Abstract
Orexins (‘hypocretins’) are peptides produced by neurons of the hypothalamus that project to structures implicated in reward and emotion processing. Converging evidence demonstrates functional roles of orexin signaling in arousal, sleep/wakefulness, and motivated behaviors for natural and drug rewards. Suvorexant, a dual orexin receptor antagonist (DORA), recently received approval from the U.S. Food and Drug Administration (FDA) to treat insomnia. In Experiment 1, rats self-administered cocaine under a progressive-ratio (PR) schedule of reinforcement and effects of suvorexant on motivation to self-administer cocaine were measured. In Experiment 2, effects of suvorexant on cocaine reward were assessed using a place conditioning paradigm, and 50-kHz ultrasonic vocalizations (USVs) were also recorded to track changes in hedonic reactivity to cocaine. To rule out potentially confounding effects of suvorexant-induced somnolence, locomotor activity was also measured. In Experiment 3, effects of suvorexant on cocaine-evoked elevations in ventral striatal dopamine were examined. Data reveal that suvorexant: (i) reduced the number of cocaine infusions earned during PR self-administration, (ii) attenuated initial positive hedonic reactivity to cocaine and prevented cocaine place preference, (iii) did not affect cocaine-induced hyperlocomotion and (iv) reduced cocaine-induced elevations in extracellular ventral striatal dopamine. The present study examined the therapeutic potential of suvorexant in rodent models of cocaine use disorder. These results contribute towards a growing literature supporting therapeutic roles of orexin receptor antagonists in treating substance use disorders.
Keywords: Addiction, Affect, Dopamine, Orexin, Self-Administration, Ultrasonic Vocalizations
Introduction
Illicit drug use remains a major problem in the United States costing approximately $181 billion annually and leading to deteriorated lifestyles for the drug user as well as friends and families of drug users. Psychostimulants such as cocaine cause rapid and long-lasting changes in brain reward circuitry principally by elevating synaptic levels of DA (Heal et al 2014). Cocaine administration transiently causes euphoria and subjective positive affect. It is generally believed that the positive affective state following cocaine administration functions as a reward signal which is conducted in part through mesolimbic DA transmission—afferents within the ventral tegmental area (VTA) transmit DA to post-synaptic targets within ventral striatum. Pharmacological interventions that work to reduce positive affect following cocaine use may prove useful by reducing the rewarding value of the drug.
Orexins (‘hypocretins’) are peptides produced within the hypothalamus that innervate monoaminergic nuclei of the brainstem, including VTA, locus coruleus, and dorsal raphe nucleus (Darwinkel et al 2014; Peyron et al 1998). Orexins exert excitatory effects by signaling through two G-protein coupled receptors (OX1R, OX2R). Accordingly, orexin transmission participates in various behavioral states including arousal, sleep/wakefulness, and motivation to retrieve natural and drug rewards (Borgland et al 2009; de Lecea et al 1998; Mahler et al 2012; Sakurai et al 1998; Smith et al 2009). Systemically-administered OX1R antagonists reduce morphine-conditioned place preference (Harris et al 2005), block cued and stress-induced reinstatement of cocaine-seeking (Boutrel et al 2005; Smith et al 2009) and reduce self-administered cocaine (Brodnik et al 2015; Muschamp et al 2014). Dual orexin receptor antagonists also reduce cocaine-seeking behavior in part through augmenting cocaine-evoked elevations in ventral striatal DA (Prince et al 2015). Moreover, signaling via OX1Rs appears to selectively modulate motivation for high-incentive rewards, such as cocaine and high-fat food but not normal chow (Borgland et al 2009). Recently, selective OX2R antagonism was shown to reduce escalation in self-administered heroin under extended-access conditions (Schmeichel et al 2015). Orexin transmission thus appears to mediate aspects of the rewarding and reinforcing properties of abused drugs through both receptor subtypes, and antagonists reliably reduce the rewarding properties of cocaine in preclinical models.
Converging anatomical and functional reports highlight the significance of orexin transmission within the mesolimbic reward circuit for addiction-related psychiatric disorders (Calipari and España 2012). Orexins provide both direct and feed-forward excitatory input to dopaminergic neurons of the VTA (Fadel and Deutch 2002; Korotkova et al 2003; Muschamp et al 2014). Intra-VTA application of the orexin-A peptide increases cocaine-seeking and enhances cocaine-evoked increases in DA transmission to ventral striatal targets (España et al 2010). Blockade of orexin transmission appears to diminish the reinforcing effects of cocaine by attenuating cocaine-induced mesolimbic DA transmission at its origin in the VTA.
The goal of the present study was to evaluate the therapeutic potential of suvorexant, a clinically-available dual orexin receptor antagonist, in rodent models of cocaine use disorder. Specifically, we used a self-administration model to study the effects of suvorexant on cocaine-seeking. We further assessed effects of suvorexant on conditioned cocaine reward and on hedonic processing of cocaine using conditioned place preference (CPP) and through recording positively-valenced 50-kHz ultrasonic vocalizations (USVs), respectively. Next, we measured the effects of suvorexant on cocaine-induced locomotor activity. Finally, we performed in vivo fast scan cyclic voltammetry to assess effects of suvorexant on cocaine-evoked elevations of ventral striatal DA.
