Abstract
The advent of next-generation sequencing has dramatically decreased the cost for whole-genome sequencing and increased the viability for its application in research and clinical care. The Personal Genome Project (PGP) provides unrestricted access to genomes of individuals and their associated phenotypes. This resource enabled the Critical Assessment of Genome Interpretation (CAGI) to create a community challenge to assess the bioinformatics community’s ability to predict traits from whole genomes. In the CAGI PGP challenge, researchers were asked to predict whether an individual had a particular trait or profile based on their whole genome. Several approaches were used to assess submissions, including ROC AUC (area under receiver operating characteristic curve), probability rankings, the number of correct predictions, and statistical significance simulations. Overall, we found that prediction of individual traits is difficult, relying on a strong knowledge of trait frequency within the general population, whereas matching genomes to trait profiles relies heavily upon a small number of common traits including ancestry, blood type, and eye color. When a rare genetic disorder is present, profiles can be matched when one or more pathogenic variants are identified. Prediction accuracy has improved substantially over the last 6 years due to improved methodology and a better understanding of features.
Keywords: biomedical informatics, community challenge, critical assessment, genome, genome interpretation, open consent, personal genome project (PGP), phenotype
1 INTRODUCTION
Sequencing of whole genomes has improved our ability to build new methods for predicting phenotype from genotype. These new methods will be instrumental when applied in the clinic to achieve precision medicine. Community challenges are a mechanism to assess how well the scientific community can solve certain problems in an unbiased manner (Friedberg, Wass, Mooney, & Radivojac, 2015). As part of the Critical Assessment of Genome Interpretation (CAGI) challenges (CAGI, 2016), we developed a genome-to-phenotype matching challenge to assess how well individual traits and phenotypic profiles could be predicted from whole-genome sequences. Using open consent model genomes (from individuals who are comfortable sharing them without any promises of privacy, confidentiality, or anonymity) (Ball et al., 2014; PGP, 2016), we asked the community to predict traits and/or profiles for whole genomes for which the matched phenotypes had not yet been publicly released (but were available to and had been held back by challenge organizers). We have iterated on this process four times over the past 6 years, in 2010, 2011, 2013, and 2015, with only slight deviations on the experimental design each year. Our aim was to provide an opportunity for researchers to compare new methods and approaches for interpreting whole-genome sequences into phenotype predictions.
The Personal Genome Project (PGP) is a research study that produces freely available scientific resources that bring together genomic, environmental, and human trait data donated by volunteers (Ball et al., 2014). More about the project PGP can be found in Ball et al. (2014) and PGP (2016). The PGP follows an open consent model to human genome sequencing and was the reason our prediction challenge was possible (Ball et al., 2014). On the PGP recruitment and enrollment website, individuals provide a public profile of their phenotypes by answering a number of trait questions in different categories. Selected individuals then have their samples collected and sequenced, and their assembled genomes made available on the PGP Website along with their survey answers. The PGP CAGI organizers decided that as genomes and phenotype profiles were released, the matching key between the genomes and the phenotypes would remain undisclosed to allow submitters to blindly predict matches between available profiles and released genomes. Note that the trait data are self-reported by PGP participants using the PGP project website and are updated on the CAGI challenge website at the beginning of the challenge.
The PGP challenge has been held in 2011, 2013, and 2015. In this manuscript, we present the methodologies of the challenge submissions as well as an assessment of each submitter’s ability to predict individual traits and to match whole genomes to phenotype profiles. We also assess whether we observe improvement over the 6 years of hosting this challenge. Using simulations, we show that the best groups made predictions that are statistically better than random submissions. We also show that in order to obtain accurate predictions of individual traits, an estimate of the statistical priors must be known. We then show that 5%–10% of the PGP profiles can be matched based on a small number of common features including ancestry, blood type, and eye color. In specific cases, when a rare genetic disorder was present, profiles could be matched to a genome when one or more pathogenic variants were identified.
Moreover, through the four iterations of PGP challenges, we found that challenges are a good measure of our standing in predicting phenotypes from whole-genome sequences. The authors found it of interest that, at best, only 20%–25% of whole genomes (the best correct matching prediction in PGP 2015) could be matched to profiles derived from participant reported phenotype, suggesting that our ability to identify individuals or individual traits from a genome is far less accurate than one might expect based on our significant advances in genome interpretation approaches over the last two decades.
2 MATERIALS AND METHODS
In this section, we first give an overview of the four iterations of CAGI PGP challenges (i.e., PGP 2010, PGP 2011, PGP 2013, and PGP 2015), and then discuss in detail each iteration of the challenge, including data, submitters’methods, and assessment methods.
2.1 Overview
In this subsection, we briefly introduce the four iterations of PGP challenges, with comparisons of the four PGP challenges shown in Table 1.
TABLE 1.
Comparison of PGP 2015, PGP 2013, PGP 2011, and PGP 2010
| PGP 2015 | PGP 2013 | PGP 2013 (assessed) | PGP 2011 | PGP 2010 | |
|---|---|---|---|---|---|
| Genomes | 23 | 77 | 50 | 10 | 10 |
| Phenotype profiles | 101 | 291 | 183 | - | - |
| Traits | 239 | 239 | 239 | 40 | 40 |
| Submissions | 5 | 16 | 16 | 4 | 1 |
| Groups | 4 | 5 | 5 | 4 | 1 |
Notes: PGP 2010 and PGP 2011 requested submitters predict each of the 40 traits, and PGP 2013 and PGP 2015 requested submitters match genomes to entire profiles. Note in 2013, 27 genomes and 108 profiles were trivially removable as unmatchable since 27 profiles had published 23 and Me data and 108 profiles had no indicated sample taken on the PGP website. This is shown in the two columns of PGP 2013, in which column “PGP 2013 (assessed)” shows the information of data used to do the assessment.
In 2010, challenge participants were asked to predict 32 binary traits of the “PGP10,” which were the first 10 individuals sequenced by the PGP project. The survey answers were withheld until after the submission deadline to ensure blinded predictions by the submitters. Predictors for 2010 were told how many predictions they got right, but not which predictions they got right, and thus were essentially blind to the results. The 2010 experiment was deemed a trial run, and as the data remained unspoiled, it was repeated in 2011 (PGP 2011) with the same data. Only those results of PGP 2011 are reported here. In total, there were four submissions from four groups for that challenge. One of the submissions used a novel methodology, which generated a patent application (Chen et al., 2014).
