Abstract
Objectives
Dysregulation of the cell cycle has been observed and implicated as an etiologic factor in a range of human malignancies, but remains relatively unstudied in neuroendocrine tumors (NETs). We evaluated expression of key proteins involved in cell cycle regulation, in a large cohort of NETs.
Methods
We evaluated immunohistochemical expression of CDKN1B, CDKN1A, CDKN2A, CDK2, CDK4, CDK6, cyclin D1 (CCND1), cyclin E1 (CCNE1) and phospho-RB1 in a cohort of 267 patients with NETs. We then explored associations between cell cycle protein expression, mutational status, histologic features, and overall survival.
Results
We found that high expression of CDK4, CDK6, CCND1, and phospho-RB1 was associated with higher proliferative index, as defined by MKI67. We additionally observed a trend toward shorter overall survival associated with low expression of CDKN1B. This association appeared strongest in SINETs (multivariate hazard ratio, 2.04; 95% confidence interval, 1.06–3.93; P = 0.03). We found no clear association between CDKN1B mutation and protein expression.
Conclusions
Our results suggest that dysregulation and activation of the CDK4 / CDK6-CCND1− phospho-RB1 axis is associated with higher proliferative index in NETs. Investigation of the therapeutic potential of CDK4/CDK6 inhibitors in higher grade NETs is warranted.
Keywords: CDKN1B (p27 / Kip1), CDK4/CDK6, CCND1, phospho-RB1, neuroendocrine tumors, proliferation
INTRODUCTION
The diagnosed incidence of neuroendocrine tumors (NETs) in United States has nearly quintupled in the past few decades, from 1.09 to 5.25 cases per 100,000 each year.1 Although the clinical course of well-differentiated NETs is relative indolent compared to other epithelial malignancies, the prognosis of patients with metastatic NETs remains poor.2 Recent studies have demonstrated that treatment with somatostatin analogs, VEGF pathway inhibitors, and MTOR inhibitors can slow tumor progression in patients with metastatic disease.3–8 However, these agents only rarely cause tumor regression. A more complete understanding of the mechanisms driving tumor growth is needed to identify better therapeutic agents for this indication.
Signaling pathways regulating the cell cycle play a key role in driving the growth of a number of human malignancies.9 A key regulator of the cell cycle is the retinoblastoma protein 1 (RB1). RB1 inhibits the activity of E2F transcription factors; this inhibitory activity is blocked by phosphorylation, which occurs at several different phases of the cell cycle.10,11 Phosphorylation of RB1 is regulated by cyclin D1 (CCND1) early in the cell cycle, and by cyclin E1 (CCNE1) later in the cell cycle. The activity of CCND1 and CCNE1 is dependent on cyclin dependent kinases, including the CDK4/CDK6 complex and CDK2. Cyclin dependent kinases are regulated by cyclin dependent kinase inhibitors (CDK’s), including CDKN1B, CDKN1A, and CDKN2A.
Expression of cell cycle pathway components has been associated with clinical outcomes in a number of human malignancies. High expression of phosphorylated RB1 (phospho-RB1), for example, has been found to be associated with reduced overall survival (OS) in advanced squamous cell carcinoma of head and neck.12 Overexpression of CCND1 has been found to predict poor survival in primary ovarian serous carcinomas,13 periampullary adenocarcinoma,14 extremity soft-tissue sarcomas15 and lung adenocarcinoma.16 Reduced nuclear expression of CDKN1B has been associated with adverse patient outcome in lung cancer, prostate cancer, breast cancer, and esophageal squamous cell carcinoma, among other malignancies.17 Inhibition of activated cell cycle pathways has also resulted in therapeutic benefit. The selective CDK4/CDK6 inhibitor palbociclib (PD0332991), for example, was recently approved by US Food and Drug Administration (FDA) for treatment of advanced, hormone receptor–positive breast cancer.18
The role of cell cycle regulatory proteins in NETs remains relatively unexplored. In small intestine neuroendocrine tumors (SINETs), recurrent mutations and hemizygous deletions have been reported in the cyclin dependent kinase inhibitor CDKN1B.19–21 Loss of CDKN1B has been associated with poor prognosis in a range of NETs.22,23 Overexpression of phospho-RB1, as well as CDK4, has been reported in pancreatic NET.24
To more fully understand the potential role of cell cycle regulators in NETs, we undertook a study broadly evaluating expression of cell cycle regulatory components in a large cohort of NETs. We focused on expression of the key regulator phospho-RB1 and on two key upstream cyclin-cyclin dependent kinase regulatory complexes: CDK4/CDK6-CCND1 and CDK2-CCNE1. We additionally evaluated expression of the cyclin dependent kinase inhibitors: CDKN1B, CDKN1A, and CDKN2A. We then explored associations between expression of these components, clinical and pathologic features of the tumors, and survival.
PATIENTS AND METHODS
Patients and Clinical Data
All patients with confirmed diagnosis of NETs, and the corresponding clinical data were obtained from Dana-Farber Cancer Institute database from 1991 to 2012. All patients provided written informed consents for their tissue blocks for molecular analysis in this study. The study protocol was approved by the institutional review board of Dana-Farber Cancer Institute. Clinical and outcome information was collected from medical records, or obtained from the Social Security Death Index if that was not available.
Construction of Tissue Microarrays
Four tissue microarray (TMA) blocks comprising 336 resection specimens NETs including 267 primary tumor samples, 34 paired metastases and 35 metastases only, from a total of 302 patients, were constructed. As previously described, three representative tissue cores (0.6-mm diameter) were taken from individual formalin-fixed paraffin-embedded tissues respectively and arranged into recipient paraffin blocks.25 Four-μm thick sections were cut from each TMA blocks for immunohistochemical staining.
