Abstract
Although epidemiological research has shown an increase in drinking following stressors and trauma, limited paradigms have been validated to study the relationship between stress and drinking in the human laboratory. The current study developed a progressive ratio (PR) operant procedure to examine the effects of psychosocial stress on alcohol craving and several alcohol‐motivated behaviors in persons with alcohol use disorder. Current heavy, nontreatment‐seeking drinkers (N = 30) were media‐recruited and completed a comprehensive assessment of recent drinking, mood and health. Participants were admitted to the clinical research unit and underwent 4‐day, physician‐monitored alcohol abstinence. On days 4 and 5, participants underwent the Trier Social Stress Test or a neutral session in random order followed by the alcohol‐motivated response (AMR) procedure in which subjects worked for money or alcohol under a PR operant procedure. Subjects received earned money vouchers or alcohol at the conclusion of the session. The Trier Social Stress Test increased alcohol craving and rate of responding and decreased the number of changeovers between alcohol versus money reinforcers on the PR schedule. There was a positive relationship between alcohol craving and drinks earned during the stress session. This novel paradigm provides an experimental platform to examine motivation to drink without confounding by actual alcohol ingestion during the work session, thereby setting the stage for future studies of alcohol interventions.
Keywords: alcohol craving, alcohol‐motivated responding, alcohol use disorder, human laboratory, stress
Introduction
There is increasing interest in the development and refinement of experimental paradigms to study the effects of alcohol on humans using well‐controlled laboratory protocols (Litten et al. 2016). To date, these experimental procedures have been somewhat limited and relied predominantly on characterizing participants' responses to alcohol cues or consumption in the laboratory. For example, many investigators have used alcohol challenge paradigms in which fixed doses of alcohol are administered to research participants and effects are measured on subjective, physiological, psychomotor and cognitive effects (e.g., O'Connor et al. 1998; McCaul et al. 2000; Roche et al. 2014; Ray et al. 2016). Other research has focused on alcohol cue procedures in which the taste, smell and sight of alcohol are used to elicit subjective and physiological responses (e.g., McCaul et al. 1989; Ramirez et al. 2015). It has proven more challenging to develop validated, reliable procedures in which heavy drinkers self‐administer alcohol in the laboratory. To date, most of these models have presented subjects with a choice between alcohol and an alternative reinforcer, and subjects have simply selected their preferred reinforcer without a ‘work’ component in the paradigm (e.g., Davidson et al. 1999; O'Malley et al. 2002; Drobes et al. 2004). Recently, Oberlin et al. (2015) employed a pseudo self‐administration task in which subjects were told that button presses earned delivery of either beer or Gatorade flavor sprays plus alcohol infusion; however, in actuality, only flavor sprays were contingent on presses and ethanol was delivered as a fixed‐dose infusion.
While this earlier research has yielded interesting and important findings, the alcohol field could benefit from the development of human laboratory paradigms, in which the motivation to work for alcohol and actual drinking behaviors can be analyzed independently and in tandem. A paradigm that isolates alcohol seeking from alcohol consumption could be of high utility in a variety of areas of alcohol research, including stress. Although the association of stress and heavy, hazardous alcohol use has been supported by large epidemiological studies (Keyes et al. 2011) as well as investigations of relapse following alcohol treatment (Brown et al. 1990; Noone et al. 1999), limited experimental paradigms have been validated to study the relationship between stress and drinking in humans. Current strategies range from diaries and interactive voice response reporting in the natural environment (Cooney et al. 1997; Ayer et al. 2011; Higley et al. 2011; Sinha et al. 2011; Kwako et al. 2015) to behavioral economic models using alcohol purchase tasks (Owens et al. 2015), and mock alcohol taste tests (Thomas et al. 2011) in the human laboratory.
There is a large preclinical literature using operant behavior models to study stress effects on reward motivation for a variety of reinforcers including sweetened beverages and highly palatable foods, as well as alcohol and other drugs. Interestingly, in rodents, chronic social defeat stress has been shown to decrease responding for sweet‐tasting solutions (Bergamini et al. 2016), but increase responding for alcohol (Caldwell & Riccio 2010; Riga et al. 2014) and cocaine (Wang et al. 2016). One of the more commonly employed operant paradigms for assessing reward motivated behavior has been the progressive ratio (PR) schedule in which various measures of response speed and perseverance as well as reinforcer choice are available to characterize stressor effects. In human laboratory studies, PR schedules have been successfully employed to assess the relative reinforcing effects of stimulants (Griffiths et al. 1989; Stoops et al. 2010) and other commonly abused drugs (Comer et al. 1997; Haney et al. 1997). This paradigm has been less frequently used to study alcohol reward (Barrett et al. 2006; Setiawan et al. 2011) and, to our knowledge, has not been used to examine stress effects on alcohol‐motivated behaviors.
The current study developed a PR operant procedure to study motivation to drink in persons with alcohol use disorder (AUD) in the human laboratory; this procedure was used to examine the effects of psychosocial stress on alcohol craving and several alcohol‐motivated behaviors.
Materials and Methods
Recruitment and assessment
Participants were media‐recruited and completed screening for study eligibility by telephone with a research assistant. Eligible participants attended an in‐person assessment session and provided informed consent. Assessment included alcohol and other drug history, nicotine history, psychiatric status, medical history and physical examination, routine laboratory tests, breath alcohol level test, urine toxicology test and pregnancy testing for females of childbearing potential. A negative pregnancy test was required for all women of childbearing potential. In addition to participants' self‐reports of recent drinking obtained using the 90‐day Timeline Followback method (Sobell & Sobell 1992), levels of phosphatidylethanol (United States Drug Testing Laboratories) in blood were used as a biomarker of recent heavy drinking at assessment (Viel et al. 2012). We used the United States Drug Testing Laboratories threshold of 8 ng/ml to verify recent heavy alcohol drinking.