By 2013, PGP had made a website available that included all the participants, their survey answers, and limited information about the status of the sequencing of their genome, including whether a sample had been collected from that individual and was awaiting sequencing. Since the survey answers were now available publicly, the challenge was changed to a blinded matching challenge where groups were asked to match a genome sequence to a phenotypic “profile.” These profiles were a list of “yes” or “no” answers from the survey questions, based on whether the individual considered her/himself to have each of 239 phenotypes. Seventy-seven phenotype profiles were released that had a matched genome, whereas a number of other profiles were released for which the matched genome was not yet sequenced. The true matches between the profiles and genomes were kept undisclosed by PGP for the duration of the challenge, and challenge participants were asked to submit the probabilities each genome had of matching to each profile. In total, 16 submissions from five groups were received. In addition, there was an optional challenge in PGP 2013, which was to predict individual traits from genomes.
In 2015, the 2013 matching challenge experiment was repeated, and 23 genomes and 101 phenotype profiles were released. We received five submissions from four different teams. Assessment was performed similarly to that of 2013 and the results from both challenges were compared.
Below and in the Supp. Material, we describe the submissions of independently submitted challenge predictors who were willing to share their methods. These identified groups are named and the list is as follows.
Group1: the group of Rachel Karchin at Johns Hopkins University;
Group2: the group of Silvio Tosatto at the University of Padova;
Group3: the group of John Moult at the University of Maryland;
Group4: the group of Julian Gough at the University of Bristol; and
Group5: the group of Mario Stanke at the University of Greifswald.
To be consistent, we have also named four submitters, who declined to be included or were otherwise unreachable, as Group6, Group7, Group8, and Group9. For groups that had multiple submissions, we numbered them by adding _1, _2, _3, and so on after group name and provided a description of their differences.
2.2 PGP 2011
Data provided in the PGP challenge for 2011 included genomes of 10 participants and 32 binary traits. The traits are listed in Table 2. The challenge was aimed at predicting the values of each binary trait based on genome sequence. In total, there were four submissions for the binary trait prediction challenge and the methodologies of the two identified methods and the assessment methods are described below.
TABLE 2.
List of binary traits used for prediction in PGP 2011
| Trait ID | Trait name |
|---|---|
| 1 | Asthma |
| 2 | Crohn’s disease |
| 3 | Ulcerative colitis |
| 4 | Irritable bowel syndrome |
| 5 | Rheumatoid arthritis |
| 6 | Type II diabetes |
| 7 | Coronary artery disease |
| 8 | Long QT syndrome |
| 9 | Hypertrophic cardiomyopathy |
| 10 | Glaucoma |
| 11 | Color blindness |
| 12 | Bipolar disorder |
| 13 | Celiac disease |
| 14 | Psoriasis |
| 15 | Lupus |
| 16 | Breast cancer |
| 17 | Prostate cancer |
| 18 | Migraine |
| 19 | Lactose intolerance |
| 20 | Dyslexia |
| 21 | Autism |
| 22 | Osteoporosis |
| 23 | Incontinence |
| 24 | Kidney stones |
| 25 | Varicose veins |
| 26 | Sleep apnea |
| 27 | Tongue rolling (tube) |
| 28 | Phenylthiocarbamide tasting |
| 29 | Blood type: has A antigen? |
| 30 | Blood type: has B antigen? |
| 31 | Blood type: is Rh(D) positive? |
| 32 | Absolute pitch |
2.2.1 Submissions
In 2011, we received both identified submissions from Group1 and Group2 and anonymous submissions from Group6. Their methodologies are described in Supp. Material.
2.2.2 Assessments
We used several different approaches to evaluate the performances of the four submissions from PGP 2011, which included area under the curve (AUC) of receiver operator characteristics (ROC) curve (Fawcett, 2006) and simulations based on probability permutation. Each submission was assessed for accuracy of predicting the 40 binary traits and ROC and precision recall (PR) curves were generated. The ROC AUC was used as a mechanism to rank submissions. In order to assess statistical significance of a submission, a simulation-based permutation test was performed. Each submission was permuted 10,000 times and the resulting ROC AUC was determined and compared with the submission AUC. Details of each assessing method are discussed as follows.
ROC AUC
The ROC AUC is one of the most effective methods to evaluate the performance of prediction results (Fawcett, 2006). The ROC AUC of the prediction output of each submission was calculated based on the predicted probabilities and the true binary answers of 320 (10 genomes × 32 traits) genome trait pairs. We also plotted the PR curves based on these probabilities and the true answers. The ROC curves and PR curves were generated from the JAVA package “jstatplotter.jar” (developed by Kevin Van Bui at the University of Pittsburgh).
Simulations
We designed and developed simulations to test the hypothesis that permuted data derived from the actual submission could exceed the accuracy of the submitting group, and we used this to estimate the P value. The simulations were conducted in the following steps:
Generate 10,000 random results based on randomized permutation of the probabilities of the 320 genome and trait pairs of a submission;
Calculate the AUCs of the randomized results;
Calculate the distance of submission’s AUC from the average AUCs of the permuted results;
- Calculate P values based on the following equation:
(1)
Scoring
We also scored each submission based on the statistical analysis mentioned above, that is, ROC AUC and AUC distance from simulated results. By adding the two ranks of each submission together, we ranked the four submissions.
2.3 PGP 2013
Data provided by CAGI for this challenge included 77 genomes and 291 phenotypic profiles, 214 of which were decoys (i.e., they did not match any genome). The phenotype profiles included a list of self-reported binary traits (e.g., asthma, breast cancer, lung cancer, colon polyps, and melanoma) and additional phenotypic and genetic information provided by PGP participants. Each profile included 239 traits (list of trait names that could be found in Supp. Material). The challenge required matching each genome to the appropriate phenotypic profile. There were 16 submissions from five groups. Each submission was a TSV file that contained probabilities for each participant genome to the 291 challenge profiles. Twenty-seven of the 77 genomes had additional genotypic data from 23 and Me, and predictions on these were considered to be trivial and were removed from the analysis, which left 50 genomes being considered. The submissions and assessments are described as follows.
2.3.1 Submissions
In 2013, we received both identified submissions from Group1, Group2, and Group3 as well as anonymous submissions from Group7 and Group8. Group1 and Group2 are groups that participated in PGP 2011, whereas Group3 was a new submitter group in PGP 2013. Their methodologies are described in Supp. Material, with overview shown in Table 3.
TABLE 3.