Immunohistochemistry and Evaluation
For immunohistochemical analysis, the following antibodies were used: Anti-CDKN1B rabbit polyclonal (1:200, Santa Cruz Biotechnology, Dallas, Texas), Anti-CDKN2A mouse monoclonal (1μg/mL, Roche Diagnostics, Indianapolis, Ind.), Anti-CDK2 mouse monoclonal (1:1000, Novus Biologicals, Littleton, Colo), Anti-CDK4 rabbit monoclonal (1:100, Abcam, Cambridge, Mass), Anti-CDK6 mouse monoclonal (1:50, Abcam), Anti-CCND1 rabbit monoclonal (1:100, Abcam), Anti-CDKN1A mouse monoclonal (1:50, BD Biosciences, San Diego, Calif), Anti-CCNE1 mouse monoclonal (1:200, Thermo Fisher Scientific, Rockford, Ill.), Anti-Phospho-RB1 rabbit monoclonal (Ser807/811, 1:100, Cell Signaling Technologies, Danvers, Mass.), and Anti-MKI67 mouse monoclonal (1:100, Dako, Glostrup, Denmark). Tissue microarrays were deparaffinized in xylene and rehydrated ethanol and heated for 17 minutes in citrate buffer (pH 6.0, BioGenex Laboratories, San Ramon, Calif) using a pressure cooker for antigen retrieval. Endogenous peroxidase and nonspecific protein binding were blocked using Dual Endogenous Enzyme Block reagent (Dako) and Protein Block reagent (Dako) for 10 min and 20 min respectively. The sections were incubated with primary antibodies at 4°C overnight and detected with streptavidin-peroxidase (BioGenex) and 3,3’-diaminobenzidine (Dako). Colorectal cancers known to be positive for all ten molecules in this study were used as positive controls,26–34 and these antibodies list in this paragraph corresponding isotypes were used as negative controls (Supplemental Table 1).
A single pathologist (Z.R.Q) reviewed and interpreted the staining TMA, who was blinded to the clinical and pathologic data. As described previously MKI67 labeling index (LI) was evaluated by counting the percentage of positive tumor cells in areas of highest nuclear labeling through high-power fields (×400)35 and cut-off values were taken from American Joint Committee on Cancer staging system (seventh edition).36
Tumor expression levels of CDKN1A, CDKN2A, CDK2, CCND1, CCNE1, and phospho-RB1 were determined by averaging the percentages of immunoreactive (positive staining) tumor cells in three tissue cores.37 The median percentage for each marker, based on analysis of all tumors in the cohort, was used as the cut-off to define low versus high expression. Cut-off percentages were as follows: CDKN1A (> 30%), CDKN2A (≥ 1%), CDK2 (≥ 1%), CCND1 (≥ 1%), CCNE1 (≥ 1%), and phospho-RB1 (≥ 1%). Other three markers expression, CDK4, CDK6, and CDKN1B, were scored by applying a semiquantitative immunoreactivity method (H-score) as described previously.38 In brief, overall staining intensity was scored as 0 (absent), 1 (weak), 2 (moderate), and 3 (strong) in three tissue cores and percentage of positive cells was scored as 0–100%. Multiplication of intensity score and the percentage resulted in an H score ranging from 1 to 300 for each tissue core. The overall H score for each tumor was calculated by averaging the H scores in three tissue cores. The median H score for each marker was used as the cut-off to define low versus high expression. Cut-off H scores were as follows: CDKN1B (> 210), CDK4 (> 150), and CDK6 (> 210).
We selected 30 cases randomly from this cohort and stained on whole sections for each marker, and compared expression level of these markers on whole sections with those on TMA according to different cut-off mentioned above for concordance. The expression level of all markers’ scores showed good consistency in whole sections and TMA except for phospho-RB1, so we had to stain expressions of phospho-RB1 of all cases on whole sections. The evaluation of phospho-RB1 expression level on whole sections was followed as mentioned above.
Random selection of around 100 tumors was reviewed for each marker by a second blind pathologist (Y.M.). The concordance scores between the two observers were consistent as follow: CDKN1B (κ = 0.77; n= 108; 95% confidence interval [CI], 0.63–0.91), CDKN2A (κ = 0.76; n = 107; 95% CI, 0.67–0.85), CDK2 (κ = 0.66; n = 101; 95% CI, 0.28–1.00), CDK4 (κ = 0.78; n = 102; 95% CI, 0.68–0.87), CDK6 (κ = 0.82; n = 103; 95% CI, 0.74–0.90), CCND1 (κ = 0.85; n = 102; 95% CI, 0.63–1.00), CDKN1A (κ = 0.74; n = 108, 95% CI, 0.64–0.85), CCNE1 (κ = 0.78; n = 106; 95% CI, 0.66–0.91), and Phospho-RB1 (κ = 0.67; n = 104, 95% CI, 0.56–0.77).
Mutation of CDKN1B
CDKN1B sequencing and mutation status was assessed as previously reported.19
Statistical Analysis
The χ2 test or Fisher’s exact test when applicable was performed to determine associations between the categorized expression status and the tumor subgroups. The Spearman rank order correlation was used to analyze the pairwise correlation among expressions of proteins and their associations with MKI67 LI status. Overall survival (OS) was defined as from the date of patient’s initial diagnosis to the date of patient death. Survival curves were estimated using the Kaplan-Meier method. Multivariate survival analyses were performed using Cox proportional hazards regression models, which included tumor primary site, sex, age at diagnosis, M stage and MKI67 LI (as a surrogate for grade). Statistical significance was defined as a two-sided P < 0.05. All statistical analyses were conducted by statistician (S.Z.) using SAS software (version 9.4; SAS Institute, Cary, NC).
RESULTS
Clinicopathologic Characteristics
Our cohort included primary tumors from a total of 267 patients with NETs comprising 165 SINETs (61.8%), 26 pancreatic NETs (PNETs) (9.7%), and 76 NETs (28.5%) of other origins (including: appendix, colon, lung, ovary, paraganglioma, parathyroid, pheochromocytoma, rectum, stomach, thymus, and unknown primary) (Table 1). We additionally evaluated 34 paired primary and metastatic lesions. The mean age at diagnosis was 53.2 years (range, 19.5–86.4 years). Eighty-three patients (31.1%) had metastatic disease. Older age, advanced stage (M1), and higher proliferative index MKI67 LI were associated with shorter OS.