Materials and Methods
Animals
Adult male Sprague-Dawley rats (Charles River; Horsham, PA, USA) arrived at Temple University’s vivarium, were pair-housed and given food and water ad libitum. Rats acclimated to the vivarium for at least one week before beginning experiments. For Experiment 1, rats were singly-housed following jugular vein catheterization surgery and were placed on a reverse 12-hour:12-hour light:dark cycle with lights turning off at 9:00 AM. For Experiment 2, rats were pair-housed throughout the experiment. For Experiment 3, mice (Charles River; Horsham, PA, USA) were used and were housed in cages of 2–5 mice per cage until surgery and were provided food and water ad libitum. All experimental procedures were approved by Institutional Animal Care and Use Committees of Temple University and Drexel University.
Drugs
For all Experiments, suvorexant (Selleckchem; Munich, Germany) was dissolved in 100% dimethyl sulfoxide (DMSO) through vortexing and ultrasonication and was administered at a fixed volume (100 μL, i.p.). For all Experiments, cocaine hydrochloride (Sigma; St. Louis, MO, USA) was dissolved in 0.9% physiological saline.
Experimental Procedures
Experimental designs can be found in Table 1.
Table 1.
Experimental designs for cocaine self-administration (Experiment 1), CPP with 50-kHz USV recording and cocaine-induced locomotor activity (Experiment 2), and in vivo fast-scan cyclic voltammetry on cocaine-evoked dopamine levels in ventral striatum (Experiment 3).
| Experiment | Assay/Paradigm | Group Design | Suvorexant Pretreatment | Cocaine Acute Treatment | Dependent Measures and Significance |
|---|---|---|---|---|---|
| Experiment 1 | Self-Administration | Within | Veh | N/A | Infusions* Correct Reponses |
| 3.0 mg/kg | |||||
| 10.0 mg/kg | |||||
| 30.0 mg/kg | |||||
| Experiment 2 | Conditioned Place Preference (CPP) | Between | Veh | Sal | Δ Preference† Δ USV Score† |
| 30.0 mg/kg | |||||
| Veh | 10.0 mg/kg | ||||
| 30.0 mg/kg | |||||
| Locomotor Activity | Between | Veh | Sal | Activity Count | |
| 10.0 mg/kg | |||||
| 30.0 mg/kg | |||||
| Experiment 3 | Fast-Scan Cyclic Voltammetry | Between | Veh | 10.0 mg/kg | Dopamine§ |
| 30.0 mg/kg |
Designates significance versus “100” or “0”—the points of no-change—following suvorexant pretreatment.
Designates significance versus vehicle-pretreated group.
Designates lack of significant cocaine-evoked effect following suvorexant pretreatment.
Experiment 1: Cocaine Self-Administration
For jugular vein catheterization surgery to permit cocaine self-administration in Experiment 1, rats were anesthetized with isoflurane gas (5% induction, 2–3% maintenance) and an aseptic environment was maintained throughout. Once under surgical anesthesia, a permanent indwelling catheter (Camcaths; Cambridgeshire, United Kingdom) was implanted in the right jugular vein of the rat and was connected to a stainless steel exit port of the rat’s mid-scapular region. Incisions were closed with 9 mm wound clips and treated post-operatively with antibiotic ointment. Rats recovered from surgery for 5–7 days before beginning cocaine self-administration training.
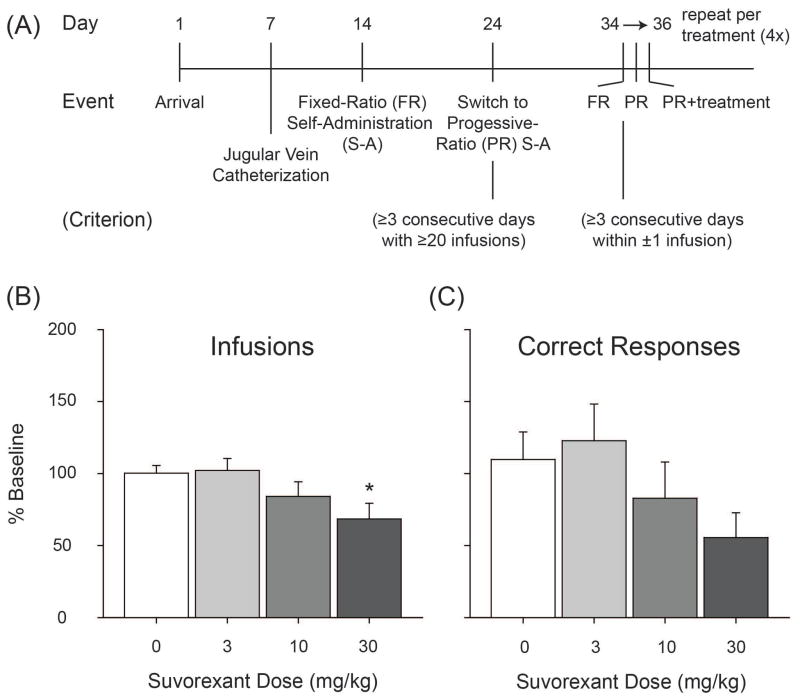
For all self-administration sessions, rats were moved to sound-attenuating, ventilated behavioral chambers (MED Associates; St. Albans, VT, USA) after receiving a 100 μL experimenter-administered infusion of heparinized saline to aide in maintaining catheter patency. Rats were weighed daily and connected to polyurethane tubing via the stainless steel exit port, which was enclosed within a metal spring leash, attached to a fluid swivel and connected to a syringe pump for drug delivery. Cocaine infusion duration was adjusted each session based on rat bodyweight, averaging around 3 seconds, and dose was maintained at ~0.36mg/kg/infusion for all infusions. For fixed-ratio (FR) reinforcement, session duration was 2-hours or 60 infusions, and the intertrial-interval (ITI) was 20-seconds. For progressive-ratio (PR) reinforcement, session duration was 4-hours, 60 infusions or an absence of responding for 90 minutes, and the ITI was 30-seconds. Rats began cocaine self-administration training on a FR-1 schedule of reinforcement, and criterion for advancement was ≥ 20 infusions for 3 consecutive sessions. Rats were then moved to a PR schedule of reinforcement, where infusions were rewarded upon performing an incrementing number of lever-press operant responses for subsequent infusions (e.g., 1, 1, 2, 4, 6, 9, 12, 15…), and criterion for advancement was 3 consecutive sessions where the number of infusions was maintained within ± 1 infusion relative to prior-day performance. Rats were then moved to the treatment phase of self-administration where a 3-day repeating block of sessions consisting of (1) FR-1, (2) PR without suvorexant pretreatment, and (3) PR with suvorexant pretreatment was used. A summary timeline of events can be found in Figure 1A.