Overview of methodology of PGP 2013 submissions
| Group7 | Group2 | Group3 | Group1 | Group8 | |
|---|---|---|---|---|---|
| Nongenomic information | None | None | None | None | Posts from PGP forum |
| Ancestry | No | No | 1000 Genomes Project data | No | DIYDodecad 2.1 |
| Traits used | 107 traits from training set | All | Mendelian traits | All | Traits self-reported by certain participants |
| Filter variants for each trait | 200 | Selected nsSNPs from top 10 genes | In-house methods | HGMD and GWAS hits | N/A |
| Mathematical model | Naive Bayes | Correlation | Bayesian | Bayesian network | Trait specific |
| Parameter estimation | Estimate odds ratio of each variant from training set | Search PubMed in estimating disease similarity | From training set and guesswork | Literature and guesswork | N/A |
2.3.2 Assessments
As noted by one team (Group8), there were 108 profile that could be readily identified as being decoys, because the PGP website reported that no blood or saliva samples had been collected for these participants (Ball et al., 2014; PGP, 2016). However, only this team explicitly used such information to exclude these profiles in the prediction, which gave them an advantage that was not based on the merits of their genetic interpretation. Thus, we assessed the submissions only based on the 50 genomes without 23 and Me data, and we excluded the 108 profile decoys in question from our evaluation, leaving 183. We used different approaches to evaluate the performances of the five different submissions, which included correct predictions and probability rankings.
Correct prediction
For this assessment, we considered a prediction for a genome to be correct if the trait profile assigned to the highest probability by the predictor was the correct trait profile for that individual. We counted the number of correct predictions by the following steps: (1) for each genome, determine which trait profile was assigned the highest probability; (2) for each genome, determine if this highest-ranked trait profile was correct; and (3) count for how many genomes, from a total of 50, the highest-ranked trait profile was the correct one. We also calculated the correct prediction rate by dividing the number of correct prediction by the total number of genomes, that is, 50.
Mean ranking of the true match
We calculated the mean ranking of the true match phenotype profile in each of 50 participants with genomes in each submission by the following steps: (1) rank all probabilities of the 183 phenotype profiles for a genome/participant; (2) identify the rank of the correct match for each participant; and (3) take the mean of the identified ranks of the correct matches.
Scoring
We also scored each submission based on the statistical analysis including criteria mentioned above, that is, the number/rate of correct predictions and the mean ranking of true matches. By adding the ranking numbers for the two above-mentioned scoring criteria together as the overall score of each submission, we got the overall placement of all 16 submissions.
2.4 PGP 2015
The CAGI PGP challenge in 2015 was similar to 2013. We asked submitters to match 23 genomes to 101 phenotype profiles. The phenotype profiles remained largely unchanged from 2013. In CAGI 2015, there were 239 binary traits, which included both Mendelian traits and “complex” traits. The challenge included 78 additional decoy phenotype profiles that did not match any genome. The submissions and assessment methods are described below.
2.4.1 Submissions
We received five submissions from four teams for the PGP Challenge of CAGI 2015. Each submission was formatted as a TSV file and contained probabilities for each participant genome to 101 profiles. The prediction methods of the identified submissions were from Group1, who applied an improved version of the method used in the 2013 challenge, Group4 and Group5. The latter two groups (i.e., Group4 and Group5) were new participants in the CAGI PGP challenges. We also received anonymous submissions from Group9. Their methods are described in Supp. Material.
2.4.2 Assessments
We assessed the 2015 challenge using the assessing methods from the 2011 challenge and the 2013 challenge, which include ROC AUC, simulations, correct prediction, mean ranking of true match, with additional description for PGP 2015 introduced as below. Also, we included several other comparative analyses including an analysis of prediction performance of each participant, and an analysis of ancestry, blood type, and eye color of the participants in the challenge (Supp. Material).
ROC AUC
In PGP 2015, the ROC AUC of the prediction output of each submission was calculated based on the predicted probabilities and the true binary answers of 2,323 (23 genomes × 101 phenotype profiles) genome phenotype profile pairs. We also plotted the PR curves based on these probabilities and the true answers.
Correct prediction
For the assessment of PGP 2015, we also considered a prediction for a genome to be correct if the trait profile assigned the highest probability by the predictor was the correct trait profile for that individual. We computed the number and rate of correct predictions using the similar steps of the ones in PGP 2013, but with the total number of genomes as 23.
Mean ranking of the true match
We also calculated the mean ranking of the true match phenotype profile in each of 23 participants with genomes in each submission by the similar steps as the ones in PGP 2013, that is, (1) rank all probabilities of the 101 phenotype profiles for a genome/participant; and (2) identify the rank of the correct match for each participant; and (3) take the mean of the identified ranks of the correct matches.
Simulations
We also designed and developed simulations to test the hypothesis that permuted data derived from the actual submission could exceed the accuracy of the submitting group, and we used this to estimate a P value. The simulations were conducted in the similar steps of the ones in PGP 2011, that is, (1) generate 10,000 random results based on randomized permutation of the probabilities of the 2,323 genome and phenotype profile pairs of a submission; (2) calculate the AUCs of the randomized results; (3) calculate the distance of submission’s AUC from the average AUCs of the permuted results; and (4) calculate P values based on Equation (1).
Scoring
We also scored each submission based on the statistical analysis including criteria mentioned above, that is, ROC AUC, the number/rate of correct predictions, the mean ranking of true matches, and simulations. By adding the ranking numbers for the four above-mentioned scoring criteria together as the overall score of each submission, we got the overall placement of all five submissions.
3 RESULTS
Below are the assessment results of PGP 2011, PGP 2013, and PGP 2015.
