Abstract
Objective
Deficits in memory have been suggested as an influential mechanism of anhedonia, because while pleasant experiences may be enjoyed in-the-moment, the cognitive processes involved in reporting anticipated or remembered enjoyable experiences is thought to be impaired. This study will determine whether any aspects of memory, including visual memory, verbal memory, or working memory, are significantly predictive of anhedonia in a sample of schizophrenia, psychotic bipolar disorder, and healthy controls.
Methods
The study included 38 individuals with schizophrenia, 19 individuals with bipolar disorder with psychosis, and 43 age-matched healthy controls. All participants completed a self-report social and physical anhedonia questionnaire along with a cognitive screening battery, which assessed the domains of attention/vigilance, working memory, verbal learning, visual learning, and reasoning and problem solving.
Results
Anhedonia scores were regressed onto domain scores to determine which areas of cognition uniquely predicted level of anhedonia in each group. For the schizophrenia group, physical anhedonia was significantly predicted by worse visual memory performance. The regression models did not find significant cognitive predictors of physical or social anhedonia in the bipolar disorder or control groups.
Conclusions
This study found a significant relationship between visual memory and physical anhedonia in schizophrenia patients that was not present in a sample of psychotic bipolar patients or healthy controls, adding to an accumulating body of evidence that visual memory is related to anhedonia in schizophrenia. This relationship may be explained by underlying abnormalities in the orbitofrontal cortex in schizophrenia.
Keywords: Memory, Cognition, Negative Symptoms, MATRICS Consensus Cognitive Battery
Introduction
Although schizophrenia is often characterized by positive symptoms, negative and cognitive symptoms are also quite prominent in this population (Capleton, 1996). Anhedonia has been studied extensively in schizophrenia (Burbridge & Barch, 2007; Herbener & Harrow, 2002; Herbener, Harrow, & Hill, 2005), (Herbener Rosen, Khine, & Sweeney, 2007) and is a particularly enduring trait that is often resistant to treatment (Horan, Kring, & Blanchard, 2006). Cognitive dysfunction is also commonly found in schizophrenia; a recent meta-analysis of cognitive performance found that individuals with schizophrenia performed significantly worse than healthy controls across all cognitive domains, including verbal memory, speed of processing, working memory, attention, and visual memory (Fatouros-Bergman, Cervenka, Flyckt, Edman, & Farde, 2014). In their meta-analysis, Fatouros-Bergman and colleagues (2014) assessed cognitive domains based on the Measurement and Treatment Research to Improve Cognition in Schizophrenia (Nuechterlein & Green, 2006; Nuechterlein et al., 2008), as it has been backed by the National Institutes of Health and the U.S. Food and Drug Administration.
Deficits in memory have been suggested as an influential mechanism of anhedonia (Herbener et al., 2007; Horan, Green, Kring, & Nuechterlein, 2006). This is because although consummatory, or in-the-moment, pleasure remains largely intact in schizophrenia, individuals with schizophrenia have a diminished ability to rate an experience in the past or future as enjoyable (Gard, Kring, Gard, Horan, & Green, 2007). Support for this comes from Burbridge and Barch (2007), who found that working memory moderated the relationship between state and trait anhedonia in schizophrenia, such that in individuals with better working memory, in-the-moment emotional responses were consistent with reported emotional experiences, whereas in people with poor working memory, their in-the-moment emotional experiences were intact but their reported emotional experiences were impaired. Cohen and colleagues (2011) outlined several explanations for this anhedonia paradox that address trait and state anhedonia, with trait referring to ongoing deficits in the ability to recall or anticipate enjoyment, and state referring to more transient deficits of this nature. The encoding-retrieval deficit theory suggests that although pleasant experiences may be enjoyed in-the-moment, there is a breakdown in forming or accessing the memory of these enjoyable experiences. Strauss and Gold (2012) recently argued that the anhedonia paradox is likely related to impairments in cognitive processes involved in reporting anticipated or remembered pleasurable experiences, such as encoding and retrieval. If this encoding-retrieval deficit theory is true, then a measure of memory should significantly correlate with anhedonia.
