Abstract
Objective
To evaluate whether a mobile health application that employs elements of social game design could compliment medical care for unresolved concussion symptoms.
Design
Phase I and Phase II (open-label, non-randomized, ecological momentary assessment methodology).
Setting
Outpatient concussion clinic.
Participants
Youth, aged 13–18 years, with concussion symptoms 3+ weeks after injury; Phase I: n = 20; Phase II: n = 19.
Interventions
Participants received standard of care for concussion. The experimental group also used a mobile health application as a gamified symptoms journal.
Outcome measures
Phase I: feasibility and satisfaction with intervention (7-point Likert scale, 1 high). Phase II: change in SCAT-3 concussion symptoms (primary), depression and optimism.
Results
Phase 1: A plurality of participants completed the intervention (14 of 20) with high use (110 +/− 18% play) and satisfaction (median +/− interquartile range (IQR) = 2.0+/− 0.0). Phase II: Groups were equivalent on baseline symptoms, intervention duration, gender distribution, days since injury and medication prescription. Symptoms and optimism improved more for the experimental than for the active control cohort (U = 18.5, p = 0.028, effect size r = 0.50 and U = 18.5, p = 0.028, effect size r = 0.51, respectively).
Conclusions
Mobile apps incorporating social game mechanics and a heroic narrative may promote health management among teenagers with unresolved concussion symptoms.
Keywords: Rehabilitation, intervention, children, concussion, mobile health, gaming
The World Health Organization task force named concussion a public health problem in 2004 [1], yet treatment options for concussion, or mild Traumatic Brain Injury (mTBI), remain limited today. The American Academy of Neurology recommends limiting cognitive and physical effort and prohibiting sports involvement until a concussed individual is asymptomatic without medication [2]; however, this level of physical, cognitive and social inactivity represents a lifestyle change with its own risk factors, including social isolation, depression and increased incidence of suicidal ideology [3]. In addition, cognitive rest often involves limiting screen stimulation associated with popular modes of interpersonal interaction, such as text messaging, social networking on digital platforms (e.g. Facebook, Twitter, Instagram) and multiplayer video gaming, thereby blocking common avenues for social connection [4]. The impact of social connections on health has been well established within the field of network science [5], but these findings have been slow to change traditional health-care practices. A critical need exists for interventions that might improve concussion symptoms faster while mitigating risks associated with prescribed inactivity and providing avenues for continued social connection.
Youth are especially at risk with regard to concussion [6]. The rate of reported concussion in high school athletes has doubled since 2005 [7], and youth demonstrate more prolonged recovery from symptoms [8–10]. Concussion symptoms can include a variety of complaints: headaches, confusion, depression, sleep disturbance, fatigue, irritability, agitation, anxiety, dizziness, difficulty concentrating or thinking clearly, sensitivity to light and noise [11], impaired cognitive function [12–14] and impaired postural control [15,16]. These symptoms create the need to manage academic and physical activity adjustments prescribed to treat the injury. Lack of resolution of such symptoms poses real-life consequences for educational progress and psychosocial–behavioural development among youth.
Mobile health platforms have been used to track symptoms, aid treatment and provide support among individuals with concussion and other diagnoses [17–20]. Framing such mobile health platforms as games has been proposed as a creative way to treat health issues [4,21]. In particular, the potential to engage social support through multiplayer interactive mechanics and reframe the work of health recovery as a personally relevant, heroic narrative using games has been noted as a promising new area for exploration in health care [4,22]. We sought to evaluate the use of one such mobile health application (app), using social, game-like interactive mechanics, as a way to complement the medical management of unresolved concussion symptoms.
The app we evaluated, called SuperBetter, was designed to apply principles of positive psychology [23,24], social interaction and gameful design—an approach that aims to evoke the psychological strengths of game play such as ‘optimism, creativity, courage, and determination’ in non-game applications and reality [21]—to personal challenges like recovery from concussion [4,21]. Specifically, this app reframes factors that might negatively or positively impact health, respectively, as ‘bad guys’ (e.g. bright lights, lack of sleep) or ‘power ups’ (e.g. wearing sunglasses or avoiding bright lights, resting when symptoms are mild vs. once they become severe). Furthermore, this app incorporates social mechanics by allowing participant to invite supportive individuals as ‘allies’ with the ability to view logged activity regarding their health journey and send encouraging prompts through the app. For instance, a player might report that they battled the headache bad guy and did or did not conquer this personal bad guy on a specific day; an ally viewing this post could send an encouraging message or a virtual ‘achievement award’ to the player to explicitly recognize the work the player has committed thus far to recovery; after a few days of play, the app enables the player to graph the time course of their progress toward vanquishing the headache bad guy as well as revisit supportive messages they have received throughout their battles. Using this framework of bad guys, power ups and allies, SuperBetter has been shown previously to improve depression in adults [22].