3.1 PGP 2011
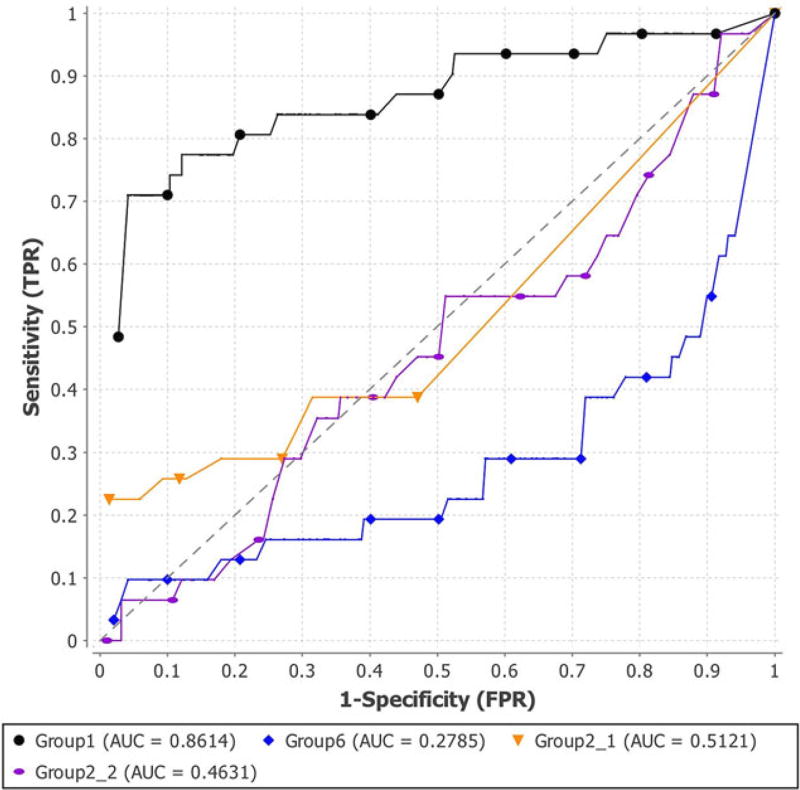
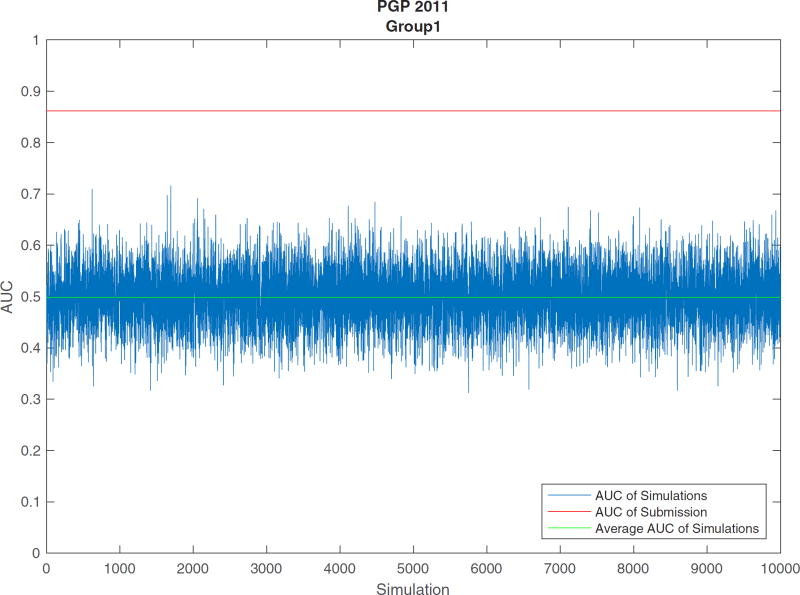
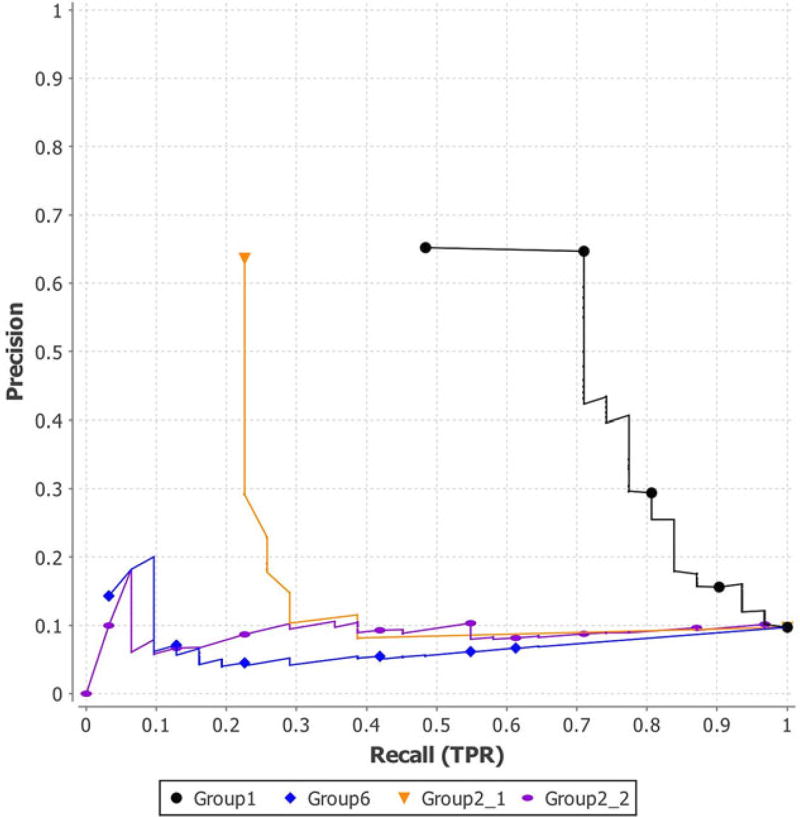
The assessment results of PGP 2011 are shown in Figures 1–3 and Tables 4 and 5. Figure 1 is the ROC curves of the four binary prediction submissions of PGP 2011. Submissions of Group2_1, Group2_2, Group6, and Group1 have AUCs of 0.5121, 0.4631, 0.2785, and 0.8614, respectively, as shown in the second column of Table 4. Figure 2 shows the PR curves of the four submissions of PGP 2011. The simulation results of PGP 2011 are shown in the third and fourth columns of Table 4 and Figure 3. In Figure 3, the red line is the AUC of the submission from Group1, the green line is the average AUC of all the 10,000 permuted results for the Group1’s submission, and the blue lines are the AUCs of all simulations. Note that only the Group1’s submission is significant as it has a P value smaller than 1.0 × 10−5. Table 5 shows the overall placement of all the four submissions based on their AUC and simulation results.
FIGURE 1.
ROC AUC curves of the four submissions of PGP 2011
FIGURE 3.
AUC comparison of simulative results and submission for the best submission from Group1 of PGP 2011
TABLE 4.
Comparison of AUC, simulation results for the four submissions of PGP 2011
| AUC | AUC distance | P value | |
|---|---|---|---|
| Group1 | 0.8614 | 0.3611 | < 1.0 × 10−5 |
| Group2_1 | 0.5121 | 0.0227 | 0.3158 |
| Group2_2 | 0.4631 | −0.0336 | 0.7332 |
| Group6 | 0.2785 | −0.2201 | 0.9999 |
TABLE 5.
Ranking of the four PGP 2011 submissions based on several metrics
| Ranking via AUC |
Ranking via AUC distance |
Overall score |
Overall placement |
|
|---|---|---|---|---|
| Group1 | 1 | 1 | 2 | 1 |
| Group2_1 | 2 | 2 | 4 | 2 |
| Group2_2 | 3 | 3 | 6 | 3 |
| Group6 | 4 | 4 | 8 | 4 |
FIGURE 2.
PR curves of the four submissions of PGP 2011
3.2 PGP 2013
The assessment results of PGP 2013 are shown in Tables 6–8.
