Abstract
Dacrymycetes, sister to Agaricomycetes, is a noteworthy lineage for studying the evolution of wood-decaying basidiomycetes; however, its species diversity and phylogeny are largely unknown. Species of Dacrymycetes previously used in molecular phylogenetic analyses are mainly derived from the Northern Hemisphere, thus insufficient knowledge exists concerning the Southern Hemisphere lineages. In this study, we investigated the species diversity of Dacrymycetes in New Zealand. We found 11 previously described species, and eight new species which were described here: Calocera pedicellata, Dacrymyces longistipitatus, D. pachysporus, D. stenosporus, D. parastenosporus, D. cylindricus, D. citrinus, and D. cyrtosporus. These eight newly described species and seven of the known ones, namely, Calocera fusca, C. cf. guepinioides, C. lutea, Dacrymyces flabelliformis, D. intermedius, D. subantarcticensis, and Heterotextus miltinus, have rarely or never been recorded from the Northern Hemisphere. In a molecular-based phylogeny, these New Zealand strains were scattered throughout the Dacrymycetaceae clade. Sequences obtained from specimens morphologically matching C. guepinioides were separated into three distant clades. Because no obvious morphological differences could be discerned between the specimens in each clade and no sequence exists from the type specimen, a C. guepinioides s.str. clade could not be determined. This survey of dacrymycetous species in the Southern Hemisphere has increased taxon sampling for phylogenetic analyses that can serve as a basis for the construction of a stable classification of Dacrymycetes.
Keywords: Dacrymycetes, New Zealand, phylogeny, Southern Hemisphere, taxonomy
INTRODUCTION
Dacrymycetes, one of the early-diverging wood decomposers in Basidiomycota, is sister to Agaricomycetes. Although consequently a noteworthy lineage for studying the evolution of wood-decaying basidiomycetes, its species diversity and phylogeny remain poorly understood. Morphology-based classifications of dacrymycetous species from the 1960s and 1970s (McNabb 1964, 1965a, 1965b, 1965c, 1965d, 1965e, 1966, 1973, Lowy 1971, Reid 1974) are only recently beginning to be reassessed using DNA-based phylogenies. To date, the species used for molecular phylogenetic analyses have been mainly collected from the Northern Hemisphere (Weiß & Oberwinkler 2001, Shirouzu et al. 2007, 2009, 2013a); consequently, insufficient knowledge exists about the phylogenetic relationships of the Southern Hemisphere Dacrymycetes. The major host trees of dacrymycetous species in the Northern Hemisphere belong to Pinaceae and Fagaceae, whereas forests in the Southern Hemisphere are characterised by families such as Nothofagaceae, Myrtaceae, Podocarpaceae, and Araucariaceae. Conifers in the Southern Hemisphere have different evolutionary histories than those in the Northern Hemisphere (Leslie et al. 2012). In some Agaricomycetes mushrooms, distributed species or lineages are different between the hemispheres (e.g. Coetzee et al. 2001, Hosaka et al. 2008). Because of the dissimilarities of host trees and geographical background, Dacrymycetes distributed in the Southern Hemisphere are predicted to include phylogenetically different lineages from those in the Northern Hemisphere.
The species diversity of Dacrymycetes from the Southern Hemisphere has been described in taxonomic studies by McNabb (McNabb 1964, 1965a, 1965b, 1965c, 1965d, 1965e, 1966, 1973) and Lowy (1971). Nevertheless, many dacrymycetous species from the Southern Hemisphere have not been included in any molecular phylogenetic analysis and samples have not been preserved for DNA extraction. Because it tends to degrade with time (e.g. Erkens et al. 2008, Hosaka & Uno 2013), DNA is difficult to obtain from specimens collected more than 50 years ago, therefore field collection of fresh material is needed. The acquisition of newly collected specimens from the Southern Hemisphere will help remove the current geographic bias in taxon sampling and will likely improve our understanding of phylogenetic relationships within Dacrymycetes.
In this study, field expeditions were conducted in New Zealand to collect dacrymycetous fruiting bodies as an initial step in the investigation of Dacrymycetes species in the Southern Hemisphere. We then conducted a molecular phylogenetic analysis and taxonomic classification of New Zealand Dacrymycetes and compared species compositions between Southern and Northern Hemispheres.
MATERIALS AND METHODS
Fruiting body collection and identification
From 2011 to 2015, fruiting bodies of Dacrymycetes were collected at 74 sites in the North and South Islands of New Zealand. For species identification, collected specimens were morphologically examined with a stereomicroscope and a light microscope (Shirouzu et al. 2009). Genus- and species-level identifications were conducted according to a classification system based on morphological characteristics (Olive 1958, McNabb 1965a, d, 1973, Lowy 1971, McNabb & Talbot 1973, Reid 1974, Oberwinkler 1993, 2014, Shirouzu et al. 2009). Although some genera based on these criteria are not monophyletic (Shirouzu et al. 2013a), we retained those generic concepts because no phylogenetic-based classification system has yet been established for Dacrymycetes. In similar situations, new species have been described according to the traditional system based on morphological criteria (Shirouzu et al. 2009, 2013b, Wu et al. 2011, Delivorias et al. 2012).
Fruiting bodies were dried with a food dehydrator (58 °C for 12 h) and deposited in the Fungal and Plant Disease Collection (PDD) in New Zealand and the National Museum of Nature and Science (TNS) in Japan. Pure cultures were isolated from fresh fruiting bodies by multi-basidiospore isolation on 2.5 % malt agar (MA; Nissui, Tokyo, Japan) plates and preserved in sealed vials containing cornmeal agar (0.2 % CMA, Nissui) + MA medium (0.2 % CMA (8.5 g), 2.5 % MA (22.5 g), 1 g yeast extract, and 1 L distilled water). The isolated cultures were deposited in the International Collection of Micro-organisms from Plants (ICMP) in New Zealand (Table 1).
Table 1.
Specimen, culture, and sequence accession numbers and localities of samples used in molecular phylogenetic analyses.
| Name | Locality | Specimen no.1 | Culture no.2 | DDBJ accession no. |
|
|---|---|---|---|---|---|
| LSU | ITS | ||||
| Calocera arborea | Brazil | INPA 241458 (holotype) | – | AB723514 | – |
| Brazil | INPA 241457 | – | AB723513 | – | |
| Calocera bambusicola | Taiwan | Wu 9910-12 | – | – | FJ195751 |
| Calocera cornea | New Zealand | PDD 104991 | ICMP 20465 | LC131362 | LC131403 |
| New Zealand | PDD 107847 | ICMP 21223 | LC131363 | LC131404 | |
| Japan | TNS-F-21061 | MAFF 241186 | AB472722 | – | |
| Japan | TNS-F-21065 | MAFF 241188 | AB472725 | – | |
| USA | – | CBS 125.84 | AB472739 | – | |
| Canada | – | CBS 124.84 | AB472738 | AB712437 | |
| Calocera fusca | New Zealand | PDD 107930 | – | LC131364 | LC131405 |
| New Zealand | PDD 107972 | ICMP 21238 | LC131365 | LC131406 | |
| Calocera glossoides (= Dacryomitra pusilla) | Germany | FO38346 | – | AJ406406 | – |
| Calocera cf. guepinioides | New Zealand | PDD 105005 | ICMP 20480 | LC131366 | LC131407 |
| New Zealand | PDD 105033 | ICMP 20502 | LC131367 | LC131408 | |
| New Zealand | PDD 107874 | ICMP 21226 | LC131368 | LC131409 | |
| New Zealand | PDD 107929 | ICMP 21231 | LC131369 | LC131410 | |
| New Zealand | PDD 107969 | ICMP 21236 | LC131370 | LC131411 | |
| New Zealand | PDD 107981 | ICMP 21240 | LC131371 | LC131412 | |
| Calocera lutea | New Zealand | PDD 107841 | ICMP 21221 | LC131372 | LC131413 |
| New Zealand | PDD 107842 | ICMP 21222 | LC131373 | LC131414 | |
| Australia | – | CBS 291.82 | AB712379 | AB712438 | |
| Calocera pedicellata | New Zealand | PDD 107830 | – | LC131374 | LC131415 |
| New Zealand | PDD 107925 (holotype) | ICMP 21230 | LC131375 | LC131416 | |
| Calocera sinensis | Taiwan | Wu 0703-6 | – | – | FJ195754 |
| Taiwan | JCH 070726 | – | – | FJ195755 | |
| Calocera viscosa | Japan | TNS-F-15704 | MAFF 240119 | AB299048 | AB712439 |
| Canada | – | CBS 292.82 | AB472740 | – | |
| Cerinomyces albosporus | Japan | TNS-F-15706 | MAFF 240121 | AB299050 | AB712440 |
| Cerinomyces canadensis | Japan | TNS-F-21034 | MAFF 241162 | AB472696 | AB712441 |
| Japan | TNS-F-21035 | MAFF 241163 | AB472697 | – | |
| Cerinomyces ceraceus | USA | – | HHB-8969 | AB712422 | AB712442 |
| Cerinomyces crustulinus | Canada | – | TUFC 30545 | AB712423 | AB712443 |