Different aspects of memory have been studied in their association with anhedonia, visual, verbal, and working memory. Previous studies have found visual memory to be associated with anhedonia in a community sample of individuals at high-risk for schizophrenia using socially anhedonic individuals (Cohen, Leung, Saperstein, & Blanchard, 2006). Specifically, the high-risk group differed significantly from controls on tasks of visual memory recall and visual-spatial construction. Notably, they did not find any group differences on other measures of memory such as verbal or working memory. On the other hand, Brebion and colleagues (2012) found that in patients with schizophrenia, greater levels of anhedonia were associated with fewer false recognition errors for visual stimuli; however, this was likely because individuals with anhedonia tended to endorse fewer items overall.
Studies that have examined the relationship between verbal memory and negative symptoms in schizophrenia have yielded mixed results. For example, Brazo and colleagues (2002) found that patients with schizophrenia with primarily negative symptoms exhibited worse verbal recall and recognition compared to controls, whereas patients with primarily positive symptoms had comparable verbal memory to the healthy participants. However, Vaz and Heinrichs (2002) found that memory-impaired schizophrenia patients tended to exhibit worse positive symptoms than their memory-unimpaired counterparts, whereas the two groups did not differ on their negative symptom severity. The literature may be mixed because different scales have been used to assess negative symptoms across studies. While Brazo et al. (2002) used the Positive and Negative Syndrome Scale (Kay, Fiszbein, & Opler, 1987), Vaz & Heinrichs (2002) chose the Brief Psychiatric Rating Scale (Overall, J. E., & Gorham, D. R., 1962). One noteworthy difference between these two measures is that the BPRS negative symptoms scale does not include anhedonia, while the PANSS does. Therefore, examination of more specific components of negative symptoms, such as anhedonia, would be useful to help clarify the association with verbal memory.
Visual working memory has also been studied in the context of anhedonia in schizophrenia. Szendi and colleagues (2006) found that greater anhedonia was associated with worse visual working memory in a sample of male patients with schizophrenia. Giráldez and colleagues (2000) found that in a community sample, individuals with high levels of schizotypy, characterized by social anhedonia, flat affect, and social isolation, performed worse on a task of visual working memory than individuals with low levels of schizotypy.
Overall, evidence for the association between visual, verbal, and working memory and anhedonia in schizophrenia remains equivocal and requires further study. Knowing which types of memory are related to anhedonia may assist in the development of interventions that can specifically target anhedonia in schizophrenia. The purpose of the present study is to test the encoding-retrieval deficit theory (Cohen et al., 2011; Strauss & Gold, 2012), which suggests that while pleasant experiences may be enjoyed in-the-moment, the cognitive processes involved in reporting anticipated or remembered enjoyable experiences is impaired. In order to do so, this study will determine whether any aspects of memory, including visual memory, verbal memory, or working memory, are significantly predictive of anhedonia. It was hypothesized that measures of visual, verbal, and working memory would significantly predict greater levels of anhedonia in schizophrenia, while other cognitive domains would not. This extends the work of previous studies by comparing clinical samples of patients with schizophrenia and bipolar disorder with psychosis, along with a healthy control sample. The inclusion of two psychiatric groups provides a valuable comparison between different disorders on the schizophrenia and psychosis spectrum. This also extends previous research by including both social and physical anhedonia measures, in order to clarify the level of specificity in the relationship between cognition and anhedonia.
Method
Participants
The present study included 38 individuals with schizophrenia and 19 individuals with bipolar disorder with psychosis, recruited from the University of Illinois Medical District and surrounding area. In addition, 43 age-matched healthy controls were recruited from the community. All study participants were between 21–60 years of age, and all patient participants met criteria for schizophrenia or bipolar disorder with psychosis based on a consensus diagnosis obtained from the research team using the Structured Clinical Diagnostic Interview for DSM-IV TR (First, Spitzer, Gibbon, & Williams, 2002). Exclusion criteria for this study were current neurological conditions, seizure disorders, and substance dependence.
Basic demographic information was collected for all participants, including age, gender, race, and intellectual ability. Group comparisons on these variables can be found in Table 1. Notably, there were no group differences found in age or gender breakdown, but the three groups differed on racial makeup and premorbid intellectual ability.
Table 1.