All participants (21–60 years old) were non‐treatment seeking, had a current DSM 5 AUD, were actively drinking at least 50% above NIAAA recommended weekly guidelines (women >10 drinks/week and men >20 drinks/week), and had at least five binge drinking episodes in the past 30 days. Based on the MINI International Neuropsychiatric Interview (Sheehan 2014), participants were excluded for a current DSM 5 mood, anxiety or psychosis diagnosis or current treatment with a psychiatric medication. Additional study exclusions included serious medical conditions; current substance use disorder except alcohol or tobacco; current illicit drug use other than marijuana; current hormonal birth control for women; history of any seizure disorders; history of serious alcohol withdrawal symptoms (e.g., seizures, hallucinations) or history of inpatient, medicated alcohol withdrawal management; Clinical Inventory of Withdrawal Assessment – Alcohol Revised (CIWA‐Ar, Sullivan et al. 1989) score ≥12 at assessment; and elevation of aspartate aminotransferase or alanine aminotransferase exceeding five times the upper limit of normal. The protocol was approved by The Johns Hopkins University School of Medicine Internal Review Board. All participants participated in a brief alcohol intervention prior to discharge.
Medically supervised alcohol withdrawal and monitored abstinence
On admission day 1, participants were admitted to the Johns Hopkins Bayview Clinical Research Unit (CRU) to ensure alcohol abstinence and monitor for alcohol withdrawal symptoms using the CIWA‐Ar and medical staff observation. For the first 24 hours, all participants received an intravenous line with D5W NS 1000 ml with MVI adult Inj 10 ml, thiamine Inj 100 mg, Folic acid Inj 1 mg, Magnesium sulfate Inj 2 g and infused at 84 ml/hour. Participants had access on request to over‐the‐counter products for mild aches and pains and diarrhea. If systolic blood pressure was greater than 180 and/or diastolic blood pressure greater than 105, atenolol 25 mg was available; however, no participants required treatment. Also, none of the participants in this study developed a CIWA score ≥ 12, and thus, no participant received benzodiazepines or other medications to treat symptoms of alcohol withdrawal. Twice daily at 8 am and 8 pm, participants completed mood [i.e., Beck Depression Inventory II (Beck et al. 1996), Beck Anxiety Inventory (Beck et al. 1988)] and cravings scales [Alcohol Urge Questionnaire (Bohn et al. 1995), Obsessive–Compulsive Drinking Scale (OCDS, Anton et al. 1995), Visual Analog Scale—Alcohol (VAS‐A), Visual Analog Scale—nicotine (VAS‐Nic)].
In our sample of heavy drinkers, 73% were current smokers. Participants who smoked cigarettes were allowed to smoke ad lib in the designated smoking room in the CRU facility, including smoking prior to and upon return from study procedures. The participants had the option to receive a transdermal patch (21 mg) on session days.
Alcohol‐motivated responding sessions
Participants received a brief practice session with the alcohol‐motivated response (AMR) computer procedure during the initial CRU days; no reinforcers were available. On study days 4 and 5, participants were randomized to undergo an active or neutral stress procedure followed by the AMR session. Based on IRB‐required information in the informed consent document, participants were aware that one of the two sessions would be a stress procedure. Female participants of childbearing potential were tested for pregnancy prior to each session.
When participants arrived in the session room, they were given details of the Trier Social Stress Test (TSST) (Kirschbaum et al. 1993; Stephens et al. 2016). Each participant completed two tasks, (1) a 5‐minute speech for a job interview for the position of a hospital administrator and (2) a 5‐minute mental arithmetic task in which they were asked to repeat a four‐digit number after the tester, repeatedly subtract 13 from it, and call out each answer. During the session, two confederates in lab coats were seated across from the participant; one of the confederates pretended to be filming the participant with a video camera. The neutral session (no stress) was similar to above except participants did not undergo the stressor but merely rested or read magazines for the period corresponding to the stress period in the active session. In a mixed effects model adjusted for session order, we observed a significant increase in mean log cortisol in the stress compared with the neutral session (P = 0.001); cortisol effects did not differ as a function of session order (P = 0.476).
Immediately following conclusion of the TSST or neutral period, subjects received a sample of the alcohol beverage (0.5 standard drink) and then began the AMR procedure. Participants were seated in front of a computer screen on which session parameters were displayed graphically, including number of responses and type of reinforcer for current PR, number of drinks earned (shown as shot glasses), number of money reinforcers earned (shown as dollar signs) and amount of time remaining in the session (in digital format). Under the PR schedule, the response requirement (i.e., number of mouse clicks) increased with each completed, earned reinforcer; the initial response required 400 mouse clicks and the requirement increased by 120% for each new requirement. Participants could earn half of a standard drink of their preferred alcoholic beverage for each completed reinforcer in which they selected to work for alcohol. A monetary reinforcer ($1.00) was included in the procedure to permit examination of relative rates and distribution of responses between the two reinforcers. Subjects could earn a maximum of 10 reinforcers during each session. Participants were able to switch back and forth between reinforcer type, but the response requirement reset to the current value and a 2‐second delay was imposed at each switch. AMR sessions lasted for 60 minutes or until no response was made for 10 minutes, whichever came first. If the session was terminated, subjects did not gain access to alcohol until the end of the 60‐minute session.