The study had two aims: 1) To evaluate whether the app would be feasible for use by youth with unresolved concussion symptoms as a complement to standard medical care (Phase I); and 2) To assess whether recovery profiles differed between youth who augmented medical care with the app and those who received medical care alone (Phase II). We hypothesized youth with concussion could successfully use the app; success was defined as having a plurality of participants complete the intervention with better than neutral satisfaction rating and no reported unresolvable barriers to app use. We also hypothesized that symptoms would improve more for those who were able to use the app in conjunction with medical care then for those who received medical care alone.
Methods
Setting
Outpatient pediatric sports medicine clinic, Cincinnati Children’s Hospital Medical Center
Design
Institutional Review Board approved this trial of Phase I feasibility and Phase II efficacy (non-randomized, open label, controlled) using ecological momentary assessment methodologies [20,25] for assessment and intervention.
Participants
Phase I
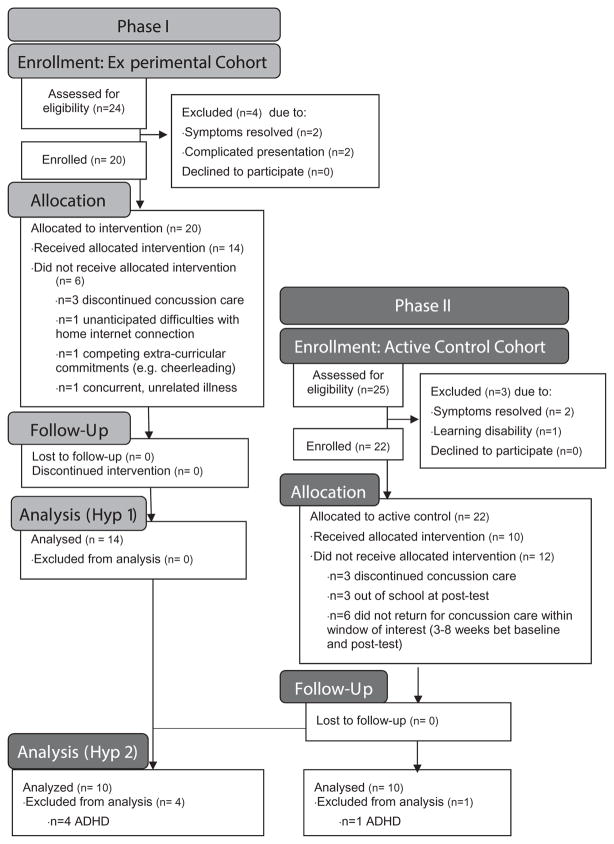
Clinic patients (aged 13–18 years) with physician-diagnosed concussion and unresolved symptoms at 3 weeks to 12 months post injury between 13 August 2014 and 9 December 2014 were screened and recruited as a convenience sample. Follow-up ended on 7 January 2015. Participants were excluded for pre-morbid learning disabilities, concurrent illness/injury at pre-test and complicated or atypical symptom presentation per treating clinician (e.g. symptoms incongruent with cognitive load). Per the funded protocol, we enrolled up to 20 participants within the specified period of grant funding. Enrollees (14 female/6 male; age mean 15. 6+/−1.6, range 13–18 years) received standard medical care plus app use. All 20 participants were included in the Phase I analysis (see CONSORT flow diagram, Figure 1) in which we assessed feasibility of combining app use with medical care as well as satisfaction with intervention (7-point Likert scale; 1 high).
Figure 1.
CONSORT flow diagram.
Phase II
Following completion of the Phase I study, we recruited 22 additional youth (17 female/5 male; age mean 15.6 +/− 1.7, range 13–18 years) from the concussion clinic as a convenience sample into an active control cohort to receive standard medical care. Enrollment occurred from 7 January 7 to 10 June 2015 and from 16 September 16 to 7 October 2015 (Note: no recruitment during summer 2015 due to school vacation). Follow-up lasted through 4 November 2015. Recruitment ended when we achieved a number of active control enrollees comparable to the number of previously enrolled experimental participants from Phase I. Using the following inclusion/exclusion criteria, nine active control participants from the active control cohort and 10 experimental participants from the Phase I cohort were included in an open-label, non-randomized Phase II analysis (see CONSORT flow diagram, Figure 1):
Symptom score of at least four points on Sports Concussion Assessment Tool-3 (SCAT-3 [26]) pre-test. Post-test occurred 3–8 weeks after pre-test. Both pre-and post-tests occurred while school was in session.