TABLE 1.
Clinicopathologic Characteristics of the Patient Cohort and Associations With Overall Survival
| Variable | No. of Patients (%) | No. of Events | Univariate HR |
95% CI | P | Multivariate HR |
95% CI | P |
|---|---|---|---|---|---|---|---|---|
| Age, median (range), y | 53.2 (19.5–86.4) | 71 | 1.067 | 1.044–1.090 | <0.0001 | 1.061 | 1.037–1.085 | <0.0001 |
| Sex | ||||||||
| Male | 131 (49.1) | 36 | 1 | reference | 1 | reference | ||
| Female | 136 (50.9) | 35 | 0.844 | 0.526–1.353 | 0.48 | 0.975 | 0.603–1.575 | 0.92 |
| Primary Site | ||||||||
| SINETs | 165 (61.8) | 47 | 1 | reference | 1 | reference | ||
| PNETs | 26 (9.7) | 8 | 1.344 | 0.633–2.852 | 0.44 | 1.141 | 0.443–2.941 | 0.78 |
| Others | 76 (28.5) | 16 | 0.876 | 0.495–1.548 | 0.65 | 1.123 | 0.536–2.354 | 0.76 |
| MKI67 LI | ||||||||
| <2% | 153 (57.3) | 34 | 1 | reference | 1 | reference | ||
| 3–20% | 98 (36.7) | 28 | 1.115 | 0.675–1.841 | 0.67 | 1.312 | 0.758–2.271 | 0.33 |
| >20% | 15 (5.6) | 9 | 5.312 | 2.495–11.310 | <0.0001 | 6.603 | 2.658–16.401 | <0.0001 |
| Stage | ||||||||
| M0 | 184 (68.9) | 35 | 1 | reference | 1 | reference | ||
| M1 | 83 (31.1) | 36 | 1.914 | 1.197-.060 | 0.007 | 2.055 | 1.097–3.859 | 0.02 |
The multivariate Cox regression adjusted for tumor primary site, age at diagnosis, gender, MKI67 LI and stage.
CI indicates confidence interval; HR, hazard ratio; LI, labeling index; NETs, neuroendocrine tumors; SINETs, small intestinal neuroendocrine tumors; PNETs, pancreatic neuroendocrine tumors; Others, other neuroendocrine tumors including appendix, colon, lung, ovary, paraganglioma, parathyroid, pheochromocytoma, rectum, stomach, thymus, and unknown primary.
Expression of Cell Cycle Regulators in NETs
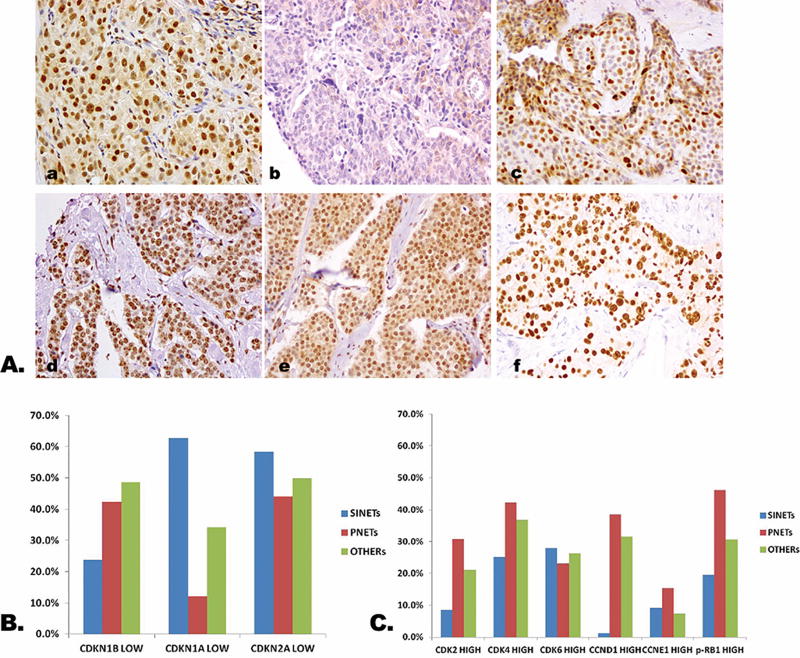
Expression of the cell cycle regulatory pathway proteins was evaluated in all 267 primary tumors and 34 paired metastases. As anticipated CDKN1B, CDKN1A, CDKN2A, CDK2, CDK4, CDK6, CCND1, CCNE1, and phospho-RB1 were detected in cell nucleus (Fig. 1A).
FIGURE 1.
Cell cycle protein expression in NETs. A, Representative expression of cell cycle protein in NETs. a: High CDKN1B expression; b: Low CDKN1B expression; c: High phospho-RB1 expression; d: High CDK4 expression; e: High CDK6 expression; f: High CCND1 expression (×400). B, Expression of inhibitory cell cycle proteins according to tumor subtype. Low expression of CDKN1B was more common in PNETs (42.3%) and other NETs (48.7%) than in SINETs (23.8%). Low expression of CDKN1A was more common in SINETs (62.8%) than in other NETs (34.2%) and PNETs (12.0%). C, Expression of activating cell cycle proteins according to tumor subtype. High expression of phospho-RB1 was more common in PNETs (46.2%) than in other NETs (30.7%) and SINETs (19.6%). High expression of CDK2 and CCND1 was also more common in PNETS (30.8% and 38.5%, respectively) than in SINETs (8.5% and 1.2%, respectively). NETs indicates neuroendocrine tumors; OTHERs, other neuroendocrine tumors including appendix, colon, lung, ovary, paraganglioma, parathyroid, pheochromocytoma, rectum, stomach, thymus, and unknown primary; p-, phospho; P, phosphorylated; PNETs, pancreatic neuroendocrine tumor; SINETs, small intestinal neuroendocrine tumors.
