Abstract
Based on molecular and morphological data we investigated the taxonomy and phylogeny of the ectomycorrhizal genus Tricholoma in northern Europe. Our phylogenetic tree confirmed the presence of at least 72 well circumscribed species within the region. Of these, three species, viz. T. boreosulphurescens, T. bryogenum and T. ilkkae are described as new to science, based on morphological, distributional, ecological and molecular data. Several other terminal branches represent putative cryptic taxa nested within classical species or species groups. Molecular type studies and/or designation of sequenced neotypes are needed in these groups, before the taxonomy can be settled. In general our phylogenetic analysis supported previous suprageneric classification systems, but with some substantial changes. Most notably, T. virgatum and allies were found to belong to sect. Tricholoma rather than sect. Atrosquamosa, while T. focale was found to be clearly nested in sect. Genuina rather than in sect. Caligata. In total, ten sections are accepted, with five species remaining unassigned. The combination of morphological and molecular data showed pileus colour, pileipellis structure, presence of clamp connections and spore size to be rather conservative characters within accepted sections, while the presence of a distinct ring, and especially host selection were highly variable within these.
Keywords: Agarics, biogeography, cryptic species, ectomycorrhizal fungi, host selection, morphological traits, phylogeny, Tricholomataceae
INTRODUCTION
The genus Tricholoma is a classic genus of agarics already proposed as a section by Fries (1821) and subsequently erected as a genus by Staude (1857).
Over the years more than 850 species epithets have been published or combined in the genus. Many of these have since been transferred to other genera, including Lepista, Leucopaxillus, Lyophyllum and Melanoleuca based on various deviations, mainly in microscopic characters. Molecular studies have supported the segregation of most of these more modern genera, and Tricholoma in its narrow circumscription (e.g. Noordeloos & Christensen 1999) is supported as a monophyletic genus of ectomycorrhizal fungi within the Tricholomataceae (e.g. Moncalvo et al. 2002). According to Ryberg & Matheny (2012), the genus seems to have segregated from its ancestral clade some 60–90 million years ago in the late Cretaceous, possibly with Pinaceae as mycorrhizal partners. A recent study (Sánchez-García et al. 2014) surveyed in depth the Tricholomataceae based on several molecular markers and concluded that only the genera Albomagister, Corneriella, Dennisiomyces, Leucopaxillus, Pseudotricholoma, Porpoloma s.str. and Tricholoma belong to the family, while other previously contained genera should be assigned to families, including the Lyophyllaceae and a poorly resolved residual Tricholomatoid clade. Of the genera included in the Tricholomataceae, also Porpoloma s.str. is proven ectomycorrhizal, while a biotrophic lifestyle is indicated to be probable in Albomagister and Pseudotricholoma. Only the latter genus is known to occur in Europe, were Pseudotricholoma metapodium is widespread.
Tricholoma has a worldwide distribution (Tedersoo et al. 2010), but seems to be most prominent in temperate and subtropical zones in both the southern and northern hemisphere. All known species are known or supposed to be ectomycorrhizal (Ryberg & Matheny 2011), mainly with trees in the Pinaceae, Betulaceae and Fagaceae, but the genus also contains species that are associated with Eucalyptus, Dryas and Helianthemum (Bougher 1996, Christensen & Heilmann-Clausen 2013). Some species form dual ectomycorrhizal and monotropoid associations linking trees and monotropoid plants (Leake et al. 2004). The centre of species richness appears to be in North America. According to Bessette et al. (2013), more than 100 species are reported from this continent while 63 to 88 species are listed from Europe (Riva 1988, Bon 1991, Kirby 2012). Several species are described or reported from Japan, New Zealand and Australia (e.g. Hongo 1988, Bougher 1996, Orlovich & Cairney 2004), but the overview of the species diversity in these regions is fragmentary due to the lack of modern comprehensive treatments.
Tricholoma species show limited microscopic variation, and are characterized by hyaline, subglobose to oblong spores, simple pileipellis structures and lack of well-differentiated sterile elements, including cystidia. Hence, species identification and partly also the infrageneric classification has mainly been based on macromorphology. Singer (1986) divided the genus in four subgenera, mainly based on pileipellis structure and the presence or absence of clamp connections. The four subgenera were further divided into nine sections, of which three (Leucorigida, Iorigida and Adusta) do not belong to the genus in the current circumscription. In their treatment of the genus, Noordeloos & Christensen (1999) accepted the four subgenera suggested by Singer (1986), but with a more narrow definition of sections, especially in subg. Tricholoma, in which seven sections were accepted. Slightly deviating classification systems have been proposed by other authors, including Bon (1984a, 1991). For a more throughout evaluation see Riva (1988) and Christensen & Heilmann-Clausen (2013).
Despite their attractive fruit bodies, and a long mycological tradition, the overall taxonomy in Tricholoma is still poorly resolved in Europe. Molecular data have been used to study the taxonomy and phylogeny of some species groups (e.g. Comandini et al. 2004, Jargeat et al. 2010, Ota et al. 2012, Moukha et al. 2013), typically resulting in the identification of cryptic diversity within previously accepted species. Simultaneously, several species have been proposed in recent years without a published test of taxonomic placement based on molecular markers (e.g. Kalamees 2001, Musemeci & Contu 2008, Ferrarese & Zaffalon 2010, Ludwig 2012), adding to taxonomic confusion in the genus.
Recently, Christensen & Heilmann-Clausen (2013) monographed the genus in northern Europe, backed by sequences of the nuclear ribosomal internal transcribed spacer (ITS). The main objective of the present paper is to present the results of the underlying scientific studies in a coherent form, with the following specific aims:
to evaluate the monophyly of proposed subgenera and sections as defined by Singer (1986) and Noordeloos & Christensen (1999);
to investigate the congruence between ITS and morphology in hypothesized sections; and
to resolve the taxonomical delimitation of Tricholoma species occurring in northern Europe, with a consideration of possibly related taxa occurring in other continents, especially North America.
MATERIALS AND METHODS
Studied material
Specimens studied for this paper were mainly collected by the first two authors during collection trips throughout Europe, since the early 1990s. It was the intention to obtain representative collections of all species present in northern Europe according to modern identification books (Gulden 1969, 1992, Noordeloos & Christensen 1999, Christensen & Heilmann-Clausen 2012), but in addition a number of species recorded from southern Europe were included. Generally, several collections of each species were included in the analysis, and if possible, specimens from different geographical regions were selected. In addition to own material we have studied a number of collections, including type-specimens from various public and private herbaria throughout Europe, and for the phylogenetic analyses selected relevant and trustworthy ITS sequences were downloaded from GenBank and Unite. Finally, we included a few original ITS sequences kindly provided by Tor Erik Brandrud (Norway) and Sven-Gunnar Ryman (Sweden).
Scoring of morphological characters
Macromorphological characters were mainly scored on fresh material or more rarely photographs (for details on studied collections see Christensen & Heilmann-Clausen 2013). Morphological characters were described according to the standard terminology published by Knudsen & Vesterholt (2008), while colours were recorded according to Kornerup & Wanscher (1974). Microscopical characters were recorded from rehydrated specimens in 2 % KOH or 5–10 % NH3. From each collection a minimum of 20 randomly selected spores were measured, avoiding obviously malformed or unripe spores. For this study, data on pileus and gill colour, pileus surface texture, presence of a ring-zone, spore size and the presence/absence of clamp connections were explored in more detail, but many other characters were described for accepted species in Christensen & Heilmann-Clausen (2013).
Molecular & phylogenetic methods
DNA was extracted from dried specimens by the CTAB-chloroform method described by Gardes & Bruns (1993). Usually, one lamella was taken with a flamed pair of forceps from the specimens. The internal transcribed spacer (ITS) region was amplified with the primer combination ITS1-F and ITS4 (White et al. 1990, Gardes & Bruns 1993). The PCR products were sequenced by Europhins Genomics (previously MWG-Biotech) or Macrogen (www.macrogen.com). Forward and reverse strands were sequenced using ITS1F or ITS5 (White et al. 1990) and ITS4 as sequencing primers. If sequencing of either the ITS-1 or ITS-2 region proved difficult, additional sequencing was performed using ITS2 and ITS3 (White et al. 1990) as sequencing primers. Sequence contigs were assembled using Sequencher (v. 3.1). Alignment was done with MAFFT (online v. 7) using the settings G-INS-i (Katoh et al. 2005), with minor manual adjustments in Se-Al (Rambaut 1996) for some sequences with incomplete ends or internal gaps. Two alignments were produced, one containing all sequences in the dataset, and one based on a reduced dataset containing only one representative of each of 72 end-clusters accepted to represent described or putative species present in northern Europe. For each alignment Maximum Likelihood phylogeny estimates were produced with RAxML v. 8.1.16 (Stamatakis et al. 2008) with 1 000 fast bootstrap replicates and GTR + CAT base substitution model. Both alignments were also subjected to bayesian phylogenetic analyses using MrBayes v. 3.2 (Ronquist et al. 2011) using the GTR+I+gamma model (nst = 6, rates = invgamma) with two independent runs of 4 chains for 5 000 000 generations with sampling every 1 000th generation. Trees from the last 1 000 000 generations from each run (2 000 trees from each analysis) were summed in a consensus tree with branch frequencies corresponding to bayesian posterior probabilities.
Sequences used in this study are listed in Table 1 including source information, geographic origin, and accession numbers. Alignments can be obtained from the first author.
Table 1.
