guttules per cell; wall c. 0.4–0.6 μm thick, not swelling.
Asexual morph. Conidiomata acervular, 1–4 mm diam, blackish, scattered or occasionally confluent, with central or eccentric stromatic column; at maturity covered by black discharged conidial masses; usually conspicuous. Conidiophores (17–)26–37(–45) × (4.0–)4.8–6.5(–7.7) μm (n = 36), cylindrical to lageniform, simple, rarely branched at the base, smooth, subhyaline to pale brown. Conidiogenous cells annellidic with distinct annellations, integrated. Conidia (15–)19–23(–28.5) × (9.5–)12–14.5(–17.2) μm, l/w = (1.2–)1.4–1.8(–2.6) (n = 905), unicellular, hyaline when immature, brown to blackish when mature, broadly ellipsoid to broadly pip-shaped, truncate with distinct scar at the base, densely multiguttulate, thick-walled; wall c. 0.7–1.1 μm thick, with distinct ornamentation on the inside of the wall consisting of irregular confluent verrucae 0.5–1.5(–2.6) μm diam, with 0.8–1.1 μm wide gelatinous sheath.
Habitat & Host range — Dead corticated twigs and branches of Juglans spp. attached to the tree.
Distribution — Europe, Asia; common, particularly as asexual morph.
Additional specimens examined (all on corticated branches of Juglans regia except where noted). AUSTRIA, Kärnten, St. Margareten im Rosental, 20 June 2015, H. Voglmayr (WU 35960, culture D142); St. Margareten im Rosental, near Stariwald, 6 Dec. 1998, W. Jaklitsch W.J. 1279 (WU 35961); ibid., 21 July 2000, W. Jaklitsch W.J. 1500 (WU 35959); St. Margareten im Rosental, Wograda, 16 June 1995, W. Jaklitsch W.J. 647 (WU 35962); ibid., 7 July 2013, W. Jaklitsch (WU 35963); St. Margareten im Rosental, Zugland, 21 Apr. 2000, W. Jaklitsch W.J. 1450 (BPI 843622, culture CBS 121083); Niederösterreich, Orth an der Donau, near Uferhaus, on corticated branches of Juglans nigra, soc. J. appendiculata, 5 Mar. 2003, H. Voglmayr (WU 35964); Schönberg am Kamp, Olbersdorf, Dienbach-Tal, 25 Mar. 1984, A. Hausknecht (WU 16103); Oberösterreich, St. Willibald, Geitzedt, 6 Aug. 2016, H. Voglmayr (WU 35966); Wien, Floridsdorf, Neu-Stammersdorf, 14 Apr. 2013, W. Jaklitsch (WU 35967, culture MC1). – CZECH REPUBLIC, Morava, Hranice, 23 July 1915, F. Petrak, Flora Bohemiae et Moraviae exsiccata 761 (BPI 614906); ibid, Aug., F. Petrak, Kryptogamae exsiccatae 2411 (BPI 614907). – FRANCE, Beaumont, on Juglans, Aug. 1852, Herb. M.R. Tulasne (PC 0723587); Chatenay, on Juglans, Feb. 1854, Herb. M.R. Tulasne (PC 0723583); ibid., Feb. 1858, Herb. M.R. Tulasne (PC 0723584, PC 0723585); Meudon (Clamart), on Juglans, Feb. 1854, Herb. M.R. Tulasne (PC 0723586); Gillancourt, on Juglans, Oct. 1852, Herb. M.R. Tulasne (PC 0723579); without place and date, on Juglans (PC 0723582). – GERMANY, Reichardtshausen, without date, L. Fuckel, Fungi rhenani 595 (BPI 614908). – RUSSIA, Circassia, Krasnaja Poljana, 6 July 1909, J. Serebrianiko, in Tranzschel & Serebrianiko, Mycotheca Rossica 94 (BPI 614910). – SPAIN, Asturias, Saliencia, 3 June 2013, W. Jaklitsch & H. Voglmayr (WU 35968, culture MC3). – UKRAINE, Odessa, Adrianovka, near Ovidiopolin, 28 Oct. 1898, Kulikovski, in Jaczewski et al., Fungi Rossiae exsiccati 299 (BPI 614909).
Notes — Melanconium juglandinum is the first epithet unequivocally applicable to this taxon. No type specimen could be traced at B (R. Lücking, pers. comm.), and therefore specimen WU 35965, which contains the sexual and asexual morph and for which sequence data are available, is here designated as neotype.
In PC no type collection of Melanconis carthusiana is extant. In the original description Tulasne (1856) cited a single collection ‘prope vicum S. Laurentii Carthusianorum’ (i.e. Saint-Laurent-du-Pont north of Grenoble, France), without a collection date. In Tulasne & Tulasne (1863), 1855 is mentioned as collection year for this specimen, and they noted that after publication they found the sexual morph also on specimens from Bellemont, Isère (collected in August 1852) and Chatenay near Paris (collected in February 1858). In PC no collection from St Laurent is extant, but there are two collections from Chatenay and one from Beaumont corresponding to the data given in Tulasne & Tulasne (1863), which, however, are no types. As these collections are in poor condition, we do not select a neotype from them. To stabilise the nomenclatural connection of both names, we select the neotype specimen of Melanconium juglandinum also as neotype of M. carthusiana.
Corda (1839) nicely illustrated conidiomata, conidia and conidiophores under Melanconium juglandis, a younger synonym. The annellidic conidiogenesis was studied in detail by Belisario & Onofri (1995) by light and scanning electron microscopy. Juglanconis juglandina has been proven to be a virulent pathogen of Juglans spp. (Belisario 1999), being the causal agent of the European black pustular dieback of walnut. Compared to the asexual morph which is very common and conspicuous, the sexual morph has been infrequently found in fully developed condition.