Expression patterns varied to some extent according to tumor subtype. High expression of phospho-RB1, for example, was more common in PNETs (46.2%) than in other NETs (30.7%) and SINETs (19.6%). High expression of other activating components, including CDK2 and CCND1 was also more common in PNETS (30.8% and 38.5%, respectively) than in SINETs (8.5% and 1.2%, respectively) (Fig. 1B). Low expression of the inhibitory protein CDKN1B was more common in PNETs (42.3%) and other NETs (48.7%) than in SINETs (23.8%). In contrast, low expression of CDKN1A was more common in SINETs (62.8%) than in other NETs (34.2%) and PNETs (12.0%) (Fig. 1C). We did not find statistically significant differences in expression of any of the markers when we compared expression between 34 pairs of primary tumors and matched metastases.
Mutation of CDKN1B in SINETs
Based on prior reports of CDKN1B mutations in SINETs, we examined potential associations between mutational status and immunohistochemical expression. CDKN1B mutation data was available in 55 SINET samples, from 52 patients. Samples included 42 cases with only primary tumors, 6 cases from 3 patients with primary tumor and their matched metastasis, and 7 cases with only metastatic lesions. Somatic mutation of CDKN1B was present in 6 samples from 6 patients, comprising 5 primary tumors and 1 unmatched metastatic lesion. CDKN1B mutation was not clearly associated with protein expression: 3/6 CDKN1B-mutated SINETs demonstrated CDKN1B low expression and 12/48 CDKN1B-wildtype SINETs demonstrated low expression (Fisher's exact test P = 0.21). CDKN1B mutation in SINETs was not associated with survival.
Intra-pathway Correlations Between Cell Cycle Regulators Expression
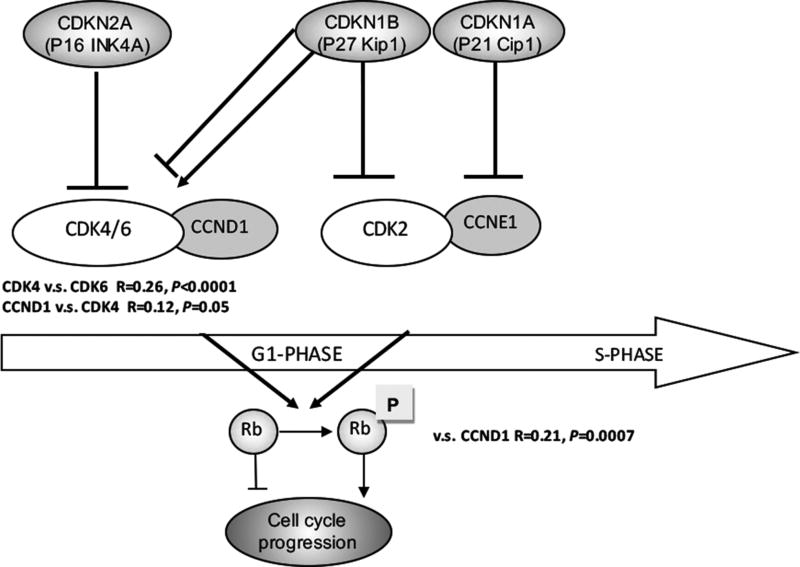
We observed strong intra-pathway associations between regulatory proteins in the CDK4/CDK6-CCND1-RB1 pathway. In this pathway, the CDK4/CDK6 complex is known to phosphorylate CCND1, which in turn phosphorylates RB1. We identified an association between expression of phospho-RB1 and CCND1(R = 0.21, P = 0.0007). Expression of CCND1 was in turn associated with expression of CDK4 (R = 0.12, P = 0.05), which was associated with expression of CDK6 (R = 0.26, P < 0.0001). We did not identify associations between expression of phospho-RB1 and CCNE1 or CDK2, nor did we identify associations between expression of phospho-RB1 and the cyclin dependent kinase inhibitors CDKN1B, CDKN2A, or CDKN1A (Fig. 2).
FIGURE 2.
Association between expression of cell cycle proteins in NETs according to pathway. Raw immunoreactivity scores were used for correlation analysis. CCND1 expression was positively correlated with expression of CDK4 (R = 0.12, P = 0.05) and phospho-RB1 (R = 0.21, P = 0.0007). CDK6 expression was positively correlated with expression of CDK4 (R = 0.26, P < 0.0001). NETs indicates neuroendocrine tumors; P, phosphorylated.
Correlations Between Expression of Cell Cycle Regulators and MKI67
Expression of regulatory proteins in the CDK4/CDK6-CCND1-phospho-RB1 pathway was associated with tumor proliferative index. The strongest association with proliferative index was observed with expression of phospho-RB1 (P < 0.0001) (Table 2). We additionally found that high expression of CDK4, CDK6, and CCND1was associated with higher proliferative index (MKI67 expression). Low expression of the cyclin dependent kinase inhibitor CDKN1B also appeared to be more somewhat more common in higher grade tumors, though the difference was not statistically significant after multiple testing adjustments. Expression of CDK2 and CCNE1were not associated with tumor grade.
TABLE 2.
