Abstract
Purpose
Exercise training promotes skeletal muscle mitochondrial biogenesis and an increase in maximal oxygen consumption. Primary myotubes retain some metabolic properties observed in vivo but it is unknown whether this includes exercise-induced mitochondrial adaptations. The goal of this study was to test if primary myotubes from exercise-trained women have higher mitochondrial content and maximal oxygen consumption compared to untrained women.
Methods
Six trained and nine untrained Caucasian women participated in this study. Muscle biopsies from the vastus lateralis muscle of the right leg were obtained and primary muscle cells were isolated. Maximal respiration rates, mitochondrial mRNA and protein content, and succinate dehydrogenase activity were measured in skeletal muscle and primary myotubes from trained and untrained women.
Results
Trained women, compared to untrained women, had higher maximal whole-body oxygen consumption (+18%, P = 0.03), in vivo maximal skeletal muscle oxidative capacity measured with near infrared spectroscopy (+48%, P < 0.01), and maximal oxygen consumption in permeabilized muscle fibers (+38%, P = 0.02), which coincided with higher protein levels of muscle mitochondrial enzymes. Primary myotubes from trained women had higher maximal oxygen consumption (+38%, P = 0.03) suggesting that some elements of exercise-induced metabolic programming persists ex vivo. Consistent with this idea, myotubes from trained women had higher mRNA levels of transcriptional regulators of mitochondrial biogenesis in addition to higher protein levels of mitochondrial enzymes.
Conclusion
These data suggest the existence of an “exercise metabolic program”, where primary myotubes isolated from exercise-trained individuals exhibit greater mitochondrial content and oxidative capacity compared to untrained individuals. These myotubes may be a useful model to study molecular mechanisms relevant to exercise adaptations in human skeletal muscle.
Keywords: Human satellite cells, mitochondria, oxidative capacity, exercise
Introduction
Exercise training promotes a broad range of skeletal muscle adaptations including increased oxidative capacity (14–16). Exercise-induced increase in skeletal muscle oxidative capacity is largely driven by an increase in mitochondrial mass known as mitochondrial biogenesis. These adaptations shift skeletal muscle substrate preference during exercise to fatty acids which promotes glycogen sparing and an increase in exercise endurance (16). In addition to improving exercise performance, these adaptations likely play a role in the health benefits of regular physical activity (20). The transcriptional machinery that may mediate exercise induced adaptations in muscle mitochondria have been extensively studied (1,18,22,23) but we are currently limited in our ability to use genetic or pharmacologic approach to explore exercise-induced mechanisms in human skeletal muscle.
Primary muscle cells isolated from human biopsy are a common tool used to understand molecular mechanisms that underlie skeletal muscle metabolic processes (3). For example, primary myotubes derived from patients with type 2 diabetes exhibit impaired insulin-stimulated glucose transport and decreased glycogen synthesis compared to myotubes derived from lean control subjects (12). Primary myotubes derived from obese individuals maintain “metabolic programming” seen in vivo such as reduced fatty acid oxidation (21), insulin signaling (4,21), autophagic flux (5), altered energy metabolism (21), and altered redox status (9). The ability of these cells to maintain the metabolic phenotype of their donor ex vivo make them useful for gain- or loss-of-function studies performed in human cells.
While multiple studies have utilized human primary myotubes to examine how obesity affects skeletal muscle metabolism, less is known if skeletal muscle adaptations promoted by exercise training also persists ex vivo. In one study, exercise-induced alterations in skeletal muscle mitochondrial content persisted in myotubes, but they did not perform functional assays to assess oxidative capacity (7). In another study, exercise training enhanced capacity for myotubes to oxidize some but not all substrates, but their exercise training protocol did not promote a robust increase in mitochondrial biogenesis in muscle biopsy samples (6). Thus, it is unclear whether human primary myotubes are valid models to study exercise-induced skeletal muscle mitochondrial adaptations. Observations that primary myotubes maintain the “exercise metabolic programming” would be of interest especially in light of recent efforts to identify the molecular transducer of physical activity (20). The objective of this study was to compare oxidative capacity of primary myotubes derived from skeletal muscle biopsies from endurance trained or untrained individuals. Therefore, we recruited exercise-trained or untrained women and examined whole-body oxidative capacity, maximal oxygen consumption in muscle biopsies, and maximal oxygen consumption in myotubes derived from these biopsies. Based on classic detraining studies that show a rapid decline in exercise capacity after cessation of regular exercise (8), we initially hypothesized that these myotubes would not maintain the increased oxidative capacity that would be observed in the muscle biopsy samples.