Sequences included in this study. For sequences retrieved from genbank or unite, only accession numbers and country of origin is given, for new sequences obtained for this study, year of collection, locality and fungarium data is given. Species names are given as in Fig. 1.
| Species | Voucher | Collection year | Locality | Origin of sequence | Herbarium | Herbarium no. | Unite accession no | GenBank accession no. | Notes |
|---|---|---|---|---|---|---|---|---|---|
| Hypsizygus marmoreus | HM561970 | Malaysia | GenBank | HM561970 | Outgroup | ||||
| T. ‘Sp. Mex1’ | AB510472 | Mexico | GenBank | AB510472 | |||||
| T. acerbum | AF377247 | Norway | GenBank | AF377247 | |||||
| JV99-638 | 1999 | Denmark, Jylland, Elbæk Skov | This study | C | C-F-41483 | UDB001474 | LT000005 | ||
| MC00-204 | 2000 | Slovenia, Central Slovenia, Vino | This study | C | C-F-96223 | UDB002361 | LT000134 | ||
| T. aestuans | JV02-540 | 2002 | Denmark, Jylland, Sønder Herreds Plantage | This study | C | C-F-40955 | UDB000779 | LT000006 | |
| MC94-008 | 1994 | Denmark, Jylland, Hårup Sande | This study | C | C-F-59265 | LT000007 | |||
| MC97-072 | 1997 | Sweden, Medelpad, Harrån | This study | C | C-F-58885 | UDB001434 | LT000153 | neotype | |
| T. ‘aff. sejunctum’ | JN021102 | Canada, Ontario | GenBank | JN021102 | |||||
| T. ‘aff. virgatum’ | MC05-201 | 2005 | Nepal, Mustang, Kunjo | This study | C | C-F-96250 | UDB002370 | LT000115 | |
| T. albobrunneum | MC99-060 | 1999 | France, Provence | This study | C | C-F-96268 | UDB001444 | LT000077 | |
| UDB001218 | Sweden | Unite | UDB001218 | ||||||
| UDB018044 | Estonia | Unite | UDB018044 | ||||||
| T. album | MC01-201 | 2001 | Slovenia, Ljubljana | This study | C | C-F-96234 | UDB001413 | LT000135 | |
| MC95-159 | 1995 | Denmark, Jylland, Skivum Krat | This study | C | C-F-96254 | LT000008 | |||
| UDB011580 | Estonia | Unite | UDB011580 | ||||||
| T. anatolicum | AB510358 | Turkey | GenBank | AB510358 | |||||
| AB699646 | Morocco | GenBank | AB699646 | ||||||
| T. apium | JHC95-049 | 1995 | Sweden, Medelpad, Borgsjö, Bergåsen SÖ | This study | C | C-F-35189 | UDB001467 | LT000154 | |
| JV00-215 | 2000 | Denmark, Jylland, Skagen Klitplantage | This study | C | C-F-41884 | UDB001685 | LT000009 | ||
| MC98-034 | 1998 | Norway, Hedmark, Hornmoen | This study | C | C-F-59207 | LT000118 | |||
| T. argyraceum | JHC95-112 | 1995 | Denmark, Sjælland, Geel Skov | This study | C | C-F-35092 | UDB000780 | LT000010 | |
| JHC96-244 | 1996 | Denmark, Sjælland, København, Botanisk Have | This study | C | C-F-96212 | UDB000781 | LT000011 | ||
| JHC97-092 | 1997 | Sweden, Värmland, Långban S of Lesjöfors | This study | C | C-F-96213 | UDB000782 | LT000155 | ||
| JHC97-174 | 1997 | Sweden, Medelpad, Bräcke, Grötingen | This study | C | C-F-96215 | UDB001692 | LT000156 | ||
| MC03-251 | 2003 | Slovakia, Presov, Snina | This study | C | C-F-96245 | UDB001419 | LT000127 | ||
| MEN9491 | 1994 | The Netherlands, Groningen, Verhildersum near Leens | This study | L | L0374886 | UDB000785 | LT000198 | epitype | |
| T. arvernense | MC95-102 | 1995 | Sweden, Medelpad, Borgsjö | This study | C | C-F-59014 | LT000157 | ||
| MC98-020 | 1998 | Norway, Hedmark, Hornmoen | This study | C | C-F-59200 | UDB002362 | LT000119 | ||
| MC98-120 | 1998 | France, Franche-Comte, St. Sifolene | This study | C | C-F-59255 | UDB001438 | LT000078 | ||
| T. ‘atrosquamosum’ | AF349701 | USA, Califonia | GenBank | AF349701 | |||||
| T. atrosquamosum | O-F159872 | 2005 | Norway, Hordaland, Granvin, Urdanes NR | This study | O | O159872 | LT222019 | ||
| O-F188799 | 2003 | Norway, Møre og Romsdal, Norddal | This study | O | O188799 | LT222024 | |||
| O-F64018 | 2010 | Norway, Buskerud, Øvre Eiker, Gommerud, Vestfossen | This study | O | O-64018 | LT000120 | |||
| TEB55008 | 2008 | Norway, Aust-Agder, Evje & Hornnes, Dåsvassdalen, Husefjell SW | This study | TEB | LT222031 | ||||
| T. ‘atroviolaceum’ | AY750166 | USA, Washington | GenBank | AY750166 | |||||
| T. ‘aurantium’ | AF377233 | USA, Califonia | GenBank | AF377233 | |||||
| T. aurantium | MC96-303 | 1996 | Italy, Toscana, Cipressa di Agnese | This study | C | C-F-59329 | UDB001470 | LT000100 | |
| MC97-227 | 1997 | Denmark, Møn, Møns Klint | This study | C | C-F-59330 | UDB001471 | LT000012 | ||
| T. ‘bakamatsutake’ | AB036898 | Japan | GenBank | AB036898 | |||||
| AB856037 | Japan | GenBank | AB856037 | ||||||
| T. basirubens | MC01-209 | 2001 | Croatia, Primorsko-Goranska, Krk | This study | C | C-F-96240 | LT000001 | ||
| TL5303 | 1998 | Sweden, Öland, Halltorps Haga | This study | C | C-F-38408 | LT000158 | |||
| T. batschii | AF377238 | USA, Califonia | GenBank | AF377238 | |||||
| MC01-200 | 2001 | Croatia, Istarska, Kanegra | This study | C | C-F-96233 | UDB001412 | LT000002 | ||
| UDB011587 | Estonia | Unite | UDB011587 | ||||||
| T. bonii | AM181413 | Estonia | GenBank | AM181413 | |||||
| JHC91-721 | 1991 | Denmark, Anholt | This study | C | C-F-96201 | UDB000811 | LT000013 | ||
| LUG-F8450 | 1996 | Italy, Probe Brallo, Pavia | This study | LUG | LUG F 8450 | UDB000790 | LT000101 | holotype | |
| MEN96112 | 1996 | Italy, Trento, Spera val Campanella | This study | L | L0354472 | UDB000791 | LT000102 | ||
| T. boreosulphurescens | IK971187 | 1997 | Finland, Outer Ostrobothnia, Tervola, Peura, Raemäki | This study | H | H6002040 | LT000199 | ||
| JF908737 | Finland | GenBank | JF908737 | ||||||
| SAE9507 | 1995 | Sweden, Medelpad, Stöde, V. Västansjö, Kockerabäcken | This study | C | C-F-59441 | UDB001475 | LT000159 | ||
| TROM-F21089 | 2007 | Norway, Troms, Storfjord, Lullesletta | This study | TROM | OF21089 | LT222032 | |||
| O-F187683 | 2004 | Norway, Finnmark, Alta, Kålfjordsbotten | This study | O | O187683 | LT222023 | holotype | ||
| T. borgsjoeënse | JHC95-067 | 1995 | Sweden, Medelpad, Borgsjö, Julåsen | This study | C | C-F-96211 | LT000160 | ||
| JV95-307 | 1995 | Sweden, Medelpad, Borgsjö, Julåsen | This study | C | C-F-96219 | UDB000807 | LT000161 | ||
| TEB22606 | 2006 | Norway, Oppland, Nord-Aurdal, Mjølkebekken nordre | This study | TEB | LT222030 | ||||
| T. boudieri | MC01-600 | 2001 | Slovenia, Ljubljana | This study | C | C-F-90092 | LT000136 | epitype | |
| MC95-317 | 1995 | Denmark, Jylland, Moesgaard Skov | This study | C | C-F-59305 | UDB001428 | LT000014 | ||
| T. bresadolanum | CL94-166 | 1994 | Sweden, Öland, Haltorp Hage | This study | C | C-F-59442 | UDB000792 | LT000162 | |
| MC96-264 | 1996 | Italy, Toscana, Monte Soldano | This study | C | C-F-59341 | UDB000549 | LT000103 | ||
| MC96-265 | 1996 | Italy, Toscana, Monte Soldano | This study | C | C-F-59342 | UDB000550 | LT000104 | ||
| TRgmb00652 | 2006 | Italy, Sardegna, Sassari, Calangianus, Baldo | This study | TR | TRgmb00652 | LT000105 | |||
| T. bryogenum | MC97-101 | 1997 | Sweden, Jämtland, Brunflo | This study | C | C-F-59167 | AY462034 | holotype | |
| O-F160040 | 2006 | Norway, Oppland, Øystre Slidre, Heggnes | This study | O | OF160040 | LT222020 | |||
| O-F52108 | 1979 | Norway, Nord-Trøndelag, Levanger, Ytterøya | This study | O | OF52108 | LT222026 | |||
| TROM-F6702 | 1995 | Norway, Troms, Storfjord, Lullesletta | This study | TROM | OF6702 | LT222034 | |||
| T. ‘caligatum’ | AF309520 | Costa Rica | GenBank | AF309520 | |||||
| T. caligatum | JV07-451 | 2007 | Spain, Valencia, E of Gandía, N of Els | This study | LT000152 | ||||
| T. ‘caligatum’ | KC152249 | Mexico | GenBank | KC152249 | |||||
| T. caligatum | KC565866 | Algeria | GenBank | KC565866 | |||||
| PH99-519 | 1999 | France, Provence | This study | C | C-F-96274 | UDB000793 | LT000079 | ||
| T. ‘cedrotorum’ | MC99049 | 1999 | France, Provence, Massif des Cedres | This study | C | C-F-96265 | UDB001442 | LT000074 | |
| T. ‘cf. japonicum’ | JN021103 | Canada, Quebec | GenBank | JN021103 | |||||
| T. cingulatum | MC03-252 | 2003 | Slovakia, Presov, Havesova | This study | C | C-F-96246 | UDB001420 | LT000128 | |
| MC96-134 | 1996 | Denmark | This study | C | C-F-59057 | UDB000543 | LT000015 | neotype | |
| MC96-170 | 1996 | Denmark, Jylland, Borum | This study | C | C-F-59068 | UDB000544 | LT000016 | ||
| MEN95210 | 1995 | The Netherlands, Drenthe, Boekweitveentje | This study | L | LT000200 | ||||
| T. colossus | MC01-205 | 2001 | Slovenia, Ljubljana | This study | C | C-F-96238 | UDB001417 | LT000137 | |
| MC97-047 | 1997 | Sweden, Jämtland, Ysjö | This study | C | C-F-59154 | UDB001433 | LT000164 | ||
| T. columbetta | AF349693 | Norway | GenBank | AF349693 | |||||
| MC95-181 | 1995 | Denmark, Jylland, Skivum Krat | This study | C | C-F-58898 | UDB001468 | LT000017 | neotype | |
| T. ‘dryophilum’ | AF377239 | USA, Califonia | GenBank | AF377239 | |||||
| T. dulciolens | AB738883 | Sweden | GenBank | AB738883 | holotype | ||||
| AF309523 | USA, Califonia | GenBank | AF309523 | ||||||
| JF908732 | Italy | GenBank | JF908732 | ||||||
| T. equestre | MC94-027 | 1994 | Denmark, Jylland, Hoverdal Plantage | This study | C | C-F-58886 | UDB001508 | LT000018 | |
| MC95-187 | 1995 | Denmark, Jylland, Hoverdal | This study | C | C-F-96256 | LT000019 | |||
| MC96-155 | 1996 | Denmark, Jylland, Bakkerne near Ørsted | This study | C | C-F-58890 | UDB001469 | LT000020 | ||
| T. filamentosum | C-F35924 | 1996 | Sweden, Skåne, Balsberget | This study | C | C-F-35924 | UDB001506 | LT000165 | |
| JHC0-1202 | 2001 | Slovenia, Ljubljana (exhibition) | This study | C | C-F-96191 | UDB000804 | LT000138 | ||
| MC00-218 | 2000 | Slovenia, Gorizia, Idria | This study | C | C-F-96226 | LT000139 | |||
| MC03-242 | 2003 | Slovakia, Presov, Rozok | This study | C | C-F-96243 | UDB000803 | LT000129 | ||
| T. ‘flavovirens’ | AB036895 | Japan | GenBank | AB036895 | |||||
| AF458452 | USA, Oregon | GenBank | AF458452 | ||||||
| AF458453 | USA, Oregon | GenBank | AF458453 | ||||||
| AF458456 | USA, Oregon | GenBank | AF458456 | ||||||
| DQ822834 | USA, California | GenBank | DQ822834 | ||||||
| T. ‘focale’ | AF309534 | USA, Califonia | GenBank | AF309534 | |||||
| AF377236 | USA, Califonia | GenBank | AF377236 | ||||||
| T. focale | JV97-239 | 1997 | Sweden, Jämtland, Orrskäret | This study | C | C-F-27500 | UDB001501 | LT000166 | neotype |
| JV99-603 | 1999 | Denmark, Læsø, Træbakke at Holtemmen | This study | C | C-F-41444 | UDB001500 | LT000021 | ||
| MC98-600 | 1998 | Denmark, Jylland, Skagen | This study | C | C-F-96260 | UDB002364 | LT000022 | ||
| T. ‘frondosae’ | MC98-086 | 1998 | France, Franche-Comte, Winkel | This study | C | C-F-59243 | UDB001504 | LT000075 | |
| T. frondosae type I | AF349689 | USA, New Mexico | GenBank | AF349689 | |||||
| MC95-130 | 1995 | Sweden, Medelpad, Borgsjö | This study | C | C-F-59031 | LT000167 | |||
| MC97-151 | 1997 | Sweden, Jämtland, Fillstabäcken | This study | C | C-F-59188 | LT000168 | |||
| T. frondosae type II | MC00-225 | 2000 | Slovenia, Gorizia, Idria | This study | C | C-F-96227 | LT000140 | ||
| MC96-235 | 1996 | Denmark, Jylland, Mols | This study | C | C-F-59084 | UDB001509 | LT000023 | ||