TABLE 6.
AUC, correct matches, and mean ranks of each submission for PGP 2013
| Number of correct predictions out of 50 |
Mean rank | |
|---|---|---|
| Group1 | 6 | 25.4 |
| Group8_2 | 5 | 35.2 |
| Group8_4 | 5 | 37.3 |
| Group8_3 | 5 | 37.9 |
| Group8_1 | 4 | 39.8 |
| Group3_2 | 2 | 37.1 |
| Group3_3 | 2 | 45.1 |
| Group3_1 | 1 | 35.8 |
| Group2_4 | 1 | 68.5 |
| Group7_1 | 0 | 59.4 |
| Group2_1 | 0 | 66.6 |
| Group2_2 | 0 | 67.0 |
| Group2_5 | 0 | 69.2 |
| Group2_3 | 0 | 79.3 |
| Group2_6 | 0 | 84.5 |
| Group7_2 | 0 | 183.0 |
TABLE 8.
The number of reported and predicted traits for five participants without 23 and Me data in PGP 2013
| huID | Number of reported traits |
Number of predicted traits | ||
|---|---|---|---|---|
| Low risk | Mid risk | High risk | ||
| hu619F51 | 3 | 2 | 1 | 1 |
| huA05317 | 5 | 6 | 2 | 0 |
| huEA4EE5 | 7 | 2 | 0 | 0 |
| hu661AD0 | 4 | 3 | 1 | 0 |
| hu5CD2C6 | 7 | 3 | 1 | 0 |
Table 6 shows the number of correct predictions and mean ranking of true matches. We can see that the submission of Group1 had the highest number of correct matches of 6 and the best mean rank of 25.4. These are also shown in the overall ranking in Table 7. We also conducted simulations for the best submission from Group1. Specifically, for each genome, we randomly selected one phenotypic profile by uniform sampling. We made 10,000 such predictions for all 50 genomes. The largest number of genomes correctly predicted was four, implying that the observed prediction of six was statistically significant with a probability of less than 10−4. Similarly, the best average rank from simulation was 75.2, nearly three times that of the observed rank from submission of Group1. Above all, the submission from Group1 group had the highest performance in the 2013 challenge.
TABLE 7.
Ranking of the 16 submissions in the PGP 2013 challenge
| Ranking via number of correct predictions |
Ranking via mean rank |
Overall score |
Overall placement |
|
|---|---|---|---|---|
| Group1 | 1 | 1 | 2 | 1 |
| Group8_2 | 2 | 2 | 4 | 2 |
| Group8_4 | 2 | 5 | 7 | 3 |
| Group8_3 | 2 | 6 | 8 | 4 |
| Group3_2 | 6 | 4 | 10 | 5 |
| Group3_1 | 8 | 3 | 11 | 6 |
| Group8_1 | 5 | 7 | 12 | 7 |
| Group3_3 | 6 | 8 | 14 | 8 |
| Group7_1 | 10 | 9 | 19 | 9 |
| Group2_4 | 8 | 12 | 20 | 10 |
| Group2_1 | 10 | 10 | 20 | 10 |
| Group2_2 | 10 | 11 | 21 | 12 |
| Group2_5 | 10 | 13 | 23 | 13 |
| Group2_3 | 10 | 14 | 24 | 14 |
| Group2_6 | 10 | 15 | 25 | 15 |
| Group7_2 | 10 | 16 | 26 | 16 |
In addition, Group1 and Group2 submitted predictions for individual traits based on genomes to an optional challenge of PGP 2013. In the main challenge, the submission from Group1 correctly identified 27 genomes with 23 and Me data and also successfully tied five genomes to corresponding phenotypic profiles. We therefore focus on the predictions on these 32 participants/genomes. Empirically, we defined three risk categories (i.e., risk of a participant/genome having a phenotype) based on predicted probabilities: low risk with probability lying between 0.5 and 0.7, middle risk with probability lying between 0.7 and 0.9, and high risk with a probability greater than 0.9. For each participant, we counted the number of traits to which the participant provided a negative response in the survey. Generally, the submission made sparse