Abstract
Purpose
We examined the effect of electric fan use on cardiovascular and thermoregulatory responses of nine young (26 ± 3 years) and nine aged (68 ± 4 years) adults exposed to extreme heat and humidity.
Methods
While resting at a temperature of 42°C, relative humidity increased from 30 to 70% in 2% increments every 5 minutes. On randomized days, the protocol was repeated without or with fan use. Heart rate (HR), core (Tcore) and mean skin (Tsk) temperatures were measured continuously. Whole-body sweat loss (WBSL) was measured from changes in nude body weight. Other measures of cardiovascular (cardiac output), thermoregulatory (local cutaneous and forearm vascular conductance, local sweat rate), and perceptual (thermal and thirst sensations) responses were also examined.
Results
When averaged over the entire protocol, fan use resulted in a small reduction of HR (−2 beats/min, 95% CI: −8 to 3), and slightly greater Tcore (+0.05°C, 95% CI: −0.13 to 0.23) and Tsk (+0.03°C, 95% CI: −0.36 to 0.42) in young adults. In contrast, fan use resulted in greater HR (+5 beats/min, 95% CI: 0 to 10), Tcore (+0.20°C, 95% CI: 0.00 to 0.41) and Tsk (+0.47°C, 95% CI: 0.18 to 0.76) in aged adults. A greater WBSL during fan use was observed in young (+0.2 kg, 95% CI: −0.2 to 0.6) but not aged (0.0 kg, 95% CI: −0.2 to 0.2) adults. Greater local sweat rate and cutaneous vascular conductance were observed with fan use in aged adults. Other measures of cardiovascular, thermoregulatory and perceptual responses were unaffected by fan use in both groups.
Conclusion
During extreme heat and humidity, fan use elevates physiological strain in aged, but not young, adults.
Keywords: Cardiovascular, core temperature, heat wave, heart rate, sweat, vasodilation
INTRODUCTION
Prolonged periods of hot weather, such as heat waves, pose a threat to human health and well-being (14, 27). The European heat wave of 2003 is a striking example as it caused an estimated 70,000 excess deaths, with nearly 40,000 of these deaths occurring within the span of two weeks (31). During such heat waves, morbidity and mortality are often related to cardiovascular events with aged adults being disproportionately affected (20). It is well established that the heat loss responses of sweating and cutaneous vasodilation are attenuated in aged adults, potentially exposing them to greater physiological strain during hot weather. Therefore, interventions that attenuate physiological strain may prevent adverse cardiovascular events in aged adults during heat waves. Such interventions are particularly needed, as climate change, urbanization, and an aging population are exposing an increasingly greater number of individuals to the risks of heat-related morbidity and mortality (13, 23, 25).
Electric fans represent a potentially simple and sustainable cooling intervention. However, guidelines regarding their use remain controversial. For example, the World Health Organization (38–40), US Environmental Protection Agency (36), and Centers for Disease Control and Prevention (4) discourage fan use when ambient temperature exceeds 35–37°C. This recommendation is based upon the premise that, under such conditions, increasing air velocity will accelerate dry heat gain and dehydration, thus causing greater physiological strain. In contrast, agencies such as Health Canada (15) and the Institut National de Prévention et d’Éducation pour la Santé – France (17) encourage fan use during hot weather, albeit with considerations. It is noteworthy that such recommendations have been put forward despite a lack of empirical evidence to support or refute electric fan use during heat waves (12, 14).
Fan use was recently shown to delay increases in heart rate and core temperature of healthy young adults exposed to air temperatures near (36°C), and well beyond (42°C), the threshold temperature at which fan use is discouraged by public health agencies (30). Although fan use increased the rate of dry heat gain from the environment, this effect was marginal relative to a vast improvement in evaporative capacity (29). However, it is aged adults that are generally at the greatest risk of heat-related morbidity and mortality. We recently reported that fan use did not delay the increase in heart rate or core temperature of aged adults exposed to extreme heat (42°C) and humidity (11). Rather, fan use resulted in greater heart rate and core temperature during the protocol. These findings suggest that fan use may differentially affect young and older adults, although a direct comparison between groups is lacking. Furthermore, physiological responses that underlie these potential age-related differences remain unknown. The purpose of this study was to compare cardiovascular and thermoregulatory responses between young and aged adults exposed to extreme heat and humidity without and with fan use. It was hypothesized that fan use would reduce cardiovascular and thermoregulatory strain of young, but not aged, adults.
METHODS
Participants
Based on previous data in young adults (30), an effect size of 1.15 for the inflection point in core temperature was expected with fan use relative to a control, no fan, condition. Assuming an α of 0.05 and β of 0.80, 7 subjects would provide enough power to detect a statistical difference of a similar magnitude (G*Power Version 3.1.9.2). Nine young (26 ± 3 years, range: 21–30, 5 males/4 females) and nine aged (68 ± 3 years, range: 61–72, 3 males/6 females) healthy adults participated in this study. Heart rate, core temperature and whole-body sweat loss data from the aged group have been published previously (11). These data from the aged group are referred to in the current manuscript to allow for a direct comparison with young adults. All participants were non-smokers, free of known cardiovascular, respiratory, neurological or metabolic diseases and were not taking any related medications. Health status was determined by having the participants fill out a detailed medical history questionnaire, measuring their resting blood pressure and heart rate, performing a 12-lead electrocardiogram and obtaining a fasting blood sample. Participants volunteered for two study visits that were performed at the same time of day. For each visit, participants were asked to arrive at the laboratory after a 12 h fast, to refrain from strenuous physical activity for 24 h, and to refrain from caffeine and alcohol for 12 h. The study and informed consent documents were approved by Institutional Review Boards at the University of Texas Southwestern Medical Center and Texas Health Presbyterian Hospital Dallas. Written informed consent was provided by all participants prior to their participation in the study.