| MC97-158 | 1997 | Sweden, Jämtland, Kyckås | This study | C | C-F-59395 | UDB002363 | LT000169 | ||
| T. fucatum | MC97-149 | 1997 | Sweden, Jämtland, Fillstabäcken | This study | C | C-F-58980 | LT000170 | neotype | |
| MC98-023 | 1998 | Norway, Hedmark, Sørskogbygdn | This study | C | C-F-59201 | LT000121 | |||
| T. ‘fulvocastanem’ | AB289668 | Japan | GenBank | AB289668 | |||||
| DQ067895 | Thailand | GenBank | DQ067895 | ||||||
| T. fulvum | JHC03-019 | 2003 | Slovakia, Poloniny National Park, Nova Sedlica | This study | C | C-F-96193 | UDB001695 | LT000130 | |
| JHC04-251 | 2004 | Sweden, Halland, Varberg, Åkulla, Valaklitt | This study | C | C-F-96195 | UDB001700 | LT000171 | ||
| MC98-078 | 1998 | France, Franche-Comte, Winkel | This study | C | C-F-96259 | UDB002365 | LT000080 | ||
| T. guldeniae | JuV16997 | 2000 | Finland, Varsinais-Suomi, Vahto, Seppälä, Ryssänvuori | This study | TURA | UDB001701 | LT000070 | ||
| MC95-103 | 1995 | Norway, Akershus | This study | C | C-F-96251 | LT000122 | |||
| T. hemisulphureum | JV08-364 | 2008 | Estonia, Saaremaa | This study | C | C-F-96217 | LT000065 | ||
| O-F74354 | 2005 | Norway, Hordaland, Ulvik, Finse | This study | O | O74354 | LT222027 | |||
| T. ‘huronense’ | AF377229 | USA, Califonia | GenBank | AF377229 | |||||
| T. ilkkae | AB738881 | Spain | GenBank | AB738881 | |||||
| AB738882 | Spain | GenBank | AB738882 | ||||||
| MC98-602 | 1998 | Sweden, Gotland | This study | C | C-F-96261 | LT000172 | |||
| S-F173364 | 2007 | Sweden, Uppland, Gräsö par., Djupdal 3 km NE of Gräsö church | This study | UPS | F-173364 | LT222028 | |||
| S-F513823 | 2000 | Sweden, Gotland, Eksta par, Ekstastrand | This study | UPS | F-513823 | LT222029 | holotype | ||
| T. ‘imbricatum’ | AF377242 | USA, Califonia | GenBank | AF377242 | |||||
| T. imbricatum | MC94-046 | 1994 | Denmark, Jylland, Bakkerne near Ørsted | This study | C | C-F-59268 | UDB001421 | LT000024 | neotype |
| UDB000699 | Sweden | Unite | UDB000699 | ||||||
| T. ‘inamoenum’ | AF377246 | USA, Califonia | GenBank | AF377246 | |||||
| T. inamoenum | JHC95-042 | 1995 | Sweden, Medelpad, Tubbobäcken | This study | C | C-F-35182 | UDB001688 | LT000173 | neotype |
| MC95-115 | 1995 | Sweden, Medelpad, Borgsjö | This study | C | C-F-59020 | UDB001424 | LT000174 | ||
| T. inocybeoides | JHC95-072 | 1995 | Sweden, Medelpad, Borgsjö, Erikslund | This study | C | C-F-35211 | UDB000796 | LT000175 | |
| MC03-229 | 2003 | Denmark, Jylland, Århus | This study | C | C-F-96242 | UDB000783 | LT000025 | ||
| MC95-152 | 1995 | Denmark, Jylland, Aarhus | This study | C | C-F-59272 | UDB000537 | LT000026 | ||
| MC96-172 | 1996 | Denmark, Jylland, Aarhus | This study | C | C-F-59094 | UDB000538 | LT000027 | ||
| MC97-060 | 1997 | Sweden, Jämtland, Østersund | This study | C | C-F-59159 | UDB000539 | LT000176 | ||
| T. ‘japonicum’ | AB036900 | Japan | GenBank | AB036900 | |||||
| T. ‘joachimii’ | HM590876 | France | GenBank | HM590876 | |||||
| T. joachimii | MC98-603 | 1998 | Sweden, Gotland | This study | C | C-F-96262 | LT000177 | ||
| TRgmb00060 | 2005 | Italy, Trento, Baselga di Piné, Cané | This study | TR | TR gmb 00600 | LT000106 | |||
| T. ‘joachimii’ | O-F167194 | 2004 | Norway, Akershus, Bæarum, Borøya | This study | O | O167194 | LT222022 | ||
| T. josserandii | MC99-053 | 1999 | France, Provence, Massif des Cedres | This study | C | C-F-96266 | UDB000797 | LT000081 | |
| MC99-056 | 1999 | France, Provence, Massif des Cedres | This study | C | C-F-96267 | UDB000798 | LT000082 | ||
| T. lascivum | JHC03-020 | 2003 | Slovakia, Poloniny National Park, Rozok | This study | C | C-F-96194 | UDB001696 | LT000131 | |
| MC00-519 | 2000 | Denmark, Sjælland, Rude Skov | This study | C | C-F-96230 | UDB000005 | LT000028 | ||
| MC99-197 | 1999 | Denmark, Sjælland, Lellinge Skovhusvænge | This study | C | C-F-59446 | LT000029 | |||
| T. ‘leucophyllum’ | EU597086 | Canada, British Columbia | GenBank | EU597086 | |||||
| JN021108 | Canada, Ontario | GenBank | JN021108 | ||||||
| T. ‘luteomaculosum’ | AF458448 | USA, Oregon | GenBank | AF458448 | |||||
| T. ‘magnivelare’ | AF377224 | USA, Califonia | GenBank | AF377224 | |||||
| T. matsutake | AF309538 | China, Yunnan | GenBank | AF309538 | |||||
| JuV23362F | 2005 | Finland, Koillismaa, Oulanka national park, NWW of biological field station | This study | TURA | LT000071 | ||||
| MC03-600 | 2003 | Sweden, Jämtland | This study | C | C-F-96247 | LT000178 | |||
| TMU62964 | South Korea | GenBank | TMU62964 | ||||||
| T. ‘moserii’ | AF377211 | USA, Califonia | GenBank | AF377211 | |||||
| T. ‘mutabile’ | AF458445 | USA, Oregon | GenBank | AF458445 | |||||
| T. olivaceotinctum | JHC95-070 | 1995 | Sweden, Medelpad, Borgsjö, Julåsen | This study | C | C-F-35209 | UDB000526 | LT000179 | |
| KJ1993 | 1993 | Sweden, Lappland, Åsele Lappmark, Risbäck, S slope of arksjöberget | This study | S | UDB000799 | LT000180 | |||
| MC95-135 | 1995 | Sweden, Medelpad, Borgsjö | This study | C | C-F-59036 | UDB000527 | LT000181 | ||
| MC97-103 | 1997 | Sweden, Jämtland, Brunflo | This study | C | C-F-59168 | UDB000525 | LT000182 | ||
| OP1981 | 1981 | Sweden, Jämtland, Sällsjö | This study | UPS | UDB000800 | LT000183 | |||
| T. orirubens | JHC01-200 | 2001 | Slovenia, Idria, Mehke Dolina | This study | C | C-F-96189 | UDB000524 | LT000141 | |
| JHC93-261 | 1993 | Denmark, Jylland, Trelde Østerskov | This study | C | C-F-96208 | UDB000523 | LT000030 | ||
| MC03-243 | 2003 | Slovakia, Presov, Rozok | This study | C | C-F-96244 | UDB000801 | LT000132 | ||
| MC96-301 | 1996 | Italy, Toscana, Cipressa di Agnese | This study | C | C-F-59365 | UDB000522 | LT000107 | ||
| MC97-258 | 1997 | Denmark, Jylland, Moesgaard Skov | This study | C | C-F-59427 | UDB000521 | LT000031 | ||
| MC98-214 | 1998 | England, Surrey, Norbury Park | This study | C | C-F-59315 | UDB000520 | LT000202 | ||
| T. ‘palustre’ | DQ494699 | USA, Massachusetts | GenBank | DQ494699 | |||||
| T. pardinum | JHC01-201 | 2001 | Slovenia, Idria, Pringle | This study | C | C-F-96190 | UDB000802 | LT000142 | |
| T. pessundatum | JV04-482 | 2004 | Denmark, Jylland, Ålbæk Klitplantage | This study | C | C-F-43780 | UDB001502 | LT000032 | epitype |
| UDB011581 | Estonia | Unite | UDB011581 | ||||||
| T. populinum | MC00-236 | 2000 | Slovenia, Gorizia, Idria | This study | C | C-F-96229 | UDB001410 | LT000143 | |
| UDB011624 | Estonia | Unite | UDB011624 | ||||||
| T. portentosum | AF349686 | USA, Califonia | GenBank | AF349686 | |||||
| JHC04-431 | 2004 | Sweden, Halland, Hylte, Ödegärdet | This study | C | C-F-96197 | UDB001698 | LT000184 | ||
| JHC92-277 | 1992 | Denmark, Lolland, Christianssædeskovene | This study | C | C-F-96202 | UDB001686 | LT000033 | ||
| MC00-206 | 2000 | Slovenia, Central Slovenia, Vino | This study | C | C-F-96224 | UDB001409 | LT000144 | ||
| MC94-082 | 1994 | Denmark, Sjælland, Ordrup Næs | This study | C | C-F-58959 | LT000034 | |||
| MC96-156 | 1996 | Denmark, Jylland, Bakkerne near Ørsted | This study | C | C-F-59053 | UDB001429 | LT000035 | neotype | |
| MC98-116 | 1998 | France, Franche-Comte, Doubs, St.-Julien les Russey | This study | C | C-F-59262 | LT000083 | |||
| T. psammopus | MC04-600 | 2004 | Slovenia, Ljubljana | This study | C | C-F-96248 | LT000145 | ||
| MC96-345 | 1996 | Italy, Toscana, Monte Soldano | This study | C | C-F-59324 | LT000108 | |||
| MC98-048 | 1998 | Denmark, Jylland, Fløjstrup Skov | This study | C | C-F-59212 | UDB001472 | LT000036 | ||
| MC99-089 | 1999 | France, Provence | This study | C | C-F-96273 | UDB001503 | LT000084 | ||
| T. ‘quercetorum’ | MC99-044 | 1999 | Portugal, Leiria | This study | C | C-F-96263 | UDB000795 | LT000125 | |
| T. ‘ramentaceum var. pseudotriste’ | HQ184102 | France | GenBank | HQ184102 | |||||
| T. rapipes | MC03228 | 2003 | Denmark, Jylland, Stråsø Plantage | This study | C | C-F-96241 | UDB001418 | LT000037 | |
| MC98-106 | 1998 | France, Franche-Comte, Doubs, St.-Julien les Russey | This study | C | C-F-59258 | UDB001439 | LT000085 | epitype | |
| T. roseoacerbum | IK881120 | 1988 | Finland, Sompio Lapland, Sodankylä, Jeesiö-Tepsa | This study | H | H6002032 | LT000072 | ||
| IK922945 | 1992 | Finland, Uusimaa, Hanko, Koverhar, Krogars | This study | H | H6002034 | LT000073 | |||
| T. rufenum | MC96-376 | 1996 | Italy, Lazio, Monte Rufenum | This study | C | C-F-59393 | UDB001432 | LT000109 | |
| T. saponaceum | C-F23337 | 1992 | Denmark, Lolland, Ryde Skov | This study | C | C-F-23337 | UDB001499 | LT000038 | |
| JHC00-049 | 2000 | Norway, Sogn og Fjordane, Leikanger, Horpa | This study | C | C-F-96188 | UDB001693 | LT000123 | ||
| JHC03-015 | 2003 | Poloniny National Park, Stuzika | This study | C | C-F-96192 | UDB001694 | LT000133 | ||
| JHC04-429 | 2004 | Sweden, Halland, Hylte, Ödegärdet | This study | C | C-F-96196 | UDB001697 | LT000185 | ||
| JHC04-439 | 2004 | Sweden, Halland, Laholm, Blåalt | This study | C | C-F-96198 | UDB001699 | LT000186 | ||
| JHC95-165 | 1995 | Denmark, Sjælland, Sorø Sønderskov | This study | C | C-F-35147 | UDB001505 | LT000039 | ||
| JHC97-237 | 1997 | Denmark, Sjælland, Frederikskilde Skov | This study | C | C-F-96216 | UDB001689 | LT000040 | ||
| JV87-682 | 1987 | Denmark, Jylland, Fløjstrup Skov | This study | C | C-F-96218 | UDB001507 | LT000041 | ||
| MC98-059 | 1998 | France, Franche-Comte, Foret de Leval | This study | C | C-F-59217 | LT000086 | |||
| TF98-098 | 1998 | France, Doubs, Forêt Valdahon | This study | C | C-F-96276 | UDB001498 | LT000087 | ||
| T. ‘saponaceum’ | DQ494700 | USA, Massachusetts | GenBank | DQ494700 | |||||
| T. scalpturatum | JHC93-263 | 1993 | Denmark, Jylland, Trelde Østerskov | This study | C | C-F-96210 | UDB000541 | LT000042 | |
| JHC94-231 | 1994 | Denmark, Fyn, Tankefuld W. of Svendborg | This study | C | C-F-35309 | UDB000542 | LT000043 | ||
| MC00-207 | 2000 | Slovenia, Ljubljana | This study | C | C-F-96225 | LT000146 | |||
| MC95-165 | 1995 | Sweden, Jämtland, Sundsnäs | This study | C | C-F-59399 | LT000187 | neotype | ||
| T. ‘scalpturatum forma meleagroides’ | HQ184113 | France | GenBank | HQ184113 | holotype | ||||
| T. ‘scalpturatum var. atrocinctum’ | JHC93-243 | 1993 | Denmark, Jylland, Nystrup Klitplantage | This study | C | C-F-96206 | UDB000784 | LT000004 | |
| T. sciodes | MC94-007 | 1994 | Denmark, Jylland, Fløjstrup Skov | This study | C | C-F-58902 | UDB000547 | LT000044 | |
| MC95-182 | 1995 | Denmark, Jylland, Borum Skov | This study | C | C-F-96255 | UDB000548 | LT000045 | ||
| T. ‘sejunctum’ | AB036899 | Japan | GenBank | AB036899 | |||||
| AF377192 | USA, Califonia | GenBank | AF377192 | ||||||
| EU819447 | USA, Wisconsin | GenBank | EU819447 | ||||||
| T. sejunctum | MC95-187 | 1995 | Denmark, Jylland, Enemærket Skov | This study | C | C-F-58998 | LT000046 | ||
| MC96-314 | 1996 | Italy, Toscana, Cipressa di Agnese | This study | C | C-F-58979 | UDB001431 | LT000110 | ||
| T. squarrulosum | JHC93-224 | 1993 | Denmark, Jylland, Trelde Østerskov | This study | C | C-F-96205 | UDB000532 | LT000047 | |