Demographic Variables for Each Group
| Variable | SZ n=38 | BD n=17 | HC n=42 | Statistic |
|---|---|---|---|---|
| Age | 39.47 (12.86) | 43.41 (13.32) | 38.26 (12.62) | F (2, 96) = .98, p = .379 |
| Sex M / F | 20 / 18 | 6 / 11 | 19 / 23 | χ2(2) = 1.46, p = .482 |
| Race AA / C / A / H | 28 / 3 / 2 / 5 | 14 / 2 / 0 / 1 | 19 / 10 / 8 / 5 | χ2(6) = 13.63, p = .034 |
| WTAR FSIQ | 88.06 (10.84) | 88.88 (7.16) | 100.34 (14.03) | F (2, 92) = 11.76, p < .001 |
Note. Values in parentheses are standard deviations. AA=African American, C = Caucasian, A=Asian, H=Hispanic. Groups differed on WTAR FSIQ, SZ=BD<HC.
Measures
Anhedonia
As a measure of trait anhedonia, the Chapman Anhedonia scale (Chapman, Chapman, & Raulin, 1976) was administered to all participants. This self-report questionnaire is comprised of 114 true/false statements, including 40 items assessing social anhedonia, defined as the inability to enjoy interpersonal pleasures such as non-physical pleasures of being with others, talking, competing, loving, and otherwise interacting with others (e.g., “having close friends is not as important as many people say”), and 61 items assessing physical anhedonia, the inability to enjoy physical pleasures, such as touching, feeling, eating, smell, sound, temperature, sex, and movement (e.g., “beautiful scenery has always been a great delight to me”). Higher scores represent greater levels of anhedonia. Also included in this measure is a 13-item symptom validity subscale which assesses whether the performance indicates poor effort, malingering, or random responding.
Depression
Given that anhedonia is both a negative symptom of schizophrenia as well as a symptom of depression, participants were also assessed for depressive symptoms based on the five-factor model of the Positive and Negative Syndrome Scale (PANSS; Kay, Fiszbein, & Opler, 1987). This model yields scores in five domains: positive symptoms, negative symptoms, cognitive symptoms, excitement, and depression, based on previous factor analytic findings (Lindenmayer, Bernstein-Hyman, & Grochowski, 1994; Lehoux, Gobeil, Lefebvre, Maziade, & Roy, 2009). Scores from the depression scale were used to control for depressive symptoms when examining the relationship between anhedonia and cognition. While positive symptoms were assessed using this scale and reported in the description of the patient samples, they were not included in the data analyses because they were beyond the scope of this study, which was specifically to examine the nature of the relationship between anhedonia and memory deficits.
Cognition
The Wechsler Test of Adult Reading (Holdnack, 2001) was included as a measure of estimated premorbid intelligence. A full-scale intelligence quotient (FSIQ) score was predicted for each participant based on age and education norms.
In addition, all groups completed the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery (Nuechterlein & Green, 2006). This neuropsychological screening battery assesses the cognitive domains of processing speed, attention/vigilance, working memory, verbal learning, visual learning, and reasoning and problem solving using age- and education-based norms. For the present study, emphasis was placed on the cognitive domains with a visual component, specifically attention and processing speed.
Procedure
The study was approved by the University of Illinois at Chicago Institutional Review Board and signed consent was obtained from participants prior to initiation of study procedures. All participants completed the study at the University of Illinois at Chicago medical campus; healthy controls and outpatient participants were seen at the Neuropsychiatric Institute or the Psychiatric Institute, while inpatient participants were seen on the psychiatric unit of the University of Illinois Hospital. Diagnosis was determined by clinical interview, after which all participants completed the self-report anhedonia scale. Following these assessments, participants completed the cognitive screening battery, which included a measure of premorbid intellectual functioning as well as current neuropsychological functioning.
Data analysis
In order to explore the relationship between anhedonia and cognition, separate stepwise multiple regressions with backward elimination were conducted for the schizophrenia, bipolar disorder, and healthy control groups, for social and physical anhedonia separately, for a total of 6 regression analyses. Trait anhedonia scores were regressed onto MATRICS domain scores (processing speed, attention/vigilance, working memory, verbal learning, visual learning, and reasoning/problem solving) as well as age and depression scores, to determine which areas of cognition uniquely predicted level of anhedonia in each group.