Methods
Study Design
This was a cross sectional study and after pre-screening to determine eligibility, all participants completed three testing visits including 1) an initial screening visit with fasting blood collection, 2) a muscle biopsy during the second visit, and 3) at least one week after the muscle biopsy the subjects performed near infrared spectroscopy (NIRS) testing and a graded maximal oxygen consumption test on a treadmill. This study was approved by the East Carolina University Institutional Review Board and all subjects provided written informed consent prior to participating in research activities.
Participants
All participants for this study were Caucasian female that were free of any known metabolic diseases or heart conditions, non-tobacco users, not taking any medications known to alter metabolism, and either sedentary or endurance exercise trained. Training status was determined by questionnaire and inclusion in this group required women to train 4 or more days per week for 30 or more minutes per day at a moderate to vigorous intensity. The sedentary, untrained women did not participate in any planned, structured physical activity. All testing was performed during the follicular phase of the menstrual cycle.
Skeletal muscle biopsies
Skeletal muscle biopsy samples were taken from the vastus lateralis muscle of the right leg. The subjects were instructed not to exercise for ~ 48 h prior to the muscle biopsy. The skin of the muscle biopsy sight was cleansed with a povidone-iodine swab (or chlorhexidine gluconate for subjects allergic to iodine/shellfish) and then anesthetized with 5 cc of lidocaine. A small incision was made in the anesthetized skin with a scalpel and the muscle biopsy sample was aspirated through a 5 mm Bergstrom needle. Part of the muscle biopsy sample was immediately flash frozen in liquid nitrogen and stored at −80°C until subsequent analysis. Other portions of the muscle biopsy were used for the in situ respiration experiments (~10–20 mg) or placed in low glucose DMEM for primary cell culture (~50–100 mg).
Maximal oxygen consumption in permeabilized muscle fibers
A small portion of fresh muscle tissue from the biopsy sample was placed into a small plastic dish on ice containing buffer X (7.23 mM K2EGTA, 2.77 mM Ca K2EGTA, 20 mM imidazole, 20 mM taurine, 5.7 mM ATP, 14.3 mM phosphocreatine, 6.56 mM MgCl2.6H2O, and 50 mM K-MES, pH = 7.1) prior to respiratory analysis. Using fine-tipped forceps the muscle fiber bundles were separated under a MX6 Stereoscope (Leica Microsystems, USA) so that substrate availability to individual myofibers was maximized. Separated fiber bundles were permeabilized for 30 min at 4°C with saponin (30 μg/ml), followed by being washed in buffer Z (105 mM K-MES, 30 mM KCl, 10 mM K2HPO4, 5 mM MgCl2.6H2O, 0.5 mg/ml BSA, and 1 mM EGTA, pH = 7.4) for 15 min, and lastly placed in the OROBOROS Oxygraph-2k chamber for high-resolution O2 consumption measurements in buffer Z with 20 mM creatine and 10 μM blebbistatin to inhibit myosin ATPases. The chamber was hyperoxygenated to ~320 pmol and a mixed substrate respiration protocol was utilized which started with palmitoyl-CoA (50 μM) + carnitine (5mM) which was followed by sequential additions of malate (0.5 mM), ADP (4 mM), pyruvate (5 mM), glutamate (10 mM), succinate (10 mM), cytochrome c (10 μM), and two additions of FCCP (0.5 μM and 0.75 μM, respectively).
Primary cell culture
Fresh muscle was used for primary cell culture as described previously (3). Briefly, primary cells were isolated, incubated on a non-collagen coated plate to remove fibroblasts, and then transferred to and grown on a collagen coated T-25 flask. The cells were propagated and frozen down at passage two and kept frozen until experiments. All experiments were performed on cells at passage four.
Maximal oxygen consumption in skeletal muscle myotubes
Oxygen consumption rates in primary myotubes were measured with a Seahorse Flux Analyzer XFe96 (Seahorse Bioscience, Billerica, MA). Myoblasts were plated (20,000 cells/well) on a Seahorse XFe96 plate and then differentiated for 5–6 d into myotubes with differentiation media (DMEM, 2% horse serum, 0.3% BSA, 0.05% Fetuin, and 100 mg/ml Pen/Strep). On the day of the experiment, media was switched to XF Assay Medium Modified DMEM (pH = 7.4) containing added glucose (10 mM), pyruvate (200 mM), and glutamine (200 mM) for 1 h. Subsequently basal and maximal respiration rates were measured during a mitochondrial stress test using the Seahorse XFe96. The protocol for each measurement during the test included a 3 min mixing step, a 30 s wait period, followed by a 3 min respiration measurement. This “mix, wait, measure” phase of each measurement cycle was performed 3 consecutive times during the basal, oligomyin (2 μM), carbonilcyanide p-triflouromethoxyphenylhydrazone (FCCP, 1 μM), and rotenone/antimycin A (5 μM and 2 μM, respectively) phases of the mitochondrial stress test.