Juglanconis oblonga (Berk.) Voglmayr & Jaklitsch, comb. nov. — MycoBank MB819585; Fig. 7, 8
Fig. 7.

Juglanconis oblonga, sexual morph. a–g. Ectostromatic discs and ostioles in surface view; h. ectostromatic disc in side view showing protruding ostioles; i. pseudostroma in vertical section; j, k. transverse sections below ectostromatic disc; l, m. pseudostromata in transverse section, showing perithecia and indistinct whitish to light brown entostroma; n–r. mature asci; s. ascus apex; t–z1. dead ascospores. All in 3 % KOH, except t–w in water (a, b: BPI 616364; c, h: BPI 616363; d, m–q, n1–q1: NY 01926933; e, r–a1: WU 35969; f, g, k, v1–z1: TFM FPH2623; i: BPI 614960; j, l, b1–f1: BPI 614954, g1–m1: BPI 616365 (lectotype of Diaporthe juglandis); r1–u1: TFM FPH3601). — Scale bars: a = 1 mm; b–m = 0.5 mm; n–r = 20 μm; s–z1 = 10 μm.
Fig. 8.

Juglanconis oblonga, asexual morph. a, b. Conidiomata in surface view; c–e. transverse (c, d) and vertical (e) sections of conidiomata, showing central column; f–h. conidiophores (annellides); i–s1. dead conidia showing gelatinous sheath in j, k, s, d1–g1; in n1–s1 showing verruculose inner conidial wall; t1–w1. detail of inner conidial wall, showing confluent verrucae. All in 3 % KOH (a, f–h: NY 01926935; b, c, k–o, n1, t1: K(M) 200286 (lectotype); d: NY 01926937; e, d1–h1, r1: WU 35969; i, j: K(M) 200285; p–t, o1, u1: NY 00921841; u–x: TFM FPH3599; y–c1: TFM FPH2623; i1–m1, q1: BPI 614951; p1, v1: TFM FPH3601; s1, w1: BPI 614955). — Scale bars: a = 2 mm; b–e = 200 μm; f–s1 = 10 μm; t1–w1 = 2 μm.
Basionym. Melanconium oblongum Berk., Grevillea 2 (no. 22): 153. 1874.
= Diaporthe juglandis Ellis & Everh., Proc. Acad. Nat. Sci. Philadelphia 45: 448. 1893.
≡ Melanconis juglandis (Ellis & Everh.) A.H. Graves, Phytopathology 13: 311. 1923.
Typification. USA, Alabama, on corticated twigs of Juglans cinerea, without date, Peters, Herb. Berkeley no. 5250 (K(M) 200286, lectotype of Melanconium oblongum here designated; MBT374386); Massachusetts, on corticated twigs of Juglans cinerea, without date & collector, Herb. Berkeley no. 3380 (K(M) 200285, syntype of Melanconium oblongum); New York, Alcove, on corticated twigs of Juglans cinerea, 31 Aug. 1893, C.L. Shear, in Ellis & Everhart, North American Fungi 3121 (BPI 616365, lectotype of Diaporthe juglandis here designated; MBT374387; BPI 616363, NY 00921841, NYS f4634 isotypes); same data, in Shear, New York Fungi 340 (BPI 616364, isotype).
Pseudostromata 1–3 mm diam, usually distinct, circular, projecting up to 0.5 mm, without perithecial bumps. Ectostromatic disc indistinct, usually circular, greyish to brownish or black, 0.4–1.3(–2.7) mm diam, commonly concealed by densely arranged ostioles, often pulvinate. Central column yellowish, greenish to brownish grey. Entostroma indistinct. Ostioles 1–15(–25) per disc, (83–)103–163(–220) μm diam (n = 20), papillate, black, sometimes covered by distinctly white crust. Perithecia (490–)525–725(–780) μm diam (n = 31), arranged in various configurations. Asci (85–)100–132(–140) × (12.5–)14.5–18(–19) μm (n = 27), clavate to fusoid, containing 8 uni- to irregularly biseriate ascospores, ring cylindrical to funnel-shaped according to Kobayashi (1970), not seen in the herbarium specimens examined. Ascospores (17.5–)19.8–24(–28) × (6.7–)8.0–11.5(–17.5) μm, l/w = (1.5–)2–2.6(–3.3) (n = 322), hyaline, ellipsoid, broadly ellipsoid or broadly fusoid, symmetric to slightly asymmetric, straight, rarely slightly curved, constricted at the septum, without appendages; cells monomorphic to slightly dimorphic with larger upper cell, with broadly rounded to subacute ends, multiguttulate; wall c. 0.4–0.6 μm thick, not swelling.
Asexual morph. Conidiomata acervular, 1–4 mm diam, blackish, scattered or occasionally confluent, with central or eccentric stromatic column; at maturity covered by black discharged conidial masses. Conidiophores (21–)28–44.5(–55) × (4.0–)4.8–6.5(–7.5) μm (n = 30), cylindrical to lageniform, simple, rarely branched at the base, smooth, subhyaline to pale brown. Conidiogenous cells annellidic with distinct annellations, integrated. Conidia (13.7–)18–22.7(–27.7) × (7–)9.2–12(–15.5) μm, l/w = (1.3–)1.7–2.3(–3.2) (n = 1633), unicellular, hyaline when immature, brown to blackish when mature, ellipsoid, elongate to pip-shaped, sometimes slightly allantoid, truncate with distinct scar at the base, densely multiguttulate, thick-walled; wall c. 0.7–0.9 μm, with distinct ornamentation on the inner side of the wall consisting of irregular confluent verrucae 0.5–2 μm diam, with 0.6–0.8 μm wide gelatinous sheath.