No pre-existing attention-deficit/hyperactivity disorder (ADHD), which has been shown to influence concussion symptom recovery [27].
No additional injury (e.g. ankle sprain) or illness (e.g. pneumonia) occurring between pre- and post-test that resulted in parallel medical care for an issue other than concussion.
Experimental group: no self-reported barriers to compliance.
Interventions
All participants received standard of care for unresolved concussion symptoms and were treated by one physician (KL).
Experimental intervention
The app was loaded onto the mobile devices of experimental participants and set to display concussion-specific content that the research team had developed, entitled the Battle Royal Power Pack, which guided participants to track the frequency and severity of 22 concussion symptoms [26]. Using the app for symptoms tracking was similar to documenting symptoms by hand in a journal but translated the symptoms journal concept into a: a) mobile device format, b) personally relevant, heroic narrative and c) platform within which support givers could provide structured support.
Players interacted with in-app content as follows:
Symptoms were represented as bad guys (e.g. headaches, dizziness, feeling confused) and medical recommendations were represented as power ups (e.g. sleep, sunglasses, academic concussion management plan).
Participants invited allies to join their personal network in the app. Our research coordinator was always an ally. The participant had the choice to invite friends and/or family as additional allies who could view their in-app activity and could send resilience points, achievements, comments and personalized emails in response to activity.
Logged activity consisted of any in-app action (Figure 2), such as reporting that a bad guy was battled and how severe the battle was, reporting that a power up was completed, ‘liking’ a comment from an ally or posting a status update in the activity feed.
Figure 2.
Screenshots from three screens encountered during SuperBetter mobile application play (clockwise from top middle): ‘My Challenge’, ‘Superhero To-Do Today’ and ‘Epic Win’.
Participants were asked to log activity at the frequency of one logged activity per day for 5 days each week, for a target dose of 15 logged activities over the first 3 weeks between pre- and post-test. To identify non-compliance and associated barriers, we assessed the number of days the app was used and contacted participants who did not use the app for 4 consecutive days in any 7-day period to ask if they were experiencing any barriers to play. If a barrier was named, our research coordinator reviewed potential strategies to overcome the barrier (e.g. for the stated barrier of ‘having trouble remembering to play’, the strategy to overcome the barrier was to look for the daily message from the app displayed on their mobile device and to take that moment to log activity). If participants showed no additional lapse in app use, then barriers to play were considered resolved. However, if participants showed an additional lapse in app use, defined as an additional 4-day gap in any 7-day period, they were considered to have not completed the intervention and their barriers to compliance were noted.
Outcome measures
Phase I
Primary outcome measure
Number of participants completing the intervention relative to all enrolled.
Secondary measures
App use (%Play), expressed as per cent of target dose in first 3 weeks of intervention;
Satisfaction with intervention, rated on a seven-point Likert scale (1 = high, 4 = neutral, 7 = low);
Barriers to compliance.
Phase II
Primary outcome measure
Concussion symptom severity on the SCAT-3 symptom checklist score. The SCAT-3 is commonly used for concussion assessment on the field and in the clinic [28], as self-reported symptoms are a key component of concussion assessment [29]. The symptom checklist score is a sum across 22 self-report symptom ratings, each ranging in severity from absent (0) to severe (6). Test–retest reliability and receiver operating characteristic (ROC) measures have been established for adolescents (Intraclass Correlation = 0.62, 7-day interval and ROC area under curve = 0.83) [29].
Secondary outcome measures
Optimism, as measured by the Life Orientation Test–Revised (LOT-R) [30]. LOT-R scores are a sum across 10 self-rated items, with higher scores (max = 24) indicating higher optimism. The LOT-R is reliable (Cronbach’s alpha coefficient = 0.70) with marginal gender differences and no linear age trend [31].
Depression, as measured by the Center for Epidemiological Studies–Depression Child (CES-DC) [32]. The CES-DC score ranges from 0 (none) to 60 (severe) and is a sum across 20 self-report items, which are rated in terms of frequency over the past week. The CES-DC is a reliable measure of depression (Cronbach’s alpha coefficient = 0.89) among youth [33].