Succinate dehydrogenase activity
Succinate dehydrogenase (SDH) activity was measured by calculating the rate of decrease in absorbance resulting from the reduction of 2,6-dichlorophenolindophenol (DCPIP) in tissue or cell homogenate at 37°C (2). Initially, the sample was pre-incubated in 0.5 M succinate to get full activity of the enzyme (2). Following the incubation, a cuvette was filled with 1 ml of SQR medium (10 mM KH2PO4, 2 mM EDTA, 1 mg/ml BSA, pH = 7.8), 100 μg of protein of pre-incubated sample, 4 μM rotenone, 0.2 mM ATP, 10 mM succinate, and 80 μM of DCPIP. After measuring the background absorbance rate, 80 μM of decylubiquinone was added to start the reaction and the activity was measured for 5 min using a Shimadzu UV-1800 UV/Visible Scanning Spectrohotometer.
Western blotting
Muscle tissue or cells were homogenized in Lysis Buffer (C2978, Sigma-Aldrich), nutated at 4°C for 1 h, centrifuged at 4°C for 15 min at 12,000 g, and the supernatant was transferred to a new tube and stored at −80°C. Western blotting was performed as previously described (10) and samples were analyzed for the protein abundance of Complex V - ATP5A (ab14748, Abcam), Complex III – UQCRC2 (ab14745, Abcam), and Complex II - SDHB (ab14714, Abcam), and the loading control actin (A2066, Sigma-Aldrich).
Quantitative real time polymerase chain reaction
Primary myotubes were lysed in 1 ml of Trizol (ThermoFisher) and RNA was isolated. Total RNA was reverse transcribed using the IScriptTM cDNA synthesis kit and quantitative PCR was performed with SYBR Green® reagents (ThermoFisher) and the pre-validated human primers used were PGC1α, PGC1β, PPAR α, PPAR γ, CPT1β, ACADL, COX IV, TFAM, and SDH A-D. The sequence for these primers was identified online at the website human primer depot (https://primerdepot.nci.nih.gov/) and primers were ordered from ThermoFisher. Cycle numbers were normalized to the mRNA levels of GAPDH which were not different between the groups (data not shown).
Graded exercise testing
All participants performed a maximal oxygen consumption test to exhaustion on a motor driven treadmill (Cardiac Science, Bothell, WA) as previously described (11). Indirect calorimetry was used to measure oxygen consumption (ParvoMedics’ TrueOne 2400 metabolic cart, Salt Lake City, Utah). The progressive exercise protocol started with a treadmill velocity of 3.5 mph at 0% grade. Every 2 min thereafter the grade was increased 2% until volitional fatigue.
Near-infrared spectroscopy
In vivo skeletal muscle mitochondrial respiratory capacity measurements were made on the right leg of each subject using near-infrared spectroscopy (NIRS), as described previously (25). Briefly, the NIRS probe was positioned and secured on the vastus lateralis muscle of the right leg ~10 cm above the knee and a blood pressure cuff was placed around the same leg as close to the hip as possible. The NIRS protocol involved a short duration (~10–20 s) isometric contraction of the quadriceps muscle group to increase skeletal muscle oxygen consumption (mVO2) which was immediately followed by measurement of the recovery kinetics of mVO2 using a series of transient arterial occlusions. The slope of the post-exercise mVO2 was calculated for each occlusion, and the recovery kinetics of mVO2 were fit to a monoexponential curve. The data is expressed as a rate constant which is considered a direct indicator of the maximal skeletal muscle oxidative capacity.
Statistical Analysis
A t-test was used to determine statistically significant differences between untrained and endurance exercise trained women for each outcome. Statistical significance was set at P ≤ 0.05. All data are presented as mean ± S.E.M. unless otherwise noted.
Results
Participant characteristics
The untrained and endurance exercise trained women were matched for age, weight, body mass index, and blood profiles (Table 1). The exercise trained women had higher maximal oxygen consumption compared to their untrained counterparts (P = 0.03).
Table 1.