Measurements
Heart rate was obtained from an electrocardiogram (GE Healthcare, Milwaukee, WI, USA). Blood pressure was measured by automated auscultation of the brachial artery (SunTech Medical, Morrisville, NC, USA). Cardiac output was measured by inert gas rebreathing (Innovision A/S, Odense, Denmark). Core temperature was measured from an esophageal temperature probe (Mallinckrodt Medical Inc., St-Louis, MO, USA) inserted to a depth of 40 cm past the nostril. Skin temperature was measured as a weighted average of six thermocouples attached to the skin surface. Local sweat rate was measured from a ventilated sweat capsule placed on the left anterior forearm. Anhydrous compressed nitrogen was passed through the capsule at a rate of 0.3 L/min. Water content of the effluent air was measured using a capacitance hygrometer (Vaisala, Woburn, WA, USA). Local sweat rate was calculated by multiplying the water content of the effluent gas by the flow rate, normalised for the area under the capsule. Cutaneous blood flow was estimated using an integrated laser-Doppler flow probe (Perimed AB, Stockholm, Sweden) placed in close proximity to the ventilated sweat capsule. Forearm blood flow was measured from recordings of brachial artery diameter and blood velocity via duplex ultrasound (LOGIQ e, GE Healthcare, Milwaukee, WI, USA). A linear transducer (4–12 MHz), operating at an insonation angle of 60°, was placed medial to the biceps brachii, approximately 4–5 cm from the elbow joint where the best spatial resolution of the artery could be obtained. Brachial artery diameter and blood velocity were determined from 1-minute recordings at baseline and every other stage during the humidity ramp protocol. Brachial artery blood velocity was determined from Doppler ultrasound audio recordings using an intensity-weighed algorithm (DUC2 software), subsequent to demodulation of forward and reverse Doppler frequencies (3). Images of the brachial artery were used to determine diameter at end-diastole using on-screen callipers. Changes in forearm blood flow during passive heat stress measured by Doppler ultrasound are similar to those measured by venous occlusion plethysmography (2).
Thermal sensation was measured using a 13-point scale, with 0.5 increments ranging from 2.0 (“Cold”) to 8.0 (“Unbearably hot”). Thirst sensation was measured with a visual analog scale (24). In response to the question: “how thirsty do you feel right now?”, participants were asked to draw a line across a 180 mm scale which had lines intersecting at 0 mm (“not at all thirsty”) and at 125 mm (“extremely thirsty”). The participants were told they could draw their line beyond the limit of the scale, if needed. Nude body mass was measured using a scale accurate to 0.1 kg (Health o meter Professional Scales, McCook, IL, USA). Whole-body sweat loss was subsequently determined from nude body mass measurements, accounting for fluid intake and urine loss. Urine specific gravity was measured in duplicate using a handheld refractometer (Atago Inc., Bellevue, WA, USA).
Plasma hemoglobin (Hemoximeter, OSM3, Radiometer, Copenhagen, Denmark), hematrocrit (Adams Microhematocrit II, Becton, Dickinson and Company, Franklin Lakes, NJ, USA), and osmolality (Micro-Osmometer, Model 3MO plus, Advanced Instruments Inc., Norwood, MA, USA) were determined in triplicate from venous blood samples drawn into lithium-heparin tubes (BD Vacutainer, Franklin Lakes, NJ, USA). Relative changes in plasma volume from baseline were subsequently calculated from changes in hematocrit and hemoglobin (7). Plasma concentrations of cardiac troponin I were determined by chemiluminescence immunoassay (ARCHITECT i2000, Abbott Laboratories, Abbott Park, IL, USA).
Protocol
Upon arrival to the laboratory, participants provided a urine sample before weighing themselves nude. They then changed into standardized shorts (males) or shorts and a sports bra (females) and sat, semi-reclined, within a room maintained at 24°C. After a minimum of 30 min, a blood sample was drawn following which the participants were given a standardized breakfast. Participants were subsequently transferred to an environmental chamber (9 × 12 feet, Climatic Testing Systems, Hatfield, PA, USA) maintained at 42°C and an initial relative humidity of 30%. The subjects sat, semi-reclined, on a chair made of breathable fabric (Lafuma, Annecy-Le-Vieux, France). Following an initial instrumentation period within the environmental chamber (~10 min), the experimental protocol began with a 30 min baseline period. Relative humidity was subsequently increased by 2% every 5 min until a relative humidity of 70% (total time of 100 min, excluding initial baseline period). At the end of the protocol, air temperature was decreased (31.0 ± 1.2°C) and participants remained in the chamber for an additional 57 ± 3 min during which maximal skin blood flow was measured using a local skin heating protocol (data not presented). Participants subsequently exited the chamber and weighed themselves nude. On separate randomized days, participants performed the protocol without or with a 16-in fan (Dayton, Niles, IL, USA) facing them from a distance of 1 m. The fan was turned on 25 min into the baseline period and it provided an air velocity (~4 m/s) that was measured with a digital anemometer (Extech Instruments, Nashua, NH, USA) placed ~30 cm from the participant at chest level.
Data analysis
Continuously measured data were collected with acquisition hardware (Biopac, Santa Barbara, CA, USA) at a sampling frequency of 25 Hz (200 Hz for the ECG signal). Baseline data (i.e. 30% relative humidity) represent an average of the last minute of the 30 min baseline period. Similarly, the average of the last minute for each relative humidity stage was used for analyses. Blood pressure measurements were obtained within the last 2 minutes of each stage to calculate mean arterial pressure as diastolic blood pressure + 1/3 of pulse pressure. Cardiac output measurements were obtained during the 30, 52, and 70% humidity stages to calculate stroke volume as cardiac output divided by heart rate and systemic vascular resistance as mean arterial pressure divided by cardiac output. Full datasets for these variables could only be obtained in 8 young and 6 aged subjects. Cutaneous vascular conductance was calculated as cutaneous perfusion units divided by mean arterial pressure and expressed as absolute values (units/mmHg). Forearm blood flow, in ml/min, was calculated as brachial artery cross-sectional area multiplied by mean blood velocity. Forearm vascular conductance was calculated as forearm blood flow divided by mean arterial pressure and expressed in absolute values (ml/min/mmHg). Forearm vascular conductance data could only be obtained in 7 young and 6 aged subjects. The critical humidity at which elevations in heart rate and esophageal temperature occurred was determined using segmented regression analysis as previously described (11, 30). Mean body temperature was calculated as: esophageal temperature × 0.8 + mean skin temperature × 0.2.