Analysis
Analyses were completed in IBM SPSS Statistics v24.0 (Armonk, NY: IBM Corp) and Microsoft Excel 2013 (Redmond, WA: Microsoft Corporation). Due to small sample sizes with non-normal distributions, non-parametric statistics were used. Phase I analyses consisted of descriptive statistics for feasibility, utilization and satisfaction. The criterion for feasibility was defined as having a plurality of enrolled participants complete the intervention. Utilization, or %Play, was calculated as actual logged activity in the first 3 weeks divided by target dose (15 logged events). The criterion for satisfaction with the app was defined as median satisfaction rating better than neutral (4) on the Likert scale. Chi-squared tests were used to test satisfaction ratings, compare group differences in categorical baseline variables and evaluate group differences for number of responders on outcome measures. Responders were defined as participants who improved numerically from pre- to post-test. Mann–Whitney U two-sample rank-sum tests were used to compare group differences in non-categorical baseline variables and evaluate group differences on pre- to post-test change scores. Effect size (r) for change scores was calculated by dividing Wilcoxon Z by square root of the combined sample size (n = 19).
Results
Phase I
The app met criterion for feasibility, with a plurality of enrollees (14 of 20, or 70%) completing the intervention. These 14 participants had a median age of 17.0 (interquartile range (IQR) 2.0) years; demonstrated high app utilization (Table I, median %Play = 110%, IQR 22% of target dose) and reported high satisfaction (Figure 3, median Likert scale rating of 2.0, IQR 0.0). Satisfaction criteria were met, indicating the ratings observed were significantly different from what would be expected given a normal distribution with a neutral mean, Chi2 (6) = 183.3, p < 0.0001. Barriers to compliance reported by the six enrollees who did not complete the study included: discontinuation of medical care (n = 3), unanticipated difficulties with home Internet access (n = 1), competing extracurricular activity schedule (n = 1) and concomitant illness during enrollment (n = 1).
Table I.
Use, described as median (IQR) percentage of target dose (%Play) and median satisfaction ratings for the SuperBetter intervention (7-point Likert rating, 1 high) during each study phase.
| Variable | Phase I (n=14) | Phase II (n=10) |
|---|---|---|
| %Play | 110% (22%) | 113% (18%) |
| Satisfaction with intervention | 2.0 (0.0) | 2.0 (0.3) |
| Chi2 statistic, comparison with normally distributed responses around a ‘neutral’ mean | 183.3 (df = 6) | 171.5 (df = 6) |
| p value | <0.0001 | <0.0001 |
Figure 3.
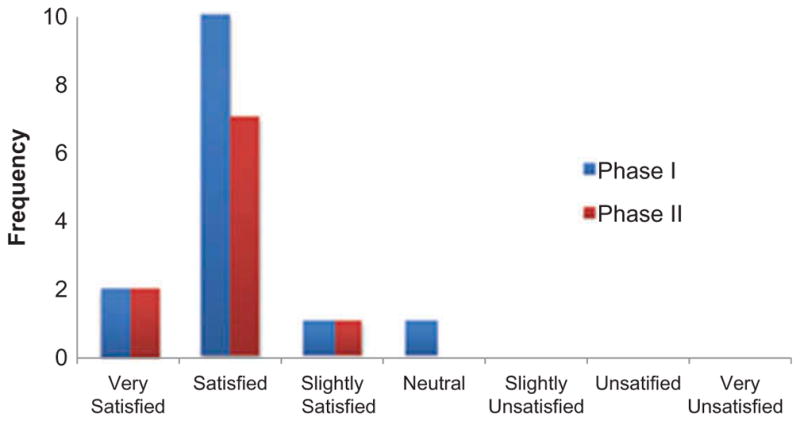
Satisfaction with app was high for both Phase I and Phase II experimental cohorts.
Phase II
Groups were not significantly different at baseline across all factors considered (Table II). App users reported high satisfaction (median 2.0, IQR 0.3) and %Play (median 113%, IQR 18%). Table III summarizes outcome measures. There were significantly more intervention ‘responders’ for SCAT-3 symptoms (p = 0.006) and optimism (p = 0.012) in the experimental group (100% and 80%, respectively) than the control cohort (44% and 22%, respectively). Furthermore, symptoms and optimism improved more for the experimental than for the active control cohort (U = 18.5, p = 0.028, effect size r = 0.50 and U = 18.5, p = 0.028, effect size r = 0.51, respectively). Experimental vs. control group medians (IQR) for symptom change were 16.0 (32.0) and −2.0 (24.0), respectively. Experimental vs. control group medians (IQR) for optimism change were 3.0 (5.0) and 0.0 (1.0), respectively. No significant differences were found between cohorts for depression responders or change in depression scores.
Table II.