| JHC93-262 | 1993 | Denmark, Jylland, Trelde Østerskov | This study | C | C-F-96209 | UDB000530 | LT000048 | ||
| JHC95-169 | 1995 | Denmark, Sjælland, Lellinge Skovhusvænge | This study | C | C-F-35151 | UDB000786 | LT000049 | ||
| MC01-202 | 2001 | Croatia, Primorsko-Goranska, Krk | This study | C | C-F-96235 | UDB001414 | LT000003 | ||
| MC01-700 | 2001 | Slovenia, Ljubljana | This study | C | C-F-96239 | UDB000528 | LT000147 | ||
| MC96-269 | 1996 | Italy, Toscana, Monte Soldano | This study | C | C-F-59343 | UDB000531 | LT000111 | ||
| MC98-081 | 1998 | France, Franche-Comte, Winkel | This study | C | C-F-59238 | UDB000529 | LT000088 | ||
| T. stans | MC95-131 | 1995 | Sweden, Medelpad, Borgsjö | This study | C | C-F-59032 | UDB001426 | LT000188 | |
| MC95-145 | 1995 | Sweden, Medelpad, Borgsjö | This study | C | C-F-59042 | UDB001427 | LT000189 | epitype | |
| MC98-018 | 1998 | Norway, Hedmark, Hornmoen | This study | C | C-F-96258 | LT000124 | |||
| T. stiparophyllum | MC95-117 | 1995 | Sweden, Medelpad, Borgsjö | This study | C | C-F-96252 | LT000190 | ||
| UDB002398 | Scotland | Unite | UDB002398 | ||||||
| UDB011582 | Estonia | Unite | UDB011582 | ||||||
| T. sudum | JV96-306 | 1996 | Denmark, Læsø, Læsø Klitplantage, Vester Højsandshoved | This study | C | C-F-96221 | UDB001684 | LT000050 | |
| MC98-601 | 1998 | Denmark, Jylland, Råbjerg Plantage | This study | C | C-F-90094 | UDB002366 | LT000051 | neotype | |
| T. sulphurescens clade 1 | MC96-296 | 1996 | Italy, Toscana, Cipressa di Agnese | This study | C | C-F-59362 | UDB000809 | LT000112 | |
| MC99-063 | 1999 | France, Provence | This study | C | C-F-96269 | UDB002367 | LT000089 | ||
| T. sulphurescens clade 2 | TRgmb00062 | 2005 | Italy, Trento, Baselga del Bondone | This study | TR | TR gmb 00062 | LT000113 | ||
| UDB011543 | Estonia | Unite | UDB011543 | ||||||
| T. ‘sulphureum’ | AF377244 | USA, Califonia | GenBank | AF377244 | |||||
| EU819448 | USA, Wisconsin | GenBank | EU819448 | ||||||
| HQ650743 | Canada, British Columbia | GenBank | HQ650743 | ||||||
| T. sulphureum clade 1 | JHC08-049 | 2008 | Sweden, Halland, Halmstad, Nissaström | This study | C | C-F-96200 | LT000191 | ||
| MC96-245 | 1996 | Denmark, Jylland, Øjesø | This study | C | C-F-59115 | AY462037 | |||
| T. sulphureum clade 2 | JHC07-236 | 2007 | Denmark, Lolland, Favrsted Skov | This study | C | C-F-96199 | LT000053 | ||
| MC01-204 | 2001 | Slovenia, Ljubljana | This study | C | C-F-96237 | UDB001416 | LT000148 | ||
| MC07-001 | 2007 | Sweden, Skåne, Drakamöllan | This study | Missing | LT000192 | ||||
| MC94-023 | 1994 | Denmark, Jylland, Kås Hoved | This study | C | C-F-58914 | AY462036 | |||
| MC95-188 | 1995 | Denmark, Jylland, Enemærket Skov | This study | C | C-F-59292 | AY462038 | |||
| MC96-162 | 1996 | Denmark, Jylland, Løvenholm Skov | This study | C | C-F-59062 | AY462035 | |||
| MC98-109 | 1998 | France, Franche-Comte, Doubs, St.-Julien les Russey | This study | C | C-F-59260 | UDB001440 | LT000090 | ||
| O-F288529 | 2008 | Norway, Oppland, Vang, Uri | This study | O | OF288529 | LT222025 | |||
| TROM-F30019 | 1996 | Norway, Troms, Storfjord, Lullesletta | This study | TROM | OF30019 | LT222033 | |||
| T. sulphureum clade 3 | AF377245 | Norway | GenBank | AF377245 | |||||
| TF06045 | 2006 | France, Doubs, Forêt de Levier | This study | C | C-F-96275 | LT000091 | |||
| T. ‘terreum’ | EU439339 | China, Yunnan | GenBank | EU439339 | |||||
| EU439340 | China, Yunnan | GenBank | EU439340 | ||||||
| T. terreum | JHC93-260 | 1993 | Denmark, Jylland, Trelde Østerskov | This study | C | C-F-96207 | UDB000536 | LT000057 | |
| JHC95-118 | 1995 | Denmark, Sjælland, Kongelunden | This study | C | C-F-35098 | LT000058 | |||
| JHC95-172 | 1995 | Denmark, Sjælland, København, Assistens Kirkegård | This study | C | C-F-35154 | UDB000812 | LT000059 | ||
| MC01-020 | 2001 | Slovenia, Ljubljana | This study | C | C-F-96232 | UDB001411 | LT000149 | ||
| MC05-200 | 2004 | Nepal, Mustang, Lete | This study | C | C-F-96249 | UDB002368 | LT000116 | ||
| MC95-119 | 1995 | Sweden, Medelpad, Borgsjö | This study | C | C-F-96253 | UDB001425 | LT000193 | ||
| MC98-209 | 1998 | Holland, Schouwen-Duiveland | This study | C | C-F-59313 | UDB000533 | LT000201 | ||
| MC99-071 | 1999 | France, Provence, Foret des Caderach | This study | C | C-F-96271 | UDB001445 | LT000092 | ||
| MC99-074 | 1999 | France, Provence, Foret des Caderach | This study | C | C-F-96272 | UDB001446 | LT000093 | ||
| MEN95192 | 1995 | Germany, Bayern, Sperberslohe near Roth | This study | L | L0374887 | UDB000813 | LT000098 | epitype | |
| O-F165767 | 2005 | Norway, Oppland, Lunner, Grua, Olsknappen | This study | O | O165767 | LT222021 | |||
| TL11317 | 1993 | Denmark, Jylland, Klim Bjerg | This study | C | C-F-96277 | UDB000808 | LT000060 | ||
| T. terreum (albinistic) | JHC93-222 | 1993 | Denmark, Jylland, Trelde Østerskov | This study | C | C-F-96204 | UDB000534 | LT000061 | |
| JV95-519 | 1995 | Denmark, Jylland, Staksrode Skov | This study | C | C-F-96220 | UDB000535 | LT000062 | ||
| T. ‘tridentinum’ | JV99-700 | 1999 | France, Provence, Petit Luberon, Massif des Cedres | This study | C | C-F-96222 | UDB000805 | LT000076 | |
| T. triste | E3754 | 1996 | Germany, Baden-Württemberg, Seedorfer Wald, Schwarzwald | This study | L | UDB000814 | LT000099 | neotype | |
| JHC97-169 | 1997 | Sweden, Jämtland, Lockna, W. of Änge | This study | C | C-F-96214 | UDB001691 | LT000194 | ||
| JuV5271F | 1990 | Estonia, Pärnu rajooni, c. 40 km S of Pärnu, Kabli | This study | TURA | LT000066 | ||||
| T. ‘ulvinenii’ | IK931613 | 1993 | Finland, Satakunta, Jämijärvi, Hämeenkangas | This study | H | H6002036 | LT000067 | ||
| JuV13229F | 1997 | Finland, Varsinais-Suomi, Dragsfjärd, Ölmos | This study | TURA | LT000068 | ||||
| JuV26740F | 2008 | Finland, Satakunta, Alastaro, Virttaankangas | This study | TURA | LT000069 | ||||
| UDB011557 | Estonia | Unite | UDB011557 | ||||||
| UDB011558 | Estonia | Unite | UDB011558 | ||||||
| UDB011559 | Estonia | Unite | UDB011559 | ||||||
| T. umbonatum type I | MC00A01 | 2000 | Denmark, Lolland, Roden Skov | This study | C | C-F-96231 | UDB002369 | LT000063 | |
| T. umbonatum type II | TRgmb00651 | 2006 | Italy, Veneto, Belluno, Meleré | This study | TR | TRgmb00651 | LT000114 | ||
| T. ustale | AF377234 | The Netherlands | GenBank | AF377234 | |||||
| JHC92-299 | 1992 | Denmark, Sjælland, Suserup Skov | This study | C | C-F-96203 | UDB000551 | LT000064 | ||
| T. ‘ustaloides’ | AF377240 | USA, Califonia | GenBank | AF377240 | |||||
| T. ustaloides | MC99-047 | 1999 | Portugal, Leiria | This study | C | C-F-96264 | UDB000816 | LT000126 | |
| MC99-067 | 1999 | France, Provence, Foret des Caderach | This study | C | C-F-96270 | UDB000815 | LT000094 | ||
| UDB011564 | Estonia | Unite | UDB011564 | ||||||
| T. vaccinum | MC00-229 | 2000 | Slovenia, Gorizia, Idria | This study | C | C-F-96228 | UDB001511 | LT000150 | |
| MC95-109 | 1995 | Sweden, Medelpad, Borgsjö | This study | C | C-F-59017 | UDB001423 | LT000195 | ||
| T. ‘venenatum’ | AF377230 | USA, Califonia | GenBank | AF377230 | |||||
| T. virgatum | JHC95-063 | 1995 | Sweden, Medelpad, Björnö, Björkviken | This study | C | C-F-35203 | UDB000546 | LT000196 | |
| MC01-203 | 2001 | Slovenia, Ljubljana | This study | C | C-F-96236 | UDB001415 | LT000151 | ||
| MC97-164 | 1997 | Sweden, Jämtland, Halåsen | This study | C | C-F-59398 | UDB000545 | LT000197 | neotype | |
| T. viridilutescens type I | MC98-061 | 1998 | France, Franche-Comte, Bois de la Brosse | This study | C | C-F-59219 | UDB001436 | LT000095 | |
| MC98-080 | 1998 | France, Franche-Comte, Winkel | This study | C | C-F-59237 | UDB001473 | LT000096 | ||
| MC98-093 | 1998 | France, Franche-Comte, Bois Lachat | This study | C | C-F-59249 | UDB001437 | LT000097 | ||
| T. viridilutescens type II | UDB011588 | Estonia | Unite | UDB011588 | |||||
| UDB011595 | Estonia | Unite | UDB011595 | ||||||
| T. ‘viridiolivaceum’ | MC96-002 | 1996 | New Zealand, Arthurs Pass National Park | This study | C | C-F-96257 | LT000117 | ||
| ‘Uncultured ectomycorrhiza’ | FJ197008 | Mexico | GenBank | FJ197008 |
TAXONOMIC PART
In total we obtained 217 novel ITS sequences for this study, while 84 published sequences were downloaded from GenBank (67) and Unite (17). The alignment contained 170 unique sequence reads, represented as terminal clusters in the phylogenetic tree based on maximum likelihood (Fig. 1). The Bayesian analyses did not contradict the ML phylogeny. Based on tree topology these were assigned to 108 putative species hypotheses, of which 27 were represented only by extra-continental sequences, while seven represented accepted species or species hypotheses only recorded from southern Europe. Of the 81 species hypotheses identified among European sequences, 72 were selected for scoring of morphological characters and evaluation of previously published infrageneric classification systems (Fig. 2).
Fig. 1.
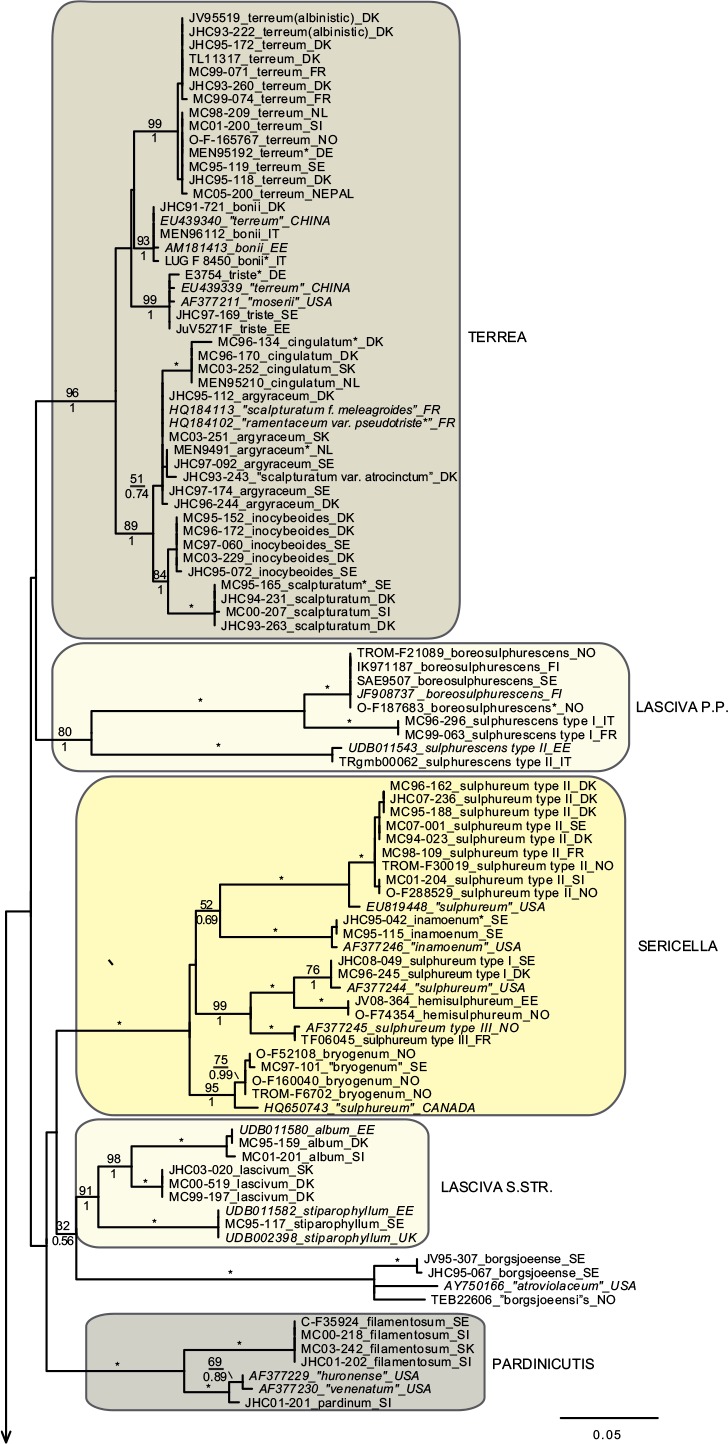
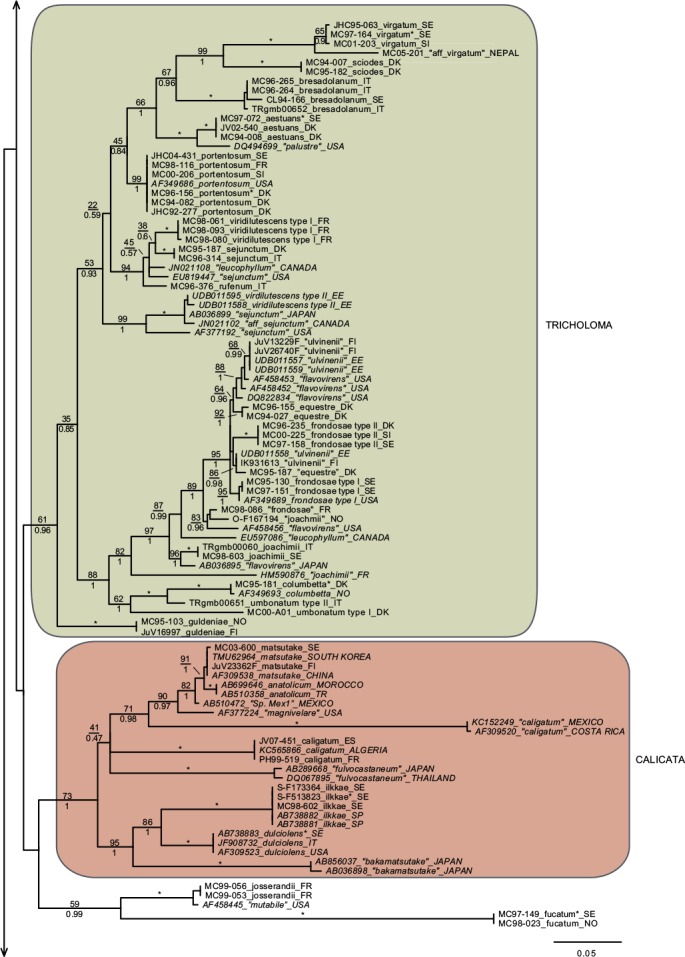
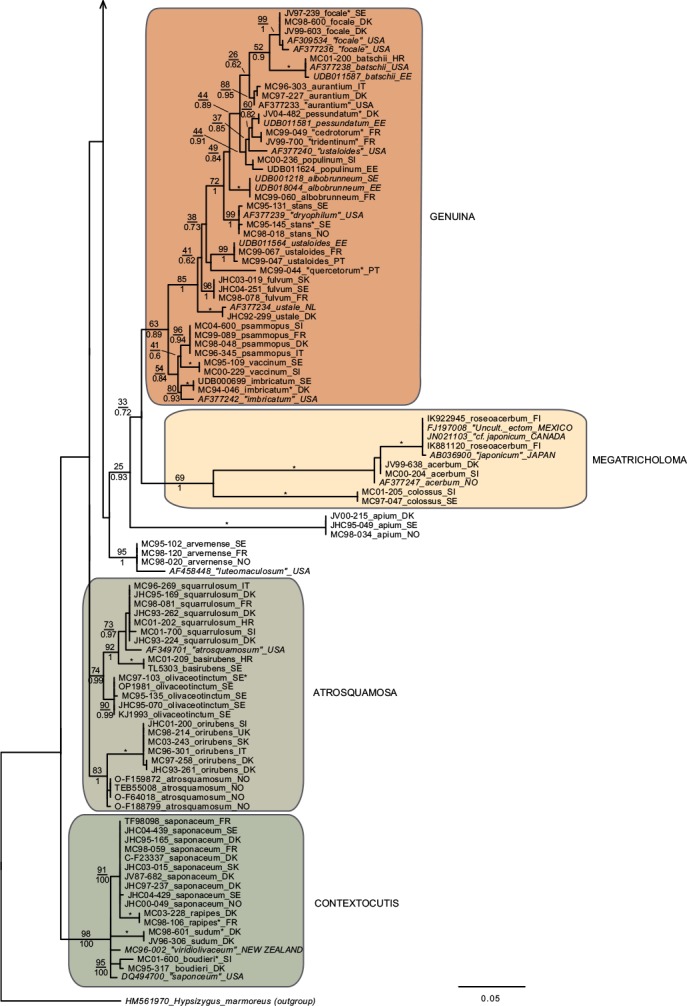
Phylogeny inferred from ITS regions for the full dataset, with branch lengths based on the Maximum Likelihood analysis. No notable differences in branching patterns were observed between the Bayesian and the Maximum Likelihood analysis. Maximum Likelihood bootstrap values are indicated above branches, while Bayesian posterior probabilities are indicated below branches. * Denotes 100 % support in both analyses. New sequences obtained for this study are indicated in regular letters, while sequences obtained from GenBank or Unite are given in italics. Species names without quotes represent our interpretation of relevant taxa as discussed in this paper. Names in quotes are not interpreted by us, but are given as in the original source, or by the collector. Hypothesized sections are indicated by background shadings with names in capital letters.
Fig. 2.
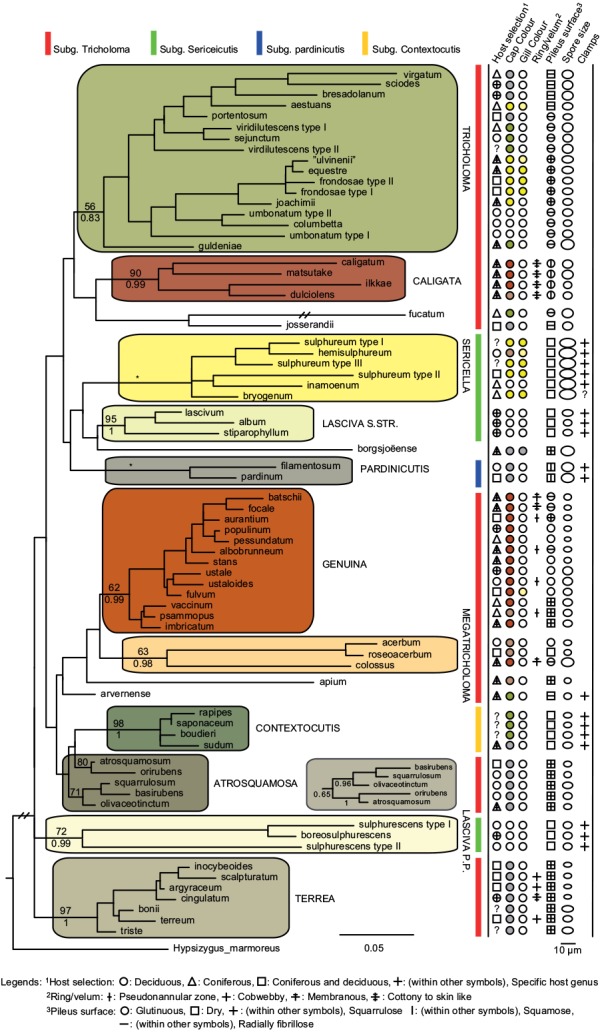
Phylogeny inferred from ITS regions for a reduced dataset, based on representative sequences for 72 well-circumscribed species or species hypotheses.Branch lengths and branching patterns are based on the Maximum Likelihood analysis. Branching patterns were similar in the Bayesian analysis, except for sect. Atrosquamosa, were the alternative configuration is shown as an insert. Maximum Likelihood bootstrap values are indicated above branches, while Bayesian posterior probabilities are indicated below branches for proposed sections indicated with background shadings and names in capital letters. * Denotes 100 % support in both analyses. Coloured bars show the affiliation to subgenera in the sense of Singer (1986). Host selection and six different morphological traits are scored using different symbols, to illustrate their distribution across the phylogeny, and to the proposed sections.