Statistical analysis
Data were analyzed using a three-way mixed model analysis of variance (ANOVA) with the non-repeated factor of age and the repeated factors of condition (control and fan) and relative humidity. When a main effect of condition or a condition by group interaction was observed, separate two-way repeated measures ANOVAs with the factors of condition and relative humidity were performed within each group. Post-hoc analyses were performed with a Holm-Sidak correction. Single time-point comparisons were analyzed using paired (within group) or independent (between groups) samples T-tests. Statistical significance was set P<0.05. Analyses were performed using commercially available software (SPSS 24, IBM Corp. and Prism 7, Graphpad Software Inc.). All variables are reported as mean ± 95% confidence intervals, except for participant characteristics which are presented as mean ± standard deviation.
RESULTS
Participant characteristics
There were no differences between groups for height (young: 168 ± 5 vs. aged: 166 ± 10 cm, P=0.46), weight (young: 70 ± 11 vs. aged: 72 ± 13 kg, P=0.79), body surface area (young: 1.79 ± 0.13 vs. aged: 1.79 ± 0.20 m2, P=0.96), resting heart rate (young: 66 ± 6 vs. aged: 68 ± 10 beats/min, P=0.53), as well as fasting total cholesterol (young: 165 ± 48 vs. aged: 199 ± 30 mg/dl, P=0.09), HDL (young: 51 ± 12 vs. aged: 53 ± 18 mg/dl, P=0.77) and blood glucose (young: 76 ± 3 vs. aged: 80 ± 15 mg/dl, P=0.33). In contrast, resting systolic (young: 113 ± 5 vs. aged: 125 ± 7 mmHg, P<0.01) and diastolic (young: 69 ± 6 vs. aged: 76 ± 7 mmHg, P=0.05) blood pressures, as well as fasting LDL (young: 87 ± 23 vs. aged: 123 ± 21 mg/dl, P<0.01) were greater in aged adults. Urine specific gravity upon arrival did not differ between groups for both the control (young: 1.015 ± 0.008 vs. aged: 1.016 ± 0.003) and fan (young: 1.017 ± 0.008 vs. aged: 1.016 ± 0.003) conditions (P=0.60).
Cardiovascular responses
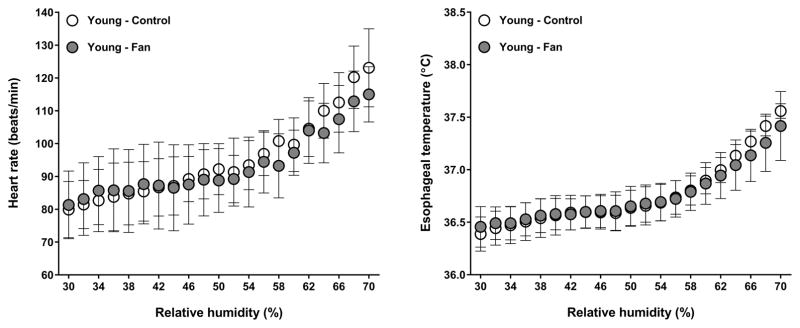
A condition by group interaction was observed for heart rate (P=0.03). As previously reported (11), heart rate was greater with fan use in aged adults (P=0.03). In contrast, heart rate did not differ between conditions in young adults (P=0.43, Fig. 1). No condition by group interaction was observed for mean arterial pressure (P=0.36), cardiac output (P=0.73), stroke volume (P=0.55), or systemic vascular resistance (P=0.78). In young adults, cardiac output and stroke volume were greater, whereas systemic vascular resistance was lower across both conditions relative to aged adults (all P≤0.01, Table 1).
Figure 1.
Heart rate (left panel) and esophageal temperature (right panel) of young adults exposed to step-wise increases in relative humidity at an ambient temperature of 42°C, without (control) and with (fan) fan use. Values are mean ± 95% confidence intervals.
Table 1.
Cardiovascular, perceptual and haematological responses of young and aged adults exposed to step-wise increases in relative humidity at a temperature of 42°C, without (CON) and with (FAN) fan use.
| Relative humidity | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| 30% | 42% | 52% | 54% | 66% | 70% | ||||||||
|
| |||||||||||||
| Young | Aged | Young | Aged | Young | Aged | Young | Aged | Young | Aged | Young | Aged | ||
| Cardiac output (L/min) | CON | 6.1 ±0.8† | 3.6 ±0.5 | - | - | 6.7 ±0.8† | 3.5 ±0.5 | - | - | - | - | 8.0 ±1.2*† | 4.1 ±0.6 |
| FAN | 6.6 ±1.2† | 3.4 ±0.7 | - | - | 7.0 ±1.5† | 4.3 ±0.7* | - | - | - | - | 8.4 ±1.6*† | 4.5 ±0.8* | |
|
| |||||||||||||
| Stroke volume (ml) | CON | 80 ±19† | 50 ±11 | - | - | 78 ±19† | 44 ±10 | - | - | - | - | 68 ±16*† | 44 ±10 |