| Taiwan | – | – | AY600248 | – | |
| Cerinomyces grandinioides | USA | – | HHB-6908 | AB712424 | AB712444 |
| Cerinomyces lagerheimii | USA | – | RLG-13487 | AB712425 | AB712445 |
| Cerinomyces pallidus | Japan | TNS-F-21064 | – | AB472724 | – |
| Belize | – | FP150848 | AB712426 | AB712446 | |
| Dacrymyces adpressus | Japan | TNS-F-21045 | MAFF 241172 | AB472707 | AB712447 |
| Japan | TNS-F-21069 | MAFF 241191 | AB472729 | – | |
| Dacrymyces ancyleus | Japan | TNS-F-21051 (holotype) | MAFF 241177 | AB472713 | AB712448 |
| Dacrymyces aureosporus | Japan | TNS-F-15711 | MAFF 240126 | AB299057 | AB712449 |
| Japan | TNS-F-21074 | MAFF 241195 | AB472734 | – | |
| Dacrymyces capitatus | Japan | TNS-F-15709 | MAFF 240124 | AB299055 | – |
| Japan | TNS-F-21062 | MAFF 241187 | AB472723 | – | |
| Canada | – | CBS 293.82 | AB472741 | AB712450 | |
| Dacrymyces chrysocomus | UK | – | CBS 280.84 | AB712427 | AB712451 |
| Dacrymyces chrysospermus | Japan | TNS-F-15712 | MAFF 240127 | AB299073 | AB712452 |
| Japan | TNS-F-21060 | MAFF 241185 | AB472721 | – | |
| Dacrymyces citrinus | New Zealand | PDD 107915 (holotype) | ICMP 21227 | LC131376 | LC131417 |
| New Zealand | PDD 107979 | ICMP 21239 | LC131377 | LC131418 | |
| Dacrymyces cylindricus | New Zealand | PDD 105052 (holotype) | ICMP 20517 | LC131378 | LC131419 |
| New Zealand | PDD 107989 | – | LC131379 | LC131420 | |
| Dacrymyces cyrtosporus | New Zealand | PDD 107952 | – | LC131380 | LC131421 |
| New Zealand | PDD 107980 (holotype) | – | LC131381 | LC131422 | |
| Dacrymyces dendrocalami | Japan | TNS-F-38903 | TUFC 13914 | AB712428 | AB712453 |
| Dacrymyces dictyosporus | USA | – | HHB-8618 | AB712429 | AB712454 |
| Dacrymyces flabelliformis | New Zealand | PDD 107863 | ICMP 21225 | LC131382 | LC131423 |
| New Zealand | PDD 107944 | ICMP 21233 | LC131383 | LC131424 | |
| New Zealand | PDD 76696 (holotype) | HHB-18308 | AB712430 | AB712455 | |
| Dacrymyces intermedius | New Zealand | PDD 107851 | ICMP 21224 | LC131384 | – |
| New Zealand | PDD 107939 | ICMP 21232 | LC131385 | – | |
| Dacrymyces lacrymalis | Japan | TNS-F-15719 | MAFF 240134 | AB299069 | AB712456 |
| Japan | TNS-F-21040 | MAFF 241167 | AB472702 | – | |
| Japan | TNS-F-21042 | MAFF 241169 | AB472704 | – | |
| Dacrymyces longistipitatus | New Zealand | PDD 107996 | ICMP 21241 | LC131386 | LC131425 |
| New Zealand | PDD 107997 (holotype) | ICMP 21242 | LC131387 | LC131426 | |
| Dacrymyces cf. microsporus | New Zealand | PDD 104992 | ICMP 20466 | LC131388 | – |
| New Zealand | PDD 104993 | ICMP 20467 | LC131389 | – | |
| Dacrymyces microsporus | Japan | TNS-F-21049 | MAFF 241175 | AB472711 | – |
| Japan | TNS-F-21050 | MAFF 241176 | AB472712 | AB712457 | |
| Dacrymyces minor | Japan | TNS-F-15720 | MAFF 240135 | AB299059 | – |
| Japan | TNS-F-15721 | MAFF 240136 | AB299063 | AB712458 | |
| Dacrymyces minutus | Japan | TNS-F-15722 | MAFF 240137 | AB299070 | – |
| Japan | TNS-F-21073 | – | AB472733 | AB712459 | |
| Dacrymyces novae-zelandiae | New Zealand | PDD 107892 | – | LC131390 | LC131427 |
| New Zealand | PDD 107953 | ICMP 21235 | LC131391 | LC131428 | |
| Japan | TNS-F-21038 | MAFF 241165 | AB472700 | AB712460 | |
| New Zealand | – | CBS 295.82 | AB472742 | – | |
| Dacrymyces pachysporus | New Zealand | PDD 105004 (holotype) | ICMP 20479 | LC131392 | LC131429 |
| New Zealand | PDD 107916 | ICMP 21228 | LC131393 | LC131430 | |
| Dacrymyces parastenosporus | New Zealand | PDD 104960 | ICMP 20433 | LC131394 | LC131431 |
| New Zealand | PDD 104963 (holotype) | ICMP 20436 | LC131395 | LC131432 | |
| Dacrymyces pinacearum | Japan | TNS-F-21056 (holotype) | MAFF 241182 | AB472718 | AB712461 |
| Dacrymyces punctiformis | Japan | TNS-F-15723 | MAFF 240138 | AB299052 | AB712462 |
| Japan | TNS-F-15725 | MAFF 240140 | AB299071 | – | |
| Dacrymyces san-augustinii | Japan | TNS-F-15726 | MAFF 240141 | AB299081 | AB712463 |
| Japan | TNS-F-21075 | MAFF 241196 | AB472735 | – | |
| Dacrymyces stenosporus | New Zealand | PDD 105018 (holotype) | >ICMP 20488 | LC131396 | LC131433 |
| New Zealand | PDD 107970 | ICMP 21237 | LC131397 | LC131434 | |
| Dacrymyces cf. stillatus | New Zealand | PDD 105038 | ICMP 20505 | LC131398 | – |
| Dacrymyces stillatus | Japan | TNS-F-15727 | MAFF 240142 | AB299061 | AB712464 |
| Japan | TNS-F-21052 | MAFF 241178 | AB472714 | – | |
| Germany | FO28136 | – | AF291309 | – | |
| Dacrymyces subalpinus | Japan | TNS-F-15730 | MAFF 240145 | AB299060 | AB712465 |
| Japan | TNS-F-21071 | MAFF 241193 | AB472731 | – | |
| Dacrymyces subantarcticensis | New Zealand | PDD 107948 | ICMP 21234 | LC131399 | LC131435 |
| New Zealand | PDD 107988 | – | LC131400 | LC131436 | |
| New Zealand | PDD 76679 (holotype) | HHB-18220 | AB712431 | AB712466 | |
| Dacrymyces subarcticus | Japan | TNS-F-21067 (holotype) | – | AB472727 | AB712467 |
| Japan | TNS-F-21076 | – | AB472736 | – | |
| Dacrymyces variisporus | Japan | TNS-F-15732 | MAFF 240147 | AB299067 | AB712470 |
| Japan | TNS-F-15733 | MAFF 240148 | AB299072 | – | |
| Dacryopinax elegans | USA | – | HHB-18731 | AB712433 | AB712471 |
| Dacryopinax indacocheae | Venezuela | – | CRM-72 | AB712434 | AB712472 |
| Dacryopinax spathularia | Japan | TNS-F-15736 | MAFF 240151 | AB299079 | – |
| Japan | TNS-F-21048 | MAFF 241174 | AB472710 | AB712473 | |
| Dacryopinax sphenocarpa | Japan | TNS-F-21046 (holotype) | MAFF 241173 | AB472708 | AB712474 |
| Japan | TNS-F-21066 | MAFF 241189 | AB472726 | – | |
| Dacryoscyphus chrysochilus | China | KUN F45014 (holotype) | – | AY604567 | – |
| Ditiola haasii | Germany | RoKi100 | – | AF291314 | – |
| Femsjonia peziziformis | Japan | TNS-F-15737 | MAFF 240152 | AB299080 | AB712476 |
| Germany | FO25100 | – | AF291330 | – | |
| Guepiniopsis buccina | Japan | TNS-F-15738 | MAFF 240153 | AB299085 | AB712477 |
| USA | AFTOL-ID 888 | – | AY745711 | DQ206986 | |
| Heterotextus miltinus | New Zealand | PDD 104962 | ICMP 20435 | LC131401 | LC131437 |
| New Zealand | PDD 107924 | ICMP 21229 | LC131402 | LC131438 | |
| New Zealand | – | ICMP 16702 | AB712436 | AB712478 | |
| Unilacryma unispora | Japan | TNS-F-15731 | MAFF 240146 | AB299074 | AB712468 |
| Japan | TNS-F-38904 | – | AB712432 | AB712469 | |
| Coprinus comatus | USA | AFTOL-ID 626 | – | AY635772 | AY854066 |
| Suillus pictus | USA | AFTOL-ID 717 | – | AY684154 | AY854069 |
Newly described species as well as specimens, cultures, and sequences obtained in this study are shown in bold.
1 PDD, Fungal and Plant Disease Collection (New Zealand).
2 ICMP, International Collection of Micro-organisms from Plants (New Zealand).
DNA sequencing and phylogenetic analysis
Fresh tissues of fruiting bodies were soaked at 4 °C in DMSO buffer (Seutin et al. 1991) containing 100 mM Tris-HCl (pH 8.0) and 0.1 M sodium sulphate (Na2SO3) until extraction. Soaked tissue samples were then ground in liquid nitrogen using a mortar and pestle. After grinding, samples were immediately transferred to 1.5 mL tubes along with 1 000 μL of 2× CTAB buffer (Doyle & Doyle 1987) followed by the addition of 0.1 M Na2SO3. Samples were incubated at 65 °C for 1 h and then centrifuged at 13 500 ×g for 5 min. The aqueous phase was transferred to a new tube and the precipitated tissue debris was discarded. After the addition of an equal volume of chloroform : isoamyl alcohol (24 : 1) and vigorous mixing for 2 min, the mixture was centrifuged at 13 500 ×g for 15 min. Using a pipette, the aqueous phase was transferred to a new tube. To c. 300 μL of the aqueous phase, 1 000 μL of 6 M sodium iodine buffer (6 M NaI, 50 mM Tris-HCl (pH 7.4), 10 mM EDTA, and 0.1 M Na2SO3) was added and mixed gently for 1 min. Twenty-five microlitres of a silica mixture prepared following the protocol of Rogstad (2003) was added to the samples. Samples were incubated at 55 °C for 1 h and then centrifuged at 13 500 ×g for c. 10 s. The supernatant was discarded and 750 μL of wash buffer (10 μL Tris-HCl (pH 7.4), 1 mM EDTA, 100 mM NaCl, and 50 % EtOH) was added and mixed briefly, followed by centrifugation at full speed for c. 5 s. This washing step was repeated twice. After washing, the samples were centrifuged at 13 500 ×g for 10 s; the remaining wash buffer was removed by pipetting, and the precipitated silica was dried at room temperature for 30 min to 1 h. Final elution was performed by adding 100 μL of ultrapure water with brief mixing, followed by incubation at 65 °C for 15 min. Samples were centrifuged at 13 500 ×g for 1 min. The supernatant layer was then transferred to a new tube and stored at -20 °C until PCR was performed.