Below we first evaluate the infrageneric classification system, and subsequently the species level taxonomy is reviewed. We have applied commonly used section names as practical labels for clades in order to guide readers. We have not intended to resolve the nomenclatural history of each section in depth, as this would require a deeper and quite time-consuming nomenclatural study on candidate section names. As long as the details of the infrageneric classification remain open, due to limited sapling of the genus in North America, and the use of only one molecular marker, we find the time unripe to perform such a study. In the section on species level taxonomy nomenclatural details are given on all species epithets.
Infrageneric classification and congruence with morphology
The phylogenetic analysis did not support a clear division of the genus in four subgenera as proposed by Singer (1986) and adopted by most subsequent authors. However, ten sections could be reasonably separated based on molecular data and scoring of morphological traits (Fig. 2), with some species remaining unclassified. Pileus colour, pileipellis structure, presence of clamp connections and spores size and shape appeared as rather constant characters supporting the validity of sections, while the presence of a distinct ring, and especially host selection was variable within sections. It is well known that the ITS region alone is poorly suited for resolving higher level phylogenies (e.g. Frøslev et al. 2005), and our infrageneric classification should be viewed as phylogenetic supported, but preliminary. However, we do trust the sections defined below as relevant hypothetical monophyletic entities that should be tested in future studies combining a global taxon sampling with multiple molecular markers.
Species with a greyish, radially fibrillose, squamulose to felty cap
Species with a dry, grey and a radially fibrillose, squamulose to felty pileipellis quite clearly represent a paraphyletic group, that we here split across four sections; Terrea, Atrosquamosa, Tricholoma and Pardinicutis (Fig. 2). Section Terrea contains species characterized by a dry, felty or squamulose pileipellis, predominantly greyish colours, and spores with a relatively high Q-value. Our concept of the section is narrow, and corresponds to stirps Terrea in Singer (1986). Most previous authors, including Noordeloos & Christensen (1999) operated with a much broader concept of the section, which included also the stirps Virgata and Atrosquamosa ss. Singer (1986). Here, we accept the latter as a separate section, although our ITS phylogeny provide only limited support for monophyly, especially in the maximum likelihood analysis (Fig. 2). The similarity in morphological traits of the assigned species is, however, striking. Species in the section are morphologically very similar to species in sect. Terrea, but tend to have spores with a higher Q-value, and are characterized by peculiar smells reminding of honey, ground pepper or cedar wood (compared to absent to farinaceous in sect. Terrea). The species belonging to stirps Virgata in the sense of Singer (1986) (i.e. T. aestuans, T. bresadolanum, T. sciodes and T. virgatum) were in our analysis deeply nested in sect. Tricholoma. As discussed later, this makes good sense morphologically. Finally, our analysis supported sect. Pardinicutis as a separate section. The members of this section are characterized by a grey, scaly pileipellis, large spores and the presence of clamp connections, a combination that has lead most modern authors to accept Pardinicutis at the subgenus level.
Species with a reddish brown cap
For the reddish brown species our ITS phylogeny showed a division in three relatively well-supported sections, sect. Caligata, sect. Genuina and sect. Megatricholoma (Fig. 2). The members of sect. Caligata are characterized by an annulate stipe, a whitish, pale brown to dark reddish brown squamose pileus, rather large spores with low Q-value, and a strong perfumed smell. All European species are associated with conifers, but according to Murata et al. (2013) basal members of the clade from other parts of the world associate with deciduous hosts. The annulate species T. focale has traditionally been included in the section (e.g. Noordeloos & Christensen 1999), but the current study shows it to be deeply nested in sect. Genuina, close to the subannulate T. batschii (Fig. 2). The species lack a perfumed smell and has no broad scales on the pileus so this makes sense morphologically. Section Megatricholoma was originally erected as a monotypic genus to accommodate T. colossus (Kost 1984). Based on detailed morphological and ontological studies the genus was by its author suggested to be only distantly related to Tricholoma s.str., a view that was challenged by Christensen & Noordeloos (1999) who recombined Megatricholoma as a section in Tricholoma. Our study supports this disposition, and gives reasonable support for the inclusion of T. acerbum and T. roseoacerbum in the section (Fig. 2). In the preliminary phylogeny presented in Christensen & Heilmann-Clausen (2013) there was no support for this, but the broader taxon sampling and more careful alignment in the current analysis has changed this. All three species share a very robust and short stipe, close lamellae, and an involute pileus margin, but T. colossus stands apart by its annulate stipe, and large spores. Section Genuina in our circumscription include species with a reddish brown and glutinous pileipellis, as well as several species with paler brown colours and/or a dry squamulose pileus. Noordeloos & Christensen (1999) divided these in sect. Imbricata (with a dry fibrillose to squamulose pileipellis) and sect. Albobrunnea (with glutinous pelipellis). Both are moderately well supported in our ITS phylogeny, but at present we prefer to treat them as entities below the section level.
Species with a smooth, white to yellowish cap
Species with a smooth, white to yellowish, dry pileipellis and presence of clamp connections were divided across four clades in our tree, which we here assign to three sections; Contextocutis, Sericella and Lasciva (Fig. 2). Members of sect. Contextocutis are characterized by reddening flesh, a soapy odour, greenish to greyish colours and small spores, and the section has long been recognized as a separate entity, often at the subgenus level (e.g. Singer 1986, Noordeloos & Christensen 1999). In contrast most previous authors have not separated Sericella and Lasciva at the section level (e.g. Bon 1984a, Singer 1986, Riva 1988, Noordeloos & Christensen 1999). However, Bon (1984a) and Riva (1988) distinguished two subsections, Sulphurea (corresponding to our sect. Sericella) and Lasciva. Our analysis do not suggest the two sections to be closely related, and they are morphologically well differentiated. Thus, species in sect. Sericella are characterized by very large spores, a strong gas-like odour and white to yellow colours, while members of sect. Lasciva have small spores, initially whitish to yellowish grey pileus colours, and a strong, complex odour combining aromatic flowery, gas-like and rancid components. A single member of sect. Sericella, i.e. T. inamoenum, was by Noordeloos & Christensen (1999) assigned to a separate sect. Inamoena, but our analysis clearly shows this section to be redundant. The species assigned to sect. Lasciva is in our analysis divided among two terminal clades (Fig. 2), one containing species with non-yellowing context close to T. lascivum, the other species with yellowing context. Based on morphological similarities, we expect that future multigene phylogenies will show the two clades to be more closely related than our current analysis proposes, and at present we prefer to keep sect. Lasciva as a single taxonomic unit.
Species with a radially fibrillose, whitish, greyish, greenish or yellow cap
The great majority of species characterized by an innately fibrillose to squamulose pileipellis and whitish, greyish, greenish or yellow colours, were joined in one terminal clade in our tree. These are here assigned to sect. Tricholoma in accordance with Noordeloos & Christensen (1999). The T. equestre group including T. columbetta and T. umbonatum form a well-supported core clade. Tricholoma columbetta has traditionally been assigned to the separate sect. Albata (e.g. Noordeloos & Christensen 1999), but this is not supported by our analysis. Two less well-supported subclades are formed by T. sejunctum and allied species, and by sect. Virgata in the sense of Singer (1986) with T. portentosum taking up an intermediate position. Tricholoma guldeniae appears to be the most deviant and basal member of the section. We previously did not consider it as a member of this section (Christensen & Heilmann-Clausen 2013), but our current phylogenetic analysis gives reasonable support for its inclusion.
For five species, viz. T. apium, T. avernense, T. borgejoensis, T. fucatum and T. josserandii, our analysis do not support a clear assignment to traditionally accepted sections (Fig. 1, 2), and appear to represent deviant lineages. These species are discussed further in the next section.
Species level taxonomy
Below we give an overview of the accepted sections, and their circumscribed species accepted by us to occur in northern Europe. We compare our results with earlier studies and comment on further taxa revealed by the analysed ITS data. For further details on the ecology, morphology and practical differentiation of discussed taxa we refer to Christensen & Heilmann-Clausen (2013).
Section Terrea
Our detailed phylogenetic tree supports the presence of seven well-differentiated terminal clades in this section, viz. T. argyraceum, T. cingulatum, T. scalpturatum, T. inocybeoides, T. terreum, T. bonii and T. triste (Fig. 1). All of these are known from northern Europe. Many authors (e.g. Huijsman 1968, Krieglsteiner 1982, Clémençon 1983, Riva 1988) accepted T. gausapatum and T. myomyces as distinct species close to T. terreum, differing by small differences in pileipellis structure, veil development and colouration. Based on our quite intensive sampling, we have found no congruence between variation in these traits and ITS phylogeny, and we doubt that T. gausapatum and T. myomyces as typically interpreted auct. are taxonomically different from T. terreum. Also material fitting with T. leucoterreum show no ITS difference to typical T. terreum, and we interpret this taxon to represent an albinistic form of T. terreum. In fact, albinism seems to be rather common in the section, with albinistic forms and varieties described also in T. cingulatum and T. scalpturatum (Hermosilla & Sánchez 1994, Bidaud & Thévenard 2003). Also T. bonii was originally described as a species with whitish colours (Basso & Candusso 1997), but the type shows 100 % ITS sequence similarity with collections with greyish pileus colours. The taxonomy and phylogeny of the species group around T. argyraceum was studied in great detail by Jargeat et al. (2010) using three molecular markers. The study found very consistent phylogenies for all markers, supporting the clear delimitation of T. argyraceum, T. inocybeoides, T. cingulatum and T. scalpturatum as biological species. Especially T. argyraceum was shown to encompass forms and varieties described to differ in colouration from pure white to strongly coloured. Two recently described species from Europe, T. urbicum and T. distantifoliaceum, have been assigned to the section. They are unknown to us, and type-material should be sequenced to compare their relatedness to more classical species. Tricholoma moseri described from North America clearly also belongs to this section, and is close to or even conspecific with T. triste, as suggested by our ITS analysis. Both species share small fruit bodies and very long elongate spores. Based on ITS data T. triste is also present in China, which is also the case for T. bonii (Fig. 1).
Section Atrosquamosa
Our analysis supports the presence of five well-circumscribed European species in this section, viz. T. atrosquamosum, T. orirubens, T. basirubens, T. squarrulosum and T. olivaceotinctum (Fig. 1). The European species fall in two distinct clades with T. squarrulosum and T. orirubens as central species, respectively. As discussed thoroughly by Christensen & Heilmann-Clausen (2009) we use the name T. atrosquamosum differently than done by Noordeloos & Christensen (1999), and apply it for a taxon close to T. orirubens, but mainly associated with conifers and lacking yellow mycelia. Conversely T. basirubens, that was first described as a variety to T. orirubens (Bon 1975), is closely related to T. squarrulosum based on our data. The section appears to be well represented, but poorly resolved in North America. Tricholoma michinganense clearly belongs to this section but appears to be poorly represented in modern literature. Bessette et al. (2013) presented three photographs labelled as T. squarrulosum, which appear to be somewhat deviant from our concept of this species, based on the slender stipe and occurrence under conifers. The included American sequence labelled as ‘T. atrosquamosum’ in our tree, is clearly deviant from included European taxa, but it is unknown if it corresponds to T. michinganense or T. squarrulosum in the sense of Bessette et al. (2013).
Section Pardinicutis
Our analysis supports five species in this section (Fig. 1), of which only two, i.e. T. filamentosum and T. pardinum, are known with certainty from Europe. Bon (1991) included a number of additional taxa in his treatment of the section, including T. tumidum, T. cookeanum and T. cedrorum, the latter two being described from Morocco. All are unknown to us. At least three North American taxa are described in this section, viz. T. huronense, T. venenatum and T. vernaticum. Our tree supports the two former as distinct species, assuming that the two sequenced specimens have been correctly labelled.
Section Caligata
Our analysis supports at least ten species in this section (Fig. 1), of which only two are known to occur in northern Europe, viz. T. matsutake and T. dulciolens. Two further species, viz. T. caligatum and T. anatolicum occur in southern Europe or adjacent regions. Kytövuori (1988) made a careful taxonomic treatment of the section in Europe, and described T. dulciolens as new to science based on collections from Fennoscandia, while T. anatolicum was recently described from Cedrus forests in Turkey (Intini et al. 2003). This species has since been recorded from Morocco (Ota et al. 2012), and might well occur in southern Europe. A fifth species, T. ilkkae, is here described as new to science. It has long been known from the Swedish island of Gotland in the Baltic Sea, but has been identified as either T. dulciolens or T. caligatum. Tricholoma ilkkae share small spores with the first mentioned species, and general colouration with the latter, so the confusion is not surprising. ITS data, however, show that T. ilkkae is clearly differentiated from both species, showing most affinity to T. dulciolens. We have studied material of the species only from Sweden, but have seen photographs of the species from Norway, and ITS sequence data show that it is also present in Spain (Murata et al. 2013) and Turkey (unpubl. data from Nicklas Bergius). Most likely it is widely distributed but rare in Europe. Quite likely, Armillaria caligata forma gracilis represent an earlier synonym, but as the name has not been combined in Tricholoma or proposed at the species level, it has limited nomenclatural relevance.
The global phylogeny of the section has been studied rather intensively (Chapela & Garbelotto 2004, Ota et al. 2012, Murata et al. 2013, Gulden et al. 2014). These studies show T. matsutake (possibly as a species complex) to be present in both Europe, Asia and North America, while T. dulciolens so far is confirmed from Europe and North America. The other recognized species appear to be restricted to smaller biogeographic regions, i.e. T. anatolicum, T. caligatum and T. ilkkae to Europe (including adjacent North Africa and Asia Minor), T. bakamatsutake, T. fulvocastaneum to eastern Asia and T. magnivelare and at least two undescribed lineages (one denoted as ‘Mexican magnivelare’ in Gulden et al. (2014), and one or two labelled as T. caligatum) in Chapela & Garbelotto (2004), to North America. These taxa are represented in our dataset, as ‘T. sp. Mex1.’ from Mexico and ‘T. caligatum’ from Mexico and Costa Rica, respectively.
Section Genuina
Our analysis supports the presence of at least 18 species in this section in Europe, which fall in two more or less well-supported subclades (Fig. 1).