Habitat & Host range — Dead corticated twigs, branches and trunks of Juglans spp.
Distribution — North America, Eastern Asia (Japan).
Additional specimens examined (all on corticated twigs of Juglans cinerea except where noted). CANADA, Ontario, Brant Co., E. of Harley, 27 June 1937, R.F. Cain (BPI 614954); Frontenac Co., Kingston, on corticated twigs of Juglans nigra, 8 June 1964, E.D. Taylor (BPI 614961); Lake Erie District, Aldsborough, 19 July 1962, G.R. Irinnell (BPI 614955); Renford Co., near Braeside, 31 Aug. 1960, G.D. Darker (BPI 614959); W of Richmond Hill, 12 Oct. 1935, H.S. Jackson (NY 01926938); Quebec, Plains d’ Abraham, 27 July 2006, H. Voglmayr (WU 35969); Gatineau Park, Nature Trail, 9 Sept. 1958, R. Arnold & J. Malvin (BPI 615124). – JAPAN, Hokkaido, Bibai, Hokkaido Forest Research Institute Experimental Forest, on corticated twigs of Juglans ailanthifolia, 24 Sept. 1964, T. Kobayashi (TFM FPH3373); Iwate-gun, Iwate Pref., Takisawa, on corticated twigs of Juglans ailanthifolia, 5 Nov. 1970, T. Kobayashi (TFM FPH3599, TFM FPH3601, NY 01926932). – USA, Connecticut, Berlin, July 1924, A.H. Graves (BPI 615128); Hamden, 16 Sept. 1922, A.H. Graves (BPI 614960); Meriden, 16 Sept. 1922, A.H. Graves (BPI 614957); Maine, North Windham, 23 July 1923, A.H. Graves (BPI 614956); Maryland, Beltsville, North Farm walnut planting, on corticated twigs of Juglans ailanthifolia, 7 July 1953, F.H. Berry (BPI 614842); Massachusetts, Conway, Baptist Hill, 10 Feb. 1980, M.E. Barr (NY 01926937); New York, Highlands of Rockland Co., 16 June 1929, A.H. Graves (BPI 614951); Putnam Co., Carmel, S. of Nichols Rd. between Gypsy Trail and Horsepound Rds., 22 June 1998, R.C. Harris (NY 01926935); Walton, Mountain Home Farm, 1 June 1924, A.E. Jenkins (BPI 614958, NY 01926934); Pennsylvania, Lancaster, on corticated twigs of Juglans nigra, 12 June 1940, J.D. Diller (BPI 614843); Vermont, Lamoille Co., Stowe, Loomis Hill Rd., 10 July 1964, H.E. Bigelow & M.E. Barr (NY 01926933); Washington D.C., Ft. Kemble Park, on corticated twigs of Juglans nigra, 8 June 1943, G.F. Gravatt (BPI 614836).
Notes — Two authentic collections of Melanconium oblongum are extant at K, of which K(M) 200286 is here selected as lectotype. The type collection of Diaporthe juglandis has been distributed in two exsiccates (Ellis & Everhart, North American Fungi 3121 and in Shear, New York Fungi 340, the latter bearing the annotation ‘These specc. are from the same collection as the type’), therefore numerous copies are present. A remaining part of the original collection has been deposited as BPI 616365; it does not bear an original label, but contains a note by Shear ‘issued in N.Y.F., Cent. III’. This note also gives the exact date of the collection, whereas the labels of the exsiccata only give the month. Of all duplicates of the original collection we examined, BPI 616365 contains the most abundant and best preserved material; it is therefore here selected as lectotype of Diaporthe juglandis.
Juglanconis oblonga, previously also known as Melanconis juglandis, has been reported as the agent of walnut dieback in North America (Graves 1923) and Japan (Kobayashi 1968). It appears to be confined to North America and Eastern Asia where it replaces the similar, closely related J. juglandina. Wehmeyer (1941) expressed some doubts about its status as a separate species and gave slightly smaller stromata (‘pustules’), shorter and less inequilateral or curved ascospores and narrower conidia as main distinctions from the European J. juglandina. However, investigation of representative collections from North America revealed a remarkable variability in conidial width, and while conidia in general are narrower in J. oblonga (typically 8.5–11 vs 12–14.5 μm in J. juglandina), there is some size overlap in certain collections. No fresh collections were available for morphological investigations, therefore it has not been possible to observe the apical ascus ring. Only few specimens containing the sexual morph were available for study, but our investigations confirmed shorter (typically 19.8–24 vs 24.3–29 μm in J. juglandina), mostly symmetric spores with monomorphic to slightly dimorphic cells. In addition, the asci are significantly shorter in J. oblonga. The Japanese collections available for study differed from North American collections by distinctly wider ascospores (11–13 μm) with rounded ends, but the sequence data demonstrated conspecificity with material from eastern North America.
Within Melanconis juglandis, Wehmeyer established var. caryae from Carya glabra (Wehmeyer 1940), and var. tiliae from Tilia americana (Wehmeyer 1941). The former differs in host and the absence of a melanconium-like asexual morph, and the latter was considered to be synonymous with the European Melanconis (now Lamproconium) desmazieri, although the American collections did not produce a lamproconium-like but a melanconium-like asexual morph. In absence of fresh collections and of DNA data, their status cannot be evaluated, but it is likely that at least the latter does not belong to Juglanconis. They are certainly not conspecific with J. oblonga.