DNA sequence data were obtained from large subunit (LSU) and internal transcribed spacer (ITS1-5.8S-ITS2, ITS) regions of nuclear rRNA. The primer combinations LR0R/LR5 (Vilgalys & Hester 1990) and ITS5/ITS4 (White et al. 1990) were used. PCR amplifications were carried out in 20 μL reaction volumes containing 1 μL genomic DNA, 1 μL dNTPs (4 mM), 1 μL of each primer (8 mM), 0.5 units of Taq polymerase (Takara, Kusatsu, Japan), 2 μL MgCl2 (25 mM), and 2 μL bovine serum albumin (10 mg/mL). Cycling parameters were 94 °C for 3 min, followed by 30 cycles of 94 °C for 1 min, 51 °C for 30 s, and 72 °C for 1 min, with a final extension at 72 °C for 15 min. PCR products were purified with ExoSAP-IT (Affymetrix, Santa Clara, CA, USA) and directly sequenced using a Big Dye Terminator Cycle Sequencing kit (Applied Biosystems, Norwalk, CT, USA) following the manufacturer’s instructions. The primers used for cycle sequencing were LR0R and LR5 (Vilgalys & Hester 1990) and ITS1 and ITS4 (White et al. 1990). The sequences determined in this study were deposited in the DNA Data Bank of Japan (DDBJ; Table 1).
Multiple sequence alignment of a combined dataset comprising the sequences obtained in this study and available sequences of Dacrymycetes and Agaricomycetes species downloaded from DDBJ was carried out with MAFFT v. 7 (mafft.cbrc.jp/ alignment/software; Katoh & Standley 2013). Poorly aligned sequence regions were removed prior to subsequent analysis. Molecular phylogenetic analysis of LSU and ITS sequences was performed in RAxML v. 8.1.15 (Stamatakis 2014) under a GTR+Γ model. The dataset was partitioned to allow different parameters for each gene region (LSU, ITS1, 5.8S, and ITS2). Maximum likelihood bootstrap percentages and the tree were obtained by simultaneously running rapid bootstrap analyses of 1 000 pseudoreplicates followed by a search for the most likely tree. The aligned dataset was uploaded to TreeBASE under ID S19007 (http://purl.org/phylo/treebase/phylows/study/TB2:S19007).
RESULTS
As a result of field collections, 441 specimens of fruiting bodies were obtained and 281 cultures were isolated. Immature or overmature fruiting bodies were omitted from subsequent observations and the molecular analysis.
Using the sequences obtained from collected samples and downloaded from DDBJ (Table 1), a phylogenetic tree was estimated in RAxML (Fig. 1). A total of 524 (LSU) and 824 (ITS) characters (including gaps) were used for the phylogenetic analysis.
Fig. 1.
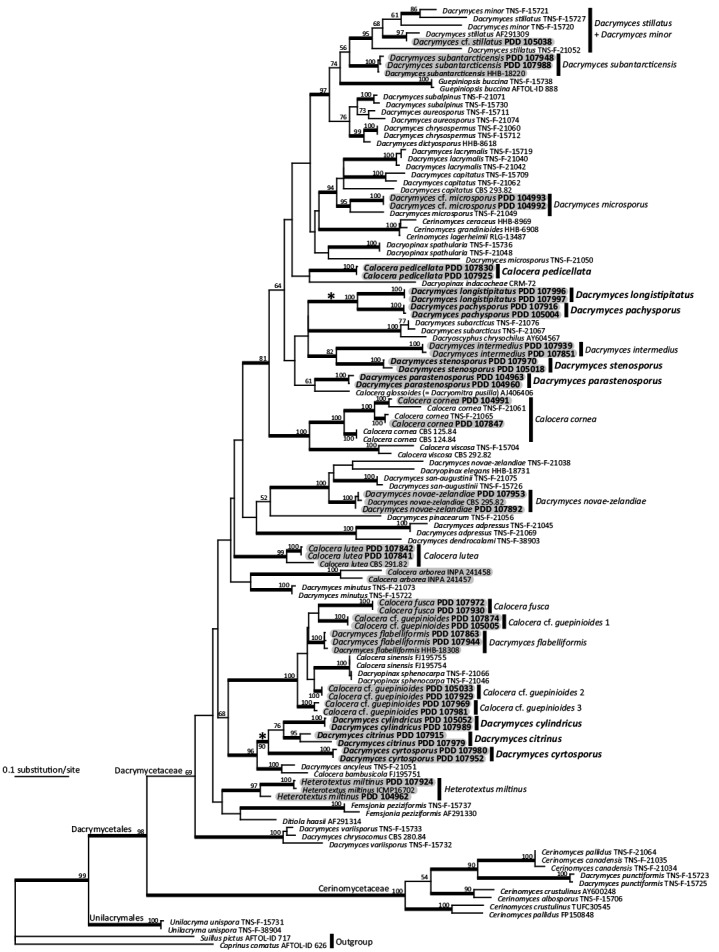
Phylogenetic tree of Dacrymycetes estimated in RAxML using concatenated LSU and ITS sequences. Maximum likelihood bootstrap percentages ≥ 50 % are shown above or below branches, with bolded branches indicating ≥ 80 % support. Newly described species and collected samples in this study are shown in bold. Southern Hemisphere strains are highlighted in grey. Asterisks denote clades comprising only New Zealand species. TreeBASE ID: S19007.
Sequences of the New Zealand samples obtained in this study were widely distributed within the Dacrymycetaceae clade, but were not found in Cerinomycetaceae and Unilacrymales clades (Fig. 1).
As described below, eight new and 11 known species were identified on the basis of morphological observations and the molecular phylogenetic analysis.
TAXONOMY
New species
Calocera pedicellata Shirouzu, sp. nov. —MycoBank MB817692; Fig. 2a, 3
Fig. 2.

Basidiocarps. a. Calocera pedicellata PDD 107925; b. Dacrymyces longistipitatus PDD 107997; c. Dacrymyces pachysporus PDD 107916; d. Dacrymyces stenosporus PDD 107970; e. Dacrymyces parastenosporus PDD 104963; f. Dacrymyces cylindricus PDD 105052; g. Dacrymyces citrinus PDD 107915; h. Dacrymyces cyrtosporus PDD 107980. — Scale bars = 5 mm.
Fig. 3.
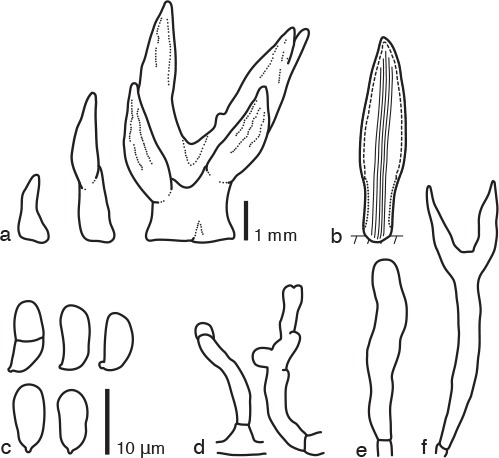
Calocera pedicellata PDD 107925. a. Basidiocarps; b. diagram showing longitudinal section of basidiocarp (solid lines: central core of compact parallel hyphae; dashed line: hymenium; dotted line: sterile marginal part); c. basidiospores; d. marginal hyphae; e. probasidium; f. basidium.
Differs from Calocera cornea by the basidiocarps consistently having stipes and by the presence of irregularly shaped terminal cells on the sterile surfaces.
Etymology. From the Latin ‘pedicellatus’ = pedicellate, referring to the stipitate basidiocarps.
Type. NEW ZEALAND, South Island, Denniston, Coalbrookdale Walk, on dead branches of a woody plant, 27 May 2015, T. Shirouzu (holotype PDD 107925; isotype TNS-F-65489, culture ex-type ICMP 21230).
DNA sequences from the holotype — LC131375 (LSU), LC131416 (ITS).
Basidiocarps scattered, cylindrical, subulate, sometimes palmate, simple or branched, stipitate-pileate, bearing cylindrical or subulate, sometimes rugose pilei, pale yellow to orange, soft-cartilaginous, 1–6 mm high, 0.5–1 mm diam, in transverse section through the pileus showing an organization into three zones, i.e. a central core of compact parallel hyphae surrounded by a zone of loosely interwoven hyphae enclosed by a hymenium. Internal hyphae branched, septate, thin- or thick-walled, hyaline, 2–5 μm diam, without clamp connections. Marginal hyphae on sterile surfaces of basidiocarps cylindrical, straight or flexuous, septate, hyaline, with irregularly shaped thin-walled terminal cells of 10–30 × 2–3 μm. Hymenium limited to the surface of the pileus, amphigenous, composed of basidia and simple cylindrical dikaryophyses. Probasidia cylindrical to clavate, pale yellow, 25–40 × 4–6 μm, without basal clamp connections, becoming bifurcate. Basidiospores cylindrical to reniform, straight or curved, with an apiculum at the base, thin-walled, hyaline, 9–12 × 4–6 μm (10.5 × 5 μm on average, n = 10), l/w 1.8–2.5 (2.1 on average), 0–1-septate.
Specimens examined. NEW ZEALAND, North Island, Tararua Forest Park, Kiriwhakapapa Road, on dead branches of a woody plant, 6 June 2015, T. Shirouzu, PDD 107959, culture ICMP 21246; South Island, Catlins Forest Park, Catlins River Track, on dead branches of Pinus radiata, 3 May 2015, T. Shirouzu, PDD 107830 (TNS-F-65488); Lake Brunner, Ara O Te Kinga, on dead branches of a woody plant, 18 May 2015, T. Shirouzu, PDD 107890, culture ICMP 21243.
Notes — Calocera pedicellata is characterised by cylindrical stipitate-pileate basidiocarps, irregularly shaped terminal cells, and small 1-septate basidiospores. This species is assigned to the genus Calocera on the basis of the presence of cylindrical basidiocarps, three-zoned internal structures, and amphigenous hymenia. The most similar species to C. pedicellata is C. cornea. These two species share the characteristics of small cylindrical basidiocarps, hyphae without clamp connections, and small 0–1-septate basidiospores (McNabb 1965a). Calocera pedicellata is distinguished from C. cornea on the basis of the characteristics of the basidiocarps consistently having stipes and by irregularly shaped terminal cells on the sterile surfaces. Calocera pedicellata is phylogenetically distant from the samples accepted here as C. cornea (Fig. 1).
Dacrymyces longistipitatus Shirouzu, sp. nov. — MycoBank MB817693; Fig. 2b, 4
Fig. 4.
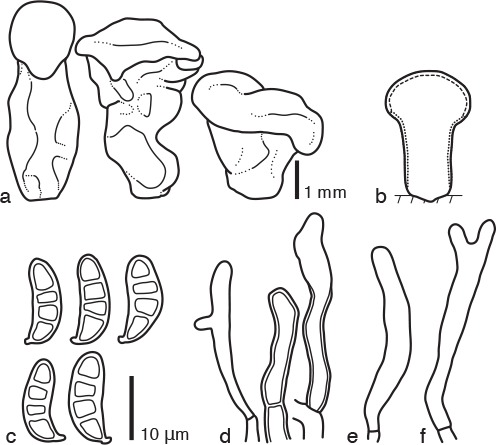
Dacrymyces longistipitatus PDD 107997. a. Basidiocarps; b. diagram showing longitudinal section of basidiocarp (dashed line: hymenium; dotted line: sterile marginal part); c. basidiospores; d. marginal hyphae; e. probasidium; f. developing basidium.