The largest subclade contains species with reddish brown colours and a glutinous pileipellis, i.e. T. focale, T. batschii, T. pessundatum, T. populinum, T. stans, T. aurantium, T. albobrunneum, T. fulvum, T. ustale and T. ustaloides, all known from northern Europe. Many authors have accepted T. pseudonictitans as a separate species close to T. fulvum, but differing by less pronounced yellow tinges in the gills and flesh of the stipe, and by the association with conifers. Our ITS data do not support this separation (JHC04-251 and MC98-078 were collected under Picea and Abies, respectively, while JHC03-109 was collected under Betula), and hence we treat T. pseudonictitans as a synonym to T. fulvum (see also Christensen & Heilmann-Clausen 2013). On the other hand, collections from southern Europe identified as T. cedretorum and T. quercetorum appear to represent distinct species based on ITS, but we have studied too little material and literature to have any opinion on the correct nomenclatural judgments regarding these. Further European taxa characterized by a reddish brown and glutinuous pileus include T. tridentinum, T. ustale var. rufoaurantiacum, T. ustaloides var. aurantiodes and T. ezcarayense. The latter taxon, T. ezcarayense, however possesses clamp connections (Hermosilla & Sánchez 1994), which are otherwise absent in the section and is probably unrelated. The North American sequences included in the tree suggest that T. aurantiacum, T. batschii, T. focale and T. stans are present also on this continent, with T. zelleri and T. dryophilum being potential synonyms to the two latter species. In contrast, the included American sequence assigned to T. ustaloides represents a distinct taxon not closely related to our concept of this species. Judging from photos and descriptions in Bessette et al. (2013) T. muricatum, T. pudorinum ined. and T. transmutans are further North American taxa in this group, with T. fulvum, T. pessundatum, T. populinum and T. ustale being also recorded as North American. Grubisha et al. (2012) investigated the phylogeography of T. populinum in Scandinavia and North America, and found no signs of recent intercontinental gene flow in this specific partner of Populus spp. Based on the molecular clock approach they estimated divergence between European and American populations to have happened between 1 and 1.7 million years ago.
A smaller, and slightly less well-supported subclade contain species with a dry, squamulose pileus. Our analysis include only three European species in this group, viz. T. psammopus, T. vaccinum and T. imbricatum, but Moreau (2011) presented and discussed two additional central-European taxa, viz. T. inodermeum and T. subfusipes. Both appear to be well delimited species, close to T. imbricatum and T. vaccinum, respectively, but with slightly different macroscopic characters (Moreau 2011) and deviant ITS data (P.-A Moreau pers. comm.). Ecologically, T. subfusipes differs from T. imbricatum by being associated with Larix rather than Pinus. A third species, T. pseudoimbricatum, described from Denmark is by us regarded as a synonym to T. imbricatum (for details see Christensen & Heilmann-Clausen 2013). The included North American sequence of T. imbricatum is quite deviant from the two European sequences, and might represent a distinct species. Both T. imbricatum and T. vaccinum are illustrated with several photographs from various American states in Bessette et al. (2013). The variation in colouration, stature and pileipellis structure is quite striking, and suggests the presence of several additional species on the continent.
Section Megatricholoma
This relatively well-supported section was not accepted in Christensen & Heilmann-Clausen (2013) but as mentioned above the present analysis has lead us to treat it in a wider sense than done previously, by including the non-annulate species T. acerbum and T. roseoacerbum beside the types species T. colossus. Thus, our concept of the section includes three well known species in Europe (Fig. 1, 2), with T. robustum representing a tentative fourth, badly known member (see Christensen & Heilmann-Clausen 2013). Tricholoma roseoacerbum appears to be remarkably widely distributed, with almost perfect ITS sequence matches connecting collections and environmental samples from Finland, Japan, Canada and Mexico. Tricholoma japonicum probably represents the oldest valid name for this species, with T. radotinense representing a further potential synonym. Also T. manzanitae described from North America belongs to this group, judging from the presentation in Bessette et al. (2013).
Section Sericella
This section contains six well-separated European end-clusters in our tree. Two additional sequences appear to represent distinct taxa occurring in Canada and the USA (Fig. 1). Across continents, only three of these can be assigned to well-known species, i.e. T. inamoenum, T. hemisulphureum and T. sulphureum, while a forth, T. bryogenum is described as new to science in this paper. Already Comandini et al. (2004) reported the presence of cryptic diversity within the section, but mainly concluded that T. bufonium, described to differ from T. sulphureum by more reddish to purplish pileus colours, could not be readily separated from T. sulphureum. Our studies partly confirm pileus colours to be poorly suited to differentiate taxa within the section (Christensen & Heilmann-Clausen 2013), but we are not convinced that the cryptic taxa detected by the phylogenetic analysis are truly indistinguishable from T. sulphureum s.str. The colours of the lamellae and basal mycelium appear to be promising characters in this respect, but we also expect differences in ecology and biogeography. At least this is the case for T. bryogenum that differs from T. sulphureum s.lat. by its habitat in boreal coniferous forests, the dull yellow colours and the whitish basal mycelium. No modern type exists of T. sulphureum, and hence it remains unknown which of the three additional lineages in our tree corresponds to T. sulphureum s.str. Hence they are labelled as type I to III in correspondence with Comandini et al. (2004) and Christensen & Heilmann-Clausen (2013). A large number of varieties have been described in T. sulphureum (see Bon 1991 for an overview). Some of these might correspond to the presently cryptic species in our tree. According to our phylogenetic tree, T. inamoenum occurs also in North America, at least based on ITS data. Another species from this continent that clearly belongs to this section is T. odorum.
Section Contextocutis (= section Rigida)
This section contains at least four European taxa in our tree, viz. T. saponaceum, T. sudum, T. rapipes (comb. nov.) and T. boudieri (Fig. 1, 2). Of these, the two latter are normally not differentiated from T. saponaceum at the species level, but we find that differences in morphology and ITS sequence data warrant their distinguishing. A large number of further varieties have been described in the section, mainly based on pileus colours and surface texture of the stipe (see Bon 1991). Our studies indicate that these characters are quite plastic characters with limited taxonomic relevance (Christensen & Heilmann-Clausen 2013). Based on the collections studied by us, T. saponaceum and T. boudieri are associated with deciduous hosts, while T. rapipes and T. sudum are associated with conifers (Christensen & Heilmann-Clausen 2013). We are far from convinced that these preferences are strict, and await future studies testing host selection and taxonomy in the group. Our current analysis indicates that our concept of T. boudieri could cover more than one species, and we would not be surprised if more dedicated studies would prove the existence of additional species in the section in Europe. The two included extralimital sequences from New Zealand and North America represent further independent species in the section, which judging from the photographs given in Besseette et al. (2013) contains several different species in North America.
Section Lasciva
In our tree this section is split across two subclades, containing a total of five species (Fig. 1, 2) in northern Europe, viz. T. lascivum, T. album, T. stiparophyllum, T. sulphurescens and T. boreosulphurescens. The latter is described as new to science in this paper. A sequence labelled a T.sulphurescens from Estonia, appears to represent a further, undescribed species. The taxonomy of the section was discussed in detail by Christensen & Noordeloos (1999) who neotypified T. lascivum, T. album and T. stiparophyllum. Tricholoma albidum and T. farinaceum in the sense of Bessette et al. (2013) appear to represent North American members of this section.
Section Tricholoma
This section contains at least 13 species in northern Europe, viz. T. virgatum, T. sciodes, T. bresadolanum, T. aestuans, T. portentosum, T. sejunctum, T. viridilutescens, T. equestre, T. frondosae, T. joachimii, T. columbetta, T. umbonatum and T. guldeniae, but several included subclades have complex ITS sequence patterns, and remain poorly resolved in our tree. This is especially the case in the T. equestre group, but also T. sejunctum/viridilutescens and T. umbonatum represent species complexes based on our phylogeny. The complex phylogeny of T. equestre s.lat. was noted previously by Horton (2002) based on North American specimens, and have been confirmed by subsequent studies, dealing with the group across the northern hemisphere (Moukha et al. 2013). Even before molecular phylogenies were available, a number of taxa were proposed but often synonymized in this group, with T. equestre, T. auratum and T. flavovirens representing classical names. Kalamees (2001) studied the group based on ecological and morphological characters. He described two new species, T. frondosae and T. ulvinenii, and at the same time assigned T. auratum and T. flavovirens as synonyms to T. equestre. The study was not supported by molecular sequences, and unfortunately we have been unsuccessful in our attempts to extract DNA from the types of the two new species. In our analysis collections labelled as T. frondosae form three groupings in the tree, but we are quite convinced that T. frondosae type I match the type, as all collections have been characterized by warm colours and small spores as emphasized in the diagnosis. Tricholoma frondosae type II have larger spores and more greenish colours and seem to represent an undescribed species. A further collection from France (MC98-086) is only distantly related to the T. equestre core group based on ITS data.
Collections labelled as T. ulvinenii fall in two distinct clusters within the poorly resolved core T. equestre clade, and it is unknown if any of these represent the type concept of this species. Collections identified as T. ulvinenii by Kuulo Kalamees (viz. UDB011557–UDB011559) are separated on both groups, indicating them to be difficult to separate based on morphological characters. In our simplified overview tree (Fig. 2) T. ulvinenii is represented by the upper terminal clade containing four collections labelled with this name, and illustrated in Christensen & Heilmann-Clausen (2013: 103).
Collections labelled as T. joachimii appear on three widely divided branches in the tree. We have not studied the type of this species, and as far we know no type sequence is available to test the correct position of this species in the phylogeny.
The included extra-liminal sequences add to the complexity of the groups. A global analysis including multiple genetic markers, and renewed attempts to sequence type collections is needed to resolve the taxonomy of the group, which also contains T. chrysophyllum, described from southern Europe (Riva 1988), and T. intermedium, described from North America. Somewhat surprisingly, the two whitish species with radially fibrillose pileipellis, T. columbetta and T. umbonatum seem to have a basal position to the T. equestre complex. As already emphasized by Christensen & Heilmann-Clausen (2013) collections labelled as T. umbonatum fall in two distinct subclades.We are quite convinced that type II, as illustrated in Christensen & Heilmann-Clausen (2013), corresponds to the original concept of this species as presented by Clémençon & Bon in Bon (1984b). Judging from Bessette et al. (2013) also T. subresplendens belongs to this species group.
The T. sejunctum/viridilutescens group is another poorly resolved subclade in sect. Tricholoma. Except for the South-European taxon T. rufenum that has a grey pileus, the European members of this group are characterized by greenish to yellowish pileus colours. In our recent monograph, we accepted only two species viz. T. sejunctum and T. viridilutescens to occur in northern Europe, but the current analysis shows that our concept of T. viridilutescens as presented in Christensen & Heilmann-Clausen (2013) circumscribes two well separated species based on ITS. These are here denoted as T. viridilutescens type I and II, respectively. Further collections from North America labelled as T. sejunctum and T. leucophyllum represent further distinct terminal branches. In our data T. viridilutescens type I is represented by two collections from France, quite close to the type locality in the Austrian Alps, and it might well represent T. viridilutescens s.str. Type II is represented by two collections from Estonia, that have high ITS similarity to collections from Canada and Japan. Tricholoma subsejunctum described from eastern North America is a relevant candidate name for these collections. We have studied the type collection of T. subsejunctum which is in poor condition and unlikely to yield usable ITS data. Tricholoma eosinobasis and T. clavocystis are additional European species described in this group and represent further candidate names (or synonyms) for T. viridilutescens type I and II. Types of the mentioned taxa have not been studied by us.
Also T. viridifucatum and T. luridum are characterized by greenish to olivaceous pileus colours and both may belong to this group, even if the squamulose stipe in the former and the greyish lamellae in the latter are deviant. We have been unsuccessful in obtaining sequence data for these two taxa. Chapon (2011) compared T. viridifucatum with a further taxon denoted as T. coryphaeum. This species might well belong to this group but could also be part of the T. equestre complex. For a nomenclatural discussion on this name see Christensen & Heilmann-Clausen (2013). Judging from descriptions and photos in Bessette et al. (2013), also the North American taxa T. davisiae and T. subluteum belong to this group, probably together with T. muscarium described from Japan (e.g. Hongo 1988). Comprehensive studies using a global sampling strategy, type studies and multiple molecular markers are needed to resolve the taxonomy of this difficult species complex.
Species with a grey or yellow, radially fibrillose dry pileus (sect. Virgata ss. Singer 1986) form a relatively well-resolved subclade in our tree, with the glutinous T. portentosum having a more distant position. According to our data, T. bresadolaum is heterogeneous in the ITS region, and in general we would not be surprised if a more comprehensive sampling would show the subclade to contain more species in Europe. Several additional species have been described in the group, including T. lilacinocinereum, T. sciodellum and T. vinaceogriseum, and some authors also distinguish T. hordum as a separate species close to T. sciodes (but see Christensen & Heilmann-Clausen 2013). The subclade seems to be richly represented in North America. Judging from Bessette et al. (2013) at least T. acris, T. argenteum, T. atrodiscus, T. palustre and T. pullum belongs here. The included sequence of the latter species is close to T. aestuans. Finally, the sequence of T. aff. virgatum from Nepal clearly represent a separate taxon close to T. virgatum.
Unassigned species
Apart from the species that are assigned to the ten hypothesized sections discussed above, five sequenced North-European species, viz. T. arvernense, T. josserandii, T. fucatum, T. borgsjoeënse and T. apium, remain unclassified at section level (Fig. 2). Despite the presence of clamp connections, T. arvernense has traditionally been assigned to the clampless sect. Tricholoma (e.g. Riva 1988, Noordeloos & Christensen 1999), but in our tree it forms an isolated cluster with a sequence identified as T. luteomaculosum from North America (Fig. 1). This species is characterized by a greyish, fibrillose to squamulose pileus and yellowing flesh and in contrast to T. arvernense, it is described to lack clamp connections (Ovrebo 1986). Smith (1942), who described the species, pointed out its similarity with T. scalpturatum, which lead Singer (1986) to regard it as a possible member of sect. Terrea. This placement is not supported by our analysis. The second unclassified species, T. josserandii, has traditionally been considered as a close relative to T. virgatum (Bon 1984a, Riva 1988), but this is disapproved by our analysis. Instead, the species clusters closely with two sequences from North America identified as T. mutabile, and more distantly so with a third unassigned species, T. fucatum, that has traditionally been assigned to sect. Tricholoma (e.g. Riva 1988, Noordeloos & Christensen 1999). All three species are characterized by slender fruit bodies with a cylindrical stipe, a radially fibrillose pileipellis and large spores. Based on photographs and descriptions in Bessette et al. (2013), the North American T. aurantio-olivaceum appears to be a close relative of T. fucatum, together with T. olivaceobrunneum. All the above species might form an evolutionary lineage worth accepting at the section level. A further deviant lineage is formed by T. borgsjoeënse that clusters closely with a sequence of T. atroviolaceum from North America. Both species share a dark grey, felty to squamulose pileus, greyish lamellae and large spores. When describing T. borgsjoeënse, Jacobsson et al. (2006) assigned the species to sect. Terrea, but the current phylogenetic analysis indicates it to be only distantly related to this section. The large spores and the quite special pileipellis structure (see Christensen & Heilmann-Clausen 2013: 20) support the isolated position among European Tricholoma species. Interestingly, our analysis points to substantial variation in the ITS region in T. borgsjoeënse, with the Norwegian collection deviating considerably from the two Swedish collections that both stem from the type locality.
Finally, T. apium appears to be isolated among the species analysed. Noordeloos & Christensen (1999) classified it in sect. Imbricata (here a part of sect. Genuina). The species do show some morphological resemblance to species in this section, and might have a basal position in it, as suggested by the maximum likelihood analysis.