Results
The MATRICS domain scores are presented in Table 2. Univariate ANOVAs revealed that groups differed significantly on all domains except reasoning/problem solving. The means in Table 2 indicate that the schizophrenia group tended to exhibit the worst performance, consistent with previous research. Figure 1 reflects significant group differences in each MATRICS domain. Anhedonia scores are also presented in Table 2; the groups did not differ on physical anhedonia but differed on social anhedonia. Notably, none of the groups exhibited elevated scores on the symptom validity scale of the anhedonia measure (Controls M = 3.21, Schizophrenia M = 3.82, Bipolar M = 2.59). Additionally, to better describe the symptom presentation of the patients in this sample, Table 2 also includes depressive and psychotic symptoms scores for the schizophrenia and bipolar groups.
Table 2.
Cognitive and Clinical Scores for Each Group
| Variable | Schizophrenia (n=38) |
Bipolar (n=17) |
Control (n=42) |
Statistic |
|---|---|---|---|---|
| Processing speed | 33.26 (12.39) | 44.76 (13.23) | 47.38 (8.84) | F (2, 96) = 16.94, p < .001 |
| Attention | 32.97 (11.75) | 40.80 (7.90) | 43.51 (9.96) | F (2, 85) = 9.33, p < .001 |
| Working memory | 31.08 (15.02) | 39.29 (12.47) | 42.62 (9.69) | F (2, 96) = 8.71, p < .001 |
| Verbal memory | 34.42 (7.28) | 37.41 (7.88) | 42.62 (9.39) | F (2, 96) = 9.77, p < .001 |
| Visual memory | 33.00 (12.87) | 41.29 (12.02) | 46.57 (10.62) | F (2, 96) = 13.29, p < .001 |
| Reasoning | 41.87 (9.57) | 44.88 (10.33) | 46.33 (8.85) | F (2, 96) = 2.29, p = .107 |
| Physical Anhedonia | 21.63 (10.62) | 16.00 (8.52) | 16.62 (14.48) | F (2. 95) = 2.08, p = .130 |
| Social Anhedonia | 16.37 (7.13) | 12.24 (7.90) | 11.33 (9.42) | F (2. 96) = 3.87, p = .024 |
| Depressive symptoms | 14.74 (2.76) | 12.59 (2.58) | 7.64 (2.72) | F (2, 96) = 70.64, p < .001 |
| Psychotic symptoms | 15.92 (3.77) | 11.41 (4.70) | 4.11 (.50) | F (2, 96) = 148.64, p < .001 |
Note. Values in parentheses are standard deviations.
Figure 1.
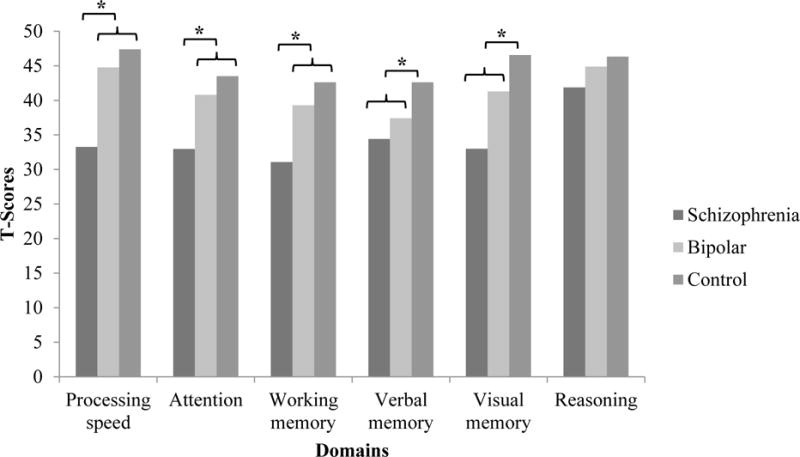
Group differences in MATRICS domain scores. Significant group differences are notated with an asterisk (*).
The partial correlations within each group between anhedonia and cognition, while controlling for depression symptoms, are shown in Table 3. As can be seen, only visual memory was significantly associated with physical anhedonia, r(33) = .40, p = .015, and only in the schizophrenia group.
Table 3.
Correlations between Cognitive Domains and Anhedonia in Each Group
| Group | Variable | Processing speed | Attention | Working Memory | Verbal Memory | Visual Memory | Reasoning |
|---|---|---|---|---|---|---|---|
| Healthy Controls | Physical Anhedonia | .101 | .282 | .145 | .078 | −.005 | .134 |
| Social Anhedonia | .122 | .232 | .152 | .004 | −.088 | .187 | |
|
| |||||||
| Schizophrenia | Physical Anhedonia | −.259 | .065 | −.199 | −.168 | −.396* | −.173 |
| Social Anhedonia | −.132 | .079 | −.110 | −.104 | −.320ˆ | −.091 | |
|
| |||||||
| Bipolar Disorder | Physical Anhedonia | −.267 | −.227 | −.270 | −.150 | −.396 | −.455 |
| Social Anhedonia | .072 | .037 | −.052 | .173 | .012 | .217 | |
Note:
Correlation is significant at the .05 level (2-tailed).