| FAN | 83 ±16† | 44 ±9 | - | - | 79 ±19† | 48 ±6 | - | - | - | - | 74 ±15† | 44 ±12 | |
|
| |||||||||||||
| MAP (mmHg) | CON | 86 ±6 | 89 ±5 | - | - | 81 ±7 | 86 ±6 | - | - | - | - | 75 ±6 | 88 ±6 |
| FAN | 85 ±6 | 87 ±4 | - | - | 82 ±6 | 84 ±4 | - | - | - | - | 78 ±7 | 89 ±9 | |
|
| |||||||||||||
| SVR (mmHg/L/min) | CON | 13.9 ±1.2† | 25.0 ±4.4 | - | - | 11.9 ±1.2*† | 24.9 ±4.4 | - | - | - | - | 9.4 ±0.9*† | 22.1 ±2.8 |
| FAN | 13.1 ±1.9† | 26.0 ±3.9 | - | - | 12.7 ±3.6† | 20.5 ±4.3* | - | - | - | - | 9.8 ±1.6*† | 20.1 ±3.3* | |
|
| |||||||||||||
| Thermal sensation (units) | CON | 5.0 ±0.3 | 4.8 ±0.4 | 5.9±0.3* | 5.9±0.3* | - | - | 6.6 ±0.4* | 7.0 ±0.5* | 7.1 ±0.6* | 7.5 ±0.4* | 7.2 ±0.5* | 7.6 ±0.4* |
| FAN | 4.9 ±0.4 | 5.3 ±0.4 | 5.6±0.4* | 6.1±0.4* | - | - | 5.9 ±0.5* | 6.7 ±0.5* | 6.7 ±0.6* | 7.3 ±0.5* | 7.1 ±0.5* | 7.5 ±0.5* | |
|
| |||||||||||||
| Thirst sensation (mm) | CON | 29 ±15 | 24 ±14 | 43±19* | 55±22* | - | - | 60 ±26* | 88 ±27* | 81 ±34* | 118 ±28* | 95 ±41* | 126 ±41* |
| FAN | 25 ±19 | 30 ±21 | 34±23 | 55±22* | - | - | 58 ±25* | 80 ±24* | 77 ±32* | 101 ±31* | 89 ±34* | 111 ±40* | |
|
| |||||||||||||
| ΔPlasma volume (%) | CON | 0 | 0 | - | - | −2.2 ±1.7 | −3.8 ±2.2* | - | - | - | - | −10.0 ±3.3* | −9.6 ±2.0* |
| FAN | 0 | 0 | - | - | −3.7 ±2.4* | −4.4 ±1.8* | - | - | - | - | −9.8 ±2.8* | −9.8 ±1.1* | |
|
| |||||||||||||
| Plasma osmolality (mosmol/kgH2O) | CON | 292 ±1 | 294 ±3 | - | - | 293 ±2 | 297 ±3 | - | - | - | - | 294 ±3 | 297 ±4 |
| FAN | 291 ±2 | 295 ±2 | - | - | 294 ±3 | 297 ±1 | - | - | - | - | 294 ±4 | 297 ±2 | |
Values are mean ± 95% confidence intervals. MAP: mean arterial pressure. SVR: systemic vascular resistance.
P<0.05 vs. baseline (i.e., 30% relative humidity) within a given condition.
P<0.05 vs. aged within a given condition.
Thermoregulatory responses
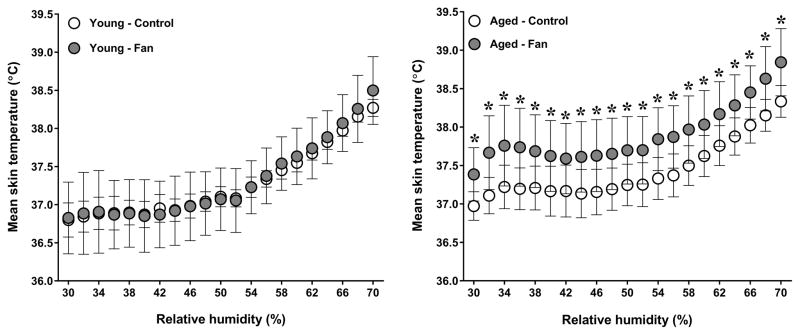
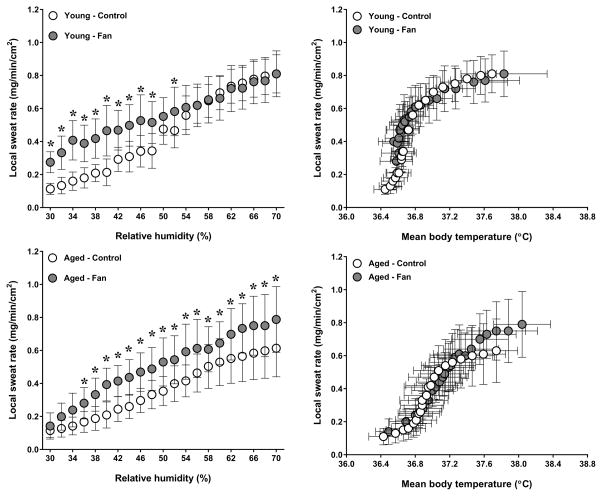
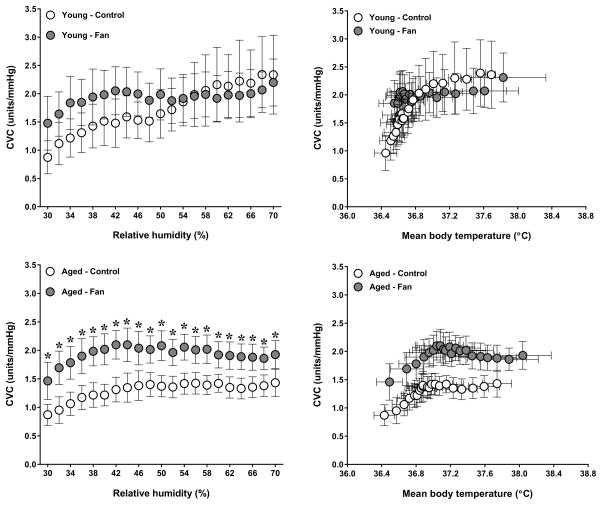
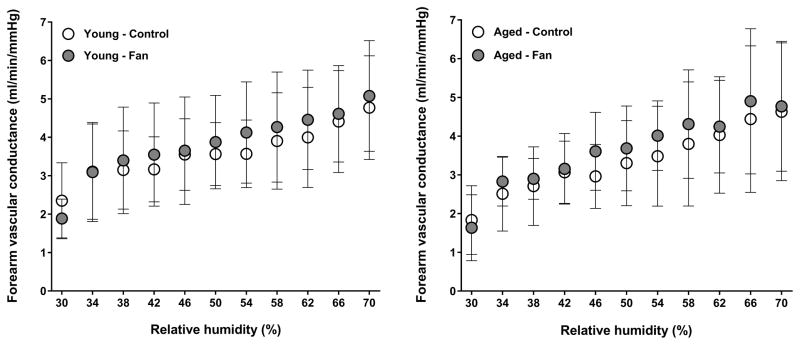
A condition by group interaction was observed for esophageal temperature (P=0.04). As previously reported (11), fan use resulted in a greater esophageal temperature in aged adults (P=0.05). In contrast, esophageal temperature was similar between conditions in young adults (P=0.58, Fig. 1). The condition by group interaction for mean skin temperature did not reach statistical significance (P=0.06). However, a main effect of condition was observed (P=0.03), as mean skin temperature was greater during fan use in aged (P<0.01) but not young (P=0.87) adults (Fig. 2). No condition by group interaction was observed for local sweat rate (P=0.44). However, a main effect of condition was observed (P<0.01) as local sweat rate was greater during fan use in both young (P<0.01) and aged (P<0.01) adults (Fig. 3). However, local sweat rates were similar between conditions when plotted as a function of mean body temperature (Fig. 3). As previously reported (11), whole-body sweat loss was similar between conditions for aged adults (P>0.99). A greater whole-body sweat loss was observed in young adults, although differences did not reach statistical significance (control: 0.8 ± 0.2 vs. fan: 1.0 ± 0.2 kg, P=0.26). The condition by group interaction for cutaneous vascular conductance did not reach statistical significance (P=0.08). However, a main effect of condition was observed (P=0.03), as cutaneous vascular conductance was greater with fan use in aged (P<0.01) but not young (P=0.34) adults (Fig. 4). However, cutaneous vascular conductance was similar between conditions when plotted as a function of mean body temperature (Fig. 4). Forearm vascular conductance did not differ between conditions for both age groups (P=0.81, Fig. 5).