Figure 1.
(A) Timeline of events to train rats in cocaine self-administration. Effects of suvorexant on the number of (B) infusions and (C) correct responses relative to prior-day baseline performance. * p < 0.05 relative to vehicle-pretreated control data. Data are presented as mean ± S.E.M. n=12.
Experiment 2: Conditioned Place Preference (CPP), 50-kHz Ultrasonic Vocalizations (USVs), and Locomotor Activity
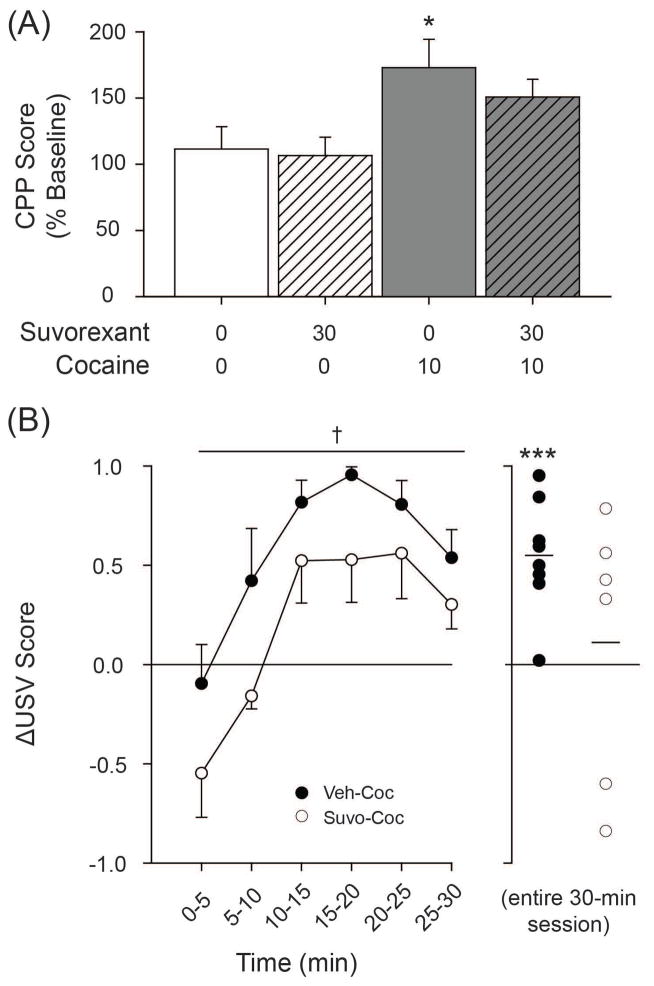
For CPP, a two-chamber apparatus with visually- and tactilely-distinguished contexts, separated by a removable partition, was used following a biased, forced-choice design. Rats (n=8/group) were first allowed to freely shuttle between the two contexts during a 30-minute pre-test to assess natural preference, and time on each Context was recorded. Eight daily, 30-minute conditioning trials proceeded. Rats were pre-treated with either suvorexant (30 mg/kg, i.p.) or vehicle followed by injections of either cocaine (10 mg/kg, i.p.) or saline vehicle and placed in Context A (non-preferred; 4 trials) or Context B (preferred; 4 trials), respectively. Lastly, rats were given a post-test for 30 minutes and time spent on each Context was recorded. Pre- and post-test times were used to calculate CPP Score.
For USV recording, a condenser microphone (CM16/CMPA; Avisoft Bioacoustics; Berlin, Germany) was suspended above each of two distinguished contexts and recorded 50-kHz USVs during first conditioning trials in Context A and Context B of CPP (described above; n=6–8/group). Audio was sampled at 192-kHz (16-bits) from an amplification unit (UltraSoundGate 116H; Avisoft Bioacoustics; Berlin, Germany) and was processed by recording software (Avisoft Bioacoustics; Berlin, Germany) on an IBM laptop. Recorded “.wav” files were analyzed offline using either RavenPro software (Cornell Lab of Ornithology, Bioacoustics Research Program; Ithaca, NY, USA) or from an XBAT/MATLAB-based auto-detection program (Barker et al 2014) for generating 50-kHz USV count data.