Baseline characteristics for Phase II participants expressed as percent of individuals in group with characteristic or median value plus interquartile range (in parentheses) and mean rank (MR).
| Characteristic | Experimental cohort (n=10) | Active control cohort (n=9) | Statistic | p value |
|---|---|---|---|---|
| Gender | Female: 7 (70%) | Female: 7 (78%) | Chi2= 0.148 | 0.701 |
| Male: 3 (30%) | Male: 2 (22%) | |||
| Medication use | Yes: 3 (30%)
|
Yes: 3 (33%)
|
Chi2= 0.024 | 0.876 |
| No: 7 (70%) | No: 6 (67%) | |||
| Prior concussions | Yes: 2 (20%)
|
Yes: 1 (11%)
|
Chi2= 0.281 | 0.596 |
| No: 8 (80%) | No: 8 (89%) | |||
| Age | 17.0 (2.0); MR: 12.0 | 15.0 (2.0): MR: 7.8 | U= 25.0 | 0.096 |
| Social network use prior to intervention | Every day: 10 (100%) | Every day: 7 (78%) | Chi2= 2.484 | 0.115 |
| Never: 0 | Never: 2 (22%) | |||
| Game play prior to intervention | Every day: 0 (0%) | Every day: 2 (22%) | Chi2=3.758 | 0.289 |
| Sometimes: 4 (40%) | Sometimes: 1 (11%) | |||
| Rarely: 4 (40%) | Rarely: 4 (44%) | |||
| Never: 2 (20%) | Never: 2 (22%) | |||
| Symptoms (SCAT-3 baseline) | 19.0 (59.0); MR: 11.3 | 17.0 (29.5); MR: 8.6 | U= 32.0 | 0.288 |
| Optimism (LOT-R baseline) | 17.0 (8.8); MR: 9.7 | 17.0 (3.5); MR: 10.4 | U= 41.5 | 0.773 |
| Depression (CES-DC baseline) | 18.0 (15.5); MR: 12.3 | 13.0 (7.0): MR: 7.5 | U= 22.5 | 0.065 |
| Days elapsed bet baseline and post-test | 30.5 (11.8); MR: 9.9 | 28.0 (19.5); MR: 10.2 | U= 43.5 | 0.902 |
| Days since injury | 30.0 (74.0); MR: 8.2 | 42.0 (12.5); MR: 12.0 | U= 27.0 | 0.141 |
Table III.
Outcome measures by group: median change with interquartile range (IQR) and mean rank (MR) or responder frequency (percentage) in cohort. Group comparison statistics are Mann-Whitney U with effect size r for pre- to post-test change scores and Chi2 for responder frequency.
| Outcome measure | Experimental (n=10) | Active control (n=9) | Statistic | p value |
|---|---|---|---|---|
| Symptoms change (SCAT-3) | 16.0 (32.0); MR:12.7 | −2.0 (24.5); MR: 7.1 | U= 18.5; r= 0.50 | 0.028 |
| Optimism change (LOT-R) | 3.0 (5.0); MR: 12.7 | 0.0 (1.0); MR: 7.1 | U= 18.5; r= 0.51 | 0.028 |
| Depression change (CES-DC) | 8.0 (17.0): MR: 11.8 | 4.0 (7.5); MR: 8.0 | U= 27.0; r= 0.34 | 0.156 |
| Responders: Symptoms | 10 (100%) | 4 (44%) | Chi2= 7.540 | 0.006 |
| Responders: Optimism | 8 (80%) | 2 (22%) | Chi2= 6.343 | 0.012 |
| Responders: Depression | 8 (80%) | 7 (78%) | Chi2= 0.014 | 0.906 |
Finally, post hoc, Spearman correlations between SCAT-3 change and optimism change were calculated and revealed no significant correlations within cohorts (app use: rho = 0.078, p = 0.830; control: rho = 0.248, p = 0.521) or across groups (rho = 0.386, p = 0.102). Furthermore, we found no significant correlation between baseline optimism and SCAT-3 change either within groups (app users: rho = 0.202, p = 0.576; control: rho = 0.298, p = 0.0.436) or across groups (rho = 0.211, p = 0.386).
Discussion
Youth were able to use the SuperBetter app in conjunction with traditional medical care for post-concussive symptoms and were satisfied with use of the app as experienced within this study (hypothesis 1). Additionally, participants who used the app to complement medical care saw more relief from concussion symptoms than those experiencing traditional medical care alone (hypothesis 2). These findings suggest that tapping into existing habits, such as mobile device and social network activity, with a gamified app is a feasible and potentially effective way to facilitate medical care among youth with concussion. To our knowledge, this is the first evidence that a mobile app, designed to reframe the concussion challenge as a personal heroic narrative and leverage social interaction mechanics, can augment concussion care for teens.