Participant Characteristics
| Untrained (N = 9) | Trained (N = 6) | P Value | |
|---|---|---|---|
| Age (y) | 25.0 ± 8.3 | 24.7 ± 4.7 | 0.93 |
| Height (m) | 1.6 ± 0.1 | 1.7 ± 0.1 | 0.09 |
| Weight (kg) | 61.7 ± 4.6 | 62.8 ± 6.6 | 0.71 |
| BMI (kg/m2) | 23.5 ± 2.7 | 22.3 ± 1.6 | 0.34 |
| Waist Circumference (cm) | 72.0 ± 5.9 | 68.7 ± 4.0 | 0.29 |
| Hip Circumference (cm) | 95.6 ± 3.8 | 93.6 ± 3.4 | 0.33 |
| Waist / Hip Ratio | 0.75 ± 0.04 | 0.73 ± 0.03 | 0.41 |
| Cholesterol (mmol/L) | 4.4 ± 0.7 | 4.8 ± 0.7 | 0.25 |
| LDL (mmol/L) | 2.4 ± 0.8 | 2.5 ± 0.9 | 0.75 |
| HDL(mmol/L) | 1.6 ± 0.4 | 1.9 ± 0.4 | 0.22 |
| Triglyceride (mmol/L) | 0.8 ± 0.4 | 0.9 ± 0.5 | 0.74 |
| Insulin (mU/L) | 7.0 ± 3.1 | 5.0 ± 1.9 | 0.19 |
| Glucose (mmol/L) | 4.7 ± 0.4 | 4.6 ± 0.6 | 0.84 |
| HOMA-IR | 1.5 ± 0.7 | 1.0 ± 0.4 | 0.21 |
| Max O2 Consumption (ml.kg−1.min−1) | 39.0 ± 7.9 | 46.2 ± 0.3 | 0.03 |
Data are presented as means ± S.D.
Maximal oxygen consumption and mitochondrial content of skeletal muscle
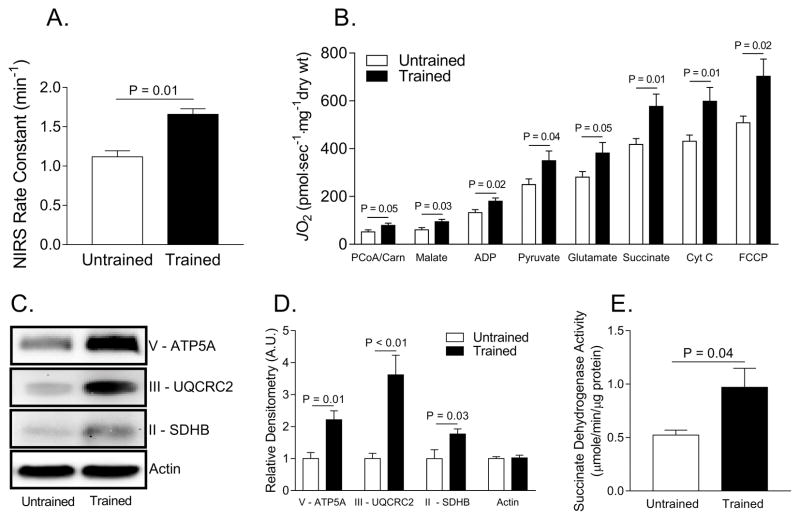
Trained women, compared to untrained women, had 48% higher skeletal muscle maximal respiration in vivo as assessed by NIRS (P = 0.01) (Figure 1A). Additionally, across a range of different substrates that were used, respiratory rates measured in permeabilized muscle fibers were higher in trained individuals compared to untrained individuals (P ≤ 0.05) (Figure 1B). The higher skeletal muscle respiration rates were due to increased mitochondrial content, as trained women had 120% higher Complex V (P=0.01), 260% higher Complex III (P<0.01), and 77% higher Complex II (succinate dehydrogenase, SDH) protein abundance (P=0.03) as well as 85% higher SDH activity (P=0.04) (Figure 1C–E).
Figure 1. Exercise-trained women have higher skeletal muscle maximal oxygen consumption than untrained women.
A. Skeletal muscle near infrared spectroscopy (NIRS) rate constant between untrained and trained women. B. High resolution oxygen consumption measurements in permeabilized muscle fibers.C. Representative Western blot images of mitochondrial Complex V, III, and II. D. Relative densitometry quantification of Western blot images pictured in C. E. Succinate dehydrogenase activity measured in muscle homogenate. Abbreviations: Adenosine diphosphate (ADP), adenosine triphosphate synthase alpha subunit 1 (ATP5A), carbonilcyanide p-triflouromethoxyphenylhydrazone (FCCP), carnitine (carn), cytochrome c (Cyt c), palmitoyl coenzyme A (PCoA), succinate dehydrogenase (SDH), ubiquinol-cytochrome c reductase core protein II (UQCRC2).