At baseline prior to the start of the session and at 10‐minute intervals during the AMR session, subjects completed the Tiffany Brief Craving Scale (Tiffany et al. 2000). Items included the following: "How badly would you like an alcoholic drink right now?" and "Rate your current desire to drink alcohol." Using a VAS, subjects rated each item from 0 (not at all) to 10 (extremely or very strong).
After 60 minutes, participants self‐administered earned drinks and received a voucher for earned money. The maximum amount of alcohol that could be earned in one session was 5 standard drinks (SDs). Because subjects drank their preferred alcohol, actual alcohol content was standardized to ensure equivalency across subjects for the amount of absolute alcohol that could be earned. For participant safety, drinking was paced to ensure that individuals could not exceed 0.5 SD every 5 minutes or the total consumption of all 5 SDs in a 50‐minute period. Subjects also were provided their preferred mixer and snacks.
Statistical analyses
We first summarized baseline characteristics, including demographics and drinking measures collected at assessment. The arithmetic mean and standard deviation of continuous variables were calculated. We also tabulated the frequency and percentage of the gender and race distribution. We did not observe any differences as a function of sex in any of our measures, and therefore, sex was not included as a covariate in our analyses.
To test stress effects on alcohol craving, we compared the VAS scores of the Tiffany Brief Craving Scale between the stress and neutral AMR sessions. To account for the repeated measures during the sessions, we constructed mixed effect models with random intercept. The VAS score was the dependent variable, and the independent variables were session type and categorical time points. The contrast test between the sessions was performed after the model was solved. We also calculated the peak VAS score for each subject and each session, and then performed paired t‐tests to compare the VAS peak scores between the stress and neutral sessions.
We tested stress effects on AMR. The outcomes of interest were: total number of alcohol drinks earned; total amount of money earned; total number of responses during the 60‐minute session; distribution of responses for alcohol versus money; response rate (calculated as total session time divided by number of clicks; the unit is millisecond/click) and the number of changeovers between alcohol and money. We constructed mixed effect models to test within‐subject differences between sessions on these measures, which were dependent variables in the models. The independent variables include session type and session randomization order. To remove the order effect further, we also stratified the subjects by session order and tested each sub‐set of data. We also tested the number of changeovers during the first reinforcer of the session using Fisher's exact test.
Lastly, we tested the correlation between craving measure and AMR measures. We ran simple correlations between VAS score peak or area under the curve and number of alcohol drinks earned, response rate, and number of changeovers. Pearson's coefficients and P values were calculated for each correlation.
Results
Participant characteristics
As summarized in Table 1, mean age of the participants was mid‐30s, 70% were male and 57% were Black. On average subjects drank 8.2 SDs on a drinking day. Subjects drank on approximately two‐thirds of available days and drank at binge levels (four or more drinks for women, five or more drinks for men) on approximately half of all available days during the 90 days preceding the assessment visit. Mean Alcohol Use Disorders Identification Test scores indicated a high severity of alcohol‐related problems, and almost half of the participants were diagnosed with severe AUD. Because we excluded participants with mood or anxiety disorders, mean Beck Depression Inventory II Scores and Beck Anxiety Inventory scores were low.
Table 1.
Demographic and psychosocial characteristics of study participants (n = 30)
| Variable | n (%) |
|---|---|
| Sex | |
| Female | 9 (30) |
| Male | 21 (70) |
| Race | |
| African American or Black | 17 (56.7) |
| White | 10 (33.3) |
| Other | 3 (10) |
| Alcohol Use Disorder Severity | |
| Mild | 6 (20) |
| Moderate | 11 (36.7) |
| Severe | 13 (43.3) |
| Mean (standard deviation) | |
| Age | 35.1 (11) |
| Alcohol Consumption | |
| Drinks/Drinking day | 8.2 (3.7) |
| Drinking days/90 days | 64.8 (18.8) |
| Binge days/90 days | 46.4 (24.3) |
| Psychological Assessment Scores | |
| BAI Score | 7.1 (6.6) |
| BDI Score | 9.2 (7.3) |
BAI = Beck Anxiety Inventory; BDI = Beck Depression Inventory II.
Stress effects on alcohol craving
As shown in Figure 1, although alcohol craving increased during both stress and neutral sessions, there was a more rapid and elevated increase in participants' ratings of alcohol craving during the stress compared with neutral session. Overall, in the mixed effect model, mean VAS ratings of alcohol cravings were significantly higher in the stress compared with the neutral session (P = 0.032). Additionally, peak craving scores were significantly greater in the stress session (P = 0.043). When stratified by session order, the increase in alcohol craving following stress was significant only among those subjects who received the neutral session prior to the stress session (P = 0.016).
Figure 1.