Figure 2.
Mean skin temperatures of young (left panel) and aged (right panel) adults exposed to extreme heat and humidity without (control) and with (fan) fan use. Values are mean ± 95% confidence intervals. *, P<0.05 between conditions.
Figure 3.
Local forearm sweat rate plotted as a function of relative humidity (left panels) and mean body temperature (right panels) in young (top panels) and aged (bottom panels) adults exposed to extreme heat and humidity without (control) and with (fan) fan use. Values are mean ± 95% confidence intervals. *, P<0.05 between conditions.
Figure 4.
Local forearm cutaneous vascular conductance plotted as a function of relative humidity (left panels) and mean body temperature (right panels) in young (top panels) and aged (bottom panels) adults exposed to extreme heat and humidity without (control) and with (fan) fan use. Values are mean ± 95% confidence intervals. *, P<0.05 between conditions.
Figure 5.
Whole-limb forearm vascular conductance of young (left panels) and aged (right panels) adults exposed to extreme heat and humidity without (control) and with (fan) fan use. Values are mean ± 95% confidence intervals.
Thermal and thirst sensations
Thermal and thirst sensations increased during the protocol (Table 1), although no condition by group interactions were observed for these variables (both P≥0.19).
Blood parameters
A decrease in plasma volume and an increase in plasma osmolality occurred during the protocol (Table 1), without any condition by group interaction for both variables (both P≥0.84). Of all the blood samples analyzed for cardiac troponin I, detectable levels (>0.010 ng/ml) were only observed in 4 samples obtained from aged adults. However, troponin I levels for each of these 4 samples (mean of 0.013 ± 0.002 ng/ml) were below the 99th percentile clinical reference limit (≤0.036 ng/ml), and thus the observed changes were inconsequential.
Inflection points
As previously reported (11), the relative humidity at which elevations in heart rate and esophageal temperature occurred did not differ between the control and fan conditions in aged adults. Likewise, the relative humidity inflection points for heart rate (control: 54 ± 4 vs. fan: 57 ± 3%, P=0.11) and esophageal temperature (control: 60 ± 1 vs. fan: 62 ± 3%, P=0.14) did not differ between conditions in young adults.
Overall effect of fan use
When averaged over the entire protocol, the mean difference between fan and control conditions (negative value indicating a reduction and positive value indicating an increase with fan use relative to the control condition) for heart rate was −2 beats/min [95% CI: −8 to 3] in young adults and +5 beats/min [95% CI: 0 to 10] in aged adults. For esophageal temperature, it was +0.05°C [95% CI: −0.13 to 0.23] for young adults and +0.20°C [95% CI: 0.00 to 0.41] in aged adults. For mean skin temperature, it was +0.03°C [95% CI: −0.36 to 0.42] in young adults and +0.47°C [95% CI: 0.18 to 0.76] in aged adults. For whole-body sweat loss it was +0.2 kg [95% CI: −0.2 to 0.6] in young adults and 0.0 kg [95% CI: −0.2 to 0.2] in aged adults.
DISCUSSION
This study provides a direct comparison of cardiovascular and thermoregulatory responses between healthy young and aged adults exposed to extreme heat and humidity, without and with electric fan use. As previously reported (11), fan use resulted in greater heart rate and core temperature responses in aged adults. In contrast, fan use had little effect in young adults. Fan use also increased mean skin temperature in aged, but not young adults. These age-related differences could be due to a reduced sweating capacity in aged adults. A greater, albeit not statistically significant, sweat loss was observed with fan use in young adults. No such increase occurred in aged adults. Overall, these results demonstrate that fan use affects young and aged adults differently during exposure to extreme heat and humidity.
By increasing air velocity across the skin surface, fan use results in a greater rate of dry heat exchange and evaporative heat loss between the body and the environment (18). These rates are determined by the skin-to-air temperature gradient (dry heat exchange) or the skin-to-air vapor pressure gradient (evaporative heat loss), as well as the heat transfer coefficient which is primarily dependent upon air velocity (9). In terms of dry heat exchange, fan use will increase the rate of heat transfer from the body to the environment when air temperature is lower than skin temperature (~34–35°C). However, fan use will increase the rate of heat gain by the body from the environment when air temperature exceeds skin temperature. Greater heat gain from the environment, and therefore the potential for increased physiological strain, is the main reason why public health agencies discourage fan use if air temperature exceeds 35–37°C (4, 36, 38, 39). However, such recommendations ignore the fact that fan use also improves evaporative capacity, particularly in humid environments (29). In young adults, fan use delayed the increase in heart rate and core temperature during exposure to air temperatures near (36°C) and well above (42°C) the threshold (e.g., 35–37°C) at which fan use is discouraged (30). Despite causing a greater rate of dry heat gain, fan use provided a “protective” effect by elevating evaporative capacity and maintaining sweating efficiency at greater levels (29). This delayed the humidity at which heat gain from the environment could no longer be balanced by evaporative heat loss (29).