Maximal oxygen consumption of primary myotubes
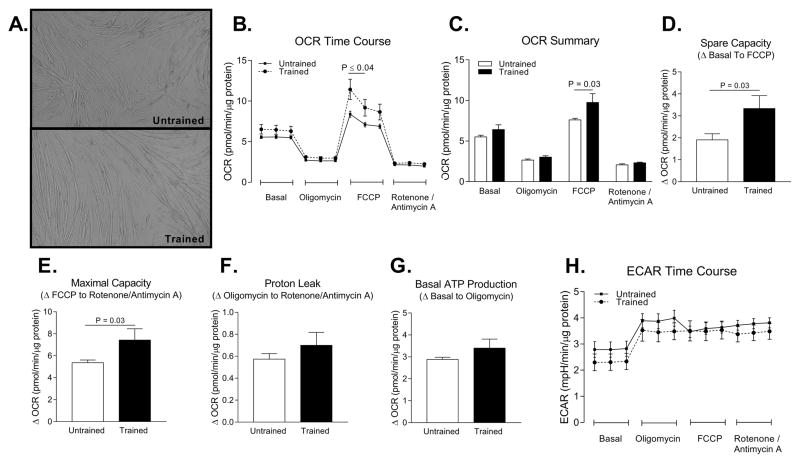
Primary muscle cells from untrained and endurance trained women differentiated into myotubes at similar rates and their gross morphology appeared normal (Figure 2A). Primary myotubes from endurance trained women had 38% higher maximal FCCP stimulated oxygen consumption compared to untrained women (P = 0.03) (Figure 2B–E). There was no statistically significant difference (all P > 0.05) in proton leak, basal ATP production, or extracellular acidification rate between trained and untrained primary myotubes (Figure 2F–H).
Figure 2. Myotubes from endurance trained women have higher maximal oxygen consumption compared to myotubes from untrained women.
A. Representative images of differentiated myotubes from untrained and trained women. B. Time course of oxygen consumption rates (OCR) during a mitochondrial stress test in myotubes from untrained and trained women. C. Average OCR during each phase of the mitochondrial stress test. D. Spare capacity of myotubes (OCRFCCP – OCRbasal). E. Maximal capacity of myotubes (OCRFCCP – OCRrotenone/antimycin A). F. Proton leak of myotubes (OCRoligomycin – OCRrotenone/antimycin A). G. Basal ATP production (OCRbasal – OCRoligomycin). H. Extracellular acidification rate (ECAR) time course during mitochondrial stress test. Abbreviation: carbonilcyanide p-triflouromethoxyphenylhydrazone (FCCP)
Mitochondrial mRNA and protein, and succinate dehydrogenase activity in myotubes
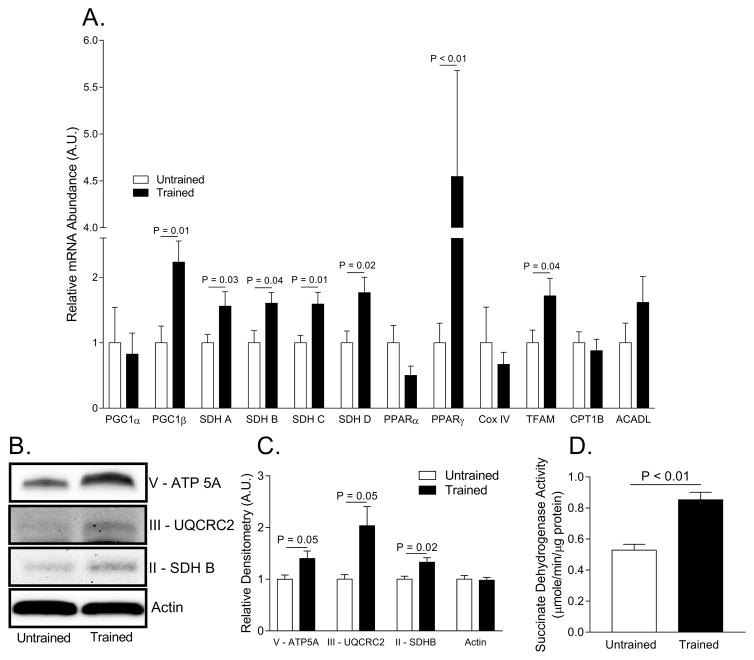
Primary myotubes from endurance trained women had an epigenetic “exercise metabolic program” as evidenced by higher mRNA abundance of several genes associated with mitochondrial biogenesis including PGC1β (+223%), TFAM (+72), PPAR γ (+455%), SDHA (+56%), SDHB (+60%), SDHC (+59%), and SDHD (+77%) (Figure 3A). The persistence of this epigenetic signature in primary myotubes raised in vitro likely attributed to the 40% higher Complex V (P = 0.05), 100% higher Complex III (P = 0.05), and 33% higher Complex II (P = 0.02) protein abundance along with the 61% higher SDH activity in primary myotubes (P < 0.01) (Figure 3B–D).