Use of the app in combination with standard care also appeared to promote optimism more than standard care alone, regardless of optimism levels at start of the study. We propose that the gameful [21] and/or social interactive design of SuperBetter were effective to improve optimism among users without requiring high optimism at the start of the intervention to ensure participant adoption. These data provide evidence that gameful, social design is possible for use in health care, is successful deployed in this particular mobile app, and is impactful even among those who do not start out optimistic at the commencement of an intervention. Further study is needed to understand the interplay between optimism and recovery in neurorehabilitation for youth, as well as for the general population.
Several potential mechanisms may explain how app use reduces symptom severity and increases optimism. The app may serve to remind participants of concussion management recommendations during intervals between clinic visits. The inclusion of our clinical coordinator as an ally provided a connection to the medical team that may improve patient ‘buy-in’ to medical care and/or compliance with medical recommendations. The encouragement to recruit a small number of high-quality allies may help the participant cope with their illness by guiding them to tap into their social network for support. The cognitive reframing of their concussion recovery journey as a heroic narrative, and the specific constructive actions of game play, may increase optimism and reduce learned helplessness [21] by returning a locus of control to the individual coping with illness. Whether app use increases medical compliance or improves optimism by enhancing social support, positive self-thinking or other constructive coping strategies require further study. However, qualitative feedback from app users provides support for the theories, specifically that: 1) Reframing recovery as a heroic narrative helped them cope with the symptoms of their injury; and 2) Recruiting close allies, combined with positive messaging within the app, helped them feel less isolated in their recovery journey. An early play-tester of the app noted: ‘It’s HARD to ask for help when you’re sick, especially if you are used to being able to take care of yourself. You fear becoming a burden, of sounding like you are constantly complaining, of running out of goodwill from your friends and family. The SuperBetter game provides a framework, through missions, that allows friends and family to have concrete, actionable ways to help me, and provides me with a way to focus on what I CAN do, instead of what I CAN’T. It lets me see, and lets loved ones see, exactly what I go through on a daily basis, and to recognize that those are, in fact, epic wins. It allows me to participate in my life and healing instead of just sitting around waiting to get better’.
Because app use and game play inherently involve active repetition of some mental or physical task, they provide an opportunity, as McGonigal (2011 & 2015) has said, to harness the work and attention that players willingly devote to interactive play in order to improve health [4,21]. The implementation of the SuperBetter app as a youth concussion intervention is a concrete demonstration of this principle. Specifically, pairing the social, mobile app SuperBetter with traditional medical care appears to improve outcomes and optimism for youth with unresolved concussion symptoms. More study is needed to investigate ways that leveraging interactive media may complement medical care and promote health outcomes among youth with concussion and the general population.
Study limitations
Study limitations include small sample size, single testing site and lack of blinding or random assignment to treatment groups. In addition, we did not address technical barriers to participation or potentially clinically important qualitative differences between cohorts (e.g. mechanism of injury, types of concussion symptoms). Finally, findings may not be generalizable to youth with ADHD or adults with concussion, as representatives of these populations were not studied.
Conclusions
SuperBetter improved symptoms and optimism relative to standard medical care alone in a non-randomized, open-label trial. Mobile apps that employ social game mechanics, and that reframe health recommendations as steps within a patient’s personal, heroic narrative, may promote health management among teenagers with unresolved concussion symptoms.
Footnotes
Declaration of interest
Funding was provided by NIH-NICHD SBIR grant 1R43HD075638-01A1. (Clinicaltrials.gov Identifier: NCT01398566). SuperBetter was developed under the auspices of SuperBetter, LLC and is now owned by Cherry Street Innovation, both for-profit organizations. JM founded SuperBetter LLC and is Chief Scientific Officer of Cherry Street Innovation. LWC has served as a Science Advisor to SuperBetter Labs, LLC, on a pro bono basis. No other authors have any conflict to report.