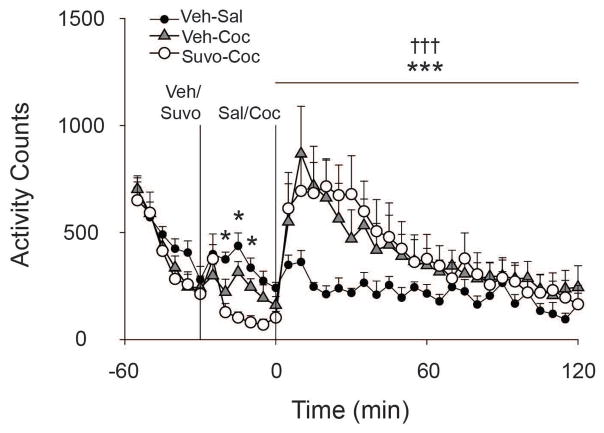
For the locomotor activity assay, rats were placed individually into activity chambers and allowed to acclimate for 30 minutes. Activity was measured as beam breaks collected in 5-minute time bins for 180 minutes. Baseline activity was recorded for 30 minutes, followed by suvorexant pretreatment (30.0 mg/kg, i.p.) or vehicle (0.1 mL, i.p.) and a subsequent activity recording for 30 minutes. An acute cocaine (10.0 mg/kg, i.p.) or saline (1.0 mL/kg, i.p.) injection was administered and activity recorded for the following 120 minutes. Digiscan DMicro system (Accuscan, Inc.; Columbus, OH, USA) measured ambulatory activity as consecutive beam breaks resulting from horizontal movement and non-ambulatory activity as repetitive-beam breaks.
Experiment 3: In Vivo Fast-Scan Cyclic Voltammetry in Ventral Striatum
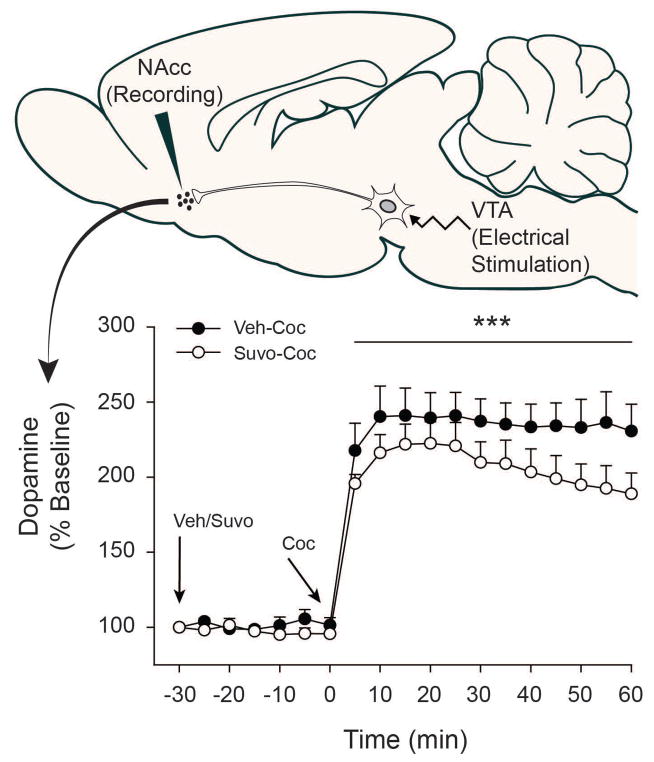
Mice for in vivo voltammetry (n=5/group) were anesthetized with isoflurane, placed into a stereotaxic apparatus and subsequently implanted with a carbon fiber microelectrode aimed at the ventral striatum (AP: +1.3, ML: +1.3, DV: −4.5) and a Ag/AgCl reference electrode placed in contralateral cortex. A bipolar stimulating electrode (Plastics One; Roanoke, VA, USA) aimed at the VTA (AP: −3.0, ML: +1.1, DV: −4.0) was lowered in 100 μM increments until a 0.5-s, 60-Hz monophasic (4 ms; 250 μA) stimulation train elicited a robust DA response. Stimulation trains were delivered every 5 mins for at least 30 mins until DA release reached stability (three consecutive collections within 10%). Once stability was achieved, mice were injected with suvorexant (30mg/kg, i.p.) and subsequent changes in DA release recorded for 30 mins. Following the last collection, mice were injected with 10 mg/kg cocaine i.p. (6 mg/ml) and the change in DA release was recorded for at least 60 mins.
For data acquisition, the electrode potential was linearly scanned (−0.4 to 1.2 V and back to −0.4 V vs Ag/AgCl) and cyclic voltammograms were recorded at the carbon fiber electrode every 100 ms with a scan rate of 400 V/s using a voltammeter/amperometer (Chem-Clamp, Dagan Corporation; Minneapolis, MN, USA). The magnitude of electrically-evoked DA release was monitored. DA overflow curves were analyzed as previously described for DA release (peak concentrations of DA) and uptake (tau) using Demon Voltammetry and Analysis software written in Labview language (National Instruments; Austin, TX, USA; Yorgason et al 2011).
Statistical Analyses
For self-administration data, Infusions and Correct Responses were expressed as % Baseline relative to prior-day performance. One-way repeated-measures ANOVAs were used with Drug Treatment as the within-subjects factor (0, 3, 10, 30 mg/kg of suvorexant). Bonferonni-corrected contrast analyses proceeded for all suvorexant-pretreated groups (3, 10, 30 mg/kg) against vehicle-pretreated (0 mg/kg) control data.
For CPP data, CPP Score was expressed as % Baseline relative to pre-test time (s) in Context B. A one-way ANOVA was used with Treatment Group as the between-groups factor (Veh-Sal, Suvo-Sal, Veh-Coc, Suvo-Coc), and Bonferonni-corrected contrasts were conducted against the Veh-Sal control group.
For USV analyses, standardized change scores (“Δ USV Score”) were used to normalize data for parametric assessment and to assess changes in 50-kHz USVs following initial exposure to cocaine or saline using the formula [(B-A)/(A+B)]. For between-groups analysis, independent samples t-tests were conducted against “0”—the point of no-change. For within-session analyses, a two-way mixed ANOVA examining Treatment Group by Time was conducted.