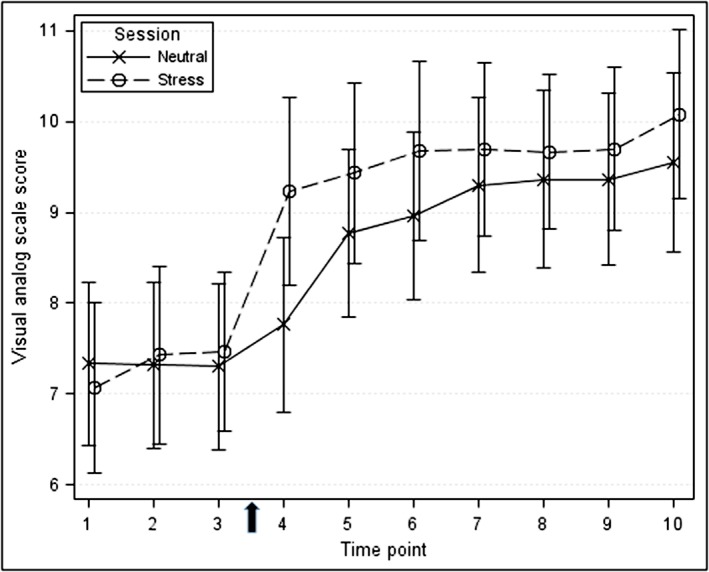
Alcohol craving at baseline and during the alcohol‐motivated responding sessions. Participants rated current craving on a visual analog scale from 0 (not at all) to 10 (extremely or very strong). Scores during the neutral session are shown in blue, and scores during the stress session are shown in red. The arrow on the x‐axis shows completion of the Trier Social Stress Test during the stress session. Time points are mean and standard error
Stress effects on alcohol‐motivated responding
Differences between the stress and neutral sessions were examined on four measures of AMR: number of drinks earned, rate of responding, total number of alcohol‐directed responses (mouse clicks), and the number of changeovers between alcohol and money reinforcers. Figure 2 shows the distribution of number of drinks (panel a) and number of changeovers between reinforcers (panel b) for the stress and neutral sessions.
Figure 2.
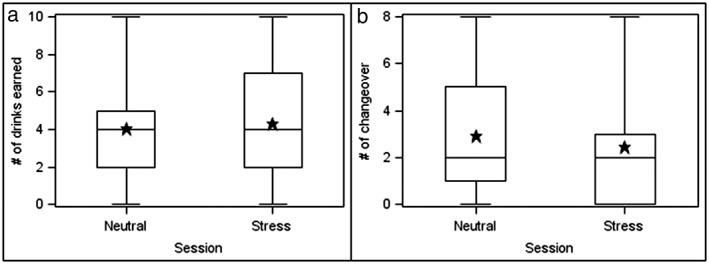
Alcohol‐motivated behaviors as a function of session type. Panel A shows the number of drinks earned, and Panel B shows the number of changeovers between the alcohol and money reinforcers. Stars are means across all subjects, the upper and lower limits of the boxes are the 25 and 75 percentile. The vertical lines end at the most extreme data values
Number of drinks earned
From the mixed‐effects model, no significant difference was observed between the number of alcohol drinks earned during the stress compared with the neutral session (P = 0.183). However, among participants who received the neutral session followed by the active TSST session, there was a trend for subjects to earn more drinks following the TSST (M = 4.18) than following the neutral session (M = 3.29) (P = 0.083). No difference in the number of drinks earned was observed when participants received the active TSST session followed by the neutral session. Additionally, there was no interaction of stress condition and session order.
Total number of alcohol‐directed responses
There was a significant interaction between session type (stress versus neutral) and session order (N/S versus S/N) (P = 0.035) on the total number of alcohol‐directed responses. Specifically, the number of alcohol‐directed responses was significantly higher in the stress compared with neutral session (P = 0.045) for subjects who received the neutral session first and the stress session second. There was no significant difference in the number of alcohol‐directed responses during the stress versus neutral session for subjects who received the stress session first and the neutral session second.
Number of changeovers
From the mixed‐effects model, participants made significantly fewer changeovers during the stress compared with the neutral session (difference in adjusted mean number of changeovers =1.8, P = 0.012). Much of this effect was accounted for by changeovers while subjects were responding for their first reinforcer. During the neutral session, 11 of the 30 subjects switched back and forth between reinforcer type (alcohol versus money), whereas only three subjects switched during the stress session. Using Fisher's Exact Test, the number of changeovers during the first reinforcer was significantly lower following the stress compared with the neutral session (P = 0.030).
In line with these overall findings, when participants were stratified by session order, subjects who received the neutral session before the stress session had fewer changeovers following the TSST as compared with the neutral session (P = 0.004). There was no difference in the number of changeovers in the neutral and stress sessions for those participants who received the TSST before the neutral session. Additionally, there was no interaction of stress condition and session order.
Response rate
Figure 3 shows the mean response rate across all reinforcer opportunities for the stress and neutral sessions. Participants responded significantly faster throughout the stress compared with the neutral session. The adjusted means from the mixed‐effect model of the stress session (4.82) and neutral session (4.55) are significantly different (P < 0.001). Response rate did not differ as a function of session order or the type of reinforcer (alcohol versus money) in either the stress or neutral session (all P > 0.10).
Figure 3.