Correlation is trending significant at the .06 level (2-tailed).
Cognitive Predictors of Physical Anhedonia
For the schizophrenia regression, the only significant predictor contained in the final model was visual memory. The model was reached in one step, and was statistically significant, R2 = .135, F(1, 31) = 4.69, p = .038. Physical anhedonia was primarily predicted by worse visual memory performance, β = −.37, t(31) = −2.17, p = .038, and accounted for approximately 14% of the variance of physical anhedonia.
For the healthy control group, the prediction model was not significant, R2 = .002, F(1, 38) = .06, p = .814, and none of the individual cognitive domains predicted physical anhedonia. The prediction model was also not significant in the bipolar disorder group, R2 = .041, F(1, 13) = .51, p = .489, and none of the individual cognitive domains predicted physical anhedonia.
Cognitive Predictors of Social Anhedonia
The MATRICS domain scores of processing speed, attention/vigilance, working memory, verbal learning, visual learning, and reasoning/problem solving, along with depression and age, were also used in a stepwise multiple regression analysis to predict social anhedonia for each of the three diagnostic groups. For the schizophrenia regression, the model was significant, R2 = .263, F(2, 31) = 5.17, p = .012, and accounted for approximately 26% of the variance of social anhedonia. Higher social anhedonia scores were primarily predicted by higher depression scores, β = .385, t(31) = 2.42, p = .022, and younger age, β = −.330, t(31) = −2.07, p = .048.
The prediction model was also significant in the bipolar disorder group, R2 = .445, F(1, 14) = 10.43, p = .007, and accounted for approximately 45% of the variance of social anhedonia. However, as with the schizophrenia group, higher social anhedonia scores were primarily predicted by greater depression scores, β = .667, t(31) = 3.23, p = .007. The mode was not significant in the healthy control group, R2 = .009, F (1, 38) = .33, p = .569. Notably, none of the cognitive variables were predictive of social anhedonia in any of the groups.
Discussion
The present study aimed to predict trait physical and social anhedonia in schizophrenia, bipolar disorder, and healthy controls using the cognitive domains of processing speed, attention, working memory, verbal memory, visual memory and reasoning. The schizophrenia group performed worse on all cognitive domains compared to the other groups. Our main findings concern the relationship between the cognitive domain of visual memory and physical anhedonia. Within the schizophrenia group, physical anhedonia was significantly predicted by visual memory, but this relationship was not observed for the psychotic bipolar disorder or healthy control groups. None of the cognitive variables were found to predict social anhedonia in the schizophrenia, bipolar disorder, or control groups.
The results of the present study are consistent with the previous finding of an association between anhedonia and visual memory in a community sample of anhedonic individuals at high-risk for schizophrenia (Cohen et al., 2006), and a finding in a sample of male patients with schizophrenia that greater anhedonia was associated with worse visual working memory (Szendi et al., 2006). Furthermore, our results support the encoding-retrieval deficit theory that posits that memory impairment is an important mechanism involved in anhedonia (Strauss & Gold, 2012). However, the support from the present study is limited to visual memory, as we did not find an association with either verbal memory, or working memory, which was reported previously to be associated with anhedonia (Burbridge & Barch, 2007). However, Cohen and colleagues (2006) also did not find a significant association between anhedonia and verbal or working memory in their study. Thus, our study adds to an accumulating body of evidence that visual memory is related to anhedonia in schizophrenia.