For locomotor activity, a between-groups one-way ANOVA was used to analyze total activity during minutes 0 to 120 (post-cocaine). Tukey’s HSD post-hoc tests were used for pairwise comparisons, and an additional series of independent samples Bonferroni-corrected t-tests were used to examine locomotor differences within each 5-minute time bin during the pre-drug phase (−30 to 0 minutes).
For voltammetry analyses, % Dopamine was expressed as % Baseline relative to evoked dopamine release from VTA stimulation (recorded every 5 minutes for 30 minutes prior to drug pretreatment). Two-way ANOVAs were used examining % Dopamine with Treatment Group and Time as factors in 5-minute bins for time prior to or following acute cocaine administration.
For contrasts on self-administration, CPP and USV data, Type I error rate (α) within each family of comparisons was maintained at 0.05.
Results
Experiment 1: Suvorexant Decreases Cocaine-Seeking (Figure 1)
Repeated-measures ANOVAs examining Infusions [F(3, 8) = 2.286, n.s.] or Correct Responses [F(3, 8) = 1.679, n.s.] by Drug Treatment were not significant although contrast analyses revealed that the high dose of suvorexant (30.0 mg/kg) caused a significant reduction in Infusions earned relative to prior-day performance [|t(11)| = 2.892, p < 0.05] and had a minor effect at reducing the number of Correct Responses [|t(11)| = 2.583, p = 0.075].
Experiment 2: Suvorexant Prevents Conditioned Cocaine Reward and Attenuates Initial Hedonic Reactivity to Cocaine (Figures 2 and 3, Supplemental Figure 1)
Figure 2.
Effects of suvorexant on (A) CPP Score and (B) Δ USV Score. † p = 0.053 main effect of Drug Type. *** p < 0.001 compared to “0” in (B). Data in (A) are presented as mean ± S.E.M. n=6–8/group.
Figure 3.
Effects of suvorexant on cocaine-induced locomotor activity. Solid vertical line indicates pretreatment with either suvorexant (30 mg/kg) or vehicle at −30 minutes. Solid vertical line indicates acute treatment with either cocaine (10 mg/kg) or vehicle at 0 minutes. * p < 0.05, ** p < 0.01, *** p < 0.001 indicates activity count difference between Veh-Sal and Suvo-Coc groups. † p < 0.05, †† p < 0.01, ††† p < 0.001 indicates activity count difference between Veh-Sal and Veh-Coc groups. Data are presented as mean ± S.E.M. n=8/group.
A between-groups one-way ANOVA examining CPP Score revealed a significant main effect of Treatment Group [F(3, 28) = 3.635, p < 0.05]. Contrast analyses revealed that CPP Score from the Veh-Coc group was significantly greater than the Veh-Sal control group [t(14) = 2.600, p < 0.05], but that CPP Score from the Suvo-Coc group was not different from the Veh-Sal control group [t(14) = 1.662, n.s.].
One-sample t-tests examining Δ USV Score found that positive affective reactivity to cocaine was significant for the vehicle-pretreated group [t(7) = 5.461, p < 0.001] but not for the suvorexant-pretreated group [t(5) = 0.408, n.s.] versus “0”—the point of no-change—when examining USVs across the entire 30-minute session. For within-session analyses, a two-way mixed model ANOVA examining Δ USV Score did not find a significant interaction between Treatment Group and Time but did find a significant main effect of Time [F(5, 60) = 11.415, p < 0.001] and a marginally-significant main effect of Treatment Group [F(1, 12) = 4.600, p = 0.053]. No differences between Veh-Coc and Suvo-Coc groups were found when examining 50-kHz USV duration (MVeh-Coc = 57.5 ms, MSuvo-Coc = 59.4 ms) or bandwidth (MVeh-Coc = 45.0 kHz, MSuvo-Coc = 34.5 kHz) following systemic cocaine injection.
A one-way ANOVA examining activity counts during minutes 0 to 120 revealed a significant main effect of Treatment Group [F(2, 72) =11.640, p < 0.001]. Post-hoc tests revealed significantly greater activity from minutes 0 to 120 in cocaine-treated groups irrespective of pretreatment compared to the vehicle-pretreated, acute saline-treated group [Veh-Coc: p < 0.001; Suvo-Coc: p < 0.001]. T-tests within each 5-minute bin revealed significantly lower activity at minutes -20,-15 and -10 [all p < 0.05] in the Suvo-Coc animals relative to Veh-Sal control animals.
Experiment 3: Suvorexant Reduces Cocaine-Induced Elevations in Ventral Striatal Dopamine (Figure 4, Supplemental Figure 2)
Figure 4.
Dopamine levels (% Baseline) in ventral striatum between Veh-Coc and Suvo-Coc groups. Arrows indicate points of vehicle/suvorexant injection (−30 min; 30 mg/kg, i.p.) and cocaine injection (0 min; 10 mg/kg, i.p.). * p < 0.05, ** p < 0.01, *** p < 0.001 relative to Veh-Coc group. Data are presented as mean ± S.E.M. n=5/group.
For time bins prior to acute cocaine injection (−30 to 0 minutes), a two-way ANOVA examining % Dopamine revealed a non-significant interaction [F(5, 48) = 0.598, n.s.], a non-significant main effect of Time [F(5, 48) = 0.242, n.s.] and a trend towards significance in main effect of Treatment Group [F(1, 48) = 3.726, p = 0.06]. No significant pairwise comparisons were found. Following acute cocaine injection (0 to 60 minutes), a two-way ANOVA examining % Dopamine revealed neither a significant interaction between Treatment Group and Time [F(11, 96) = 0.167, n.s.] nor a main effect of Time [F(11, 96) = 0.597, n.s.]. There was, however, a significant main effect of Treatment Group indicating greater % Dopamine in the Veh-Coc group compared to Suvo-Coc group [F(1, 96) = 20.072, p < 0.001]. No significant effects of suvorexant on cocaine’s propensity to block the dopamine transporter (tau) were observed [main effect of Treatment Group during the post-cocaine phase: F(1, 96) = 0.902, n.s.].
