Abstract
The degradation of cartilage in the human body is impacted by aging, disease, genetic predisposition, and continued insults resulting from daily activity. The burden of cartilage defects (osteoarthritis, rheumatoid arthritis, intervertebral disc damage, knee replacement surgeries, etc.) is daunting in light of substantial economic and social stresses. This review strives to broaden the scope of regenerative medicine and tissue engineering approaches used for cartilage repair by comparing and contrasting the anatomical and functional nature of the meniscus, articular cartilage (AC), and nucleus pulposus (NP). Many review papers have provided detailed evaluations of these cartilages and cartilage-like tissues individually, but none have comprehensively examined the parallels and inconsistencies in signaling, genetic expression, and extracellular matrix (ECM) composition between tissues. For the first time, this review outlines the importance of understanding these three tissues as unique entities, providing a comparative analysis of anatomy, ultrastructure, biochemistry, and function for each tissue. This novel approach highlights the similarities and differences between tissues, progressing research toward an understanding of what defines each tissue as distinctive. The goal of this paper is to provide researchers with the fundamental knowledge to correctly engineer the meniscus, AC, and NP without inadvertently developing the wrong tissue function or biochemistry.
Keywords: Articular Cartilage, Meniscus, Nucleus Pulposus, Development, Tissue Engineering
Introduction
The meniscus, articular cartilage (AC), and nucleus pulposus (NP) are all significant tissues in the progression of pathologies such as osteoarthritis (OA) (Loeser et al., 2012), rheumatoid arthritis (RA) (Goldring, 2003), meniscus tears (Fox et al., 2015), and degenerative disc diseases (Tian et al., 2013). What defines these three tissues as unique compared to other tissues, and subject to regenerative approaches, is their overall avascularity, inability to heal properly in vivo, and difficult clinical and translational remediation (Fox et al., 2015; Hunziker, 2002; Tian et al., 2013). Because these tissues assume similar functions (distribution and transfer of weight across surfaces) and are composed of similar cell types (fibrochondrocytes, chondrocytes, and chondrocyte-like cells), it may be presumed that regenerative approaches would also be similar. The purpose of this paper is to thoroughly explicate upon these parallels, illustrating how these tissues appear comparable, but have far ranging disparities in their development, anatomy, tissue and cell structure, and function. For researchers interested in tissue engineering and regenerative medicine approaches, this review provides a compare and contrast analysis between the meniscus, AC, and NP.
Developing the appropriate tissue is not only subject to achieving a specific cell phenotype, but also to regulating extracellular matrix (ECM) composition and production levels (Shine et al., 2009), vascularization and innervation (Johnson et al., 2001), growth factors (Pei et al., 2002), proper molecular signaling (Zhang et al., 2014), and ability to correctly respond to pressure/tension stimulation (Zhang et al., 2016a). Each tissue exhibits differing levels of ultrastructure, composed of varying cellular components, ECM, and levels of oxygen distribution; the meniscus with red-red, red-white, and white-white zones (Fox et al., 2015), AC with four layers (superficial, transitional, deep, and calcified) (Becerra et al., 2010), and NP with central and peripheral regions (Roberts et al., 1995). As surgical procedures become less invasive and more easily conducted (Frank and Cole, 2013), the field of regenerative medicine will see increased opportunities for tissue explants (Musumeci et al., 2014). This review offers an assessment of anatomical differences between these three tissues to better understand their commonality and diversity, providing the reader with the knowledge of more efficient differentiation studies in the meniscus, AC, and NP. By increasing the quality of cartilage and cartilage-like grafts, the graft/host homology should allow for more efficient assimilation and comparable functionality thereby limiting tissue explant deterioration (Chen et al., 2012; Jackson, 2015;).
Vascular, Neural, and Basic Anatomy
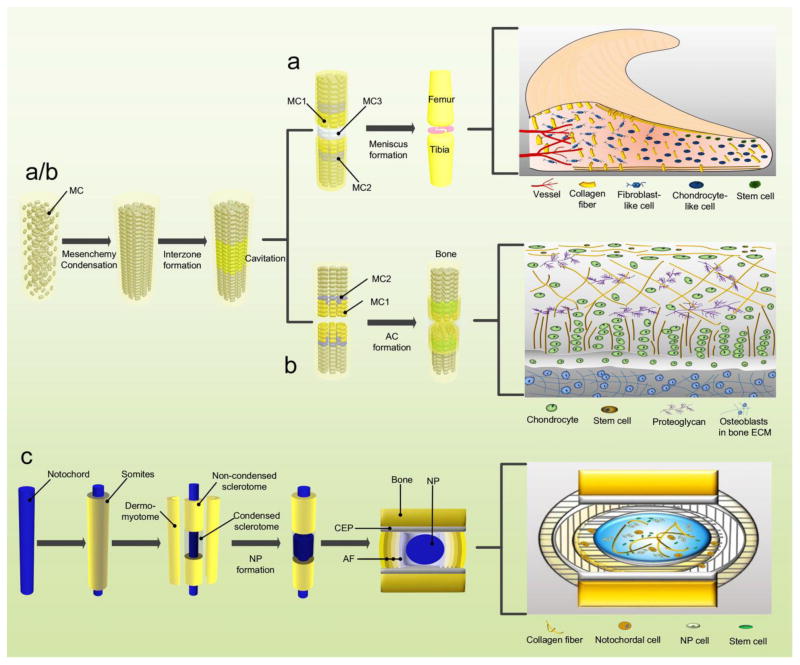
The uniqueness of the meniscus, AC, and NP as discrete cartilage-like tissues begins in development, through site-dependent signaling and extraneous environmental stimuli casting the shape, and terminates in the vascularization and innervation that directly impacts the layering of each tissue. Signaling, vascularization, innervation, stem cell source, and location in the body all present slight nuances to tissue development; these distinctions direct the subset of cells that will eventually populate the tissue.
Meniscus
Deriving from a condensation of mesenchymal cells within the intermediate layer, called interzone cells, the meniscus develops its typical shape from the eighth to tenth week of conception (Gardner and O’Rahilly, 1968). The immature menisci are rife with cells and blood vessels, with a blood supply through the whole menisci (Clark and Ogden, 1983); as the fetus develops, cellularity continues to decrease in the menisci, while the collagen content continues to increase in a circumferential arrangement (Clark and Ogden, 1983). Besides partial vascularization in the periphery provided by branches of the popliteal artery, the meniscus comparatively is a tissue without blood vessels. Antiangiogenic factors are important not only for the development but also for the maintenance of avascular zones in the meniscus. The antiangiogenic peptide endostatin/collagen XVIII was detected in the menisci of both human fetus and adult; however, in the adult, endostatin/collagen XVIII mainly existed in the inner two-thirds avascular region of the meniscus, whereas, in the fetus, endostatin/collagen XVIII was mainly distributed in the outer one-third (Pufe et al., 2004). Blood supply for the lateral meniscus ranges from the peripheral 10% to 25% and 10% to 30% for the medial meniscus, functioning significantly for self-healing (Danzig et al., 1983). The rest of the meniscus absorbs nutrition through synovial diffusion or joint motion.
The nerve fibers following the blood supply are detected mainly in the peripheral vascular area of the meniscus (Kennedy et al., 1982). The mechanoreceptors within the menisci could convert mechanical stimulation into a unique electrical nerve impulse. Three morphologically distinguishing mechanoreceptors have been found within the human meniscus: “Ruffini endings, Pacinian corpuscles, and Golgi tendon organs”, particularly in the meniscal horns (Zimny et al., 1988). It is believed that proprioception can be acquired from free nerve endings (nociceptors) (Mine et al., 2000) stimulated on the anterior and posterior horns in the process of knee flexion and extension (O’Connor, 1984; O’Connor and McConnaughey, 1978). (Figure 1A) (Table 1)
Fig. 1.
Development of the meniscus (A), AC (B), and NP (C). Abbreviations: AF: annulus fibrosus; CEP: cartilaginous endplate; MC: mesenchymal cells; MC1: MC committed to form superficial layer of AC; MC2: MC with chondrogenic fate; MC3: MC with meniscal fate.
Table 1.
Characterization of meniscus.
| Characterization | Embryology and Development | Reference |
|---|---|---|
| Origination | Interzone cells | (Gardner and O'Rahilly, 1968) |
| Shape formation | Between the 8th and 10th week of gestation | (Gardner and O'Rahilly, 1968) |
| Component switch | Increase in collagen content but decrease in cellularity and vascularity with the development of fetus | (Clark and Ogden, 1983) |
| Gross Anatomy | ||
| Medial meniscus | “C”-shaped; 39.8±3.7 mm long and 9.5±0.7 mm wide; anterior horn is attached to the tibia anterior to the ACL; posterior horn is attached immediately anterior to the attachment of the PCL; peripheral border merges with the knee joint capsule | (Fox et al., 2015, Proffen et al., 2012) |
| Lateral meniscus | “O”-shaped; 33.3±3.5 mm long and 9.8±0.7 mm wide; anterior horn is attached to the intercondylar fossa adjacent to the broad attachment site of the ACL; posterior horn is attached to the PCL and medial femoral condyle | (Fox et al., 2015, Proffen et al., 2012) |
| Vascular and Neural Anatomy | ||
| Blood supply | Peripheral 10–25% are vascular for LM and 10–30% for MM | (Danzig et al., 1983) |
| Intrinsic innervation | Most abundant on the periphery and the anterior and posterior horns | (Kennedy et al., 1982; Zimny et al., 1988) |
| Cell Property and Phenotype | ||
| Outer 2/3 region | Elongated fibroblast-like cells | (Melrose et al., 2005) |
| Inner 1/3 region | Rounded chondrocyte-like cells | (Melrose et al., 2005) |
| Superficial region | Flattened and fusiform progenitor cells | (Declercq et al., 2012) |
| Cell density | Vary with regions: 200–2800 cells/mm2 | (Lin et al., 2002) |
| Phenotypic marker | C1QR; CA12; COL1A1; COL1A2; ESTs; FLJ20831; HPCAL1; LIMK2; PDLIM1 | (Ochi et al., 2003) |
| Matrix Microenvironment | ||
| Water | 72% of wet weight and content is higher in the posterior areas | (Herwig et al., 1984) |
| Collagen | 22% of wet weight | (Herwig et al., 1984) |
| Outer 2/3 region | Type I collagen (80% by dry weight) and other collagen variants (e.g., types II, III, IV, VI, and XVIII) (<1%) | (Fox et al., 2012; Fox et al., 2015) |
| Inner 1/3 region | Type II (60%) and type I collagen (40%) | (Cheung, 1987) |
| Proteoglycan | 1–2% of dry weight; the major PG (aggrecan) and other smaller PGs (e.g., decorin, biglycan, fibromodulin, and lubricin) | (Ghosh and Taylor, 1987) |
| Glycoprotein | Type VI collagen, link protein, fibronectin, thrombospondin, elastin, and chondromodulin-I | (Fujii et al., 2013; Höpker et al., 1986; McDevitt and Webber, 1990) |
| Tissue Function | ||
| Primary role | Transferring vertical compressive load into circumferential “hoop” stresses | (Ghosh et al., 1975) |
| Secondary role | Shock absorption, stability, lubrication, nutrition, and proprioception to the knee joint | (Fithian et al., 1990; Renström and Johnson, 1990) |
Abbreviations: ACL: anterior cruciate ligament; C1QR: complement component C1q receptor; CA12: carbonic anhydrase XII; COL1A2: collagen, type I, alpha 2; FLJ20831: hypothetical protein FLJ20831; HPCAL1: hippocalcin-like 1; LIMK2: LIM domain kinase 2; LM: lateral meniscus; MM: medial meniscus; PCL: posterior cruciate ligament; PDLIM1: PDZ and LIM domain 1 (elfin); PG: proteoglycan.
Articular Cartilage
Like the meniscus, the AC also originates from the interzone (Archer et al., 2003). Recent evidence indicates that a continuous influx of GDF5 (growth differentiation factor 5) positive cells contributes to joint development (Ray et al., 2015; Shwartz et al., 2016). With the upregulation of unique molecules such as Wnt9A (Wingless-Type MMTV Integration Site Family, Member 9A), GDF5, Erg (ets related gene), Gli3 (GLI Family Zinc Finger 3), CD44 (cluster differentiation 44), and type IIA/I collagen (Iwamoto et al., 2007; Koyama et al., 2008; Pacifici et al., 2006), cavitation appears within the interzone (Archer et al., 2003). In the meantime, the joint capsule, consisting of the outer ligaments and the inner synovium, promotes the connection of the two cartilaginous constituents (Merida-Velasco et al., 1997). From the top surface of the AC, chondrocyte size becomes larger toward the secondary ossification center, ending with calcified and vascularized hypertrophic cells (Hunziker et al., 2007). The mature AC consists of four sequential layers; superficial, transitional (middle), deep (radial), and calcified zones (Becerra et al., 2010). The tidemark is a transition zone between the non-calcified and calcified layer (Meirer et al., 2011). Due to its avascular and aneural properties, AC depends on diffusion to acquire its nutrition and oxygen supply, which results in limitations in self-repairing capacity. (Figure 1B) (Table 2)
Table 2.