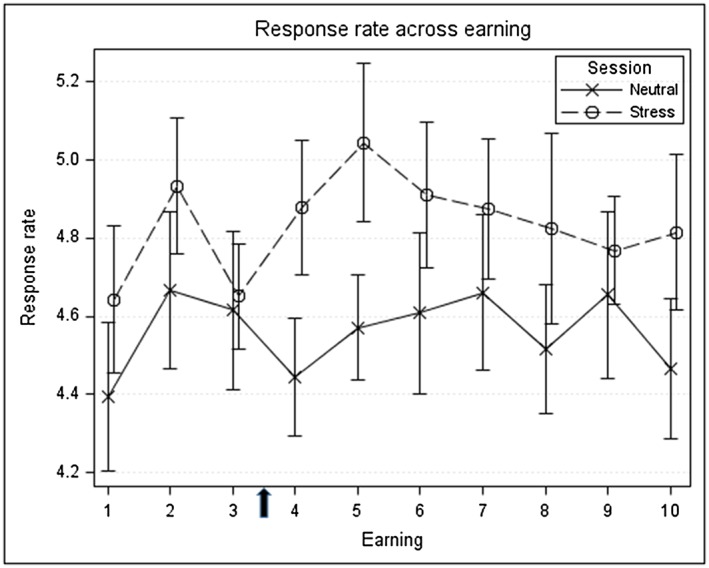
Response rate (clicks/second) across reinforcement opportunities as a function of session type. Rates during the neutral session are shown in blue, and rates during the stress session are shown in red. Points show mean and standard error
Relationships between alcohol craving and number of drinks earned during the stress session
There was a significant relationship between the magnitude of alcohol craving following stress and the number of drinks earned during that session. Specifically, VAS area under the curve (r = 0.366; P = 0.047) and peak score (Figure 4; r = 0.447; P = 0.013) were positively related to drinks earned. The higher the craving the greater the number of alcohol drinks earned during the session.
Figure 4.
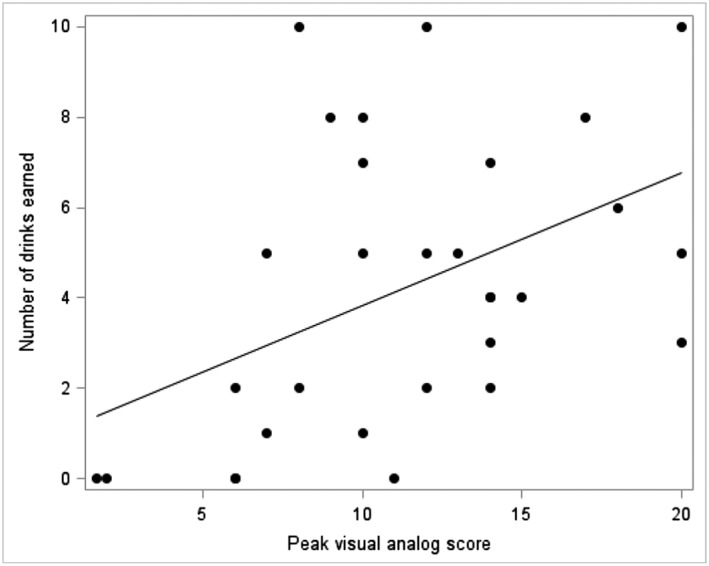
Relationship between alcohol craving and number of drinks earned during the stress session (r = 0.447; P = 0.013). Data points represent individual subjects. Alcohol craving was measured as peak visual analog score during the stress alcohol‐motivated response session
Discussion
We have demonstrated the utility of an alcohol‐motivated PR response procedure to study stress effects on alcohol‐related behaviors in heavy drinkers with AUD. Using the TSST as the provocateur, we observed stress effects on alcohol craving and motivated responding under controlled human laboratory conditions. Specifically, alcohol craving was increased in the stress compared with neutral session. Subjects responded faster for alcohol and were less likely to switch between reinforcer types (alcohol versus money) following stress. Although we did not observe an overall difference in the number of earned drinks between the two session types, the magnitude of alcohol craving following stress was related to the number of alcohol drinks earned during the stress session.
Interestingly, there was a strong order effect on stress responsivity. We anticipated that this might be an important factor and randomized session order to counterbalance potential effects. Because the stress and neutral sessions were conducted on separate days, the observed order effect may result from the rapidly changing neurochemical milieu during early alcohol abstinence in persons with AUD. It also may highlight the role of stressor predictability, which induces anticipatory anxiety as a motivator to drink. Subjects have no way to predict the condition (stress versus neutral) of the first session. However, once the first session is completed, the condition for the second session is known, potentially increasing anticipatory anxiety and thereby enhancing the impact of the active stress condition. These types of order effects when not adequately taken into account may contribute to the challenge of reproducibility in human studies of stress and alcohol, and definitely warrant consideration in the design of future research.
In the current experimental paradigm, participants responded faster for the reinforcer and made fewer switches between reinforcers following stress. The hyper‐focused and ultra‐repetitive behaviors of rapid mouse clicking with limited or no changeovers following the stress condition are consistent with the emergence of habitual behaviors and the activation of habit forming neurocircuitry reported in preclinical and clinical studies (Schwabe and Wolf, 2011; Corbit & Janak 2016). These alcohol‐induced behavioral changes, which most likely result from alterations in neural circuitry, may contribute to a weakening of volitional control and a failure to abstain from alcohol. It has also been proposed that this switch is involved in the transition from casual to heavy, hazardous drinking (Barker & Taylor 2014; McKim et al. 2016).
As outlined in the introduction, a number of paradigms have been developed in an effort to explore these behaviors in the human laboratory. Most recently, behavioral economics has provided new insights and approaches to these investigations across a variety of addictive substances (Amlung et al. 2016). Recently, Amlung & MacKillop (2014) have used these approaches to examine the effects of stress and alcohol cues on alcohol‐motivated behaviors in heavy drinkers. Using the TSST to induce stress, they observed increases in the incentive value of alcohol in an alcohol purchase task (i.e., the amount of money participants are willing to spend to acquire alcohol) and an alcohol multiple choice procedure (i.e., the choice between immediate alcohol and different values of delayed money). These paradigms rely on subjects' choices of drink versus money as a metric of their incentive to drink following stress.