predictions on disease risk for these 32 participants compared with reported number of positive traits. For all 32 genomes correctly predicted in the main challenge, the submitters predicted that each genome carried, on average, an additional 2.4 low-risk traits, 1.1 middle-risk traits, and 0.2 high-risk traits, on top of the average 11.2 reported phenotype traits. Table 8 lists the number of reported and predicted traits for five participants without 23 and Me data. From the table, we can see that the predictions estimated that each participant potentially carried 4.4 additional traits on top of the reported traits.
3.3 PGP 2015
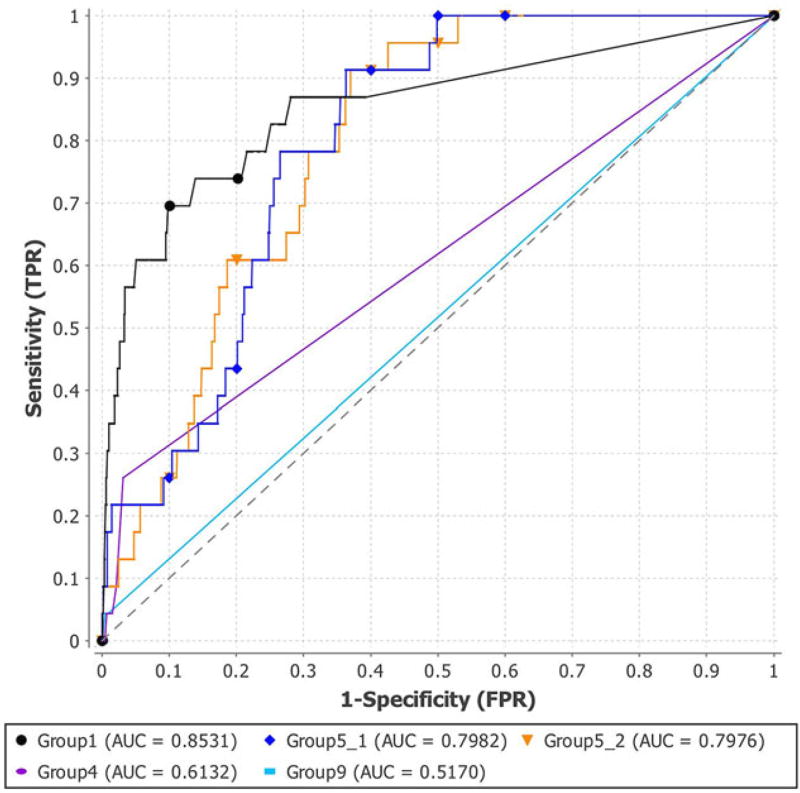
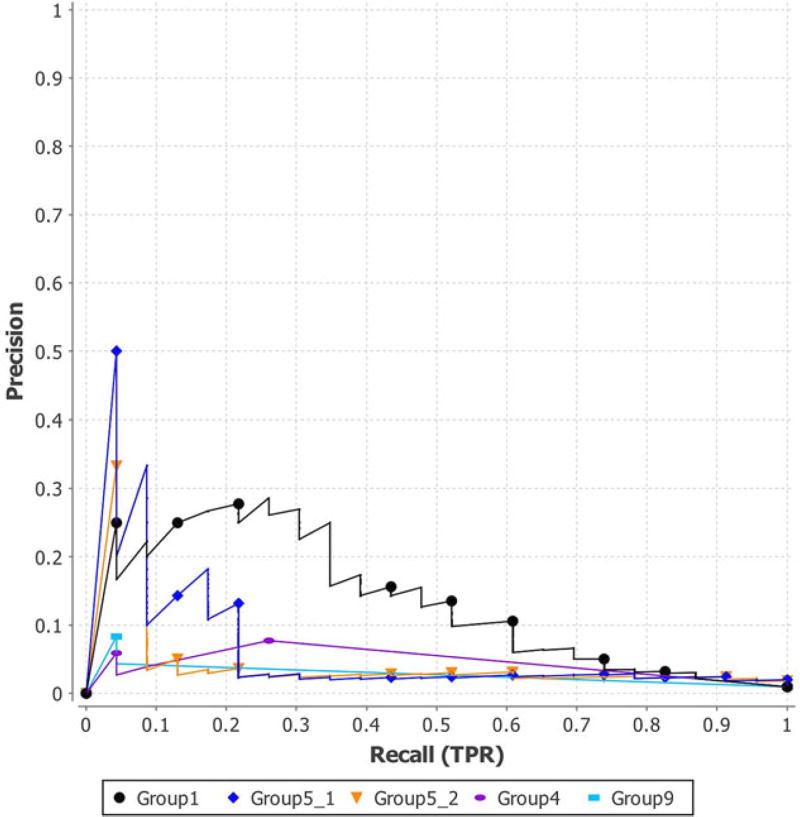
The assessment results of PGP 2015 are shown in Figures 4–6 and in Tables 9 and 10. Figure 4 and the second column of Table 9 show the ROC curves and AUC of all the five submissions. We observed that the submission of Group1 had the highest AUC of 0.8531, whereas Group5_1 and Group5_2 had AUCs of 0.7982 and 0.7976, respectively. The AUC of the predictions of Group4 and Group9 were lower (i.e., 0.5170 and 0.6132). In addition, Figure 5 shows the PR curves of the five submissions. The figure demonstrates the higher performance of the submissions of Group1 and Group5.
FIGURE 4.
ROC AUC curves of the five submissions of PGP 2015
FIGURE 6.
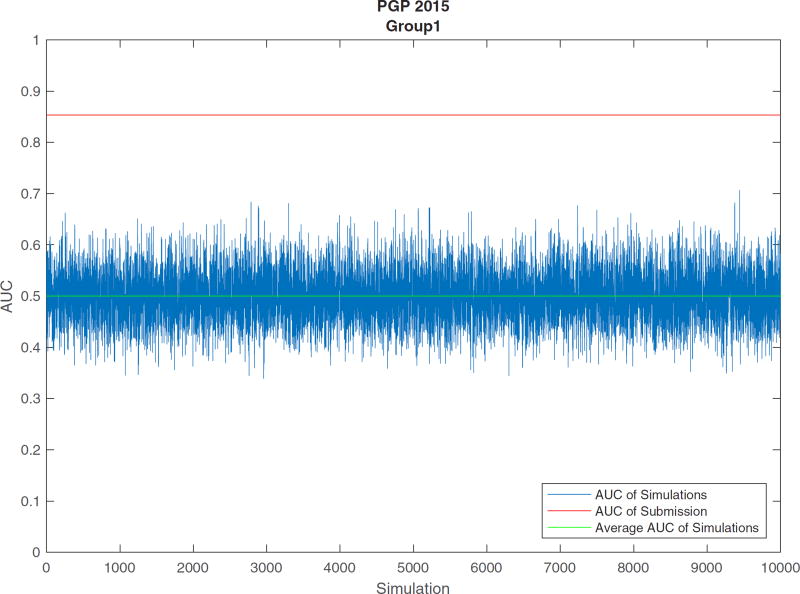
AUC comparison of simulative results and submission for the best submission from Group1 of PGP 2015
TABLE 9.
AUC, correct matches, mean ranks, and simulation results of each submission for PGP 2015
| AUC | Number of correct predictions out of 23 |
Mean rank | AUC distance | P value | |
|---|---|---|---|---|---|
| Group1 | 0.8531 | 5 | 16.93 | 0.3534 | < 1.0 × 10−5 |
| Group5_1 | 0.7982 | 3 | 23.65 | 0.2981 | < 1.0 × 10−5 |
| Group5_2 | 0.7976 | 3 | 21.54 | 0.2971 | < 1.0 × 10−5 |
| Group4 | 0.6132 | 1 | 39.59 | 0.1134 | 0.0001 |
| Group9 | 0.5170 | 1 | 49.30 | 0.017 | 0.0387 |
TABLE 10.
Ranking of the five submissions of PGP 2015
| Ranking via AUC |
Ranking via number of correct predictions |
Ranking via mean rank |
Ranking via AUC distance |
Overall score | Overall placement |
|
|---|---|---|---|---|---|---|
| Group1 | 1 | 1 | 1 | 1 | 4 | 1 |
| Group5_1 | 2 | 2 | 3 | 2 | 9 | 2 |
| Group5_2 | 3 | 2 | 2 | 3 | 10 | 3 |
| Group4 | 4 | 4 | 4 | 4 | 16 | 4 |
| Group9 | 5 | 4 | 5 | 5 | 19 | 5 |
FIGURE 5.
PR curves of the five submissions of PGP 2015
The last two columns of Table 9 and Figure 6 show the simulation results. From Figure 6, we observed that the AUC of Group1’s submission was higher than the permuted results. The last two columns of Table 9 show the distances between the submissions’ AUCs and the average simulated AUCs, and the P values demonstrating significant differences from the permuted results. From these two columns, we can see that all the submissions were significantly different from random prediction, especially submissions from Group1 and Group5, with small P values (smaller than 1.0 × 10−5).
The third column of Table 9 shows the results of the correct predictions for each submission. From the column, we can see that Group1 got five correct predictions out of 23 true matches, whereas Group5 had three correct predictions. Other submissions had one correct prediction.