Characterization of articular cartilage.
| Characterization | Embryology and Development | Reference |
|---|---|---|
| Origination | Interzone cells | (Archer et al., 2003) |
| Developing AC | 3–4 layers that show a distinct cell shape and size | (Hunziker et al., 2007) |
| Mature AC | Superficial, middle, deep, and calcified layers | (Becerra et al., 2010) |
| Gross Anatomy | ||
| AC thickness | 2.4±0.4 mm at the medial femoral condyle and 3.0±0.4 mm at the medial tibial plateau | (Quinn et al., 2013) |
| Zonal Organization | ||
| Superficial | Flattened chondrocytes, low quantity of PGs, and high quantity of collagen fibrils arranged parallel to AC surface | (Schumacher et al., 1994) |
| Middle | Rounded chondrocytes, the highest level of PGs among the four zones, and a random arrangement of collagen | (Lorenzo et al., 1998) |
| Deep | Chondrocyte columns arrayed along the axis of fibrils, which is perpendicular to the underlying bone | (Schmid and Linsenmayer, 1985) |
| Calcified | Partly mineralized and acting as the transition between cartilage and the underlying subchondral bone | (Schmid and Linsenmayer, 1985) |
| Tidemark | The transition zone between the non-calcified and calcified normal AC | (Meirer et al., 2011) |
| Vascular and Neural Anatomy | ||
| Blood supply | Avascular | (Buckwalter, 1983) |
| Innervation | No nerve supply | (Buckwalter, 1983) |
| Cell Property and Phenotype | ||
| Chondrocytes | The sole cell in AC | (Buckwalter and Mankin, 1998) |
| Superficial zone | Progenitor/stem cell | (Muinos-Lopez et al., 2012) |
| Cell density | 1.4×104 cells/mm3 | (Stockwell, 1971) |
| Phenotypic marker | COMP; CYTL1; FBLN1; GDF10; HIF-1/2α; IBSP; MGP | (Minogue et al., 2010a&b; Rutges et al., 2010; Wang et al., 2016) |
| Matrix Microenvironment | ||
| Water | 65–80% of wet weight | (Buckwalter and Mankin, 1998) |
| Collagen | 10–20% of wet weight; 90–95% type II collagen with a small percentage of types I, IV, V, VI, IX, and XI collagen | (Buckwalter and Mankin, 1998; Hunziker, 2010) |
| Proteoglycan | 10–15% of wet weight; the major component (aggrecan) and small leucine-rich PGs (biglycan, fibromodulin, decorin, and lubricin) | (Heinegard and Oldberg, 1989; Oldberg et al., 1990) |
| Glycoprotein | Clusterin, lubricin, and chondromodulin-I | (Hiraki et al., 1991; Khan et al., 2001; Musumeci et al., 2013) |
| Tissue Function | ||
| Primary role | Load transmission and distribution, smooth articulation, lubricating, and wear-resisting structure that facilitates joint motion | (Buckwalter and Mankin, 1998) |
Abbreviations: AC: articular cartilage; COMP: cartilage oligomeric matrix protein; CYTL1: cytokine-like 1; FBLN1: fibulin 1; GDF10: growth differentiation factor 10; IBSP: integrin-binding sialoprotein; MGP: matrix gla protein; PG: proteoglycan.
The avascular nature of AC is attributable to its biochemical composition that antagonizes vascular invasion. The breakdown of the antiangiogenic barrier can cause undesirable vascular invasion of AC and irreversible cartilage degeneration. Of the components encompassed in AC, thrombospondin-1 (TSP1), chondromodulin-I (ChM-I), endostatin/collagen XVIII, secreted protein acidic and rich in cysteine (SPARC), and the type II collagen-derived N-terminal propeptide (PIIBNP) have demonstrated antiangiogenic properties in vitro and in vivo (Patra and Sandell, 2012). Additionally, tissue inhibitor of metalloproteinases-2 (TIMP2) was also present at high levels in normal articular chondrocytes as an antiangiogenic factor (Mi et al., 2012).
Nucleus Pulposus
The emergence of the intervertebral disc (IVD) begins during the third week of embryogenic development (Rodrigues-Pinto et al., 2014). The axial mesoderm, or notochord, goes through two transitions – first a mesenchymal to epithelial transition (MET) (to allow for correct formation of the neural tube and somites) and then an epithelial to mesenchymal transition (EMT) (to allow for appropriate differentiation) (Hay, 2005; Nakaya and Sheng, 2008). At week four, when the cells readapt this mesenchymal phenotype, the somites that surround the notochord begin to associate into new layers: the dermomyotome (muscle and skin), non-condensed sclerotome (vertebral bodies), and condensed sclerotome (annulus fibrosus, AF) (Rodrigues-Pinto et al., 2014) (Figure 1C). At week five, the dermomyotome begins to dissociate from the notochord, leaving only the notochord and sclerotome cells (Peacock, 1951).
In the sixth and seventh week, notochord cells start their migration to the central portions of the condensed sclerotome (Rodrigues-Pinto et al., 2014) (Figure 1C). By the tenth week, the notochord derived cells, confined within the condensed sclerotome, will begin transition into large, immature NP cells (Figure 1C) (Rodrigues-Pinto et al., 2014; Smith et al., 2011). The notochord’s transition into the NP, as well as the direction of other mesenchymal cell populations during development, is controlled through Brachyury (T), Sonic hedgehog (Shh), Noggin (Nog), transforming growth factor beta (TGFβ), and other signaling molecules (Chan et al., 2014). The Shh-dependent expression of Paired box 1 and 9 (Pax1/9) synergistically regulate vertebral column development (Peters et al., 1999), whereas TGFβ is involved in the differentiation of the sclerotome into AF cells (Hayes et al., 2011).
Few blood vessels are available for mature discs and they mainly exist in the longitudinal ligaments alongside the disc and in young cartilaginous endplates (CEP) which are branches of the spinal artery (Crock et al., 1988; Roberts et al., 1995). The disc acquires most nutrition through diffusion via the CEP or from the restricted blood supply in the outer layers of the AF. Fas ligand, a type II transmembrane protein of the tumor necrosis factor family, expressed by normal NP cells, could cause apoptosis in vascular endothelial cells and subsequently inhibit blood vessel infiltration (Sun et al., 2013). Additionally, Nog and chondroitin sulfate released from notochordal cells inhibited angiogenesis by suppressing vascular endothelial growth factor signaling (Cornejo et al., 2015). Nerves in the discs, either accompanying the vessels or occurring independently, are branches of the sinuvertebral nerve or the gray rami communicantes (Johnson et al., 2001; Raj, 2008). (Table 3)
Table 3.
Characterization of nucleus pulposus.
| Characterization | Embryology and Development | Reference |
|---|---|---|
| Origination | Mesodermal somites | (Peacock, 1951; Rodrigues-Pinto et al., 2014) |
| Shape formation | The tenth week of embryonic development | (Peacock, 1951; Rodrigues-Pinto et al., 2014) |
| Gross Anatomy | ||
| Property | Gelatinous | (Maroudas et al., 1975) |
| Microenvironment | Avascular, hypoxia, low pH, low nutrition, low cellularity, high GAG content, and type II collagen | (Agrawal et al., 2007, Rajpurohit et al., 2002) |
| Vascular and Neural Anatomy | ||
| Blood supply | Avascular | (Crock et al., 1988; Roberts et al., 1995) |
| Innervation | No innervation | (Crock et al., 1988; Roberts et al., 1995) |
| Cell Property and Phenotype | ||
| NP cell | Smaller (10 μm), round, and chondrocyte-like cells | (Hunter et al., 2003; Hunter et al., 2004) |
| Notochordal cell | Large (25–85 μm) and vacuolated | (Hunter et al., 2004; Risbud et al., 2015) |
| NP progenitor cell | Tie2+ and GD2+ positive | (Sakai et al., 2012) |
| Cell density | 6000 cells/mm3 | (Maroudas et al., 1975) |
| Phenotypic marker | HIF1/2α, GLUT1, KRT 18/19, CA-3/12, CD24, A2M | # |
| Matrix Microenvironment | ||
| Water | 80% of the wet weight | (Raj, 2008) |
| Collagen | About 20% (type II collagen) of dry weight and small amounts of types VI, IX, and XI collagen | (Eyre and Muir, 1977; Sive et al., 2002) |
| Proteoglycan | 15% of wet weight; the major PG (aggrecan) and smaller amounts (decorin, biglycan, and lumican) | (Inkinen et al., 1998; Malrose et al., 2001; Raj, 2008) |
| Glycoprotein | Elastin, fibronectin, laminin, lubricin, link protein, and chondromodulin-I | ## |
| Tissue Function | ||
| Primary role | Absorb the loads and equalize the compressive stress on the vertebral CEP | (Pattappa et al., 2012) |
Abbreviations: A2M: alpha-2-macroglobulin; CEP: cartilaginous end-plate; GAG: glycosaminoglycan. GLUT1: glucose transporter 1; HIF1α: hypoxia-inducible factor 1 alpha; KRT18: keratin 18;
Cell Property and Phenotype
The physical environment (oxygen and nutrient supply, microenvironment composition, and biomechanical stress) directly modifies the shape and function of cells within the meniscus, AC, and NP. Each cell population demonstrates an exclusive phenotype within these cartilage-like tissues, having distinctive cell surface profiles, progenitor cell lines, and responses to injury or inflammation.
Meniscus
The cells in the meniscus were initially classified as “chondrocytes, fibroblasts, or cells of intermediate morphology” (Ghadially et al., 1978). However, the characteristic description of meniscus cells seems disputed in the literature, with various terms being applied, such as fibrocytes, fibroblasts, meniscus cells, fibrochondrocytes, and chondrocytes (Nakata et al., 2001). Despite the diverse terminology used, the inner zone cells are apparently round to oval shaped and display a distinct cell associated matrix (CAM) including a mass of cartilaginous type II collagen and a lower, but remarkable, quantity of type I collagen and aggrecan. These properties lead meniscus cells to be termed “fibrochondrocytes” in comparison with hyaline chondrocytes that produce primarily type II collagen and aggrecan (Melrose et al., 2005). In contrast, the cells in the outer portion of the tissue were named fibroblast-like cells due to their similarity to fibroblasts in appearance and behavior; they are encircled in the ECM predominantly by type I collagen, have fewer glycoproteins, and less type III and type V collagen (Melrose et al., 2005). However, mRNA levels of SRY (Sex Determining Region Y)-Box 9 (SOX9), an important transcription factor of type II collagen synthesis and chondrogenesis (Lefebvre and de Crombrugghe, 1998), were similar in the meniscus between the inner and outer regions (Upton et al., 2006).
A third cell group, characterized as CD34+ and identified in the outer area of the meniscus (with the majority of meniscal cells exhibiting a CD34−/CD31− phenotype), is flat and fusiform-like shaped without cell extensions (Verdonk et al., 2005). This cell group has been proposed to be specific progenitor cells for therapeutic and regenerative purposes (Declercq et al., 2012). As CD34 is regarded as a marker of mesenchymal stem cells (MSCs) (Kopher et al., 2010) which express smooth muscle actin (SMA) (Cai et al., 2001), CD34+ and SMA+ meniscus cells might participate in the reparative process of pathological menisci (Declercq et al., 2012). α-SMA+ cells were reported to align with collagen fibers in a meniscus crevice three weeks after injury (Kambic et al., 2000) thus indicating their involvement in the differentiation process.
Articular Cartilage
Distributed throughout the matrix, chondrocytes comprise less than 5% wet weight of the AC (Buckwalter and Mankin, 1998). The chondrocyte and its pericellular matrix (PCM) together constitute the chondron, which is recognized as the main structural, functional, and metabolic unit of the AC (Poole, 1997). Investigations show that the cells harvested from the surface of postnatal bovine or mouse AC have stem cell traits such as a high capability of colony formation and expression of MSC markers (Dowthwaite et al., 2004; Hattori et al., 2007), acquiring and expressing chondrogenic phenotypes after multiple passages (Yasuhara et al., 2011). The existence of stem cells in the superficial zone of human AC has also been verified by their positive reaction to TGFβs (Dowthwaite et al., 2004; Hattori et al., 2007), such as boosting production of proteoglycan 4 (PRG4) proteins [also named superficial zone proteins (SZPs) or lubricin] and cartilage matrix aggrecan and type II collagen (Muinos-Lopez et al., 2012). The SOX9 protein is necessary but not sufficient for induction and maintenance of chondrocytic phenotypes; it may act in concert with SOX5 and SOX6, to induce transcription of type II collagen and aggrecan (Ikeda et al., 2004). It is worth noting that SOX9 expression does not correlate with type II collagen expression in AC cells (Aigner et al., 2003) and has been shown to suppress type II collagen transcription in de-differentiated chondrocytes (Kypriotou et al., 2003).
Multiple techniques evaluating either genetic or surface protein expression have been implemented to distinguish AC from cells of the meniscus and NP. In studying AC and NP cells, AC cells were identified positive for fibulin-1 (FBLN1) and integrin-binding sialoprotein (IBSP), with minor NP expression. Interestingly, sources of more compromised, degraded NP expressed higher levels of FBLN1, purporting potential problems for cross-tissue regeneration approaches (Minogue et al., 2010b). Later studies by Minogue involved more genome analysis but with a switch from bovine NP tissue to human. Analysis of human NP cells revealed similar findings for FBLN1 and IBSP, but also revealed novel markers, cytokine-like-1 (CYTL1) and GDF10 (Minogue et al., 2010a). These factors were shown to be increased more than 100-fold in AC compared to NP cells. Other studies have suggested cartilage oligomeric matrix protein (COMP) and matrix gla protein (MGP) as possible identification markers distinguishing AC cells. Again comparing AC and NP cells, AC more highly expressed COMP and MGP (Rutges et al., 2010). COMP is known to be associated as a biomarker for OA cartilage turnover (Pearle et al., 2005) , but it is also suggested that COMP may play a role in suppressing vascularization (Rutges et al., 2010).
Nucleus Pulposus
In early childhood, the cells within the NP are shaped like those that make up the embryonic notochord (large – 25–85 μm, intracellular vacuole-like structures, “immature” mitochondria, and large endoplasmic reticulum) (Hunter et al., 2004; Risbud et al., 2015). In humans, it is reported that these large vacuolated notochordal cells decrease during the first decade of life, gradually being replaced by smaller (around 10 μm in diameter) and non-vacuolated round cells in the NP (Hunter et al., 2003). The mature NP cells have morphological similarities with articular chondrocytes, even being termed “chondrocyte-like cells” (Risbud et al., 2015; Urban and Roberts, 1995). However, a small proportion of cells which express notochordal biomarkers persisting until maturity (Stosiek et al., 1988) and retaining a distinct phenotype (Clouet et al., 2009; Minogue et al., 2010a) makes NP cells distinct from articular chondrocytes.
The importance of notochordal cells has been demonstrated in the synthesis of functional ECM and in the survival of chondrocyte-like cells. Connective tissue growth factor (CTGF/CCN2), one of the growth factors synthesized by notochordal cells, stimulated the proliferation of chondrocyte-like cells and the production of type II collagen and aggrecan (Erwin et al., 2006). Furthermore, the secretome of notochordal cells could protect chondrocyte-like cells from apoptosis (inhibiting caspases-3 and -9 and favoring aggrecan and type II collagen expression) (Erwin et al., 2011). A recent study reported that the expression of CTGF/CCN2 in notochordal cells could be controlled by oxygen tension (Tran et al., 2013). Thus NP degeneration can be initiated with the gradual disappearance of notochordal cells during skeletal maturation.