Figure 3. Evidence for greater mitochondrial content in myotubes from trained women than in untrained women.
A. Relative mRNA abundance of mitochondrial enzymes and transcriptional regulators of mitochondrial biogenesis. Cycle numbers were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). B. Representative Western blot images of mitochondrial enzymes Complex V, III, and II. C. Relative densitometry quantification of Western blot images pictured in B. D. Succinate dehydrogenase activity measured in cell homogenate. Abbreviations: Adenosine triphosphate synthase alpha subunit 1 (ATP5A), carnitine palmitoyltransferase 1B (CPT1B), cytochrome c oxidase subunit IV (Cox IV), long chain acyl-CoA dehydrogenase (ACADL), mitochondrial transcription factor (TFAM), peroxisome proliferator-activated receptor gamma coactivator 1 alpha or beta (PGC1α, PGC1β), peroxisome proliferator-activated receptor alpha or gamma (PPARα or γ), succinate dehydrogenase A–D (SDH A–D), ubiquinol-cytochrome c reductase core protein II (UQCRC2).
Discussion
The goal of this study was to test if primary myotubes derived from exercise-trained women exhibit greater mitochondrial content and oxidative capacity compared to myotubes obtained from untrained women. We initially hypothesized that these myotubes would not demonstrate “exercise metabolic programming” based on the following reasons. First, primary myoblasts themselves do not undergo muscle contraction during exercise, so it would be reasonable to assume that they may not be subject to the exercise-induced signals that promote skeletal muscle mitochondrial biogenesis. Second, even if these cells may initially demonstrate greater oxidative capacity, we predicted that such adaptation would fade away while cells are cultured and propagated ex vivo for a month. However, we show that primary muscle cells isolated from exercise-trained skeletal muscle and grown in vitro maintain greater oxidative capacity compared to untrained donors cells. Our findings are in line with previous observations demonstrating that some exercise adaptations persist in primary myotubes grown in vitro (6,7). Together, these data suggest that primary myotubes isolated from exercise-trained individuals maintain the “exercise metabolic program” for enhanced oxidative metabolism ex vivo.
Skeletal muscle primary cells, also known as resident satellite cells, are located outside the muscle sarcolemma layer but beneath the basement lamina and are quiescent mono-nucleated myogenic cells. These cells are activated during normal muscle maintenance or when muscle is damaged, both of which will signal the cells to differentiate in vivo to replace old or damaged muscle. The close proximity of primary cells with myofibers is important for myofiber-primary cell communication, which can occur via cell-adhesion proteins (17), diffusible growth factors or myokines (26), or mechanical, chemical, and electrical activity (27). From the present work it is apparent that repeated sessions of exercise results in adaptive responses in these primary muscle cells. It is not clear whether these adaptations are triggered by communication from the proximate myofibers, or whether they are results of changes in systemic circulating factors that occur with exercise.
Fifty years ago, John Holloszy made the seminal discovery that exercise training promotes skeletal muscle mitochondrial biogenesis and increases oxidative capacity (13,14). A number of studies since then explored the molecular mechanisms that regulate skeletal muscle mitochondrial biogenesis (1,18,22,23). In myotubes from trained vs. untrained individuals, mRNA abundance for TFAM, PGC1β, and PPARγ were elevated, suggesting that transcriptional machinery similar to those activated in the myofibers potentially play a role in the increased respiratory capacity in these cells. Surprisingly, we did not find a difference in PGC1α mRNA abundance, a transcriptional coactivator that is often referred to as a “master” regulator of mitochondrial biogenesis. Prior work has shown that in mouse skeletal muscle, PGC1α is not required for exercise induced mitochondrial biogenesis (24), so it is possible that exercise metabolic programming in these myotubes occurs independent of PGC1α. Another possibility is that PGC1α was only transiently increased with exercise, and that the sustenance of greater mitochondrial mass in myotubes does not require persistently elevated PGC1α. Regardless of the transcriptional mechanism, we found that primary myotubes from trained women had higher mRNA and protein levels of SDH, a classic marker of mitochondrial content. The enzyme SDH is a critical component of fatty acid oxidation and is involved in both the electron transport chain and Krebs cycle as its function is to catalyze the oxidation of succinate to fumarate in the Krebs cycle in parallel with the reduction of ubiquinone to ubiquinol in the electron transport chain. The increased levels of SDH likely increased the capacity for fatty acid oxidation which in turn contributed to the greater maximal oxygen consumption we observed.