Associations Between Expression of Cell Cycle Proteins and MKI67 LI in NETs
| No. of Positive Cases (%) According to MKI67 LI | |||||
|---|---|---|---|---|---|
|
|
|||||
| Variable | G1 (N = 153) | G2 (N = 98) | G3 (N = 15) | Total (N = 266) | P |
| CDKN1B | 110 (72.4) | 60 (61.2) | 8 (53.3) | 178 (67.2) | 0.03 |
| CDKN1A | 70 (46.4) | 52 (53.1) | 10 (66.7) | 132 (50.0) | 0.13 |
| CDKN2A | 74 (49.3) | 41 (41.8) | 5 (33.3) | 120 (45.6) | 0.14 |
| CDK2 | 20 (13.1) | 13 (13.3) | 4 (26.7) | 37 (13.9) | 0.48 |
| CDK4 | 30 (19.9) | 43 (43.9) | 7 (46.7) | 80 (30.3) | <0.0001 |
| CDK6 | 32 (21.1) | 36 (36.7) | 4 (26.7) | 72 (27.2) | 0.02 |
| CCND1 | 11 (7.2) | 19 (19.4) | 6 (40.0) | 36 (13.5) | <0.0001 |
| CCNE1 | 10 (6.5) | 10 (10.2) | 3 (20.0) | 23 (8.6) | 0.1 |
| Phospho-RB1 | 16 (10.5) | 42 (43.8) | 9 (60) | 67 (25.5) | <0.0001 |
Association Between Expression of Cell Cycle Regulators and Clinical Outcomes
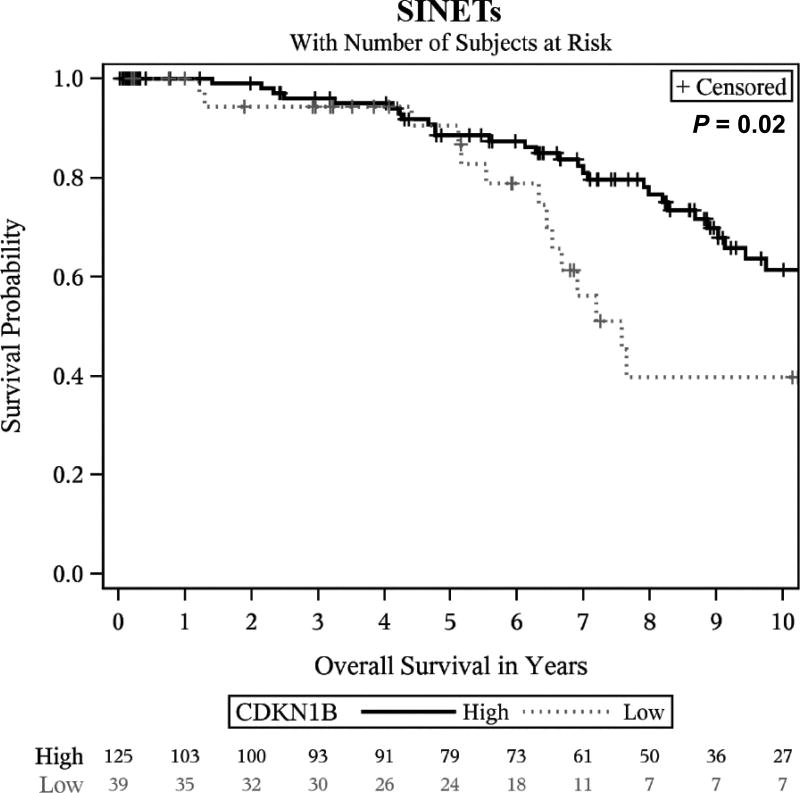
We next evaluated whether expression of cell cycle markers had prognostic significance. Among the cohort of 267 patients with evaluable primary tumors, 71 patients died with a median follow-up time of 6 years. In a multivariate analysis, only low expression of CDKN1B showed a potential association with shorter OS (HR, 1.72; 95% CI, 1.02–2.9; P = 0.04) after multiple testing adjustment. The association between survival and low expression of CDKN1B appeared to be strongest in SINETs (HR, 2.04; 95% CI, 1.06–3.93; P = 0.03; Fig. 3); Clear survival associations were not observed in other tumor subtypes.
FIGURE 3.
Overall survival in patients with SINETs according to CDKN1B expression. Survival curves were estimated using the Kaplan-Meier method, and statistical significance was defined as a two-sided P < 0.05. Low expression of CDKN1B was associated with shorter survival in SINETs (P = 0.02). SINETs indicates small intestinal neuroendocrine tumors.
DISCUSSION
In this study, we performed a comprehensive analysis of cell cycle protein expression in a large cohort of NETs. We found that high expression of activating proteins in the CDK4/CDK6-CCND1− phospho-RB1 pathway were strongly correlated with each other and were also associated with proliferative index. We observed a trend toward shorter overall survival and loss of CDKN1B expression, which appeared to be strongest in SINETs.
Our findings suggest that activation of the CDK4/CDK6-CCND1 pathway, with subsequent phosphorylation of RB1, may play a role in driving proliferation in NETs. Our observations are consistent with other studies, which have found that high expression of phospho-RB1 is associated with an increasing degree of tumor aggressiveness in a variety tumors, including pancreatic adenocarcinoma,39 breast and ovarian cancer,40 synovial sarcoma,41 head and neck squamous cell carcinoma.12 High expression of CDK4/CDK6 and/or high expression of CCND1 were also found to be associated with more aggressive behavior and/or poor survival in breast cancer,42,43 primary ovarian serous carcinomas,13 papillary carcinoma,44 meningiomas,45 periampullary adenocarcinoma,14 extremity soft-tissue sarcomas,15 laryngeal squamous cell carcinomas,46 esophageal adenocarcinoma,47 and lung adenocarcinoma.16
While studies are limited, our findings are consistent with previous observations in NETs. Tang et al found a correlation between high expression of phospho-RB1 and high expression of both CDK4 (R = 0.55; P = 0.01) and CCND1 (R = 0.51; P = 0.03) in 92 PNETs. In this study, the growth of two pancreatic neuroendocrine tumor cell lines was inhibited with a CDK4/CDK6 inhibitor.24 Taken together with this prior study, our observations suggest that NETs with high- or intermediate-grade may have an activated CDK4/CDK6-phospho-RB1 pathway and may therefore be candidates for treatment with CDK4/CDK6 inhibitors.