Like many other tissues, tissue-specific stem cells were also identified in the IVD (Blanco et al., 2010). A subpopulation of cells distinguished by expression of Tie2+ and GD2+ was shown to be multipotent in the NP tissues because of their ability for differentiation to both mesenchymal and NP lineages (Sakai et al., 2012). The similarity between articular chondrocytes and NP cells, such as sharing common markers, Sox9, type II collagen, and aggrecan (Sive et al., 2002), facilitates a hypothesis that differentiation of MSCs to NP cells with a chondrocyte-like phenotype would be enough to imitate the IVD environment. Interestingly, anabolism of type I collagen and catabolism of type II collagen in the NP may diminish the differentiation into NP cells and ECM biosynthesis of transplanted stem cells (Tao et al., 2016). However, this view has been questioned in a study that determined that AC and NP cells synthesized an obviously different ECM in terms of the ratio of proteoglycan to collagen (Mwale et al., 2004). Moreover, a report showed that, compared to the AC, aggrecan in the NP was highly enriched with keratan sulfate and less aggregated with smaller, more degraded fragments (Donohue et al., 1988). In addition, autologous chondrocyte implantation (harvested from the AC) of the same rabbit’s IVD led to hyaline-like cartilage formation (Gorensek et al., 2004).
Matrix Microenvironment
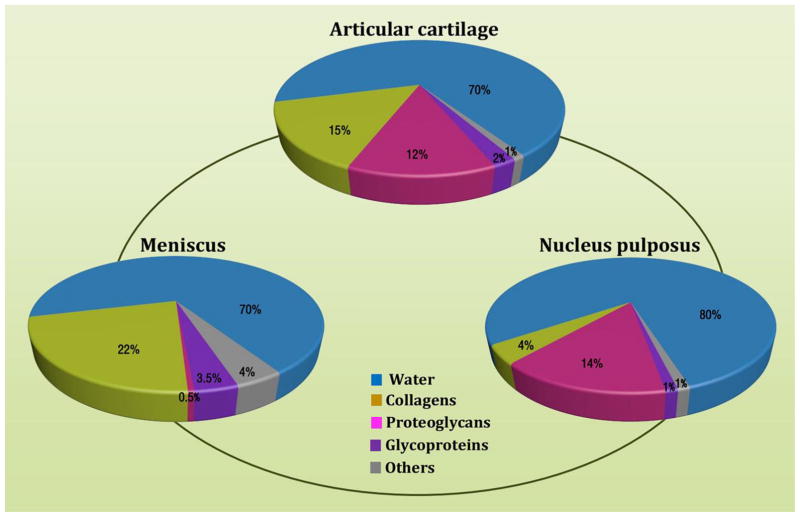
Evaluation of the meniscus, AC, and NP through water, collagen, proteoglycan, and glycoprotein content illustrates significant compositional changes between tissues. Figure 2 develops a gradient, starting with a low hydrated, low aggrecan, high collagen tissue, the meniscus, and progressing toward a more highly hydrated, high aggrecan, low collagen tissue, the NP. This section provides further detail about the unique tissue-specific environment of the meniscus, AC, and NP. In developing a high-quality tissue graft, the microenvironment must be sufficiently capable of handling biomechanical, oxidative, and matrix remodeling stresses, while retaining proper genome expression.
Fig. 2.
ECM composition of the meniscus, AC, and NP.
Meniscus
Water, constituting 72% of the wet weight in mature meniscus, contributes to hydraulic pressures (HP) by binding with proteoglycans to overcome the friction drag of forcing fluid flow through the meniscus (Fox et al., 2012; Herwig et al., 1984). Collagen, another major matrix component, constitutes up to 22% of the wet weight in the meniscus, primarily for type I collagen (Eyre and Wu, 1983; Herwig et al., 1984; McDevitt and Webber, 1990). The unique collagen fiber arrangement in the meniscus, oriented circumferentially in the deeper layers and more radially in the superficial region (Aspden et al., 1985; Fithian et al., 1990; Skaggs et al., 1994), contributes a vertical compressive load transferred into circumferential “hoop” stresses (Ghosh et al., 1975).
Proteoglycans, constituting 1–2% of the dry weight in mature meniscus, initiate hydration for the resistance of compressive loads (Ghosh and Taylor, 1987). The density in the meniscus is significantly diverse at the sample site and is patient age dependent (Fithian et al., 1990). As the major proteoglycan in human menisci, aggrecan largely contributes to the viscoelastic compressive properties by binding with chondroitin sulfate and keratan sulfate of glycosaminoglycan (GAG) (Herwig et al., 1984). Regarding glycoproteins, fibronectin, constituting 8–13% of the dry weight in the meniscus, takes part in tissue repair, embryogenesis, and cell migration/adhesion (Fox et al., 2012). Elastin, which accounts for less than 0.6% of the dry weight in the meniscus (Höpker et al., 1986), most likely interacts directly with collagen to provide resiliency to the tissue (Fithian et al., 1990). Link protein (LP) can stabilize proteoglycan-hyaluronic acid aggregates that are situated around the collagen bunches in the interterritorial matrix (Fife, 1985). ChM-I, a 25 kDa glycoprotein, is involved in the inhibition of endothelial cell proliferation (Hiraki et al., 1997). Larger amounts of ChM-I in the inner meniscus inhibited endothelial cell proliferation, suggesting that ChM-I may be a key antiangiogenic factor for maintaining the avascularity of the inner meniscus (Fujii et al., 2013).
Articular Cartilage
Water, constituting 65–80% of the wet weight of the AC, is approximately 15% more concentrated at the surface than in the deeper zone (Buckwalter and Mankin, 1998). Collagens make up about 10–20% of the wet weight of the AC (Pearle et al., 2005). Of at least 15 distinguishing types of collagen in the AC, type II collagen accounts for 90–95% of the collagens in AC matrix (Eyre et al., 1978). Despite contributing only a minor proportion, types I, IV, V, VI, IX, and XI collagen help create and maintain a fibril meshwork formed by type II collagen (Buckwalter and Mankin, 1998; Hunziker, 2010). This meshwork helps withstand the swelling pressure produced by proteoglycans and supply the tissue’s tensile strength. Types I and III collagen are undetectable in healthy AC, but the expression is upregulated in degeneration (Gouttenoire et al., 2004). Type VI collagen is the primary element of the PCM and solely identified within the PCM in adult AC (Poole et al., 2001), despite the fact that it is ubiquitous in AC ECM of the newborn (Poole, 1997).
Proteoglycans, constituting 10–15% of the wet weight of the AC, are the second largest category of macromolecules in the matrix (Oldberg et al., 1990; Pearle et al., 2005). The major proteoglycan (aggrecan) and small leucine-rich proteoglycans [biglycan, fibromodulin, decorin, aspirin, and parathyroid hormone-like hormone (PTHLH)] (arcOGEN Consortium. et al., 2012; Heinegard and Oldberg,1989; Kizawa et al., 2005) interact with type II collagen and regulate fibril formation to modify the tissue structure and characters. In the AC, the distribution and arrangement of these matrix components are not even. For example, compared to the surface with flattened chondrocytes, a relatively small quantity of proteoglycans and high amounts of collagen fibrils are arranged parallel to the articular surface (Schumacher et al., 1994); the middle zone, on the contrary, has round chondrocytes, the largest quantity of proteoglycans among the four areas, and a random arrangement of collagen (Lorenzo et al., 1998). The deep zone is distinguished by collagen fibrils accompanied by columns of chondrocytes which are perpendicular to the underlying bone (Schmid and Linsenmayer, 1985). The calcified zone is mineralized to some extent and serves as a transformation between cartilage and the underlying subchondral bone (Schmid and Linsenmayer, 1985).
Glycoprotein concentration in the AC decreases depending on disease states (Noyes and Stabler, 1989). During fetal development, AC chondrocytes have been shown to express α6β1 integrins, which associate with laminin in the ECM and promote cell proliferation, differentiation, and polarization (Durr et al., 1996). Through development and into adulthood, laminin becomes less important as a glycoprotein for sustaining the AC, while clusterin begins to play a more important role. Clusterin, excreted from chondrocytes of the superficial zone, activates the complementary pathway, resulting in immune response, cell death, and potentially tissue destruction (Khan et al., 2001). For daily load-bearing activities, lubricin, another important glycoprotein excreted from chondrocytes of the superficial zone, is responsible for reducing friction within the joint; a decrease in lubricin is also associated with OA progression (Musumeci et al., 2013). ChM-I is expressed specifically in cartilage as a functional matrix component (Hiraki et al., 1991). ChM-1 null mice exhibit retarded chondrocyte maturation in the periosteal callus, aberrant cartilage formation during fracture repair (Yukata et al., 2008), and marked reduction in bone remodeling (Nakamichi et al., 2003). A recent study suggested that ChM-1 governed stable chondrocyte phenotypes and maintained cartilage homeostasis possibly by inhibiting hypoxia inducible factor-2 alpha (HIF-2α) induced catabolic activity (Zhang et al., 2016b).
Nucleus Pulposus
Water, constituting about 80% of the wet weight in the NP compared to 70% of the wet weight in the AF (Choi et al., 2015; Raj, 2008), along with type II collagen, allows NP to be elastic and deform under stress. In the IVD, the outer AF contains highly organized type I collagen fibers (about 70% of dry weight), which becomes progressively richer in type II collagen fibers toward the inner AF and the central gelatinous NP (Eyre and Muir, 1977). The primary collagen in the NP is type II (about 20% of dry weight), while types VI, IX, and XI only occur in small amounts (Sive et al., 2002). The arrangement of collagen fibers within the disc is random, interspersing throughout the ECM environment (Inoue, 1981).
Proteoglycans constitute around 14% of the wet weight in the NP and about 5% of the wet weight in the AF (Raj, 2008). Unlike collagen that mainly contributes to the tensile strength of the disc, proteoglycans are the primary components resisting compression and providing resilience (Greenwald et al., 1978). In young discs, the major macromolecules are chondroitin sulfate A and C, which are strongly hydrophilic and promote disc viscosity (Freeman and Meachim, 1979); during the early 20s, however, these macromolecules start to break down into smaller molecules, such as chondroitin sulfate B and keratan sulfate, which bind less water (Holm et al., 1981). Of them, aggrecan is responsible for sustaining tissue hydration (Bogduk and Twomey, 1987; Johnstone and Bayliss, 1995) via osmotic pressure supplied by chondroitin and keratan sulfate chains (Urban et al., 1979). Despite expression of aggrecan and type II collagen in both chondrocytes and NP cells, the ratio of GAG to hydroxyproline was reported to be around 27:1 in young adult NP and about 2:1 in the AC (Mwale et al., 2004).
For glycoprotein, elastin by dry weight in nondegenerated human disc was 2% on average with no site dependent difference (Cloyd and Elliott, 2007). Elastic fibers, aligned with fibrillin-rich microfibrils in the disc (Yu et al., 2007), are important for the recovery of collagen fibers after deformation. Fibronectin plays a key role in matrix organization by interacting with integrins such as α5β1 on cell surfaces, as well as ECM compositions such as collagen, fibrin, and heparin sulfate proteoglycans (Hynes and Yamada, 1982). Lubricin found in both NP and AF is suggestive of its role in inter-lamellar tribology (Shine et al., 2009). As with the AC, three LPs have also been detected in human IVD (Mort et al., 1985; Tengblad et al., 1984). The largest, LP1, is the predominant form in immature discs, whereas the smallest form (LP3), a proteolytic cleaved product of LP1 and LP2 (Mort et al., 1985), is more abundant in mature discs (Pearce et al., 1989). ChM-I was detected in both the ECM and chondrocytes in the zone of hypertrophic cartilage, the zone of proliferative cartilage, and the zone of resting cartilage in human fetal discs as well as in the AF, NP, and CEP in human mature discs (Takao et al., 2000).
Collagen Network
Examining microenvironments of the meniscus, AC, and NP, it is important to understand how posttranslational modifications, enzyme activity during expansion, and changes in gene expression can alter the composition of the tissues produced. Since collagen meshwork plays an important role in the biomechanical characteristics of cartilage (Bastiaansen-Jenniskens et al., 2008), the collagen meshwork should be a focus in cartilage engineering and regeneration (Maroudas and Venn, 1977; Maroudas, 1976). Modifications in the collagen network involving hydroxypryidinoline, percent of hydroxylysine (Hyl), and pentosidine content are all important in determining the function of the tissue. Hydroxypryidinoline and Hyl are both more highly expressed in type II collagen fibers (Bank et al., 2002), showing a greater percentage in the AC than the meniscus and NP; these modifications are also involved in cross-linking collagen fibers together, increasing the mechanical integrity of the tissue (Bastiaansen-Jenniskens et al., 2008). Pentosidine, an advanced-glycation end-product (AGE) found on collagen fibers, allows for more collagen aggregates, but increases during aging and can result in stiff and brittle cartilage (Brama et al., 1999; Duance et al., 1998).
In alginate microbead-expanded cells from the meniscus, AC, and NP, levels of matrix synthesis and degradation proteins change, e.g. procollagenlysine 2-oxoglutarate 5-dioxygenase 3 (PLOD3), matrix metalloproteinase 13 (MMP13), serpin peptidase inhibitor, clade H (heat shock protein 47), member 1 (SERPINH1), and a disintegrin-like and metalloprotease (reprolysin type) with thrombospondin type 1 motif, 2 (ADAMTS2), from levels observed in native meniscus, AC, and NP tissues (Vonk et al., 2010). Vonk et al. (Vonk et al., 2010) measured genes including proteoglycans, collagens, and enzymes, in collagen synthesizing or degradation (16 genes are assessed in Table 4). Each meniscal, AC, and NP cell displays a specific mode of aggrecan (ACAN), biglycan (BGN), α1 (I) procollagen (COL1A1), and α1 (II) procollagen (COL2A1). Lysyl hydroxylation levels also changed during expansion of cells (Vonk et al., 2010). The results of this study indicate that expanding cells can drastically alter the characteristics of tissues produced when compared to the in vivo condition. Two specific examples include a 15–150 fold increase in MMP13 expression and decrease of lysyl hydroxylation within the meniscus, AC, and NP tissue (Bastiaansen-Jenniskens et al., 2008; Vonk et al., 2010). MMP13 is also known to increase in OA affected tissue (Kevorkian et al., 2004). Changes associated with type I and II collagen ratios, amount of remodeling present, and specific posttranslational modification in each group were all seen during cellular expansion (Vonk et al., 2010).
Table 4.