The fourth column of Table 9 shows the mean ranking of the true match phenotype profile for each of the 23 genomes/participant. From this column, we can see that Group1 obtained the true matches in the mean ranking of 16.93 of 101 for the 23 genomes, and Group5’s submissions had 21.54 and 23.65 out of 101.
In addition, we also added the ranking of the correct matches among the five submissions to get the ranking of each participant/genome. From the results shown in the first two columns of Supp. Table S1, the best predicted participant was participant 14 (with a very small average ranking as compared with other participants), and the worst predicted participants were participants 16 and 21. We also listed profile information of each participant in the order of average ranking of each participant (Supp. Table S1). From the table, participant 14 was the only person with AB+ blood type among the 23 participants. In addition, from the ancestry analysis in Supp. Figure S1, we can see the two worst predicted participants are in the native American ancestry group, whereas the best predicted participants are across different groups.
3.4 Comparisons of PGP challenges
Here, we compare the three PGP challenges, that is, PGP 2015, PGP 2013, and PGP 2011. From Table 1, we can see that PGP 2013 had more genomes and phenotype profiles, as well as more submissions, when compared with PGP 2011 and PGP 2015. From Tables 6 and 9, we can see that the highest proportion of correct predictions was in PGP 2015, with five out of 23 correct (around 21.74%), as compared with PGP 2013, where the best prediction was 6 out of 50 correct (around 12.00% correct). The top-ranked submission from Group1 in PGP 2015 had improved prediction performance with respect to the top-ranked submission, also from Group1, in PGP 2013, from 12.00% to 21.74% in terms of correct prediction. From the overall placements of submissions in three challenges in Tables 5, 7, and 10, we can see Group1 group had the best performance over the three challenges.
4 DISCUSSION
We live in an era of genetic testing, either performed in the clinic or by direct-to-consumer (DTC) genetics services. Many results of genetic testing are difficult to interpret (Hudson, Javitt, Burke, Byers, & ASHG Social Issues Committee, 2007), though this is not always made sufficiently clear to patients or customers. It is important to understand the accuracy and limitations of currently available methodologies for interpreting the relationship between a patient’s genetic information and phenotypic traits.
4.1 PGP 2011
In the assessment of PGP 2011, we focused primarily on assessing binary trait predictions. Interestingly, one team had a vastly higher ROC AUC than the other teams. We hypothesized that Group1’s submission was superior due to their accurate modeling of the overall rates of traits in the general population, a challenging problem due to the lack of available data and the educated guesswork that goes into building predicted population rates. This is an important issue and needs to be addressed in order to make accurate genomic predictions. Further, while AUC is relatively high, overall precision remains low for all teams, suggesting that individual trait prediction is still very difficult. The prediction of Group1 was, by our assessment, the only statistically significant submission.
4.2 PGP 2013
Four groups (i.e., Group1, Group2, Group3, and Group7) used systematic approaches to modeling the probability of a trait given genomic data. One group (i.e., Group8) found participants with self-reported traits and then searched the genetic data for specific genotypes of these traits. Their mathematical model contained multiple unknown parameters, such as variant frequency and penetrance. For many of the phenotypes, no accurate estimation or sufficient data exits for such parameters. This difficulty caused three groups to resort to intuitive guesswork when developing the models, which is hard to justify and which varied from group to group.
Interestingly, one team revealed that for certain participants, several discrepancies were observed between the provided traits and what genomic data could indicate. Such discrepancies, among other difficulties mentioned above, could have reduced the performance of complex models built for this challenge. On the other hand, some simple models, such as the Naive Bayesian method, may have been insufficient to capture weak statistical signal embedded in the highly heterogeneous data.
The two top performing groups successfully matched six and five genomes to their phenotypic profiles, although they used distinct approaches: one used a Bayesian network and the other used trait-specific models. The relatively accurate results from the submission of Group8_2 can be significantly attributed to the elimination of unlikely phenotypic profiles using additional information available from manual sleuthing on the Internet, including PGP participant discussion forums. Therefore, a straightforward way to improve the prediction for this challenge may be to apply the Bayesian network modeling (used by the top performing group, i.e., Group1) to curated data (which can be obtained through the similar approach used in the submission from Group8_2).
4.3 PGP 2015
In our assessment, we also analyzed and compared ancestry, blood type, and eye color of the 101 participants (Supp. Material). These features are likely the source of a number of identified matches and appear to be driving predictive factors when identifying participants. For instance, the participant whom most teams correctly identified had the rare AB+ blood type. This feature could be one of the main factors in identification for this participant.
5 CONCLUSION
We show that the ability to predict a broad profile of phenotypes from genotype is improving, and highly statistically significant, but still lacks the accuracy to be definitive. We also show that several major factors, including blood type and ancestry, increase the rate of correctly identifying individuals when compared with identification methods using rare traits. Notably, Group1, which performed well throughout each of the experiments, improved from predicting six out of 50 correctly (12%) in 2013 to five out of 23 (~21%) in 2015, a more than twofold improvement in performance. While these results are limited by small dataset, it is clear that we can still greatly improve our ability to definitively match an individual’s phenotype with their genotype. In the future, we would like to include more genomes and trait profiles in the challenge and hope to see improvement of prediction accuracy.
Supplementary Material
Acknowledgments
The authors would like to thank anonymous predictors and other CAGI PGP participants for their contributions to the challenges. The CAGI experiment coordination is supported by NIH U41 HG007346 and the CAGI conference by NIH R13 HG006650. This work was also funded by NIH funding R01LM0009722, UL1TR000423, R01MH105524, U01 HG008473 and NSF funding 1458443.
Footnotes
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
References
- 1000 Genomes Project Consortium. A map of human genome variation from population-scale sequencing. Nature. 2010;467(7319):1061–1073. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adzhubei I, Jordan DM, Sunyaev SR. Predicting functional effect of human missense mutations using PolyPhen-2. Current Protocols in Human Genetics. 2013;(Unit7.20) doi: 10.1002/0471142905.hg0720s76. Chapter 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander DH, Novembre J, Lange K. Fast model-based estimation of ancestry in unrelated individuals. Genome Research. 2009;19:9. doi: 10.1101/gr.094052.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstee DJ. Red cell genotyping and the future of pretransfusion testing. Blood. 2009;114(2):248–256. doi: 10.1182/blood-2008-11-146860. [DOI] [PubMed] [Google Scholar]
- Ball MP, Bobe JR, Chou MF, Clegg T, Estep PW, Lunshof JE, Church GM. Harvard Personal Genome Project: Lessons from participatory public research. Genome Medicine. 2014;6(2):10. doi: 10.1186/gm527. (1–7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodmer W, Bonilla C. Common and rare variants in multifactorial susceptibility to common diseases. Nature Genetics. 2008;40(6):695–701. doi: 10.1038/ng.f.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdett T, Hall PN, Hastings E, Hindorff LA, Junkins HA, Klemm AK, Welter D. The NHGRI-EBI Catalog of published genome-wide association studies. 2015 doi: 10.1093/nar/gkw1133. Retrieved from http://www.ebi.ac.uk/gwas. [DOI] [PMC free article] [PubMed]
- CAGI (Critical Assessment of Genome Interpretation) Welcome to the CAGI experiment. 2016 Retrieved from genomeinterpretation.org.