The mechanism by which visual memory may be associated with anhedonia may be that individuals with schizophrenia have impairments in visualizing previously obtained rewarding stimuli. This argument is consistent with the suggestion by Cohen et al. (2011), but here it is specifically applied to visual memory. In terms of potential neural mechanisms underlying this impairment, the orbitofrontal cortex (OFC) is a likely candidate. This brain region receives sensory input from all five sensory modalities (Carmichael & Price, 1995; Rolls, 2004), and contains neurons that represent and update the reward value of stimuli (Rolls, 1996; Rolls, 2000; Rolls, 2002; Rolls, 2004). Neurons in the OFC receive projections from the ventral temporal lobe ‘what’ pathway and have been shown to respond to visual images and objects based on their reward value (Rolls, 1996; Thorpe, Rolls, & Maddison, 1983). Human neuroimaging studies have found that feeding a person to satiety decreases the OFC activation in response to that specific food, and that OFC activation correlates with subjective pleasantness of the food (Kringelbach, O’Doherty, Rolls, & Andrews, 2003; O’Doherty et al., 2000). OFC activation has also been reported in response to the relative value of stimuli that have acquired emotional significance via association with a primary reinforcer, such as monetary rewards (Elliott, Newman, Longe, & Deakin, 2003). Many studies have reported abnormalities in the OFC in schizophrenia (Kuperberg et al., 2003; Nakamura et al., 2007; Nakamura et al., 2008; Takayanagi et al., 2010). Furthermore, generalized white matter reductions in OFC subregions have been reportedly associated with high negative symptoms in schizophrenia (Sanfilipo et al., 2000). Thus, alterations in the OFC could underlie the association between anhedonia and visual memory in schizophrenia.
This study examined both social and physical anhedonia to introduce specificity into the relationship between cognition and anhedonia. The social anhedonia scale assessed enjoyment from interpersonal interactions, while the physical anhedonia scale measured enjoyment from activities involving touch, taste, smell, sound, temperature, sex, and movement. If the OFC is indeed involved in the relationship between anhedonia and visual memory in schizophrenia, then this further supports the specificity of our findings for physical but not social anhedonia. Given that the OFC receives input from all of the sensory modalities, then the measure reflective of enjoyment from sensory experiences would best capture the association between anhedonia and visual memory.
Limitations
While there was no overall group difference in physical anhedonia, examination of the means revealed that the bipolar disorder group scored similar to the control group, whereas the schizophrenia group scored the highest. Thus, it is not clear whether there is an absence of a relationship between visual memory and physical anhedonia, or whether there is a floor effect of physical anhedonia in the bipolar disorder and control groups. In addition, while groups were well-matched on age and gender, there were group differences in race composition and premorbid IQ. Another limitation is that in the correlational analyses, a Bonferroni correction was not applied, and therefore the potential for multiple comparison errors exists. However, the pattern reflected by the correlational analysis is consistent with the results of the regression analysis, thereby reducing the likelihood of multiple comparison errors. Finally, the lack of significant results for the bipolar disorder group may be due to the smaller size of this group.
In conclusion, we found a significant relationship between visual memory and physical anhedonia in a sample of schizophrenic patients that was not present in a sample of psychotic bipolar patients or healthy controls. We suggest that this relationship may be explained by underlying abnormalities in the orbitofrontal cortex in the schizophrenia group. Future research should examine a larger sample of bipolar disorder patients, and should also determine whether memory for other primary sensory modalities (i.e. touch, taste and smell) is also associated with anhedonia.
Acknowledgments
This work was supported in part by PHS grant (NIH) R01MH094358 (RPS). The authors would like to thank all the individuals who participated in this study.