Relative expression levels of 16 genes in freshly isolated chondrocytes from the meniscus, AC, and NP.
| Gene | Meniscus | Articular Cartilage | Nucleus Pulposus |
|---|---|---|---|
| ACAN | b, c | a, c | a, b |
| BGN | b | a, c | b |
| COL1A1 | b | a, c | b |
| COL2A1 | - | - | - |
| SERPINH1 | - | - | - |
| PLOD1 | - | - | - |
| PLOD2 | b, c | a, c | a, b |
| PLOD3 | b | a, c | b |
| LOX | b, c | a, c | a |
| P4HA1 | b, c | a, c | b |
| P4HA2 | - | - | - |
| P4HA3 | - | - | - |
| ADAMTS2 | - | - | - |
| ADAMTS3 | b | a, c | b |
| MMP13 | - | - | - |
| MMP14 | - | - | - |
The expression levels of genes related to synthesis and degradation of the ECM normalized for three housekeeping genes (Vonk et al., 2010). Abbreviations: ACAN: aggrecan; BGN: biglycan; COL1A1: α1(I) procollagen; COL2A1: α1(II)procollagen; SERPINH1: serpin peptidase inhibitor, clade H (heat shock protein 47), member 1; PLOD1, 2, and 3: procollagenlysine 2-oxoglutarate 5-dioxygenase 1, 2, and 3; Lox: lysyl oxidase; P4HA1, 2, and 3: procollagen-proline, 2-oxoglutarate 4-dioxygenase (proline 4-hydroxylase), alpha polypeptide 1, 2, and 3; ADAMTS2, and 3: a disintegrin-like and metalloprotease (reprolysin type) with thrombospondin type 1 motif, 2, and 3; MMP13, and 14: matrix metallopeptidase 13, and 14.
Significantly different compared to meniscus
Significantly different compared to AC
Significantly different compared to NP
- Not significantly different compared to other cartilage types
Biomechanics and Function
Developing a functional tissue requires biomechanical stimulation that invokes proper cell-ECM signaling, gene activation, and ultimately, ECM remodeling. Because some functions of the meniscus (stability), AC (articulation), and NP (compression) are unique to their location, biomechanical stimulation needs to be specifically targeted for each tissue, involving regulating fluid flow mechanisms, ECM organization, and autocrine/paracrine signaling.
Meniscus
Elaborating on biomechanical properties is very important for acknowledging meniscus functionality in situ. The biomechanical function of the meniscus is determined by its fibrocartilaginous structures and semilunar shape as well as its relationship to the surrounding tissues, including load transmission (King, 1936) and load bearing functions (Fairbanks, 1948). Load transmission stems from the wedge shape of the meniscus, on which the hoop stresses from circumferentially oriented collagen fibers balance the shear force from radially oriented collagen fibers when a load is applied (Aspden et al., 1985; Shrive, 1974; Shrive et al., 1978); through this mechanism, the meniscus faces compressive, shear, and tensile forces. The posterior horns of the meniscus carry more load than the anterior horns though both connect to the tibial plateau by intertwining collagen fibers which convey forces from the meniscus to the tibial plateau (Gao et al., 1998). Either total or partial meniscectomy (Baratz et al., 1986) and subsequent malalignment of the joint would decrease the contact areas and increase the peak stresses in the knee joints (Bargar et al., 1980). The meniscus also functions to increase joint stability and congruity by virtue of its unique concave surface that can accommodate the convexity of the femoral condyles (Renström and Johnson, 1990; Walker and Erkman, 1975; Warren et al., 1986).
Articular Cartilage
In 1970, for the first time, Kempson et al. characterized the correlation between biochemical composition and mechanical parameters of human femoral head cartilage and found that the two-second creep modulus strongly correlated with chondroitin and keratan sulfates, but weakly correlated with collagen content, indicating that the compressive stiffness of human AC was mainly determined by both GAGs rather than by collagen (Kempson et al., 1970). The positive correlation between sulfated GAG and the equilibrium shear and equilibrium aggregate modulus was further confirmed by other research groups (Jurvelin et al., 1988; Treppo et al., 2000; Williamson et al., 2001). During maturation, external forces assist to regulate innate mechanical properties via matrix adjustment (Responte et al., 2012), such as compressive and shear strain, stress, hydrostatic pressure, and fluid flow, which are assigned to the anisotropic, zonal organization of AC matrix (Wong and Carter, 2003). A recent report suggested that mechanical motion induced PRG4 expression in the superficial zone of articular cartilage (Ogawa et al., 2014). Age-dependent and regional variation were found in the compressive and tensile properties of bovine fetal, newborn, and adult cartilage tissues (Williamson et al., 2003). Evidence indicates the physiological magnitude of stresses in AC (Hodge et al., 1989; Afoke et al., 1987), such as hydrostatic pressure and compression, varied from 3 to 10 MPa with a frequency of 1 Hz (Waters et al., 1988). In human AC, PCM has a significantly lower modulus than that of the ECM (Darling et al., 2010). PCM has an important influence on the stress-strain environment of the chondrocytes that potentially varies with the depth of AC (Alexopoulos et al., 2003). AC deformation under compressive loading is highly dependent on the relative mechanical properties of the chondrocytes, PCM, and ECM (Choi et al., 2007).
Nucleus Pulposus
As a critical factor for the flexibility and stability of the spine, NP mechanics is primarily dependent on compressive and shear stresses in vivo (Nerurkar et al., 2010). The main function of the NP is to absorb the loads acting on the spine and redistribute them radially to the inner layers of the AF and vertically to the cartilaginous endplates (Nixon, 1986; Pattappa et al., 2012). The swelling pressure in human discs was approximately 0.1–0.2 MPa in the supine position (Wilke et al., 2001) but increased up to 2.3 MPa when lifting a heavy weight (Wilke et al., 1999). In degenerated discs, the fragmentation of aggrecan increased but its effective negative charge decreased, resulting in a decrease of intradiscal pressure (Sato et al., 1999) and the ability to retain water under compressive forces (Lee et al., 2013), which led to a reduction of disc height (Iatridis et al., 2013; Vergroesen et al., 2014).
For all human spines tested, proteoglycan and collagen contents could be used to predict the correlation between equilibrium hydration and swelling pressure (Urban and McMullin, 1988). Proteoglycan content was reported with an age- and site-dependent decrease and was lowest in the L5-S1 disc (Urban and McMullin, 1988) and/or L4/L5 (Adams et al., 1996). Age-related degenerative changes were also found in a switch of size and pressure of the NP and AF; with a decrease of the diameter and pressure of the NP region, the width of the AF and the height of compressive “stress peaks” increased, indicating that anatomic changes within the AF and cartilaginous endplate led to a shift of load from the NP to AF (Adams et al., 1996).
Conclusions/Perspectives
This review highlights the similarities and differences between the meniscus, AC, and NP. The vascular, neural, and basic anatomy overview depicts the complex layering that each tissue possesses, ranging from levels of nutrient supply and oxygen distribution to sensitization and proprioception. Each tissue is a unique construct whose cellular ultrastructure and genetic expression further detail its functionality. The cellular composition, along with MSC populations which react distinctly under differentiation conditions, further validates the inconsistencies between tissues. Figure 2 also analyzes the ECM compositional changes between each tissue, showing changes in arrangement between the meniscus, AC, and NP, which are responsible for unique biomechanical stresses of each tissue varying from joint articulation, to gliding, to compression.
Despite the fundamental knowledge provided in this review paper for developing an ideal cartilage or cartilage-like tissue, there are critical and distinct molecular signaling pathways governing tissue regeneration. Regulation of signaling during development in the meniscus (TGFβ and insulin-like growth factor I) (Pazin et al., 2014), the AC (Wnt9A, GDF5, Erg, Gli3, CD44, type IIA collagen, and type I collagen) (Iwamoto et al., 2007; Koyama et al., 2008; Pacifici et al., 2006), and the NP [Brachyury (T), Shh, Nog, and TGFβ] (Chan et al., 2014) all provide insight toward achieving a tissue-specific cell population. Each of the tissues relies on a variety of disparate signaling pathways to achieve maturation; refinement of these pathways can better progress tissue engineering and regenerative medicine approaches. Research to produce better tissue constructs needs to involve a more adequate understanding of the surface expression of host progenitor cells, including CD34+/CD31− cells in the meniscus (Declercq et al., 2012), FBLN1, IBSP, CYTL1, and GDF10 expressing cells in the AC (Minogue et al., 2010a), and Tie2+ and GD2+ cells in the NP (Sakai et al., 2012). Biomechanical stimuli needed in vitro to sustain a tissue-specific, functional population need more defined parameters for each tissue type. While the inner and outer portions of the meniscal cells may respond to variable levels of hydrostatic and tensile strain (Spilker et al., 1992), articular chondrocytes need a specific balance of mechanical loading, potentially different for chondrocytes within any one of the four layers (Jortikka et al., 1997). Likewise, NP cells in culture should be evaluated on matrix synthesis and degradation protein expression correlating to induced pressure gradients (Millward-Sadler et al., 2004).
More serious concerns for tissue engineering are realized during cellular expansion (Vonk et al., 2010), thus understanding how to better regulate cellular responses to in vitro stresses is crucial in directing cells toward a specific tissue. Compared to two-dimensional conventional culture, decellularized extracellular matrix (dECM) deposited by stem cells is a three-dimensional nanofibrous scaffold that may alleviate problems of cell senescence during ex vivo expansion (Pei et al., 2011b). Using synovium-derived stem cells (SDSCs) to deposit a dECM, it has been demonstrated that SDSC expansion on this substrate increases cell proliferation and chondrogenic capacity (He et al., 2009); likewise, bone marrow-derived stem cells (BMSCs) as a donor cell for a dECM can increase BMSC proliferation and osteogenic differentiation capacity during expansion (Pei et al., 2011a), indicating that a tissue-specific stem cell might provide a unique microenvironment for a lineage-specific tissue regeneration (Pizzute et al., 2015). For example, SDSCs are tissue-specific stem cells (Jones and Pei, 2012) and currently available research suggests that SDSCs may mimic the regulatory role of notocordal cells for NP regeneration (Shoukry et al., 2013), which might explain how dECM from SDSCs promotes NP rejuvenation (He and Pei, 2012; Pei et al., 2012).
This review hopes to encourage regenerative medicine research through presenting the differences between each tissue, but also explaining levels of commonality that may be utilized for future tissue engineering. The goal is to provide clarity in creating meniscus, AC, and NP tissue that can be produced, not only in high quantity, but also with high biomechanical and functional quality.
Acknowledgments
We thank Suzanne Danley for editing the manuscript and Quincy Hathaway for valuable comments and revision. This project was partially supported by Research Grants from the Musculoskeletal Transplant Foundation and the National Institutes of Health (R03AR062763-01A1, R01AR067747-01A1) (to M.P.), Natural Science Foundation of Shanghai City, China (15ZR1414000, to P.F.), and Natural Science Foundation of China (81601889, to S.C.).
Footnotes
Author Disclosure Statement
No competing financial interests exist.
Contributor Information
Song Chen, Stem Cell and Tissue Engineering Laboratory, Department of Orthopaedics and Division of Exercise Physiology, West Virginia University, Morgantown, WV 26506-9196, USA. Department of Orthopaedics, Changzheng Hospital, Second Military Medical University, Shanghai 200003, People’s Republic of China.
Peiliang Fu, Department of Orthopaedics, Changzheng Hospital, Second Military Medical University, Shanghai 200003, People’s Republic of China.
Haishan Wu, Department of Orthopaedics, Changzheng Hospital, Second Military Medical University, Shanghai 200003, People’s Republic of China.
Ming Pei, Stem Cell and Tissue Engineering Laboratory, Department of Orthopaedics and Division of Exercise Physiology, West Virginia University, Morgantown, WV 26506-9196, USA.