Our experimental paradigm complements other approaches in several notable ways. First, our protocol separately measures alcohol craving, alcohol‐motivated behaviors and alcohol consumption. Using the AMR procedure, we can study these behaviors independently and in interaction. Similar to the behavioral economic models, the current procedure not only quantifies choice of alcohol versus money but also provides a behavioral work component that may further quantify the strength of motivation driving the choice. Alcohol craving, motivation to drink and the number of drinks actually consumed are overlapping but distinct behavioral concepts. This distinction is most frequently observed in the context of alcohol treatment. During the stress of alcohol withdrawal and following other external stressors, patients may have very high alcohol urges and motivation to drink but they successfully resist drinking during their treatment and recovery (DiClemente 2016). Human experimental paradigms are needed that better quantify the interactions of urge, choice and consumption to more accurately capture the human experience of problematic drinking, treatment and recovery.
A second strength of the AMR procedure is the dissociation of choice and work from consumption. Our AMR prevents acute alcohol intoxication from altering the abstinence‐based measures that are under investigation. In many of the other human‐alcohol administration models, participants drink alcohol throughout the session, thus, outcome measures reflect both participants' initial alcohol motivation at abstinence and their motivation in the presence of increasing levels of intoxication, thereby confounding these two measurement types. Third, the AMR procedure prevents alcohol ingestion from confounding measurement of potential risk biomarkers (e.g., corticotropin releasing factor (CRF) and brain‐derived neurotrophic factor (BDNF)) that may be correlated with motivation to drink in the absence but not the presence of a blood alcohol level. Fourth, this model also provides a translational opportunity between preclinical and human research (Kaminski et al. 2008); there are parallels with preclinical appetitive models as well as models using second order and chained schedules of reinforcement. For instance, in the current AMR procedure, participants were rewarded following completion of each response requirement with a symbol on the computer screen of which reinforcer had just been earned (either a shot glass or dollar sign).
Finally, this design provides a platform to study a range of both provocateurs of and interventions for alcohol‐related behaviors. While we focused this study on social stress, other widely studied provocateurs could include sexual stimuli, alcohol cues (olfactory, visual and tastes), drinking context (solo versus group; bar versus lab setting) and other types of stressors (e.g. imagery and virtual reality). The platform also offers the ability to determine how various behavioral and pharmacologic interventions can differentially decrease alcohol craving, motivated responding and consumption. This broader scope of information provided by the paradigm may be helpful in making decisions about which pharmacological agents should advance to clinical trials (Litten et al. 2016). The design could become even more powerful if incorporated into a functional imaging platform where the strength of connectivity between the prefrontal cortex and mesolimbic systems could be simultaneously assessed with the AMR measures. Such research has the potential to identify new pharmacotherapy targets for alcohol treatments. Finally, the AMR measures may serve as potent predictors of clinical outcomes for persons undergoing treatment. For example, it is plausible that rate of responding and strength of habitual alcohol seeking as measured by changeover patterns would be powerful predictors of relapse following care.
There also are several limitations of the current study that should be noted. First, the study had a relatively small sample, but despite this limitation, significant stress relationships were observed. Second, it is important to note that this is not a relapse model of human drinking since participants were not treatment seekers, and therefore had no motivation to limit or stop drinking. If subjects had been motivated to refrain from or limit drinking, we would expect to see more pronounced behavioral differences between the neutral and stress sessions. Third, for subject safety, we capped the number of drinks that subjects could earn within the session and the speed of actual drinking following the AMR session. Thus, we created a ceiling in drink opportunities that may have further limited the differences between neutral and stress sessions. Although the TSST is the most widely validated and widely used social stress procedure in the human literature, 15–20% of participants do not respond. Using a more potent stressor (e.g., virtual reality combat or physical assault videos) may produce more pronounced session differences but raises ethical concerns in human research. It also should be noted that Human Subjects Protection required disclosure of the use of stress procedures, thus possibly introducing anticipatory anxiety into the paradigm. Despite these procedural restrictions necessitated by participant safety, it is striking that we were able to detect stress effects in this model. Finally, this study was conducted only in persons with AUD; thus, we do not know whether the observed relationships would generalize to social drinkers. Clarification of the specificity of stress effects on alcohol use in hazardous versus social drinkers will be an important direction for future research. A recent naturalistic study has provided evidence of differential stress effects as a function of drinking status prior to stressor onset. Specifically, in a study of directly exposed survivors across 10 disasters, North et al. (2011) found an overall AUD prevalence of 19%; however, only 0.3% of the survivors developed new‐onset AUD. Almost all AUDs in the sample were a continuation of or relapse to pre‐disaster alcohol misuse.
In summary, we have translated a traditional preclinical operant paradigm to study humans with AUD in the laboratory. Our work points out both procedural strengths and some pitfalls (e.g., order effects and drinking limits). In this study, we employed social stress as our provocateur and demonstrated some important relationships that have been challenging to demonstrate in humans using other paradigms. Going forward, the AMR model should provide a useful new platform on which a variety of provocateurs and interventions can be studied.
Acknowledgements
Support: This research was supported by NIH grants U01‐AA020890 (Co‐PIs: Wand and McCaul), The Kenneth Lattman Foundation and K05AA020342 (PI: Wand). This publication also was made possible by the Johns Hopkins Institute for Clinical and Translational Research (ICTR), which is funded in part by grant number UL1 TR 001079 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the Johns Hopkins ICTR, NCATS or NIH.
Authors Contribution
MEMc and GSW were responsible for the study concept, design and conduct. EMW contributed to the development of the PR procedure and assisted with data collection. XX assisted with data analysis and interpretation of findings. MEMc and GSW wrote the manuscript. All authors critically reviewed content and approved the final version for publication.