References
- Brazo P, Marie RM, Halbecq I, Benali K, Segard L, Delamillieure P, Dollfus S. Cognitive patterns in subtypes of schizophrenia. European Psychiatry : The Journal of the Association of European Psychiatrists. 2002;17(3):155–162. doi: 10.1016/s0924-9338(02)00648-x. doi:S092493380200648X [pii] [DOI] [PubMed] [Google Scholar]
- Brebion G, Ohlsen RI, Bressan RA, David AS. Source memory errors in schizophrenia, hallucinations and negative symptoms: A synthesis of research findings. Psychological Medicine. 2012;42(12):2543–2554. doi: 10.1017/S003329171200075X. [DOI] [PubMed] [Google Scholar]
- Burbridge JA, Barch DM. Anhedonia and the experience of emotion in individuals with schizophrenia. Journal of Abnormal Psychology. 2007;116(1):30–42. doi: 10.1037/0021-843X.116.1.30. doi:2007-01891-003 [pii] [DOI] [PubMed] [Google Scholar]
- Capleton RA. Cognitive function in schizophrenia: Association with negative and positive symptoms. Psychological Reports. 1996;78(1):123–128. doi: 10.2466/pr0.1996.78.1.123. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Sensory and premotor connections of the orbital and medial prefrontal cortex of macaque monkeys. The Journal of Comparative Neurology. 1995;363(4):642–664. doi: 10.1002/cne.903630409. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP, Raulin ML. Scales for physical and social anhedonia. Journal of Abnormal Psychology. 1976;85(4):374–382. doi: 10.1037//0021-843x.85.4.374. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Leung WW, Saperstein AM, Blanchard JJ. Neuropsychological functioning and social anhedonia: Results from a community high-risk study. Schizophrenia Research. 2006;85(1–3):132–141. doi: 10.1016/j.schres.2006.03.044. doi:S0920-9964(06)00168-X [pii] [DOI] [PubMed] [Google Scholar]
- Cohen AS, Najolia GM, Brown LA, Minor KS. The state-trait disjunction of anhedonia in schizophrenia: Potential affective, cognitive and social-based mechanisms. Clinical Psychology Review. 2011;31(3):440–448. doi: 10.1016/j.cpr.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Elliott R, Newman JL, Longe OA, Deakin JF. Differential response patterns in the striatum and orbitofrontal cortex to financial reward in humans: A parametric functional magnetic resonance imaging study. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2003;23(1):303–307. doi: 10.1523/JNEUROSCI.23-01-00303.2003. doi:23/1/303 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatouros-Bergman H, Cervenka S, Flyckt L, Edman G, Farde L. Meta-analysis of cognitive performance in drug-naive patients with schizophrenia. Schizophrenia Research. 2014;158(1–3):156–162. doi: 10.1016/j.schres.2014.06.034. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition. Biometrics Research 2002 [Google Scholar]
- Gard DE, Kring AM, Gard MG, Horan WP, Green MF. Anhedonia in schizophrenia: Distinctions between anticipatory and consummatory pleasure. Schizophrenia Research. 2007;93(1–3):253–260. doi: 10.1016/j.schres.2007.03.008. doi:S0920-9964(07)00125-9 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giráldez S, Caro M, Rodrigo A, Piñeiro M, González J. Assessment of essential componernts of schizotypy using neurocognitive measures. Psychology in Spain. 2000;4(1):183–194. [Google Scholar]
- Herbener ES, Harrow M. The course of anhedonia during 10 years of schizophrenic illness. Journal of Abnormal Psychology. 2002;111(2):237–248. [PubMed] [Google Scholar]
- Herbener ES, Harrow M, Hill SK. Change in the relationship between anhedonia and functional deficits over a 20-year period in individuals with schizophrenia. Schizophrenia Research. 2005;75(1):97–105. doi: 10.1016/j.schres.2004.12.013. doi:S0920-9964(04)00474-8 [pii] [DOI] [PubMed] [Google Scholar]
- Herbener ES, Rosen C, Khine T, Sweeney JA. Failure of positive but not negative emotional valence to enhance memory in schizophrenia. Journal of Abnormal Psychology. 2007;116(1):43–55. doi: 10.1037/0021-843X.116.1.43. doi:2007-01891-004 [pii] [DOI] [PubMed] [Google Scholar]
- Holdnack HA. Wechsler test of adult reading: WTAR. San Antonio: The Psychological Corporation; 2001. [Google Scholar]
- Horan WP, Green MF, Kring AM, Nuechterlein KH. Does anhedonia in schizophrenia reflect faulty memory for subjectively experienced emotions? Journal of Abnormal Psychology. 2006;115(3):496–508. doi: 10.1037/0021-843X.115.3.496. doi:2006-09167-011 [pii] [DOI] [PubMed] [Google Scholar]