In the current study, we compared primary myotubes isolated and differentiated from muscle biopsies from untrained and trained female subjects. While our data demonstrate greater mitochondrial mass and oxidative capacity in myotubes from trained vs. untrained women, we cannot rule out a possibility that these differences came from genetic differences that predisposed these subjects to be untrained/trained. In other words, the cross-sectional nature of this study does not allow us to conclude that the differences in the myotubes came strictly from the effect of exercise training. In our future studies we will investigate whether similar differences can be observed between pre- and post-exercise training. It is worth noting that all of our experiments were carried out at passage four. Prior work has shown that the metabolic characteristics of myotubes gradually fade after each passage (7,19). Thus, it would be of use to identify how many passages the “exercise metabolic program” can persist in these myotubes.
In conclusion, we show that primary myotubes isolated and differentiated from muscle biopsies of exercise trained women exhibit greater oxidative capacity than those from untrained sedentary women. These results suggest that primary myotubes from endurance trained skeletal muscle have an imprinted epigenetic “exercise metabolic program” that persists after being propagated in vitro. These properties make these primary myotubes an intriguing human model to study the molecular mechanism for exercise adaptations.
Acknowledgments
This project was supported by grants NIH DK107397, DK109888, DK095774 (to K.F.), HL125695 (to J.M.M.), DK110656 (to P.D.N.), HL129632 (to T.E.R.), DK109556, RWXE2148, and AHA 16POST30980047 (to T.D.H.). We have no conflict of interest to report. The results of the present study do not constitute endorsement by ACSM and are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
We would like to thank Angela H. Clark for helping collect blood samples.
Footnotes
Conflict of Interest
We have no conflict of interest to report. The results of the present study do not constitute endorsement by ACSM and are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
References
- 1.Arany Z, Lebrasseur N, Morris C, et al. The Transcriptional Coactivator PGC-1β Drives the Formation of Oxidative Type IIX Fibers in Skeletal Muscle. Cell Metab. 2007;5(1):35–46. doi: 10.1016/j.cmet.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Barrientos A. In vivo and in organello assessment of OXPHOS activities. Methods. 2002;26(4):307–316. doi: 10.1016/S1046-2023(02)00036-1. [DOI] [PubMed] [Google Scholar]
- 3.Berggren JR, Tanner CJ, Houmard JA. Primary cell cultures in the study of human muscle metabolism. Exerc Sport Sci Rev. 2007;35(2):56–61. doi: 10.1249/JES.0b013e31803eae63. [DOI] [PubMed] [Google Scholar]
- 4.Bikman BT, Zheng D, Reed MA, Hickner RC, Houmard JA, Dohm GL. Lipid-induced insulin resistance is prevented in lean and obese myotubes by AICAR treatment. AJP Regul Integr Comp Physiol. 2010;298(6):R1692–R1699. doi: 10.1152/ajpregu.00190.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bollinger LM, Powell JJS, Houmard JA, Witczak CA, Brault JJ. Skeletal muscle myotubes in severe obesity exhibit altered ubiquitin-proteasome and autophagic/lysosomal proteolytic flux. Obesity (Silver Spring) 2015;23(6):1185–1193. doi: 10.1002/oby.21081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bourlier V, Saint-Laurent C, Louche K, et al. Enhanced Glucose Metabolism Is Preserved in Cultured Primary Myotubes From Obese Donors in Response to Exercise Training. J Clin Endocrinol Metab. 2013;98(9):3739–3747. doi: 10.1210/jc.2013-1727. [DOI] [PubMed] [Google Scholar]
- 7.Covington JD, Myland CK, Rustan AC, Ravussin E, Smith SR, Bajpeyi S. Effect of serial cell passaging in the retention of fiber type and mitochondrial content in primary human myotubes. Obesity (Silver Spring) 2015;23(12):2414–2420. doi: 10.1002/oby.21192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coyle EF, Martin WH, Sinacore DR, Joyner MJ, Hagberg JM, Holloszy JO. Time course of loss of adaptations after stopping prolonged intense endurance training. J Appl Physiol. 1984;57(6):1857–1864. doi: 10.1152/jappl.1984.57.6.1857. [DOI] [PubMed] [Google Scholar]