A second observation in our study was a trend toward decreased overall survival in patients with decreased CDKN1B, particularly in SINETS. CDKN1B encodes a cyclin-dependent kinase inhibitor that binds to and suppresses cyclin-CDK complexes, including cyclin E −CDK2 and cyclin D −CDK4/CDK6, thereby interrupting phosphorylation of the retinoblastoma protein 1/E2F pathway and arresting tumor cells in G1 phase.9,11,48,49 Mutations in CDKN1B have been reported in SINETs, but not in other neuroendocrine tumor subtypes, suggesting that CDKN1B may play a particularly important role in this neuroendocrine tumor subtype.
We did not observe a clear association between mutation of CDKN1B and protein expression, an observation that is consistent with a prior study, which similarly did not find correlation between expression of CDKN1B with CDKN1B mutation in 200 patients with SINETs. We did, however, observe a trend toward decreased overall survival and loss of CDKN1B expression. Our observation is consistent with observations in other malignancies,49 as well as in other recent studies in gastroenteropancreatic NETs (GEP-NETs).22,23 In the largest of these studies on NETs, loss of CDKN1B expression was also found to be a predictor of poor prognosis in total 327 patients with GEP-NETs.22 Interestingly, in our cohort, the association appeared to be strongest in SINETS.
Limitations of our study include retrospective nature and use of paraffin-embedded tissue to evaluate protein expression using IHC. Due to lack of uniformity in IHC staining protocols and scoring cutoff values for each marker, the rates of CDK4/CDK6-CCND1-phospho-RB1 overexpression in NETs may be different in different studies. In addition, protein expression alone may not reliably predict activation of certain pathway. In an ongoing study of LEE0011 (a high selective CDK4/CDK6 inhibitor) in patients with CDK4/CDK6 pathway activated tumors, having a tumor with CDK4/CDK6 amplification or mutation, CCND1/CCND3 amplification or CDKN2A mutation was used as one of the important inclusion criteria indicating activation of CDK4/CDK6-CCND1-phospho-RB1 pathway (NCT02187783). Sequencing techniques alone, however, are not suitable for detecting phosphorylation status of specific proteins. A second clinical trial of Palbociclib in metastatic PNET is using IHC to detect overexpression of cell cycle markers (CDK4, and/or phospho-RB1, and/or CCND1) to represent activation of this pathway (NCT02806648).
In conclusion, we have performed a large-scale study evaluating the expression of key cell cycle regulatory proteins in NETs, and correlating expression levels with clinical features and outcomes. Our results are consistent with prior studies suggesting that loss of CDKN1B expression is an adverse prognostic factor in NET, and suggest that loss of CDKN1B expression may play a particularly important role in small intestine NET. Our results further suggest that activation of the CDK4/CDK6-CCND1-phospho-RB1 pathway is associated with higher proliferative index in NETs. Therapeutic agents targeting this pathway, particularly currently available CDK4/CDK6 inhibitors, may have a beneficial role in NETs with higher proliferative index, and clinical studies in this setting are warranted.
Supplementary Material
Acknowledgments
The authors are grateful the Gitta and Saul Kurlat Fund for Neuroendocrine Tumor Research, Jane Dybowski Fund for Neuroendocrine Cancer, McIntyre Family Fund for Neuroendocrine Tumor Research, Lipson Family Fund, Goldhirsh-Yellin Foundation Fund for Neuroendocrine Tumor Research, and the Murphy Family Fund for Carcinoid Tumor Research
Funding: This work was supported by U.S. National Institutes of Health (NIH) grants R01 CA151532 to M.H.K.; P50 CA127003 to C.S.F.; R35 CA197735 to S.O.; and K07 CA190673 to R.N.. This work was also supported by Medical Oncology Translation Grant from the Department of Medical Oncology, Dana-Farber Cancer Institute to Z.R.Q.. Y.S. is supported by the grants from National Natural Science Foundation of China No.81402016, Beijing Municipal Natural Science Foundation No.7152140 and Beijing Nova Program XXJH2015B098. K.K. is supported by a grant from Program for Advancing Strategic International Networks to Accelerate the Circulation of Talented Researchers from Japanese Society for the Promotion of Science. L.L. is supported by a scholarship grant from Chinese Scholarship Council and a fellowship grant from Huazhong University of Science and Technology.
Abbreviations
- CI
confidence interval
- FDA
Food and Drug Administration
- GEP-NETs
gastroenteropancreatic neuroendocrine tumors
- HR
hazard ratio
- LI
labeling index
- MTOR
mechanistic target of rapamycin
- NETs
neuroendocrine tumors
- OS
overall survival
- phospho-RB1
phosphorylated retinoblastoma protein 1
- PNETs
pancreatic neuroendocrine tumors
- SINETs
small intestinal neuroendocrine tumors
- TMA
tissue microarray
- VEGF
vascular endothelial growth factor
Footnotes
Contributions: All authors contributed to review and revision. Study concept and design: Y.S., Z.R.Q., M.H.K. Acquisition of data: all coauthors. Analysis and interpretation of data: Y.S., Z.R.Q., S.Z., C.S.F., S.O., M.H.K.. Manuscript writing: Y.S., Z.R.Q., M.H.K. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: Y.S., Z.R.Q., S.Z.. Final approval of manuscript: all authors. Study supervision: Z.R.Q., S.O., M.H.K..
Use of standardized official symbols: We use HUGO (Human Genome Organisation)-approved official symbols for genes and gene products; including CDKN1B, CDKN1A, CDKN2A, CDK2, CDK4, CDK6, CCND1, CCNE1, RB1 and MKI67; all of which are described at www.genenames.org. Gene names are italicized, and gene product names are non-italicized.
Disclosure: The authors have declared no conflicts of interest.