- Horan WP, Kring AM, Blanchard JJ. Anhedonia in schizophrenia: A review of assessment strategies. Schizophrenia Bulletin. 2006;32(2):259–273. doi: 10.1093/schbul/sbj009. doi:sbj009 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler L. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2) doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, O’Doherty J, Rolls ET, Andrews C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cerebral Cortex (New York, N.Y.: 1991) 2003;13(10):1064–1071. doi: 10.1093/cercor/13.10.1064. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, Fischl B. Regionally localized thinning of the cerebral cortex in schizophrenia. Archives of General Psychiatry. 2003;60(9):878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- Lehoux C, Gobeil MH, Lefebvre AA, Maziade M, Roy MA. The five-factor structure of the PANSS: A critical review of its consistency across studies. Clinical Schizophrenia & Related Psychoses. 2009;3(2):103–110. [Google Scholar]
- Lindenmayer JP, Bernstein-Hyman R, Grochowski S. Five-factor model of schizophrenia. initial validation. The Journal of Nervous and Mental Disease. 1994;182(11):631–638. doi: 10.1097/00005053-199411000-00006. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Nestor PG, Levitt JJ, Cohen AS, Kawashima T, Shenton ME, McCarley RW. Orbitofrontal volume deficit in schizophrenia and thought disorder. Brain: A Journal of Neurology. 2008;131(Pt 1):180–195. doi: 10.1093/brain/awm265. doi:awm265 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Nestor PG, McCarley RW, Levitt JJ, Hsu L, Kawashima T, Shenton ME. Altered orbitofrontal sulcogyral pattern in schizophrenia. Brain : A Journal of Neurology. 2007;130(Pt 3):693–707. doi: 10.1093/brain/awm007. doi:130/3/693 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF. MATRICS consensus cognitive battery. Los Angeles: MATRICS Assessment, Inc; 2006. [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Marder SR. The MATRICS consensus cognitive battery, part 1: Test selection, reliability, and validity. The American Journal of Psychiatry. 2008;165(2):203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F, Kobal G, Ahne G. Sensory-specific satiety-related olfactory activation of the human orbitofrontal cortex. Neuroreport. 2000;11(4):893–897. doi: 10.1097/00001756-200003200-00046. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The brief psychiatric rating scale. psychological report. 1962;10:799–812. [Google Scholar]
- Rolls ET. The functions of the orbitofrontal cortex. In: stuss DT, knight RT, editors. principles of frontal lobe function. Oxford University Press; 2002. [Google Scholar]
- Rolls ET. The orbitofrontal cortex. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 1996;351(1346):1433–43. doi: 10.1098/rstb.1996.0128. discussion 1443-4. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The orbitofrontal cortex and reward. Cerebral Cortex (New York, N.Y.: 1991) 2000;10(3):284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- Rolls ET. Convergence of sensory systems in the orbitofrontal cortex in primates and brain design for emotion. The Anatomical Record. Part A, Discoveries in Molecular, Cellular, and Evolutionary Biology. 2004;281(1):1212–1225. doi: 10.1002/ar.a.20126. [DOI] [PubMed] [Google Scholar]
- Sanfilipo M, Lafargue T, Rusinek H, Arena L, Loneragan C, Lautin A, Wolkin A. Volumetric measure of the frontal and temporal lobe regions in schizophrenia: Relationship to negative symptoms. Archives of General Psychiatry. 2000;57(5):471–480. doi: 10.1001/archpsyc.57.5.471. [DOI] [PubMed] [Google Scholar]
- Strauss GP, Gold JM. A new perspective on anhedonia in schizophrenia. The American Journal of Psychiatry. 2012;169(4):364–373. doi: 10.1176/appi.ajp.2011.11030447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szendi I, Kiss M, Racsmany M, Boda K, Cimmer C, Voros E, Janka Z. Correlations between clinical symptoms, working memory functions and structural brain abnormalities in men with schizophrenia. Psychiatry Research. 2006;147(1):47–55. doi: 10.1016/j.pscychresns.2005.05.014. doi:S0925-4927(05)00216-7 [pii] [DOI] [PubMed] [Google Scholar]
- Takayanagi Y, Takahashi T, Orikabe L, Masuda N, Mozue Y, Nakamura K, Suzuki M. Volume reduction and altered sulco-gyral pattern of the orbitofrontal cortex in first-episode schizophrenia. Schizophrenia Research. 2010;121(1–3):55–65. doi: 10.1016/j.schres.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Thorpe SJ, Rolls ET, Maddison S. The orbitofrontal cortex: Neuronal activity in the behaving monkey. Experimental Brain Research. 1983;49(1):93–115. doi: 10.1007/BF00235545. [DOI] [PubMed] [Google Scholar]
- Vaz S, Heinrichs RW. Schizophrenia and memory impairment: Evidence for a neurocognitive subtype. Psychiatry Research. 2002;113(1–2):93–105. doi: 10.1016/s0165-1781(02)00246-9. [DOI] [PubMed] [Google Scholar]