Differs from Dacrymyces capitatus by the basidiocarps having longer stipes and by its thicker-walled basidiospores.
Etymology. From the Latin ‘longus’ = long and ‘stipitatus’ = stipitate, referring to the basidiocarps with long stipes.
Type. NEW ZEALAND, North Island, Coromandel Forest Park, Rangihau Track, on dead branches of a broad-leaved tree, 22 June 2015, T. Shirouzu (holotype PDD 107997; isotype TNS-F-65501, culture ex-type ICMP 21242).
DNA sequences from the holotype — LC131387 (LSU), LC131426 (ITS).
Basidiocarps scattered, cylindrical to turbinate, simple, stipitate-pileate, bearing a cylindrical to subglobose, sometimes discoid pileus, pale yellow to pale olive, firm-gelatinous to soft-cartilaginous, 2–6 mm high, 1–3 mm diam. Internal hyphae branched, septate, thin-walled, hyaline, 2–4 μm diam, without clamp connections. Marginal hyphae on sterile surfaces of basidiocarps cylindrical, straight or flexuous, septate, hyaline, with irregularly shaped thin- or slightly thick-walled terminal cells of 20–30 × 3–4 μm. Hymenium limited to the surface of the pileus, amphigenous, composed of basidia. Probasidia cylindrical to clavate, pale yellow, 30–40 × 3–4 μm, without basal clamp connections, becoming bifurcate. Basidiospores cylindrical to reniform, curved, with an apiculum at the base, thick-walled, hyaline to pale yellow, 12–15 × 4–5 μm (14 × 4.5 μm on average, n = 10), l/w 2.4–3.8 (3 on average), 0–3-septate.
Specimens examined. NEW ZEALAND, North Island, Coromandel Forest Park, Rangihau Track, on dead branches of a woody plant, 22 June 2015, T. Shirouzu, PDD 107996 (TNS-F-65500), culture ICMP 21241; Whenuakite Block, on dead branches of a conifer, 22 June 2015, T. Shirouzu, PDD 107995; South Island, Westland Tai Poutini National Park, Fox Glacier, on dead branches of a woody plant, 16 May 2015, T. Shirouzu, PDD 107885; Lake Brunner, Ara O Te Kinga, on dead branches of a woody plant, 18 May 2015, T. Shirouzu, PDD 107889.
Notes — Dacrymyces longistipitatus is characterised by cylindrical to turbinate stipitate-pileate basidiocarps, irregularly shaped slightly thick-walled terminal cells, and thick-walled 3-septate basidiospores. This species is similar to D. capitatus and D. dacryomitriformis in having stipitate-pileate basidiocarps, hyphae lacking clamp connections, and 3-septate basidiospores. Compared with D. longistipitatus, D. capitatus has shorter-stiped basidiocarps and thinner-walled basidiospores (McNabb 1973). Dacrymyces longistipitatus is phylogenetically distant from specimens accepted here as D. capitatus (Fig. 1). In contrast to D. longistipitatus, D. dacryomitriformis has simple or sparingly branched dikaryophyses, relatively long probasidia (35–60 × 3.5–5 μm), and thin-walled basidiospores with thick septa (McNabb 1973).
Dacrymyces pachysporus Shirouzu, sp. nov. — MycoBank MB817694; Fig. 2c, 5
Fig. 5.
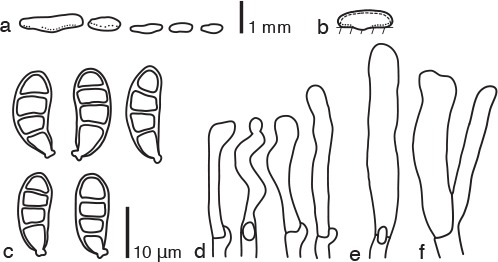
Dacrymyces pachysporus PDD 105004. a. Basidiocarps; b. diagram showing longitudinal section of basidiocarp (dashed line: hymenium; dotted line: sterile marginal part); c. basidiospores; d. marginal hyphae; e. probasidium; f. developing basidium and dikaryophysis.
Differs from Dacrymyces sichuanensis by the presence of longer basidiospores and the absence of branched dikaryophyses.
Etymology. From the Greek ‘pachy’ = thick and ‘sporus’ = spore, referring to the thick-walled basidiospores.
Type. NEW ZEALAND, South Island, Nelson Lakes National Park, Lake Lotoiti, on dead branches of Leptospermum sp. or Kunzea sp., 8 May 2014, T. Shirouzu (holotype PDD 105004; isotype TNS-F-65506, culture ex-type ICMP 20479).
DNA sequences from the holotype — LC131392 (LSU), LC131429 (ITS).
Basidiocarps scattered or gregarious, sometimes coalesced, pustulate to pulvinate, sessile, orange, firm-gelatinous, 0.5–1 mm high, 0.5–2 mm diam. Internal hyphae branched, septate, thin-walled, hyaline, 2–3 μm diam, with clamp connections. Marginal hyphae on sterile surfaces of basidiocarps cylindrical, straight or flexuous, septate, hyaline, with irregularly shaped thin-walled terminal cells of 30–45 × 3–5 μm. Hymenium limited to the ventral surface of the basidiocarp, amphigenous, composed of basidia and simple cylindrical dikaryophyses. Probasidia cylindrical to clavate, pale yellow, 35–55 × 5 μm, with basal clamp connections, becoming bifurcate. Basidiospores cylindrical to reniform, straight or curved, with an apiculum at the base, thick-walled, hyaline to pale yellow, 16–19 × 6–7 μm (17 × 6 μm on average, n = 10), l/w 2.3–3.2 (2.8 on average), 0–3-septate.
Specimens examined. NEW ZEALAND, South Island, Victoria Forest Park, Mt Haast Route, on dead branches of a woody plant, 22 May 2015, T. Shirouzu, PDD 107916 (TNS-F-65507), culture ICMP 21228.
Notes — Dacrymyces pachysporus is characterised by its small pustulate to pulvinate basidiocarps, hyphae with clamp connections, and long thick-walled 0–3-septate basidiospores. This species is similar to D. sichuanensis and D. stillatus in having small pustulate to pulvinate sessile basidiocarps and 0–3-septate thick-walled basidiospores. Dacrymyces sichuanensis has shorter basidiospores (12.5–15.6 × 4.5–6.5 μm, Liu & Fan 1990) and branched dikaryophyses, the latter discerned based on a line drawing in Liu & Fan (1990). Dacrymyces stillatus has no clamp connections on hyphae (McNabb 1973, Shirouzu et al. 2009). Dacrymyces pachysporus is also similar to D. punctiformis in having small pustulate to pulvinate sessile basidiocarps and clamp connections on hyphae, but D. punctiformis has thin-walled smaller basidiospores (10–15 × 3.5–5 μm, as Dacrymyces tortus, McNabb 1973; 7–13 × 4–6 μm, Shirouzu et al. 2009). Samples accepted here as D. punctiformis and D. stillatus are phylogenetically distant from D. pachysporus (Fig. 1).
Dacrymyces stenosporus Shirouzu, sp. nov. — MycoBank MB817695; Fig. 2d, 6
Fig. 6.
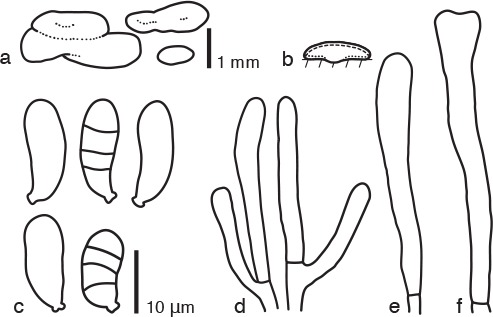
Dacrymyces stenosporus PDD 105018. a. Basidiocarps; b. diagram showing longitudinal section of basidiocarp (dashed line: hymenium; dotted line: sterile marginal part); c. basidiospores; d. marginal hyphae; e probasidium; f. developing basidium.
Differs from Dacrymyces lacrymalis by its longer basidiospores.
Etymology. From the Greek ‘stenos’ = narrow and ‘sporus’ = spore, referring to the slender basidiospores.
Type. NEW ZEALAND, South Island, Nelson Lakes National Park, Lake Rotoroa, on dead branches of Coprosma robusta, 9 May 2014, T. Shirouzu (holotype PDD 105018; isotype TNS-F-65510, culture ex-type ICMP 20488).
DNA sequences from the holotype — LC131396 (LSU), LC131433 (ITS).
Basidiocarps scattered or gregarious, sometimes coalesced, pulvinate to irregularly discoid, sometimes gyrose, sessile, pale to orange yellow, firm-gelatinous, 1–2 mm high, 1–5 mm diam. Internal hyphae branched, septate, thin-walled, hyaline, 2–3 μm diam, without clamp connections. Marginal hyphae on sterile surfaces of basidiocarps cylindrical, straight or flexuous, septate, hyaline, with cylindrical thin-walled terminal cells of 20–30 × 2–3 μm. Hymenium limited to the ventral surface of the basidiocarp, amphigenous, composed of basidia and simple cylindrical dikaryophyses. Probasidia cylindrical to clavate, pale yellow, 30–40 × 4 μm, without basal clamp connections, becoming bifurcate. Basidiospores cylindrical to reniform, curved, with an apiculum at the base, thin-walled, hyaline to pale yellow, 13–17 × 5–6 μm (15 × 5.5 μm on average, n = 10), l/w 2.5–3.2 (2.8 on average), 0–3-septate.
Specimens examined. NEW ZEALAND, North Island, Tongariro National Park, Rotopounamu Walk, on dead branches of a broad-leaved tree, 18 June 2015, T. Shirouzu, PDD 107990; Whanganui National Park, Pipiriki, on dead branches of a broad-leaved tree, 11 June 2015, T. Shirouzu, PDD 107970 (TNS-F-65511), culture ICMP 21237; South Island, Fiordland National Park, Kepler Track, on dead branches of a woody plant, 10 May 2015, T. Shirouzu, PDD 107852; Kahurangi National Park, Wangapeka Track, on dead branches of a woody plant, 10 May 2014, T. Shirouzu, PDD 105022, culture ICMP 20491.