Discussion
Results from the present study suggest that suvorexant, a clinically-available dual orexin receptor antagonist (DORA), reduces cocaine-seeking in a preclinical cocaine self-administration model (Figure 1). These results corroborate a recent report showing that either single or dual orexin receptor antagonism reduces cocaine-seeking using progressive-ratio schedule of reinforcement (Brodnik et al 2015; Prince et al 2015). Moreover, intra-VTA OX1R antagonism reduces cocaine infusions earned during self-administration in rats (España et al 2010; Muschamp et al 2014), suggesting that orexinergic transmission to and signaling within the VTA is critical for the reinforcing properties of cocaine. While studies have shown that orexins can induce food consummatory behavior (Sakurai et al 1998), signaling via OX1Rs preferentially modulates motivation to consume highly-salient rewards (Borgland et al 2009; Martin-Fardon and Weiss 2014). Furthermore, significant effects of orexin receptor blockade on drug infusions are usually observed when rodents are placed on a progressive-ratio schedule of reinforcement and not under fixed-ratio access conditions (e.g. España et al 2010; Hutcheson et al 2011; Mahler et al 2013; Smith et al 2009). A separate line of evidence reveals a critical role for signaling via OX2Rs in mediating escalation of drug-taking in self-administering rats using an extended-access design (Schmeichel et al 2015). OX2Rs additionally potentiate VTA neuronal activity by enhancing pre-synaptic glutamate transmission (Borgland et al 2008). Orexins normally excite VTA DA neurons through somatodendritic OX1Rs (Korotkova et al 2003) as well as by heteroceptors on glutamatergic terminals in VTA (Borgland et al 2006). Mechanistically, our data support that suvorexant, a clinically-available DORA, may occlude cocaine-evoked striatal DA release by reducing excitability of VTA DA neurons. Notably, OX1Rs in NAcc positively regulate phasic DA release when tested ex vivo, but our study revealed a selective effect of systemic suvorexant on cocaine-evoked DA changes and not on NAcc DA dynamics before cocaine injection (Patyal et al 2012). Results from the present study add to a growing literature detailing receptor-specific aspects of targeting OX1Rs and OX2Rs alone or in combination (Khoo and Brown 2014).
Results from the present study further suggest that suvorexant modestly attenuates cocaine CPP (Figure 2A). An earlier report demonstrated that systemic OX1R antagonism significantly attenuated morphine CPP (Harris et al 2005), while other studies find that systemic OX1R antagonism attenuates expression of amphetamine CPP and reduces motivation for conditioned cocaine reinforcement (Hutcheson et al 2011; Shaw et al 2016). CPP assesses reward following passive contextual conditioning, and thus orexin transmission may only be modestly recruited for development of conditioned reward. Additionally, orexin peptide levels in plasma and cerebrospinal fluid are highest during active hours (i.e. when in dark lighting) and lowest during inactive hours (Blouin et al 2013; Kiyashchenko et al 2002; Lee et al 2005; Mileykovskiy et al 2005). As CPP training and testing took place during the rats’ inactive hours, effects of orexin transmission blockade may have been relatively weak compared to transmission blockade during active hours.
Ultrasonic vocalizations (USVs) have been used in preclinical models of substance use disorders to characterize changes in affective processing upon drug administration as well as during withdrawal and relapse (Barker et al 2014; Barker et al 2015; Knutson et al 2002). Experimenter- and self-administered cocaine robustly evoke 50-kHz USVs in rats (Barker et al 2010; Maier et al 2012; Mu et al 2009; Williams and Undieh 2010). Further, while 50-kHz USVs elicited following cocaine injection belong to fixed-frequency, frequency-modulated and “trill” categories, 22-kHz USVs associated with negative affective states are typically monotonic and thus classified predominantly as fixed-frequency USVs. Results from the present study suggest that DORAs are capable of reducing affective reactivity to cocaine as indicated by an augmentation of Δ USV Score (Figure 2B, Supplemental Figure 1). In vivo voltammetry data from previous investigations (Prince et al 2015) as well as the present study (Figure 4, Supplemental Figure 2) find that single or dual orexin receptor antagonists reduce cocaine-evoked elevations in ventral striatal DA. A separate line of evidence finds that 50-kHz USVs following cocaine injection are modulated in part by mesolimbic DA transmission—systemic or direct blockade of DA signaling in the nucleus accumbens (NAcc) reduces 50-kHz USVs elicited by psychostimulant injection (Thompson et al 2006; Williams and Undieh 2010; Wright et al 2013). Furthermore, systemic OX1R antagonism decreases reward sensitivity in mice performing an operant task for brain stimulation reward in an intracranial self-stimulation paradigm (ICSS). This may indicate that blocking orexin signaling pharmacologically alters hedonic state (Muschamp et al 2014). Results from the present study support the idea that dopaminergic signaling along the mesolimbic pathway is critical for positive affect associated with cocaine administration, and that blockade of orexin transmission decreases activity of this pathway and subjective experience following cocaine.