McCaul, M. E. , Wand, G. S. , Weerts, E. M. , and Xu, X. (2018) A paradigm for examining stress effects on alcohol‐motivated behaviors in participants with alcohol use disorder. Addiction Biology, 23: 836–845. doi: 10.1111/adb.12511.
References
- Amlung M, MacKillop J (2014) Understanding the effects of stress and alcohol cues on motivation for alcohol via behavioral economics. Alcohol Clin Exp Res 38:1780–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amlung M, Vedelago L, Acker J, Balodis I, MacKillop J (2016) Steep delay discounting and addictive behavior: a meta‐analysis of continuous associations. Addiction . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton RF, Moak DH, Latham P (1995) The obsessive compulsive drinking scale: a self‐rated instrument for the quantification of thoughts about alcohol and drinking behavior. Alcohol Clin Exp Res 19:92–99. [DOI] [PubMed] [Google Scholar]
- Ayer LA, Harder VS, Rose GL, Helzer JE (2011) Drinking and stress: an examination of sex and stressor differences using IVR‐based daily data. Drug Alcohol Depend 115:205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, Taylor JR (2014) Habitual alcohol seeking: modeling the transition from casual drinking to addiction. Neurosci Biobehav Rev 47:281–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett SP, Tichauer M, Leyton M, Pihl RO (2006) Nicotine increases alcohol self‐administration in non‐dependent male smokers. Drug Alcohol Depend 81:197–204. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA (1988) An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol 56:893. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK (1996) Beck Depression Inventory‐II, pp. 78204–72498: San Antonio, TX. [Google Scholar]
- Bergamini G, Cathomas F, Auer S, Sigrist H, Seifritz E, Patterson M, Gabriel C, Pryce CR (2016) Mouse psychosocial stress reduces motivation and cognitive function in operant reward tests: a model for reward pathology with effects of agomelatine. Eur Neuropsychopharmacol 26:1448–1464. [DOI] [PubMed] [Google Scholar]
- Bohn MJ, Krahn DD, Staehler BA (1995) Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res 19:600–606. [DOI] [PubMed] [Google Scholar]
- Brown SA, Vik PW, McQuaid JR, Patterson TL, Irwin MR, Grant I (1990) Severity of psychosocial stress and outcome of alcoholism treatment. J Abnorm Psychol 99:344. [DOI] [PubMed] [Google Scholar]
- Caldwell EE, Riccio DC (2010) Alcohol self‐administration in rats: modulation by temporal parameters related to repeated mild social defeat stress. Alcohol 44:265–274. [DOI] [PubMed] [Google Scholar]
- Comer S, Collins E, Fischman M (1997) Choice between money and intranasal heroin in morphine‐maintained humans. Behav Pharmacol 8:677–690. [DOI] [PubMed] [Google Scholar]
- Cooney NL, Litt MD, Morse PA, Bauer LO, Gaupp L (1997) Alcohol cue reactivity, negative‐mood reactivity, and relapse in treated alcoholic men. J Abnorm Psychol 106:243. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Janak PH (2016) Habitual alcohol seeking: neural bases and possible relations to alcohol use disorders. Alcohol Clin Exp Res 40:1380–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson D, Palfai T, Bird C, Swift R (1999) Effects of naltrexone on alcohol self‐administration in heavy drinkers. Alcohol Clin Exp Res 23:195–203. [PubMed] [Google Scholar]
- DiClemente CC (2016) Alcohol relapse and change needs a broader view than counting drinks. Alcohol Clin Exp Res in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobes DJ, Anton RF, Thomas SE, Voronin K (2004) Effects of naltrexone and nalmefene on subjective response to alcohol among non‐treatment‐seeking alcoholics and social drinkers. Alcohol Clin Exp Res 28:1362–1370. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Bigelow GE, Liebson IA (1989) Reinforcing effects of caffeine in coffee and capsules. J Exp Anal Behav 52:127–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Comer S, Ward AS, Foltin R, Fischman M (1997) Factors influencing marijuana self‐administration by humans. Behav Pharmacol 8:108–112. [PubMed] [Google Scholar]
- Higley AE, Crane NA, Spadoni AD, Quello SB, Goodell V, Mason BJ (2011) Craving in response to stress induction in a human laboratory paradigm predicts treatment outcome in alcohol‐dependent individuals. Psychopharmacology (Berl) 218:121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski BJ, Goodwin AK, Wand G, Weerts EM (2008) Dissociation of alcohol‐seeking and consumption under a chained schedule of oral alcohol reinforcement in baboons. Alcohol Clin Exp Res 32:1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Hatzenbuehler ML, Hasin DS (2011) Stressful life experiences, alcohol consumption, and alcohol use disorders: the epidemiologic evidence for four main types of stressors. Psychopharmacology (Berl) 218:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH (1993) The 'Trier Social Stress Test': a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 28:76–81. [DOI] [PubMed] [Google Scholar]
- Kwako LE, Schwandt ML, Sells JR, Ramchandani VA, Hommer DW, George DT, Sinha R, Heilig M (2015) Methods for inducing alcohol craving in individuals with co‐morbid alcohol dependence and posttraumatic stress disorder: Behavioral and physiological outcomes. Addict Biol 20:733–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten RZ, Falk DE, Ryan ML, Fertig JB (2016) Discovery, development, and adoption of medications to treat alcohol use disorder: goals for the phases of medications development. Alcohol Clin Exp Res 40:1368–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaul ME, Turkkan JS, Stitzer ML (1989) Conditioned opponent responses: effects of placebo challenge in alcoholic subjects. Alcohol Clin Exp Res 13:631–635. [DOI] [PubMed] [Google Scholar]