Notes — Dacrymyces stenosporus is characterised by its pulvinate to irregularly discoid basidiocarps and slender 0–3-septate basidiospores. This species is similar to D. lacrymalis, D. minor, D. neoalbidus, and D. subantarcticensis in having pulvinate sessile basidiocarps, hyphae without clamp connections, and 0–3-septate thin-walled basidiospores. Dacrymyces lacrymalis (10–15.5 × 4.5–6 μm, McNabb 1973; 9.5–15 × 3.5–6 μm, Shirouzu et al. 2009) and D. subantarcticensis (10–13 × 4.5–6 μm, Burdsall & Laursen 2004) have shorter basidiospores, and D. minor is characterized by having smaller basidiocarps (0.5–2 mm diam, McNabb 1973; 1–2 mm diam, Shirouzu et al. 2009). Dacrymyces subantarcticensis and samples accepted here as D. lacrymalis and D. minor are phylogenetically distant from D. stenosporus (Fig. 1). Dacrymyces neoalbidus has white fruiting bodies and larger basidiospores (21–22 × 5–6 μm, as Dacrymyces albidus, Kobayasi 1954, 1955).
Dacrymyces parastenosporus Shirouzu, sp. nov. — MycoBank MB817696; Fig. 2e, 7
Fig. 7.
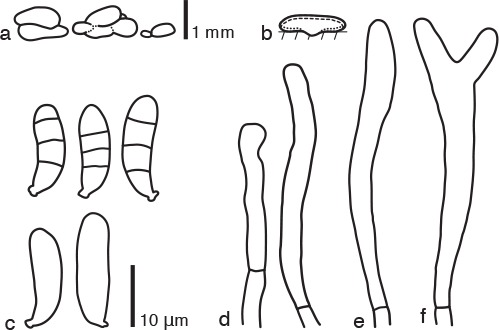
Dacrymyces parastenosporus PDD 104963. a. Basidiocarps; b. diagram showing longitudinal section of basidiocarp (dashed line: hymenium; dotted line: sterile marginal part); c. basidiospores; d. marginal hyphae; e. probasidium; f. developing basidium.
Differs from D. stenosporus by having longer probasidia.
Etymology. From the Greek ‘para’ = near and the epithet ‘stenosporus’, referring to its similarity to D. stenosporus.
Type. NEW ZEALAND, South Island, Arthur’s Pass National Park, Waimakariri River, on dead branches of a woody plant, 4 May 2014, T. Shirouzu (holotype PDD 104963; isotype TNS-F-65509, culture ex-type ICMP 20436).
DNA sequences from the holotype — LC131395 (LSU), LC131432 (ITS).
Basidiocarps scattered or gregarious, coalesced, pustulate to pulvinate, gyrose, sessile, orange yellow, firm-gelatinous, 0.5–1 mm high, 1–4 mm diam. Internal hyphae branched, septate, thin-walled, hyaline, 2–5 μm diam, without clamp connections. Marginal hyphae on sterile surfaces of basidiocarps cylindrical, straight or flexuous, septate, hyaline, with cylindrical thin-walled terminal cells of 20–30 × 2–3 μm. Hymenium limited to the ventral surface of the basidiocarp, amphigenous, composed of basidia and simple cylindrical dikaryophyses. Probasidia cylindrical to clavate, pale yellow, 40–50 × 5 μm, without basal clamp connections, becoming bifurcate. Basidiospores cylindrical, straight or slightly curved, with an apiculum at the base, thin-walled, hyaline to pale yellow, 14–17 × 4–6 μm (16 × 5 μm on average, n = 10), l/w 2.8–4.3 (3.4 on average), 0–3-septate.
Specimens examined. NEW ZEALAND, North Island, Tararua Forest Park, Kiriwhakapapa Road, on dead branches of a broad-leaved tree, 6 June 2015, T. Shirouzu, PDD 107962; South Island, Craigieburn Forest Park, Dracophyllum Flat Track, on dead branches of Pinus radiata, 4 May 2014, T. Shirouzu, PDD 104960 (TNS-F-65508), culture ICMP 20433; Fiordland National Park, Lake Hauroko, on dead branches of a broad-leaved tree, 8 May 2015, T. Shirouzu, PDD 107843; Victoria Forest Park, Waimakariri Valley, on dead branches of a broad-leaved tree, 19 May 2015, T. Shirouzu, PDD 107895.
Notes — Dacrymyces parastenosporus is characterised by its pustulate to pulvinate basidiocarps and slender 0–3-septate basidiospores. This species is similar to Dacrymyces stenosporus, but the latter species has shorter probasidia (30–40 × 4 μm). These two species are phylogenetically distant from one another (Fig. 1).
Dacrymyces cylindricus Shirouzu, sp. nov. — MycoBank MB817697; Fig. 2f, 8
Fig. 8.
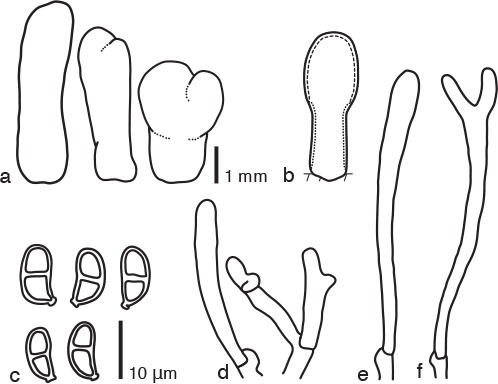
Dacrymyces cylindricus PDD 105052. a. Basidiocarps; b. diagram showing longitudinal section of basidiocarp (dashed line: hymenium; dotted line: sterile marginal part); c. basidiospores; d. marginal hyphae; e. probasidium; f. developing basidium.
Differs from Dacrymyces ancyleus by the presence of smaller thick-walled basidiospores.
Etymology. From the Latin ‘cylindricus’ = cylindrical, referring to the shape of the basidiocarps.
Type. NEW ZEALAND, South Island, Kahurangi National Park, Kaituna Track, on dead branches of a broad-leaved tree, 15 May 2014, T. Shirouzu (holotype PDD 105052; isotype TNS-F-65492, culture ex-type ICMP 20517).
DNA sequences from the holotype — LC131378 (LSU), LC131419 (ITS).
Basidiocarps scattered, cylindrical to subulate, simple, stipitate-pileate, bearing a cylindrical to subglobose, sometimes subulate pileus, white to pale yellow, firm-gelatinous to soft-cartilaginous, 2–4 mm high, 2–3 mm diam. Internal hyphae branched, septate, thin-walled, hyaline, 2–4 μm diam, with clamp connections. Marginal hyphae on sterile surfaces of basidiocarps cylindrical, straight or flexuous, septate, hyaline, with irregularly shaped thin-walled terminal cells of 25–35 × 3 μm. Hymenium limited to the surface of the pileus, amphigenous, composed of basidia. Probasidia cylindrical to clavate, pale yellow, 40–50 × 4 μm, with basal clamp connections, becoming bifurcate. Basidiospores cylindrical to reniform, straight or curved, with an apiculum at the base, thick-walled, hyaline, 8–10 × 4–5 μm (9 × 4 μm on average, n = 10), l/w 2–2.5 (2.3 on average), 0–1-septate.
Specimens examined. NEW ZEALAND, North Island, Tararua Forest Park, Kiriwhakapapa Road, 6 June 2015, T. Shirouzu, PDD 107960, culture ICMP 21247; Tongariro National Park, Rotopou namu Walk, on dead branches of a woody plant, 18 June 2015, T. Shirouzu, PDD 107989 (TNS-F-65493); South Island, Mt Richmond Forest Park, Pelorus Bridge, on dead branches of a woody plant, 30 May 2015, T. Shirouzu, PDD 107933; Nelson Lakes National Park, Lake Rotoiti, on dead branches of a broad-leaved tree, 1 June 2015, T. Shirouzu, PDD 107945.
Notes — Dacrymyces cylindricus is characterised by its cylindrical to subulate basidiocarps, hyphae with clamp connections, and small thick-walled 1-septate basidiospores. The irregularly shaped terminal cells are also diagnostic characters of this species. Dacrymyces cylindricus has cylindrical to subulate basidiocarps, but its fruiting bodies lack the three-zoned internal structure of species in the genus Calocera. Furthermore, this new species is not placed in Dacryopinax because the pileus is cylindrical to subglobose and the hymenium is amphigenous. Consequently, this fungus should be assigned to the genus Dacrymyces. Dacrymyces cylindricus is similar to D. ancyleus and D. flabelliformis in having stipitate-pileate basidiocarps and clamp connections on hyphae. Dacrymyces ancyleus has larger thin-walled basidiospores (10.5–19.5 × 4–9 μm, Shirouzu et al. 2009). Dacrymyces flabelliformis has spathulate to flabelliform basidiocarps and larger thin-walled 0–3-septate basidiospores (12.5–14 × 5–6 μm, Burdsall & Laursen 2004). These two species are phylogenetically distant from D. cylindricus (Fig. 1).
Dacrymyces citrinus Shirouzu, sp. nov. — MycoBank MB817698; Fig. 2g, 9
Fig. 9.
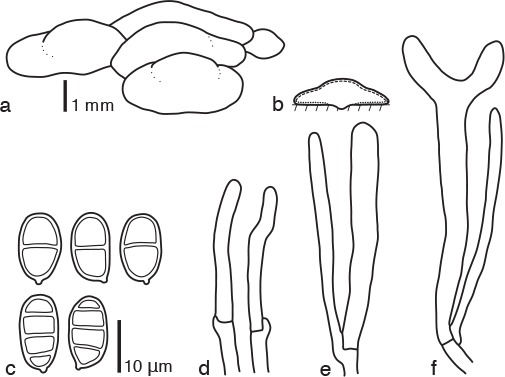
Dacrymyces citrinus PDD 107915. a. Basidiocarps; b. diagram showing longitudinal section of basidiocarp (dashed line: hymenium; dotted line: sterile marginal part); c. basidiospores; d. marginal hyphae; e. probasidium and dikaryophysis; f. developing basidium and dikaryophysis.
Differs from Dacrymyces enatus var. macrosporus by its wider basidiospores and the absence of branched dikaryophyses.
Etymology. From the Latin ‘citrinus’ = pale yellow, referring to the colour of the basidiocarps.
Type. NEW ZEALAND, South Island, Victoria Forest Park, Mt Haast Route, on dead branches of a woody plant, 22 May 2015, T. Shirouzu (holotype PDD 107915, isotype TNS-F-65490, culture ex-type ICMP 21227).
DNA sequences from the holotype — LC131376 (LSU), LC131417 (ITS).