References
- 1.Cassidy JD, Carroll L, Peloso P, Borg J, von Holst H, Holm L, Kraus J, Coronado V. Incidence, risk factors and prevention of mild traumatic brain injury: results of the who collaborating centre task force on mild traumatic brain injury. J Rehabil Med. 2004;36:28–60. doi: 10.1080/16501960410023732. [DOI] [PubMed] [Google Scholar]
- 2.Giza CC, Kutcher JS, Ashwal S, Barth J, Getchius TSD, Gioia GA, Gronseth GS, Guskiewicz K, Mandel S, Manley G, et al. Summary of evidence-based guideline update: evaluation and management of concussion in sports: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;80(24):2250–7. doi: 10.1212/WNL.0b013e31828d57dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broshek DK, De Marco AP, Freeman JR. A review of post-concussion syndrome and psychological factors associated with concussion. Brain Inj. 2015;29(2):228–37. doi: 10.3109/02699052.2014.974674. [DOI] [PubMed] [Google Scholar]
- 4.McGonigal J. Reality is broken : Why games make us better and how they can change the world. New York (NY): Penguin Press; 2011. p. 388. [Google Scholar]
- 5.Christakis NA, Fowler JH. Connected : The surprising power of our social networks and how they shape our lives. New York (NY): Little, Brown and Co; 2009. p. 338. [Google Scholar]
- 6.YEATES KO, Babikian T, Asarnow R, Bazarian JJ, Mcclung J, Shah MN, Ting Cheng Y, Flesher W, Kraus J, Boake C, et al. Mild traumatic brain injury and postconcussive symptoms in children and adolescents. J Int Neuropsychol Soc. 2010;16(6):953–60. doi: 10.1017/S1355617710000986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenthal JA, Foraker RE, Collins CL, Comstock RD. National High School Athlete Concussion Rates From 2005–2006 to 2011–2012. Am J Sports Med. 2014;42(7):1710–5. doi: 10.1177/0363546514530091. [DOI] [PubMed] [Google Scholar]
- 8.McClincy MP, Lovell MR, Pardini J, Collins MW, Spore MK. Recovery from sports concussion in high school and collegiate athletes. Brain Inj. 2006;20(1):33–9. doi: 10.1080/02699050500309817. [DOI] [PubMed] [Google Scholar]
- 9.Lovell MR, Collins MW, Iverson GL, Field M, Maroon JC, Cantu R, Podell K, Powell JW, Belza M, Fu FH. Recovery from mild concussion in high school athletes. J Neurosurg. 2003;98(2):296–301. doi: 10.3171/jns.2003.98.2.0296. [DOI] [PubMed] [Google Scholar]
- 10.Field M, Collins MW, Lovell MR, Maroon J. Does age play a role in recovery from sports-related concussion? A comparison of high school and collegiate athletes. J Pediatrics. 2003;142(5):546–53. doi: 10.1067/mpd.2003.190. [DOI] [PubMed] [Google Scholar]
- 11.Mayo Clinic Staff. Concussion [Internet] Mayo Clinic; 2011. [cited 2007 Feb 10]. Available from http://www.mayoclinic.org/diseases-conditions/concussion/symptoms-causes/dxc-20273155. [Google Scholar]
- 12.Collins MW, Iverson GL, Lovell MR, McKeag DB, Norwig J, Maroon J. On-field predictors of neuropsychological and symptom deficit following sports-related concussion. Clin J Sport Med. 2003;13(4):222–9. doi: 10.1097/00042752-200307000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Erlanger D, Kaushik T, Cantu R, Barth JT, Broshek DK, Freeman JR, Webbe FM. Symptom-based assessment of the severity of a concussion. J Neurosurg. 2003;98(3):477–84. doi: 10.3171/jns.2003.98.3.0477. [DOI] [PubMed] [Google Scholar]
- 14.Sosnoff JJ, Broglio SP, Ferrara MS. Cognitive and motor function are associated following mild traumatic brain injury. Exp Brain Res. 2008;187(4):563–71. doi: 10.1007/s00221-008-1324-x. [DOI] [PubMed] [Google Scholar]
- 15.Guskiewicz KM, Ross SE, Marshall SW. Postural Stability and Neuropsychological Deficits After Concussion in Collegiate Athletes. J Athletic Train. 2001;36(3):263–73. [PMC free article] [PubMed] [Google Scholar]
- 16.Sosnoff JJ, Broglio SP, Shin S, Ferrara MS. Previous mild traumatic brain injury and postural-control dynamics. J Athletic Train. 2011;46(1):85–91. doi: 10.4085/1062-6050-46.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pramana G, Parmanto B, Kendall PC, Silk JS. The SmartCAT: an m-health platform for ecological momentary intervention in child anxiety treatment. Telemed J E Health. 2014;20(5):419–27. doi: 10.1089/tmj.2013.0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewandowski L, Rieger B, Smyth J, Perry L, Gathje R. Measuring post-concussion symptoms in adolescents: Feasibility of ecological momentary assessment. Arch Clin Neuropsychol. 2009;24(8):791–6. doi: 10.1093/arclin/acp087. [DOI] [PubMed] [Google Scholar]