Juglanconis pterocaryae (Kuschke) Voglmayr & Jaklitsch, comb. nov. — MycoBank MB819586; Fig. 9
Fig. 9.

Juglanconis pterocaryae (TFM FPH3373). a, b. Ectostromatic discs and ostioles in surface view; c. ectostromatic disc in side view showing slightly protruding ostioles; d. pseudostromata in vertical section; e, f. transverse sections below ectostromatic disc; g. pseudostroma in transverse section, showing perithecia and indistinct entostroma; h–j. mature asci; k. ascus apex (no apical ring visible); l–z. dead ascospores (l–r with tapering, rounded to subacute gelatinous appendages); a1. conidiomata in surface view; b1. vertical section of conidioma; c1. transverse section of conidioma, showing central column; d1, e1. conidiophores (annellides) with conidia; f1. annellations of conidiophore and young conidium; g1–p1. dead conidia (showing gelatinous sheath in g1–j1; in p1 showing verrucae on inner conidial wall); q1. detail of verruculose inner conidial wall. All in 3 % KOH, except l–r, g1–j1 in water. — Scale bars: a, b, d, g, a1 = 0.5 mm; c, e, f, b1, c1 = 200 μm; h–j = 20 μm; k–z, d1–p1 = 10 μm; q1 = 1 μm.
Basionym. Melanconium pterocaryae Kuschke, Trudy Tiflissk. Bot. Sada 28: 25. 1913.
Synonym. Melanconis pterocaryae Tak. Kobay., Bull. Govt. Forest Exp. Stn Meguro 226: 24. 1970.
Typification. JAPAN, Shizuoka, Fuji, on corticated twigs of Pterocarya rhoifolia, 5 Aug. 1968, T. Kobayashi (TFM FPH2623, holotype of Melanconis pterocaryae).
Pseudostromata 1–2 mm diam, typically distinct, circular, projecting up to 0.3 mm, without perithecial bumps. Ectostromatic disc reduced to almost absent, circular, grey or brown, 0.15–0.5 mm diam, concealed by densely arranged ostioles, pulvinate. Central column poorly developed, grey to brownish grey, or absent. Entostroma indistinct. Ostioles 1–9 per disc, (79–)91–123(–135) μm diam (n = 31), plane or slightly papillate, black. Perithecia (410–)470–600(–640) μm diam (n = 14), arranged in various configurations. Asci (67.5–)79–96(–105) × (11.5–)12–14.2(–15.2) μm (n = 25), clavate to fusoid, containing 8 uni- to irregularly biseriate ascospores; ring cylindrical according to Kobayashi (1970), not seen in the herbarium specimen. Ascospores (16.5–)17.5–20(–21.5) × (5.3–)6–7(–7.5) μm, l/w = (2.5–)2.7–3.1(–3.5) (n = 51), hyaline, broadly fusoid to fusoid, symmetric to slightly asymmetric, straight or slightly curved, slightly constricted at the septum, with distinct tapering appendages having rounded to subacute tips, (1.6–)2–3.4(–4.6) μm long, (1.9–)2.2–2.5(–2.6) μm wide (n = 42); cells monomorphic to dimorphic with slightly larger upper cell, with narrowly rounded to subacute ends, multiguttulate; wall c. 0.5 μm thick, not swelling.
Asexual morph. Conidiomata acervular, 0.4–1.2 mm diam, dark brown to blackish, inconspicuous, scattered, with central or eccentric greenish yellow to grey stromatic column; at maturity covered by brown to blackish discharged conidial masses. Conidiophores (14–)17–28(–38) × (2.5–)3.5–4.7(–5.5) μm (n = 35), narrowly cylindrical to lageniform, simple or branched at the base, smooth, subhyaline to pale brown. Conidiogenous cells annellidic with distinct annellations, integrated. Conidia (10.8–)14.5–17.5(–20) × (5.2–)6.7–7.7(–8.7) μm, l/w = (1.5–)2–2.5(–3.1) (n = 100), unicellular, hyaline when immature, medium brown when mature, narrowly ellipsoid to elongate, rarely pip-shaped, often truncate with scar at the base, densely multiguttulate, thick-walled; wall c. 0.6–0.8 μm, with faint ornamentation on the inside of the wall consisting of small irregular confluent verrucae 0.5–0.8(–1.5) μm diam, with c. 0.5–0.9 μm wide gelatinous sheath.
Habitat & Host range — Dead corticated twigs and branches of Pterocarya spp. attached to the tree.
Distribution — Asia (Georgian Republic, Iran, Japan).
Notes — Melanconium pterocaryae was described from the Georgian Republic (Abkhazia) from Pterocarya fraxinifolia, but no type collection could be traced and no collections from the original host were available for morphological investigations and for DNA sequencing. Kobayashi (1970) described Melanconis pterocaryae from Pterocarya rhoifolia collected in Japan as sexual morph of Melanconium pterocaryae. However, the conidial sizes of the Japanese collection were slightly narrower than those given in the protologue of Melanconium pterocaryae (14–21 × 6–10 μm vs 14–19 × 8–12 μm), which was confirmed in the present study. Riedl & Ershad (1977) also reported narrower conidia (12–15.5 × 6.5–9.5 μm) from an Iranian collection on the original host, Pterocarya fraxinifolia, and we therefore consider these size differences to be within the range of intraspecific variability.