Basidiocarps scattered or gregarious, sometimes coalesced, pustulate to pulvinate, sessile, pale yellow to yellow, firm-gelatinous, 0.5–1 mm high, 1–5 mm diam. Internal hyphae branched, septate, thin-walled, hyaline, 2–5 μm diam, with clamp connections. Marginal hyphae on sterile surfaces of basidiocarps cylindrical, straight or flexuous, septate, hyaline, with cylindrical thin-walled terminal cells of 20–40 × 2–5 μm. Hymenium limited to the ventral surface of the basidiocarp, amphigenous, composed of basidia and simple cylindrical dikaryophyses. Probasidia cylindrical to clavate, pale yellow, 35–45 × 5–6 μm, with basal clamp connections, becoming bifurcate. Basidiospores cylindrical to reniform, straight, with an apiculum at the base, thick-walled, hyaline to pale yellow, 11–14 × 7–9 μm (13 × 8 μm on average, n = 10), l/w 1.5–2 (1.7 on average), 0–3-septate.
Specimens examined. NEW ZEALAND, North Island, Tararua Forest Park, Waiohine Gorge, on dead branches of a broad-leaved tree, 5 June 2015, T. Shirouzu, PDD 107949; Whanganui National Park, Atene Viewpoint Walk, on dead branches of a woody plant, 12 June 2015, T. Shirouzu, PDD 107979 (TNS-F-65491), culture ICMP 21239; South Island, Fiordland National Park, Lake Hauroko, on dead branches of a broad-leaved tree, 7 May 2015, T. Shirouzu, PDD 107837; Kahurangi National Park, Wangapeka Track, on dead branches of Leptospermum scoparium, 31 May 2015, T. Shirouzu, PDD 107934.
Notes — Dacrymyces citrinus is characterised by the presence of pulvinate yellow basidiocarps, hyphae with clamp connections, and wide, thick-walled, 3-septate basidiospores. This species is similar to D. enatus var. macrosporus, D. paraphysatus, D. sichuanensis, and D. pachysporus in having pulvinate basidiocarps, hyphae with clamp connections, and 3-septate thick-walled basidiospores. Dacrymyces enatus var. macrosporus has thinner basidiospores (11–15.5 × 4.5–6.5 μm), branched dikaryophyses, and dark basidiocarps (McNabb 1973). Dacrymyces paraphysatus has longer basidiospores (13.5–21 × 5–7 μm) and branched dikaryophyses (McNabb 1973). Dacrymyces sichuanensis has smaller basidiocarps (1–2 mm diam), narrower basidiospores (12.5–15.6 × 4.5–6.5 μm), and branched dikaryophyses as discerned from a line drawing in Liu & Fan (1990). Dacrymyces pachysporus has smaller basidiocarps (0.5–2 mm diam), longer basidiospores (16–19 × 6–7 μm), and irregularly shaped terminal cells (Fig. 4). Dacrymyces citrinus is phylogenetically distant from D. pachysporus (Fig. 1). Some specimens of D. citrinus have slightly slender basidiospores (e.g. 13–14 × 6–7 μm, l/w 1.9–2.3, PDD 107979) but are phylogenetically indistinguishable from those with wider spores (Fig. 1).
Dacrymyces cyrtosporus Shirouzu, sp. nov. — MycoBank MB817699; Fig. 2h, 10
Fig. 10.
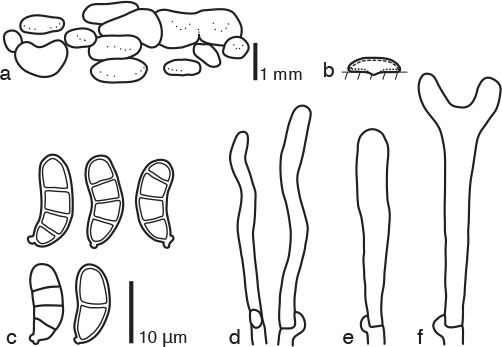
Dacrymyces cyrtosporus PDD 107980. a. Basidiocarps; b. diagram showing longitudinal section of basidiocarp (dashed line: hymenium; dotted line: sterile marginal part); c. basidiospores; d. marginal hyphae; e. probasidium; f. developing basidium.
Differs from D. sichuanensis by the absence of branched dikaryophyses.
Etymology. From the Greek ‘cyrto’ = bent or curved and ‘sporus’ = spore, referring to the curved basidiospores.
Type. NEW ZEALAND, North Island, Whanganui National Park, Atene Viewpoint Walk, on dead branches of a woody plant, 13 June 2015, T. Shirouzu (holotype PDD 107980; isotype TNS-F-65495).
DNA sequences from the holotype — LC131381 (LSU), LC131422 (ITS).
Basidiocarps scattered or gregarious, sometimes coalesced, pustulate to pulvinate, sessile, pale yellow to olive, firm-gelatinous, 0.5 mm high, 0.5–2 mm diam. Internal hyphae branched, septate, thin-walled, hyaline, 2–3 μm diam, with clamp connections. Marginal hyphae on sterile surfaces of basidiocarps cylindrical, straight or flexuous, septate, hyaline, with cylindrical thin-walled terminal cells of 15–45 × 2–3 μm. Hymenium limited to the ventral surface of the basidiocarp, amphigenous, composed of basidia and simple cylindrical dikaryophyses. Probasidia cylindrical to clavate, hyaline, 30–50 × 5–6 μm, with basal clamp connections, becoming bifurcate. Basidiospores cylindrical, curved, with an apiculum at the base, thick-walled, hyaline, 13–15 × 5–6 μm (14 × 5.5 μm on average, n = 10), l/w 2.2–3 (2.6 on average), 0–3-septate.
Specimens examined. NEW ZEALAND, North Island, Tararua Forest Park, Kiriwhakapapa Road, on dead branches of a woody plant, 6 June 2015, T. Shirouzu, PDD 107957, culture ICMP 21245; Tararua Forest Park, Waiohine Gorge, on dead branches of a broad-leaved tree, 5 June 2015, T. Shirouzu, PDD 107951, culture ICMP 21244; PDD 107952 (TNS-F-65494); South Island, Fiordland National Park, Borland Nature Walk, on dead branches of a woody plant, 12 May 2015, T. Shirouzu, PDD 107867.
Notes — Dacrymyces cyrtosporus is characterised by its pustulate to pulvinate basidiocarps, hyphae with clamp connections, and curved thick-walled 3-septate basidiospores. This species is similar to D. enatus var. macrosporus, D. paraphysatus, D. sichuanensis, D. pachysporus, and D. citrinus in having pulvinate basidiocarps, hyphae with clamp connections, and 3-septate thick-walled basidiospores. However, D. sichuanensis has branched dikaryophyses as discerned from a line drawing in Liu & Fan (1990). Dacrymyces enatus var. macrosporus has larger, dark basidiocarps (3–4 mm diam) and branched dikaryophyses (McNabb 1973). Dacrymyces paraphysatus has branched dikaryophyses and yellowish brown, larger basidiospores (13.5–21 × 5–7 μm, McNabb 1973). Dacrymyces pachysporus has irregularly shaped terminal cells (Fig. 5) and longer basidiospores (16–19 × 6–7 μm). Dacrymyces citrinus has larger basidiocarps (1–5 mm diam) and wider, straight basidiospores (11–14 × 7–9 μm). Dacrymyces cyrtosporus is phylogenetically distant from D. pachysporus and D. citrinus (Fig. 1).
Known species
Calocera cornea (Batsch) Fr., Stirp. Agri Fems. 5: 67. 1827
Type locality. Germany.
Specimens examined. NEW ZEALAND, South Island, Fiordland National Park, Lake Hauroko, on dead branches of a woody plant, 8 May 2015, T. Shirouzu, PDD 107847, culture ICMP 21223; Granville Ecological Area, Granville Road, on dead branches of a woody plant, 6 May 2014, T. Shirouzu, PDD 104991, culture ICMP 20465.
Notes — Calocera cornea was morphologically identified with reference to McNabb (1965a), Reid (1974), and Shirouzu et al. (2009). The sequences obtained in this study formed a clade with Japanese (TNS-F-21061, 21065) and North American (CBS 124.84, 125.84) strains identified as C. cornea (Fig. 1). Calocera cornea is a common species of Dacrymycetes and has been recorded worldwide (McNabb 1965a, Lowy 1971, Reid 1974, Shirouzu et al. 2009). The geographical and phylogenetic distributions of C. cornea seem wide and diverse, suggesting that it could be a species complex.
Calocera fusca Lloyd, Mycol. Writings 7 (75): 1357. 1925
Type locality. Canterbury, New Zealand.
Specimens examined. NEW ZEALAND, North Island, Whanganui National Park, Pipiriki, on dead branches of a broad-leaved tree, 11 June 2015, T. Shirouzu, PDD 107972, culture ICMP 21238; South Island, Mt Richmond Forest Park, Pelorus Bridge, on dead branches of a broad-leaved tree, 30 May 2015, T. Shirouzu, PDD 107930.
Notes — Calocera fusca was morphologically identified with reference to McNabb (1965a). This species has also been recorded from the Juan Fernández Islands (McNabb 1965a). The sequence obtained in this study is the first DNA sequence data provided for C. fusca.
Calocera cf. guepinioides Berk., London J. Bot. 4: 61. 1845
Type locality. Swan River, West Australia.
Specimens examined. NEW ZEALAND, North Island, Kaimanawa Forest Park, Clements Mill Road, on dead branches of a woody plant, 16 June 2015, T. Shirouzu, PDD 107981, culture ICMP 21240; Tararua Forest Park, Waiotauru Track, on dead branches of a woody plant, 8 June 2015, T. Shirouzu, PDD 107969, culture ICMP 21236; South Island, Kahurangi National Park, Heaphy Track, on dead branches of a woody plant, 28 May 2015, T. Shirouzu, PDD 107929, culture ICMP 21231; Mt Aspiring National Park, Haast Pass Lookout, on dead branches of a woody plant, 15 May 2015, T. Shirouzu, PDD 107874, culture ICMP 21226; Nelson, Fringed Hill, on dead branches of a woody plant, 12 May 2014, T. Shirouzu, PDD 105033, culture ICMP 20502; Nelson Lakes National Park, Lake Rotoiti, on dead branches of a woody plant, 8 May 2014, T. Shirouzu, PDD 105005, culture ICMP 20480.