References
- Adams MA, McNally DS, Dolan P. ‘Stress’ distributions inside interbertebral discs. The effects of age and degeneration. J Bone Joint Surg [Br] 1996;78:965–972. doi: 10.1302/0301-620x78b6.1287. [DOI] [PubMed] [Google Scholar]
- Afoke NY, Byers PD, Hutton WC. Contact pressures in the human hip joint. J Bone Joint Surg [Br] 1987;69:536–541. doi: 10.1302/0301-620X.69B4.3611154. [DOI] [PubMed] [Google Scholar]
- Aigner T, Gebhard PM, Schmid E, Bau B, Harley V, Poschl E. SOX9 expression does not correlate with type II collagen expression in adult articular chondrocytes. Matrix Biol. 2003;22:363–372. doi: 10.1016/s0945-053x(03)00049-0. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Guttapalli A, Narayan S, Narayan S, Albert TJ, Shapiro IM, Risbud MV. Normoxic stabilization of HIF-1alpha drives glycolytic metabolism and regulates aggrecan gene expression in nucleus pulposus cells of the rat intervertebral disk. Am J Physiol Cell Physiol. 2007;293:C621–631. doi: 10.1152/ajpcell.00538.2006. [DOI] [PubMed] [Google Scholar]
- Alexopoulos LG, Haider MA, Vail TP, Guilak F. Alterations in the mechanical properties of the human chondrocyte pericellular matrix with osteoarthritis. J Biomech Eng. 2003;125:323–333. doi: 10.1115/1.1579047. [DOI] [PubMed] [Google Scholar]
- arcOGEN Consortium.; arcOGEN Collaborators. Zeggini E, Panoutsopoulou K, Southam L, Rayner NW, Day-Williams AG, Lopes MC, Boraska V, Esko T, Evangelou E, Hoffman A, Houwing-Duistermaat JJ, Ingvarsson T, Jonsdottir I, Jonnson H, Kerkhof HJ, Kloppenburg M, Bos SD, Mangino M, Metrustry S, Slagboom PE, Thorleifsson G, Raine EV, Ratnayake M, Ricketts M, Beazley C, Blackburn H, Bumpstead S, Elliott KS, Hunt SE, Potter SC, Shin SY, Yadav VK, Zhai G, Sherburn K, Dixon K, Arden E, Aslam N, Battley PK, Carluke I, Doherty S, Gordon A, Joseph J, Keen R, Koller NC, Mitchell S, O’Neill F, Paling E, Reed MR, Rivadeneira F, Swift D, Walker K, Watkins B, Wheeler M, Birrell F, Ioannidis JP, Meulenbelt I, Metspalu A, Rai A, Salter D, Stefansson K, Stykarsdottir U, Uitterlinden AG, van Meurs JB, Chapman K, Deloukas P, Ollier WE, Wallis GA, Arden N, Carr A, Doherty M, McCaskie A, Willkinson JM, Ralston SH, Valdes AM, Spector TD, Loughlin J. Identification of new susceptibility loci for osteoarthritis (arcOGEN): a genome-wide association study. Lancet. 2012;380:815–823. doi: 10.1016/S0140-6736(12)60681-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer CW, Dowthwaite GP, Francis-West P. Development of synovial joints. Birth Defects Res C Embryo Today. 2003;69:144–155. doi: 10.1002/bdrc.10015. [DOI] [PubMed] [Google Scholar]
- Aspden RM, Yarker YE, Hukins DW. Collagen orientations in the meniscus of the knee joint. J Anat. 1985;140:371–380. [PMC free article] [PubMed] [Google Scholar]
- Bank RA, Verzijl N, Lafeber FP, Tekoppele JM. Putative role of lysyl hydroxylation and pyridinoline cross-linking during adolescence in the occurrence of osteoarthritis at old age. Osteoarthritis Cartilage. 2002;10:127–134. doi: 10.1053/joca.2001.0487. [DOI] [PubMed] [Google Scholar]
- Baratz ME, Fu FH, Mengato R. Meniscal tears: The effect of meniscectomy and of repair on intra-articular contact areas and stress in the human knee. Am J Sports Med. 1986;14:270–275. doi: 10.1177/036354658601400405. [DOI] [PubMed] [Google Scholar]
- Bargar WL, Moreland JR, Markolf KL, Shoemaker SC, Amstutz HC, Grant TT. In vivo stability testing of post-meniscectomy knees. Clin Orthop Relat Res. 1980;150:247–252. [PubMed] [Google Scholar]
- Bastiaansen-Jenniskens YM, Koevoet W, de Bart AC, van der Linden JC, Zuurmond AM, Weinans H, Verhaar JA, van Osch GJ, Degroot J. Contribution of collagen network features to functional properties of engineered cartilage. Osteoarthritis Cartilage. 2008;16:359–366. doi: 10.1016/j.joca.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Becerra J, Andrades JA, Guerado E, Zamora-Navas P, Lopez-Puertas JM, Reddi AH. Articular cartilage: structure and regeneration. Tissue Eng Part B Rev. 2010;16:617–627. doi: 10.1089/ten.TEB.2010.0191. [DOI] [PubMed] [Google Scholar]
- Blanco JF, Graciani IF, Sanchez-Guijo FM, Muntion S, Hernandez-Campo P, Santamaria C, Carrancio S, Barbado MV, Cruz G, Gutierrez-Cosio S, Herrero C, San Miguel JF, Brinon JG, del Canizo MC. Isolation and characterization of mesenchymal stromal cells from human degenerated nucleus pulposus: comparison with bone marrow mesenchymal stromal cells from the same subjects. Spine (Phila Pa 1976) 2010;35:2259–2265. doi: 10.1097/BRS.0b013e3181cb8828. [DOI] [PubMed] [Google Scholar]
- Bogduk N, Twomey LT. Clinical anatomy of the lumbar spine. 1. New York, NY: Churchill Livingstone Inc; 1987. pp. 130–138. [Google Scholar]
- Brama PA, Tekoppele JM, Bank RA, van Weeren PR, Barneveld A. Influence of different exercise levels and age on the biochemical characteristics of immature equine articular cartilage. Equine Vet J Suppl. 1999;31:55–61. doi: 10.1111/j.2042-3306.1999.tb05314.x. [DOI] [PubMed] [Google Scholar]
- Buckwalter JA, Mankin HJ. Articular cartilage: tissue design and chondrocyte-matrix interactions. Instr Course Lect. 1998;47:477–486. [PubMed] [Google Scholar]
- Buckwalter JA. Articular cartilage. Instr Course Lect. 1983;32:349–370. [PubMed] [Google Scholar]
- Cai D, Marty-Roix R, Hsu HP, Spector M. Lapine and canine bone marrow stromal cells contain smooth muscle actin and contract a collagen-glycosaminoglycan matrix. Tissue Eng. 2001;7:829–841. doi: 10.1089/107632701753337762. [DOI] [PubMed] [Google Scholar]
- Chan WC, Au TY, Tam V, Cheah KS, Chan D. Coming together is a beginning: the making of an intervertebral disc. Birth Defects Res C Embryo Today. 2014;102:83–100. doi: 10.1002/bdrc.21061. [DOI] [PubMed] [Google Scholar]
- Chen J, Jing L, Gilchrist CL, Richardson WJ, Fitch RD, Setton LA. Expression of laminin isoforms, receptors, and binding proteins unique to nucleus pulposus cells of immature intervertebral disc. Connect Tissue Res. 2009;50:294–306. [PMC free article] [PubMed] [Google Scholar]
- Chen S, Fu P, Cong R, Wu H, Pei M. to minimize hypertrophy in cartilage engineering and regeneration. Gene Dis. 2015;2:76–95. doi: 10.1016/j.gendis.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung HS. Distribution of type I, II, III and V in the pepsin solubilized collagens in bovine menisci. Connect Tissue Res. 1987;16:343–356. doi: 10.3109/03008208709005619. [DOI] [PubMed] [Google Scholar]
- Choi H, Johnson ZI, Risbud MV. Understanding nucleus pulposus cell phenotype: a prerequisite for stem cell based therapies to treat intervertebral disc degeneration. Curr Stem Cell Res Ther. 2015;10:307–316. doi: 10.2174/1574888x10666150113112149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JB, Youn I, Cao L, Leddy HA, Gilchrist CL, Setton LA, Guilak F. Zonal changes in the three-dimensional morphology of the chondron under compression: the relationship among cellular, pericellular, and extracellular deformation in articular cartilage. J Biomech. 2007;40:2596–2603. doi: 10.1016/j.jbiomech.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CR, Ogden JA. Development of the menisci of the human knee joint. Morphological changes and their potential role in childhood meniscal injury. J Bone Joint Surg [Am] 1983;65:538–547. [PubMed] [Google Scholar]
- Clouet J, Grimandi G, Pot-Vaucel M, Masson M, Fellah HB, Guigand L, Cherel Y, Bord E, Rannou F, Weiss P, Guicheux J, Vinatier C. Identification of phenotypic discriminating markers for intervertebral disc cells and articular chondrocytes. Rheumatology (Oxford) 2009;48:1447–1450. doi: 10.1093/rheumatology/kep262. [DOI] [PubMed] [Google Scholar]
- Cloyd JM, Elliott DM. Elastin content correlates with human disc degeneration in the anulus fibrosus and nucleus pulposus. Spine (Phila Pa 1976) 2007;32:1826–1831. doi: 10.1097/BRS.0b013e3181132a9d. [DOI] [PubMed] [Google Scholar]
- Cornejo MC, Cho SK, Giannarelli C, Iatridis JC, Purmessur D. Soluble factors from the notochordal-rich intervertebral disc inhibit endothelial cell invasion and vessel formation in the presence and absence of pro-inflammatory cytokines. Osteoarthritis Cartilage. 2015;23:487–496. doi: 10.1016/j.joca.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crock HV, Goldwasser M, Yoshizawa H. Vascular anatomy related to the intervertebral disc. In: Ghosh P, editor. Biology of the intervertebral disc. CRC Press; Boca Raton, FL, USA: 1988. pp. 109–133. [Google Scholar]
- Danzig L, Resnick D, Gonsalves M, Akeson WH. Blood supply to the normal and abnormal menisci of the human knee. Clin Orthop Relat Res. 1983;172:271–276. [PubMed] [Google Scholar]
- Darling EM, Wilusz RE, Bolognesi MP, Zauscher S, Guilak F. Spatial mapping of the biomechanical properties of the pericellular matrix of articular cartilage measured in situ via atomic force microscopy. Biophys J. 2010;98:2848–2856. doi: 10.1016/j.bpj.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Declercq HA, Forsyth RG, Verbruggen A, Verdonk R, Cornelissen MJ, Verdonk PC. CD34 and SMA expression of superficial zone cells in the normal and pathological human meniscus. J Orthop Res. 2012;30:800–808. doi: 10.1002/jor.21582. [DOI] [PubMed] [Google Scholar]
- Donohue PJ, Jahnke MR, Blaha JD, Caterson B. Characterization of link protein(s) from human intervertebral disc tissues. Biochem J. 1988;251:739–747. doi: 10.1042/bj2510739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowthwaite GP, Bishop JC, Redman SN, Khan IM, Rooney P, Evans DJ, Haughton L, Bayram Z, Boyer S, Thomson B, Wolfe MS, Archer CW. The surface of articular cartilage contains a progenitor cell population. J Cell Sci. 2004;117:889–897. doi: 10.1242/jcs.00912. [DOI] [PubMed] [Google Scholar]
- Duance VC, Crean JK, Sims TJ, Avery N, Smith S, Menage J, Eisenstein SM, Roberts S. Changes in collagen cross-linking in degenerative disc disease and scoliosis. Spine (Phila Pa 1976) 1998;23:2545–2551. doi: 10.1097/00007632-199812010-00009. [DOI] [PubMed] [Google Scholar]
- Durr J, Lammi P, Goodman SL, Aigner T, von der Mark K. Identification and immunolocalization of laminin in cartilage. Exp Cell Res. 1996;222:225–233. doi: 10.1006/excr.1996.0028. [DOI] [PubMed] [Google Scholar]
- Erwin WM, Ashman K, O’Donnel P, Inman RD. Nucleus pulposus notochord cells secrete connective tissue growth factor and up-regulate proteoglycan expression by intervertebral disc chondrocytes. Arthritis Rheum. 2006;54:3859–3867. doi: 10.1002/art.22258. [DOI] [PubMed] [Google Scholar]
- Erwin WM, Islam D, Inman RD, Fehlings MG, Tsui FW. Notochordal cells protect nucleus pulposus cells from degradation and apoptosis: implications for the mechanisms of intervertebral disc degeneration. Arthritis Res Ther. 2011;13:R215. doi: 10.1186/ar3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre DR, Brickley-Parsons DM, Glimcher MJ. Predominance of type I collagen at the surface of avian articular cartilage. FEBS Lett. 1978;85:259–263. doi: 10.1016/0014-5793(78)80468-2. [DOI] [PubMed] [Google Scholar]
- Eyre DR, Muir H. Quantitative analysis of types I and II collagens in human intervertebral discs at various ages. Biochim Biophys Acta. 1977;492:29–42. doi: 10.1016/0005-2795(77)90211-2. [DOI] [PubMed] [Google Scholar]
- Eyre DR, Wu JJ. Collagen of fibrocartilage: a distinctive molecular phenotype in bovine meniscus. FEBS Lett. 1983;158:265–270. doi: 10.1016/0014-5793(83)80592-4. [DOI] [PubMed] [Google Scholar]
- Fairbanks TJ. Knee joint changes after meniscectomy. J Bone Joint Surg [Br] 1948;30:664–670. [PubMed] [Google Scholar]
- Fife RS. Identification of link proteins and a 116,000-Dalton matrix protein in canine meniscus. Arch Biochem Biophys. 1985;240:682–688. doi: 10.1016/0003-9861(85)90076-1. [DOI] [PubMed] [Google Scholar]
- Fithian DC, Kelly MA, Mow VC. Material properties and structure-function relationships in the menisci. Clin Orthop Relat Res. 1990;252:19–31. [PubMed] [Google Scholar]
- Fox AJ, Bedi A, Rodeo SA. The basic science of human knee menisci: structure, composition, and function. Sports Health. 2012;4:340–351. doi: 10.1177/1941738111429419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AJ, Wanivenhaus F, Burge AJ, Warren RF, Rodeo SA. The human meniscus: A review of anatomy, function, injury, and advances in treatment. Clin Anat. 2015;28:269–287. doi: 10.1002/ca.22456. [DOI] [PubMed] [Google Scholar]
- Frank RM, Cole BJ. Complex cartilage cases in the athletic patient: advances in malalignment, instability, articular defects, and meniscal insufficiency. Phys Sportsmed. 2013;41:41–52. doi: 10.3810/psm.2013.11.2035. [DOI] [PubMed] [Google Scholar]
- Freeman MAR, Meachim G. Ageing and degeneration. In: Freeman MAR, editor. Adult articular cartilage. 2. London: Pitman; 1979. pp. 487–543. [Google Scholar]
- Fujii M, Furumatsu T, Yokoyama Y, Kanazawa T, Kajiki Y, Abe N, Ozaki T. Chondromodulin-I derived from the inner meniscus prevents endothelial cell proliferation. J Orthop Res. 2013;31:538–543. doi: 10.1002/jor.22257. [DOI] [PubMed] [Google Scholar]
- Fujita N, Miyamoto T, Imai J, Hosogane N, Suzuki T, Yagi M, Morita K, Ninomiya K, Miyamoto K, Takaishi H, Matsumoto M, Morioka H, Yabe H, Chiba K, Watanabe S, Toyama Y, Suda T. CD24 is expressed specifically in the nucleus pulposus of intervertebral discs. Biochem Biophys Res Commun. 2005;338:1890–1896. doi: 10.1016/j.bbrc.2005.10.166. [DOI] [PubMed] [Google Scholar]