In the original description of Melanconis pterocaryae, Kobayashi (1970) mentioned absence of ascospore appendages. However, re-investigation of the type in water mounts revealed the presence of small tapering appendages with rounded to subacute tips, whereas in KOH the appendages were faint and disappearing quickly.
Melanconium ershadii Riedl, in Riedl & Ershad, Sydowia 29 (1–6): 163. 1977 (1976–77). — Fig. 10
Fig. 10.

Melanconium ershadii (W 1978-03131, holotype). a, b. Conidiomata in surface view; c. transverse section of conidioma, showing reduced central column; d–g. conidiophores (annellides) with conidia; h–y. dead conidia surrounded by gelatinous sheath. All in 3 % KOH. — Scale bars: a = 1 mm; b = 400 μm; c = 200 μm; d–y = 10 μm.
Holotype. IRAN, S Gorgan, Nahār Khorān, on corticated twigs of Pterocarya rhoifolia, 21 Apr. 1974 (given as 24 Apr. 1974 in Riedl & Ershad 1977), H. Riedl & D. Ershad (W 1978-03131).
Sexual morph unknown. Conidiomata acervular, 0.3–0.8 mm diam, dark brown to blackish, flat, inconspicuous, scattered, with small central or eccentric whitish to light grey stromatic column; at maturity sometimes covered by brown to blackish discharged conidial masses. Conidiophores (20–)24–39(–46) × (2.5–)3–4(–5) μm (n = 22), narrowly cylindrical to lageniform, simple or branched at the base, smooth, hyaline. Conidiogenous cells annellidic with few indistinct annellations, integrated. Conidia (7.5–)9.5–11.5(–13.3) × (4–)4.7–5.7(–6.8) μm, l/w = (1.2–)1.7–2.4(–3) (n = 100), unicellular, hyaline when immature, pale to medium brown when mature, very variable in shape from subglobose, narrowly ellipsoid, allantoid, pip-shaped to elongate, often truncate with scar at the base, with few faint guttules, thin-walled; wall smooth, c. 0.4 μm, without ornamentation on the inside of the wall, with c. 0.5 μm wide gelatinous sheath.
Habitat & Host range — Dead corticated twigs of Pterocarya fraxinifolia.
Distribution — Only known from Iran.
Notes — Melanconium ershadii and M. pterocaryae share the same host, Pterocarya fraxinifolia, and the former was reported to differ from the latter in flatter conidiomata and shorter conidia (10.5–11.5 × 5.5–6.2 μm; Riedl & Ershad 1977). This was confirmed by re-investigation of the type. No sexual morph is known and no cultures and sequence data are available, which currently makes an appropriate phylogenetic placement impossible. Whereas the annellidic conidiation and the unicellular brown conidia are melanconium-like, we do not think that it belongs to Juglanconis. Important differences concern the consistently hyaline conidiophores with few indistinct annellations (vs at least partly light brown conidiophores with distinct annellations in Juglanconis), the entirely smooth inner conidial wall (vs at least finely verrucose in Juglanconis), and the highly variable, and commonly irregular, shape of the conidia. These characters indicate that Melanconium ershadii may rather belong to Melanconis s.str., which needs to be confirmed by sequence data.
Key to species of Juglanconis
NB: For observation of ascospore appendages the ascospores should be mounted in water, as in KOH the appendages usually disappear quickly.
1. Ascospores with hyaline terminal appendages; conidia light to medium brown at maturity, inner surface of conidial wall with finely verruculose ornamentation............... 2
1. Ascospores without appendages; conidia dark brown at maturity, inner surface of conidial wall with distinct verruculose ornamentation.................................3
2. Ascospore appendages cylindrical with truncate ends; most conidia distinctly longer than 20 μm, variable in shape from pip-shaped, narrowly ellipsoid, elongate to suballantoid; on Juglans in Europe..................J. appendiculata
2. Ascospore appendages tapering, with rounded to subacute ends; most conidia distinctly shorter than 20 μm, narrowly ellipsoid to elongate; on Pterocarya spp. in Asia ...........................................J. pterocaryae
3. Ascospores (20.5–)24.3–29(–36.5) μm long, distinctly inequilateral, commonly curved, cells dimorphic; conidia in average usually wider than 12 μm; on Juglans spp. in Europe and Asia.............................J. juglandina
3. Ascospores (17.5–)19.8–24(–28) μm long, mostly symmetrical, only rarely curved, cells monomorphic to slightly dimorphic; conidia in average usually narrower than 12 μm; on Juglans spp. in North America and Eastern Asia (Japan).................................. J. oblonga
DISCUSSION
Molecular phylogeny, species delimitation and barcoding
The molecular phylogenetic analyses reveal that Juglanconis is a genus distinct from Melanconis, which cannot be classified within any existing family (Fig. 1–3). Therefore, we consider it justified to describe the family Juglanconidaceae for it. In the LSU tree (Fig. 1) the genus receives low (52 %) MP and no ML bootstrap support, whereas in the ITS-LSU tree (Fig. 2) bootstrap support rises to 100 % (MP) or 70 % (ML), which shows that the LSU alone does not contain sufficient information for providing a sound resolution of all phylogenetic relationships within Diaporthales. This has been also shown for other groups of Diaporthales like Stilbospora (Voglmayr & Jaklitsch 2014), which appeared as paraphyletic in the LSU tree but was resolved as a highly supported monophylum in trees obtained from other markers (ITS, rpb2, tef1). Similarly, also other families like Gnomoniaceae and Melanconidaceae receive only low to medium support in the LSU analyses (Fig. 1), but become highly supported in multilocus analyses (Sogonov et al. 2008). Unfortunately, for most representatives of Diaporthales no sequence data are available apart from ITS and LSU, therefore it has not been possible to perform a multilocus phylogenetic analyses which includes a representative taxon sampling. However, the genus becomes well supported upon addition of the ITS, and also the similar morphological and ecological traits provide good characters for generic and familial delimitation.