Notes — These specimens were morphologically identified with reference to McNabb (1965a). Phylogenetic analysis separated the sequences obtained from the samples into three clades (Calocera cf. guepinioides 1, 2, and 3; Fig. 1). The specimens constituting each clade could not be morphologically distinguished, and the true clade of C. guepinioides could be not confirmed because DNA from the type strain was not included in this study. Calocera guepinioides has already been recorded from New Zealand (McNabb 1965a). This species has originally been described from Western Australia; the inclusion of samples from such areas is critically needed in phylogenetic and taxonomic studies.
Calocera lutea (Massee) McNabb, New Zealand J. Bot. 3: 46. 1965
Type locality. Tasmania, Australia.
Specimens examined. NEW ZEALAND, South Island, Fiordland National Park, Lake Hauroko, on dead branches of a woody plant, 8 May 2015, T. Shirouzu, PDD 107841, culture ICMP 21221; PDD 107842, culture ICMP 21222.
Notes — Calocera lutea, originally described from Tasmania, was morphologically identified with reference to McNabb (1965a). This species has already been recorded from New Zealand (McNabb 1965a). The sequences obtained in this study formed a clade with an Australian strain (CBS 291.82; Fig. 1). Seifert (1983) has reported that a decomposition test using the Australian strain of C. lutea revealed features of white rot, but our specimens collected in New Zealand showed characteristics of brown rot, such as brown discoloration and cracking into roughly cubical pieces of wood.
Dacrymyces flabelliformis Burds. & Laursen, Mem. New York Bot. Gard. 89: 109. 2004
Type locality. Auckland Islands, New Zealand.
Specimens examined. NEW ZEALAND, South Island, Fiordland National Park, Borland Nature Walk, on dead branches of a broad-leaved tree, 12 May 2015, T. Shirouzu, PDD 107863, culture ICMP 21225; Nelson Lakes National Park, Lake Rotoiti, on dead branches of a broad-leaved tree, 1 June 2015, T. Shirouzu, PDD 107944, culture ICMP 21233.
Notes — Dacrymyces flabelliformis was morphologically identified with reference to the original description (Burdsall & Laursen 2004). The sequences obtained in this study were closely related to the ex-type strain collected from New Zealand (HHB-18308; Fig. 1). This species is presumably endemic to New Zealand.
Dacrymyces intermedius L.S. Olive, Bull. Torrey Bot. Club 85: 108. 1958
Type locality. Tahiti.
Specimens examined. NEW ZEALAND, South Island, Fiordland National Park, Kepler Track, on dead branches of a woody plant, 10 May 2015, T. Shirouzu, PDD 107851, culture ICMP 21224; Kahurangi National Park, Wangapeka Track, on dead branches of a broad-leaved tree, 31 May 2015, T. Shirouzu, PDD 107939, culture ICMP 21232.
Notes — Dacrymyces intermedius, originally described from Tahiti, was morphologically identified with reference to the original description (Olive 1958) and that in McNabb (1973). The species has not been reported from any other regions of the world, and no other DNA sequence data are available.
Dacrymyces cf.microsporus P. Karst., Bidrag Kannedom Finlands Natur Folk 48: 459. 1889
Type locality. Mustiala, Finland.
Specimens examined. NEW ZEALAND, South Island, Granville Ecological Area, Granville Road, on dead branches of a woody plant, 6 May 2014, T. Shirouzu, PDD 104992, culture ICMP 20466; PDD 104993, culture ICMP 20467.
Notes — These specimens were morphologically identified with reference to McNabb (1973) and Shirouzu et al. (2009). The sequences obtained from the New Zealand specimens were related to that of a Japanese strain (TNS-F-21049); however, a second Japanese strain (TNS-F-20150), although morphologically similar, was genetically distinct (Fig. 1). Sequences from the type specimen or authentically identified specimens from the type locality are needed to clarify the taxonomy of this species.
Dacrymyces novae-zelandiae McNabb, New Zealand J. Bot. 11: 493. 1973
Type locality. Auckland, New Zealand.
Specimens examined. NEW ZEALAND, North Island, Tararua Forest Park, Kiriwhakapapa Road, on dead branches of a broad-leaved tree, 6 June 2015, T. Shirouzu, PDD 107953, culture ICMP 21235; South Island, Greymouth, Point Elizabeth, on dead branches of a broad-leaved tree, 18 May 2015, T. Shirouzu, PDD 107892.
Notes — Dacrymyces novae-zelandiae, described on the basis of a New Zealand type, was morphologically identified with reference to the original description (McNabb 1973). The sequences obtained in this study were closely related to a New Zealand strain (CBS 295.82) collected near the type locality, but a morphologically similar Japanese strain was genetically distinct (TNS-F-21038; Fig. 1). This species is presumably endemic to New Zealand.
Dacrymyces cf. stillatus Nees, Syst. Mycol. 2: 250. 1822
Type locality. Europe.
Specimens examined. NEW ZEALAND, South Island, Farewell Spit, on dead branches of a woody plant, 13 May 2014, T. Shirouzu, PDD 105038, culture ICMP 20505.
Notes — This specimen was morphologically identified with reference to McNabb (1973), Reid (1974), and Shirouzu et al. (2009). The sequence obtained in this study was very close to that from a German strain (AF291309; Weiß & Oberwinkler 2001) and close to but distinct from Japanese strains identified as D. stillatus (TNS-F-15727) and D. minor (TNS-F-15720,15721; Fig. 1). According to McNabb (1973), D. stillatus can be distinguished from D. minor by its larger basidiocarps and thicker-walled basidiospores. Dacrymyces stillatus is a common species of Dacrymycetes and has been recorded worldwide (Lowy 1971, McNabb 1973, Reid 1974, Shirouzu et al. 2009).
Dacrymyces subantarcticensis Burds. & Laursen, Mem. New York Bot. Gard. 89: 107. 2004
Type locality. Campbell Island, New Zealand.
Specimens examined. NEW ZEALAND, North Island, Tongariro National Park, Rotopounamu Walk, on dead branches of a woody plant, 18 June 2015, T. Shirouzu, PDD 107988; South Island, Nelson Lakes National Park, Lake Rotoiti, on dead branches of a woody plant, 1 June 2015, T. Shirouzu, PDD 107948, culture ICMP 21234.
Notes — Dacrymyces subantarcticensis was morphologically identified with reference to the original description (Burdsall & Laursen 2004). The sequences obtained in this study were closely related to the type strain collected from Campbell Island (HHB-18220; Fig. 1). This species is presumably endemic to New Zealand.
Heterotextus miltinus (Berk.) McNabb, New Zealand J. Bot. 3: 220. 1965
Type locality. Tasmania, Australia.
Specimens examined. NEW ZEALAND, South Island, Arthur’s Pass National Park, Waimakariri River, on dead branches of Nothofagus solandri, 4 May 2014, T. Shirouzu, PDD 104962, ICMP 20435; Denniston, Coalbrookdale Walk, on dead branches of a broad-leaved tree, 27 May 2015, T. Shirouzu, PDD 107924, culture ICMP 21229.
Notes — Heterotextus miltinus, originally described from Tasmania, was morphologically identified with reference to McNabb (1965d). This species has already been recorded from New Zealand (McNabb 1965d). The sequences referred to H. miltinus in this study were genetically somewhat divergent but in a close sister relationship (Fig. 1). One of the isolates exactly matched a New Zealand strain (ICMP 16702, isolated from PDD 89156) from the North Island (Fig. 1).
DISCUSSION
Dacrymycetes species in the Southern Hemisphere
The phylogenetic hypothesis of Dacrymycetes was updated by the addition of eight new taxa as well as specimens referable to previously described species with no available DNA sequence data, namely, C. fusca, C. cf. guepinioides, and D. intermedius. Two monophyletic groups, one comprising D. longistipitatus and D. pachysporus and the other consisting of D. cylindricus, D. citrinus, and D. cyrtosporus, were each composed only of New Zealand species (Fig. 1). These clades might be unique lineages useful for characterisation of the dacrymycetous mycoflora of New Zealand.
Although specimens identified as Dacrymyces cf. stillatus, Dacrymyces cf. microsporus, and C. cornea were morphologically and phylogenetically related to strains from the Northern Hemisphere, unique species characterising New Zealand or the Southern Hemisphere Dacrymycetes were also collected in this study. The eight newly described taxa as well as seven known species, i.e., C. fusca, C. cf. guepinioides, C. lutea, D. flabelliformis, D. intermedius, D. subantarcticensis, and H. miltinus – which have been collected from New Zealand, Australia, Tahiti, and the Juan Fernández Islands (McNabb 1965a, d, 1973, Burdsall & Laursen 2004), have rarely or never been reported from the Northern Hemisphere. These known species were identified on the basis of morphology with the exception of D. flabelliformis and D. subantarcticensis, for which sequences from type specimens or authentically identified specimens from type localities are lacking. We believe that these eight new and seven known species reflect the unique Dacrymycetes mycoflora in the Southern Hemisphere and complement existing knowledge of the species diversity of this class.
Two new species, C. pedicellata and D. parastenosporus, were collected from dead branches of Pinus radiata, a conifer introduced from the west coast of the United States. We believe, however, that these dacrymycetous species are native to New Zealand, as they have never been reported from the original habitats of P. radiata and were additionally found on dead branches of unidentified local trees in the collection sites.
Morphologically indistinguishable species
Among the newly described taxa, six species – D. citrinus, D. cylindricus, D. cyrtosporus, D. longistipitatus, D. pachysporus, and D. pedicellata – were morphologically and phylogenetically distinct from other species. Two new species, D. stenosporus and D. parastenosporus, were morphologically similar to each other, but were described as two different species because they were phylogenetically distant from one another (Fig. 1). Dacrymyces parastenosporus can be distinguished from D. stenosporus in having longer probasidia. Although the size of probasidia has not been considered to be a significant criterion compared with characteristics such as shape and size of basidiocarps, basidiospores, and marginal hyphae, it might be a useful feature to distinguish some dacrymycetous species.
The molecular phylogenetic analysis separated the sequences obtained from C. cf. guepinioides specimens into three clades (C. cf. guepinioides 1, 2, and 3; Fig. 1). These specimens share the morphological features of small and typically spathulate basidiocarps, 1–3-septate spores, and clamp connections on hyphae that characterize C. guepinioides (McNabb 1965a). The clade corresponding to C. guepinioides s.str. could not be identified because no morphological differences were found among the three clades and no sequence exists from the type specimen. This species displays wide variation in the shape of basidiocarps (McNabb 1965a) and therefore might be separated into two or more species.