In the current study, we did not observe a beneficial or detrimental effect of fan use in young adults. The relative humidity inflection points for heart rate and core temperature were slightly delayed with fan use, but the magnitude of difference between conditions (~2–3%) is likely negligible. These findings are in contrast to the aforementioned study in young adults (30). In the previous study, longer humidity stages were employed (7.5 min vs. 5 min in the current study), participants wore a t-shirt and shorts (vs. shorts only, and sports bra for females, in the current study) and were seated behind a barrier during the control condition (vs. unobstructed airflow in the current study). Regardless of these differences, the findings of the current study do not support the notion that fan use is detrimental for young adults at 42°C, which is well above the air temperature limit for fan use recommended by major public health agencies (4, 36, 38, 39).
The primary objective of the current study was to compare the effect of fan use between young and aged adults exposed to extreme heat and humidity. As previously reported (11), fan use also had little effect upon the relative humidity inflection points for heart rate and core temperature in aged adults, but resulted in greater heart rate and core temperature during most of the protocol. This was due to a transient increase in these variables when the fan was turned on, a response which was not observed in young adults (Fig. 1). A likely explanation for this aged-related difference relates to sweating capacity. In young adults, fan use resulted in a greater sweat loss, although differences did not reach statistical significance. It should be noted that one young adult exhibited a greater sweat loss during the control condition that was more than 2-fold greater than the mean value of the young group (1.7 kg vs. 0.7 kg). If the data from this participant are removed, a statistically greater sweat loss is observed with fan use in young adults (fan: 1.1 ± 0.2 kg vs. control: 0.7 ± 0.1 kg, P<0.01). Interestingly, fan use elevated local sweat production in aged adults. Local sweat rate should be viewed as a measure of sudomotor activity, as the capsule and constant flow of dry gas ensure full evaporation of the sweat produced. The greater local sweat rate with fan use in aged adults is likely related to a greater thermal drive, as esophageal and mean skin temperatures were elevated with fan use. In fact, local sweat rate was similar between conditions when plotted as a function of mean body temperature (Fig. 3). Nonetheless, whole-body sweat loss was similar between conditions (mean difference: 0.0 kg, 95% CI: −0.2 to 0.2). This highlights the fact that local measures of sweat production are not always representative of whole-body sweat production (5, 28). A lack of increase in whole-body sweat loss with fan use could be related to a diminished sweating capacity in aged adults (16, 22). Under the conditions employed, a diminished sweating capacity would not allow for greater evaporation to counter an elevated rate of dry heat gain caused by fan use.
The implications for the greater heart rate and core temperature observed during fan use in aged adults remain unclear. The magnitude of differences was relatively small and fan use had little to no effect upon other cardiovascular and thermoregulatory responses. Two exceptions include a greater mean skin temperature and local cutaneous vascular conductance with fan use in aged adults. The greater mean skin temperature could be related to an initially greater rate of dry heat gain that was not offset by greater sweat evaporation. As for local cutaneous vascular conductance, it is interesting to note that it was elevated during the fan condition, yet forearm (whole-limb) vascular conductance was similar between conditions. Similarly to local sweat rate, local measures of cutaneous vascular conductance are possibly less affected by changes in environmental conditions as the area of measurement is covered. Local cutaneous vascular conductance therefore represents a measure of vasomotor activity, which was increased by fan use in aged adults due to a greater thermal drive. In fact, cutaneous vascular conductance was similar between conditions when plotted as a function of mean body temperature (Fig. 4). Nonetheless, greater vasomotor activity did not translate into greater forearm vascular conductance, which was measured from the arm exposed to the environmental conditions. Although forearm vascular conductance reflects the dilation that occurs in all vascular beds of the arm, the increase in this variable during heat exposure is primarily related to dilation of skin blood vessels (6, 8). It is therefore possible that local measures of cutaneous vascular conductance do not always reflect what is occurring at the whole-body level, or at least the limb. Changes in plasma volume and osmolality, as well as perceptual measures of thermal and thirst sensations were unaffected by fan use in both groups. Finally, no evidence of cardiac damage (quantified by troponin I levels) was detected for either group during each condition. Taken together, the findings of the current study suggest that fan use had little to no effect upon cardiovascular, thermoregulatory, hematological and perceptual responses beyond a ~5 beats/min greater heart rate and ~0.2°C greater core temperature in aged adults. Future studies are needed to determine the implication(s) of these findings.
Considerations
The environmental conditions employed represent an important consideration of the current study (10). These conditions were chosen to provide a background of high physiological heat strain against which to evaluate the effect of fan use. These conditions rarely occur in reality, although conditions of 45°C and ~50% relative humidity were reported during a 2015 heat wave in Pakistan and India. Using the conceptual heat balance equation, Jay et al. (18) modeled the environmental conditions at which fan use would theoretically: a) be beneficial, b) provide marginal benefit, or c) be detrimental in young and aged adults. Even prior to beginning the step-wise increases in humidity, the baseline environmental conditions (42°C, 30% relative humidity) of the current study were close to the zone of marginal benefit in young adults and were well within this zone for aged adults. At the humidity levels when inflections in heart rate (~50–55% relative humidity) and core temperature (~60–65% relative humidity) were observed, environmental conditions were within the zone where fan use was modeled to be detrimental for both young and aged adults (10). The current results therefore provide some validation for the guidelines put forward by Jay et al. (18). Future studies are needed to examine the effect of fan use at more common environmental conditions experienced during heat waves, particularly for more prolonged exposures and perhaps with supplemental skin wetting to account for potential age-related reductions in sweating capacity. Moreover, the efficacy of other low-cost, energy-efficient cooling interventions for mitigating physiological strain should be examined. Such studies should be performed in the populations at greatest risk of heat-related morbidity and mortality (1). The interaction between cooling interventions and medications that may interfere with cardiovascular and thermoregulatory responses during heat exposure are also needed (14).