- McCaul ME, Wand GS, Eissenberg T, Rohde CA, Cheskin LJ (2000) Naltrexone alters subjective and psychomotor responses to alcohol in heavy drinking subjects. Neuropsychopharmacology 22:480–492. [DOI] [PubMed] [Google Scholar]
- McKim TH, Shnitko TA, Robinson DL, Boettiger CA (2016) Translational research on habit and alcohol. Curr Addict Rep 3:37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noone M, Dua J, Markham R (1999) Stress, cognitive factors, and coping resources as predictors of relapse in alcoholics. Addict Behav 24:687–693. [DOI] [PubMed] [Google Scholar]
- North CS, Ringwalt CL, Downs D, Derzon J, Galvin D (2011) Post‐disaster course of alcohol use disorders in systematically studied survivors of 10 disasters. Arch Gen Psychiatry 68:173–180. [DOI] [PubMed] [Google Scholar]
- Oberlin BG, Dzemidzic M, Tran SM, Soeurt CM, O'Connor SJ, Yoder KK, Kareken DA (2015) Beer self‐administration provokes lateralized nucleus accumbens dopamine release in male heavy drinkers. Psychopharmacology (Berl) 232:861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor S, Morzorati S, Christian J, Li T (1998) Clamping breath alcohol concentration reduces experimental variance: application to the study of acute tolerance to alcohol and alcohol elimination rate. Alcohol Clin Exp Res 22:202–210. [PubMed] [Google Scholar]
- O'Malley SS, Krishnan‐Sarin S, Farren C, Sinha R, Kreek M (2002) Naltrexone decreases craving and alcohol self‐administration in alcohol‐dependent subjects and activates the hypothalamo–pituitary–adrenocortical axis. Psychopharmacology (Berl) 160:19–29. [DOI] [PubMed] [Google Scholar]
- Owens MM, Ray LA, MacKillop J (2015) Behavioral economic analysis of stress effects on acute motivation for alcohol. J Exp Anal Behav 103:77–86. [DOI] [PubMed] [Google Scholar]
- Ramirez JJ, Monti PM, Colwill RM (2015) Brief and extended alcohol‐cue‐exposure effects on craving and attentional bias. Exp Clin Psychopharmacol 23:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Bujarski S, Roche DJ (2016) Subjective response to alcohol as a research domain criterion. Alcohol Clin Exp Res 40:6–17. [DOI] [PubMed] [Google Scholar]
- Riga D, Schmitz L, van der Harst JE, van Mourik Y, Hoogendijk W, Smit AB, De Vries TJ, Spijker S (2014) A sustained depressive state promotes a guanfacine reversible susceptibility to alcohol seeking in rats. Neuropsychopharmacology 39:1115–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche DJ, Palmeri MD, King AC (2014) Acute alcohol response phenotype in heavy social drinkers is robust and reproducible. Alcohol Clin Exp Res 38:844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setiawan E, Pihl RO, Cox SM, Gianoulakis C, Palmour RM, Benkelfat C, Leyton M (2011) The effect of naltrexone on alcohol's stimulant properties and self‐administration behavior in social drinkers: influence of gender and genotype. Alcohol Clin Exp Res 35:1134–1141. [DOI] [PubMed] [Google Scholar]
- Sheehan DV (2014) MINI International Neuropsychiatric Interview 7.0.0 for DSM‐V.
- Sinha R, Fox HC, Hong KA, Hansen J, Tuit K, Kreek MJ (2011) Effects of adrenal sensitivity, stress‐and cue‐induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Arch Gen Psychiatry 68:942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB (1992) Timeline Follow‐back: A technique for assessing self‐reported ethanol consumption In: Litten RZ, Allen JP. eds. Measuring Alcohol Consumption: Psychosocial and Biological Methods, pp. 41–72. Humana Press: Totowa, NJ. [Google Scholar]
- Stephens MAC, Mahon PB, McCaul ME, Wand GS (2016) Hypothalamic–pituitary–adrenal axis response to acute psychosocial stress: effects of biological sex and circulating sex hormones. Psychoneuroendocrinology 66:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Glaser PE, Hays LR, Rush CR (2010) Intranasal cocaine functions as reinforcer on a progressive ratio schedule in humans. Eur J Pharmacol 644:101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM (1989) Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA‐Ar). Br J Addict 84:1353–1357. [DOI] [PubMed] [Google Scholar]
- Thomas SE, Bacon AK, Randall PK, Brady KT, See RE (2011) An acute psychosocial stressor increases drinking in non‐treatment‐seeking alcoholics. Psychopharmacology (Berl) 218:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany ST, Carter BL, Singleton EG (2000) Challenges in the manipulation, assessment and interpretation of craving relevant variables. Addiction 95:177–187. [DOI] [PubMed] [Google Scholar]
- Viel G, Boscolo‐Berto R, Cecchetto G, Fais P, Nalesso A, Ferrara SD (2012) Phosphatidylethanol in blood as a marker of chronic alcohol use: A systematic review and meta‐analysis. Int J Mol Sci 13:14788–14812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Bastle RM, Bass CE, Hammer RP, Neisewander JL, Nikulina EM (2016) Overexpression of BDNF in the ventral tegmental area enhances binge cocaine self‐administration in rats exposed to repeated social defeat. Neuropharmacology 109:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]