- 9.Fisher-Wellman KH, Weber TM, Cathey BL, et al. Mitochondrial respiratory capacity and content are normal in young insulin-resistant obese humans. Diabetes. 2014;63(1):132–141. doi: 10.2337/db13-0940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Funai K, Cartee GD. Contraction-stimulated glucose transport in rat skeletal muscle is sustained despite reversal of increased PAS-phosphorylation of AS160 and TBC1D1. J Appl Physiol. 2008;105(6):1788–1795. doi: 10.1152/japplphysiol.90838.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heden TD, Liu Y, Kearney ML, et al. Prior exercise and postprandial incretin responses in lean and obese individuals. Med Sci Sports Exerc. 2013;45(10):1897–1905. doi: 10.1249/MSS.0b013e318294b225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henry RR, Abrams L, Nikoulina S, Ciaraldi TP. Insulin Action and Glucose Metabolism in Nondiabetic Control and NIDDM Subjects: Comparison Using Human Skeletal Muscle Cell Cultures. Diabetes. 1995;44(8):936–946. doi: 10.2337/diab.44.8.936. [DOI] [PubMed] [Google Scholar]
- 13.Holloszy JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem. 1967;242(9):2278–2282. [PubMed] [Google Scholar]
- 14.Holloszy JO. Biochemical adaptations to exercise: aerobic metabolism. Exerc Sport Sci Rev. 1973;1:45–71. [PubMed] [Google Scholar]
- 15.Holloszy JO. Muscle metabolism during exercise. Arch Phys Med Rehabil. 1982;63(5):231–234. [PubMed] [Google Scholar]
- 16.Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol. 1984;56(4):831–838. doi: 10.1152/jappl.1984.56.4.831. [DOI] [PubMed] [Google Scholar]
- 17.Irintchev A, Zeschnigk M, Starzinski-Powitz A, Wernig A. Expression pattern of M-cadherin in normal, denervated, and regenerating mouse muscles. Dev Dyn. 1994;199(4):326–337. doi: 10.1002/aja.1001990407. [DOI] [PubMed] [Google Scholar]
- 18.Lin J, Wu H, Tarr PT, et al. Transcriptional co-activator PGC-1α drives the formation of slow-twitch muscle fibres. Nature. 2002;418(6899):797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 19.Nehlin JO, Just M, Rustan AC, Gaster M. Human myotubes from myoblast cultures undergoing senescence exhibit defects in glucose and lipid metabolism. Biogerontology. 2011;12(4):349–365. doi: 10.1007/s10522-011-9336-5. [DOI] [PubMed] [Google Scholar]
- 20.Neufer PD, Bamman MM, Muoio DM, et al. Understanding the Cellular and Molecular Mechanisms of Physical Activity-Induced Health Benefits. Cell Metab. 2015;22(1):4–11. doi: 10.1016/j.cmet.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 21.Paran CW, Verkerke ARP, Heden TD, et al. Reduced efficiency of sarcolipin-dependent respiration in myocytes from humans with severe obesity. Obesity (Silver Spring) 2015;23(7):1440–1449. doi: 10.1002/oby.21123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parisi MA, Clayton DA. Similarity of human mitochondrial transcription factor 1 to high mobility group proteins. Science. 1991;252(5008):965–969. doi: 10.1126/science.2035027. [DOI] [PubMed] [Google Scholar]
- 23.Picca A, Lezza AMS. Regulation of mitochondrial biogenesis through TFAM–mitochondrial DNA interactions. Mitochondrion. 2015;25:67–75. doi: 10.1016/j.mito.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Rowe GC, El-Khoury R, Patten IS, Rustin P, Arany Z. PGC-1α is Dispensable for Exercise-Induced Mitochondrial Biogenesis in Skeletal Muscle. In: Dzeja P, editor. PLoS One. 7. Vol. 7. 2012. p. e41817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryan TE, Brophy P, Lin C-T, Hickner RC, Neufer PD. Assessment of in vivo skeletal muscle mitochondrial respiratory capacity in humans by near-infrared spectroscopy: a comparison with in situ measurements. J Physiol. 2014;592(Pt 15):3231–3241. doi: 10.1113/jphysiol.2014.274456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tatsumi R, Allen RE. Active hepatocyte growth factor is present in skeletal muscle extracellular matrix. Muscle Nerve. 2004;30(5):654–658. doi: 10.1002/mus.20114. [DOI] [PubMed] [Google Scholar]
- 27.Tatsumi R, Sheehan SM, Iwasaki H, Hattori A, Allen RE. Mechanical stretch induces activation of skeletal muscle satellite cells in vitro. Exp Cell Res. 2001;267(1):107–114. doi: 10.1006/excr.2001.5252. [DOI] [PubMed] [Google Scholar]