References
- 1.Yao JC, Hassan M, Phan A, et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 2.Vinik AI, Woltering EA, Warner RR, et al. NANETS consensus guidelines for the diagnosis of neuroendocrine tumor. Pancreas. 2010;39:713–734. doi: 10.1097/MPA.0b013e3181ebaffd. [DOI] [PubMed] [Google Scholar]
- 3.Narayanan S, Kunz PL. Role of Somatostatin Analogues in the Treatment of Neuroendocrine Tumors. Hematol Oncol Clin North Am. 2016;30:163–177. doi: 10.1016/j.hoc.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yao JC, Fazio N, Singh S, et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet. 2016;387:968–977. doi: 10.1016/S0140-6736(15)00817-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pavel ME, Hainsworth JD, Baudin E, et al. Everolim us plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet. 2011;378:2005–2012. doi: 10.1016/S0140-6736(11)61742-X. [DOI] [PubMed] [Google Scholar]
- 7.Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501–513. doi: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]
- 8.Yao JC, Lagunes DR, Kulke MH. Targeted therapies in neuroendocrine tumors (NET): clinical trial challenges and lessons learned. Oncologist. 2013;18:525–532. doi: 10.1634/theoncologist.2012-0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santo L, Siu KT, Raje N. Targeting Cyclin-Dependent Kinases and Cell Cycle Progression in Human Cancers. Semin Oncol. 2015;42:788–800. doi: 10.1053/j.seminoncol.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 10.Burkhart DL, Sage J. Cellular mechanisms of tumour suppression by the retinoblastoma gene. Nat Rev Cancer. 2008;8:671–682. doi: 10.1038/nrc2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du W, Searle JS. The rb pathway and cancer therapeutics. Curr Drug Targets. 2009;10:581–589. doi: 10.2174/138945009788680392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beck TN, Kaczmar J, Handorf E, et al. Phospho-T356RB1 predicts survival in HPV-negative squamous cell carcinoma of the head and neck. Oncotarget. 2015;6:18863–18874. doi: 10.18632/oncotarget.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bilyk OO, Pande NT, Buchynska LG. Analysis of p53, p16(INK4a), pRb and Cyclin D1 expression and human papillomavirus in primary ovarian serous carcinomas. Exp Oncol. 2011;33:150–156. [PubMed] [Google Scholar]
- 14.Tomazic A, Pegan V, Ferlan-Marolt K, Pleskovic A, Luzar B. Cyclin D1 and bax influence the prognosis after pancreatoduodenectomy for periampullary adenocarcinoma. Hepatogastroenterology. 2004;51:1832–1837. [PubMed] [Google Scholar]
- 15.Kim SH, Lewis JJ, Brennan MF, Woodruff JM, Dudas M, Cordon-Cardo C. Overexpression of cyclin D1 is associated with poor prognosis in extremity soft-tissue sarcomas. Clin Cancer Res. 1998;4:2377–2382. [PubMed] [Google Scholar]
- 16.Lee E, Jin D, Lee BB, et al. Negative effect of cyclin D1 overexpression on recurrence-free survival in stage II–IIIA lung adenocarcinoma and its expression modulation by vorinostat in vitro. BMC Cancer. 2015;15:982. doi: 10.1186/s12885-015-2001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wander SA, Zhao D, Slingerland JM. p27: a barometer of signaling deregulation and potential predictor of response to targeted therapies. Clin Cancer Res. 2011;17:12–18. doi: 10.1158/1078-0432.CCR-10-0752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Leary B, Finn RS, Turner NC. Treating cancer with selective CDK4/6 inhibitors. Nat Rev Clin Oncol. 2016;13:417–430. doi: 10.1038/nrclinonc.2016.26. [DOI] [PubMed] [Google Scholar]
- 19.Francis JM, Kiezun A, Ramos AH, et al. Somatic mutation of CDKN1B in small intestine neuroendocrine tumors. Nat Genet. 2013;45:1483–1486. doi: 10.1038/ng.2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maxwell JE, Sherman SK, Li G, et al. Somatic alterations of CDKN1B are associated with small bowel neuroendocrine tumors. Cancer Genet. 2015 doi: 10.1016/j.cancergen.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karpathakis A, Dibra H, Pipinikas C, et al. Prognostic Impact of Novel Molecular Subtypes of Small Intestinal Neuroendocrine Tumor. Clin Cancer Res. 2016;22:250–258. doi: 10.1158/1078-0432.CCR-15-0373. [DOI] [PubMed] [Google Scholar]
- 22.Kim HS, Lee HS, Nam KH, Choi J, Kim WH. p27 Loss Is Associated with Poor Prognosis in Gastroenteropancreatic Neuroendocrine Tumors. Cancer Res Treat. 2014;46:383–392. doi: 10.4143/crt.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grabowski P, Schrader J, Wagner J, et al. Loss of nuclear p27 expression and its prognostic role in relation to cyclin E and p53 mutation in gastroenteropancreatic neuroendocrine tumors. Clin Cancer Res. 2008;14:7378–7384. doi: 10.1158/1078-0432.CCR-08-0698. [DOI] [PubMed] [Google Scholar]
- 24.Tang LH, Contractor T, Clausen R, et al. Attenuation of the retinoblastoma pathway in pancreatic neuroendocrine tumors due to increased cdk4/cdk6. Clin Cancer Res. 2012;18:4612–4620. doi: 10.1158/1078-0432.CCR-11-3264. [DOI] [PubMed] [Google Scholar]