- Gao J, Wei X, Messner K. Healing of the anterior attachment of the rabbit meniscus to bone. Clin Orthop Relat Res. 1998;348:246–258. [PubMed] [Google Scholar]
- Gardner E, O’Rahilly R. The early development of the knee joint in staged human embryos. J Anat. 1968;102:289–299. [PMC free article] [PubMed] [Google Scholar]
- Ghadially FN, Thomas I, Yong N, Lalonde JM. Ultrastructure of rabbit semilunar cartilages. J Anat. 1978;125:499–517. [PMC free article] [PubMed] [Google Scholar]
- Ghosh P, Ingman AM, Taylor TK. Variations in collagen, non-collagenous proteins, and hexosamine in menisci derived from osteoarthritic and rheumatoid arthritic knee joints. J Rheumatol. 1975;2:100–107. [PubMed] [Google Scholar]
- Ghosh P, Taylor TK. The knee joint meniscus. A fibrocartilage of some distinction. Clin Orthop Relat Res. 1987;224:52–63. [PubMed] [Google Scholar]
- Goldring SR. Pathogenesis of bone and cartilage destruction in rheumatoid arthritis. Rheumatology (Oxford) 2003;42(Suppl 2ii):11–16. doi: 10.1093/rheumatology/keg327. [DOI] [PubMed] [Google Scholar]
- Gorensek M, Jaksimovic C, Kregar-Velikonja N, Gorensek M, Knezevic M, Jeras M, Pavlovcic V, Cor A. pulposus repair with cultured autologous elastic cartilage derived chondrocytes. Cell Mol Biol Lett. 2004;9:363–373. [PubMed] [Google Scholar]
- Gouttenoire J, Valcourt U, Ronziere MC, Aubert-Foucher E, Mallein-Gerin F, Herbage D. Modulation of collagen synthesis in normal and osteoarthritic cartilage. Biorheology. 2004;41:535–542. [PubMed] [Google Scholar]
- Greenwald RA, Moy WW, Seibold J. Functional properties of cartilage proteoglycans. Semin Arthritis Rheum. 1978;8:53–67. doi: 10.1016/0049-0172(78)90034-3. [DOI] [PubMed] [Google Scholar]
- Hattori S, Oxford C, Reddi AH. Identification of superficial zone articular chondrocyte stem/progenitor cells. Biochem Biophys Res Commun. 2007;358:99–103. doi: 10.1016/j.bbrc.2007.04.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay ED. The mesenchymal cell, its role in the embryo, and the remarkable signaling mechanisms that create it. Dev Dyn. 2005;233:706–720. doi: 10.1002/dvdy.20345. [DOI] [PubMed] [Google Scholar]
- Hayes AJ, Benjamin M, Ralphs JR. Extracellular matrix in development of the intervertebral disc. Matrix Biol. 2001;20:107–121. doi: 10.1016/s0945-053x(01)00125-1. [DOI] [PubMed] [Google Scholar]
- Hayes AJ, Isaacs MD, Hughes C, Caterson B, Ralphs JR. Collagen fibrillogenesis in the development of the annulus fibrosus of the intervertebral disc. Eur Cell Mater. 2011;22:226–241. doi: 10.22203/ecm.v022a18. [DOI] [PubMed] [Google Scholar]
- He F, Chen X, Pei M. Reconstruction of an in vitro tissue-specific microenvironment to rejuvenate synovium-derived stem cells for cartilage tissue engineering. Tissue Eng Part A. 2009;15:3809–3821. doi: 10.1089/ten.TEA.2009.0188. [DOI] [PubMed] [Google Scholar]
- He F, Pei M. Rejuvenation of nucleus pulposus cells using extracellular matrix deposited by synovium-derived stem cells. Spine (Phila Pa 1976) 2012;37:459–469. doi: 10.1097/BRS.0b013e31821fcc64. [DOI] [PubMed] [Google Scholar]
- Heinegard D, Oldberg A. Structure and biology of cartilage and bone matrix noncollagenous macromolecules. FASEB J. 1989;3:2042–2051. doi: 10.1096/fasebj.3.9.2663581. [DOI] [PubMed] [Google Scholar]
- Herwig J, Egner E, Buddecke E. Chemical changes of human knee joint menisci in various stages of degeneration. Ann Rheum Dis. 1984;43:635–640. doi: 10.1136/ard.43.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraki Y, Inoue H, Iyama K, Kamizono A, Ochiai M, Shukunami C, Iijima S, Suzuki F, Kondo J. Identification of chondromodulin I as a novel endothelial cell growth inhibitor. Purification and its localization in the avascular zone of epiphyseal cartilage. J Biol Chem. 1997;272:32419–32426. doi: 10.1074/jbc.272.51.32419. [DOI] [PubMed] [Google Scholar]
- Hodge WA, Carlson KL, Fijan RS, Burgess RG, Riley PO, Harris WH, Mann RW. Contact pressures from an instrumented hip endoprosthesis. J Bone Joint Surg [Am] 1989;71:1378–1386. [PubMed] [Google Scholar]
- Holm S, Maroudas A, Urban JP, Selstam G, Nachemson A. Nutrition of the intervertebral disc: solute transport and metabolism. Connect Tissue Res. 1981;8:101–119. doi: 10.3109/03008208109152130. [DOI] [PubMed] [Google Scholar]
- Höpker WW, Angres G, Klingel K, Komitowski D, Schuchardt E. Changes of the elastin compartment in the human meniscus. Virchows Arch A Pathol Anat Histopathol. 1986;408:575–592. doi: 10.1007/BF00705337. [DOI] [PubMed] [Google Scholar]
- Hunter CJ, Matyas JR, Duncan NA. The notochordal cell in the nucleus pulposus: a review in the context of tissue engineering. Tissue Eng. 2003;9:667–677. doi: 10.1089/107632703768247368. [DOI] [PubMed] [Google Scholar]
- Hunter CJ, Matyas JR, Duncan NA. The functional significance of cell clusters in the notochordal nucleus pulposus: survival and signaling in the canine intervertebral disc. Spine (Phila Pa 1976) 2004;29:1099–1104. doi: 10.1097/00007632-200405150-00010. [DOI] [PubMed] [Google Scholar]
- Hunziker EB, Kapfinger E, Geiss J. The structural architecture of adult mammalian articular cartilage evolves by a synchronized process of tissue resorption and neoformation during postnatal development. Osteoarthritis Cartilage. 2007;15:403–413. doi: 10.1016/j.joca.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10:432–463. doi: 10.1053/joca.2002.0801. [DOI] [PubMed] [Google Scholar]
- Hunziker EB. The structure of articular cartilage. In: Archer C, Ralphs J, editors. Regenerative Medicine and Biomaterials for the Repair of Connective Tissues. Woodhead Publishing; Sawston, Cambridge, UK: 2010. pp. 83–105. [Google Scholar]
- Hynes RO, Yamada KM. Fibronectins: multifunctional modular glycoproteins. J Cell Biol. 1982;95:369–377. doi: 10.1083/jcb.95.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inkinen RI, Lammi MJ, Lehmonen S, Puustjarvi K, Kaapa E, Tammi MI. Relative increase of biglycan and decorin and altered chondroitin sulfate epitopes in the degenerating human intervertebral disc. J Rheumatol. 1998;25:506–514. [PubMed] [Google Scholar]
- Iatridis JC, Nicoll SB, Michalek AJ, Walter BA, Gupta MS. Role of biomechanics in intervertebral disc degeneration and regenerative therapies: what needs repairing in the disc and what are promising biomaterials for its repair? Spine J. 2013;13:243–262. doi: 10.1016/j.spinee.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda T, Kamekura S, Mabuchi A, Kou I, Seki S, Takato T, Nakamura K, Kawaguchi H, Ikegawa S, Chung UI. The combination of SOX5, SOX6, and SOX9 (the SOX trio) provides signals sufficient for induction of permanent cartilage. Arthritis Rheum. 2004;50:3561–3573. doi: 10.1002/art.20611. [DOI] [PubMed] [Google Scholar]
- Inoue H. Three-dimensional architecture of lumbar intervertebral discs. Spine (Phila Pa 1976) 1981;6:139–146. doi: 10.1097/00007632-198103000-00006. [DOI] [PubMed] [Google Scholar]
- Iwamoto M, Tamamura Y, Koyama E, Komori T, Takeshita N, Williams JA, Nakamura T, Enomoto-Iwamoto M, Pacifici M. Transcription factor ERG and joint and articular cartilage formation during mouse limb and spine skeletogenesis. Dev Biol. 2007;305:40–51. doi: 10.1016/j.ydbio.2007.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AR. Notochordal Nucleus Pulposus Cells: Prospective Strategies for Intervertebral Disc Repair and Regeneration. Curr Tissue Eng. 2015;4:77–85. [Google Scholar]
- Johnson EF, Chetty K, Moore IM, Stewart A, Jones W. The distribution and arrangement of elastic fibres in the intervertebral disc of the adult human. J Anat. 1982;135:301–309. [PMC free article] [PubMed] [Google Scholar]
- Johnson WE, Evans H, Menage J, Eisenstein SM, El Haj A, Roberts S. Immunohistochemical detection of Schwann cells in innervated and vascularized human intervertebral discs. Spine (Phila Pa 1976) 2001;26:2550–2557. doi: 10.1097/00007632-200112010-00007. [DOI] [PubMed] [Google Scholar]
- Johnstone B, Bayliss MT. The large proteoglycans of the human intervertebral disc. Changes in their biosynthesis and structure with age, topography, and pathology. Spine (Phila Pa 1976) 1995;20:674–684. doi: 10.1097/00007632-199503150-00008. [DOI] [PubMed] [Google Scholar]
- Jones BA, Pei M. Synovium-derived stem cells: a tissue-specific stem cell for cartilage engineering and regeneration. Tissue Eng Part B Rev. 2012;18:301–311. doi: 10.1089/ten.TEB.2012.0002. [DOI] [PubMed] [Google Scholar]
- Jortikka MO, Inkinen RI, Tammi MI, Parkkinen JJ, Haapala J, Kiviranta I, Helminen HJ, Lammi MJ. Immobilisation causes longlasting matrix changes both in the immobilised and contralateral joint cartilage. Ann Rheum Dis. 1997;56:255–261. doi: 10.1136/ard.56.4.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurvelin J, Saamanen AM, Arokoski J, Helminen HJ, Kiviranta I, Tammi M. Biomechanical properties of the canine knee articular cartilage as related to matrix proteoglycans and collagen. Eng Med. 1988;17:157–162. doi: 10.1243/emed_jour_1988_017_042_02. [DOI] [PubMed] [Google Scholar]
- Kambic HE, Futani H, McDevitt CA. Cell, matrix changes and alpha-smooth muscle actin expression in repair of the canine meniscus. Wound Repair Regen. 2000;8:554–561. doi: 10.1046/j.1524-475x.2000.00554.x. [DOI] [PubMed] [Google Scholar]
- Kempson GE, Muir H, Swanson SA, Freeman MA. Correlations between stiffness and the chemical constituents of cartilage on the human femoral head. Biochim Biophys Acta. 1970;215:70–77. doi: 10.1016/0304-4165(70)90388-0. [DOI] [PubMed] [Google Scholar]
- Kennedy JC, Alexander IJ, Hayes KC. Nerve supply of the human knee and its functional importance. Am J Sports Med. 1982;10:329–335. doi: 10.1177/036354658201000601. [DOI] [PubMed] [Google Scholar]
- Kevorkian L, Young DA, Darrah C, Donell ST, Shepstone L, Porter S, Brockbank SM, Edwards DR, Parker AE, Clark IM. Expression profiling of metalloproteinases and their inhibitors in cartilage. Arthritis Rheum. 2004;50:131–141. doi: 10.1002/art.11433. [DOI] [PubMed] [Google Scholar]
- Khan IM, Salter DM, Bayliss MT, Thomson BM, Archer CW. Expression of clusterin in the superficial zone of bovine articular cartilage. Arthritis Rheum. 2001;44:1795–1799. doi: 10.1002/1529-0131(200108)44:8<1795::AID-ART316>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- King D. The function of semilunar cartilages. J Bone Joint Surg [Am] 1936;18:1069–1076. [Google Scholar]
- Kizawa H, Kou I, Iida A, Sudo A, Miyamoto Y, Fukuda A, Mabuchi A, Kotani A, Kawakami A, Yamamoto S, Uchida A, Nakamura K, Notoya K, Nakamura Y, Ikegawa S. An aspartic acid repeat polymorphism in asporin inhibits chondrogenesis and increases susceptibility to osteoarthritis. Nat Genet. 2005;37:138–144. doi: 10.1038/ng1496. [DOI] [PubMed] [Google Scholar]
- Kopher RA, Penchev VR, Islam MS, Hill KL, Khosla S, Kaufman DS. Human embryonic stem cell-derived CD34+ cells function as MSC progenitor cells. Bone. 2010;47:718–728. doi: 10.1016/j.bone.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama E, Shibukawa Y, Nagayama M, Sugito H, Young B, Yuasa T, Okabe T, Ochiai T, Kamiya N, Rountree RB, Kingsley DM, Iwamoto M, Enomoto-Iwamoto M, Pacifici M. A distinct cohort of progenitor cells participates in synovial joint and articular cartilage formation during mouse limb skeletogenesis. Dev Biol. 2008;316:62–73. doi: 10.1016/j.ydbio.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kypriotou M, Fossard-Demoor M, Chadjichristos C, Ghayor C, de Crombrugghe B, Pujol JP, Galera P. SOX9 exerts a bifunctional effect on type II collagen gene (COL2A1) expression in chondrocytes depending on the differentiation state. DNA Cell Biol. 2003;22:119–129. doi: 10.1089/104454903321515922. [DOI] [PubMed] [Google Scholar]
- Lee H-Y, Han L, Roughley PJ, Grodzinsky AJ, Ortiz C. Age-related nanostructural and nanomechanical changes of individual human cartilage aggrecan monomers and their glycosaminoglycan side chairs. J Struct Biol. 2013;181:264–273. doi: 10.1016/j.jsb.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre V, de Crombrugghe B. Toward understanding SOX9 function in chondrocyte differentiation. Matrix Biol. 1998;16:529–540. doi: 10.1016/s0945-053x(98)90065-8. [DOI] [PubMed] [Google Scholar]
- Lin BY, Richmond JC, Spector M. Contractile actin expression in torn human menisci. Wound Repair Regen. 2002;10:259–266. doi: 10.1046/j.1524-475x.2002.10410.x. [DOI] [PubMed] [Google Scholar]
- Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–1707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo P, Bayliss MT, Heinegard D. A novel cartilage protein (CILP) present in the mid-zone of human articular cartilage increases with age. J Biol Chem. 1998;273:23463–23468. doi: 10.1074/jbc.273.36.23463. [DOI] [PubMed] [Google Scholar]
- Maroudas A, Stockwell RA, Nachemson A, Urban J. Factors involved in the nutrition of the human lumbar intervertebral disc: cellularity and diffusion of glucose in vitro. J Anat. 1975;120:113–130. [PMC free article] [PubMed] [Google Scholar]