NEW COMBINATIONS
Tricholoma rapipes (Krombh.) Heilm.-Claus. & Mort.Chr., comb. nov. — Mycobank MB816908
Basionym. Agaricus rapipes Krombh. (1836: 22).
Description in Christensen & Heilmann-Clausen (2013).
DIAGNOSES AND DESCRIPTIONS OF NEW SPECIES
Tricholoma ilkkae Mort.Chr., Heilm.-Claus., Ryman & Niclas Bergius, sp. nov. — MycoBank MB816909; Fig. 3a, b
Fig. 3.

Fruitbodies of Trichloma species. a. Tricholoma ilkkae (holotype); b. Tricholoma ilkkae, older specimens (UPS-F173364); c. Tricholoma bryogenum (holotype); d. Tricholoma boreosulphurescens (holotype). — Scale bars = 1 cm.
Etymology. Latin ‘ilkkae’ in honour of the Finnish mycologist Ilkka Kutövuori.
Holotype. SWEDEN, Gotland, Eksta par, Ekstastrand, coniferous forest dominated by Pinus sylvestris, with scattered Picea abies on old beach ridges, 21 Sept. 2000, leg. Svengunnar Ryman 9080 (UPS, F-513823).
Diagnosis — A medium-sized to large Tricholoma, with a distinct ring, and fawn to dark brick, confluent fibrillose patches on the pileus and girdles on the stipe. Mycorrhizal with Pinus and possibly Picea.
Pileus 40–100 mm, at first hemispherical to convex with involute margin, later convex to flattened, often with low, broad umbo, in central part soon breaking up into appressed, ± confluent scales, which are fawn, orange brown to dark brick, on a cream to straw yellow background; scales gradually or more abruptly thinning out towards the marginal zone, which is typically white to straw yellow or pale mouse grey; margin in young fruit bodies shaggy due to remnants of veil, but soon ± smooth to somewhat felty-costate. Lamellae emarginate, with even to somewhat eroded edges, whitish, with age sometimes with orange brown spots, rather close to medium spaced. Stipe 50–100 × 15–25 mm, cylindrical or tapering downwards, often somewhat rooting, with a distinct, persistent, cuff-like, cottony-woolly ring, whitish and granulose or slightly fibrillose above ring, below ring with irregular, fawn, orange brown to dark brick confluent girdles and patches on a whitish background, basal part occasionally with a weak greenish tinge. Flesh whitish; smell sweetish, perfumed fruity, similar to Inocybe corydalina or Hemipholiota heteroclita; taste unknown. Spores 4.5–6.7 × 3.9–5.5 μm, average 5.1–6.0 × 4.4–4.9 μm, predominantly broadly ellipsoid, Q = 1.0–1.5, average 1.15–1.31. Basidia 30–40 × 6–8 μm, 4-spored. Cheilocystidia not observed. Pileipellis cutis made up of cylindrical, warm brown hyphae, 50–300 × 5–15(–20) μm, pigment not incrusting. Clamp connections absent.
Ecology & Habitat — Ectomycorrhizal with Pinus and possibly Picea, mainly in forests on calcareous ground.
Known distribution — Central Sweden (holotype), Norway, Spain and Turkey; most likely widespread in Europe.
Additional material examined. SWEDEN, Gotland, Eksta Par., Ekstastrand, coniferous forest on old beach walls, 9 Oct. 1998 (MC98-602, C-F-96261); ibid., 18 Sept. 2000 (UPS-F013888); ibid., associated with T. aurantium, T. fracticum, Hydnum albidum, Hygrophorus latitabundus and Sarcodon fuligineoviolaceous, 3 Oct. 2009, Irene Anderson & Michael Krikorev (MKR 091003-4, IMG: 100/1208-11); ibid., 29. Sept. 2011 (TF2011-201); Uppland, Gräsö par., Djupdal 3 km NE of Gräsö church (Grid: RN1648667 x 6697072), in needle bed under Picea abies in old Picea/Pinus forest, 2 Oct. 2007, Gillis Aronsson (UPS-F173364); Uppland, Börstil par., the turnaround on NW Tvärnö (Grid: RN1648788 x 6681279), under Picea abies and Pinus sylvestris in older, grass-dominated forest on old slag heap, 13 Sept. 2007 (UPS-F173264); ibid., 24 Sept. 2009, Gillis Aronsson (UPS-F173265).
Notes — According to the phylogenetic analysis the new species is close to T. dulciolens. Both species share small spores, but T. ilkkae is easily distinguished from T. dulciolens by the shorter stem and much darker pileus scales and stipe girdles. Another similar species is T. caligatum, which is distinguished by larger spores and by slightly darker, more contrasting pileus scales and stipe girdles. In addition, the two species differ in habitat and distribution, as T. caligatum seems to be a strictly Mediterranean species, in contrast to T. ilkkae, which so far is known from more temperate environments. Finally, T. matsutake differs by duller colours, larger fruit bodies and larger spores. Armillaria caligata var. gracilis, as illustrated by Bresadola (1927), matches well with T. ilkkae, but we don’t know if authentical material exists that could prove this. A potential synonymy will not have nomenclatural consequences as Armillaria caligata var. gracilis has never been combined as a species epithet.
Tricholoma bryogenum Mort.Chr., Heilm.-Claus. & Vauras, sp. nov. — MycoBank MB816910; Fig. 3c
Etymology. From Greek ‘βρύον’ (moss) and ‘γεννώ’ (born), referring to the habitat in mossy Picea-forests.
Holotype. SWEDEN, Jämtland, Brunflo, under Picea abies on rich soil, among mosses, 4 Sept. 1997, Morten Christensen MC97-101 (C-F59167).
Diagnosis — A small to medium-sized Tricholoma, with dull yellow colours on stipe, lamellae and pileus margin and a strong, chemical smell. Differing from the closely related T. sulphureum by the duller colours, white basal mycelium and by the occurrence in mossy Picea forests.
Pileus 30–100 mm, at first conical, bell-shaped or convex, soon low convex to plane, with or without a low umbo, smooth, glossy, at margin whitish chrome to pale chrome, with age and towards centre darker, pinkish buff to ochraceous orange. Lamellae adnate to deeply emarginate, rather broad and thick, medium spaced to rather distant, lemon yellow to lemon chrome or honey, more saturated than the margin of the pileus. Stipe 50–130 × 8–25 mm, cylindrical or slightly club-shaped, smooth or more often distinctly fibrillose, at base often with white tomentum, straw yellow, pale yellow to light chrome, darkest and most yellow towards base, with age often duller, pale cream to cream, with a ± fibrillose brownish covering; basal mycelium whitish to faintly yellowish. Flesh rather firm, coloured more or less like the surface; smell strong, tar- or gas-like as in T. sulphureum, after cutting more farinaceous; taste unpleasant, mild, farinaceousrancid to slightly bitter. Spores 8.2–14.4 × 4.7–8.4 μm, average 9.4–12.3 × 5.6–7.5 μm, ellipsoid to elongate or amygdaliform, Q = 1.3–2.0, average 1.62–1.69. Basidia 35–60 × 7.5–10.0 μm, mainly 2-spored. Cheilocystidia not observed. Pileipellis an interwoven cutis with individual hyphal elements generally 50–150 × 3–6 μm; subpellis poorly differentiated. Clamp connections not observed, apparently absent.
Ecology & Habitat — Ectomycorrhizal with Picea and possibly Pinus, mainly in rich, mixed forests on calcareous soils. Most records are from moist, eutrophic depressions, or spring-fed slopes with abundant bryophytes, but there are also some records from drier soils.
Known distribution — Central Sweden, Norway and Finland; most likely widespread in Fennoscandia, and possibly in the mountains of central Europe.
Additional material examined. FINLAND, Koillismaa, Kuusamo, Iivaara, E slope, S of Saunakunnas, near Isokorpi, forest with mainly Picea abies and scattered Pinus sylvestris, Alnus incana and Betula, eutrophic depression, 29 Aug. 2007, Jukka Vauras (25068, TURA); Perä-Pohjanmaa, Rovaniemi rural commune, Jaatila, Jaatilanvaara, near Kylmäojao brook, fairly rich, gently W-sloping, spring-fed forest with Picea abies, Betula, Alnus incana, Populus tremula and Pinus sylvestris, 11 Aug. 1999, Jukka Vauras (15082F, TURA); ibid., 19 Aug. 1999, Jukka Vauras (15223F, TURA).
Notes — The new species is distinguished from T. sulphureum mainly by its habitat, the dull yellow colours and the whitish basal mycelium. The difference in coloration is distinct even in exsiccata, which are typically pale buff in T. bryogenum, but cinnamon to greyish brown in T. sulphureum. A further difference may be the absence of clamp connections in T. bryogenum, but we are not certain if this character difference is truly stable. Tricholoma bryogenum is quite similar to T. odorum described from North America, but the latter taxon has more crowded lamellae.
Tricholoma boreosulphurescens Mort.Chr. & Heilm.-Claus., sp. nov. — MycoBank MB816911; Fig. 3d
Etymology. From latin ‘borealis’ (northern) combined with the species epithet of Tricholoma sulphurescens, a closely related and morphologically almost similar relative with a southern distribution in Europe.
Holotype. NORWAY, Finnmark, Alta, Kåfjordsbotten, S of Hesteskovattnet, under Betula, 18 Aug. 2004, Per Marstad 197-04 (O-F187683).
Diagnosis — A medium-sized to large Tricholoma, with whitish colours and strongly yellowing context. Mycorrhizal with Betula and possibly Picea in boreal and subalpine forests on calcareous soils. Morphologically very similar to T. sulphurescens, but with substantial differences in the mitochondrial ITS region and a different ecology and distribution range.
Pileus 30–100 mm, at first bell-shaped to convex, soon low convex to plane or slightly depressed, often irregularly wavy, with or without a low umbo, dry and dull, very finely velutinate, without radial structure, white when young, becoming pale chrome, ochraceous or yellowish brown with age, especially in central part, strongly yellowing when touched, after some time fading to ochre. Lamellae adnate to emarginate, medium broad, medium spaced to rather crowded, whitish to cream or pale chrome, with age becoming lemon yellow to honey, especially near the edges or when damaged. Stipe 50–100 × 10–20 mm, ± cylindrical, mostly widened at base, more rarely tapering, smooth, but mostly finely floccose to squamulose at top, at base often velutinate, at first white to whitish chrome, staining lemon yellow to pale chrome, especially when touched, slowly fading to clay buff reddish brown. Flesh rather firm, white to cream, staining lemon yellow to sulphur yellow after cutting; smell strong, at first recalling lemons, then complex nauseating, combining aromatic flowery, gas-like and rancid components; taste first mild, but after a while somewhat acrid to bitter. Spores 4.5–7.6 × 3.9–6.0 μm, average 5.6–6.4 × 4.2–5.1 μm, predominantly broadly ellipsoid, Q = 1.0–1.5, average 1.20–1.25. Basidia 25–35 × 5.5–8 μm, mainly 4-spored. Cheilocystidia not observed. Pileipellis an irregularly interwoven cutis with individual hyphal elements generally 50–200 × 4–10 μm; subpellis poorly differentiated. Clamp connections present at some septa.
Ecology & Habitat — Ectomycorrhizal with Betula and possibly Picea on calcareous soils in rich, mixed Picea dominated forests and in subalpine Betula forests near the timber line.
Known distribution — Seemingly with an eastern distribution in Fennoscandia; known from several localities in the northern part of Finland, but only from scattered localities in Sweden and Norway. Probably distributed eastwards in Russia, and perhaps even present in other parts of Europe, e.g. in subalpine forests in central European mountain chains.
Additional material examined. FINLAND, Outer Ostrobothnia (PeP/Obu), Tervola, Peura, Raemäki, E of the forest road to Syvälampi, between the pond Pikku-Ruuntana and Raemäenjänkä, S-sloping, grass-herb spruce forest with spring-fed depressions on calcareous ground, 11 Oct.1997, Ilkka Kytövuori 97-1187 (H6002040); Koillismaa, Kuusamo, Oulanka National Park, N of the biological field station, E of Puukkosuo, herb rich forest with Picea abies, Pinus sylvestris, Betula, Populus tremula and Salix, eutrophic depression with Daphne mezereum, Filipendia ulmaria, Goodyera repens, Cirsium helenoides and Elymus caninus, 4 Sept. 2005, Jukka Vauras (23414F, TURA); Koillismaa, Kuusamo, Oulanka National Park, Ampumavaara, E of Puukkosuo, S of the main road, margin of eutrophic depression with Picea abies, Pinus sylvestris, Alnus incana, Betula and Salix. 30 Aug. 2007, Emanuele Campo & Jukka Vauras (25089F, TURA).
Notes — Tricholoma sulphurescens has long been known as rare but easily identified species characterized by whitish colours and strongly yellowing context. While working with the volume on Tricholoma in Fungi of Northern Europe (Christensen & Heilmann-Clausen 2013) we realized that collections from boreal to subalpine Fennoscandia represented a clearly different lineage, than collections from southern Europe that are typically associated with Fagus and Quercus, on warm calcareous soils. Since T. sulphurescens was originally described from Italy (Bresadola 1905) we here describe the new species as T. boreosulphurescens emphasizing its boreal distribution. The new species is very similar to T. sulphurescens in all important morphological characters. Our updated phylogeny presented here strongly indicates the presence of a third cryptic taxon in the group represented by one collection from Italy and one from a boreonemoral forest with Quercus and Tilia in Estonia. It remains to be determined which of the two non-boreal lineages corresponds to the type specimen of T. sulphurescens originally described by Bresadola, and the degree to which they are separable based on morphological or ecological characters.
DISCUSSION
With the present study we have provided a first comprehensive phylogenetically supported taxonomic overview of the genus Tricholoma in northern Europe. Based on this we consider sections Caligata, Atrosquamosa and Terrea as rather well evaluated taxonomically on the European continental scale. All three sections have been sampled intensively in this or other studies, and we would be surprised if future studies will change fundamentally with the species delimitations presented here and elaborated in more detail by Christensen & Heilmann-Clausen (2013). For all other sections our sampling is limited and additional European species are likely to occur, not least in southern Europe. The sections Genuina, Contextocutis, Sericella and Tricholoma in particular are in need of further phylogenetic studies with T. equestre s.lat., T. sulphureum s.lat. and T. viridilutescens/sejunctum representing species complexes with considerable cryptic diversity. These are all represented across the northern hemisphere and future studies addressing these two groups should apply a comprehensive sampling strategy and apply multiple genetic markers to unravel the complex phylogeography of both groups.
Many Tricholoma species appear to have a circumboreal distribution based on the data presented in our study. At least T. aurantium, T. batschii, T. bonii, T. dulciolens, T. focale, T. frondosae, T. inamoenum, T. matsutake, T. portentosum, T. roseoacerbum T. stans and T. triste have almost exact ITS similarity across two or three continents, and according to Jargeat et al. (2010) the same applies for T. argyraceum and T. cingulatum. Most extreme in this respect is T. roseoacerbum, which according to our data, is present in Finland, Canada, Japan and Mexico. In Europe it is considered a rarity (Riva 1988, Christensen & Heilmann-Clausen 2013), making the wide distribution particularly intriguing. The above-mentioned species with an intercontinental distribution are all associated with widely distributed boreal host tree genera: T. dulciolens and T. inamoenum are primarily associated with Picea, T. frondosae with Populus, T. cingulatum with Salix, while T. aurantium and T. argyraceum have a broad host selection. The remaining species are associated primarily or exclusively with Pinus. Thus, none of the species associated exclusively with nemoral deciduous hosts, including Fagus and Quercus occurs across continents based on our data. Grubisha et al. (2012), investigated in more detail the phylogeography of T. populinum and found substantial divergence between North American and Fennoscandian populations, pointing to a reproductive isolation established 1–1.7 million years ago. Similar studies investigating the phylogeography of the apparently circumboreal species mentioned above would be interesting.