- Carter H, Douville C, Stenson PD, Cooper DN, Karchin R. Identifying Mendelian disease genes with the Variant Effect Scoring Tool. BMC Genomics. 2013;14(Suppl 3):S3. doi: 10.1186/1471-2164-14-S3-S3. (1–16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Douville C, Wang C, Niknafs N, Yeo G, Beleva-Guthrie V, Karchin R. A probabilistic model to predict clinical phenotypic traits from genome sequencing. PLoS Computational Biology. 2014;10(9):e1003825. doi: 10.1371/journal.pcbi.1003825. (1–11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper DN, Krawczak M. Human Gene Mutation Database. Human Genetics. 1996;98(5):629. doi: 10.1007/s004390050272. [DOI] [PubMed] [Google Scholar]
- Douville C, Masica DL, Stenson PD, Cooper DN, Gygax DM, Kim R, Karchin R. Assessing the pathogenicity of insertion and deletion variants with the Variant Effect Scoring Tool (VEST-Indel) Human Mutation. 2016;37(1):28–35. doi: 10.1002/humu.22911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exome Aggregation Consortium (ExAC) ExAC Browser. Cambridge, MA: 2015. Retrieved from http://exac.broadinstitute.org. [Google Scholar]
- Fang H, Gough J. dcGO: Database of domain-centric ontologies on functions, phenotypes, diseases and more. Nucleic Acids Research. 2013;41(Database issue):D536–D544. doi: 10.1093/nar/gks1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett T. An introduction to ROC analysis. Pattern Recognition Letters. 2006;27:861–874. [Google Scholar]
- Friedberg I, Wass MN, Mooney SD, Radivojac P. Ten simple rules for a community computational challenge. PLoS Computational Biology. 2015;11(4):e1004150. doi: 10.1371/journal.pcbi.1004150. (1–5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage BF, Eby C, Johnson JA, Deych E, Rieder MJ, Ridker PM, McLeod HL. Use of pharmacogenetic and clinical factors to predict the therapeutic dose of warfarin. Clinical Pharmacology & Therapeutics. 2008;84(3):326–331. doi: 10.1038/clpt.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Perez A, Lopez-Bigas N. Improving the assessment of the outcome of nonsynonymous SNVs with a consensus deleteriousness score, Condel. The American Journal of Human Genetics. 2011;88(4):440–449. doi: 10.1016/j.ajhg.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindorff LA, MacArthur J, Morales J, Junkins HA, Hall PN, Klemm AK, Manolio TA. A catalog of published genome-wide association studies. 2015 Retrieved from http://www.genome.gov/gwastudies.
- Hindorff LA, Sethupathy P, Junkins HA, Ramos EM, Mehta JP, Collins FS, Manolio TA. Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(23):9362–9367. doi: 10.1073/pnas.0903103106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson K, Javitt G, Burke W, Byers P ASHG Social Issues Committee. ASHG statement on direct-to-consumer genetic testing in the United States. The American Journal of Human Genetics. 2007;81(3):635–637. doi: 10.1097/01.AOG.0000292086.98514.8b. [DOI] [PubMed] [Google Scholar]
- Karolchik D, Hinrichs AS, Kent WJ. The UCSC Genome Browser. Current Protocols in Bioinformatics. 2009;(Unit 1.4) doi: 10.1002/0471250953.bi0104s17. Chapter 1. [DOI] [PubMed] [Google Scholar]
- McKusick VA. Mendelian inheritance in man and its online version, OMIM. The American Journal of Human Genetics. 2007;80(4):588–604. doi: 10.1086/514346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng PC, Henikoff S. SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Research. 2003;31(13):3812–3814. doi: 10.1093/nar/gkg509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oates ME, Stahlhacke J, Vavoulis DV, Smithers B, Rackham OJ, Sardar AJ, Gough J. The SUPERFAMILY 1.75 database in 2014: A doubling of data. Nucleic Acids Research. 2015;43(Database issue):D227–D233. doi: 10.1093/nar/gku1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PGP (Personal Genome Project) Personal Genome Project. Harvard: 2016. Retrieved from personalgenomes.org. [Google Scholar]
- Rich-Edwards JW, Colditz GA, Stampfer MJ, Willett WC, Gillman MW, Hennekens CH, Manson JE. Birthweight and the risk for type 2 diabetes mellitus in adult women. Annals of Internal Medicine. 1999;130(4 Pt 1):278–284. doi: 10.7326/0003-4819-130-4_part_1-199902160-00005. [DOI] [PubMed] [Google Scholar]
- Shihab HA, Gough J, Cooper DN, Stenson PD, Barker GL, Edwards KJ, Gaunt TR. Predicting the functional, molecular, and phenotypic consequences of amino acid substitutions using hidden Markov models. Human Mutation. 2013;34(1):57–65. doi: 10.1002/humu.22225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennessen JA, Bigham AW, O’Connor TD, Fu W, Kenny EE, Gravel S NHLBI Exome Sequencing Project. Evolution and functional impact of rare coding variation from deep sequencing of human exomes. Science. 2012;337(6090):64–69. doi: 10.1126/science.1219240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The International HapMap Consortium. The International HapMap Project. Nature. 2003;426:789–796. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- Walsh S, Liu F, Wollstein A, Kovatsi L, Ralf A, Kosiniak-Kamysz A, Kayser M. The HIrisPlex system for simultaneous prediction of hair and eye colour from DNA. Forensic Science International: Genetics. 2013;7:98–115. doi: 10.1016/j.fsigen.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Wang K, Li M, Hakonarson H. ANNOVAR: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Research. 2010;38(16):e164. doi: 10.1093/nar/gkq603. (1–7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue P, Melamud E, Moult J. SNPs3D: Candidate gene and SNP selection for association studies. BMC Bioinformatics. 2006;7:166. doi: 10.1186/1471-2105-7-166. (1–15) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.