- Maroudas A, Venn M. Chemical composition and swelling of normal and osteoarthrotic femoral head cartilage. II. Swelling. Ann Rheum Dis. 1977;36:399–406. doi: 10.1136/ard.36.5.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroudas AI. Balance between swelling pressure and collagen tension in normal and degenerate cartilage. Nature. 1976;260:808–809. doi: 10.1038/260808a0. [DOI] [PubMed] [Google Scholar]
- McDevitt CA, Webber RJ. The ultrastructure and biochemistry of meniscal cartilage. Clin Orthop Relat Res. 1990;252:8–18. [PubMed] [Google Scholar]
- Meirer F, Pemmer B, Pepponi G, Zoeger N, Wobrauschek P, Sprio S, Tampieri A, Goettlicher J, Steininger R, Mangold S, Roschger P, Berzlanovich A, Hofstaetter JG, Streli C. Assessment of chemical species of lead accumulated in tidemarks of human articular cartilage by X-ray absorption near-edge structure analysis. J Synchrotron Radiat. 2011;18:238–244. doi: 10.1107/S0909049510052040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melrose J, Ghosh P, Taylor TK. A comparative analysis of the differential spatial and temporal distributions of the large (aggrecan, versican) and small (decorin, biglycan, fibromodulin) proteoglycans of the intervertebral disc. J Anat. 2001;198:3–15. doi: 10.1046/j.1469-7580.2001.19810003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melrose J, Smith S, Cake M, Read R, Whitelock J. Comparative spatial and temporal localisation of perlecan, aggrecan and type I, II and IV collagen in the ovine meniscus: an ageing study. Histochem Cell Biol. 2005;124:225–235. doi: 10.1007/s00418-005-0005-0. [DOI] [PubMed] [Google Scholar]
- Merida-Velasco JA, Sanchez-Montesinos I, Espin-Ferra J, Merida-Velasco JR, Rodriguez-Vazquez JF, Jimenez-Collado J. Development of the human knee joint ligaments. Anat Rec. 1997;248:259–268. doi: 10.1002/(SICI)1097-0185(199706)248:2<259::AID-AR13>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Mi M, Shi S, Li T, Holz J, Lee YJ, Sheu TJ, Liao Q, Xiao T. TIMP2 deficient mice develop accelerated osteoarthritis via promotion of angiogenesis upon destabilization of the medial meniscus. Biochem Biophys Res Comm. 2012;423:366–372. doi: 10.1016/j.bbrc.2012.05.132. [DOI] [PubMed] [Google Scholar]
- Millward-Sadler SJ, Wright MO, Flatman PW, Salter DM. ATP in the mechanotransduction pathway of normal human chondrocytes. Biorheology. 2004;41:567–575. [PubMed] [Google Scholar]
- Mine T, Kimura M, Sakka A, Kawai S. Innervation of nociceptors in the menisci of the knee joint: an immunohistochemical study. Arch Orthop Trauma Surg. 2000;120:201–204. doi: 10.1007/s004020050044. [DOI] [PubMed] [Google Scholar]
- Minogue BM, Richardson SM, Zeef LA, Freemont AJ, Hoyland JA. Characterization of the human nucleus pulposus cell phenotype and evaluation of novel marker gene expression to define adult stem cell differentiation. Arthritis Rheum. 2010a;62:3695–3705. doi: 10.1002/art.27710. [DOI] [PubMed] [Google Scholar]
- Minogue BM, Richardson SM, Zeef LA, Freemont AJ, Hoyland JA. Transcriptional profiling of bovine intervertebral disc cells: implications for identification of normal and degenerate human intervertebral disc cell phenotypes. Arthritis Res Ther. 2010b;12:R22. doi: 10.1186/ar2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mort JS, Caterson B, Poole AR, Roughley PJ. The origin of human cartilage proteoglycan link-protein heterogeneity and fragmentation during aging. Biochem J. 1985;232:805–812. doi: 10.1042/bj2320805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muinos-Lopez E, Rendal-Vazquez ME, Hermida-Gomez T, Fuentes-Boquete I, Diaz-Prado S, Blanco FJ. Cryopreservation effect on proliferative and chondrogenic potential of human chondrocytes isolated from superficial and deep cartilage. Open Orthop J. 2012;6:150–159. doi: 10.2174/1874325001206010150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musumeci G, Castrogiovanni P, Leonardi R, Trovato FM, Szychlinska MA, Di Giunta A, Loreto C, Castorina S. New perspectives for articular cartilage repair treatment through tissue engineering: A contemporary review. World J Orthop. 2014;5:80–88. doi: 10.5312/wjo.v5.i2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musumeci G, Trovato FM, Pichler K, Weinberg AM, Loreto C, Castrogiovanni P. Extra-virgin olive oil diet and mild physical activity prevent cartilage degeneration in an osteoarthritis model: an in vivo and in vitro study on lubricin expression. J Nutr Biochem. 2013;24:2064–2075. doi: 10.1016/j.jnutbio.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Mwale F, Roughley P, Antoniou J. Distinction between the extracellular matrix of the nucleus pulposus and hyaline cartilage: a requisite for tissue engineering of intervertebral disc. Eur Cell Mater. 2004;8:58–63. doi: 10.22203/ecm.v008a06. discussion 63–54. [DOI] [PubMed] [Google Scholar]
- Nakamichi Y, Shukunami C, Yamada T, Aihara K, Kawano H, Sato T, Nishizaki Y, Yamamoto Y, Shindo M, Yoshimura K, Nakamura T, Takahashi N, Kawaguchi H, Hiraki Y, Kato S. Chondromodulin I is a bone remodeling factor. Mol Cell Biol. 2003;23:636–644. doi: 10.1128/MCB.23.2.636-644.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata K, Shino K, Hamada M, Miyama T, Shinjo H, Horibe S, Tada K, Ochi T, Yoshikawa H. Human meniscus cell: characterization of the primary culture and use for tissue engineering. Clin Orthop Relat Res. 2001;391(Suppl):S208–218. [PubMed] [Google Scholar]
- Nakaya Y, Sheng G. Epithelial to mesenchymal transition during gastrulation: an embryological view. Dev Growth Differ. 2008;50:755–766. doi: 10.1111/j.1440-169X.2008.01070.x. [DOI] [PubMed] [Google Scholar]
- Nerurkar NL, Elliott DM, Mauck RL. Mechanical design criteria for intervertebral disc tissue engineering. J Biomech. 2010;43:1017–1030. doi: 10.1016/j.jbiomech.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon J. Intervetebral disc mechanics: a review. J R Soc Med. 1986;79:100–104. doi: 10.1177/014107688607900211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyes FR, Stabler CL. A system for grading articular cartilage lesions at arthroscopy. Am J Sports Med. 1989;17:505–513. doi: 10.1177/036354658901700410. [DOI] [PubMed] [Google Scholar]
- Ochi K, Daigo Y, Katagiri T, Saito-Hisaminato A, Tsunoda T, Toyama Y, Matsumoto H, Nakamura Y. Expression profiles of two types of human knee-joint cartilage. J Hum Genet. 2003;48:177–182. doi: 10.1007/s10038-003-0004-8. [DOI] [PubMed] [Google Scholar]
- O’Connor BL, McConnaughey JS. The structure and innervation of cat knee menisci, and their relation to a “sensory hypothesis” of meniscal function. Am J Anat. 1978;153:431–442. doi: 10.1002/aja.1001530306. [DOI] [PubMed] [Google Scholar]
- O’Connor BL. The mechanoreceptor innervation of the posterior attachments of the lateral meniscus of the dog knee joint. J Anat. 1984;138:15–26. [PMC free article] [PubMed] [Google Scholar]
- Ohshima H, Urban JP, Bergel DH. Effect of static load on matrix synthesis rates in the intervertebral disc measured in vitro by a new perfusion technique. J Orthop Res. 1995;13:22–29. doi: 10.1002/jor.1100130106. [DOI] [PubMed] [Google Scholar]
- Oldberg A, Antonsson P, Hedbom E, Heinegard D. Structure and function of extracellular matrix proteoglycans. Biochem Soc Trans. 1990;18:789–792. doi: 10.1042/bst0180789. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Kozhemyakina E, Hung HH, Grodzinsky AJ, Lassar AB. Mechanical motion promotes expression of Prg4 in articular cartilage via multiple CREB-dependent, fluid flow shear stress-induced signaling pathways. Genes Dev. 2014;28:127–139. doi: 10.1101/gad.231969.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacifici M, Koyama E, Shibukawa Y, Wu C, Tamamura Y, Enomoto-Iwamoto M, Iwamoto M. Cellular and molecular mechanisms of synovial joint and articular cartilage formation. Ann N Y Acad Sci. 2006;1068:74–86. doi: 10.1196/annals.1346.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra D, Sandell LJ. Antiangiogenic and anticancer molecules in cartilage. Expert Rev Mol Med. 2012;14:e10. doi: 10.1017/erm.2012.3. [DOI] [PubMed] [Google Scholar]
- Pattappa G, Li Z, Peroglio M, Wismer N, Alini M, Grad S. Diversity of intervertebral disc cells: phenotype and function. J Anat. 2012;221:480–496. doi: 10.1111/j.1469-7580.2012.01521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazin DE, Gamer LW, Capelo LP, Cox KA, Rosen V. Gene signature of the embryonic meniscus. J Orthop Res. 2014;32:46–53. doi: 10.1002/jor.22490. [DOI] [PubMed] [Google Scholar]
- Peacock A. Observations on the prenatal development of the intervertebral disc in man. J Anat. 1951;85:260–274. [PMC free article] [PubMed] [Google Scholar]
- Pearce RH, Mathieson JM, Mort JS, Roughley PJ. Effect of age on the abundance and fragmentation of link protein of the human intervertebral disc. J Orthop Res. 1989;7:861–867. doi: 10.1002/jor.1100070612. [DOI] [PubMed] [Google Scholar]
- Pearle AD, Warren RF, Rodeo SA. Basic science of articular cartilage and osteoarthritis. Clin Sports Med. 2005;24:1–12. doi: 10.1016/j.csm.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Pei M, He F, Kish VL. Expansion on extracellular matrix deposited by human bone marrow stromal cells facilitates stem cell proliferation and tissue-specific lineage potential. Tissue Eng Part A. 2011a;17:3067–3076. doi: 10.1089/ten.tea.2011.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei M, Li JT, Shoukry M, Zhang Y. A review of decellularized stem cell matrix: a novel cell expansion system for cartilage tissue engineering. Eur Cell Mater. 2011b;22:333–343. doi: 10.22203/ecm.v022a25. discussion 343. [DOI] [PubMed] [Google Scholar]
- Pei M, Seidel J, Vunjak-Novakovic G, Freed LE. Growth factors for sequential cellular de- and re-differentiation in tissue engineering. Biochem Biophys Res Commun. 2002;294:149–154. doi: 10.1016/S0006-291X(02)00439-4. [DOI] [PubMed] [Google Scholar]
- Pei M, Shoukry M, Li J, Daffner SD, France JC, Emery SE. Modulation of in vitro microenvironment facilitates synovium-derived stem cell-based nucleus pulposus tissue regeneration. Spine (Phila Pa 1976) 2012;37:1538–1547. doi: 10.1097/BRS.0b013e31825150bf. [DOI] [PubMed] [Google Scholar]
- Peters H, Wilm B, Sakai N, Imai K, Maas R, Balling R. Pax1 and Pax9 synergistically regulate vertebral column development. Development. 1999;126:5399–5408. doi: 10.1242/dev.126.23.5399. [DOI] [PubMed] [Google Scholar]
- Pizzute T, Lynch K, Pei M. Impact of tissue-specific stem cells on lineage-specific differentiation: a focus on the musculoskeletal system. Stem Cell Rev. 2015;11:119–132. doi: 10.1007/s12015-014-9546-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole AR, Kojima T, Yasuda T, Mwale F, Kobayashi M, Laverty S. Composition and structure of articular cartilage: a template for tissue repair. Clin Orthop Relat Res. 2001;391(Suppl):S26–33. doi: 10.1097/00003086-200110001-00004. [DOI] [PubMed] [Google Scholar]
- Poole CA. Articular cartilage chondrons: form, function and failure. J Anat. 1997;191:1–13. doi: 10.1046/j.1469-7580.1997.19110001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power KA, Grad S, Rutges JP, Creemers LB, van Rijen MH, O’Gaora P, Wall JG, Alini M, Pandit A, Gallagher WM. Identification of cell surface-specific markers to target human nucleus pulposus cells: expression of carbonic anhydrase XII varies with age and degeneration. Arthritis Rheum. 2011;63:3876–3886. doi: 10.1002/art.30607. [DOI] [PubMed] [Google Scholar]
- Proffen BL, McElfresh M, Fleming BC, Murray MM. A comparative anatomical study of the human knee and six animal species. Knee. 2012;19:493–499. doi: 10.1016/j.knee.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pufe T, Petersen WJ, Miosge N, Goldring MB, Mentlein R, Varoga DJ, Tillmann BN. Endostatin/collagen XVIII--an inhibitor of angiogenesis--is expressed in cartilage and fibrocartilage. Matrix Biol. 2004;23:267–276. doi: 10.1016/j.matbio.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Quinn TM, Häuselmann HJ, Shintani N, Hunziker EB. Cell and matrix morphology in articular cartilage from adult human knee and ankle joints suggests depth-associated adaptations to biomechanical and anatomical roles. Osteoarthritis Cartilage. 2013;21:1904–1912. [PubMed] [Google Scholar]
- Raj PP. Intervertebral disc: anatomy-physiology-pathophysiology-treatment. Pain Pract. 2008;8:18–44. doi: 10.1111/j.1533-2500.2007.00171.x. [DOI] [PubMed] [Google Scholar]
- Rajpurohit R, Risbud MV, Ducheyne P, Vresilovic EJ, Shapiro IM. Phenotypic characteristics of the nucleus pulposus: expression of hypoxia inducing factor-1, glucose transporter-1 and MMP-2. Cell Tissue Res. 2002;308:401–407. doi: 10.1007/s00441-002-0563-6. [DOI] [PubMed] [Google Scholar]
- Ray A, Singh PN, Sohaskey ML, Harland RM, Bandyopadhyay A. Precise spatial restriction of BMP signaling is essential for articular cartilage differentiation. Development. 2015;142:1169–1179. doi: 10.1242/dev.110940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renström P, Johnson RJ. Anatomy and biomechanics of the menisci. Clin Sports Med. 1990;9:523–538. [PubMed] [Google Scholar]