Higher classification in Dacrymycetes
Familial and generic classifications in Dacrymycetes are based on morphological criteria such as the shape and internal hyphal structure of basidiocarps, the position of the hymenium, and presence or absence of developed marginal hyphae (McNabb 1964, 1965a, 1965b, 1965c, 1965d, 1965e, 1966, 1973, McNabb & Talbot 1973, Reid 1974, Jülich 1981). However, this morphology-based classification has often conflicted with the results of molecular phylogenetic analyses, and Calocera, Cerinomyces, Dacrymyces, and Dacryopinax have been shown to be non-monophyletic genera (Fig. 1; Shirouzu et al. 2009, 2013a). As a result, Dacrymycetaceae and Cerinomycetaceae, the two families in Dacrymycetales, are also revealed to be non-monophyletic in various phylogenetic trees. No useful phenotypic features have been found for classification of families and genera that reflect their phylogenetic relationships.
The phylogenetic heterogeneity of the studied genera and families became even more obvious upon the addition of the sequences of New Zealand specimens. The polyphyletic nature of Dacrymyces and Calocera was particularly evident (Fig. 1). The genus Dacrymyces is mainly characterised by sessile pulvinate, turbinate, or sometimes stipitate basidiocarps, a homogeneous intra-structure of fruiting bodies, and an amphigenous hymenium (McNabb 1973), but its delineation has often been obscure (e.g. Reid 1974). The results of molecular phylogenetic analyses have supported this ambiguity (Shirouzu et al. 2007, 2009, 2013a), and Dacrymyces appears to be the most phylogenetically scattered genus in the Dacrymycetales clade (Fig. 1). The genus Calocera is characterised by cylindrical basidiocarps, a three-zoned intra-structure of fruiting bodies, and an amphigenous hymenium (McNabb 1965a). Because previous studies have demonstrated the sister relationship of C. cornea and C. viscosa (Weiß & Oberwinkler 2001, Shirouzu et al. 2007, 2009), the genus Calocera has been considered to be a monophyletic taxon. In the present phylogenetic tree, however, many of the Calocera species used in this study, such as C. arborea, C. bambusicola, C. fusca, C. cf. guepinioides, C. glossoides, C. lutea, C. pedicellata, and C. sinensis, were found to be dispersed throughout the Dacrymycetaceae clade (Fig. 1), suggesting the convergent evolution of calocera-like cylindrical basidiocarps in this family.
Our field investigations in New Zealand have improved the current knowledge of the diversity and phylogeny of Southern Hemisphere Dacrymycetes. In this class, however, taxon sampling is still insufficient to estimate a reliable phylogeny and establish a higher classification system (Shirouzu et al. 2013a). In addition, a recent study has suggested the existence of hidden dacrymycetous lineages that rarely or perhaps never produce visible fruiting bodies – the structures providing almost all morphological criteria used for classification purposes (Shirouzu et al. 2016). To unveil the whole range of phylogenetic diversity of Dacrymycetes, mycelium strains not associated with basidiocarps as well as lineages with visible fruiting bodies must be incorporated. Our survey of the diversity of Dacrymycetes in the Southern Hemisphere has increased taxon sampling and thus improves the reliability of phylogenetic analyses that can serve as a basis for establishing a stable classification of Dacrymycetes.
Acknowledgments
We are grateful to Drs Renee Johansen, Peter Buchanan, Roy E. Halling, Teresa Lebel, and Gregory Bonito for their help during field trips. This work was supported by the Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for JSPS Fellows (25-9680), Young Scientists (A) (24680085), and Scientific Research (B) (24300314).
REFERENCES
- Burdsall HH, Laursen GA. 2004. Fungi of New Zealand’s subarctic islands I: Two new species of Dacrymyces (Basidiomycota). In: Miller OK, Cripps CL. (eds), Fungi in forest ecosystems: systematics, diversity and ecology: 107–111. New York Botanical Garden, USA. [Google Scholar]
- Coetzee MPA, Wingfield BD, Bloomer P, et al. 2001. Phylogenetic relationships of Australian and New Zealand Armillaria species. Mycologia 93: 887–896. [Google Scholar]
- Delivorias P, Gonou-Zagou Z, Kapsanaki-Gotsi E. 2012. A new species of Guepiniopsis (Dacrymycetes) from Greece. Sydowia 64: 19–27. [Google Scholar]
- Doyle JJ, Doyle JL. 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemistry Bulletin 19: 11–15. [Google Scholar]
- Erkens RHJ, Cross H, Maas JW, et al. 2008. Assessment of age and greenness herbarium specimens as predictors for successful extraction and amplification of DNA. Blumea 53: 407–428. [Google Scholar]
- Hosaka K, Castellano MA, Spatafora JW. 2008. Biogeography of Hysterangiales (Phallomycetidae, Basidiomycota). Mycological Research 112: 448–462. [DOI] [PubMed] [Google Scholar]
- Hosaka K, Uno K. 2013. Assessment of the DNA quality in mushroom specimens: a recovery of the whole ITS sequence from fragmented DNA of the type specimen. Bulletin of the National Museum of Nature and Science. Series B, Botany 39: 53–60. [Google Scholar]
- Jülich W. 1981. Higher taxa of Basidiomycetes. Bibliotheca Mycologica 85: 1–845. [Google Scholar]
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Molecular Biology and Evolution 30: 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayasi Y. 1954. Monographic studies of Japanese Tremellaceous fungi VI. Nagaoa 4: 36–47. [Google Scholar]
- Kobayasi Y. 1955. Correction of scientific name. Nagaoa 5: 60. [Google Scholar]
- Leslie AB, Beaulieu JM, Rai HS, et al. 2012. Hemisphere-scale differences in conifer evolutionary dynamics. Proceedings of the National Academy of Sciences of the United States of America 109: 16217–16221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Fan L. 1990. New species and new variety of Dacrymycetaceae in China. Acta Mycologica Sinica 9: 12–19. [Google Scholar]
- Lowy B. 1971. Tremellales. Flora Neotropica 6: 1–153. [Google Scholar]
- McNabb RFR. 1964. Taxonomic studies in the Dacrymycetaceae I. Cerinomyces Martin. New Zealand Journal of Botany 2: 415–424. [Google Scholar]
- McNabb RFR. 1965a. Taxonomic studies in the Dacrymycetaceae II. Calocera (Fries) Fries. New Zealand Journal of Botany 3: 31–58. [Google Scholar]
- McNabb RFR. 1965b. Taxonomic studies in the Dacrymycetaceae III. Dacryopinax Martin. New Zealand Journal of Botany 3: 59–72. [Google Scholar]
- McNabb RFR. 1965c. Taxonomic studies in the Dacrymycetaceae IV. Guepiniopsis Patouillard. New Zealand Journal of Botany 3: 159–169. [Google Scholar]
- McNabb RFR. 1965d. Taxonomic studies in the Dacrymycetaceae V. Heterotextus Lloyd. New Zealand Journal of Botany 3: 215–222. [Google Scholar]
- McNabb RFR. 1965e. Taxonomic studies in the Dacrymycetaceae VI. Femsjonia Fries. New Zealand Journal of Botany 3: 223–228. [Google Scholar]
- McNabb RFR. 1966. Taxonomic studies in the Dacrymycetaceae VII. Ditiola Fries. New Zealand Journal of Botany 4: 546–558. [Google Scholar]
- McNabb RFR. 1973. Taxonomic studies in the Dacrymycetaceae VIII. Dacrymyces Nees ex Fries. New Zealand Journal of Botany 11: 461–524. [Google Scholar]
- McNabb RFR, Talbot PHB. 1973. Holobasidiomycetidae: Exobasidiales, Brachybasidiales, Dacrymycetales. In: Ainsworth GC, Sparrow FK, Sussman AS. (eds), The fungi, Vol. IV B: 317–325. Academic Press, USA. [Google Scholar]
- Oberwinkler F. 1993. Genera in a monophyletic group: The Dacrymycetales. Mycologia Helvetica 6: 35–72. [Google Scholar]
- Oberwinkler F. 2014. Dacrymycetes. In: McLaughlin DJ, Spatafora JW. (eds), The Mycota, systematics and evolution, vol. 7A: 357–372. Springer, Germany. [Google Scholar]
- Olive LS. 1958. The lower Basidiomycetes of Tahiti (continued). Bulletin of the Torrey Botanical Club 85: 89–110. [Google Scholar]
- Reid DA. 1974. A monograph of the British Dacrymycetales. Transactions of the British Mycological Society 62: 433–494. [Google Scholar]
- Rogstad SH. 2003. Plant DNA extraction using silica. Plant Molecular Biology Reporter 21: 463a–463g. [Google Scholar]
- Seifert KA. 1983. Decay of wood by the Dacrymycetales. Mycologia 75: 1011–1018. [Google Scholar]
- Seutin G, White BN, Boag PT. 1991. Preservation of avian blood and tissue samples for DNA analyses. Canadian Journal of Zoology 69: 82–90. [Google Scholar]
- Shirouzu T, Hirose D, Oberwinkler F, et al. 2013a. Combined molecular and morphological data for improving phylogenetic hypothesis in Dacrymycetes. Mycologia 115: 1110–1125. [DOI] [PubMed] [Google Scholar]
- Shirouzu T, Hirose D, Tokumasu S. 2007. Sequence analyses of the 28S rRNA gene D1/D2 region suggest Dacrymyces (Heterobasidiomycetes, Dacrymycetales) is polyphyletic. Mycoscience 48: 388–394. [Google Scholar]
- Shirouzu T, Hirose D, Tokumasu S. 2009. Taxonomic study of the Japanese Dacrymycetes. Persoonia 23: 16–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirouzu T, Ishikawa NK, Hirose D, et al. 2013b. A new Amazonian species of Calocera with dendroid and multi-headed basidiocarp. Mycoscience 54: 252–256. [Google Scholar]
- Shirouzu T, Uno K, Hosaka K, et al. 2016. Early-diverging wood-decaying fungi detected using three complementary sampling methods. Molecular Phylogenetics and Evolution 98: 11–20. [DOI] [PubMed] [Google Scholar]
- Stamatakis A. 2014. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilgalys R, Hester M. 1990. Rapid genetic identification and mapping of enzymatically amplified DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiß M, Oberwinkler F. 2001. Phylogenetic relationships in Auriculariales and related groups – hypotheses derived from nuclear ribosomal DNA sequences. Mycological Research 105: 403–415. [Google Scholar]
- White TJ, Bruns T, Lee S, et al. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, et al. (eds), PCR protocols: 315–322. Academic Press, USA. [Google Scholar]
- Wu S-H, Shih K, Yu S-Y. 2011. Calocera bambusicola sp. nov. and C. sinensis newly recorded from Taiwan. Mycotaxon 115: 163–169. [Google Scholar]