Wehmeyer (1941) questioned the status of Juglanconis juglandinum and J. oblongum as distinct species, but the molecular phylogenetic analyses of the multilocus matrix (Fig. 3) clearly reveal them as distinct species, which also differ morphologically by ascospore and conidial characters (see notes at the respective species).
The comparison of the different markers used for multilocus analyses (Table 3) showed that the tef1 fragment containing introns 4 and 5 is the best suited marker for species resolution within Juglanconis, which is in line with other investigations on Diaporthales (e.g. Voglmayr et al. 2012, Voglmayr & Jaklitsch 2014, Wang et al. 2014, Udayanga et al. 2014, 2015), and it should be adopted as barcoding marker for the group. On the other hand, the ITS which is the primary barcoding locus for fungi (Schoch et al. 2012) is amongst the poorest performing markers, which is clearly due to the comparatively low number (13) of informative characters. This is in line with what is observed in other ascomycete lineages like Hypocreales (e.g. Jaklitsch et al. 2013, Jaklitsch & Voglmayr 2015). In phylogenetic multilocus analyses within closely related species of Diaporthales, tef1 should be combined with other informative markers like ms204, cal and tub2, whereas for higher-level relationships the rpb2 may be more suitable, as the sequenced fragment consists of a coding region, which facilitates a better alignment.
Host range, distribution and other species
The genus Juglanconis appears to be confined to hosts from Juglandaceae (Fagales). Three species (J. appendiculata, J. juglandina and J. oblonga) are so far known from the genus Juglans and one (J. pterocariae) from Pterocarya. In contrast to e.g. Stegonsporium (Voglmayr & Jaklitsch 2008, 2014), which is highly host specific and where European plantations of North American Acer hosts consistently also harbour the North American S. acerinum, the genus Juglanconis appears to be less host specific. In Europe, J. appendiculata and J. juglandina are occurring on the indigenous Juglans regia as well as on the introduced North American J. nigra, which has been planted and become widely naturalised in Central European alluvial forests. Juglanconis oblonga has been confirmed from J. cinerea and J. nigra in North America and from J. ailanthifolia in Japan, and it has been recorded from several additional Juglans species (see Farr & Rossman 2016), but these records need to be critically evaluated by morphological and DNA data.
Additional Juglanconis species may be hidden within the Juglandaceae, especially in America and Eastern Asia, the biodiversity centres of Juglandaceae, which are still largely understudied except for the few economically important Juglans species. It cannot be ruled out that Melanconis juglandis var. caryae also belongs to Juglanconis, but no specimens were available for study. It has been described from Carya alba, and differs from J. oblonga primarily in hyaline conidia sized 10.5–14 × 5–7 μm; in addition, hyaline beta-conidia of size 2–2.5 × 0.8–1 μm were recorded (Wehmeyer 1940). Already Wehmeyer (1940) assumed that Melanconis juglandis var. caryae may represent a distinct species, and considering the results of recent molecular phylogenetic investigations of corticolous Diaporthales with similar ecology (e.g. Mejía et al. 2008, 2011a, b, Voglmayr & Jaklitsch 2008, 2014, Voglmayr et al. 2012, Walker et al. 2014), which revealed a much higher species biodiversity than previously perceived, we are convinced that it represents a distinct species. However, DNA sequence data as well as detailed morphological investigations are necessary to evaluate its generic affiliation. Wehmeyer (1936) also transferred Melanconiella pallida, a species growing on Carya spp., to Melanconis. This species differs from all Juglanconis species in dark brown ascospores. We have not been able to investigate this species morphologically, and in the absence of DNA sequence data its generic affiliation cannot be evaluated, but its morphological features indicate that it may not belong to Juglanconis.
The current investigations once again show that the traditional generic classification in Diaporthales needs to be critically re-evaluated by detailed morphological and molecular phylogenetic analyses. Even supposedly well-studied areas and hosts still harbour undescribed, morphologically distinct species. Our results also confirm that the ITS-LSU rDNA region, which has mostly been used for phylogenetic analyses in Diaporthales, commonly does not contain sufficient information for satisfactory phylogenetic resolution, and should be supplemented by additional suitable single-copy markers like tef1, cal, tub2, ms204 and rpb2.
Acknowledgments
We thank Enrique Rubio for providing fresh material, Jack Fournier for providing his detailed documentation of a French collection of J. appendiculata, Robert Lücking from B for information on specimens of Melanconium juglandinum, and the fungarium curators of BPI, K, NY, NYS, PC, TFM, W, and Walter Till at WU for sending and managing collections. The financial support by the Austrian Science Fund (FWF; project P27645-B16) is gratefully acknowledged.