- Responte DJ, Lee JK, Hu JC, Athanasiou KA. Biomechanics-driven chondrogenesis: from embryo to adult. FASEB J. 2012;26:3614–3624. doi: 10.1096/fj.12-207241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson SM, Knowles R, Tyler J, Mobasheri A, Hoyland JA. Expression of glucose transporters GLUT-1, GLUT-3, GLUT-9 and HIF-1alpha in normal and degenerate human intervertebral disc. Histochem Cell Biol. 2008;129:503–511. doi: 10.1007/s00418-007-0372-9. [DOI] [PubMed] [Google Scholar]
- Risbud MV, Guttapalli A, Stokes DG, Hawkins D, Danielson KG, Schaer TP, Albert TJ, Shapiro IM. Nucleus pulposus cells express HIF-1 alpha under normoxic culture conditions: a metabolic adaptation to the intervertebral disc microenvironment. J Cell Biochem. 2006;98:152–159. doi: 10.1002/jcb.20765. [DOI] [PubMed] [Google Scholar]
- Risbud MV, Guttapalli A, Tsai TT, Lee JY, Danielson KG, Vaccaro AR, Albert TJ, Gazit Z, Gazit D, Shapiro IM. Evidence for skeletal progenitor cells in the degenerate human intervertebral disc. Spine (Phila Pa 1976) 2007;32:2537–2544. doi: 10.1097/BRS.0b013e318158dea6. [DOI] [PubMed] [Google Scholar]
- Risbud MV, Schoepflin ZR, Mwale F, Kandel RA, Grad S, Iatridis JC, Sakai D, Hoyland JA. Defining the phenotype of young healthy nucleus pulposus cells: recommendations of the Spine Research Interest Group at the 2014 annual ORS meeting. J Orthop Res. 2015;33:283–293. doi: 10.1002/jor.22789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts S, Eisenstein SM, Menage J, Evans EH, Ashton IK. Mechanoreceptors in intervertebral discs. Morphology, distribution, and neuropeptides. Spine (Phila Pa 1976) 1995;20:2645–2651. doi: 10.1097/00007632-199512150-00005. [DOI] [PubMed] [Google Scholar]
- Rodrigues-Pinto R, Richardson SM, Hoyland JA. An understanding of intervertebral disc development, maturation and cell phenotype provides clues to direct cell-based tissue regeneration therapies for disc degeneration. Eur Spine J. 2014;23:1803–1814. doi: 10.1007/s00586-014-3305-z. [DOI] [PubMed] [Google Scholar]
- Rutges J, Creemers LB, Dhert W, Milz S, Sakai D, Mochida J, Alini M, Grad S. Variations in gene and protein expression in human nucleus pulposus in comparison with annulus fibrosus and cartilage cells: potential associations with aging and degeneration. Osteoarthritis Cartilage. 2010;18:416–423. doi: 10.1016/j.joca.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Sakai D, Nakamura Y, Nakai T, Mishima T, Kato S, Grad S, Alini M, Risbud MV, Chan D, Cheah KS, Yamamura K, Masuda K, Okano H, Ando K, Mochida J. Exhaustion of nucleus pulposus progenitor cells with ageing and degeneration of the intervertebral disc. Nat Commun. 2012;3:1264. doi: 10.1038/ncomms2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Kikuchi S, Yonezawa T. In vivo intradiscal pressure measurement in healthy individuals and in patients with ongoing back problems. Spine (Phila Pa 1976) 1999;24:2468–2474. doi: 10.1097/00007632-199912010-00008. [DOI] [PubMed] [Google Scholar]
- Schmid TM, Linsenmayer TF. Immunohistochemical localization of short chain cartilage collagen (type X) in avian tissues. J Cell Biol. 1985;100:598–605. doi: 10.1083/jcb.100.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher BL, Block JA, Schmid TM, Aydelotte MB, Kuettner KE. A novel proteoglycan synthesized and secreted by chondrocytes of the superficial zone of articular cartilage. Arch Biochem Biophys. 1994;311:144–152. doi: 10.1006/abbi.1994.1219. [DOI] [PubMed] [Google Scholar]
- Shine KM, Simson JA, Spector M. Lubricin distribution in the human intervertebral disc. J Bone Joint Surg [Am] 2009;91:2205–2212. doi: 10.2106/JBJS.H.01344. [DOI] [PubMed] [Google Scholar]
- Shoukry M, Li J, Pei M. Reconstruction of an in vitro niche for the transition from intervertebral disc development to nucleus pulposus regeneration. Stem Cells Dev. 2013;22:1162–1176. doi: 10.1089/scd.2012.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrive N. The weight-bearing role of the menisci of the knee. J Bone J Joint [Br] 1974;56:381. [Google Scholar]
- Shrive NG, O’Connor JJ, Goodfellow JW. Load bearing in the knee joint. Clin Orthop Relat Res. 1978;131:279–287. [PubMed] [Google Scholar]
- Shwartz Y, Viukov S, Krief S, Zelzer E. Joint Development Involves a Continuous Influx of Gdf5-Positive Cells. Cell Rep. 2016;15:2577–2587. doi: 10.1016/j.celrep.2016.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sive JI, Baird P, Jeziorsk M, Watkins A, Hoyland JA, Freemont AJ. Expression of chondrocyte markers by cells of normal and degenerate intervertebral discs. Mol Pathol. 2002;55:91–97. doi: 10.1136/mp.55.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaggs DL, Warden WH, Mow VC. Radial tie fibers influence the tensile properties of the bovine medial meniscus. J Orthop Res. 1994;12:176–185. doi: 10.1002/jor.1100120205. [DOI] [PubMed] [Google Scholar]
- Smith LJ, Nerurkar NL, Choi KS, Harfe BD, Elliott DM. Degeneration and regeneration of the intervertebral disc: lessons from development. Dis Model Mech. 2011;4:31–41. doi: 10.1242/dmm.006403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spilker RL, Donzelli PS, Mow VC. A transversely isotropic biphasic finite element model of the meniscus. J Biomech. 1992;25:1027–1045. doi: 10.1016/0021-9290(92)90038-3. [DOI] [PubMed] [Google Scholar]
- Stockwell RA. The interrelationship of cell density and cartilage thickness in mammalian articular cartilage. J Anat. 1971;109:411–421. [PMC free article] [PubMed] [Google Scholar]
- Stosiek P, Kasper M, Karsten U. Expression of cytokeratin and vimentin in nucleus pulposus cells. Differentiation. 1988;39:78–81. doi: 10.1111/j.1432-0436.1988.tb00083.x. [DOI] [PubMed] [Google Scholar]
- Sun Z, Wan ZY, Guo YS, Wang HQ, Luo ZJ. FasL on human nucleus pulposus cells prevents angiogenesis in the disc by inducing Fas-mediated apoptosis of vascular endothelial cells. Int J Clin Exp Pathol. 2013;6:2376–2385. [PMC free article] [PubMed] [Google Scholar]
- Takao T, Iwaki T, Kondo J, Hiraki Y. Immunohistochemistry of chondromodulin-I in the human intervertebral discs with special reference to the degenerative changes. Histochem J. 2000;32:545–550. doi: 10.1023/a:1004150211097. [DOI] [PubMed] [Google Scholar]
- Tao Y, Zhou X, Liu D, Li H, Liang C, Li F, Chen Q. Proportion of collagen type II in the extracellular matrix promotes the differentiation of human adipose-derived mesenchymal stem cells into nucleus pulposus cells. Biofactors. 2016;42:212–223. doi: 10.1002/biof.1266. [DOI] [PubMed] [Google Scholar]
- Tengblad A, Pearce RH, Grimmer BJ. Demonstration of link protein in proteoglycan aggregates from human intervertebral disc. Biochem J. 1984;222:85–92. doi: 10.1042/bj2220085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Yuan W, Fujita N, Wang J, Wang H, Shapiro IM, Risbud MV. Inflammatory cytokines associated with degenerative disc disease control aggrecanase-1 (ADAMTS-4) expression in nucleus pulposus cells through MAPK and NF-kappaB. Am J Pathol. 2013;182:2310–2321. doi: 10.1016/j.ajpath.2013.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran CM, Fujita N, Huang BL, Ong JR, Lyons KM, Shapiro IM, Risbud MV. Hypoxia-inducible factor (HIF)-1alpha and CCN2 form a regulatory circuit in hypoxic nucleus pulposus cells: CCN2 suppresses HIF-1alpha level and transcriptional activity. J Biol Chem. 2013;288:12654–12666. doi: 10.1074/jbc.M112.448860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treppo S, Koepp H, Quan EC, Cole AA, Kuettner KE, Grodzinsky AJ. Comparison of biomechanical and biochemical properties of cartilage from human knee and ankle pairs. J Orthop Res. 2000;18:739–748. doi: 10.1002/jor.1100180510. [DOI] [PubMed] [Google Scholar]
- Upton ML, Chen J, Setton LA. Region-specific constitutive gene expression in the adult porcine meniscus. J Orthop Res. 2006;24:1562–1570. doi: 10.1002/jor.20146. [DOI] [PubMed] [Google Scholar]
- Urban JP, Maroudas A, Bayliss MT, Dillon J. Swelling pressures of proteoglycans at the concentrations found in cartilaginous tissues. Biorheology. 1979;16:447–464. doi: 10.3233/bir-1979-16609. [DOI] [PubMed] [Google Scholar]
- Urban JP, McMullin JF. Swelling pressures of the lumbar intervertebral discs: influence of age, spinal level, composition, and degeneration. Spine (Phila Pa 1976) 1988;13:179–187. doi: 10.1097/00007632-198802000-00009. [DOI] [PubMed] [Google Scholar]
- Urban JP, Roberts S. Development and degeneration of the intervertebral discs. Mol Med Today. 1995;1:329–335. doi: 10.1016/s1357-4310(95)80032-8. [DOI] [PubMed] [Google Scholar]
- Verdonk PC, Forsyth RG, Wang J, Almqvist KF, Verdonk R, Veys EM, Verbruggen G. Characterisation of human knee meniscus cell phenotype. Osteoarthritis Cartilage. 2005;13:548–560. doi: 10.1016/j.joca.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Vergroesen P-PA, van der Veen AJ, van Royen BJ, Kingma I, Smit TH. Intradiscal pressure depends on recent loading and correlates with disc height and compressive stiffness. Eur Spine J. 2014;23:2359–2368. doi: 10.1007/s00586-014-3450-4. [DOI] [PubMed] [Google Scholar]
- Vonk LA, Kroeze RJ, Doulabi BZ, Hoogendoorn RJ, Huang C, Helder MN, Everts V, Bank RA. Caprine articular, meniscus and intervertebral disc cartilage: an integral analysis of collagen network and chondrocytes. Matrix Biol. 2010;29:209–218. doi: 10.1016/j.matbio.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Walker PS, Erkman MJ. The role of the menisci in force transmission across the knee. Clin Orthop Relat Res. 1975;109:184–192. doi: 10.1097/00003086-197506000-00027. [DOI] [PubMed] [Google Scholar]
- Wang P, Zhang F, He Q, Wang J, Shiu HT, Shu Y, Tsang WP, Liang S, Zhao K, Wan C. Flavonoid Compound Icariin Activates Hypoxia Inducible Factor-1alpha in Chondrocytes and Promotes Articular Cartilage Repair. PloS one. 2016;11:e0148372. doi: 10.1371/journal.pone.0148372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren R, Arnoczky SP, Wickiewicz TL. Anatomy of the knee. In: Nicholas JA, Hershman EB, editors. The Lower Extremity and Spine in Sports Medicine. St. Louis, MO: Mosby; 1986. pp. 657–694. [Google Scholar]
- Waters RL, Lunsford BR, Perry J, Byrd R. Energy-speed relationship of walking: standard tables. J Orthop Res. 1988;6:215–222. doi: 10.1002/jor.1100060208. [DOI] [PubMed] [Google Scholar]
- Wilke HJ, Neef P, Caimi M, Hoogland T, Claes LE. New in vivo measurements of pressures in the intervertebral disc in daily life. Spine (Phila Pa 1976) 1999;24:755–762. doi: 10.1097/00007632-199904150-00005. [DOI] [PubMed] [Google Scholar]
- Wilke HJ, Neef P, Hinz B, Seidel H, Claes L. Intradiscal pressure together with anthropometric data – a data set for the volidation of models. Clin Biomech (Bristol, Avon) 2001;16(Suppl 1):S111–126. doi: 10.1016/s0268-0033(00)00103-0. [DOI] [PubMed] [Google Scholar]
- Williamson AK, Chen AC, Masuda K, Thonar EJ, Sah RL. Tensile mechanical properties of bovine articular cartilage: variations with growth and relationships to collagen network components. J Orthop Res. 2003;21:872–880. doi: 10.1016/S0736-0266(03)00030-5. [DOI] [PubMed] [Google Scholar]
- Williamson AK, Chen AC, Sah RL. Compressive properties and function-composition relationships of developing bovine articular cartilage. J Orthop Res. 2001;19:1113–1121. doi: 10.1016/S0736-0266(01)00052-3. [DOI] [PubMed] [Google Scholar]
- Wong M, Carter DR. Articular cartilage functional histomorphology and mechanobiology: a research perspective. Bone. 2003;33:1–13. doi: 10.1016/s8756-3282(03)00083-8. [DOI] [PubMed] [Google Scholar]
- Yasuhara R, Ohta Y, Yuasa T, Kondo N, Hoang T, Addya S, Fortina P, Pacifici M, Iwamoto M, Enomoto-Iwamoto M. Roles of beta-catenin signaling in phenotypic expression and proliferation of articular cartilage superficial zone cells. Lab Invest. 2011;91:1739–1752. doi: 10.1038/labinvest.2011.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Tirlapur U, Fairbank J, Handford P, Roberts S, Winlove CP, Cui Z, Urban J. Microfibrils, elastin fibres and collagen fibres in the human intervertebral disc and bovine tail disc. J Anat. 2007;210:460–471. doi: 10.1111/j.1469-7580.2007.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yukata K, Matsui Y, Shukunami C, Takimoto A, Goto T, Nishizaki Y, Nakamichi Y, Kubo T, Sano T, Kato S, Hiraki Y, Yasui N. Altered fracture callus formation in chondromodulin-I deficient mice. Bone. 2008;43:1047–1056. doi: 10.1016/j.bone.2008.08.111. [DOI] [PubMed] [Google Scholar]
- Zhang X, Prasadam I, Fang W, Crawford R, Xiao Y. Chondromodulin-1 ameliorates osteoarthritis progression by inhibiting HIF-2alpha activity. Osteoarthritis cartilage. 2016b;24:1970–1980. doi: 10.1016/j.joca.2016.06.005. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen S, Pei M. Biomechanical signals guiding stem cell cartilage engineering: from molecular adaption to tissue functionality. Eur Cell Mater. 2016a;30:59–78. doi: 10.22203/ecm.v031a05. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Pizzute T, Pei M. A review of crosstalk between MAPK and Wnt signals and its impact on cartilage regeneration. Cell Tissue Res. 2014;358:633–649. doi: 10.1007/s00441-014-2010-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimny ML, Albright DJ, Dabezies E. Mechanoreceptors in the human medial meniscus. Acta Anat (Basel) 1988;133:35–40. doi: 10.1159/000146611. [DOI] [PubMed] [Google Scholar]