Significance
Prolonged periods of hot weather pose a risk to human health and well-being (14, 27). Although air-conditioning is arguably the most effective cooling intervention, access to air-conditioning is not universal and economical concerns limit its use by those who do have access (35). Reliance upon air-conditioning also places significant burden on the electrical grid, which can cause brown-outs or black-outs during periods of extreme heat, and it adds a substantial thermal load to the environment (32, 33). Electric fans represent a potentially more sustainable option to minimize the health effects of prolonged hot weather (14). A few studies have considered the modulating effect of fan use on mortality and morbidity during heat waves (19, 21, 26, 34, 37). However, these studies could not provide conclusive evidence regarding the effect of fan use, and there is currently no evidence to support or refute their use during heat waves (12, 14). Together with recent studies (11, 18, 29, 30), the current results represent an initial step towards understanding the consequence of fan use on cardiovascular and thermoregulatory responses in young and aged adults exposed to extreme heat and humidity. Such studies are particularly needed as climate change, urbanization and a rapidly aging population are placing an increasingly greater number of individuals at risk of heat-related morbidity and mortality (13, 23, 25).
Conclusion
The current study compared the effect of electric fan use on cardiovascular and thermoregulatory responses between young and aged adults exposed to extreme heat (42°C) and humidity (30–70%). The main finding was that fan use resulted in a greater heart rate and core temperature in aged, but not young, adults throughout the exposure. This finding could be related to differences in sweating capacity, as fan use elevated whole-body sweat loss in young, but not aged, adults. Overall, the current study demonstrates that fan use differentially affects young and aged adults during exposure to extreme heat and humidity. The implications of these findings to support or refute electric fan use during heat waves remain to be determined.
Acknowledgments
This study was supported by the National Institutes of Health (GM-068865) and Department of Defense (W81XWH-12-1-0152). D.G. was supported by a Postdoctoral Fellowship from the Natural Sciences and Engineering Research Council of Canada. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. The authors have no conflicts of interest to disclose. The results of the present study do not constitute endorsement by ACSM.
This study was supported by the National Institutes of Health (GM-068865) and Department of Defense (W81XWH-12-1-0152). D.G. was supported by a Postdoctoral Fellowship from the Natural Sciences and Engineering Research Council of Canada. The authors thank Naomi Kennedy and Amy Adams for their contributions to the study. We would also like to thank Dr. John R. Halliwill for the development and use of the DUC2 software. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicts of interest to disclose. The results of the present study do not constitute endorsement by ACSM.
References
- 1.Bouchama A, Dehbi M, Mohamed G, Matthies F, Shoukri M, Menne B. Prognostic factors in heat wave related deaths: a meta-analysis. Arch Intern Med. 2007;167(20):2170–6. doi: 10.1001/archinte.167.20.ira70009. [DOI] [PubMed] [Google Scholar]
- 2.Brothers RM, Wingo JE, Hubing KA, Crandall CG. Methodological assessment of skin and limb blood flows in the human forearm during thermal and baroreceptor provocations. J Appl Physiol. 2010;109(3):895–900. doi: 10.1152/japplphysiol.00319.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buck TM, Sieck DC, Halliwill JR. Thin-beam ultrasound overestimation of blood flow: how wide is your beam? J Appl Physiol. 2014;116(8):1096–104. doi: 10.1152/japplphysiol.00027.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. [accessed November 24, 2016];Extreme Heat Prevention Guide. Available from: https://www.cdc.gov/disasters/extremeheat/heat_guide.html.
- 5.Cotter JD, Patterson MJ, Taylor NA. The topography of eccrine sweating in humans during exercise. Eur J Appl Physiol. 1995;71(6):549–54. doi: 10.1007/BF00238559. [DOI] [PubMed] [Google Scholar]
- 6.Detry JM, Brengelmann GL, Rowell LB, Wyss C. Skin and muscle components of forearm blood flow in directly heated resting man. J Appl Physiol. 1972;32(4):506–11. doi: 10.1152/jappl.1972.32.4.506. [DOI] [PubMed] [Google Scholar]
- 7.Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol. 1974;37(2):247–8. doi: 10.1152/jappl.1974.37.2.247. [DOI] [PubMed] [Google Scholar]
- 8.Edholm OG, Fox RH, Macpherson RK. The effect of body heating on the circulation in skin and muscle. J Physiol. 1956;134(3):612–9. doi: 10.1113/jphysiol.1956.sp005669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gagge AP, Gonzales RR. Mechanisms of heat exchange. In: Fregley MJ, Blatteis CM, editors. Handbook of Physiology. Section 4: Environmental Physiology. New York, NY: Oxford University press; 1996. pp. 45–84. [Google Scholar]
- 10.Gagnon D, Crandall CG. Electric fan use during heat waves: Turn off for the elderly? Temperature (Austin) 2017;4(2):1–3. doi: 10.1080/23328940.2017.1295833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gagnon D, Romero SA, Cramer MN, Jay O, Crandall CG. Cardiac and Thermal Strain of Elderly Adults Exposed to Extreme Heat and Humidity With and Without Electric Fan Use. JAMA. 2016;316(9):989–91. doi: 10.1001/jama.2016.10550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta S, Carmichael C, Simpson C, et al. Electric fans for reducing adverse health impacts in heatwaves. Cochrane Database Syst Rev. 2012;7:CD009888. doi: 10.1002/14651858.CD009888.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haines A, Kovats RS, Campbell-Lendrum D, Corvalan C. Climate change and human health: impacts, vulnerability, and mitigation. Lancet. 2006;367(9528):2101–9. doi: 10.1016/S0140-6736(06)68933-2. [DOI] [PubMed] [Google Scholar]
- 14.Hajat S, O’Connor M, Kosatsky T. Health effects of hot weather: from awareness of risk factors to effective health protection. Lancet. 2010;375(9717):856–63. doi: 10.1016/S0140-6736(09)61711-6. [DOI] [PubMed] [Google Scholar]
- 15.Health Canada. Extreme heat events guidelines: Technical guide for health care workers. Water Air and Climate Change Bureau, Healthy Environments and Consumer Safety Branch; 2011. pp. 1–158. [Google Scholar]
- 16.Inoue Y, Shibasaki M. Regional differences in age-related decrements of the cutaneous vascular and sweating responses to passive heating. Eur J Appl Physiol Occup Physiol. 1996;74(1–2):78–84. doi: 10.1007/BF00376498. [DOI] [PubMed] [Google Scholar]
- 17.[Institute of National Health Prevention and Education], France. [accessed November 24, 2016];Heat waves and extreme heat: acting to prevent risks. Available from: http://inpes.santepubliquefrance.fr/10000/themes/evenement_climatique/canicule/canicule-agir.asp.