Suvorexant contributes to somnolence and typically decreases locomotor activity in animals (Winrow et al 2011). Results from the present study support that suvorexant reduces activity but likely does not interfere with behavioral task performance when an operandum is employed (i.e., during cocaine self-administration). It is possible that suvorexant-associated sedation contributed to the observed reduction in infusions earned during progressive-ratio cocaine self-administration, but data reveal that all but one suvorexant-pretreated rat did indeed self-administer cocaine (Supplemental Figure 3). Notably, systemic cocaine elicited comparable hyperlocomotor responses irrespective of drug pretreatment (Figure 3). Taken together, it is unlikely that the reported decrease in motivation to self-administer cocaine was due to suvorexant-induced hypolocomotor effects.
Together, our results support the possibility that DORAs may promote abstinence from drug taking in human cocaine users. We demonstrate that suvorexant attenuates both the hedonic as well as the motivational properties associated with cocaine use. These effects are likely due to a reduction in cocaine-evoked forebrain DA transmission by suvorexant. It should be noted that suvorexant is currently used as a sleep aid and thus, if investigated clinically for treating substance use disorders, may elicit sedation that could interfere with normal waking hours. Future studies are needed to more thoroughly characterize the effectiveness of DORAs in preclinical models of addiction relapse and to determine circuits responsible for the effects of suvorexant on motivation versus affective processing.
Supplementary Material
Acknowledgments
The authors would like to acknowledge generous grant support from NIDA to individual investigators (T32DA007237, TAG/SJS; R00DA031767, JWM; R01DA031900, RAE) as well as to the Center for Substance Abuse (P30DA013429, Ellen M. Unterwald PhD). Additionally, the authors acknowledge technical assistance from Dr. Sara Ward, director of the Behavioral Core Facility of the Center for Substance Abuse Research. The authors collectively have no competing financial interests.
Footnotes
Authors Contribution.
TAG and SJS designed experiments, collected/analyzed data and wrote the manuscript under advisory of JWM. DJB analyzed ultrasonic vocalization data sets. JKS and RAE conducted in vivo voltammetry and collected/analyzed corresponding data sets. Tables.
References
- Barker DJ, Herrera C, West MO. Automated detection of 50-kHz ultrasonic vocalizations using template matching in XBAT. J Neurosci Methods. 2014;236:68–75. doi: 10.1016/j.jneumeth.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Simmons SJ, West MO. Ultrasonic Vocalizations as a Measure of Affect in Preclinical Models of Drug Abuse: A Review of Current Findings. Curr Neuropharmacol. 2015;13:193–210. doi: 10.2174/1570159X13999150318113642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Root DH, Ma S, Jha S, Megehee L, Pawlak AP, West MO. Dose-dependent differences in short ultrasonic vocalizations emitted by rats during cocaine self-administration. Psychopharmacology (Berl) 2010;211:435–442. doi: 10.1007/s00213-010-1913-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Simmons SJ, Servilio LC, Bercovicz D, Ma S, Root DH, Pawlak AP, West MO. Ultrasonic vocalizations: evidence for an affective opponent process during cocaine self-administration. Psychopharmacology (Berl) 2014;231:909–918. doi: 10.1007/s00213-013-3309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blouin AM, Fried I, Wilson CL, Staba RJ, Behnke EJ, Lam HA, Maidment NT, Karlsson KAE, Lapierre JL, Siegel JM. Human hypocretin and melanin-concentrating hormone levels are linked to emotion and social interaction. Nat Commun. 2013;4:1547. doi: 10.1038/ncomms2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Storm E, Bonci A. Orexin B/hypocretin 2 increases glutamatergic transmission to ventral tegmental area neurons. European Journal of Neuroscience. 2008;28:1545–1556. doi: 10.1111/j.1460-9568.2008.06397.x. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, Chou J, Chen BT, Bonci A. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci. 2009;29:11215–11225. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de Lecea L. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci U S A. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodnik ZD, Bernstein DL, Prince CD, España RA. Hypocretin receptor 1 blockade preferentially reduces high effort responding for cocaine without promoting sleep. Behav Brain Res. 2015;291:377–384. doi: 10.1016/j.bbr.2015.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, España RA. Hypocretin/orexin regulation of dopamine signaling: implications for reward and reinforcement mechanisms. Front Behav Neurosci. 2012;6:54. doi: 10.3389/fnbeh.2012.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwinkel A, Stanic D, Booth LC, May CN, Lawrence AJ, Yao ST. Distribution of orexin-1 receptor-green fluorescent protein- (OX1-GFP) expressing neurons in the mouse brain stem and pons: Co-localization with tyrosine hydroxylase and neuronal nitric oxide synthase. Neuroscience. 2014;278:253–264. doi: 10.1016/j.neuroscience.2014.08.027. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- España RA, Oleson EB, Locke JL, Brookshire BR, Roberts DC, Jones SR. The hypocretin-orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. Eur J Neurosci. 2010;31:336–348. doi: 10.1111/j.1460-9568.2009.07065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111:379–387. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Heal DJ, Gosden J, Smith SL. Dopamine reuptake transporter (DAT) “inverse agonism”--a novel hypothesis to explain the enigmatic pharmacology of cocaine. Neuropharmacology. 2014;87:19–40. doi: 10.1016/j.neuropharm.2014.06.012. [DOI] [PubMed] [Google Scholar]