- 19.Suffoletto B, Wagner AK, Arenth PM, Calabria J, Kingsley E, Kristan J, Callaway CW. Mobile phone text messaging to assess symptoms after mild traumatic brain injury and provide self-care support: a pilot study. J Head Trauma Rehabil. 2013;28(4):302–12. doi: 10.1097/HTR.0b013e3182847468. [DOI] [PubMed] [Google Scholar]
- 20.Stone AA, Shiffman S, Atienza AA, Nebeling L. The science of real-time data capture. New York (NY): Oxford University Press; 2007. [Google Scholar]
- 21.McGonigal J. SuperBetter: A revolutionary approach to getting stronger, happier, braver and more resilient–Powered by the Science of Games. New York (NY): The Penguin Press; 2015. p. 466. [Google Scholar]
- 22.Roepke AM, Jaffee SR, Riffle OM, McGonigal J, Broome R, Maxwell B. Randomized controlled trial of superbetter, a smart-phone-based/internet-based self-help tool to reduce depressive symptoms. Games Health J. 2015;4(3):235–46. doi: 10.1089/g4h.2014.0046. [DOI] [PubMed] [Google Scholar]
- 23.Seligman MEP, Csikszentmihalyi M. Flow and the foundations of positive psychology. Dordrecht, Netherlands: Springer; 2014. Positive psychology: An introduction; pp. 279–98. [Google Scholar]
- 24.Seligman MEP, Steen TA, Park N, Peterson C. Positive psychology progress: Empirical validation of interventions. Am Psychol. 2005;60(5):410–21. doi: 10.1037/0003-066X.60.5.410. [DOI] [PubMed] [Google Scholar]
- 25.Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annu Rev Clin Psychol. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- 26.Guskiewicz KM, Register-Mihalik J, McCrory P, McCrea M, Johnston K, Makdissi M, Dvorák J, Davis G, Meeuwisse W. Evidence-based approach to revising the SCAT2: introducing the SCAT3. Br J Sports Med. 2013;47(5):289–93. doi: 10.1136/bjsports-2013-092225. [DOI] [PubMed] [Google Scholar]
- 27.Elbin RJ, Kontos AP, Kegel N, Johnson E, Burkhart S, Schatz P. Individual and combined effects of LD and ADHD on computerized neurocognitive concussion test performance: evidence for separate norms. Arch Clin Neuropsychol. 2013;28(5):476–84. doi: 10.1093/arclin/act024. [DOI] [PubMed] [Google Scholar]
- 28.Broglio SP, Cantu RC, Gioia GA, Guskiewicz KM, Kutcher J, Palm M, McLeod TCV. National athletic trainers’ association position statement: Management of sport concussion. J Athletic Train. 2014;49(2):245–65. doi: 10.4085/1062-6050-49.1.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chin EY, Nelson LD, Barr WB, McCrory P, McCrea MA. Reliability and validity of the sport concussion assessment tool-3 (SCAT3) in high school and collegiate athletes. Am J Sports Med. 2016;44(9):2276–85. doi: 10.1177/0363546516648141. [DOI] [PubMed] [Google Scholar]
- 30.Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): A reevaluation of the life orientation test. J Personal Soc Psychol. 1994;67(6):1063–78. doi: 10.1037/0022-3514.67.6.1063. [DOI] [PubMed] [Google Scholar]
- 31.Glaesmer H, Rief W, Martin A, Mewes R, Brähler E, Zenger M, Hinz A. Psychometric properties and population-based norms of the life orientation test revised (LOT-R) Br J Health Psychol. 2012;17(2):432–45. doi: 10.1111/j.2044-8287.2011.02046.x. [DOI] [PubMed] [Google Scholar]
- 32.Faulstich ME, Carey MP, Ruggiero L, Enyart P, Gresham F. Assessment of depression in childhood and adolescence: an evaluation of the Center for Epidemiological Studies Depression Scale for Children (CES-DC) Am J Psychiatry. 1986;143(8):1024–7. doi: 10.1176/ajp.143.8.1024. [DOI] [PubMed] [Google Scholar]
- 33.Fendrich M, Weissman MM, Warner V. Screening for depressive disorder in children and adolescents: validating the Center for Epidemiologic Studies Depression Scale for Children. Am J Epidemiol. 1990;131(3):538–51. doi: 10.1093/oxfordjournals.aje.a115529. [DOI] [PubMed] [Google Scholar]