Regarding the higher level taxonomy, our study has provided support for several classical sections accepted in Tricholoma, but with some modifications. Most importantly our data showed T. focale to be a member of sect. Genuina, rather than sect. Caligata, while T. sciodes and allied taxa were shown to belong to sect. Tricholoma rather than to sections Terrea or Atrosquamosa. While ITS appears to be a stable marker for species delimitations in Tricholoma (Mouhamadou et al. 2008, Jargeat et al. 2010) there are no reasons to believe that the region can resolve higher taxonomic relationships at a sufficiently detailed level (e.g. Frøslev et al. 2005). Hence the here suggested infrageneric classification should be viewed as preliminary, and we encourage further studies using multiple molecular markers to investigate the infrageneric phylogeny of the genus. As the majority of known species in Tricholoma occurs in North America it is obvious that a careful sampling of North American taxa should be part of such a study, but even Asia, Australia, New Zealand and southern South America host Tricholoma species that are highly relevant to include in future attempts to unravel the biodiversity, evolution and phylogeography of this important ectomycorrhizal genus.
Acknowledgments
Irene Andersson, Niclas Bergius, Tor Erik Brandrud, Gro Gulden, Liz Holden, Claes Ingvert, Thomas S. Jeppesen, Kuulo Kalamees, Tommy Knutsson, Michael Krikorev, Lasse Kosonen, Ilkka Kytövuori, Christian Lange, Perry Larsen, Thomas Læssøe, Jens Maarbjerg, Michal Mikšik, Pierre-Arthur Moreau, Siw Muskos, Johan Nitare, Machiel Noordeloos, Clark Ovrebo, Scott Redhead, Alfredo Riva, Svengunnar Ryman, Sigvard Svensson, Jukka Vauras and Jan Vesterholt† are thanked for valuable discussions, for giving us the opportunity to study their interesting collections and for allowing us to use their sequence data. The curators of the herbaria C, E, H, K, L, LIP, LUG, M, O, OULU, S, TAA and UPS are thanked for arranging loans. We want to thank the J. E. Lange and the Flora Agaricina Danica Foundations for supporting collection trips outside Denmark, while UNITE is thanked for supporting the sequencing of collections.
REFERENCES
- Basso MT, Candusso M. 1997. Tricholoma bonii. Documents Mycologiques 27, 107: 61–71. [Google Scholar]
- Bessette AE, Bessette AR, Roody WC, et al. 2013. Tricholomas of North America. University of Texas Press, US. [Google Scholar]
- Bidaud A, Thévenard G. 2003. Tricholoma cingulatum var. alboflavescens var. nov. Documents Mycologiques 32, 127-128: 69–74. [Google Scholar]
- Bon M. 1975. Tricholomes de France (3′me tome: sections Atrosquamosa et Equetria, ss. sect. Albata). Documents Mycologiques 5, 18: 111–164. [Google Scholar]
- Bon M. 1984a. Les Tricholomes de France et d’Europe occidentale. Encyclopedie Mycologique 36 Paris. [Google Scholar]
- Bon M. 1984b. Novitates – Validations, nouvelles combinaisons et espèces. Documents Mycologiques 14, 56: 22. [Google Scholar]
- Bon M. 1991. Flore Mycologique d’Europe, vol. 2, Les Tricholomes et ressemblants. St Valerysur-Somme, France. [Google Scholar]
- Bougher NL. 1996. Diversity of ectomycorrhizal fungi associated with eucalypts in Australia. In: Brundrett M, Dell B, Malajczuk N, et al. (eds), Mycorrhizas for plantation forestry in Asia: 8–15. Australian Centre for International Agricultural Research, Australia. [Google Scholar]
- Bresadola G. 1905. Hymenomycetes novi vel minus cogniti. Annales Mycologici 3: 159–164. [Google Scholar]
- Bresadola G. 1927. Iconographia Mycologica, 2: pl. 51–100. Mediolani, Italy. [Google Scholar]
- Chapela IH, Garbelotto M. 2004. Phylogeography and evolution in matsutake and close allies inferred by analyses of ITS sequences and AFLPs. Mycologia 96: 730–41. [DOI] [PubMed] [Google Scholar]
- Chapon P. 2011. Les Tricholomes du groupe ‘Fucatum’. Bulletin Mycologique et Botanique Dauphiné-Savoie 200: 79–90. [Google Scholar]
- Christensen M, Heilmann-Clausen J. 2009. Two new boreal species of Tricholoma from Fennoscandia. Mycotaxon 107: 431–440. [Google Scholar]
- Christensen M, Heilmann-Clausen J. 2012. Tricholoma (Fr.) P. Kumm. In: Vesterholt J, Knudsen H. (eds), Funga Nordica. 2nd edn.: 494–510. Nordsvamp, Copenhagen, Denmark. [Google Scholar]
- Christensen M, Heilmann-Clausen J. 2013. The genus Tricholoma. Fungi of Northern Europe, vol. 4 Svampetryk, Denmark. [Google Scholar]
- Christensen M, Noordeloos ME. 1999. Notulae ad floram agaricinam neerlandicam – Tricholoma. Persoonia 17: 295–317. [Google Scholar]
- Clémençon H. 1983. Die Erdritterlinge und ihre nächst verwandten Arten aus der Gattung Tricholoma, Sektion Tricholoma. Mycologica Helvetica 1: 17–30. [Google Scholar]
- Comandini O, Haug I, Rinaldi AC, et al. 2004. Uniting Tricholoma sulphureum and T. bufonium. Mycological Research 108: 1162–1171. [DOI] [PubMed] [Google Scholar]
- Ferrarese GG, Zaffalon C. 2010. A new Tricholoma of the section Atrosquamosa: Tricholoma urbicum sp. nov. Micologia e Vegetazione Mediterranea 25: 119–128. [Google Scholar]
- Fries E. 1821. Systema Mycologicum. Lund, Sweden. [Google Scholar]
- Frøslev TG, Matheny PB, Hibbett D. 2005. Lower level relationships in the mushroom genus Cortinarius (Basidiomycota, Agaricales): A comparison of RPB1, RPB2, and ITS phylogenies. Molecular Phylogenetics and Evolution 37: 602–618. [DOI] [PubMed] [Google Scholar]
- Gardes M, Bruns TD. 1993. ITS primers with enhanced specificity for basidiomycetes – application to the identification of mycorrhizae and rusts. Molecular Ecology 2: 113–118. [DOI] [PubMed] [Google Scholar]
- Grubisha LC, Levsen N, Olson MS, et al. 2012. Intercontinental divergence in the Populus-associated ectomycorrhizal fungus, Tricholoma populinum. New Phytologist 194: 548–560. [DOI] [PubMed] [Google Scholar]
- Gulden G. 1969. Musseronflora. Universitetsforlaget, Norway. [Google Scholar]
- Gulden G. 1992. Tricholoma (Fr.) Staude. In Hansen L, Knudsen H. (eds), Nordic Macromycetes, vol. 2: 183–191. Nordsvamp, Copenhagen, Denmark. [Google Scholar]
- Gulden G, Trudell S, Frøslev TG, et al. 2014. Species of Tricholoma section Caligatum in Newfoundland and Labrador. Omphalina 5, 6: 5–9. [Google Scholar]
- Hermosilla CE, Sánchez J. 1994. Aportaciones a un posible catálogo de Tricholoma Fr. Belarra 10–11: 71–78. [Google Scholar]
- Hongo T. 1988. On the genus Tricholoma of Japan. Transactions of the Mycological Society of Japan 29: 441–447. [Google Scholar]
- Horton TR. 2002. Molecular approaches to ectomycorrhizal diversity studies: variation in ITS at a local scale. Plant and Soil 244: 29–39. [Google Scholar]
- Huijsman HSC. 1968. Observations sur les Tricholomataceae 1. Schweizerische Zeitschrift für Pilzkunde 46: 143–153. [Google Scholar]
- Intini M, Dogan HH, Riva A. 2003. Tricholoma anatolicum spec. nov.: a new member of the matsutake group. Micologia e Vegetatione Mediterranea 18: 135–142. [Google Scholar]
- Jacobsson S, Muskos S, Larsson E. 2006. Tricholoma borgsjoeënse, a new species from a boreal coniferous forest in Fennoscandia. Mycotaxon 95: 195–200. [Google Scholar]
- Jargeat P, Martos F, Carriconde F, et al. 2010. Phylogenetic species delimitation in ectomycorrhizal fungi and implications for barcoding: the case of the Tricholoma scalpturatum complex. Molecular Ecology 19: 5216–5230. [DOI] [PubMed] [Google Scholar]
- Kalamees K. 2001. Taxonomy and ecology of the species of the Tricholoma equestre group in the Nordic and Baltic countries. Folia Cryptogamica Estonica 38: 13–23. [Google Scholar]
- Katoh K, Kuma K, Toh H, et al. 2005. MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucleic Acids Research 33: 511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby G. 2012. The genus Tricholoma in Britain. Private print, UK. [Google Scholar]
- Knudsen H, Vesterholt J. (eds). 2008. Funga Nordica. Nordsvamp, Copenhagen, Denmark. [Google Scholar]
- Kornerup A, Wanscher JH. 1974. Farver i Farver. Politikens Forlag, Denmark. [Google Scholar]
- Kost G. 1984. Megatricholoma nov. gen. Eine neue agaricoide Gattung mit verwandtschaftlichen Beziehungen zu Arten anderer Organisationsstufen der Homobasidiomyzeten. Sydowia 37: 53–74. [Google Scholar]
- Krieglsteiner GJ. 1982. Über einige neue, seltene, kritische Makromyzeten in der Bundesrepublik Deutschland. III. Zeitschrift für Mykologie 48: 44. [Google Scholar]
- Krombholz JV. 1836. Naturgetreue Abbildungen und Beschreibungen der Essbare, Schadlichen und Verdachtigen Schwämme, vol. 4 Prague. [Google Scholar]
- Kytövuori I. 1988. The Tricholoma caligatum group in Europe and North Africa. Karstenia 28: 65–77. [Google Scholar]
- Leake JR, McKendrick SL, Bidartondo M, et al. 2004. Symbiotic germination and development of the mycoheterotroph Monotropa hypopitys in nature and its requirement for locally distributed Tricholoma spp. New Phytologist 163: 405–423. [DOI] [PubMed] [Google Scholar]
- Ludwig E. 2012. Pilzkompendium, band 3. Fungicon-verlag, Germany. [Google Scholar]
- Moncalvo JM, Vilgalys R, Redhead SA, et al. 2002. One hundred seventeen clades of euagarics. Molecular Phylogenetics and Evolution 23: 357–400. [DOI] [PubMed] [Google Scholar]
- Moreau PA. 2011. Deux tricholomes peu connus retrouvés en Savoie: Tricholoma inodermeum et T. subfusipes. Bulletin Mycologique et Botanique Dauphiné-Savoie 200: 21–34. [Google Scholar]
- Mouhamadou B, Carriconde F, Gryta H, et al. 2008. Molecular evolution of mitochondrial ribosomal DNA in the fungal genus Tricholoma: barcoding implications. Fungal Genetics and Biology 45: 1219–1226. [DOI] [PubMed] [Google Scholar]
- Moukha S, Férandon C, Beroard E, et al. 2013. A molecular contribution to the assessment of the Tricholoma equestre species complex. Fungal Biology 117: 145–155. [DOI] [PubMed] [Google Scholar]
- Murata H, Ota Y, Yamaguchi M, et al. 2013. Mobile DNA distributions refine the phylogeny of ‘matsutake’ mushrooms, Tricholoma sect. Caligata. Mycorrhiza 23: 447–461. [DOI] [PubMed] [Google Scholar]
- Musumeci E, Contu M. 2008. Tricholoma clavocystis (Agaricomycetes, Basidiomycota), una nuova specie della sezione Tricholoma osservata in Svizzera. Bollettino dell’Associazione Micologica ed Ecologica Romana 73–74: 47–55. [Google Scholar]
- Noordeloos ME, Christensen M. 1999. Tricholoma. In: Bas C, Kuyper TW, Noordeloos ME, et al. (eds), Flora Agaricina Neerlandica, Vol. 4: 107–148. Balkema, The Netherlands. [Google Scholar]
- Orlovich DA, Cairney JWG. 2004. Ectomycorrhizal fungi in New Zealand, current perspectives and future directions. New Zealand Journal of Botany 42: 721–738. [Google Scholar]
- Ota Y, Yamanaka T, Murata H, et al. 2012. Phylogenetic relationship and species delimitation of matsutake and allied species based on multilocus phylogeny and haplotype analyses. Mycologia 104: 1369–1380. [DOI] [PubMed] [Google Scholar]
- Ovrebo CL. 1986. Three new species of Tricholoma with a description of Tricholoma luteomaculosum. Mycologia 78: 418–425. [Google Scholar]
- Rambaut A. 1996. Se-Al: Sequence Alignment Editor. http://tree.bio.ed.ac.uk/software/seal/ [Google Scholar]
- Riva A. 1988. Tricholoma (Fr.) Staude. Fungi Europaei, vol. 3, Libreria Giovanni Biella, Italy. [Google Scholar]
- Ronquist F, Teslenko M, Van der Mark P, et al. 2012. MrBayes v. 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryberg M, Matheny PB. 2011. Asynchronous origins of ectomycorrhizal clades of Agaricales. Proceedings of the Royal Society B 279: 2013–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-García M, Matheny PB, Palfner G, et al. 2014. Deconstructing the Tricholomataceae (Agaricales) and introduction of the new genera Albomagister, Corneriella, Pogonoloma and Pseudotricholoma. Taxon 63: 993–1007. [Google Scholar]
- Singer R. 1986. The Agaricales in modern taxonomy. 4th ed. Koeltz Scientific Books, Germany. [Google Scholar]
- Smith AH. 1942. New and unusual Agarics from Michigan - III. Papers of the Michigan Academy of Sciences. 27: 57–74. [Google Scholar]
- Stamatakis A, Hoover P, Rougemont J. 2008. A rapid bootstrap algorithm for the RAxML web server. Systematic Biology 57, 5: 758–771. [DOI] [PubMed] [Google Scholar]
- Staude F. 1857. Die Schwämme Mitteldeutschlands, in besondere des Herzogthums. Coburg, Germany. [Google Scholar]
- Tedersoo L, May TW, Smith ME. 2010. Ectomycorrhizal lifestyle in fungi: global diversity, distribution, and evolution of phylogenetic lineages. Mycorrhiza 20: 217–263. [DOI] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, et al. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, et al. (eds), PCR protocols: A guide to methods and applications: 315–322. Academic Press, Inc., US. [Google Scholar]