REFERENCES
- Barr ME. 1978. The Diaporthales in North America, with emphasis on Gnomonia and its segregates. Mycologia Memoir 7: 1–232. [Google Scholar]
- Belisario A. 1999. Cultural characteristics and pathogenicity of Melanconium juglandinum. European Journal of Forest Pathology 29: 317–322. [Google Scholar]
- Belisario A, Onofri S. 1995. Conidiogenesis and morphology of Melanconium juglandinum. Mycological Research 99: 1059–1062. [Google Scholar]
- Carbone I, Kohn LM. 1999. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553–556. [Google Scholar]
- Castlebury LA, Rossman AY, Jaklitsch WJ, et al. 2002. A preliminary overview of the Diaporthales based on large subunit nuclear ribosomal DNA sequences. Mycologia 94: 1017–1031. [PubMed] [Google Scholar]
- Corda AKJ. 1839. Icones fungorum hucusque cognitorum 3. Calve, Prague. [Google Scholar]
- Crous PW, Groenewald JZ, Risede JM, et al. 2004. Calonectria species and their Cylindrocladium anamorphs: species with sphaeropedunculate vesicles. Studies in Mycology 50: 415–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Hoog GS, Gerrits van den Ende AHG. 1998. Molecular diagnostics of clinical strains of filamentous basidiomycetes. Mycoses 41: 183–189. [DOI] [PubMed] [Google Scholar]
- Fan X, Du Z, Liang Y, et al. 2016. Melanconis (Melanconidaceae) associated with Betula spp. in China. Mycological Progress 15: 40. [Google Scholar]
- Farr DF, Rossman AY. 2016. Fungal databases - fungus-host distributions. Systematic Mycology and Microbiology Laboratory, ARS, USDA: Retrieved November 16, 2016, from http://nt.ars-grin.gov/fungaldatabases/. [Google Scholar]
- Glass NL, Donaldson G. 1995. Development of primer sets designed for use with PCR to amplify conserved genes from filamentous ascomycetes. Applied and Environmental Microbiology 61: 1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves AH. 1923. The Melanconis disease of the butternut (Juglans cinerea L.). Phytopathology 13: 411–435. [Google Scholar]
- Grove WB. 1937. British stem- and leaf-fungi (Coelomycetes) 2. Cambridge University Press, Cambridge, UK. [Google Scholar]
- Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis. Program for Windows 95/98/NT. Nucleic Acids Symposium Series 41: 95–98. [Google Scholar]
- Jaklitsch WM, Komon M, Kubicek CP, et al. 2005. Hypocrea voglmayrii sp. nov. from the Austrian Alps represents a new phylogenetic clade in Hypocrea/Trichoderma. Mycologia 97: 1365–1378. [DOI] [PubMed] [Google Scholar]
- Jaklitsch WM, Samuels GJ, Ismaiel A, et al. 2013. Disentangling the Trichoderma viridescens complex. Persoonia 31: 112–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch WM, Stadler M, Voglmayr H. 2012. Blue pigment in Hypocrea caerulescens sp. nov. and two additional new species in sect. Trichoderma. Mycologia 104: 925–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch WM, Voglmayr H. 2014. Persistent hamathecial threads in the Nectriaceae, Hypocreales: Thyronectria revisited and re-instated. Persoonia 33: 182–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch WM, Voglmayr H. 2015. Biodiversity of Trichoderma (Hypocreaceae) in Southern Europe and Macaronesia. Studies in Mycology 80: 1–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T. 1968. Notes on Japanese species of the genus Melanconium. Transactions of the Mycological Society of Japan 9: 1–11. [Google Scholar]
- Kobayashi T. 1970. Taxonomic studies of Japanese Diaporthaceae with special reference to their life-histories. Bulletin of the Government Forest Experimental Station Meguro 226: 1–242. [Google Scholar]
- Liu YL, Whelen S, Hall BD. 1999. Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Molecular Biology and Evolution 16: 1799–1808. [DOI] [PubMed] [Google Scholar]
- Mejía LC, Castlebury LA, Rossman AY, et al. 2008. Phylogenetic placement and taxonomic review of the genus Cryptosporella and its synonyms Ophiovalsa and Winterella (Gnomoniaceae, Diaporthales). Mycological Research 112: 23–35. [DOI] [PubMed] [Google Scholar]
- Mejía LC, Castlebury LA, Rossman AY, et al. 2011a. A systematic account of the genus Plagiostoma (Gnomoniaceae, Diaporthales) based on morphology, host-associations, and a four-gene phylogeny. Studies in Mycology 68: 211–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejía LC, Rossman AY, Castlebury LA, et al. 2011b. New species, phylogeny, host-associations and geographic distribution of genus Cryptosporella (Gnomoniaceae, Diaporthales). Mycologia 103: 379–399. [DOI] [PubMed] [Google Scholar]
- Müller E, Von Arx JA. 1962. Die Gattungen der didymosporen Pyrenomyceten. Beiträge zur Kryptogamenflora der Schweiz 11, 2: 1–922. [Google Scholar]
- Müller K. 2004. PRAP – calculation of Bremer support for large data sets. Molecular Phylogenetics and Evolution 31: 780–782. [DOI] [PubMed] [Google Scholar]
- Norphanphoun C, Hongsanan S, Doilom M, et al. 2016. Lamproconiaceae fam. nov. to accommodate Lamproconium desmazieri. Phytotaxa 270: 89–102. [Google Scholar]
- O’Donnell K, Cigelnik E. 1997. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Molecular Phylogenetics and Evolution 7: 103–116. [DOI] [PubMed] [Google Scholar]
- Petrak F. 1919. Mykologische Notizen. I. Annales Mycologici 17: 59–100. [Google Scholar]
- Petrak F. 1938. Beiträge zur Kenntnis der Gattung Hercospora mit besonderer Berücksichtigung ihrer Typusart Hercospora tiliae (Pers.) Fr. Annales Mycologici 36: 44–60. [Google Scholar]