- 18.Jay O, Cramer MN, Ravanelli NM, Hodder SG. Should electric fans be used during a heat wave? Appl Ergon. 2015;46(Pt A):137–43. doi: 10.1016/j.apergo.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 19.Kaiser R, Rubin CH, Henderson AK, et al. Heat-related death and mental illness during the 1999 Cincinnati heat wave. Am J Forensic Med Pathol. 2001;22(3):303–7. doi: 10.1097/00000433-200109000-00022. [DOI] [PubMed] [Google Scholar]
- 20.Kenney WL, Craighead DH, Alexander LM. Heat waves, aging, and human cardiovascular health. Med Sci Sports Exerc. 2014;46(10):1891–9. doi: 10.1249/MSS.0000000000000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kilbourne EM, Choi K, Jones TS, Thacker SB. Risk factors for heatstroke. A case-control study. JAMA. 1982;247(24):3332–6. [PubMed] [Google Scholar]
- 22.Larose J, Boulay P, Sigal RJ, Wright HE, Kenny GP. Age-related decrements in heat dissipation during physical activity occur as early as the age of 40. PLoS One. 2013;8(12):e83148. doi: 10.1371/journal.pone.0083148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luber G, McGeehin M. Climate change and extreme heat events. Am J Prev Med. 2008;35(5):429–35. doi: 10.1016/j.amepre.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 24.Mack GW, Weseman CA, Langhans GW, Scherzer H, Gillen CM, Nadel ER. Body fluid balance in dehydrated healthy older men: thirst and renal osmoregulation. J Appl Physiol. 1994;76(4):1615–23. doi: 10.1152/jappl.1994.76.4.1615. [DOI] [PubMed] [Google Scholar]
- 25.McMichael AJ, Woodruff RE, Hales S. Climate change and human health: present and future risks. Lancet. 2006;367(9513):859–69. doi: 10.1016/S0140-6736(06)68079-3. [DOI] [PubMed] [Google Scholar]
- 26.Naughton MP, Henderson A, Mirabelli MC, et al. Heat-related mortality during a 1999 heat wave in Chicago. Am J Prev Med. 2002;22(4):221–7. doi: 10.1016/s0749-3797(02)00421-x. [DOI] [PubMed] [Google Scholar]
- 27.Patz JA, Frumkin H, Holloway T, Vimont DJ, Haines A. Climate change: challenges and opportunities for global health. JAMA. 2014;312(15):1565–80. doi: 10.1001/jama.2014.13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poirier MP, Gagnon D, Kenny GP. Local versus whole-body sweating adaptations following 14 days of traditional heat acclimation. Appl Physiol Nutr Metab. 2016;41(8):816–24. doi: 10.1139/apnm-2015-0698. [DOI] [PubMed] [Google Scholar]
- 29.Ravanelli NM, Gagnon D, Hodder SG, Havenith G, Jay O. The biophysical and physiological basis for mitigated elevations in heart rate with electric fan use in extreme heat and humidity. Int J Biometeorol. 2017;61(2):313–23. doi: 10.1007/s00484-016-1213-0. [DOI] [PubMed] [Google Scholar]
- 30.Ravanelli NM, Hodder SG, Havenith G, Jay O. Heart rate and body temperature responses to extreme heat and humidity with and without electric fans. JAMA. 2015;313(7):724–5. doi: 10.1001/jama.2015.153. [DOI] [PubMed] [Google Scholar]
- 31.Robine JM, Cheung SL, Le Roy S, et al. Death toll exceeded 70,000 in Europe during the summer of 2003. C R Biol. 2008;331(2):171–8. doi: 10.1016/j.crvi.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Salamanca F, Georgescu M, Mahalov A, Moustaoui M, Wang M. Anthropogenic heating of the urban environment due to air conditioning. J Geophys Res Atmos. 2014;119:5949–65. [Google Scholar]
- 33.Salamanca F, Georgescu M, Mahalov A, Moustaoui M, Wang M, Svoma BM. Assessing summertime urban air conditioning consumption in a semiarid environment. Environ Res Lett. 2013;8(3):034022. [Google Scholar]
- 34.Semenza JC, Rubin CH, Falter KH, et al. Heat-related deaths during the July 1995 heat wave in Chicago. N Engl J Med. 1996;335(2):84–90. doi: 10.1056/NEJM199607113350203. [DOI] [PubMed] [Google Scholar]
- 35.Sheridan SC. A survey of public perception and response to heat warnings across four North American cities: an evaluation of municipal effectiveness. Int J Biometeorol. 2007;52(1):3–15. doi: 10.1007/s00484-006-0052-9. [DOI] [PubMed] [Google Scholar]
- 36.United States Environmental Protection Agency. Excessive heat events guidebook. 2006. pp. 1–60. [Google Scholar]
- 37.Vandentorren S, Bretin P, Zeghnoun A, et al. August 2003 heat wave in France: risk factors for death of elderly people living at home. Eur J Public Health. 2006;16(6):583–91. doi: 10.1093/eurpub/ckl063. [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization. Heat-Health Action Plans. 2008. pp. 1–58. [Google Scholar]
- 39.World Health Organization. Public health advice on preventing health effects of heat. 2011. pp. 1–37. [Google Scholar]
- 40.World Meteorological Organization, World Health Organization. Heatwaves and Health: Guidance on Warning-System Development. 2015. pp. 1–114. [Google Scholar]