- 25.Qian ZR, Ter-Minassian M, Chan JA, et al. Prognostic significance of MTOR pathway component expression in neuroendocrine tumors. J Clin Oncol. 2013;31:3418–3425. doi: 10.1200/JCO.2012.46.6946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fredersdorf S, Burns J, Milne AM, et al. High level expression of p27(kip1) and cyclin D1 in some human breast cancer cells: inverse correlation between the expression of p27(kip1) and degree of malignancy in human breast and colorectal cancers. Proc Natl Acad Sci U S A. 1997;94:6380–6385. doi: 10.1073/pnas.94.12.6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palmqvist R, Stenling R, Oberg A, Landberg G. Prognostic significance of p27(Kip1) expression in colorectal cancer: a clinico-pathological characterization. J Pathol. 1999;188:18–23. doi: 10.1002/(SICI)1096-9896(199905)188:1<18::AID-PATH311>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 28.Valassiadou KE, Stefanaki K, Tzardi M, et al. Immunohistochemical expression of p53, bcl-2, mdm2 and waf1/p21 proteins in colorectal adenocarcinomas. Anticancer Res. 1997;17:2571–2576. [PubMed] [Google Scholar]
- 29.Tada T, Watanabe T, Kazama S, et al. Reduced p16 expression correlates with lymphatic invasion in colorectal cancers. Hepatogastroenterology. 2003;50:1756–1760. [PubMed] [Google Scholar]
- 30.Yamamoto H, Monden T, Ikeda K, et al. Coexpression of cdk2/cdc2 and retinoblastoma gene products in colorectal cancer. Br J Cancer. 1995;71:1231–1236. doi: 10.1038/bjc.1995.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao P, Hu YC, Talbot IC. Expressing patterns of p16 and CDK4 correlated to prognosis in colorectal carcinoma. World J Gastroenterol. 2003;9:2202–2206. doi: 10.3748/wjg.v9.i10.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arber N, Hibshoosh H, Moss SF, et al. Increased expression of cyclin D1 is an early event in multistage colorectal carcinogenesis. Gastroenterology. 1996;110:669–674. doi: 10.1053/gast.1996.v110.pm8608874. [DOI] [PubMed] [Google Scholar]
- 33.Salh B, Bergman D, Marotta A, Pelech SL. Differential cyclin-dependent kinase expression and activation in human colon cancer. Anticancer Res. 1999;19:741–748. [PubMed] [Google Scholar]
- 34.Yasui W, Kuniyasu H, Yokozaki H, Semba S, Shimamoto F, Tahara E. Expression of cyclin E in colorectal adenomas and adenocarcinomas: correlation with expression of Ki-67 antigen and p53 protein. Virchows Arch. 1996;429:13–19. doi: 10.1007/BF00196815. [DOI] [PubMed] [Google Scholar]
- 35.Kulke MH, Siu LL, Tepper JE, et al. Future directions in the treatment of neuroendocrine tumors: consensus report of the National Cancer Institute Neuroendocrine Tumor clinical trials planning meeting. J Clin Oncol. 2011;29:934–943. doi: 10.1200/JCO.2010.33.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 37.Park GC, Lee M, Roh JL, et al. Phospho-Rb (Ser780) as a biomarker in patients with cervical lymph node metastases from an unknown primary tumour: a retrospective cohort study. Clin Otolaryngol. 2013;38:313–321. doi: 10.1111/coa.12138. [DOI] [PubMed] [Google Scholar]
- 38.Sion-Vardy N, Freedman J, Lazarov I, Bolotin A, Ariad S. p27kip1 Expression in non-small cell lung cancer is not an independent prognostic factor. Anticancer Res. 2010;30:3699–3704. [PubMed] [Google Scholar]
- 39.Trevino JG, Verma M, Singh S, et al. Selective disruption of rb-raf-1 kinase interaction inhibits pancreatic adenocarcinoma growth irrespective of gemcitabine sensitivity. Mol Cancer Ther. 2013;12:2722–2734. doi: 10.1158/1535-7163.MCT-12-0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seviour EG, Sehgal V, Lu Y, et al. Functional proteomics identifies miRNAs to target a p27/Myc/phospho-Rb signature in breast and ovarian cancer. Oncogene. 2016;35:691–701. doi: 10.1038/onc.2014.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vlenterie M, Hillebrandt-Roeffen MH, Schaars EW, et al. Targeting Cyclin-Dependent Kinases in Synovial Sarcoma: Palbociclib as a Potential Treatment for Synovial Sarcoma Patients. Ann Surg Oncol. 2016;23:2745–2752. doi: 10.1245/s10434-016-5341-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dai M, Zhang C, Ali A, et al. CDK4 regulates cancer stemness and is a novel therapeutic target for triple-negative breast cancer. Sci Rep. 2016;6:35383. doi: 10.1038/srep35383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aaltonen K, Amini RM, Landberg G, et al. Cyclin D1 expression is associated with poor prognostic features in estrogen receptor positive breast cancer. Breast Cancer Res Treat. 2009;113:75–82. doi: 10.1007/s10549-008-9908-5. [DOI] [PubMed] [Google Scholar]
- 44.Pesutic-Pisac V, Punda A, Gluncic I, Bedekovic V, Pranic-Kragic A, Kunac N. Cyclin D1 and p27 expression as prognostic factor in papillary carcinoma of thyroid: association with clinicopathological parameters. Croat Med J. 2008;49:643–649. doi: 10.3325/cmj.2008.5.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alama A, Barbieri F, Spaziante R, et al. Significance of cyclin D1 expression in meningiomas: a preliminary study. J Clin Neurosci. 2007;14:355–358. doi: 10.1016/j.jocn.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 46.Capaccio P, Pruneri G, Carboni N, et al. Cyclin D1 protein expression is related to clinical progression in laryngeal squamous cell carcinomas. J Laryngol Otol. 1997;111:622–626. doi: 10.1017/s0022215100138149. [DOI] [PubMed] [Google Scholar]
- 47.Ismail A, Bandla S, Reveiller M, et al. Early G(1) cyclin-dependent kinases as prognostic markers and potential therapeutic targets in esophageal adenocarcinoma. Clin Cancer Res. 2011;17:4513–4522. doi: 10.1158/1078-0432.CCR-11-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bretones G, Delgado MD, Leon J. Myc and cell cycle control. Biochim Biophys Acta. 2015;1849:506–516. doi: 10.1016/j.bbagrm.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 49.Chu IM, Hengst L, Slingerland JM. The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat Rev Cancer. 2008;8:253–267. doi: 10.1038/nrc2347. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.