- Riedl H, Ershad D. 1977. Mykologische Ergebnisse einer Sammelreise in den Iran im Frühjahr 1974 – I. Sydowia 29: 155–169. [Google Scholar]
- Riethmüller A, Voglmayr H, Göker M, et al. 2002. Phylogenetic relationships of the downy mildews (Peronosporales) and related groups based on nuclear large subunit ribosomal DNA sequences. Mycologia 94: 834–849. [DOI] [PubMed] [Google Scholar]
- Rossman AY, Farr DF, Castlebury LA. 2007. A review of the phylogeny and biology of the Diaporthales. Mycoscience 48: 135–144. [Google Scholar]
- Schoch CL, Seifert KA, Huhndorf S, et al. 2012. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for fungi. Proceedings of the National Academy of Sciences of the United States of America 109: 6241–6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestro D, Michalak I. 2012. raxmlGUI: a graphical front-end for RAxML. Organisms Diversity & Evolution 12: 335–337. [Google Scholar]
- Sogonov MV, Castlebury LA, Rossman AY, et al. 2008. Leaf-inhabiting genera of the Gnomoniaceae, Diaporthales. Studies in Mycology 62: 1–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis E. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690. [DOI] [PubMed] [Google Scholar]
- Stiller JW, Hall BD. 1997. The origin of red algae: implications for plastid evolution. Proceedings of the National Academy of Sciences of the United States of America 94: 4520–4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung GH, Sung JM, Hywel-Jones NL, et al. 2007. A multigene phylogeny of Clavicipitaceae (Ascomycota, Fungi): identification of localised incongruence using a combinational bootstrap approach. Molecular Phylogenetics and Evolution 44: 1204–1223. [DOI] [PubMed] [Google Scholar]
- Swofford DL. 2002. PAUP* 4.0b10: phylogenetic analysis using parsimony (*and other methods). Sinauer Associates, Sunderland, Massachusetts. [Google Scholar]
- Thiers B. 2016. Index Herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium; http://sweetgum.nybg.org/ih/. [Google Scholar]
- Tulasne ELR. 1856. Note sur l’appareil reproducteur multiple des Hypoxylées (DC) ou Pyrénomycètes (Fr). Annales des Sciences Naturelles, Botanique, sér. 4, 5: 107–118. [Google Scholar]
- Tulasne ELR, Tulasne C. 1863. Selecta Fungorum Carpologia: Xylariei-Valsei-Spaeriei. 2. Imperial Typograph, Paris. [Google Scholar]
- Udayanga D, Castlebury LA, Rossman AY, et al. 2014. Insights into the genus Diaporthe: phylogenetic species delimitation in the D. eres species complex. Fungal Diversity 67: 203–229. [Google Scholar]
- Udayanga D, Castlebury LA, Rossman AY, et al. 2015. The Diaporthe sojae species complex: Phylogenetic re-assessment of pathogens associated with soybean, cucurbits and other field crops. Fungal Biology 119: 383–407. [DOI] [PubMed] [Google Scholar]
- Vilgalys R, Hester M. 1990. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr H, Akulov OY, Jaklitsch WM. 2016. Reassessment of Allantonectria, phylogenetic position of Thyronectroidea, and Thyronectria caraganae sp. nov. Mycological Progress 15: 921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr H, Jaklitsch WM. 2008. Prosthecium species with Stegonsporium anamorphs on Acer. Mycological Research 112: 885–905. [DOI] [PubMed] [Google Scholar]
- Voglmayr H, Jaklitsch WM. 2011. Molecular data reveal high host specificity in the phylogenetically isolated genus Massaria (Ascomycota, Massariaceae). Fungal Diversity 46: 133–170. [Google Scholar]
- Voglmayr H, Jaklitsch WM. 2014. Stilbosporaceae resurrected: generic reclassification and speciation. Persoonia 33: 61–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr H, Rossman AY, Castlebury LA, et al. 2012. Multigene phylogeny and taxonomy of the genus Melanconiella (Diaporthales). Fungal Diversity 57: 1–44. [Google Scholar]
- Walker DM, Castlebury LA, Rossman AY, et al. 2012. New molecular markers for fungal phylogenetics: two genes for species-level systematics in the Sordariomycetes (Ascomycota). Molecular Phylogenetics and Evolution 64: 500–512. [DOI] [PubMed] [Google Scholar]
- Walker DM, Lawrence BR, Wooten JA, et al. 2014. Five new species of the highly diverse genus Plagiostoma (Gnomoniaceae, Diaporthales) from Japan. Mycological Progress 13: 1057–1067. [Google Scholar]
- Wang X, Zang R, Yin Z, et al. 2014. Delimiting cryptic pathogen species causing apple Valsa canker with multilocus data. Ecology and Evolution 4: 1369–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehmeyer LE. 1936. Cultural life histories of Melanconis and Pseudovalsa. II. Mycologia 28: 528–541. [Google Scholar]
- Wehmeyer LE. 1940. Cultural histories of Melanconis and Pseudovalsa. IV. Mycologia 32: 321–330. [Google Scholar]
- Wehmeyer LE. 1941. A revision of Melanconis, Pseudovalsa, Prosthecium and Titania. University of Michigan Studies, Scientific Series 14: 1–161. [Google Scholar]
- Werle E, Schneider C, Renner M, et al. 1994. Convenient single-step, one tube purification of PCR products for direct sequencing. Nucleic Acids Research 22: 4354–4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, et al. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, et al. (eds), PCR protocols: A guide to methods and applications: 315–322. Academic Press, San Diego. [Google Scholar]
- Wiens JJ. 1998. Combining datasets with different phylogenetic histories. Systematic Biology 47: 568–581. [DOI] [PubMed] [Google Scholar]