- Hutcheson DM, Quarta D, Halbout B, Rigal A, Valerio E, Heidbreder C. Orexin-1 receptor antagonist SB-334867 reduces the acquisition and expression of cocaine-conditioned reinforcement and the expression of amphetamine-conditioned reward. Behav Pharmacol. 2011;22:173–181. doi: 10.1097/FBP.0b013e328343d761. [DOI] [PubMed] [Google Scholar]
- Khoo SY, Brown RM. Orexin/hypocretin based pharmacotherapies for the treatment of addiction: DORA or SORA? CNS Drugs. 2014;28:713–730. doi: 10.1007/s40263-014-0179-x. [DOI] [PubMed] [Google Scholar]
- Kiyashchenko LI, Mileykovskiy BY, Maidment N, Lam HA, Wu MF, John J, Peever J, Siegel JM. Release of hypocretin (orexin) during waking and sleep states. J Neurosci. 2002;22:5282–5286. doi: 10.1523/JNEUROSCI.22-13-05282.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Burgdorf J, Panksepp J. Ultrasonic vocalizations as indices of affective states in rats. Psychol Bull. 2002;128:961–977. doi: 10.1037/0033-2909.128.6.961. [DOI] [PubMed] [Google Scholar]
- Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci. 2003;23:7–11. doi: 10.1523/JNEUROSCI.23-01-00007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25:6716–6720. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Smith RJ, Aston-Jones G. Interactions between VTA orexin and glutamate in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2013;226:687–698. doi: 10.1007/s00213-012-2681-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Smith RJ, Moorman DE, Sartor GC, Aston-Jones G. Multiple roles for orexin/hypocretin in addiction. Prog Brain Res. 2012;198:79–121. doi: 10.1016/B978-0-444-59489-1.00007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier EY, Abdalla M, Ahrens AM, Schallert T, Duvauchelle CL. The missing variable: ultrasonic vocalizations reveal hidden sensitization and tolerance-like effects during long-term cocaine administration. Psychopharmacology (Berl) 2012;219:1141–1152. doi: 10.1007/s00213-011-2445-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, Weiss F. Blockade of hypocretin receptor-1 preferentially prevents cocaine seeking: comparison with natural reward seeking. Neuroreport. 2014;25:485–488. doi: 10.1097/WNR.0000000000000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–798. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu P, Fuchs T, Saal DB, Sorg BA, Dong Y, Panksepp J. Repeated cocaine exposure induces sensitization of ultrasonic vocalization in rats. Neurosci Lett. 2009;453:31–35. doi: 10.1016/j.neulet.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschamp JW, Hollander JA, Thompson JL, Voren G, Hassinger LC, Onvani S, Kamenecka TM, Borgland SL, Kenny PJ, Carlezon WA., Jr Hypocretin (orexin) facilitates reward by attenuating the antireward effects of its cotransmitter dynorphin in ventral tegmental area. Proc Natl Acad Sci U S A. 2014;111:E1648–1655. doi: 10.1073/pnas.1315542111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patyal R, Woo EY, Borgland SL. Local hypocretin-1 modulates terminal dopamine concentration in the nucleus accumbens shell. Front Behav Neurosci. 2012;6:82. doi: 10.3389/fnbeh.2012.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince CD, Rau AR, Yorgason JT, España RA. Hypocretin/Orexin regulation of dopamine signaling and cocaine self-administration is mediated predominantly by hypocretin receptor 1. ACS Chem Neurosci. 2015;6:138–146. doi: 10.1021/cn500246j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richarson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:1. doi: 10.1016/s0092-8674(02)09256-5. page following 696. [DOI] [PubMed] [Google Scholar]
- Schmeichel BE, Barbier E, Misra KK, Contet C, Schlosburg JE, Grigoriadis D, Williams JP, Karlsson C, Pitcairn C, Heilig M, Koob GF, Vendruscolo LF. Hypocretin receptor 2 antagonism dose-dependently reduces escalated heroin self-administration in rats. Neuropsychopharmacology. 2015;40:1123–1129. doi: 10.1038/npp.2014.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw JK, Ferris MJ, Locke JL, Brodnik ZD, Jones SR, España RA. Hypocretin/orexin knock-out mice display disrupted behavioral and dopamine responses to cocaine. Addict Biol. 2016 doi: 10.1111/adb.12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, See RE, Aston-Jones G. Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. Eur J Neurosci. 2009;30:493–503. doi: 10.1111/j.1460-9568.2009.06844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson B, Leonard KC, Brudzynski SM. Amphetamine-induced 50 kHz calls from rat nucleus accumbens: a quantitative mapping study and acoustic analysis. Behav Brain Res. 2006;168:64–73. doi: 10.1016/j.bbr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Williams SN, Undieh AS. Brain-derived neurotrophic factor signaling modulates cocaine induction of reward-associated ultrasonic vocalization in rats. J Pharmacol Exp Ther. 2010;332:463–468. doi: 10.1124/jpet.109.158535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winrow CJ, Gotter AL, Cox CD, Doran SM, Tannenbaum PL, Breslin MJ, Garson SL, Fox SV, Harrell CM, Stevens J, Reiss DR, Cui D, Coleman PJ, Renger JJ. Promotion of sleep by suvorexant-a novel dual orexin receptor antagonist. J Neurogenet. 2011;25:52–61. doi: 10.3109/01677063.2011.566953. [DOI] [PubMed] [Google Scholar]
- Wright JM, Dobosiewicz MR, Clarke PB. The role of dopaminergic transmission through D1-like and D2-like receptors in amphetamine-induced rat ultrasonic vocalizations. Psychopharmacology (Berl) 2013;225:853–868. doi: 10.1007/s00213-012-2871-1. [DOI] [PubMed] [Google Scholar]
- Yorgason JT, España RA, Jones SR. Demon voltammetry and analysis software: analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J Neurosci Methods. 2011;202:158–164. doi: 10.1016/j.jneumeth.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.